Highlights
-
•
Analysis of biorepository of craniopharyngioma data shows robust genomic sequencing resources, but lack of information on functional and quality of life (QoL) outcomes.
-
•
Patient and family survey demonstrates treatment and outcome priorities at time of initial diagnosis and recurrence.
-
•
Patients and families suggest prioritization of more effective prospective treatments that may improve quality-of-life even if increased risk of recurrence.
Keywords: Craniopharyngioma, Quality of life, Molecular sequencing, Neuroendocrine
Abstract
Introduction
Craniopharyngioma is a rare, low-grade tumor located in the suprasellar region of the brain, near critical structures like the pituitary gland. Here, we concurrently investigate the status of clinical and genomic data in a retrospective craniopharyngioma cohort and survey-based data to better understand patient-relevant outcomes associated with existing therapies and provide a foundation to inform new treatment strategies.
Methods
Clinical, genomic, and outcome data for a retrospective cohort of patients with craniopharyngioma were collected and reviewed through the Children's Brain Tumor Network (CBTN) database. An anonymous survey was distributed to patients and families with a diagnosis of craniopharyngioma to understand their experiences throughout diagnosis and treatment.
Results
The CBTN repository revealed a large proportion of patients (40 – 70%) with specimens that are available for sequencing but lacked relevant quality of life (QoL) and functional outcomes. Frequencies of reported patient comorbidities ranged from 20 to 25%, which is significantly lower than historically reported. Survey results from 159 patients/families identified differences in treatment considerations at time of diagnosis versus time of recurrence. In retrospective review, patients and families identified preference for therapy that would improve QoL, rather than decrease risk of recurrence (mean 3.9 vs. 4.4 of 5) and identified endocrine issues as having the greatest impact on patients’ lives.
Conclusions
This work highlights the importance of prospective collection of QoL and functional metrics alongside robust clinical and molecular correlates in individuals with craniopharyngioma. Such comprehensive measures will facilitate biologically relevant therapeutic strategies that also prioritize patient needs.
Introduction
Craniopharyngioma is a rare, benign tumor located in the sellar/parasellar region with low histological grade (WHO 1). The tumors are classified histopathologically into two distinct subtypes: the adamantinomatous subtype, which occurs in pediatric and adult populations, and the papillary subtype, which occurs almost exclusively in the adult population between ages 60-70. The adamantinomatous subtype is characterized by molecular mutations in exon 3 of the β-catenin gene, while the papillary subtype most frequently carries alterations in BRAF V600E [1]. The tumor develops in about 0.5-2 individuals per million, representing 2–5% of all pediatric central nervous system (CNS) tumors [2].
Although histologically benign and slow growing, craniopharyngiomas are particularly challenging to treat because of a balancing act between high rates of recurrence and a location in the brain closely adjacent to delicate and extremely important structures like the optic chiasm and hypothalamus. Because of this, there is historic and ongoing variability and lack of agreement on the most effective and safe treatments for craniopharyngioma, and thus it currently lacks a “gold standard” approach. Historically, initial treatment has prioritized resection to the greatest extent possible, with the goal of alleviating symptoms and avoiding recurrence. However, studies have shown that aggressive gross total resection (GTR) carries a high risk of damage to adjacent brain structures, resulting in significant and chronic complications, most commonly affecting vision and neuroendocrine function [3], [4], [5]. Radiotherapy, as an adjuvant or alternative to surgery, is associated with similar risk of pituitary damage, as well as added complications such as vasculopathy, radiation-induced malignancy, and heightened risk of recurrence [6], [7], [8]. Given these risks, there have been efforts to transition to more targeted treatment approaches that decrease the possibility of damage and associated complications. For example, endoscopic endonasal surgery (EES) offers a less invasive surgical alternative to the typical transcranial approach with potential for decreased mortality and morbidity [9]. In addition, intracystic therapies including interferon therapies and minimally invasive reservoir insertion provide plausible options for cystic shrinkage while minimizing neuroendocrine injury [11]. While these less-invasive therapies may improve event free survival (EFS) and reduce disease progression for certain tumors, transcranial surgeries remain necessary in more advanced tumors with vascular involvement [10], [11], [12].
With any of these treatment approaches, craniopharyngiomas are associated with high rates of visual, endocrine, neurologic and psychological complications. Neuroendocrine injury is commonly exacerbated in patients following surgical resection and leads to considerably high rates of deficiencies in growth hormone, thyroid stimulating hormone, glucocorticoid, and vasopressin that commonly require some form of lifelong hormone replacement [13]. Hypothalamic involvement of the tumor can lead to hypothalamic syndrome and/or hypothalamic obesity, depending on the degree of damage to the hypothalamus from the tumor or treatments [14]. Along with these complications, patients often face obesity and weight regulation issues that are recalcitrant to treatment [15,16]. These tumor-related side effects have appropriately received increasing attention and consideration in treatment, given the significant potential impact on immediate and long-term quality of life (QoL) of patients. In select studies that have followed craniopharyngioma survivors for 10-20 years after treatment, patients scored significantly lower on standardized surveys assessing QoL. Specific issues include impacts on physical issues such as vision, weight, and hormone changes; social and behavioral concerns such as attention and learning difficulties, difficulty with peer relationships; and, challenges with emotional regulation, physical appearance, and body image [17], [18], [19].
