Abstract
The objective of the present study was to characterize the difference in 10-year carcinoid-specific survival (CSS) and disease-free survival (DFS) among patients with resected pulmonary typical carcinoid (TC) and atypical carcinoid (AC). Patients diagnosed with pulmonary carcinoid tumors (PCT) between January 1, 1997, and December 31, 2016, were identified. All patients underwent video-assisted thoracoscopic surgery or thoracotomy with thoracic lymphadenectomy. Cumulative CSS was estimated using the Kaplan-Meier model. The analysis of hazard ratios (HRs) and 95% confidence intervals (CIs) was performed using univariate and multivariate Cox proportional hazards models. A total of 404 patients with PCT were included in the present study. The 10-year CSS and DFS rates of patients with AC were significantly worse than those of patients with TC (49.1 vs. 86.8% and 52.2 vs. 92.6%, respectively; P<0.001). In the CSS multivariate analysis, older age and lymph node involvement (HR, 2.45; P=0.022) were associated with worse survival in AC, while age, male sex, M1 stage, cigarette smoking and inadequate N2 lymphadenectomy were associate with worse survival in TC. In the recurrence multivariate analysis, N1-3 stage (HR, 2.62; 95% CI, 1.16-5.95; P=0.018) and inadequate N2 lymphadenectomy (HR, 2.13; 95% CI, 1.04-4.39; P=0.041) were associated with an increase in recurrence in AC, while male sex (HR, 3.72; 95% CI, 1.33-10.42; P=0.010) and M1 stage (HR, 14.93; 95% CI, 4.77-46.77; P<0.001) were associated with an increase in recurrence in TC. In conclusion, patients with AC tumors had significantly worse CSS and DFS rates compared with patients with TC. The degree of nodal involvement in AC was a prognostic marker, in contrast to that in TC. Inadequate lymphadenectomy increased the risk of recurrence in AC and mortality in TC, although surgical approaches did not have a significant impact. The present study therefore emphasizes the importance of mediastinal nodal dissection in patients with PCTs.
Keywords: pulmonary carcinoid tumor, typical carcinoid, atypical carcinoid, prognostic factors, surgery, lymphadenectomy
Introduction
Neuroendocrine tumors (NETs) are a spectrum of malignancies that originate from the neuroendocrine Kulchitsky cells located in the bronchial epithelium (1). These tumors have distinct patterns of behavior, ranging from indolent to highly aggressive patterns, and include large cell neuroendocrine carcinoma and small cell lung cancer (SCLC). Among the NETs, pulmonary carcinoid tumors (PCTs) are a rare subgroup of tumors with an estimated age-adjusted incidence of 1.35 cases per 100,000 individuals in the USA (2), accounting for 20–25% of all NETs. The World Health Organization classifies PCTs into two subtypes depending on the microscopic findings of mitosis and necrosis (3). Typical carcinoid (TC) has fewer than 2 mitoses per 2 mm2 and a lack of necrosis, while atypical carcinoid (AC) has 2–10 mitoses per 2 mm2 and/or foci of necrosis.
As surgical resection remains the mainstay of treatment for PCT, multiple retrospective studies can be found in the literature reporting the outcomes of these patients, as well as therapeutic and prognostic factors that are implicated with disease outcomes (1,3). However, PCTs have patterns of biological behavior that are different in comparison to other types of non-SCLC (NSCLC), and due to this, the surgical approach, in general, can be more aggressive for PCT than for other types of NSCLC. For example, surgery for concomitant N2 disease and resection for patients with distant metastatic disease can be considered more often in PCT than in NSCLC. Additionally, the long-term prognosis of patients with PCT, as well as the effect of adjuvant therapies, under these circumstances is not entirely clear and needs to be better evaluated. The pattern of recurrence (local, regional and distant) for PCT is also different, considering the latency of these tumors. Hence, the relationship between these patterns and survival outcomes should be further analyzed through long-term follow-up.
