Abstract
Background
Exploring the brain reorganization patterns associated with language recovery would promote the treatment of global aphasia. While functional near-infrared spectroscopy (fNIRS) has been widely used in the study of speech and language impairment, its application in the field of global aphasia is still limited.
Aims
We aimed to identify cortical activation patterns of patients with global aphasia during naming and repetition tasks.
Methods and procedures
We recruited patients with post-stroke aphasia from the Department of Rehabilitation Medicine at Huashan Hospital. These individuals were diagnosed with global aphasia without cognitive impairments, as assessed by speech-language pathology evaluations. Age- and sex-matched healthy controls were recruited from the greater Shanghai area. During fNIRS measurement, patients and healthy controls completed the picture-naming and phrase repetition task. Cortical activation patterns on each of these language tasks were then compared between groups.
Outcomes and results
A total of nine patients with global aphasia and 14 healthy controls were included in this study. Compared with the healthy subjects, patients with global aphasia showed increased activation in the left Broca's area, middle temporal gyrus (MTG), superior temporal gyrus (STG), and pre-motor and supplementary motor cortex (SMA) (p < 0.05) in the picture-naming task. Furthermore, the latency of the oxyhemoglobin (HbO) concentration in the left supramarginal gyrus (SMG) region had a strong negative correlation with their score of the naming task (p < 0.01). In the phrase repetition task, decreased activation was detected in the left SMA and SMG (p < 0.05) of patients relative to controls.
Conclusion
The left SMG plays a critical role in the language function of patients with global aphasia, especially in their abilities to name and repeat. fNIRS is a promising approach to revealing the changes in brain activities in patients with aphasia, and we believe it will contribute to a deeper understanding of the neurological mechanisms and the establishment of a novel treatment approach for global aphasia.
Keywords: global aphasia, fNIRS, picture naming, repetition phrase, left SMG
Introduction
Aphasia is a common functional disorder following a stroke and affects about 24–50% of all stroke survivors (1). In an observational study, global aphasia was the most commonly observed aphasia (33.33%) (2). Global aphasia is the most severe form of post-stroke aphasia and is characterized by severe impairments in all language modalities. The common specific injury sites include Broca's and Wernicke's areas, which can arise after massive strokes (3–5). Although some patients with global aphasia do not have cognitive deficits (6), they cannot express their thoughts correctly and appropriately, and their comprehension is severely restricted. The pattern of language impairment in patients with global aphasia is still controversial. For instance, Sarno proposed that patients with global aphasia retain no propositional language abilities (7). However, the measurements that they used emphasized too much on expressive language production and language comprehension to accurately reflect the language function of patients with severe aphasia (8). Several studies utilizing computerized visual communication showed that patients with global aphasia retain a degree of language ability (9–11). To date, there are still difficulties in assessing the residual language ability of patients with global aphasia.
The promotion of functional neuroimaging has led to a deeper understanding of patients with global aphasia. Functional magnetic resonance imaging (fMRI) is the most common imaging approach, which has helped reveal the damage pattern of brain networks and the mechanism underlying treatment-induced plasticity. For instance, researchers have found that the most consistently active regions during semantic word processing in patients with global aphasia are the left posterior temporal and right posterior parietal cortex. The recovery of the lexical-semantic system was associated predominantly with the activations of these regions (12). Resting-state fMRI (rs-fMRI) showed that patients with global aphasia had decreased interhemispheric and intra-hemispheric connectivity and enhanced connection between language network and cerebellar structures (13). These studies show that the utilization of neuroimaging techniques is feasible for research on global aphasia. Nevertheless, certain disadvantages of fMRI may limit its application in post-stroke aphasia (14). For example, motion artifacts will interfere with the neuron activation signals. In addition, the loud noise during scanning can affect language processing. Individuals with electronic medical implants are unsuited for an fMRI study. All of these factors affect the generalizability of the study.
Despite functional near-infrared spectroscopy (fNIRS) being a mature and feasible neuroimaging method, it is only now becoming an increasingly popular technique. Within the activated cortical region, oxyhemoglobin (HbO) increases and deoxyhemoglobin (HbR) decreases, and the changes in HbO and HbR can be measured by NIR light to reflect cortical activation. Two irreplaceable advantages of fNIRS for language studies are ecological validity and compatibility with other techniques (15). fNIRS can be compatible with facial and jaw movements for broader application in language production processes (16, 17). Moreover, it does not produce a magnetic field and is silent and comfortable for the user. Despite the feasibility and advantage, its application in aphasia research is restricted, especially for patients with global aphasia. In reality, for the past 20 years, although the number of published articles related to fNIRS has doubled every 3.5 years, only five of them have included individuals with aphasia. Moreover, more focus is placed on altered language functional patterns in patients with mild or moderate aphasia, and individuals with severe or global aphasia have not been included in prior studies to date. Systematic reviews also call for more articles to be published in areas related to aphasia and fNIRS (15).
