Summary
Characterization of double-stranded (ds)RNAs is relevant to the understanding of viral replication and immune sensing. Here, we provide a protocol describing the use of anti-dsRNA antibodies for immunofluorescence and immunoblotting in virus-infected insect cells, which can also be applied to tissues and other organisms. We describe the procedures to prepare insect cells for viral infection, followed by RNA extraction and in vitro production of synthetic dsRNA controls. We then detail the steps for dsRNA detection by immunoblotting and immunofluorescence.
For complete details on the use and execution of this protocol, please refer to de Faria et al. (2022).1
Subject areas: Cell Biology, Immunology, Microbiology, Microscopy, Molecular Biology, Antibody
Graphical abstract

Highlights
-
•
Protocol to detect dsRNAs in viral-infected insect cells using anti-dsRNA antibodies
-
•
Immunoblotting and immunofluorescence techniques to detect dsRNAs
-
•
Guidance for protocol optimization and selection of the most suitable antibody
-
•
Alternatives provided to deal with background and to improve signal-to-noise ratios
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Characterization of double-stranded (ds)RNAs is relevant to the understanding of viral replication and immune sensing. Here, we provide a protocol describing the use of anti-dsRNA antibodies for immunofluorescence and immunoblotting in virus-infected insect cells, which can also be applied to tissues and other organisms. We describe the procedures to prepare insect cells for viral infection, followed by RNA extraction and in vitro production of synthetic dsRNA controls. We then detail the steps for dsRNA detection by immunoblotting and immunofluorescence.
Before you begin
Our protocol describes the detection and characterization of virus-derived dsRNAs in Drosophila S2 cells by immunofluorescence and immunoblotting, but these techniques can be applied to other cells or tissues (Figure 1). The antibodies used here were previously shown to detect dsRNA in cells infected by different viruses.2,3,4 We have also described procedures for RNA extraction and in vitro dsRNA synthesis that are complementary to the main protocol. In our model system of viral infection, we use two viruses: a positive stranded RNA virus (Drosophila C Virus, DCV) and a linear double-stranded DNA virus (Invertebrate Iridescent virus 6, IIV-6). Of note, DCV is a picorna-like virus that directly generates dsRNAs as a byproduct of its replication strategy but, in contrast, production of dsRNA is driven by the host RNA polymerase II in cells infected by IIV-6.1
Figure 1.
Overview of the detection of dsRNAs by immunofluorescence (IFA) and immunoblotting
Steps and reagents required for the detection of dsRNA in virus-infected cells by immunoblot (left) or immunofluorescence (right) are shown in the figure.
Different versions of anti-dsRNA antibodies (e.g., K1, J2, 9D5) are available, including recombinant versions. Testing different antibodies that recognize dsRNA is important to reveal the most suitable reagent for each virus-cell combination and the choice of detection technique to be used. Here, we show standardization and results for immunofluorescence and immunoblotting using two different antibodies that recognize dsRNA: J2 and 9D5. The successful detection of dsRNA may require additional optimization of the techniques described here.
Institutional permissions
Here, we describe protocols for dsRNA analysis in Drosophila cells, that do not require special Institutional permissions. On the other hand, experiments on vertebrates or higher invertebrates must be performed in accordance with Institutional Animal Care and National guidelines.
Cell culture for immunofluorescence or RNA extraction
Timing: 2–3 days (for step 1)
This part of the protocol briefly describes the culture of Drosophila S2 cells and virus infections.
-
1.Thaw and Cultivate S2 cells:
-
a.Prepare an aliquot of complete Schneider’s medium (10% FBS, 1× Penicillin/Streptomycin, 1× GlutaMAX).
-
b.Take a vial of S2 cells stored in liquid Nitrogen and place into a holder in a 37°C water bath until most of the tube content is thawed.
-
c.Add 8 mL of complete Schneider’s medium into a 15 mL conical tube.
-
d.Clean the S2 cell vial with 70% ethanol and dry the tube with a lint-free wipe.
-
e.Transfer the cells into the conical tube and centrifuge at 200 g for 5 min at room temperature (25 ± 3°C).
-
f.Remove the supernatant and add 6 mL of complete Schneider’s medium.
-
g.Homogenize with a 10 mL serological pipette and transfer the content to a culture flask with 25 cm2 of surface.
-
h.Incubate S2 cells in a BOD incubator (Bio-Oxygen Demand) at 25°C–28°C for 2–3 days and then sub cultivate S2 cells at a passage of ∼1,0 × 106 cells/mL.
-
a.
Note: S2 cells are maintained in culture for a maximum of 6–10 passages. Virus stocks were produced in Drosophila cells as described elsewhere.1,5 IIV-6 was grown in S2 cells and DCV in Drosophila Kc167 cells. Both viruses were tittered in S2 cells.
CRITICAL: Check the biosafety procedures of your institution before working with viruses.
RNA extraction from S2 cells
Timing: 2 days (for step 2)
RNA extraction is an essential step for dsRNA immunoblotting since the quality of input RNAs determine the outcome of dsRNA detection. During the procedure, it is essential to keep samples at low temperatures (4°C–8°C) to avoid formation of dsRNA that was not present in the cells prior to extraction.
-
2.Prepare S2 cell culture for viral infections:
-
a.Count S2 cells in a Neubauer chamber and transfer 107 cells into 15 mL conical tubes. Use cell densities between 3 and 6 × 106 cells/mL.
-
b.Add 108 pfu (plaque-forming units) of IIV-6 (10 pfu/cell) or 107 pfu of DCV (1 pfu/cell) to the tube. As a mock infected control, transfer the equivalent amount of medium into S2 cells.
-
c.Incubate for 1 h at 25°C on a rotating shaker or manually rocking cells every 10 min.
-
d.Centrifuge cells at 200 g for 5 min at room temperature.
-
e.Remove the medium and add fresh complete Schneider’s medium into cells and resuspend them at density of 1 × 106 cells/mL with a 10 mL serological pipette.
-
f.Homogenize and transfer 2 mL of S2 cell suspension into each well in 6-well plates.
-
g.Incubate S2 cells at 25°C.
-
a.
-
3.Homogenizing cells in TRIzol:
-
a.At each desired time point post infection, remove plates with infected cells from the incubator and resuspend cells in the media using a micropipette.
-
b.Transfer cell suspension (about 2 mL) into fresh 2 mL microtube and centrifuge at 600 g for 5 min at 4°C.
-
c.Remove the supernatant by quickly inverting the microtube in an appropriate container for virus disposal. Immediately, transfer the pellets to ice.
-
d.Add 500 μL of TRIzol (Invitrogen) to each cell pellet and homogenize by pipetting up and down. Keep the tube on ice.
-
e.Vortex samples for 10–20 s and incubate on ice for 5–10 min.
-
a.
Pause point: At this point, samples can be stored at −80°C before further processing.
CRITICAL: TRIzol (Phenol + guanidine) and Chloroform are toxic and should be handled with caution using protective equipment and using a chemical hood. All solutions with residual TRIzol contaminants must be disposed in proper containers.
-
4.Recover RNAs using TRIzol:
-
a.Add 100 μL of Chloroform into each sample and homogenize on the vortex for at least 20 s.
-
b.Incubate on ice for 5 min and then centrifuge at 12,000 g for 15 min at 4°C to separate aqueous (RNA) and TRIzolic (DNA + proteins) phases.
-
c.Take fresh 1.5 mL microtubes and add 10 μg of glycogen into each tube.
-
d.Transfer the upper aqueous phase into the fresh tube containing glycogen.
-
e.Add an equal volume of isopropanol to each sample and homogenize by inversion the microtubes.Incubate samples overnight (18 ± 2 h) at −20°C.
-
f.Centrifuge samples at 12,000 g for 10 min at 4°C and remove the supernatant by inverting the microtube into an appropriate container for chemical disposal.
-
g.Add 1 mL of 75% ethanol at 25°C and homogenize by gently tapping on the microtube 5 times. Centrifuge samples at 7,500 g for 7 min at 4°C.
-
h.Remove the ethanol by inverting the microtube into an appropriate container for chemical disposal.
-
i.Place the tube upside down on a towel paper to drain the excess of ethanol.
-
j.Air dry the pellet and add 10 μL of nuclease-free H2O to the pellet. Homogenize by pipetting up and down and then incubate for 30 min on ice.