With the risks of available treatment options and the severity of long-term clinical deficits, patients and families are often faced with a variety of uncertainties and challenging decisions regarding treatment. Recent work has focused on multi-institutional efforts to examine trends in diagnosis, treatment and survival of pediatric patients. The Children's Brain Tumor Network (CBTN) is a consortium consisting of over 25 domestic and international institutions that provides a biorepository of clinical, histological, and genomic data for pediatric patients diagnosed with CNS tumors. This consortium contributes to large public, NIH-supported databases and is a rich resource for researchers. The Pacific Pediatric Neuro-Oncology Consortium (PNOC) similarly offers an international clinical trial consortium dedicated to precision-based medicine approaches for pediatric CNS tumors and with collection of clinical data and associated biospecimens. In 2019, PNOC and CBTN joined efforts to establish a craniopharyngioma working group to assess gaps in treatment/outcomes of patients and develop novel therapy strategies. Initial work looked at existing genomic and clinical data in the CBTN cohort, while attaining patient and family survey data in parallel. In consideration of both clinical and patient-centric outcomes, we present the results of this preliminary review, with a focus on potential opportunities for improvements in the data collection and development of treatment strategies through the lens of challenges faced by patients and families with pediatric craniopharyngioma. The outcomes of these separate analyses are integrally intertwined and provide valuable insight into prospective advances in management and data collection that can translate to improved outcomes.
Methods
This report consists of two components: analysis of an existing pediatric craniopharyngioma patient cohort from the CBTN database and a survey of patients and families affected by pediatric craniopharyngioma. All work was done according to institutional regulatory board approval procedures and when appropriate, patients and/or legal representatives were consented prior to inclusion. CBTN data was accessioned under Children's Hospital of Philadelphia (C)OP IRB 09-007316 and survey data was collected under CHOP IRB 20-018189.
CBTN cohort
In December of 2020, the CBTN biorepository was queried and identified a total of 124 patients with a primary diagnosis of craniopharyngioma enrolled between 2012-2020. From this cohort, data variables including demographics, details on initial diagnosis and progression(s) (new tumor growth after a partial resection) or recurrence(s) (new tumor growth following a complete resection), treatments, and survival data (event-free survival [EFS] and OS) were collected.
Any patients with tumor location and/or pathology that were inconsistent with a diagnosis of craniopharyngioma (i.e., location outside of the suprasellar/midline structures) underwent individual review. In these cases, source documentation for pathology and imaging were reviewed by two study team members (FM, CK) to confirm discrepancies with craniopharyngioma and determine appropriateness for patient inclusion in the cohort.
Survey development & distribution
In Fall 2020, an anonymous REDCap (Research Electronic Data Capture) survey for patients and families was developed to gather data about patient and family experiences of those affected by pediatric craniopharyngioma (CK, FM, SM, JC in collaboration with a family-based foundation focused on pituitary tumors, Raymond A Wood Foundation [RAWF]). The survey was divided into four sections: (1) patient experience at the time of diagnosis; (2) patient experience after diagnosis and long-term; (3) current hormonal and/or metabolic problems; and (4) tumor recurrence. Complication data were collected using a self-report approach, which aligns with previously validated sleep complication data collection in craniopharyngioma [20].
The survey was restricted to respondents 18 years or older, including adult survivors of pediatric craniopharyngioma, adult patients with craniopharyngioma, and adult caregivers of patients with pediatric craniopharyngioma. It was distributed and advertised on social media, on foundation and consortia websites (RAWF, Dragon Master's Foundation, CBTN), and in electronic or paper format via mail collection at a single site (British Columbia Children's Hospital). The purpose, risks, benefits, consent and confidentiality of the study were explained on the welcome page before participants entered the survey. Survey responses were collected between April 28 to July 1, 2021.
Statistical analyses
CBTN Cohort – Summary statistics were utilized to review clinical and genomic data available in the CBTN cohort. Chi-Square Paired Tests and unpaired t-tests were used to compare the frequency of reported functional outcomes and complications in the CBTN cohort to previously reported frequencies in published studies.
Survey Cohort – Binary (yes/no) responses, multi-choice, or Likert scale questions were collected within the survey, dependent of the type of information being queried (supplemental methods). Likert-type scales were utilized to determine degree of concern or impact on patient and family, i.e., level of concern (0 = no concern, 5 = very concerned) and level of importance (0 = no importance, 5 = very important) at different timepoints for tumor and treatment parameters.
Where applicable, an alpha of 0.05 to determine statistical significance was utilized. SAS 9.4 [21] or R 4.1 [22] were used for all statistical analyses.
Results
CBTN cohort highlights availability of molecular resources balanced with gaps in critical QoL and functional outcomes
Of the 124 patients with primary craniopharyngioma, (median age 7.7 years, 56% female, age range 1-24 years), 10 were excluded due to inconclusive craniopharyngioma diagnosis based on review of pathology source documents. An additional 39 patients were excluded from analysis for lack of documented follow-up data, leaving a total of 75 patients to include in analyses (Fig. 1). Twenty-eight of the 75 (37%) had a documented pathology classification of adamantinomatous subtype, with the remaining 47 (63%) documented as “craniopharyngioma” with unspecified subtype. Seventy-four (99%) patients received surgery, 26 (35%) received radiation, and 4 (5%) received chemotherapy at some point in their treatment course. At time of diagnosis, 45 (60%) had a gross/near total resection and 24 (32%) initially underwent partial surgery. Of those that had partial resection, 8 later required gross/near total resection due to tumor recurrence or progression. Twenty-six (35%) patients in the cohort reported requiring multiple surgeries with some undergoing up to 4 operations throughout their treatment course. Over half of this craniopharyngioma cohort experienced a tumor recurrence (n = 22, 29%) or progression (n = 17, 23%). Median event-free survival (defined as time to progression, recurrence, or death) was 1.8 years.
Fig. 1.
Flowchart depicting available clinical and demographic data of the craniopharyngioma patient cohort from CBTN.