In conclusion, fNIRS has the potential to significantly advance the field of rehabilitation for global aphasia. However, before it can be used as a therapeutic intervention, it is crucial to understand the activation patterns captured by fNIRS that are exhibited by patients with global aphasia. Therefore, this study applied fNIRS to capture different cortical activation in patients with global aphasia and healthy controls during the process of naming and repetition. We hypothesized that patients with global aphasia would show distinct cortical activation patterns from healthy controls. More specifically, we expected patients with global aphasia to show increased activation in the left hemisphere and decreased activation in other areas of the cortex relative to healthy controls. Therefore, in the present study, fNIRS was utilized to explore the differential cortical activation patterns between patients with global aphasia and healthy controls during the process of naming and repetition, which may provide a novel perspective on the hemodynamic response during language processing in these patients.
Materials and methods
Participants
A total of 14 older healthy individuals with a mean age of 57.61 years (SD: 8.66, age range: 49–77 years) and nine individuals with chronic left-hemisphere stroke-induced global aphasia (mean age; 58 ± 8.15 years, age range: 48–76 years; mean duration post onset: 18.67 ± 6.38 months, range: 9–30 months) were recruited from greater Shanghai area from January 2020 to September 2021. Handedness was assessed with the Edinburgh Inventory (18). All included subjects were native Mandarin speakers. The clinical characteristics between the two groups showed no difference (except for C-WAB). Supplementary Table 1 provides additional details on the clinical characteristics of each participant.
Participants in the stroke group were diagnosed with global aphasia based on the Western Aphasia Battery (Simplified Chinese version) (WAB-AQ; mean: 13.34 ± 6.92, range: 2.6–21.7), and the Non-Language-based Cognitive Assessment (NLCA) was used to exclude patients who had cognitive impairment (19) (mean: 74.11 ± 2.92, range: 70–79). Table 1 presents detailed demographic and clinical information of patients in the global aphasia group. All participants provided written informed consent, and the study was approved by the ethics committee of Huashan Hospital [CHiCTR2000038808]. Supplementary Table 2 provides additional details on WAB scores and lesions for each patient.
Table 1.
Demographics for individuals with global aphasia.
| Patient | Age (years) | Sex | Dominant hand | Time since stroke (months) | Stroke type | C-WAB | NLCA |
|---|---|---|---|---|---|---|---|
| PA1 | 52 | Male | Right | 22 | Infarction | 6.3 | 73 |
| PA2 | 55 | Male | Right | 18 | Infarction | 4.7 | 70 |
| PA3 | 48 | Male | Right | 12 | Infarction | 21.7 | 71 |
| PA4 | 68 | Male | Right | 30 | Hemorrhage | 12.7 | 76 |
| PA5 | 55 | Male | Right | 9 | Hemorrhage | 13 | 73 |
| PA6 | 56 | Female | Right | 14 | Infarction | 19.8 | 75 |
| PA7 | 54 | Male | Right | 21 | Infarction | 19.3 | 72 |
| PA8 | 58 | Male | Right | 26 | Hemorrhage | 2.6 | 79 |
| PA9 | 76 | Female | Right | 16 | Hemorrhage | 20 | 78 |
Experimental procedure
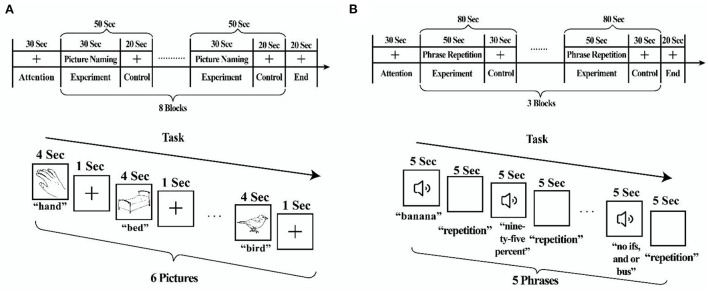
During fNIRS scanning, participants completed two runs of picture-naming and phrase repetition tasks in consecutive order (Figure 1). In a practice session before the fNIRS scanning session, tasks were performed to familiarize the participants.
Figure 1.
fNIRS behavioral tasks. (A) Picture-naming task. (B) Phrase repetition task.
The picture-naming task (20) comprised 48 pictures from the S&V database of black and white line drawings (21). The task paradigm was a periodic block design with the experimental block (30 s) and control block (20 s). Each block was repeated eight times for an overall task time of 7.5–8 min/run. For the experimental blocks, participants were shown six pictures (e.g., hand) and asked to name them aloud. For the control blocks, participants were shown a black fixation crosshair. Responses during the picture-naming task were recorded to ensure accuracy.
The phrase repetition task (22) comprised 15 phrases selected from the Western Aphasia Battery or Boston Diagnostic Aphasia Examination. The task paradigm was a periodic block design with the experimental block (50 s) and control block (30 s). Each block was repeated three times for an overall task time of 5 min/run. For the experimental blocks, participants were asked to listen to five series of words carefully through earphones (e.g., banana) and subsequently had to repeat the phrases out aloud. For the control blocks, they were shown a black fixation crosshair. Supplementary Table 3 provides additional details on behavioral data for each participant.
In this experiment, continuous-wave fNIRS measurements (NirScan, Huichuang, China) were utilized to capture the HbO signals and HbR signals of the participants' scalp and cortex. The sampling frequency was 11 Hz, and the wavelengths were 730 and 850 nm.