-
k.Quantify samples using a Nanodrop or equivalent strategy, such as Qubit fluorometers.Note: Nucleic acids have light maximum absorbance at the UV wavelength (260 nm). TRIzol extraction may interfere with these ratios, as the solution has absorbance in the 230 nm and 270 nm wavelengths. Therefore, 260/280 and 260/230 absorbance ratios in the range of 1.8–2.0 indicate good overall purity of the nucleic acid preparation. Refer to the TRIzol protocol in case of low yields or low 260/280 and 260/230 ratios.
 CRITICAL: RNA pellets should not be overdried as indicated by the appearance of a white color. It is essential that the pellet maintains a transparent gel-like appearance. To avoid formation of inter- and intra-molecular dsRNAs, from this step forward keep sample on ice. RNA samples can be stored at −80°C until further processing.
CRITICAL: RNA pellets should not be overdried as indicated by the appearance of a white color. It is essential that the pellet maintains a transparent gel-like appearance. To avoid formation of inter- and intra-molecular dsRNAs, from this step forward keep sample on ice. RNA samples can be stored at −80°C until further processing.
-
a.
In vitro production of synthetic dsRNAs
Timing: 3 days (for step 5)
For dsRNA immunoblotting, synthetic dsRNA control can be very helpful to analyze the efficiency of the technique, including limits of detection and direct comparison between anti-dsRNA antibodies.
-
5.Generating PCR products containing the T7 RNA polymerase promoter sequence:
-
a.Design and order primers to amplify your gene of interest and add the T7 RNA polymerase promoter to both forward and reverse primer sequences.
-
b.Using a plasmid containing a desired sequence, such as Green Fluorescent Protein (GFP) or Firefly Luciferase (FLuc), perform 10 × 50 μL PCR reactions using primers containing the T7 promoter (refer to PCR reaction master mix and PCR cycling conditions for reaction set up).Note: A ladder of dsRNA can be generated using this strategy. In this case, sequences with different lengths are required to produce the ladder.
-
c.Run a small aliquot of the PCR on an agarose gel to verify whether there is a single band at the right molecular size, before you proceed to the next step.PCR reaction master mix
Reagent Amount Plasmid DNA template (eGFP sequence) 1–10 ng Platinum Taq DNA Polymerase (5 U/μL) 0.2 μL Primer eGFP_F 10 μM 0.5 μL Primer eGFP_R 10 μM 0.5 μL 10× Buffer 5 μL MgCl2 1.5 μL dNTP mix (10 mM each) 1 μL DEPC-treated H2O Add to 50 μL Note: Prepare a master reaction with total volume of 500 μL. Briefly mix on vortex, spin and distribute 50 μL in 0.2 mL microtubes.PCR cycling conditionsSteps Temperature Time Cycles Initial Denaturation 95°C 3 min 1 Denaturation 95°C 30 s 25–35 cycles Annealing 55°C 30 s Extension 72°C 1 min Final extension 72°C 6 min 1 Hold 4°C forever
-
a.
-
6.Purify PCR products:
-
a.Transfer the PCR reactions into a single 1.5 mL microtube.
-
b.Add an equal volume of Phenol:Chloroform:Isoamyl alcohol (pH 8–8.5).
-
c.Vortex for 15 s and incubate 5 min at room temperature.
-
d.Centrifuge at 13,000 g for 5 min at room temperature.
-
e.Transfer the upper aqueous phase into a fresh 1.5 mL microtube containing 10 μg of glycogen.
-
f.Add 1/10 of total volume of 3 M Sodium acetate (pH 5.5) and homogenize.
-
g.Add 100% ethanol equivalent to 2.5–3 times the total volume of the aqueous phase and homogenize.
-
h.Incubate overnight at −20°C.
-
i.Centrifuge at 16,000 g for 30 min at 4°C.
-
j.Remove the supernatant and wash the pellet with 70% ethanol.
-
k.Centrifuge at 16,000 g for 5 min at 4°C.
-
l.Remove the ethanol and invert the tube onto a towel paper to drain excess of ethanol.
-
m.Air dry the pellet, add 20 μL of nuclease-free H2O to the purified PCR product, homogenize pipetting up and down and incubate 30 min on ice.
-
n.Quantify the DNA using a Nanodrop or equivalent.
-
a.
Note: Purified DNA with Phenol: Chloroform have 260/280 and 260/230 ratios of 1.8 or higher.
CRITICAL: Phenol: Chloroform should be handled with caution using gloves, preferentially in a chemical ventilated hood.
-
7.In vitro transcription of dsRNAs using the MEGAscript T7 kit.
-
a.Thaw and homogenize the nucleoside triphosphates (NTPs): Adenine (ATP), Cytosine (CTP), Guanine (GTP) and Uridine (UTP).
-
b.Thaw and homogenize the 10× buffer.
-
c.In a 0.2 mL tube, mix NTPs, 10× buffer, H2O, Enzyme mix and DNA template (100 ng of purified PCR product) for a 20 μL reaction.
-
d.Incubate for 16 h at 37°C.
-
e.Add 1 μL of DNase from MEGAscript kit into the T7 reaction tube and incubate 15 min at 37°C.
-
f.Add 159 μL of nuclease-free H2O and 20 μL of 3 M Sodium acetate (pH 5.5) to each 0.2 mL tube from T7 transcription.
-
g.Transfer the sample to a 1.5 mL microtube and add 200 μL of Acid Phenol: Chloroform, pH 4.5.
-
h.Vortex for 15 s and incubate for 5 min at room temperature.
-
i.Centrifuge at 13,000 g for 5 min at 4°C.
-
j.Transfer the upper aqueous phase into a fresh 1.5 mL microtube containing 10 μg of glycogen.
-
k.Add 100% ethanol equivalent to 2.5–3 times the total volume of the aqueous phase and homogenize.
-
l.Incubate overnight at −20°C.
-
m.Centrifuge at 16,000 g for 30 min at 4°C.
-
n.Remove the supernatant and wash the pellet with 75% ethanol.
-
o.Centrifuge at 16,000 g for 5 min at 4°C.
-
p.Remove the ethanol and place the inverted tube onto a towel paper to drain excess of ethanol.
-
q.Air dry the pellet and add 20 μL of annealing buffer (10 mM TRIS-HCl pH 8.0, 100 mM NaCl, 1 mM EDTA) into each sample, homogenize pipetting up and down and incubate 30 min on ice.
-
a.
Note: For a 1 M TRIS-HCl pH 8.0 solution, dissolve 12.11 g of Tris base in 80 mL of DEPC-treated H2O and adjust the pH with concentrated HCl. To prepare EDTA at 0.5 M (pH 8.0), add 18.61 g of disodium EDTA-2H2O to 80 mL of DEPC-treated H2O. Adjust the pH to 8.0 with NaOH stirring as EDTA will go into solution only with pH 8.0. Autoclave to sterilize.
-
8.Anneal the T7 products to form dsRNA:
-
a.Heat the T7 synthesized RNA samples for 5 min at 95°C and place them at room temperature allowing them to slowly cool down.
-
b.Keep dsRNAs on ice.
-
c.Quantify dsRNA using a Nanodrop, selecting the RNA measurement set up.Note: High quality purified dsRNAs have 260/280 and 260/230 ratios of 2.0 or above.Note: A 20 μL reaction using MEGAscript T7 kit can generate on the order of 100 μg of RNA.
-
d.Make aliquots with the desired amount of dsRNA and store at −80°C for up to 1 year.
-
a.
-
9.Checking the dsRNA size and quality:
-
a.Use 1 μg aliquots of the in vitro synthesized dsRNAs.
-
b.Bring the sample volumes to 6 μL and add 3 μL of 6× DNA loading Dye.
-
c.Take one of the aliquots and heat at 95°C for 5 min. Immediately, transfer the sample onto ice.
-
d.Make a 2% agarose gel stained with Sybr safe and prepared in 1× TBE (running buffer).
-
e.Load the agarose gel with a DNA ladder and the RNA samples.
-
f.Run at 80 V and develop the image in a UV imager.
-
a.