There has been a lack of clinical data in these subgroups of patients, thus making identification of prognostic factors and treatment recommendations challenging, since there have been a number of inconsistent findings regarding the prognostic factors associated with recurrence and survival in patients with resected PCT, especially in those cases that are considered as advanced disease. Due to these challenges, a retrospective study was conducted of the Health Science Research (HSR) Study Data Management System (Mayo Clinic, AZ, USA) to characterize the prognostic factors associated with recurrence, survival in resected PCT tumors and the effect of adjuvant therapies.
Patients and methods
Patients and protocols
Patients with resected PCT were analyzed. All patients underwent video-assisted thoracoscopic surgery or thoracotomy with thoracic lymphadenectomy. Data for patients histologically diagnosed with PCT between January 1, 1997, and December 31, 2016, were retrieved from the lung cancer cohort, where all primary lung cancer patients has been enrolled and followed prospectively (4). Patients with multiple primary lung cancer, non-surgical treatment and non-carcinoid specific death were excluded. Eventually, 404 patients were included in the study. The selection of patients with PCT is shown in Fig. 1.
Figure 1.
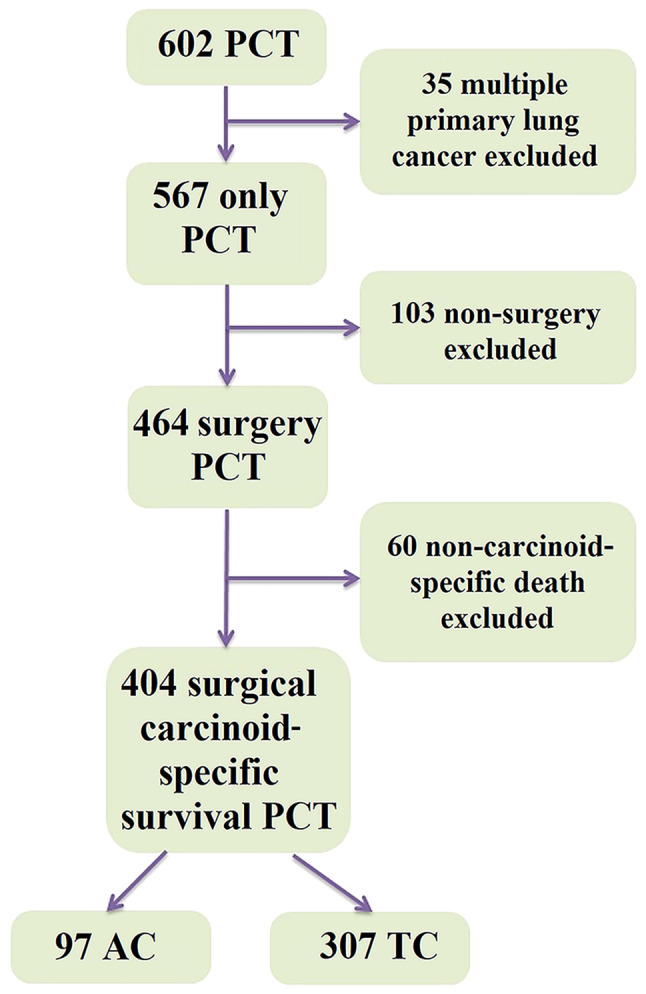
Selection of patients with PCT. AC, atypical carcinoid; PCT, pulmonary carcinoid tumors; TC, typical carcinoid.
The clinicopathological characteristics of the patients were collected, including age, sex, BMI, Tumor-Node-Metastasis (TNM) stage, tumor size, tumor lobe location, smoking status, family history of cancer, surgical type and comorbidity. Patients were restaged according to the eighth edition of the TNM staging system of the American Joint Committee on Cancer (5). Classification of smoking was based on the Adult Tobacco Use Information in National Health Interview Survey (6). Adequate mediastinal lymphadenectomy consisted of node resection and mapping (American Thoracic Society map) of at least three N2 groups (7). For the purposes of the present study, if the number of assessed N2 groups was <3, then this was defined as inadequate mediastinal lymphadenectomy. The diagnosis of recurrence was made with a combination of CT images, and/or biopsy of the new suspected site of disease. The survival data of each patient was collected through electronic medical notes, registration database, next-of-kin reports, death certificates, obituary documents filed in the patients' medical records, The Mayo Clinic Institutional Tumor Registry and the Social Security Death Index website (https://socialsecuritydeathindex-search.com).