Many of the patients in this cohort were listed as having associated biospecimen collections that were eligible for genomic sequencing, including proteomics, single cell-, RNA- and whole genome sequencing. However, these analyses were infrequently completed and/or reported through CBTN. Notably, only 9 of 29 eligible patients had single cell sequencing completed, 21 of 47 eligible patients had RNA sequencing completed, and 26 of 41 eligible patients had WGS completed. Most patients had genomic sequencing available or had associated biospecimen collections that were eligible for genomic sequencing (Fig. 1). Thirty-seven patients (37%) lacked RNA sequencing data and 33 (44%) lacked whole genome sequencing data. Of these, 26 (70%) were eligible for RNA sequencing and 15 (45%) were eligible for whole genome sequencing. Twenty-two patients (29%) had paired initial and recurrent tumor samples available for potential or available sequencing (Fig. 1).
There was also a lack of data related to functional and QoL outcomes in the CBTN craniopharyngioma patient cohort. The database includes information on patients’ other medical conditions at time of initial diagnosis, including those secondary to tumor (i.e., visual deficits, endocrinopathies, focal neurologic deficits, and hydrocephalus). However, 29 of 75 (39%) patients were missing documented medical comorbidities, being listed as "unavailable," "none documented," or "other medical condition, not otherwise specified" in the database. No additional details were available on the type of other medical condition(s) for this categorization.
Of the 46 patients with one or more documented comorbidities, visual deficits were the most prevalent (n = 24), followed by neurologic conditions (n = 21) and neuroendocrine conditions (n = 20), all present at time of diagnosis (Table 1). An additional 12 patients were listed as having some other medical condition that was not further specified. The frequency of reported comorbidities in the CBTN cohort was much less than previously reported in published literature when compared to previous reports. Frequencies of comorbidities in the CBTN cohort ranged from 27-30% (visual: 32%; neuroendocrine: 27%; neurologic: 28%), while those reported in prior publications range from 50% to greater than 90%, depending on the specific comorbidity (Table 1). Furthermore, for these patients with documented comorbidities, the severity and change with any progressions, recurrences, or treatments is not adequately detailed in the CBTN database.
Table 1.
Proportions of patients with medical comorbidities. The proportion of patients with reported visual, neuroendocrine, and/or neurologic comorbidities (n = 66) as compared to historically reported proportions of medical comorbidities in previously published studies (p < 0.001).
| Comorbities | CBTN (% reported comorbidities) | Previous Analyses | Previous Analyses (% reported comorbidities) |
|---|---|---|---|
| Visual | 32% | Wijnen et al. [36] | 78% |
| Tan et al. [37] | 71% | ||
| Gautier et al. [38] | 75% | ||
| Lo et al. [39] | 80% | ||
| Neuroendocrine | 27% | Wijnen et al.[36] | 92% |
| Tan et al. [37] | 94% | ||
| Gautier et al. [38] | 64% | ||
| Lo et al. [39] | 87% | ||
| Neurologic | 28% | Porletti et al. [40] | 49% |
| Pereira et al. [41] | 49% | ||
| Zada et al. [42] | 57% |
Survey demographics and initial treatment decision-making
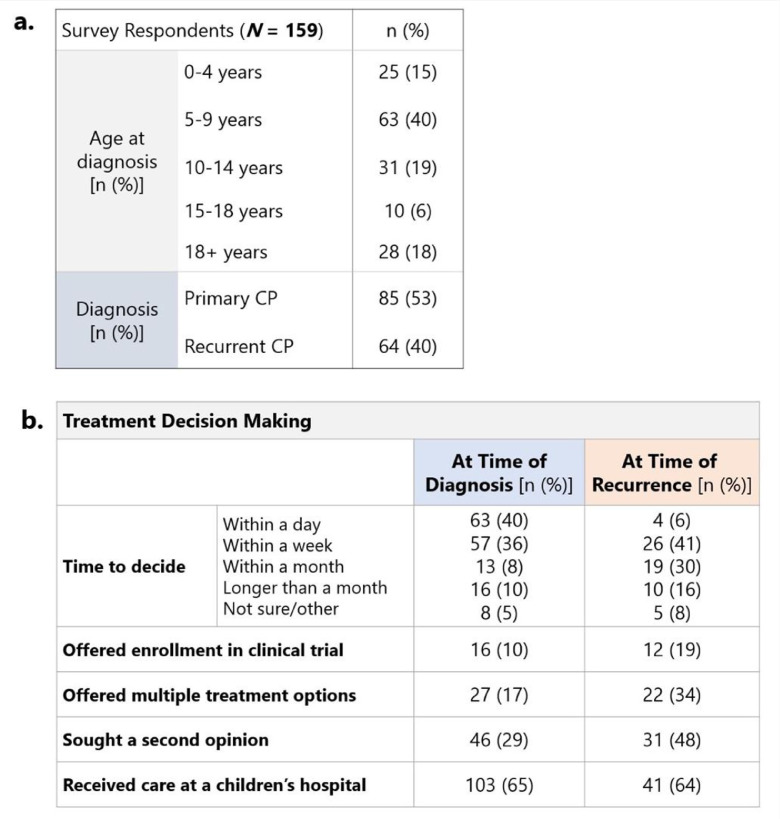
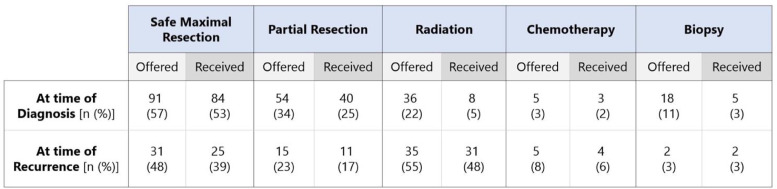
A total of 231 participants initiated the survey and 159 completed the entire form (69% survey completion rate). Most (n = 85, 53%) were cases of primary craniopharyngioma with 40% (n = 64) reporting craniopharyngioma recurrence. The patients were most commonly 5-9 years of age at time of diagnosis (40%), followed by 10-14 years (19%), 18+ years (18%), 0-4 years (15%) and 15-18 years (6%) (Fig. 2a). At time of initial diagnosis, 27 patients (17%) were offered multiple treatment options, while 117 (42%) were offered only one option. Most families (n = 63, 40%) had to make a treatment decision within the same day and 57 (36%) had to make a treatment decision within one week (Fig. 2b). The most common treatment received at time of diagnosis was surgery, with 84 patients (53%) undergoing maximal surgery and 40 (25%) undergoing partial surgery. An additional 8 patients (5%) received radiation and 3 (2%) received chemotherapy as their initial treatment approach (Fig. 3).