Note: dsRNAs migrate marginally more slowly than the same length of single stranded RNAs and the DNA ladder used as a reference. Other DNA dyes can replace Sybr safe for in gel staining, such as Ethidium Bromide.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-dsRNA J2 monoclonal antibody (mAb) | English and Scientific Consulting Kft | Cat# 10010200; RRID: AB_2651015 |
| Anti-dsRNA 9D5 rabbit IgG | Absolute Antibody | Cat# Ab00458-23.0, RRID: AB_2920603 |
| Anti-Lamin Dm0 | Developmental Studies Hybridoma Bank, | Cat# adl84.12 RRID: AB_528338 |
| Goat anti-mouse IgG Alexa Fluor 488 | Thermo Fisher Scientific | Cat#A-11001; RRID: AB_2534069 |
| Goat anti-rabbit IgG Alexa Fluor 546 | Thermo Fisher Scientific | Cat# A-11010; RRID: AB_2534077 |
| Anti-rabbit IgG, HRP-linked whole Ab (from donkey) | Amersham (Cytiva) | Cat# NA934; RRID: AB_772206 |
| Amersham Enhanced chemiluminescence (ECL) Mouse IgG, HRP-linked whole Ab (from sheep) | Amersham (Cytiva) | Cat# NA931; RRID: AB_772210 |
| Bacterial and virus strains | ||
| DCV | Kemp et al.5 | N/A (not applicable) |
| IIV-6 | Kemp et al.5 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | Cat# D2650 CAS Number 67-68-5 |
| TRIzol Reagent | Thermo Fisher Scientific | Cat# 15596018 |
| Acid phenol-chloroform | Thermo Fisher Scientific | Cat# AM9720 |
| Phenol:Chloroform:Isoamyl Alcohol (25:24:1, v/v) | Thermo Fisher Scientific | Cat# 15593031 |
| Hydromount medium | National Diagnostics | Cat# HS-106 |
| Hoechst 33342 DNA staining | Thermo Fisher Scientific | Cat# H-3570 CAS Number: 23491-52-3 |
| Pierce 16% Formaldehyde (wt:vol), methanol-free | Thermo Fisher Scientific | Cat# 28906 |
| SYBR Safe DNA Gel Stain | Thermo Fisher Scientific | Cat# S33102 |
| Agarose | Sigma-Aldrich | Cat# A9539 |
| SYBR Gold Nucleic Acid Gel Stain | Thermo Fisher Scientific | Cat# S11494 |
| Non-fat dry milk | Bio-Rad Laboratories | Cat# 1706404 |
| 3 M sodium acetate solution | Thermo Fisher Scientific | Cat# AM9740 |
| Isopropanol | Sigma-Aldrich | Cat# I9516 |
| Absolute ethanol (200 proof) | Sigma-Aldrich | Cat# E7023 |
| Boric acid | Sigma-Aldrich | Cat# B7901 |
| KCl | Sigma-Aldrich | Cat# P9541 |
| KH2PO4 | Sigma-Aldrich | Cat# P5655 |
| Na2HPO4 | Sigma-Aldrich | Cat# S3264 |
| NaCl | Sigma-Aldrich | Cat# S3014 |
| Glycogen | Thermo Fisher Scientific | Cat# AM9510 |
| Tween 20 | Sigma-Aldrich | Cat# P1379 |
| Triton X-100 | Sigma-Aldrich | Cat# X100-1GA |
| Ethylenediaminetetraacetic acid (EDTA) | Sigma-Aldrich | Cat# E5134 |
| Diethyl pyrocarbonate (DEPC) | Sigma-Aldrich | Cat# D5758 |
| Ammonium persulfate (APS) | Sigma-Aldrich | Cat# A3678 |
| Acrylamide | Sigma-Aldrich | Cat# A9099 |
| N,N′-Methylenebisacrylamide | Sigma-Aldrich | Cat# 294381 |
| Tetramethylenediamine (TEMED) | Sigma-Aldrich | Cat# T9281 |
| Trizma | Sigma-Aldrich | Cat# T6066 |
| Sperm Salmon DNA, sheared | Thermo Fisher Scientific | Cat# AM9680 |
| Poly-L-lysine solution | Sigma-Aldrich | Cat# P8920 |
| Concanavalin A | Sigma-Aldrich | Cat# L7647 |
| Hydrochloric acid (HCl) | Sigma-Aldrich | Cat# H1758 |
| Sodium hydroxide solution (NaOH) | Sigma-Aldrich | Cat# 415413 |
| Critical commercial assays | ||
| MEGAscript™ T7 Transcription Kit | Thermo Fisher Scientific | Cat# AM1334 |
| Penicillin-Streptomycin (100×) | Thermo Fisher Scientific | Cat# 15070063 |
| GlutaMAX | Thermo Fisher Scientific | Cat# 35050061 |
| Schneider’s Medium | Thermo Fisher Scientific | Cat# 21720024 |
| Fetal serum bovine | Thermo Fisher Scientific | Cat# 12657029 |
| Fetal serum bovine | Cytiva | Cat# SV30160 |
| Enhanced chemiluminescence (ECL) detection reagent | Amersham (Cytiva) | Cat# RPN3004 |
| RNase A/T1 Mix | Thermo Fisher Scientific | Cat# EN0551 |
| ShortCut RNase III | New England Biolabs | Cat# M0245S |
| Platinum Taq DNA Polymerase, DNA-free | Thermo Fisher Scientific | Cat# 15966005 |
| Nuclease-free H2O | Thermo Fisher Scientific | Cat# AM9932 |
| Experimental models: Cell lines | ||
| Drosophila S2∗ | Laboratory of Richard Carthew | FlyBase ID: FBtc9000011 |
| Oligonucleotides | ||
| eGFP_Foward (dsRNA synthesis): | Olmo et al.6 | taatacgactcactataggga GACCTGAAGTTCATCTGCACCA |
| eGFP_Reverse (dsRNA synthesis): | Olmo et al.6 | taatacgactcactatagggaAGTG GTTGTGGCGGATCTTGAAGT |
| Software and algorithms | ||
| Fiji (ImageJ) 1.53f51 | Schindelin et al.7 | fiji.sc/ |
| Illustrator | Adobe | www.adobe.com/ |
| Photoshop | Adobe | www.adobe.com/ |
| Primer3plus | Andreas Untergasser | primer3plus.com/ |
| Zen 3.1 Lite software | Zeiss | https://www.zeiss.com/microscopy/int/products/microscope-software/zen-lite.html |
| Other | ||
| Parafilm | Bemis Company, Inc | Cat# PM996 |
| Coverlips | Knittel | Cat# 100013 |
| 6-well plates | Sarstedt | Cat# 83.3920 |
| 12-well plates | Sarstedt | Cat# 83.3921 |
| Culture flask with 25 cm2 of surface | Sarstedt | Cat# 83.3910 |
| Fine forceps | Locally purchased | N/A |
| Hybond-N+ Membrane | Thermo Fisher Scientific | Cat# RPN303B |
| Mini-PROTEAN Tetra Cell | Bio-Rad Laboratories | Cat# 1658000 |
| Mini Trans-Blot Electrophoretic Transfer Cell | Bio-Rad Laboratories | Cat# 1703930 |
| PowerPac Universal Power Supply | Bio-Rad Laboratories | Cat# 1645070 |
| NanoDrop | Thermo Fisher Scientific | Cat# ND-ONE |
| Veriti Dx 96-well Thermal Cycler, 0.2 mL | Thermo Fisher Scientific | Cat# 4452300 |
| Confocal microscopy (Spinning Disk, LSM 780 and LSM 880 with Airyscan) | Zeiss | https://www.zeiss.com/microscopy/en/home.html |
| Fusion FX | Vilber | https://www.vilber.com/fusion-fx/ |
Materials and equipment
Microscope system
We have used different systems to image dsRNAs in infected cells, such as Nikon A1 confocal microscope (Nikon, Japan) and Zeiss Spinning Disk/LSM 780/LSM 880 confocal microscopes (Zeiss, Germany). In addition to visualizing dsRNA, separate channels can be used for imaging DNA (Hoechst), cellular or viral proteins of interest such as actin, Lamin. Replicative DNA can also be visualized by 5′-Bromo-2-deoxyuridine (BrdU) staining and may be useful to monitor DNA viruses. Images were acquired both as single and multiple z-stack images.
Note: Acquire at least 3 regions of interest on each slide from independent experiments.
Image processing
Images acquired on Zeiss Spinning Disk or LSM 780/880 confocal microscopes were analyzed in ImageJ software using FIJI plugins.7 Processed images were then exported as .tiff files and final panels assembled on Adobe PhotoShop or Illustrator.
Alternatives: Other microscope systems are also available for imaging that provide similar results. The choice of the objective lens and magnification depend on the imaging purpose. For quantification of percentage of virus infected cells using dsRNA staining in high throughput screening, antibody dilution optimization and antibody selection, images can be captured with a 10–20× magnification objective. A minimum 40× (ideally a 63×) magnification objective is required for high resolution imaging of subcellular localization of dsRNAs.