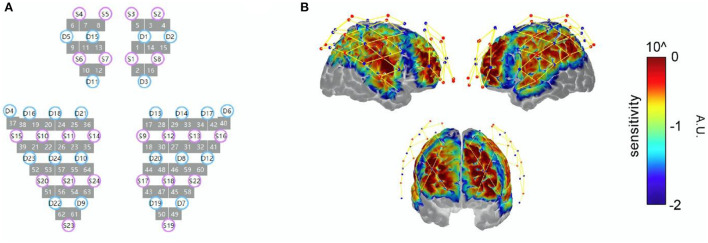
The 64-channel probe (24 sources and 24 detectors) covered the frontal, temporal, parietal, and occipital lobes bilaterally. To obtain the Montreal Neurological Institute (MNI) coordinates of each fNIRS channel (represented by the midpoint location of the source–detector pair), the spatial coordinates of the sources, detectors, and anchor points (Cz, Nz, Iz, AL, and AR) were measured using the electromagnetic 3D digitizer system (FASTRAK, Polhemus, USA). Then, the cortical sensitivity of each channel was displayed on the MNI “Colin27” brain template by using the AtlasViewer (23) (Figure 2). Supplementary Table 4 shows the exact location of each channel.
Figure 2.
(A) fNIRS probe design; sources are represented by S, while detectors are represented by D and channels with lines between S and D. The arrangement of the optodes with an interoptode distance of 30 mm. (B) Sensitivity profile; sources are displayed with red dots, while detectors are displayed with blue dots and channels with yellow lines. Results of the Monte Carlo simulation based on 1 × 10−8 photons over the frontal, parietal, temporal, and occipital lobes: colors represent the spatial sensitivity of the fNIRS measurements. Warmer colors suggest higher sensitivity to the cortex, while cooler colors suggest lower sensitivity to the cortex.
Channels were assigned to the following regions of interest (ROIs) using their average MNI coordinate location: the dorsolateral prefrontal cortex (DLPFC), Broca's area (Broca), pre-motor and supplementary motor cortex (SMA), middle temporal gyrus (MTG), superior temporal gyrus (STG), supramarginal gyrus (SMG), and angular gyrus (AG). Table 2 presents information on the channel assignment to ROI.
Table 2.
Channels assigned to regions of interest.
| ROI | Left hemisphere | Right hemisphere |
|---|---|---|
| Dorsolateral prefrontal cortex (DLPFC) | 6, 10, 11, 12 | 2, 4, 14, 16 |
| Broca's area (Broca) | 9 | 15 |
| Pre-motor and supplementary motor cortex (SMA) | 24, 25, 36 | 17, 18, 29 |
| Middle temporal gyrus (MTG) | 37 | 40 |
| Superior temporal gyrus (STG) | 38, 39 | 41, 42 |
| Supramarginal gyrus (SMG) | 52, 53, 55, 57 | 31, 46, 48, 59, 60 |
| Angular gyrus (AG) | 51 | 45, 58 |
fNIRS data analysis and statistics
All fNIRS data were analyzed using HomER2 (24), NIRS-SPM (25), and BrainNet View (26). The processing stream included converting raw data to an optical density, and a 0.01–0.2 Hz Butterworth bandpass filter was applied to remove noise. The optical density was converted to HbO and HbR by using the modified Beer–Lambert law with a differential pathlength factor of 6. In this study, we used the HbO signal to denote the results, which generally has a better signal-to-noise ratio than the HbR signal (27). To estimate the task-related cortical activation, the HbO signals of each channel were analyzed using the general linear model (GLM) with regression to a hemodynamic response function (HRF) model with time and dispersion derivatives as modified for fNIRS. Beta-values served as contrasts, which were speculated using two-sample t-tests. A p-value of < 0.05 was considered to indicate significant differences between the HbO concentration in the experimental and control condition, but significant findings at p < 0.05 level that did not survive Bonferroni adjustment were interpreted and discussed given the exploratory nature of this pilot study. Moreover, to investigate the relationship between the accuracy of picture naming and cortical activation, we applied Spearman's correlation by using the latency of HbO concentration and the accuracy of the naming score. Data were analyzed using SPSS software version 26.0 (IBM Corporation, Armonk, NY, USA).
Results
Picture naming
During the picture-naming task, patients with global aphasia showed significantly increased activation in channels 55 and 57, while significantly decreased activation was detected in channels 9, 37, and 39 in the left hemisphere. No channels in the right hemisphere showed significant differences between the two groups. Significant activations principally included classical left-hemisphere language areas. These were found in the left Broca's area, MTG, STG, and SMG. The details are shown in Figure 3 and Table 3.
Figure 3.