Fig. 2.
Demographics of patients and families represented in craniopharyngioma survey. (a) age at diagnosis and type of diagnosis (initial/recurrent) of patients represented in survey (b) responses regarding time to decide on treatment, treatment offerings, treatment considerations, and hospital care at time of diagnosis and at time of recurrence.
Fig. 3.
Proportion of survey respondents that were offered and received different treatments (full resection, partial resection, radiation, chemotherapy, biopsy) at time of initial diagnosis versus time of recurrence. Rows indicate treatment at different timepoints (initial diagnosis versus recurrence) and columns indicate type of treatment offered versus received.
Treatment options offered and received at time of recurrence differed from initial diagnosis
Patients that later experienced a recurrence were most commonly offered maximal or partial surgery at time of initial diagnosis (n = 46, 71%). At time of recurrence, 30 patients (47%) had previously undergone maximal surgery at initial diagnosis, 21 (33%) had undergone partial surgery, and 2 (3%) had received radiation or chemotherapy. For the recurrent tumor, 25 (39%) patients underwent maximal resection and 11 (17%) underwent partial resection. A greater proportion of patients that experienced recurrence were offered and received radiation (55% offered, 48% received), when compared to initial diagnosis (22% offered, 5% received). A greater percentage of patients (34% compared to 17%) were offered multiple treatment options at time of recurrence, when compared to time of initial diagnosis. Turnaround time for treatment decisions at recurrence mostly ranged from a week (n = 26, 41%) to a month (n = 19, 30%). At time of recurrence, a larger portion of patients sought a second opinion (48% compared to 29%) and were offered enrollment in a clinical trial (19% compared to 10%) (Fig. 3).
Factors influencing treatment choices consistent at diagnosis and recurrence
At time of initial diagnosis, participants most frequently reported treatment decisions based on the need for emergent intervention or on recommendations from the medical team. The majority (n = 114, 72%) of participants demonstrated a preference for “targeted treatment specific for craniopharyngioma.” Seventy-one (44%) participants preferred an option with no surgery and 53 (33%) preferred an option with no radiation (Fig. 4a). These responses were selected at similar proportions for patients with a recurrence.
Fig. 4.
Treatment Concerns and Preferences at select timepoints. (a) Reported concerns of patients and families regarding tumor, treatment options, and decision-making at initial diagnosis versus recurrence (b) Treatment considerations that most concerned patients/families at time of initial diagnosis versus recurrence ranked from most concerning (top) to least concerning (bottom).
The majority of patients received care at a dedicated children's hospital, both at time of initial diagnosis (n = 103, 65%) and at time of recurrence (n = 41, 64%) (Fig. 2b). The most frequently selected factors that guided families’ decisions regarding hospital choice were expert care (initial diagnosis 33%, recurrence 69%,) and proximity to home (initial diagnosis 29%, recurrence 33%). At time of recurrence, families also valued comfort with the clinical team (n = 27, 42%). Fifty-six (36%) participants indicated that they needed urgent care, so hospital selection was entirely dependent on the closest option. To gather more information about hospital information, families most frequently used resources provided by the clinical team that diagnosed the patient (n = 59, 38%), as well as online resources and input from family and friends (n = 30, 19%).
When asked about factors affecting treatment choice at time of recurrence, many participants reported relying on the medical team, just as they had at time of diagnosis, but a greater percentage (50% vs. 30%) reported taking into consideration the potential complications related to treatment when making this choice.
Concerns regarding tumor and treatment at time of diagnosis and recurrence
When asked about their most pressing concerns at time of initial diagnosis, participants reported feeling most worried about immediate and long-term side effects related to tumor (n = 122, 77%), followed by immediate and long-term effects related to treatment (n = 100, 63%), and making the right decision with the treatment options offered (n = 90, 57%) (Fig. 4b). Participants were generally less concerned about not having enough treatment options at both time-points (n = 48, 30%).
At time of recurrence, concerns about long-term complications of both tumor and treatment were similarly high. A large proportion (n = 65, 90%) of participants whose child experienced a recurrence reported a 4 to 5 out of 5 level of concern about long-term effects related to the tumor, as well as additional complications of treatment. Other frequently reported concerns at time of recurrence included immediate side effects of tumor (n = 42, 66%) and making the right decisions as far as treatment choices (n = 41, 64%).
Complications of craniopharyngioma tumor growth and treatment
At time of diagnosis, most participants were informed about potential visual and endocrine side effects of both the tumor (n = 93, 59%) and treatment (n = 85, 54%), and reported being most concerned about these effects and effects on neurocognition (n = 86, 54%) (Fig. 5a, b). A significant proportion of the patients (n = 146, 92%) continue to deal with hormonal or metabolic complications from either the tumor or treatment and 80 (50%) participants reported ongoing issues with vision and neurocognition that could be attributed to the tumor, treatment, or a combination of both (Fig. 5c). Of these complications, participants reported that those related to hormones and metabolism continue to have the greatest impact on QoL (n = 141, 89%).