All materials for Cell culture, RNA extraction, Western blotting (WB), Immunofluorescence analysis (IFA) and preparation for microscopy must be available in the laboratory. This includes glassware, pipettes, culture materials, microcentrifuges, incubators, RNA electrophoresis apparatus, UV detector, Nanodrop and chemiluminescence detector.
Solutions
Timing: 2 h
Timing: 2 h
Timing: 30 min
Timing: 30 min
Timing: 10 min
Timing: 30 min
Timing: 30 min
Timing: 2 h
Timing: 2 h
Timing: 2 h
Timing: 2 h
Timing: 30 min
-
•
Diethyl pyrocarbonate (DEPC)-treated H2O: add 1 mL DEPC in 1 L ddH2O.
Note: DEPC breaks down when autoclaved for 20 min. If necessary, glassware may be incubated with DEPC solution and then autoclaved to breakdown the chemical. Store DEPC-treated H2O at room temperature before use. Open the DEPC-treated H2O bottle on the culture hood, aliquot and store at 4°C up to 3 months.
Alternatives: Ready-to-use Nuclease-free water (Ambion, AM 9932) can replace DEPC-treated H2O, although is much more expensive.
CRITICAL: DEPC is carcinogenic and should be handled with care.
1× Phosphate Buffered Saline (PBS)
| Reagent | Final concentration | Amount |
|---|---|---|
| KH2PO4 | 2 mM | 0.27218 g |
| Na2HPO4 | 10 mM | 1.4196 g |
| NaCl | 137 mM | 8.00628 g |
| KCl | 2.7 mM | 0.201285 g |
| DEPC-treated H2O | N/A | Add to 1 L |
Note: Sterilize PBS by autoclaving and store at room temperature for 3 months or 4°C after first use.
Alternatives: We also performed IFA experiments with 10× ready-to-use PBS (Invitrogen) with similar results.
-
•
4% paraformaldehyde (PFA) solution: add 1 mL Pierce 16% methanol free Formaldehyde (wt:vol) in 4 mL 1× PBS.
Note: We use Molecular Grade PFA to avoid RNA degradation in IFA. 4% PFA solution should be freshly prepared on the day of use in the chemical ventilated hood.
-
•
1× PBS + 0.1% Triton X-100 solution (PBSTX): add 0.5 mL Triton X-100 in 499.5 mL of 1× PBS.
Note: Triton X-100 may require gently rocking after mixing with PBS to fully dissolve. This solution is used for permeabilization and as washing buffer in IFA. Prefer to use freshly prepared solution.
-
•
IFA blocking buffer: add 1 mL of Fetal Bovine Serum (FBS) in 9 mL of PBSTX.
Note: Blocking buffer should be freshly prepared on the day of use.
-
•
1× PBS + 0.05% Tween-20 solution (PBS-Tween): add 0.5 mL Tween-20 in 999.5 mL of 1× PBS.
Note: Tween-20 may require gently rocking after mixing with 1× PBS to fully dissolve. This solution is used as washing buffer and to prepare primary/secondary antibody solution in immunoblotting. Use freshly prepared solution for antibody dilution; store at room temperature for up to 1 month (washing buffer).
Dot blot/Immunoblotting Blocking buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Non-fat dry milk | 5% (wt:vol) | 1 g |
| Sperm Salmon DNA, sheared | 50 μg/mL | 1 mg |
| PBS-Tween | 1× | Add to 20 mL |
| Total | N/A | 20 mL |
Note: Blocking buffer should be freshly prepared on the day of use.
5× Tris-Borate-EDTA buffer (TBE)
| Reagent | Final concentration | Amount |
|---|---|---|
| TRIS base | 445 mM | 54 g |
| Boric acid | 445 mM | 27.5 g |
| 0.5 M EDTA solution (pH 8.0) | 10 mM | 20 mL |
| DEPC-treated H2O | N/A | Add to 1 L |
| Total | N/A | 1 L |
Note: Autoclave the solution for 20 min. 5× TBE may precipitate when stored for long time (6–12 months). If necessary, split the solution in 50 mL plastic conical tubes to avoid precipitation.
30% (wt:vol) Acrylamide: bisacrylamide 29/1
| Reagent | Final concentration | Amount |
|---|---|---|
| Acrylamide | 29% | 29 g |
| N,N′-methylbisacrylamide | 1% | 1 g |
| DEPC-treated H2O | N/A | Add to 100 mL |
| Total | N/A | 100 mL |
Note: Remove impurities by filtration in a 0.45 μm pore size filter. Store in dark bottles protected from light at room temperature up to 12 months.
Alternatives: We also used ROTIPHORESE®NF-Acrylamide/Bis-solution 30 (29:1) to prepare polyacrylamide gels with good efficiency.
3.5% Polyacrylamide gel
| Reagent | Final concentration | Amount |
|---|---|---|
| 30% Acrylamide: bisacrylamide 29/1 | 3.5% | 2.34 mL |
| 5× TBE | 1× | 4 mL |
| DEPC-treated H2O | N/A | 13.54 mL |
| TEMED | 0.1% (vol/vol) | 20 μL |
| 25% (wt:vol) Ammonium Persulfate (APS) | 0.125% | 100 μL |
| Total | N/A | 20 mL |
Note: To make 25% APS, add 0.25 g APS per 0.86 mL of DEPC-treated H2O and mix (APS dissolves quickly). Use freshly prepared solution.
CRITICAL: Acrylamide and TEMED are toxic and should be handled with care using gloves, in a ventilated chemical hood.
Complete Schneider’s medium
| Reagent | Final concentration | Amount |
|---|---|---|
| Schneider’s medium | 90% | 450 mL |
| Fetal Bovine Serum | 10% | 50 mL |
| 100× Penicillin/Streptomycin | 1× | 5 mL |
| 100× GlutaMAX | 1× | 5 mL |
| Total | N/A | 500 mL |
Note: Take reagents to room temperature. Store at 4°C and use within 1–2 months.
dsRNA annealing buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M Tris-HCl, pH 8.0 | 10 mM | 100 μL |
| 5 M NaCl | 100 mM | 200 μL |
| 0.5 M EDTA solution (pH 8.0) | 1 mM | 20 μL |
| Nuclease-free H2O | N/A | 9.68 mL |
| Total | N/A | 10 mL |
Note: We use nuclease-free H2O for solubilization of RNAs instead DEPEC-treated H2O. Open the nuclease-free H2O bottle on the culture hood, aliquot and store at 4°C up to 3 months.
Step-by-step method details
dsRNA detection by immunoblotting
Timing: 2–3 days
Immunoblotting is a technique that can be used to study molecular characteristics of dsRNAs such as length and number of different species as well as direct quantification. In this case, it is recommended that the RNA is first separated by electrophoresis followed by immunoblotting. A simplified method without RNA electrophoresis can also be used for rapid quantification of dsRNA by dot blotting when information about size and species is not required.
We describe below two strategies to perform immunoblot for dsRNA detection on Nylon membranes: transfer of RNAs to the membrane after gel electrophoresis or direct spotting the RNA onto the membrane. We efficiently captured dsRNA immunoblotting images with a chemiluminescence imager or using high sensitivity X-ray films, with results that may vary according to the antibody. Of note, immunoblotting using the 9D5 antibody displayed high background when we used X-ray films.
RNA separation by electrophoresis in non-denaturing polyacrylamide gel
Timing: 4–6 h
-
1.Prepare the RNA electrophoresis apparatus for running:
-
a.Prepare a homemade 3.5%–5% non-denaturing polyacrylamide gel or, alternatively, use a precast gel.
-
b.Add the non-denaturing polyacrylamide solution into the cast.
-
c.After the polyacrylamide gel has polymerized, remove the gel from the cast stand and then from the clamp assembly.
-
d.Rinse the glass plate surfaces with DEPEC-treated H2O to remove residual polyacrylamide gel.
-
e.Drain excess water.
-
f.Mount the gel in the upper chamber (2 gels for each cassette or 1 gel and an acrylic plate on the other side) with spacer plates outwards.
-
g.Place the upper chamber in the running electrophoresis tank.
-
h.Fill the upper chamber and the tank with 1× TBE running buffer. Check for leaking from the chamber to the tank.Note: Check for leaking from the upper chamber carefully.
