Abstract
We present the case of a young woman transferred to our center with acute hypoxic respiratory failure due to an obstructing subcarinal mass. We review the management and rationale of this respiratory failure at different stages of her hospital course. We describe the approach and rationale in both the intensive care unit as well as the bronchoscopy suite. Finally, we discuss how the use of a novel hybrid Y stent effectively palliated her symptoms.
Keywords: Hybrid Y stent, Extracorporeal membrane oxygenation, Malignant central airway obstruction
1. Introduction
The management of acute hypoxic respiratory failure due to an obstructing mass is clinically challenging in terms of the delivery of timely, safe, and appropriate care. Oftentimes, the symptoms precipitating the respiratory failure have gone ignored or unrecognized by the patient and physician alike, and by the time of presentation, the patient's functional reserve has been exhausted. While appropriate management actions will differ for the different phases of clinical care, coordinated efforts among teams remain crucial to achieving a positive outcome. This case discusses the management approach that was used during the acute presentation of a young patient with malignancy-induced, life-threatening hypoxemic respiratory failure. Specifically, the case highlights the use of a hybrid Y stent, for which clinical knowledge gaps exist because this device has only recently become available for use in the United States.
2. Case presentation
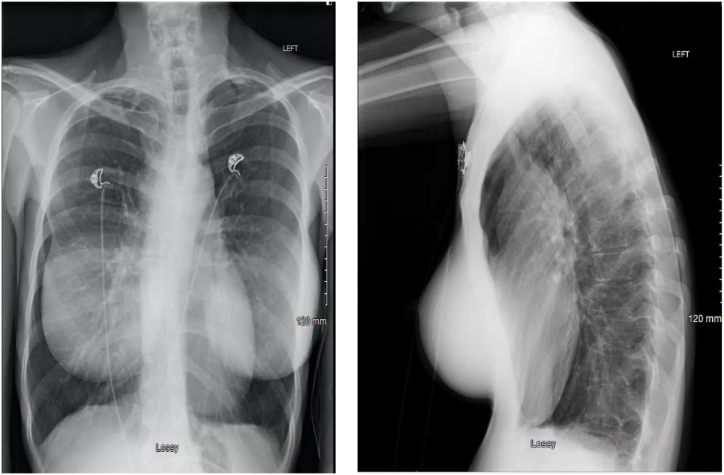
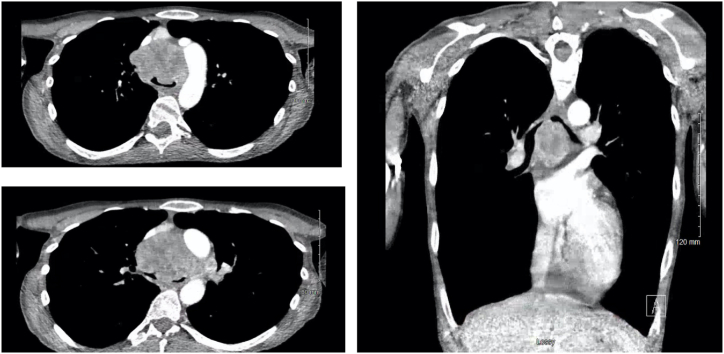
A 42-year-old female with a past medical history of multiple sclerosis was transferred under an emergent context (i.e., immediate care needed) to our institution for a life-threatening airway obstruction due to a subcarinal mass extending into the distal trachea. During a later interview, she noted that she had been unable to lie flat for the past two months because of difficulties with breathing and coughing. Recently, she had been treated for bronchitis several times without an improvement in her symptoms. A chest X-ray (CXR) was obtained one week prior to transfer, but the results were interpreted as negative (Fig. 1A and B). When a computed tomography (CT) scan was eventually obtained by her local physicians, she was found to have severe airway obstruction involving the subcarinal space and anterior mediastinum, such that both the left and right mainstem bronchi were severely occluded (Fig. 2A–C). She subsequently was transferred to a tertiary care hospital where she was admitted to the intensive care unit (ICU). The initial evaluation revealed a thin woman sitting upright in severe respiratory distress, with diffuse rhonchi and accessory muscle use on examination. Her distress improved significantly with non-invasive positive pressure ventilation (NIPPV, 18/12 cm H2O), scheduled doses of nebulized racemic epinephrine, and maintenance of her upright position. An attempt was made to lessen the work needed for her to breathe with heliox (80% helium and 20% oxygen), but ultimately heliox could not be used because of the technical constraints of the available NIPPV devices. Steroids were avoided because of suspicion of potential lymphoma.