Fig. 5.
Awareness and prioritization of patient and family concerns and patient complications in relation to tumor and therapy. Degree of awareness and concern of reported complications attributed to (a) tumor and (b) treatment. (c) Complications of either tumor or treatment that were reported as most impactful to patient at time of initial diagnosis versus recurrence. (d) Likert ratings of importance of recurrence versus QoL reported by patient/family at time of initial diagnosis, recurrence, and in retrospect to time of diagnosis (1 = not important, 5 = very important).
Participants reported sleep complications including insomnia, obstructive sleep apnea, circadian rhythm sleep disorder, or narcolepsy. Sixty (37%) patients were reported to have one or more of these complications. Overall, 26 patients (16%) had insomnia, 30 patients (19%) had obstructive sleep apnea, 16 patients (10%) had circadian rhythm sleep disorder and 11 patients (7%) had narcolepsy. A considerable proportion of patients with sleep complications reported not being treated for their respective sleep disorders (n = 21, 35%).
For families of children that experienced a tumor recurrence, questions focused on impact of recurrence on any pre-existing long-term effects of either the tumor or the treatment received at diagnosis, as well as the effect of the recurrence and its effect on the child's life. The deficits most affected or amplified by recurrence were neuroendocrine (n = 48, 75%), visual (n = 35, 55%) and neurocognitive deficits (n = 27, 42%). These same deficits were reported to have the greatest impact on the child's immediate and long-term QoL.
Balancing chance of recurrence with long-term QoL
The final section of the survey asked families to rank importance of treatment approach that would best minimize chance of recurrence versus a treatment approach that may have increased recurrence risk but increased potential for better long-term QoL. At time of initial diagnosis and before any treatment, families ranked the two treatment approaches similarly (mean 3.9 of 5). However, at time of recurrence, families reported preference for treatment options that provided potential improved long-term QoL, even if those options carried a higher risk of recurrence (mean 4.4 of 5, Fig. 5d). Similarly, families whose child experienced a recurrence commonly reported that in hindsight, they wished they had placed more importance on long-term QoL at time of initial diagnosis, rather than focusing on minimizing the chance of recurrence (mean = 4.3 of 5).
Discussion
Craniopharyngioma is a complex low-grade tumor with significant recurrence rates and potentially severe detrimental effects on long-term QoL of patients and their families. Many patients suffer from neuroendocrine, visual, and other focal neurologic complications, both from the tumor itself and the current treatment approaches. To explore the effects of these complications on pediatric patients, we concurrently analyzed a cohort from the CBTN database and survey data from patients and families affected by craniopharyngioma. Our goal was to evaluate the state of current data collection and management to provide more context and guidance for future data collection and therapy and clinical trial development.
In our initial work focusing on a CBTN cohort of patients, it was evident that patient samples lacked several measures required for a holistic analysis of individuals with craniopharyngioma. For one, patients had markedly lower levels of reported comorbidities compared to previously published studies. As such, we hypothesize that the data is subject to under-reporting and/or under-documentation of comorbidities in the patient cohort. Such gaps limit predictive capabilities and analysis of clinical, molecular and treatment correlates of functional and QoL measures. We propose such data is routinely and consistently collected on a prospective basis for this patient population. This will allow more reliable correlation between clinical and molecular characteristics, selection of new treatment strategies, and long-term QoL in this patient population that is faced with a high burden of comorbidity [5,13]. In our review of molecular sequencing of craniopharyngioma samples within CBTN, we did confirm availability of a valuable cohort of eligible sequencing data which could provide important foundation to guide future management of craniopharyngioma. Separate work is currently underway to utilize the available molecular sequencing data and complete additional sequencing on eligible samples to augment molecular characterization of the disease and validate previous findings [23]. The CBTN cohort represents one of the largest clinically and molecularly matched pediatric CNS tumor cohorts. This dataset provides unique opportunity to explore the molecular underpinnings of the disease and correlate with clinical characteristics, but even in such a large cohort, our evaluation exemplifies that improvements in data collection are needed to improve prognostication and clinical translation. Our findings provide support for the utilization of existing genomic and clinical data in the CBTN and other publicly available datasets, as well as incorporation of functional and QoL correlates, whenever feasible.