-
i.Carefully remove the comb using clean powder-free cloves.
-
j.Rinse the wells with 1× TBE using a syringe to clean debris from lanes.
-
k.Using the Nanodrop quantification, calculate and transfer 5–10 μg of total RNA into pre-cooled 1.5 mL microtubes.Note: Use nuclease-free H2O to produce equal volumes of RNA samples for all samples.
-
l.Add 6× DNA loading dye to RNA samples. Do not exceed 15 μL of total volume (RNA solution + loading dye).
-
m.Quickly spin samples in a pre-cooled centrifuge and keep samples on ice.
-
n.Load lanes in the polyacrylamide gel(s) with RNA samples.
-
o.If any, load empty wells with equal volume of 6× DNA loading dye + nuclease-free H2O.
-
p.Run gel(s) for 30 min using a voltage of 50 V and then increase to 100 V.
-
q.Suit off the power supply when Xylene cyanole dye runs for 80%–90% of the gel. This may take in 1.5–2 h.Note: A DNA ladder can be loaded on the gel and used as control for fractionation and transfer steps.
-
a.
RNA transfer to nylon membranes
Timing: 2–3 h (for step 2)
This is the critical step for successful detection of dsRNA by immunoblotting.
-
2.Perform the wet transfer:
-
a.Take 2 thick blotting filter paper and cut them off slightly larger than the size of the membrane.
-
b.Soak sponges and blotting filter paper in the 0.5× TBE (transfer buffer).
-
c.Carefully open the gel cassette, removing the short glass plate and soaking the gel with the spacer glass plate into the transfer buffer.
-
d.Take the spacer glass plate with the gel from the transfer buffer and carefully place a blotting filter paper on top of the gel, invert the montage and remove the spacer glass.
-
e.Open the transfer cassette, on the black side place the soaked sponge and then the blotting filter paper with the gel.
-
f.Soak the positively charged Nylon membrane in 0.5× TBE and then place the membrane on top of the gel, removing bubbles using a roller.
-
g.Add a small amount of transfer buffer and then place a soaked blotting filter paper on top of the membrane.
-
h.Add a soaked sponge on top of the blotting filter paper and carefully close the transfer cassette.
-
i.Place the cassette in the transfer module, with the black side of the cassette facing the black side of the module.
-
j.Fill the tank with transfer buffer and then close the lid.
-
k.In a cold room (4°C–6°C), set up the transfer.
-
l.Perform the transfer for 30–60 min using a voltage of 35 V.
-
m.Cut off the power supply, open the transfer cassette and take the membrane from the sandwich.
-
a.
Note: After RNA transfer to the membrane, stain the remaining gel for 5 min at room temperature with SYBR Gold (1:10,000 dilution in 0.5× TBE buffer) and detect the RNA bands under UV light. No RNA should be visualized in the gel, consistent with an efficiency transfer of RNAs to the membrane.
Alternatives: Before blocking, the RNAs can be crosslinked to the membrane using UV light, 120 mJ/cm2 (autocrosslink mode in a Stratalinker® UV Crosslinker).
Dot blot
Timing: 2 h
Dot blot is a simple form of immunoblotting to detect and quantify dsRNAs. This technique can be used to assess different parameters of the immunoblot, such as antibody efficiency, limit of detection, background, and buffer options.
-
3.Load samples on the Nylon membrane:
-
a.Cut off a piece of a positively charged Nylon membrane with a size of 2 cm × 10 cm, increasing another 2 cm for each new dilution (e.g., 3 samples to be blotted require a 6 cm × 10 cm membrane).
-
b.Prepare a solution of RNA in nuclease-free H2O at a concentration of 0.5 μg/μL and then prepare 10× dilutions (6–8 points in total).
-
c.Using a micropipette, slowly spot 4 μL of samples onto pre-determined locations on the Nylon membrane. Usually, spot centers are separated by 1 cm in the horizontal axis and 2 cm in the vertical axis.
-
d.Let the spots dry before proceeding to the immunoblotting.
-
a.
Alternatives: the RNAs can be crosslinked to the membrane using UV light, 120 mJ/cm2 (autocrosslink mode in a Stratalinker® UV Crosslinker).
Detection of dsRNAs by immunoblotting
Timing: 2 days (for step 4)
After transferring RNAs to Nylon membranes after gel electrophoresis or directly spotting onto membranes by Dot Blot, proceed to the immunoblotting for detection of dsRNAs.
-
4.Block non-specific sites on the membrane:
-
a.Place the RNA membrane into a container with 10 mL of PBS-Tween, with the RNA side of the membrane facing up.
-
b.Prepare 10 mL of blocking solution (50 μg/mL Sheared Salmon DNA, 1× PBS-Tween, 5% non-fat milk wt:vol).
-
c.Remove PBS-Tween from the membrane and add 10 mL of the blocking solution.
-
d.Incubate the membrane overnight, rocking at 4°C.
-
a.
-
5.Probe membranes with anti-dsRNA antibodies:
-
a.Dilute anti-dsRNA in primary antibody solution (PBS-Tween, 2% non-fat dry milk wt:vol).
-
b.Remove the blocking solution from the membrane and replace with 10 mL of primary antibody solution.
-
c.Incubate for 2 h at room temperature in a rocking shaker.
-
a.
Note: The appropriate anti-dsRNA dilution will need to be established when testing these antibodies for the first time in different cell and virus systems.
Alternatives: Incubating the primary antibody for 16 h at 4°C may increase sensitivity.
-
6.Incubate with secondary antibody:
-
a.Remove the primary antibody and transfer the solution into a 15 mL conical tube for further reuse.
-
b.Wash the membrane three times with 10 mL of PBS-Tween for 10 min at room temperature while rocking.
-
c.Prepare the secondary antibody dilution (Anti-HRP, Goat-Anti-Rabbit for 9D5 and Goat-Anti-Mouse for J2, 1:10,000 in PBS-Tween, 2% non-fat dry milk wt:vol).
-
d.Replace the PBS-Tween with 10 mL of the secondary antibody solution.
-
e.Incubate for 1 h at room temperature with agitation.
-
f.Remove secondary antibody solution.
-
g.Wash three times with 10 mL of PBS-Tween for 10 min at room temperature while rocking.
-
a.
-
7.Chemiluminescence detection:
-
a.Cut a piece of plastic and lay onto the chemiluminescence detection tray.
-
b.Remove the RNA membrane from the PBS-Tween, drain the excess of liquid using a paper towel and place the membrane on top of the plastic.
-
c.Add 250–500 μL of ECL substrate on top of the membrane and ensure the homogenous distribution throughout the membrane.
-
d.Incubate for 1 min and cover with another piece of plastic on top of the membrane and remove air bubbles.
-
e.Set the auto-exposure mode for detection of chemiluminescence on to the Fusion FX imager. Test different exposure times to find the best image.
-
f.Carefully transfer the membrane back into the western blot incubation box and wash three times with 10 mL of PBS-Tween.
-
g.Dry the membrane on top of a paper towel with the RNA faced up at room temperature.
-
h.Carefully wrap the membrane in filter paper and store at 4°C if necessary.
-
a.
Note: If the detection of dsRNA fails or displays a weak signal, please refer to troubleshooting problems 1 and 2, respectively. In the case dsRNA bands were not well resolved in 3.5% polyacrylamide gels, please refer to troubleshooting problem 3.
Immunofluorescence of dsRNA
Timing: 3–5 days
Immunofluorescence (IFA) is a technique to analyze many aspects of spatial (subcellular and tissue) distribution of dsRNA species during virus infection.
The steps below describe the procedure to perform IFA in Drosophila S2 cells. S2 cells are semi-adherent cells, and in our hands, the culture works better maintaining them as non-adherent. We usually perform IFA using coverslips, but S2 cells also adhere well in chambered cell culture slides.
-
8.Prepare S2 cell culture for viral infections:
-
a.Count and transfer 107 cells into 15 mL conical tubes. Use cell densities between 3–6 × 106 cells/mL.
-
b.Infect S2 cells with 105 pfu of IIV-6 (0.01 pfu/cell) and 107 pfu of DCV (1 pfu/cell). For mock infected controls, use the equivalent amount of complete Schneider’s media.
-
c.Incubate for 1 h at 25°C on a rotating shaker or manually rocking cells every 10 min.
-
d.Centrifuge cells at 200 g for 5 min at 4°C.