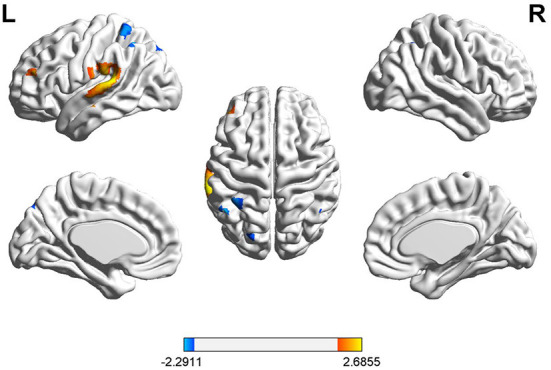
Cortical activation associated with the picture-naming task. HbO activation maps between patients with global aphasia and healthy individuals. The color bar represents the t-statistic. Warmer colors with positive values represent increases in HbO in patients with global aphasia compared with healthy individuals, while cooler colors with negative values represent opposite results (p < 0.05).
Table 3.
Exact location and intensity of the activation during the picture-naming task.
| Channel | MNI coordinates (X/Y/Z) | Cortical region | BA | Proportion | T-value | p-value |
|---|---|---|---|---|---|---|
| Ch.9 | −47/47/18 | L Broca | 45 | 56.39% | 2.37 | 0.027 |
| Ch.37 | −69/−13/−7 | L MTG | 21 | 85.44% | 2.30 | 0.032 |
| Ch.39 | −69/−34/17 | L STG | 22 | 74.15% | 2.69 | 0.014 |
| Ch.55 | −40/−50/67 | L SMG | 40 | 40.08% | −2.16 | 0.043 |
| Ch.57 | −50/−52/57 | L SMG | 40 | 89.84% | −2.29 | 0.032 |
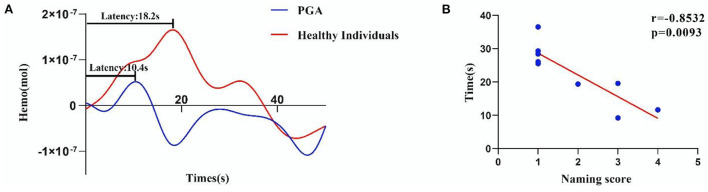
At the same time, we found that the latency of HbO concentration in the left SMG had a significant negative correlation with the naming score in patients with global aphasia (r = −0.8532, p = 0.0093) (Figure 4).
Figure 4.
Significant correlation between the naming score and latency of HbO concentration in the left SMG (r = −0.8532, p = 0.0093). (A) The average HRF of the left SMG in patients with global aphasia and healthy individuals. (B) The accuracy of HbO concentration in the left SMG was negatively correlated with the naming score (p < 0.01) (The latency of HbO concentration and the naming score in some patients are very similar (refer to Supplementary Table 5 for more detail).
Phrase repetition
During the phrase repetition task, a significant HbO decrease was found in three channels (25, 55, and 57) in the left hemisphere of patients with global aphasia relative to healthy individuals. These were detected in the left pre-motor, SMA, and SMG. Anatomical labeling and spatial probability were estimated for the significantly activated channels (Figure 5 and Table 4).
Figure 5.
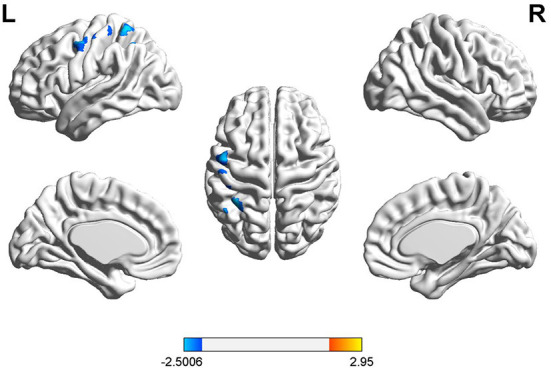
Cortical activation associated with phrase repetition task. HbO activation maps between patients with global aphasia and healthy individuals, and the color bar represents the t-statistic. Cooler colors with negative values represent decreases in HbO in patients with global aphasia compared with healthy individuals (p < 0.05).
Table 4.
Exact location and intensity of activation during phrase repetition task.
| Channel | MNI coordinates (X/Y/Z) | Cortical region | BA | Proportion | T-value | p-value |
|---|---|---|---|---|---|---|
| Ch.25 | −50/−1/55 | L SMA | 45 | 98.39% | −2.43 | 0.024 |
| Ch.55 | −40/−50/67 | L SMG | 40 | 40.08% | −2.50 | 0.021 |
| Ch.57 | −50/−52/57 | L SMG | 40 | 89.84% | −2.39 | 0.026 |
Discussion
In the present study, we used fNIRS to assess and compare the different cortical activation patterns for picture naming and phrase repetition between patients with global aphasia and healthy controls. In the naming task, patients with global aphasia demonstrated increased activation in the left Broca's area, MTG, and STG, but a decreased activation in the left SMG relative to controls. Furthermore, the latency of the HbO hemodynamic curve in the SMG region was highly negatively correlated with the naming score of patients with global aphasia. On the contrary, the SMA and SMG in the left hemisphere exhibited a decrease in cortical activation during the phrase repetition task in patients with global aphasia compared with healthy controls. Notably, we found that the left SMG may play a critical role in both tasks for patients with global aphasia.