In addition to review of the CBTN database, collection of patient and family survey data also inform on how patient-reported experiences should impact data collection. Survey responses illuminated key differences in patient and family concerns as well as reasoning behind treatment decision-making at time of diagnosis and recurrence. In general, participants were offered fewer treatment options at time of initial diagnosis and demonstrated a tendency to prioritize minimizing the possibility of recurrence, and therefore choose maximal resection as initial treatment. In contrast, participants that experienced a recurrence were offered more conservative radiation-based treatment approaches at a much higher rate, while maximal resection was less commonly offered and chosen. We do note that this could be related to under-reporting, as the overall frequency of radiation therapy is substantially different in this cohort compared to previously reported metrics [24]. Our survey results also indicate that families of patients who experienced a recurrence more frequently considered complications of treatment in decision-making, and generally reported greater concern for acute and long-term complications associated with tumor at time of recurrence. This population more commonly prioritized treatment options that considered future QoL, even if it meant a higher risk of further recurrence. As previously outlined, a large proportion of craniopharyngioma patients face long-term comorbidities associated with craniopharyngioma and these comorbidities undoubtedly impact later decision-making at time of recurrence. Our work shows that patients feel more concerned about the impact of treatment on QoL at time of recurrence than at initial diagnosis, but in retrospect there may be a higher priority on QoL than risk of recurrence at all time points. We advocate that patients and families are provided with all information regarding recurrence, as well as potential treatment complications in the short and long-term, and how those complications might affect future QoL. By providing this information along with multiple different treatment options at time of diagnosis and recurrence, providers may help families more appropriately consider the long-term implications in decision-making. The results also reflect the variability in treatment paradigms at the institutional level and support multidisciplinary conversation between oncologists, neurosurgeons, patients, and families in consideration of the long-term impacts of therapeutic approaches and inclusion of options. Anonymous patient and family survey responses from those affected by craniopharyngioma inform on priorities and concerns of this patient population at both initial diagnosis and time of recurrence. However, we recognize the retrospective nature of the survey and potential components of recall, geographic, and selection bias and/or under-reporting for distant experiences that contribute to variance. Further, patient records were not accessioned to validate or verify reports of comorbidities, complications, and management. To address concerns from retrospective collection, prospective collection is underway within a clinical trial using a combination targeted strategy for children and young adults with newly diagnosed or recurrent craniopharyngioma (PNOC029, NCT05465174). Within the trial will be an exploratory aim to collect self-reported race and ethnicity alongside patient/family experiences and neuroendocrine, visual and functional outcomes. Through self-reported, prospective collection of variables that correlate with social determinants of health combined with functional assessments, we aim to improve generalizability and reliability of findings and data collection.
Separate work has shown the significant neuroendocrine complications faced by patients: hypothyroidism, growth hormone deficiency, hypogonadism, diabetes insipidus, adrenal insufficiency, as well as obesity and obesity-related health problems [25]. The concomitant increase in conservative approaches at recurrence could possibly arise from increased concerns for impact on QoL [26,27]. While more aggressive approaches such as maximal resection may decrease risk of recurrence, these approaches commonly lead to pituitary/hypothalamic dysfunction. Conversely, less conservative approaches like subtotal resection in combination with radiotherapy, EES, or intracystic therapies can minimize pituitary and hypothalamic damage, and thus improve overall QoL in the long-term [[9], [10], [11], [12],[28], [29], [30]]. Our work demonstrates likely under-reporting of both frequency and treatment of functional and QoL outcomes. For example, only 5% of eligible patients in our cohort reported treatment for thyroid deficiency, 47% of patients underwent treatment for growth hormone deficiency and 61% of patients receiving treatment for hypogonadism [25]. Sleep disturbances, too, were self-reported in a significant proportion of patients but only 35% reported treatment for a respective sleep disorder. Reliable collection of such data is imperative to consider in the development of new therapy options and especially given risk of under-reporting in historic cohorts such as the CBTN cohort and other published data [31,32]. As such, it is key to involve endocrinologists, ophthalmologists, and other specialists in multidisciplinary teams in order to tackle this rare disease. Overall, participants indicate a preference for less aggressive treatment strategies which balance long-term impacts on mortality/morbidity, while dually minimizing the likelihood of tumor progression or recurrence may be preferred for craniopharyngioma [1,33]. Select studies have followed craniopharyngioma patients over decades and discovered higher rates of premature death, most commonly due to tumor recurrences, a secondary malignancy from radiation, or complications of acquired comorbidities, for example uncontrolled diabetes mellitus or panhypopituitarism [34,35]. These causes of death are not all obvious, and are hard to predict in initial treatment. However, these data support the long-term impact of craniopharyngioma. On the whole, the data justify consideration of treatment approaches that limit long-term injury and encourages practitioners to offer treatment options that maximize the long-term QoL of patients, even if balanced with some risk of recurrence.
Given the low mortality and high morbidity of craniopharyngioma and based on the data collected in our work, we challenge providers and researchers to consider more effective, risk-adapted approaches to help reduce the long-term visual, neurological, and neuroendocrine complications faced by patients. With the expansion of molecular sequencing methods and publicly available data and sequencing eligible biospecimens, stronger understanding of the molecularly targeted strategies and impact of such treatment strategies on functional and QoL outcomes will be imperative to inform future trials and treatments – requiring reliable collection of these data. Rigorous collection of patient-relevant outcome data in turn will allow for more targeted treatment approaches with decreased morbidity and improved QoL.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Emily Marshall: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing, Visualization. Nikhil Joshi: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing, Visualization. Julia Crowley: Data curation, Project administration, Writing – review & editing. Shana McCormack: Conceptualization, Data curation, Project administration, Writing – review & editing. Sylvia Cheng: Conceptualization, Data curation. Walter Faig: Methodology, Formal analysis. Phillip B. Storm: Data curation, Project administration, Writing – review & editing. Adam Resnick: Data curation, Project administration, Investigation, Software. Sabine Mueller: Conceptualization, Supervision, Writing – review & editing. Fatema Malbari: Conceptualization, Formal analysis, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Visualization. Cassie Kline: Conceptualization, Formal analysis, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Visualization.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
• Raymond A. Wood Foundation
• Dragon Master Foundation
• Kortney Rose Foundation
• Members of PNOC/CBTN Working Group
° Ryan Velasco
° Kamnaa Arya, MS
° Brian Rood, MD
° Todd Hankinson, MD
° Michael DuCuypere, MD, PhD
° Sandi Lam, MD, MBA
° Stewart Goldman, MD
° Lance Ballester
° Michael Prados, MD
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2022.100873.