-
e.Add fresh media to the cells and resuspend them at density of 1 × 106 cells/mL with 10 mL serological pipettes.
-
f.Homogenize and transfer 2 mL of the cell suspension into each well in 6-well plates.
-
g.Incubate S2 cells at 25°C.
-
a.
Note: Virus-derived dsRNAs are better analyzed at 72 h post infection for IIV-6 and at 24 h for DCV.
Optional: IIV-6 replicative DNA can be marked with BrdU. To mark IIV-6 DNA, 70 h post IIV-6 infection, S2 cells are homogenized and BrdU added at a final concentration of 20 μM. S2 cells are incubated for 1 h at 25°C and then transferred to coverslips for cell attachment for one hour.
-
9.Sterilize coverslips:
-
a.In the culture hood, fill 2 wells of a 6-well plate with 100% ethanol and 1 well with sterilized H2O (DEPEC-tread H2O is suitable here).
-
b.With forceps, soak 13 mm coverslips in the first well with 100% ethanol for at least 5 s.
-
c.Transfer single coverslips from 100% ethanol into sterilized double-distilled water (ddH2O) for 5 s and then to the second well with 100% ethanol.
-
d.In an inverted 6-well plate lid, place coverslips on its walls to dry. Repeat these steps for all remaining coverslips.
-
e.With forceps, drop single coverslip into wells in a 12-well plate.
-
f.Add 500 μL of Poly-L-lysine (0.01% wt:vol) or Concanavalin A (ConcA, 0.5 mg/mL) solution diluted in 1× PBS to each well of the 12-well plate. Incubate within the hood for 1 h and then wash once with 500 μL of 1× PBS.
-
a.
-
10.Transfer infected S2 cells to poly-L-Lysine- or Concanavalin A (ConcA)-treated coverslips:
-
a.At each indicated time point, remove the plates with infected cells from the incubator.
-
b.Resuspend cells into the media and homogenize.
-
c.Transfer 500 μL of the cell suspension into the wells of the 12-well plate containing the coverslips.
-
d.Incubate cells 1 h at 25°C to allow cell attachment.
-
a.
-
11.Fix and permeabilize cells:
-
a.Remove the media and fix cells with 4% formaldehyde solution (PFA) for 10–15 min at room temperature.
-
b.Wash three times with 1× PBS.
-
c.Incubate 5 min at room temperature.
-
d.Permeabilize fixed cells adding 500 μL of PBSTX into each well and incubate for 10 min at room temperature. Repeat once this action.
-
a.
Optional: After permeabilization, coverslips can be treated with RNases to confirm whether the signal originates from dsRNA. We use the RNase mix A/T1 (Thermo) and Short Cut RNase III (NEB) to verify the origin of IFA signal, as the latter digests dsRNA but the former should not affect the signal. For this step, the use of Chambered Cell Culture Slides or 24-well plates is more convenient to save reagents.
RNase A/T1 reaction
| Reagent | Amount |
|---|---|
| RNase A/T1 mix | 2 μL |
| 10× buffer | 10 μL |
| H2O | 88 μL |
Short Cut RNase III reaction
| Reagent | Amount |
|---|---|
| RNase III | 2 μL |
| 10× buffer | 10 μL |
| 10× MnCl2 | 10 μL |
| H2O | 78 μL |
-
12.Block non-specific sites and incubate with anti-dsRNA antibodies:
-
a.Remove the PBSTX and add 500 μL of IFA blocking buffer into each well and incubate for 1 h at room temperature with gentle rocking.
-
b.During this time, prepare the primary antibody solution in IFA blocking buffer.Note: The appropriate anti-dsRNA dilution will need to be established when testing these antibodies for the first time in different cell and virus systems. We tested different dilutions of anti-dsRNAs, especially 9D5 antibody that we had less information than the most cited J2 antibody.
-
c.On a Parafilm placed in the bottom of a 90 mm culture dish, add 25–50 μL of the anti-dsRNA solution for each sample (4–6 droplets/culture dish).
-
d.After blocking and using a pipette tip and forceps, take coverslips from the 12-well plate and place them face down on the anti-dsRNA droplet.
-
e.Add cotton rolls imbibed on water in the 90 mm culture dish without touching the Parafilm.
-
f.Cover the 90 mm culture dish with the lid and incubate overnight at 4°C.Optional: Anti-dsRNA antibodies can be combined with other primary antibodies for co-staining. We usually combine dsRNA detection with IIV-6 DNA staining using BrdU or nuclear envelope using Lamin0.
-
a.
-
13.Incubate with Fluorophore-conjugated antibody solution:
-
a.Add 500 μL of PBSTX into wells in a 12-well plate.
-
b.Carefully, remove coverslips from the culture dish, transfer them into 12-well plate containing PBSTX, now face up. Wash three times with PBSTX for 5 min each.
-
c.During this time, prepare the secondary antibody solution in PBSTX, adding 1:300 Goat anti-mouse IgG Alexa Fluor 488 for detection of the monoclonal antibody (mAb) J2, 1:300 Goat anti-rabbit IgG Alexa Fluor 546 for detection of 9D5 and 1:500 Hoechst for DNA staining.Note: The presence of other targets requires a combination of different Fluorophore-conjugated antibodies for visualization.
-
d.Change the Parafilm in the 90 mm culture dish, add 25–50 μL droplets of the secondary antibody solution for each sample (4–6 droplets/culture dish).
-
e.Take the coverslips from the washing solution and place the coverslips face down on the droplets.
-
f.Add cotton rolls imbibed on water in the 90 mm culture dish, cover with dish lid and wrap the culture dish with aluminum foil paper to protect from light.
-
g.Incubate for 1 h at room temperature.
-
a.
-
14.Mount slides for microscopy:
-
a.Add 500 μL of PBSTX into wells in a 12-well plate.
-
b.Carefully, remove coverslips from the culture dish, transfer them into 12-well plate, now face up. Wash three times with PBSTX for 5 min each.
-
c.Remove the PBSTX and add 500 μL of 1× PBS into wells with coverslips.
-
d.Clean microscopy slides with 70%–100% ethanol using a lint-free wipe.
-
e.Add 10 μL of Hydromount medium onto microscope slides.
-
f.Carefully place the coverslip face down onto the Hydromount droplets.
-
g.Transfer the slides to a light-protective container and store at room temperature overnight.
-
h.Using transparent nail polish, seal the rim of the coverslips and transfer the slides to a light-protective container at room temperature for nail polish drying (1–2 h).
-
i.Store slides at 4°C until imaging. For long storage, keep slides at −20°C.
-
a.
Note: We usually mount at least one coverslip for mock and another from infected cells in the same slide to prevent unwanted noise caused by differences between slides.
Alternatives: We have used different mounting media, like DAKO (Agilent Technologies) and VectaShield (Vector Laboratories) with good results. When the mounting medium have DAPI, such as VectaShield H-1200, the Hoechst addition in the secondary antibody solution must be omitted.
CRITICAL: Bubbles may form in the medium during mounting and should be avoided.
-
15.Perform wide-field fluorescence or confocal microscopy:
-
a.Select the proper objective lens and laser wavelengths to capture images.
-
b.Adjust the laser power, detector gain, resolution (number of pixels per image), single or stack acquisition and file format to save images.
-
a.
Note: Set the parameters to obtain the appropriate signal to differentiate dsRNA levels in virus infected compared to the background signal present in mock cells.
Note: If the detection of dsRNA fails or displays a weak signal in the IFA, please refer to troubleshooting problems 1 and 2, respectively.
Expected outcomes
The protocols described here are designed for dsRNA detection by IFA and immunoblotting using different antibodies (Figure 1). Overall, the 9D5 antibody was more sensitive for dsRNA detection using both IFA and immunoblotting than the J2 antibody. Using Dot blot to determine the sensitivity, 9D5 was able to detect as low as 20 pg of dsRNA while J2 required at least 200 pg for a clear signal (Figure 2). Based on the detection limit for these antibodies, we observed that DCV generates abundant dsRNAs during infection ranging from 1%–10% of the total RNA in infected cells (Figure 2). 9D5 also performed well in the detection of dsRNA in DCV infected cells by immunoblotting (Figure 3). The increased sensitivity of 9D5 compared to J2 was also evidenced by the capacity to detect dsRNAs in cells infected with IIV-6, an insect DNA virus that generates low levels of dsRNAs compared to DCV (Figure 3).1 Both 9D5 and J2 performed well at detecting dsRNA in DCV infected cells by IFA (Figure 4). Using RNases to target specific RNA species, we confirmed that the staining by 9D5 in the IFA was depleted by RNase III that degrades dsRNA but not a mix of ssRNA specific nucleases (Figure 5). Using markers for organelles, such as Lamin to stain the nuclear envelope, we observed that dsRNA accumulates in the nucleus of IIV-6 infected cells (Figure 6). It is important to note that dsRNA is an intermediate of RNA virus replication and, in many cases, can be used to show the site of virus replication by IFA. Viral sensors and antiviral defenses can also be identified by tracking dsRNA in infected cells since it is also an immunostimulatory molecule.