Cortical activation patterns for language captured by fNIRS
Our results suggest that the left Broca's area, MTG, and STG play key roles in picture naming, which is consistent with previous fNIRS studies on healthy controls (28–30), while at the same time, a retrospective multicenter study reported one cluster of anomia in the posterior STG and MTG, and the other within the Broca's area (31). Meanwhile, the results from an earlier fNIRS study revealed that the prefrontal cortex was more active during naming in patients with post-stroke aphasia than in healthy controls and those with non-aphasia (32). These results supported our finding that the left prefrontal lobe (Broca's area) is more activated during naming tasks in patients with global aphasia, while our present study showed that the over-activation was also detected in the Wernicke's area. We believe that the over-activation was attributed to the overcompensation in the phonological and semantic processes in the patients with global aphasia (33).
In this study, patients with global aphasia showed decreased activation of left SMA relative to controls when repeating automatic phrases vs. looking at a fixation cross. These results are novel because very few fNIRS studies have investigated cortical activation during automatic phrase repetition in people with aphasia (34). Our study isolated the location of brain activation during repetition in patients with global aphasia. These preliminary findings are reasonable in the context of prior fNIRS studies showing the role of the SMA in the recovery of thalamic aphasia (35). Some even suggest that the SMA is important for language recovery in aphasia given its connection to the left inferior frontal gyrus, or Broca's area through the arcuate fasciculus, a key tract for language processing (36). In conclusion, we believe that the left SMA plays a key role not only in mild or moderate aphasia but also in a global aphasia.
Left SMG plays a fundamental role in picture naming and phrase repetition
In both naming and phrase repetition tasks, we found a significant decrease in the deactivation of the left SMG. This may be related to the different degrees of the loss of “repetition ability” and “naming ability” in patients with global aphasia. This loss of language function may be similar to the phenomenon of “anomia” or “conduction aphasia” induced by direct cortical electrical stimulation (DES), which is the gold standard for defining a patient's speech cortex (37). During the picture-naming task, DES on the left SMG resulted in phonological or semantic discontinuation (38). Studies have also found that DES on the left SMG leads to conduction aphasia (39). Some researchers have found that restraining the left SMG by repetitive transcranial magnetic stimulation (rTMS) will reduce language performance (40).
Moreover, we found the latency of the left SMG was negatively correlated with the ability to name, similar to the results from a previous fMRI study (41). In a recent fNIRS study performed by Gilmore et al., the left SMG was also reported to be involved in semantic features and picture-naming tasks (42), while differences should be noted between the two studies. Gilmore et al. enrolled patients with an average AQ of 67.17 (42.20–93.20), while in our present study, patients with an average AQ of 13.34 (2.6–21.7) were included. The fact that in aphasia patients with various severity, the left SMG has been constantly found to be activated in language tasks, stressing its vital role in language function. More importantly, the left SMG activation may be occurring because it is likely the primary area of the spared cortex in individuals with global aphasia with large left hemisphere damage or not actually be a signal from the cortex (may be the scalp signal over lesion). However, this cannot be confirmed in this study because MRI was not obtained. The results of the alternate analysis (excluding the area of the frank lesion from analysis) are reported in Supplementary Tables 6, 7 and Figures 1, 2. To conclude, the left SMG was suggested to represent a general cognitive region required for language processing in various aphasic situations.
Studies have started using rTMS to stimulate either the dominant or non-dominant hemisphere in aphasia to support language recovery (43–47). The stimulation site of rTMS in patients with aphasia can be selected by using the results of fNIRS, as also reported by Chang et al. (48). Our findings are congruent with a study conducted by Ren, wherein patients with global aphasia were treated with low-frequency rTMS at the temporoparietal region (CP6), which includes the posterior STG (pSTG) and SMG in the right hemisphere. They reported an improvement in the WAB-AQ score, spontaneous speech, and repetition ability when compared to the sham groups (49). This may indicate that SMG is a potential target in noninvasive brain stimulation for the treatment of patients with global aphasia, based on the fNIRS mapping method.
Conclusion
The current study utilized fNIRS to assess brain reorganization in patients with global aphasia. We believe that the decline of language ability in patients with global aphasia may be related to the abnormal decline of the left SMG, which may play a key role in language, especially in naming and repetition ability. Our results indicate the specific brain functional pattern in patients with global aphasia, especially in naming and repetition and in facilitating the wider application of fNIRS. Moreover, these findings may be applied to better assist patients with global aphasia and are expected to contribute to the development of noninvasive brain stimulation for the treatment of such patients.