Appendix. Supplementary materials
References
- 1.Buslei R., Nolde M., Hofmann B., et al. Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol. 2005;109(6):589–597. doi: 10.1007/s00401-005-1004-x. [DOI] [PubMed] [Google Scholar]
- 2.Müller H.L. Craniopharyngioma. Handb. Clin. Neurol. 2014;124:235–253. doi: 10.1016/B978-0-444-59602-4.00016-2. [DOI] [PubMed] [Google Scholar]
- 3.Wang G., Zhang X., Feng M., Guo F. Comparing survival outcomes of gross total resection and subtotal resection with radiotherapy for craniopharyngioma: a meta-analysis. J. Surg. Res. 2018;226:131–139. doi: 10.1016/j.jss.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Akinduro O.O., Izzo A., Lu V.M., et al. Endocrine and visual outcomes following gross total resection and subtotal resection of adult craniopharyngioma: systematic review and meta-analysis. World Neurosurg. 2019;127:e656–e668. doi: 10.1016/j.wneu.2019.03.239. [DOI] [PubMed] [Google Scholar]
- 5.Wan M.J., Zapotocky M., Bouffet E., Bartels U., Kulkarni A.V., Drake JM. Long-term visual outcomes of craniopharyngioma in children. J. Neurooncol. 2018;137(3):645–651. doi: 10.1007/s11060-018-2762-3. [DOI] [PubMed] [Google Scholar]
- 6.Lucas J.T., Jr, Faught A.M., Hsu C.Y., et al. Pre- and posttherapy risk factors for vasculopathy in pediatric patients with craniopharyngioma treated with surgery and proton radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022;113(1):152–160. doi: 10.1016/j.ijrobp.2021.12.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer-Furlan A., Abi-Hachem R., Goksel B., Otero J.J., Carrau R.L., Prevedello D.M. Letter: radiation-induced malignant transformation of craniopharyngiomas. Neurosurgery. 2016;79(2):E313–E315. doi: 10.1227/NEU.0000000000001292. [DOI] [PubMed] [Google Scholar]
- 8.Hamblin R., Vardon A., Akpalu J., et al. Risk of second brain tumour after radiotherapy for pituitary adenoma or craniopharyngioma: a retrospective, multicentre, cohort study of 3679 patients with long-term imaging surveillance. Lancet Diabetes Endocrinol. 2022;10(8):581–588. doi: 10.1016/S2213-8587(22)00160-7. [DOI] [PubMed] [Google Scholar]
- 9.Koutourousiou M., Gardner P.A., Fernandez-Miranda J.C., Tyler-Kabara E.C., Wang E.W., Snyderman C.H. Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J. Neurosurg. 2013;119(5):1194–1207. doi: 10.3171/2013.6.JNS122259. [DOI] [PubMed] [Google Scholar]
- 10.Elliott R.E., Jane J.A., Jr, Wisoff J.H. Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery. 2011;69(3):630–643. doi: 10.1227/NEU.0b013e31821a872d. [DOI] [PubMed] [Google Scholar]
- 11.Lohkamp L.N., Kulkarni A.V., Drake J.M., et al. Preservation of endocrine function after Ommaya reservoir insertion in children with cystic craniopharyngioma. J. Neurooncol. 2022;159(3):597–607. doi: 10.1007/s11060-022-04099-0. [DOI] [PubMed] [Google Scholar]
- 12.Kilday J.P., Caldarelli M., Massimi L., et al. Intracystic interferon-alpha in pediatric craniopharyngioma patients: an international multicenter assessment on behalf of SIOPE and ISPN. Neuro. Oncol. 2017;19(10):1398–1407. doi: 10.1093/neuonc/nox056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z., Zhang S., Hu F. Endocrine disorder in patients with craniopharyngioma. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.737743. Published 2021 Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustig RH. Hypothalamic obesity after craniopharyngioma: mechanisms, diagnosis, and treatment. Front. Endocrinol. (Lausanne) 2011;2:60. doi: 10.3389/fendo.2011.00060. Published 2011 Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller H.L., Merchant T.E., Warmuth-Metz M., Martinez-Barbera J.P., Puget S. Craniopharyngioma. Nat. Rev. Dis. Prim. 2019;5(1):75. doi: 10.1038/s41572-019-0125-9. Published 2019 Nov 7. [DOI] [PubMed] [Google Scholar]
- 16.Yano S., Kudo M., Hide T., et al. Quality of life and clinical features of long-term survivors surgically treated for pediatric craniopharyngioma. World Neurosurg. 2016;85:153–162. doi: 10.1016/j.wneu.2015.08.059. [DOI] [PubMed] [Google Scholar]
- 17.Clark A.J., Cage T.A., Aranda D., Parsa A.T., Auguste K.I., Gupta N. Treatment-related morbidity and the management of pediatric craniopharyngioma: a systematic review. J Neurosurg. Pediatr. 2012;10(4):293–301. doi: 10.3171/2012.7.PEDS11436. [DOI] [PubMed] [Google Scholar]
- 18.Daubenbüchel A.M., Müller HL. Neuroendocrine disorders in pediatric craniopharyngioma patients. J. Clin. Med. 2015;4(3):389–413. doi: 10.3390/jcm4030389. Published 2015 Mar 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sands S.A., Milner J.S., Goldberg J., et al. Quality of life and behavioral follow-up study of pediatric survivors of craniopharyngioma. J. Neurosurg. 2005;103(4 Suppl):302–311. doi: 10.3171/ped.2005.103.4.0302. [DOI] [PubMed] [Google Scholar]
- 20.Manley P.E., McKendrick K., McGillicudy M., et al. Sleep dysfunction in long term survivors of craniopharyngioma. J. Neurooncol. 2012;108(3):543–549. doi: 10.1007/s11060-012-0859-7. [DOI] [PubMed] [Google Scholar]
- 21.SAS Institute Inc. 2013. SAS/ACCESS® 9.4 Interface to ADABAS: Reference. Cary, NC: SAS Institute Inc.