Figure 2.
Determining the sensitivity of anti-dsRNA antibodies using dot-blot
(A and B) Representative Dot blot images comparing limits of detection of anti-dsRNA 9D5 (A) and J2 (B) antibodies. Synthetic dsRNA from GFP sequence, total RNA from mock- or DCV-infected S2 cells (1 pfu/cell, 24 h post infection) were spotted onto Nylon membrane and probed with anti-dsRNA 9D5 (dilution 1/6000) and J2 (1/2000) antibodies. 9D5 was at least 10 times more sensitive than J2 to detection synthetic and DCV-derived dsRNAs.
Figure 3.
dsRNA detection in virus-infected cells using immunoblotting
Representative images of dsRNA detection by immunoblotting using anti-dsRNA 9D5. RNA samples were resolved in non-denaturing polyacrylamide gels and then transferred onto nylon membranes and probed with anti-dsRNA 9D5 (dilution 1/6000).
(A) Immunoblotting images comparing limits of detection of dsRNAs in total RNA from DCV-infected cells using anti-dsRNA 9D5.
(B) Immunoblotting images comparing detection of dsRNAs derived from mock-, IIV-6- or DCV-infected cells, and synthetic dsRNAs. IIV-6 generates moderate dsRNA levels when compared to DCV-infected cells.
Figure 4.
Optimization of antibody dilution for detection of dsRNA in virus-infected cells
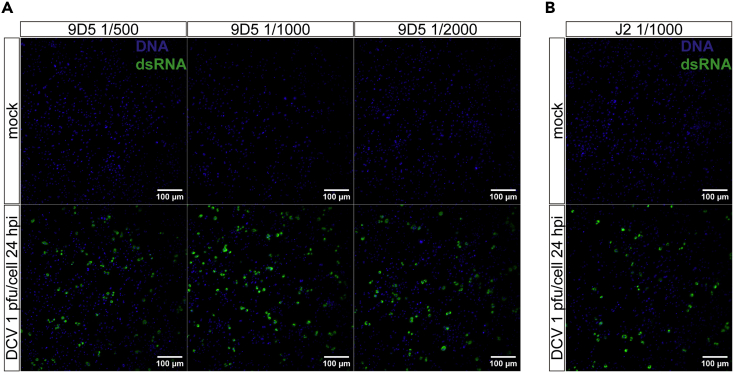
(A) Representative IFA images of dsRNAs in DCV-infected S2 cells. DCV-infected S2 cells (1 pfu/cell, 24 h post infection). For immunofluorescence, different dilutions of the anti-dsRNA 9D5 were tested, with similar results. Scale bars: 100 µm.
(B) dsRNAs from DCV-infected cells were also visualized with anti-dsRNA J2. Images were acquired in a Spinning Disk confocal microscopy (Zeiss). Scale bars: 100 µm.
Figure 5.
Testing the specificity of anti-dsRNA antibodies using RNases
(A) Representative IFA images of dsRNAs (9D5, dilution 1/2000) in mock-infected S2 cells treated with RNase III. Scale bars: 100 µm.
(B) Representative IFA images of dsRNAs in DCV-infected S2 cells (1 pfu/cell, 24 hpi) treated with RNase III. Scale bars: 100 µm.
(C) Representative IFA images of dsRNAs in DCV-infected S2 cells (1 pfu/cell, 24 hpi) treated with RNase A/T1 mix (specific single strand RNases). Images were acquired in a LSM 780 confocal microscopy (Zeiss). Scale bars: 100 µm.
Figure 6.
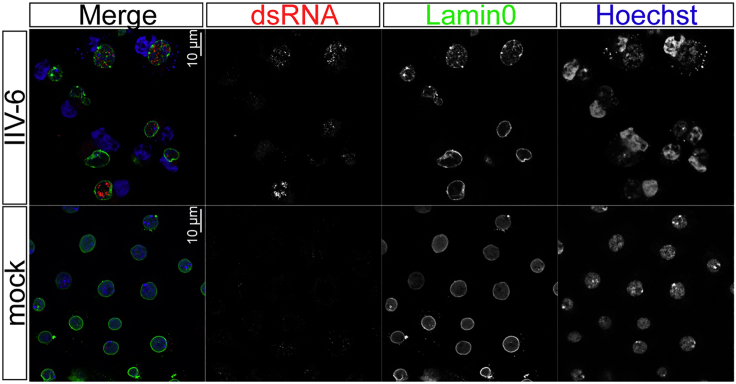
Labeling the nuclear membrane as a strategy to define the subcellular localization of dsRNA in virus-infected cells
Representative IFA images of dsRNAs (9D5, dilution 1/2000) in mock- and IIV-6-infected S2 cells (0.01 pfu/cell, 72 h post infection). Most IIV-6-derived dsRNAs accumulate within the nucleus of infected cells, marked using anti-Lamin0 antibody. Images were acquired in a LSM 880 confocal microscopy, using Airyscan mode (Zeiss). Scale bars: 10 µm.
Our protocol can be applied to infections by different types of viruses with varying genome structures although antibody selection and optimal conditions for detection may differ from our experiment. In addition, the anti-dsRNA antibodies can be used for immunoprecipitation and enrichment of dsRNA from complex samples. This step, coupled to high throughput sequencing, can also be used for identification of dsRNA species in tissues or organisms that can lead to virus discovery.
Quantification and statistical analysis
Immunofluorescence
-
1.
Open images in imageJ.
-
2.
Use ImageJ to analyze different aspects of dsRNA detection using IFA.
-
3.
For dsRNA channel, adjust Minimum and Maximum, setting same parameters for all images analyzed.
-
4.
To compare other channels, perform the same analysis of step 3.
-
5.
Set scale bar and add the scale to images.
-
6.
Convert images to RGB files.
-
7.
Mount panels and save images as TIFF.
-
8.
Use Photoshop or Illustrator to create final panels.
Immunoblotting
-
9.
Open TIFF images in ImageJ.
-
10.
Adjust Brightness and Contrast if needed.
-
11.
If required, use ImageJ to quantify bands or the presence of dsRNA in each lane from the immunoblotting.
-
12.
Export measurement data and analyze using Excel files.
-
13.
Plot results.
Note: ImageJ or Illustrator can be used to remove empty lanes or to crop the membrane to show only regions of interest. Do not alter the original data.
Limitations
Although most viruses generate dsRNA during infection, their abundance may vary considerably, which affects detection using anti-dsRNA antibodies. Using 9D5, we detected 20 pg of dsRNA in the sample, indicating that the technique is sensitive enough to detect low amounts of dsRNA (Figure 2). If we compare dsRNA detection in cells infected by IIV-6 and DCV, it is clear that the latter generates different larger amounts of dsRNA (Figure 3). In the case of IIV-6, detection of dsRNAs required many adjustments of multiplicity of infection and kinetics to make sure we observed a signal above the detection limit (Figure 3). Of note, we could not directly visualize dsRNAs using IFA or immunoblotting at 6 h post IIV-6 infection, but dsRNA precursors (sense and antisense transcripts) were detected by strand specific PCR and dsRNA immunoprecipitation (dsRIP).1 Furthermore, we detected small interfering RNAs (siRNAs) from IIV-6, that originate from dsRNA precursors recognized by the antiviral RNA interference (RNAi) pathway.1 Thus, if the virus subjected to study generates low amounts of dsRNA, a prior step of dsRNA immunoprecipitation (dsRIP) to enrich the sample for dsRNAs species can be performed before the immunoblotting.