Fig. 1.
Chest radiograph obtained 1 week prior to emergency transfer.
A. PA chest radiograph. Note the lack of clear signs of airway obstruction.
B. Lateral chest radiograph. Note the anterior mediastinal fullness and the narrowing of the tracheal airway.
Fig. 2.
CT imaging prior to transfer and 1 week after the chest radiograph.
A. (Top Left) Axial cut at level of aortic arch.
B. (Bottom Left) Axial cut just below carina.
C. (Right) Representative coronal image.
After temporizing the patient's respiratory distress, the plan was to transfer the patient to a center with interventional pulmonary expertise for more definitive management of the airway obstruction, but 24 hours after admission to our hospital, the patient developed worsening respiratory distress. Given the risk of complete airway obstruction, a decision was made to perform an awake, upright, fiberoptic intubation in the operating room with preparations made for emergent extracorporeal membrane oxygenation (ECMO) if needed. While in the upright position, the patient was moderately sedated with dexmedetomidine, and small doses of midazolam and lidocaine were administered via nebulization. She was then successfully intubated using an endotracheal tube (ETT)-loaded bronchoscope in the upright position, and proper placement of the tube was confirmed bronchoscopically. Higher positive end-expiratory pressure (PEEP) (14 cm H2O) was utilized to maintain airway patency via a pneumatic stent mechanism. Despite maintenance of higher PEEP, even brief periods in the supine position resulted in significant and prolonged desaturation events. The patient was then transferred to the bronchoscopy suite for definitive management of the airway obstruction.
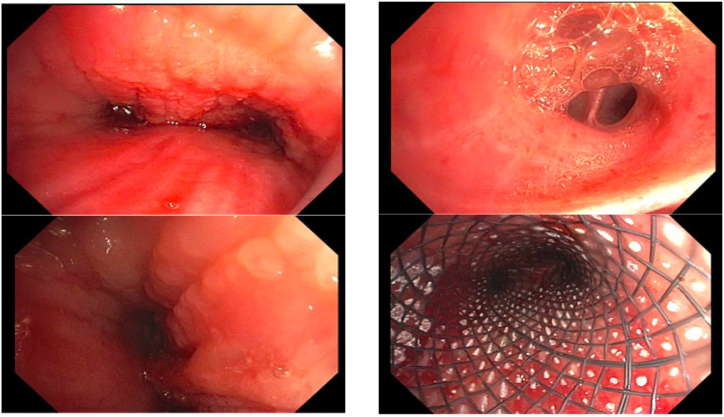
The patient arrived at the bronchoscopy suite intubated, sedated, and positioned in a 45° upright posture. Her ventilator settings at the start of the bronchoscopy procedure were notable for peak pressures of >40 cm H20 on pressure-regulated volume control (PRVC) mode to achieve tidal volumes of 450 cc. The initial PEEP value was set at 5 cm H20, but this value had to be decreased to 0 because of hypotension. Inspection bronchoscopy confirmed a bilateral mass-induced airway obstruction in the mainstem bronchi (Fig. 3A and B). The left and right mainstem bronchi were 95% and 70% occluded, respectively. Using gentle pressure, we were able to bypass the mass and confirm that the secondary carinas and more distal airways were patent and healthy in appearance. One notable feature was the presence of air bubbles and yellowish fluid spilling from the orifice of several airways, particularly the left lower lobe superior segment (Fig. 3C). The nature of the obstruction was mixed but predominantly consisted of extrinsic type compression with infiltration; therefore, aside from several cryobiopsies of the mass, we did not attempt significant tumor debulking. Initial biopsies were read as atypical cells but without definite evidence of cancer, and therefore, endobronchial ultrasound (EBUS) was performed, which led to a diagnosis of carcinoma. Once this work was complete, the ETT was removed and the patient was reintubated in an upright posture with a black Bryan rigid bronchoscope and ventilated through a jet ventilator. With the rigid scope in place, we did not have any issues with hypoxia. Next, we proceeded to place a hybrid self-expanding silicone/nitinol Y stent (Fig. 3D). The hybrid self-expanding Y stent was placed with ease in under 5 minutes.