Limitations
This study has some limitations. A disadvantage of fNIRS that is not unique to this study is that it measures signals ~1.5-cm deep into the cortex, meaning that the contribution of subcortical structures in naming tasks in global aphasia could not be captured in this study. Nevertheless, the cortical coverage in this study was greater than in other aphasia studies to date [e.g., 64 channels in this study vs. 56 channels in Gilmore et al. (42)]. Similarly, methods for managing the lesion in fNIRS studies in aphasia are still emerging. In this study, we did not conduct a structural MRI and could not map the location of participants' lesions. Thus, we were unable to confirm whether we were obtaining spurious signals from the scalp vs. the cortex when the channels were measuring an area of the frank lesion. Beyond that, our probe did not include short-separation channels, meaning that we were unable to regress out physiological noise from the hemodynamic response, while this study used Wavelet-MDL and generalized linear model (GLM)-based analysis, which can regress out some physiological noise and manage delayed activation at other regions. In addition, the results of the alternate analysis [average all of the long-separation channels together and divide all the channels by that signal to adjust for physiological noise (50, 51)] were in line with what was found in the primary analyses reported in the article; we get a similar cortical activation pattern (see Supplementary Table 8 and Figures 3, 4), and the result further confirms our scientific hypothesis that the left SMG plays a key regulatory role in the process of naming and repetition of patients with global aphasia. The use of short-separation regression channels will be an important step in the future to increase the precision of our findings. Furthermore, we were unable to carry out structural MRI, so we could not measure the scalp-to-cortex distance (i.e., greater distance, less likely capturing the signal from the cortex), which increases with age and brain injury—another factor impacting the validity of our findings that must be taken into consideration when interpreting the results. Another limitation worth discussing is that the activation we captured when contrasting picture naming with a fixation cross (silent task) could reflect engagement for speech production vs. language processing. A tighter contrast of contrasting picture naming with a control condition that also involves speech production but no naming (e.g., repeating their name or a word they can reliably say) would provide information about language processing/lexical retrieval in people with aphasia. More ecologically valid language tasks (e.g., covert word retrieval to semantic cues) can also be used in future studies to improve the measurement of various linguistic processes important for word retrieval. Despite these challenges, the results from this research pave the way for future work investigating patients with global aphasia by fNIRS, using high-density probes, and a larger sample size, together with more complex and diverse language tasks.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Huashan Hospital Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HL and JL performed the experiments, analyzed the fNIRS data, conducted all statistical analyses, and wrote all sections of the manuscript. ST, SF, and TW helped to revise the manuscript, figures, and tables. HQ and GL advised on the statistical analyses. YZ helped conceive the illustrations and revised the main body of the manuscript. RH and YW served as the primary scientific mentors to HL and were involved in experiment conceptualization, experimental procedure design, and manuscript review. All authors have declared that no competing interests exist and approved the submitted version.
Acknowledgments
The authors appreciate the participants who volunteered their time for this study.
Funding
This study was supported by the National Key R&D Program of China (No. 2018YFC2001700), the Shanghai Health and Family Planning Commission Project (No. 201840225), the Innovation Project of Shanghai Science and Technology on Yangtze River Delta Alliance (No. 20412420200), the Shanghai University of Traditional Chinese Medicine Graduate Student Innovation Project (No. Y2021061), and National Natural Science Foundation of China (No. 82272607).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1025384/full#supplementary-material
References
- 1.Flowers HL, Skoretz SA, Silver FL, Rochon E, Fang J, Flamand-Roze C, et al. Poststroke aphasia frequency, recovery, and outcomes: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2016) 97:2188–201.e8. 10.1016/j.apmr.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 2.Bohra V, Khwaja GA, Jain S, Duggal A, Ghuge VV, Srivastava A. Clinicoanatomical correlation in stroke related aphasia. Ann Indian Acad Neurol. (2015) 18:424–9. 10.4103/0972-2327.165469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarno MT, Silverman M, Sands E. Speech therapy and language recovery in severe aphasia. J Speech Hear Res. (1970) 13:607–23. 10.1044/jshr.1303.607 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz L. Sign comprehension in global aphasia. Cortex. (1978) 14:112–8. 10.1016/S0010-9452(78)80013-6 [DOI] [PubMed] [Google Scholar]
- 5.Wapner W, Gardner H. A note on patterns of comprehension and recovery in global aphasia. J Speech Hear Res. (1979) 22:765–72. 10.1044/jshr.2204.765 [DOI] [PubMed] [Google Scholar]