Another important limitation for detection of dsRNAs in virus-infected cells is that endogenous dsRNAs species can sometimes be found at very high concentrations depending on cell type and conditions. Although it is expected that virus infections generate dsRNAs at concentrations far above endogenous sources, non-infected cells are essential controls to determine the background of endogenous dsRNA detection (Figures 2, 3, 4, 5, and 6). This is specially concerning for viruses that generate low amounts of dsRNA such as IIV-6 (Figures 3 and 6). However, the detection of dsRNA in virus-infected cells does not necessarily mean that these have viral origin since endogenous dsRNA species may increase in response to the infection. Final characterization of dsRNA species from infected cells requires that these are sequenced to identify their exact origin.
Another limitation is the separation of specific dsRNA species using RNA electrophoresis in polyacrylamide gel. The manipulation of 3.5% polyacrylamide gels is complex, as the gel is fragile and can break and deform easily. Non-denaturing agarose gels can be used as an alternative for separation of dsRNAs prior to the immunoblotting, but they are less sensitive. Nevertheless, we observed better separation of individual dsRNA species in total RNAs from DCV-infected cells in the immunoblotting when using non-denaturing agarose gels.
Troubleshooting
Problem 1
Weak signal for dsRNA using IFA or immunoblotting (Related to steps 5–7, 12, and 15).
Potential solution
Each model of virus infections requires optimization of dsRNA detection, and one should always test different anti-dsRNA antibodies. Here, we report the use of 9D5 and J2 antibodies, but others can also be used such as K1 antibody. In our hands, 9D5 was more sensitive to dsRNA detection, specially using immunoblotting. In each case, check the limit detection using synthetic dsRNAs to confirm that the immunoblotting is working properly. To ensure that the detection by immunoblotting is robust, always load internal controls such as synthetic dsRNAs or RNAs derived from cells infected with viruses that generate abundant dsRNAs for immunoblotting detection of dsRNAs. For the IFA, consider the transfection of synthetic dsRNAs or dsRNA mimics such as poly I:C to analyze the efficiency of dsRNA detection in cells.
Test different times post infection and multiplicities of infection. Some viruses, like IIV-6, just generate detectable dsRNAs in late stages of virus cycle. Combining different amounts of virus and multiple times post infection will help find the optimal conditions for dsRNA detection.
Overall, we recommend using 10 μg of total RNA as input for the immunoblotting detection of dsRNAs, but higher RNA amounts can be resolved in the RNA electrophoresis to improve detection. Another option is to include a step of immunoprecipitation using anti-dsRNA antibodies (dsRIP) as an enrichment step prior to gel separation and immunoblotting.
For immunoblotting detection of dsRNAs, changes in antibody concentration, incubation times, use of ECL reagents with higher sensitivity and different settings on the chemiluminescence imager can also improve detection.
Problem 2
High background (Related to steps 5, 7 and 11–15).
Potential solution
Test different anti-dsRNA antibodies. Background during dsRNA detection may be derived from endogenous dsRNAs or non-specific staining. In our case, the anti-dsRNA J2 showed higher levels of background detection in mock S2 cells than 9D5. Since J2 detects dsRNA >40 bp and 9D5 >100 bp, an explanation is that most endogenous dsRNAs in S2 are shorter than 100 bp and, thus, better detected by J2. Alternatively, these antibodies have different degrees of non-specific staining that may vary in cell lines.
Test different concentrations of anti-dsRNA antibody for your assays to find the best signal-to-noise ratio. Adjustments in antibody concentrations that differ from those established in this protocol may be required to fine tune the detection of virus dsRNAs above cellular background levels in different systems. Also check the manufacture’s recommendations and previous publications that used these antibodies in different cell and virus systems. For the immunoblotting detection, internal controls can determine the maximum dilution of the antibody without affecting the detection limit.
Another step that affects background detection in the immunoblotting is the image acquisition system. Of note, the background detected by each antibody may be different for specific assays. For example, we acquired images of dsRNA immunoblotting using high sensitivity X-ray films. Although J2 blot showed low background in the X-ray film, 9D5 immunoblotting displayed considerably high background and we could not improve detection using this method. Then, images were efficiently captured with a chemiluminescence imager (Fusion FX), with improved signal-to-noise ratios.
The background can be a consequence of materials and reagents used to perform dsRNA detection. If the high background persists in our system, consider treating your samples with RNases. Comparing the signal in samples treated with specific RNases for dsRNAs and single stranded RNases can determine if the signal truly comes from dsRNA species.
Problem 3
Poor separation or resolution of dsRNAs with high molecular weight in the RNA electrophoresis (Related to step 1).
Potential solution
Try the RNA electrophoresis in non-denaturing agarose gels instead of polyacrylamide. However, we could not detect dsRNAs in the immunoblotting with the same efficiency when the RNA electrophoresis was done in agarose compared to polyacrylamide gels. However, the electrophoresis in agarose gel is very flexible and is a robust method to separate RNAs with high molecular weights. Changes in the types of agarose powder (e.g., low melting agarose), running buffers and transfer systems could improve the dsRNA detection by immunoblotting, although we did not test many different conditions during our set up.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, João Trindade Marques (jtm@ufmg.br).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We would like to thank all members of the Marques and Imler laboratories for assistance and discussions. We thank Francesco Bergami, Laurent Daeffler, and Juliana Alves-Silva for technical and methodological assistance. We also thank the UFMG microscopy facility (CAPI) for image acquisition. This work of the Interdisciplinary Thematic Institute IMCBio, as part of the ITI 2021–2028 program of the Institute for Advanced Studies of the University of Strasbourg, CNRS, and Inserm, was supported by IdEx Unistra (ANR-10-IDEX-0002), by the SFRI-STRAT’US project (ANR 20-SFRI-0012), and by EUR IMCBio (IMCBio ANR-17-EURE-0023) under the framework of the French Investments for the Future Program as well as from the previous Labex NetRNA (ANR-10-LABX-0036). This work has been also supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Rede Mineira de Imunobiológicos (grant REDE-00140-16), Rede Mineira de Biomoléculas (grant REDE-00125-16), Instituto Nacional de Ciência e Tecnologia de Vacinas (INCTV), Institute for Advanced Studies of the University of Strasbourg (USIAS fellowship 2019), and Fonds régionale de coopération pour la recherche FRCT2020 Région Grand-Est (ViroMod) to J.T.M. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 to J.T.M. I.J.S.d.F. was supported with fellowships by CNPq and CAPES.
Author contributions
I.J.S.d.F., J-L.I., and J.T.M. designed the experiments. I.J.S.d.F. optimized and conducted the experiments. J-L.I. and J.T.M. were responsible for funding acquisition. I.J.S.d.F. and J.T.M. wrote the paper. All authors read and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Isaque J.S. de Faria, Email: isaquejsf@gmail.com.
João T. Marques, Email: jtm@ufmg.br.
Data and code availability
All the data are available from the authors upon request.
References
- 1.de Faria I.J.S., Aguiar E.R.G.R., Olmo R.P., Alves da Silva J., Daeffler L., Carthew R.W., Imler J.-L., Marques J.T. Invading viral DNA triggers dsRNA synthesis by RNA polymerase II to activate antiviral RNA interference in Drosophila. Cell Rep. 2022;39:110976. doi: 10.1016/j.celrep.2022.110976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schönborn J., Oberstrass J., Breyel E., Tittgen J., Schumacher J., Lukacs N. Monoclonal antibodies to double-stranded RNA as probes of RNA structure in crude nucleic acid extracts. Nucleic Acids Res. 1991;19:2993–3000. doi: 10.1093/nar/19.11.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Son K.-N., Liang Z., Lipton H.L. Double-stranded RNA is detected by immunofluorescence analysis in RNA and DNA virus infections, including those by negative-stranded RNA viruses. J. Virol. 2015;89:9383–9392. doi: 10.1128/JVI.01299-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber F., Wagner V., Rasmussen S.B., Hartmann R., Paludan S.R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemp C., Mueller S., Goto A., Barbier V., Paro S., Bonnay F., Dostert C., Troxler L., Hetru C., Meignin C., et al. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J. Immunol. 2013;190:650–658. doi: 10.4049/jimmunol.1102486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olmo R.P., Ferreira A.G.A., Izidoro-Toledo T.C., Aguiar E.R.G.R., de Faria I.J.S., de Souza K.P.R., Osório K.P., Kuhn L., Hammann P., de Andrade E.G., et al. Control of dengue virus in the midgut of Aedes aegypti by ectopic expression of the dsRNA-binding protein Loqs2. Nat. Microbiol. 2018;3:1385–1393. doi: 10.1038/s41564-018-0268-6. [DOI] [PubMed] [Google Scholar]
- 7.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are available from the authors upon request.