Fig. 3.
Bronchoscopic images.
A. (Top left)Main carina. Tumor infiltration is noted.
B. (Bottom left) Left mainstem bronchus at mid bronchus.
C. (Top Right) Superior segment bronchus with suspected edema fluid.
D. (Bottom Right) Stent within trachea.
Inspection with a flexible scope following the stent insertion demonstrated excellent positioning with patent right and left airways. The rigid scope was removed and an ETT placed. Peak pressures had decreased from 40 to 45 cm H20 to 14 cm H20 with the identical ventilator settings used at the start of the case. The patient was reversed and extubated into the endoscopy suite. She was admitted to the ICU for monitoring with saturations of 100% on 2 L and could now lie flat. Rapid assessment by our clinical teams included inpatient radiation and thoracic medicine consults. She received palliative radiation to the mass the following day and was discharged home without supplemental oxygen from the hospital 4 days following her emergent transfer.
3. Discussion
3.1. Clinical discussion
This case highlights the varied management approaches adopted during the management of an airway obstruction in the ICU and bronchoscopy settings over a 36 hour period. The patient was initially effectively managed with bilevel positive airway pressure (BIPAP) therapy. This is intuitive because high PEEP levels and an upright posture create a pneumatic splint that can maintain airway patency. Another salvage therapy that was attempted was heliox at an 80%/20% mixture. Helium with its smaller atomic size offers greater potential for laminar flow and can be useful as a temporizing measure [1,2]. In this case, heliox was not a successful strategy because of the difficulties encountered in delivering the heliox mixture effectively through non-invasive ventilation. Such limitations are useful to consider and should be discussed with experienced respiratory therapists and physicians.
As the patient began to fatigue, noninvasive ventilation became less effective and invasive PAP therapy became necessary. However, the process of establishing a definitive airway in her case required considerable collaborative efforts. To minimize the risk of complete airway loss and death, an awake, fiberoptic intubation in the upright position was planned. Maintaining the patient in an awake, spontaneously breathing state served to maximize the muscular tone of the chest wall and maintain thoracic volume, thereby minimizing the tumor-related airway obstruction prior to intubation. Given the anterior location of the tumor (relative to the trachea), maintenance in an upright position likewise minimized the tumor-related airway obstruction during the intubation process. Provisions were made such that the initiation of ECMO on an emergent basis would be possible if complete airway obstruction occurred. Extracorporeal membrane oxygenation refers to the use of an artificial lung or heart to oxygenate and circulate blood. Veno-venous ECMO is most commonly used as an emergency therapy in patients with acute respiratory distress syndrome (ARDS), but it has the potential to be useful in other respiratory-related diseases. The value of ECMO in life-threatening tracheal obstruction secondary to lung cancer has been described. Clap et al. reported a case where ECMO successfully allowed for interventional pulmonary procedures in a patient with life-threatening tracheal obstruction secondary to lung cancer, which required tumor debulking and silicone Y stent placement [3]. The use of ECMO is an effective means of providing adequate oxygenation for patients with a severe airway obstruction in which all other fundamental techniques of oxygenation are likely to be unsuccessful. The use of ECMO in tertiary care centers with appropriate resources may be considered in patients with severe airway obstruction secondary to anterior neck or tracheal disease and can provide essential tissue oxygenation while definitive airway management is performed.