- 22.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A language and environment for statistical computing.https://www.R-project.org/ URL. [Google Scholar]
- 23.Petralia F., Tignor N., Reva B., et al. Integrated proteogenomic characterization across major histological types of pediatric brain cancer. Cell. 2020;183(7):1962–1985.e31. doi: 10.1016/j.cell.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zacharia B.E., Bruce S.S., Goldstein H., Malone H.R., Neugut A.I., Bruce JN. Incidence, treatment and survival of patients with craniopharyngioma in the surveillance, epidemiology and end results program. Neuro. Oncol. 2012;14(8):1070–1078. doi: 10.1093/neuonc/nos142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craven M., Crowley J.H., Chiang L., et al. A survey of patient-relevant outcomes in pediatric craniopharyngioma: focus on hypothalamic obesity. Front. Endocrinol. (Lausanne) 2022;13 doi: 10.3389/fendo.2022.876770. Published 2022 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clusmann H., Höllig A. Craniopharyngioma: the benefits of a conservative approach. Dtsch. Arztebl. Int. 2019;116(18):319–320. doi: 10.3238/arztebl.2019.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenfeld A., Pekmezci M., Barnes M.J., et al. The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J. Neurooncol. 2012;108(1):133–139. doi: 10.1007/s11060-012-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen M., Bartels U., Branson H., Kulkarni A.V., Hamilton J. Trends in treatment and outcomes of pediatric craniopharyngioma, 1975–2011. Neuro. Oncol. 2013;15(6):767–774. doi: 10.1093/neuonc/not026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merchant T.E., Edmonston D.Y., Wu S., Li Y., Boop F.A., Lustig R.H. Endocrine outcomes after limited surgery and conformal photon radiation therapy for pediatric craniopharyngioma: long-term results from the RT1 Protocol. Neuro. Oncol. 2022:noac115. doi: 10.1093/neuonc/noac115. [published online ahead of print, 2022 Apr 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao Y.J., Hassanzadeh C., Fischer-Valuck B., et al. Patterns of care and treatment outcomes of patients with Craniopharyngioma in the national cancer database. J. Neurooncol. 2017;132(1):109–117. doi: 10.1007/s11060-016-2342-3. [DOI] [PubMed] [Google Scholar]
- 31.Romigi A., Feola T., Cappellano S., et al. Sleep disorders in patients with craniopharyngioma: a physiopathological and practical update. Front. Neurol. 2022;12 doi: 10.3389/fneur.2021.817257. Published 2022 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordani R., Veneruso M., Napoli F., et al. Sleep disturbances in craniopharyngioma: a challenging diagnosis. J. Neurol. 2021;268(11):4362–4369. doi: 10.1007/s00415-021-10794-1. [published correction appears in J Neurol. 2021 Sep 24;:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller HL. Childhood craniopharyngioma – current concepts in diagnosis, therapy and follow-up. Nat. Rev. Endocrinol. 2010;6(11):609–618. doi: 10.1038/nrendo.2010.168. [DOI] [PubMed] [Google Scholar]
- 34.Visser J., Hukin J., Sargent M., Steinbok P., Goddard K., Fryer C. Late mortality in pediatric patients with craniopharyngioma. J. Neurooncol. 2010;100(1):105–111. doi: 10.1007/s11060-010-0145-5. [DOI] [PubMed] [Google Scholar]
- 35.Steinbok P. Craniopharyngioma in children: long-term outcomes. Neurol. Med. Chir. 2015;55(9):722–726. doi: 10.2176/nmc.ra.2015-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wijnen M., Olsson D.S., van den Heuvel-Eibrink M.M., et al. Excess morbidity and mortality in patients with craniopharyngioma: a hospital-based retrospective cohort study. Eur. J. Endocrinol. 2018;178(1):93–102. doi: 10.1530/EJE-17-0707. [DOI] [PubMed] [Google Scholar]
- 37.Tan T.S.E., Patel L., Gopal-Kothandapani J.S., et al. The neuroendocrine sequelae of paediatric craniopharyngioma: a 40-year meta-data analysis of 185 cases from three UK centres. Eur. J. Endocrinol. 2017;176(3):359–369. doi: 10.1530/EJE-16-0812. [DOI] [PubMed] [Google Scholar]
- 38.Gautier A., Godbout A., Grosheny C., et al. Markers of recurrence and long-term morbidity in craniopharyngioma: a systematic analysis of 171 patients. J. Clin. Endocrinol. Metab. 2012;97(4):1258–1267. doi: 10.1210/jc.2011-2817. [DOI] [PubMed] [Google Scholar]
- 39.Lo A.C., Howard A.F., Nichol A., et al. Long-term outcomes and complications in patients with Craniopharyngioma: the British Columbia cancer agency experience. Int. J. Radiat. Oncol. Biol. Phys. 2014;88(5):1011–1018. doi: 10.1016/j.ijrobp.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Poretti A., Grotzer M.A., Ribi K., Schönle E., Boltshauser E. Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev. Med. Child Neurol. 2004;46(4):220–229. doi: 10.1017/s0012162204000374. [DOI] [PubMed] [Google Scholar]
- 41.Pereira A.M., Schmid E.M., Schutte P.J., et al. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin. Endocrinol. (Oxf) 2005;62(2):197–204. doi: 10.1111/j.1365-2265.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- 42.Zada G., Kintz N., Pulido M., Amezcua L. Prevalence of neurobehavioral, social, and emotional dysfunction in patients treated for childhood craniopharyngioma: a systematic literature review. PLoS One. 2013;8(11):e76562. doi: 10.2176/nmc.ra.2015-0099. Published 2013 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.