- 6.Fedorenko E, Varley R. Language and thought are not the same thing: evidence from neuroimaging and neurological patients. Ann N Y Acad Sci. (2016) 1369:132–53. 10.1111/nyas.13046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarno MT, Levita E. Some observations on the nature of recovery in global aphasia after stroke. Brain Lang. (1981) 13:1–12. 10.1016/0093-934X(81)90124-3 [DOI] [PubMed] [Google Scholar]
- 8.McCall D, Shelton JR, Weinrich M, Cox D. The utility of computerized visual communication for improving natural language in chronic global aphasia: implications for approaches to treatment in global aphasia. Aphasiology. (2000) 14:795–826. 10.1080/026870300412214 [DOI] [Google Scholar]
- 9.Shelton JR, Weinrich M, McCall D, Cox DM. Differentiating globally aphasic patients: data from in-depth language assessments and production training using C-Vic. Aphasiology. (1996) 10:319–42. 10.1080/02687039608248415 [DOI] [Google Scholar]
- 10.Weinrich M, McCall D, Weber C, Thomas K, Thornburg L. Training on an iconic communication system for severe aphasia can improve natural language production. Aphasiology. (1995) 9:343–64. 10.1080/02687039508248209 [DOI] [Google Scholar]
- 11.Weinrich M, Steele RD, Carlson GS, Kleczewska M, Wertz RT, Baker E. Processing of visual syntax in a globally aphasic patient. Brain Lang. (1989) 36:391–405. 10.1016/0093-934X(89)90075-8 [DOI] [PubMed] [Google Scholar]
- 12.Zahn R, Drews E, Specht K, Kemeny S, Reith W, Willmes K, et al. Recovery of semantic word processing in global aphasia: a functional Mri study. Brain Res Cogn Brain Res. (2004) 18:322–36. 10.1016/j.cogbrainres.2003.10.021 [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Ren C, Fang H, Li Z, Chen P, Yang J, et al. Exploring the functional connectivity characteristics of brain networks in post-stroke patients with global aphasia: a healthy control based resting-state Fmri study. Ann Palliat Med. (2021) 10:12113–28. 10.21037/apm-21-2750 [DOI] [PubMed] [Google Scholar]
- 14.Irani F, Platek SM, Bunce S, Ruocco AC, Chute D. Functional near infrared spectroscopy (Fnirs): an emerging neuroimaging technology with important applications for the study of brain disorders. Clin Neuropsychol. (2007) 21:9–37. 10.1080/13854040600910018 [DOI] [PubMed] [Google Scholar]
- 15.Butler LK, Kiran S, Tager-Flusberg H. Functional near-infrared spectroscopy in the study of speech and language impairment across the life span: a systematic review. Am J Speech Lang Pathol. (2020) 29:1674–701. 10.1044/2020_AJSLP-19-00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi S, Telkemeyer S, Wartenburger I, Obrig H. Shedding light on words and sentences: near-infrared spectroscopy in language research. Brain Lang. (2012) 121:152–63. 10.1016/j.bandl.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 17.Scherer LC, Fonseca RP, Amiri M, Adrover-Roig D, Marcotte K, Giroux F, et al. Syntactic processing in bilinguals: an Fnirs study. Brain Lang. (2012) 121:144–51. 10.1016/j.bandl.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 18.Oldfield RC. The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia. (1971) 9:97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- 19.Wu JB Lyu ZH, Liu XJ, Li HP, Wang Q. Development and Standardization of a new cognitive assessment test battery for Chinese aphasic patients: a preliminary study. Chin Med J. (2017) 130:2283–90. 10.4103/0366-6999.215326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, et al. Overt naming fmri pre- and post-Tms: two nonfluent aphasia patients, with and without improved naming post-Tms. Brain Lang. (2009) 111:20–35. 10.1016/j.bandl.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn Mem. (1980) 6:174–215. 10.1037/0278-7393.6.2.174 [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Chen Y, Hu R, Yang L, Wang M, Zhang J, et al. Rtms treatments combined with speech training for a conduction aphasia patient: a case report with Mri study. Medicine (Baltimore). (2017) 96:e7399. 10.1097/MD.0000000000007399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aasted CM, Yücel MA, Cooper RJ, Dubb J, Tsuzuki D, Becerra L, et al. Anatomical guidance for functional near-infrared spectroscopy: Atlasviewer tutorial. Neurophotonics. (2015) 2:020801. 10.1117/1.NPh.2.2.020801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huppert TJ, Diamond SG, Franceschini MA, Boas DA. Homer: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt. (2009) 48:D280–98. 10.1364/AO.48.00D280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye JC, Tak S, Jang KE, Jung J, Jang J. Nirs-Spm: statistical parametric mapping for near-infrared spectroscopy. Neuroimage. (2009) 44:428–47. 10.1016/j.neuroimage.2008.08.036 [DOI] [PubMed] [Google Scholar]
- 26.Xia M, Wang J, He Y. Brainnet viewer: a network visualization tool for human brain connectomics. PLoS ONE. (2013) 8:e68910. 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous bold Fmri and Nirs recordings during functional brain activation. Neuroimage. (2002) 17:719–31. 10.1006/nimg.2002.1227 [DOI] [PubMed] [Google Scholar]
- 28.Moriai-Izawa A, Dan H, Dan I, Sano T, Oguro K, Yokota H, et al. Multichannel Fnirs assessment of overt and covert confrontation naming. Brain Lang. (2012) 121:185–93. 10.1016/j.bandl.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 29.Cannestra AF, Wartenburger I, Obrig H, Villringer A, Toga AW. Functional assessment of Broca's area using near infrared spectroscopy in humans. Neuroreport. (2003) 14:1961–5. 10.1097/00001756-200310270-00016 [DOI] [PubMed] [Google Scholar]