The bronchoscopic management of severe hypoxic respiratory failure due to an airway obstruction requires careful consideration of several points. If the fraction of inspired oxygen (FiO2) cannot be reduced without inducing severe hypoxia, hot therapies such as electrocautery, laser, and argon plasma coagulation (APC) cannot be used without risking airway fire. This detail must inform all other choices including choice of biopsy and debulking technique [4]. Other therapies that may prove effective include the use of vasoconstrictors as a prophylactic measure against severe bleeding. Epinephrine and phenylephrine have both been described as medications for vasoconstriction when bleeding is anticipated [[5], [6], [7]]. In this case, we viewed the nature of obstruction as mixed but primarily consisting of extrinsic compression. An initial cryobiopsy of the lesion did not yield a rapid diagnosis and triggered minor bleeding, which worsened airway pressures. Thus, we pivoted to EBUS guided fine-needle aspiration (FNA) for diagnosis and to maximize safety. Once a diagnosis was established, we used very little debulking therapy because the disease was primarily extrinsic.
Airway obstructions are generally described as being either an intrinsic, extrinsic, or mixed obstruction [8]. One other type of obstruction is often encountered, as described by Hespanhol et al. [9], which is known as “infiltration.” Infiltration is characterized by vascular engorgement, edema, and mucosal irregularity. In their mathematical modeling of anticipated success rates for therapeutic bronchoscopy, Hespanhol et al. identified the presence of infiltration as the single greatest predictor of bronchosopic treatment failure [9]. In this case, infiltration was present, with edema likely arising from obstruction of the lymphatic glands in the mediastinum. Often, such infiltration is accompanied by edema fluid present in more distal airways, which we suspect was the yellowish frothy fluid that we encountered (Fig. 3C). Airway obstruction is frequently categorized as being only mixed or extrinsic, without relative contributions from each subtype. In this case, although the disease was mixed with infiltration, the greatest contributors were extrinsic compression and infiltration. The general management algorithm for mixed obstruction is ablation therapy to destroy the tumor and reduce the airway obstruction. This works well for exophytic tumor masses, but less well for diffuse infiltrative or extrinsic obstructions. In this case, major ablative modalities would have risked destruction of a mostly intact basement membrane and therefore we focused on airway stenting to open her airway.
Stenting is the only immediately effective therapy for a life-threatening obstruction if the airway cannot be opened to >50% [10,11]. Given the involvement of the main carina and distal trachea, Y stenting was the best option, and a hybrid self-expanding metallic stent was used in contrast to conventional silicone Y stents. Previous research has shown that placement of silicone Y stents is accompanied by various risks [12,13], which can include life-threatening hypoxia from stent infolding as well as airway laceration. These adverse events can occur during advancement or rotation of the stent in attempts to seat it properly [12]. Ramon et al. noted there is a particular risk of using a silicone stent when mediastinal (particularly posterior mediastinal) necrosis is present because it may precipitate further damage to the airways [14].
The hybrid self-expanding metallic stent offers a potential solution to the challenge of securing severely narrowed airways with poor tissue integrity. The stent's ability to self-expand reduces the need for aggressive forward placement or rotation. This allows placement to be successful in smaller airways and with greater speed than a silicone Y stent. In a study comparing Dumon Y vs. self-expanding hybrid Y stents, procedures were on average 40 minutes shorter with the placement of the hybrid Y stents; additionally, 7/40 silicone Y stent procedures were unsuccessful, although the study did not identify the reasons for this failure [15]. In contrast, all hybrid Y stents were successfully placed [15].
3.2. Radiologic discussion
There are pertinent radiologic features that bear consideration in this case. First is the lack of a clear obstruction (such as right and left mainstem collapse or atelectasis) on the high-quality, two-view chest radiograph obtained one week prior to the patient's emergent transfer to our facility (Fig. 1A and B). A careful review of the lateral radiograph does show definite mediastinal fullness; however, this could readily be mistaken for a widened aortic arch rather than an anterior or middle mediastinal mass. The main trachea is also visibly narrowed on the lateral radiograph, but again this finding is somewhat subtle. Thus, in an active smoker with such characteristic features as the inability to lie flat, further imaging including a CT of the chest should be sought when an initial cause is not clear with initial imaging. Second is the radiographic appearance of the mass on the CT scan. The anterior mediastinal mass is notable and present, with a clear demonstration of the marked airway obstruction that was present (Fig. 2A–C). What is also notable is the degree of heterogeneity in the mass. The location within the mediastinum is crucial for the determination of the likely etiology of the mass, as has been noted in prior reviews [16,17]. Most anterior mediastinal masses, including teratomas, thymic cysts, foregut duplication cysts, thyroid masses, and middle mediastinal masses including lymphomas and primary pulmonary malignancies can demonstrate heterogeneity. The presence of such masses suggests the possibility of mediastinal necrosis. Thus, a careful approach is needed when choosing stents because of the risk of mediastinal injury [13].