Statistical analysis
The nominal categorical variables were analyzed by the χ2 test. The continuous variables were reported as the median and interquartile range, and were evaluated by the unpaired independent sample t-test. For the carcinoid-specific survival (CSS) analysis, the date of cancer-specific death from PCT was the observation endpoint. For the disease-free survival (DFS) analysis, the date of recurrence was the endpoint of observation. Endpoints were analyzed as time-to-event data from the date of surgery to the respective events, which were subject to censoring at the last follow-up if no events were observed. The cumulative survival was estimated using the Kaplan-Meier model and log-rank test. The analysis of hazard ratios (HRs) and 95% confidence intervals (CIs) was performed using univariate (log-rank) and multivariate Cox proportional hazards models. Factors with a P-value of <0.1 in the Cox univariate analysis (log-rank) were included in the multivariate analysis. For all other statistical analyses, P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SAS 9.3 (SAS Institute, Inc.).
Results
Characteristics of PCT
Of the 404 patients with PCT included in the present study, 307 (76.0%) consisted of TC and 97 (24.0%) of AC. The median follow-up time was 89.7 months (range, 57.5-142.3 months). Patients with AC, compared with patients with TC, were older [median age, 62.0 years (51.0-70.0 years) vs. 57.0 years (range, 47.0-67.0 years), respectively; P=0.027], with more stage IIIB-IVA cases [IIIB-IVA, 10 (10.3%) vs. 9 (2.9%) respectively; P=0.003], more lymph node involvement [N1-3, 40 (41.2%) vs. 52 (16.9%), respectively; P<0.001], and more distant metastasis [M1, 7 (7.2%) vs. 7 (2.3%), respectively; P=0.021]. The AC subgroup also underwent more extensive surgical resections than the TC subgroup [pneumonectomy, 10 (10.3%) vs. 6 (2.0%); bi-lobectomy, 7 (7.2%) vs. 15 (4.9%); P<0.001]. The recurrence rate was higher in patients with AC compared with that in patients with TC [38 (39.2%) vs. 19 (6.2%); P<0.001]. Baseline characteristics and surgical treatment details are provided in Table I.
Table I.
Characteristics of 404 surgical patients with pulmonary carcinoid tumors.
| Characteristics | Atypical carcinoid (n=97) | Typical carcinoid (n=307) | P-value |
|---|---|---|---|
| Median age (interquartile range), years | 62.0 (51.0-70.0) | 57.0 (47.0-67.0) | 0.027 |
| Sex, n (%) | 0.442 | ||
| Female | 67 (69.1) | 199 (64.8) | |
| Male | 30 (30.9) | 108 (35.2) | |
| BMI, n (%) | 0.141 | ||
| Missing | 5 (5.2) | 10 (3.2) | |
| Underweight | 2 (2.1) | 2 (0.7) | |
| Normal | 28 (28.9) | 68 (22.1) | |
| Overweight | 36 (37.1) | 111 (36.2) | |