- 30.Hull R, Bortfeld H, Koons S. Near-infrared spectroscopy and cortical responses to speech production. Open Neuroimag J. (2009) 3:26–30. 10.2174/1874440000903010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, Zhao Z, Zhang J, Wu B, Zhu Y, Chang EF, et al. Functional maps of direct electrical stimulation-induced speech arrest and anomia: a multicentre retrospective study. Brain. (2021) 144:2541–53. 10.1093/brain/awab125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakatani K, Xie Y, Lichty W, Li S, Zuo H. Language-activated cerebral blood oxygenation and hemodynamic changes of the left prefrontal cortex in poststroke aphasic patients: a near-infrared spectroscopy study. Stroke. (1998) 29:1299–304. 10.1161/01.STR.29.7.1299 [DOI] [PubMed] [Google Scholar]
- 33.Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. (2006) 129(Pt 6):1371–84. 10.1093/brain/awl090 [DOI] [PubMed] [Google Scholar]
- 34.Hara T, Abo M, Kakita K, Mori Y, Yoshida M, Sasaki N. The effect of selective transcranial magnetic stimulation with functional near-infrared spectroscopy and intensive speech therapy on individuals with post-stroke aphasia. Eur Neurol. (2017) 77:186–94. 10.1159/000457901 [DOI] [PubMed] [Google Scholar]
- 35.Obayashi S. The supplementary motor area responsible for word retrieval decline after acute thalamic stroke revealed by coupled spect and near-infrared spectroscopy. Brain Sci. (2020) 10:247. 10.3390/brainsci10040247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catani M, Dell'acqua F, Vergani F, Malik F, Hodge H, Roy P, et al. Short frontal lobe connections of the human brain. Cortex. (2012) 48:273–91. 10.1016/j.cortex.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 37.Pallud J, Rigaux-Viode O, Corns R, Muto J, Lopez C, Mellerio C, et al. Direct electrical bipolar electrostimulation for functional cortical and subcortical cerebral mapping in awake craniotomy. Practical Considerations. Neurochirurgie. (2017) 63:164–74. 10.1016/j.neuchi.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 38.Corina DP, Loudermilk BC, Detwiler L, Martin RF, Brinkley JF, Ojemann G. Analysis of naming errors during cortical stimulation mapping: implications for models of language representation. Brain Lang. (2010) 115:101–12. 10.1016/j.bandl.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quigg M, Geldmacher DS, Elias WJ. Conduction aphasia as a function of the dominant posterior perisylvian cortex. Report of two cases. J Neurosurg. (2006) 104:845–8. 10.3171/jns.2006.104.5.845 [DOI] [PubMed] [Google Scholar]
- 40.Hartwigsen G, Bzdok D, Klein M, Wawrzyniak M, Stockert A, Wrede K, et al. Rapid short-term reorganization in the language network. eLife. (2017) 6:e25964. 10.7554/eLife.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. (2003) 15:71–84. 10.1162/089892903321107837 [DOI] [PubMed] [Google Scholar]
- 42.Gilmore N, Yücel MA, Li X, Boas DA, Kiran S. Investigating language and domain-general processing in neurotypicals and individuals with aphasia - a functional near-infrared spectroscopy pilot study. Front Hum Neurosci. (2021) 15:728151. 10.3389/fnhum.2021.728151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harvey DY, Podell J, Turkeltaub PE, Faseyitan O, Coslett HB, Hamilton RH. Functional reorganization of right prefrontal cortex underlies sustained naming improvements in chronic aphasia via repetitive transcranial magnetic stimulation. Cogn Behav Neurol. (2017) 30:133–44. 10.1097/WNN.0000000000000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai PY, Wang CP, Ko JS, Chung YM, Chang YW, Wang JX. The persistent and broadly modulating effect of inhibitory Rtms in Nonfluent aphasic patients: a sham-controlled, double-blind study. Neurorehabil Neural Repair. (2014) 28:779–87. 10.1177/1545968314522710 [DOI] [PubMed] [Google Scholar]
- 45.Heiss WD, Hartmann A, Rubi-Fessen I, Anglade C, Kracht L, Kessler J, et al. Noninvasive Brain Stimulation for Treatment of Right- and Left-Handed Poststroke Aphasics. Cerebrovasc Dis. (2013) 36:363–72. 10.1159/000355499 [DOI] [PubMed] [Google Scholar]
- 46.Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. (2011) 118:40–50. 10.1016/j.bandl.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hillis AE. Aphasia: progress in the last quarter of a century. Neurology. (2007) 69:200–13. 10.1212/01.wnl.0000265600.69385.6f [DOI] [PubMed] [Google Scholar]
- 48.Chang WK, Park J, Lee JY, Cho S, Lee J, Kim WS, et al. Functional network changes after high-frequency Rtms over the most activated speech-related area combined with speech therapy in chronic stroke with non-fluent aphasia. Front Neurol. (2022) 13:690048. 10.3389/fneur.2022.690048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren C, Zhang G, Xu X, Hao J, Fang H, Chen P, et al. The effect of Rtms over the different targets on language recovery in stroke patients with global aphasia: a randomized sham-controlled study. Biomed Res Int. (2019) 2019:4589056. 10.1155/2019/4589056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franceschini MA, Fantini S, Thompson JH, Culver JP, Boas DA. Hemodynamic evoked response of the sensorimotor cortex measured noninvasively with near-infrared optical imaging. Psychophysiology. (2003) 40:548–60. 10.1111/1469-8986.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yücel MA, Lühmann AV, Scholkmann F, Gervain J, Dan I, Ayaz H, et al. Best practices for Fnirs publications. Neurophotonics. (2021) 8:012101. 10.1117/1.NPh.8.1.012101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.