This case has several limitations that must be acknowledged. First, this report is for a single patient from a single cancer institution where we have encountered a significant number of malignant central airway obstructions. Experiences at other institutions may differ from ours. Second, the hybrid self-expanding Y stent has only recently been approved by the U.S. Food and Drug Administration (FDA), so we lack long-term follow up data. It is our intention to overcome this knowledge gap by close follow up and future publications on this patient and others who have received this newer Y stent to offer more objective input into patient and provider experiences. Finally, we acknowledge that significant training in advanced bronchoscopy is crucial for the safe placement of any airway stent, but in particular, silicone Y stents. Training differs between institutions, and this may impact the general perception and use of Y stents.
In this report, we have described the ICU and bronchoscopic approaches taken to manage severe hypoxic respiratory failure due to a malignant central airway obstruction in a young woman with an otherwise reasonable health status. Importantly, this reports highlights topics for bronchoscopists to consider when selecting a treatment approach (e.g., confirmatory diagnostic tests, tumor debulking, stent placement). Furthermore, we describe the substantial advantages that we believe the hybrid self-expanding metallic Y stent has over the silicone Y stent. In this patient, the improvement in health status from one of respiratory failure necessitating consideration of the use of ECMO to a stable state in which they were breathing comfortably on room air was dramatic, and this speaks to the importance of using safe and effective endobronchial therapies in appropriately selected patients.
4. Conclusion
-
•
Malignant central airway obstruction (MCAO) is a life-threatening complication of advanced lung cancer and extrapulmonary metastatic disease, and MCAO should be suspected in all age groups because of its myriad etiologies, especially in those with a severe and unexplained cough, dyspnea, or inability to lie in a supine position; this condition must be rapidly managed to prevent complications such as pneumonia and respiratory collapse.
-
•
ICU management of an MCAO is largely focused on temporizing measures (here we applied non-invasive positive pressure ventilation, scheduled doses of nebulized racemic epinephrine, and maintenance of an upright position) and preparation for safe treatment in the procedure suite. Short-term treatment of an MCAO in the procedure suite is dictated by physician choice as well as by what treatments can be safely delivered (here tumor debulking was not used because of signs of infiltration in the obstruction, and instead a newly FDA-approved hybrid self-expanding metallic Y stent was used in contrast to conventional silicone Y stents).
-
•
We found that the hybrid self-expanding metallic Y stent could be placed with ease in under 5 minutes to treat the significant carinal obstruction, and this represented notable benefits over traditional stents in terms of speed, ease of placement, and lowered potential for tissue damage. Successful treatment of future such patients would also benefit from having ECMO on standby, ventilation equipment capable of handling heliox, and physicians well-trained in stent placement.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Editorial assistance for this publication was provided by Roswell Park's Scientific Editing and Research Communications Core (SERCC) Resource, which is supported by a National Cancer Institute (NCI) Cancer Center Support Grant (grant no. NCI P30CA016056).
Handling Editor: DR AC Amit Chopra
References
- 1.Hashemian S.M., Fallahian F. The use of heliox in critical care. Int J Crit Illn Inj Sci. 2014;4(2):138–142. doi: 10.4103/2229-5151.134153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGarvey J.M., Pollack C.V. Heliox in airway management. Emerg. Med. Clin. 2008;26(4):905–920. doi: 10.1016/j.emc.2008.07.007. (viii) [DOI] [PubMed] [Google Scholar]
- 3.Clapp N., Wu H., Marburger E., Sheikh G., Chaudry F. Management of life-threatening malignant central airway obstruction: a novel approach of using extracorporeal membrane oxygenation with tumor debulking and stenting. Respir. Med. Case Rep. 2022;39 doi: 10.1016/j.rmcr.2022.101718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanick N.M., Moh M., Seeley E.J., Benn B.S. Bilateral endobronchial masses and severe hypoxemic respiratory failure. J. Bronchology Interv. Pulmonol. 2019;26(4):e65–e67. doi: 10.1097/LBR.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 5.Sakr L., Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration. 2010;80(1):38–58. doi: 10.1159/000274492. [DOI] [PubMed] [Google Scholar]
- 6.Sharkey A.J., Brennen M.D., O'Neill M.P., Black G.W. A comparative study of the haemostatic properties and cardiovascular effects of adrenaline and ornipressin in children using enflurane anaesthesia. Acta Anaesthesiol. Scand. 1982;26(4):368–370. doi: 10.1111/j.1399-6576.1982.tb01784.x. [DOI] [PubMed] [Google Scholar]
- 7.Ivanick N., Pu C.Y. QA project: hemodynamic safety of endobronchial administration of phenylephrine for control of airway bleeding by bronchoscopy. Pulm. Pharmacol. Ther. 2020 doi: 10.1016/j.pupt.2020.101961. [DOI] [PubMed] [Google Scholar]
- 8.Gorden J.A., Ernst A. Endoscopic management of central airway obstruction. Semin. Thorac. Cardiovasc. Surg. 2009;21(3):263–273. doi: 10.1053/j.semtcvs.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Hespanhol V., Magalhães A., Marques A. Neoplastic severe central airways obstruction, interventional bronchoscopy: a decision-making analysis. J. Thorac. Cardiovasc. Surg. 2013;145(4):926–932. doi: 10.1016/j.jtcvs.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Ernst A., Feller-Kopman D., Becker H.D., Mehta A.C. Central airway obstruction. Am. J. Respir. Crit. Care Med. 2004;169(12):1278–1297. doi: 10.1164/rccm.200210-1181SO. [DOI] [PubMed] [Google Scholar]
- 11.Özgül M.A., Çetinkaya E., Seyhan E.C., Turan D., Uğur Chousein E.G., Özgül G., et al. Airway stents: a retrospective evaluation of indications, results and complications in our 10-year experience. Tuberk Toraks. 2019;67(4):272–284. doi: 10.5578/tt.68967. [DOI] [PubMed] [Google Scholar]
- 12.Huan N.C., Ng K.L., Nasaruddin M.Z., Muhammad N.A., Daut U.N., Abdul Rahaman J.A. Conservative management of airway tear as a complication of silicone endobronchial stenting in bronchomalacia secondary to endobronchial tuberculosis. Respirol. Case Rep. 2020;8(9) doi: 10.1002/rcr2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapron J., Wermert D., Le Pimpec-Barthes F., Cazes A., Pommier R., Hernigou A., et al. Bronchial rupture related to endobronchial stenting in relapsing polychondritis. Eur. Respir. Rev. 2012;21(126):367–369. doi: 10.1183/09059180.00000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramon P.P., Brichet-Martin A., Fournier C. Interventional bronchoscopy in the management of lung cancer. Rev. Mal. Respir. 2005;22(2) 8s106-11. [PubMed] [Google Scholar]
- 15.Lachkar S., Couraud S., Salaün M., Roger M., Bota S., Guisier F., et al. Self-expanding metallic Y-stent compared to silicone Y-stent for malignant lesions of the main carina: a single center retrospective study. Respir. Med. Res. 2020;78 doi: 10.1016/j.resmer.2020.100767. [DOI] [PubMed] [Google Scholar]
- 16.Azour L., Moreira A.L., Washer S.L., Ko J.P. Radiologic and pathologic correlation of anterior mediastinal lesions. Mediastinum. 2020;4:5. doi: 10.21037/med.2019.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quint L.E. Imaging of anterior mediastinal masses. Cancer Imag. 2007:S56–S62. doi: 10.1102/1470-7330.2007.9014. 7 Spec No A(Special issue A) [DOI] [PMC free article] [PubMed] [Google Scholar]