| Obese | 26 (26.8) | 116 (37.8) | |
| T stage, n (%) | 0.105 | ||
| T1 | 56 (57.7) | 216 (70.4) | |
| T2 | 30 (30.9) | 63 (20.5) | |
| T3 | 8 (8.2) | 17 (5.5) | |
| T4 | 3 (3.1) | 11 (3.6) | |
| N stage, n (%) | <0.001 | ||
| N0 | 57 (58.8) | 255 (83.1) | |
| N1 | 19 (19.6) | 25 (8.1) | |
| N2 | 20 (20.6) | 26 (8.5) | |
| N3 | 1 (1.0) | 1 (0.3) | |
| M stage, n (%) | 0.021 | ||
| M0 | 90 (92.8) | 300 (97.7) | |
| M1 | 7 (7.2) | 7 (2.3) | |
| TNM stage, n (%) | 0.003 | ||
| I–IIIA | 87 (89.7) | 298 (97.1) | |
| IIIB-IVA | 10 (10.3) | 9 (2.9) | |
| Tumor side, n (%) | 0.744 | ||
| Left | 38 (39.2) | 126 (41.0) | |
| Right | 59 (60.8) | 181 (59.0) | |
| Tumor lobe, n (%) | 0.487 | ||
| Upper | 36 (37.1) | 90 (29.3) | |
| Middle | 16 (16.5) | 65 (21.2) | |
| Lower | 43 (44.3) | 144 (46.9) | |
| Main bronchus | 2 (2.1) | 8 (2.6) | |
| Smoking status, n (%) | 0.337 | ||
| Never smoker | 48 (49.5) | 162 (52.8) | |
| Former smoker | 32 (33.0) | 109 (35.5) | |
| Current smoker | 17 (17.5) | 36 (11.7) | |
| Family history of lung cancer, n (%) | 0.593 | ||
| No | 91 (93.8) | 283 (92.2) | |
| Yes | 6 (6.2) | 24 (7.8) | |
| Family history of other cancer, n (%) | 0.494 | ||
| No | 69 (71.1) | 207 (67.4) | |
| Yes | 28 (28.9) | 100 (32.6) | |
| Surgical type, n (%) | <0.001 | ||
| Pneumonectomy | 10 (10.3) | 6 (2.0) | |
| Bi-lobectomy | 7 (7.2) | 15 (4.9) | |
| Lobectomy | 61 (62.9) | 182 (59.3) | |
| Sub-lobectomy | 17 (17.5) | 75 (24.4) | |
| Sleeve resection | 2 (2.1) | 29 (9.4) | |
| N2 lymphadenectomy, n (%) | 0.312 | ||
| Inadequate (<3 groups) | 37 (38.1) | 100 (32.6) | |
| Adequate (≥3 groups) | 60 (61.9) | 207 (67.4) | |
| Recurrence, n (%) | <0.001 | ||
| No | 59 (60.8) | 288 (93.8) | |
| Yes | 38 (39.2) | 19 (6.2) | |
| Comorbidity, n (%) | 0.158 | ||
| Missing | 0 (0.0) | 2 (0.7) | |
| No | 11 (11.3) | 21 (6.8) | |
| Yes | 86 (88.7) | 284 (92.5) |
TNM, Tumor-Node-Metastasis.
CSS analysis
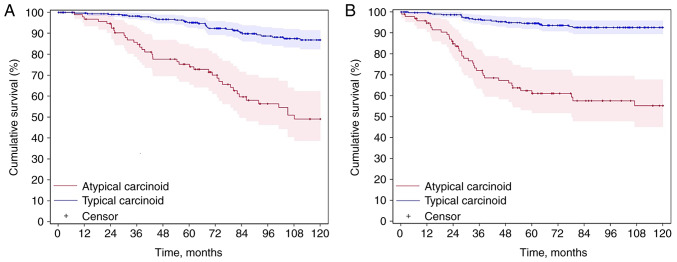
The CSS of AC was inferior to that of TC; the 10-year CSS rates were 49.1 vs. 86.8% (P<0.001), respectively (Fig. 2A). Univariate and multivariate analyses are found in Table II. For AC, univariate analysis of CSS revealed that age (HR, 1.03; 95% CI, 1.00-1.05; P=0.002), N1-3 stage (HR, 2.39; 95% CI, 1.34-4.26; P=0.002), M1 stage (HR, 2.59; 95% CI, 1.09-6.15; P=0.025), family history of lung cancer (HR, 0.16; 95% CI, 0.02-1.20; P=0.042), pneumonectomy/bi-lobectomy (HR, 2.18; 95% CI, 1.08-4.42; P=0.086) and inadequate N2 lymphadenectomy (HR, 1.78; 95% CI, 0.99-3.22; P=0.049) were all P<0.10 so were included in the multivariate analysis. For the multivariate analysis, age (HR, 1.04; 95% CI, 1.01-1.07; P=0.001) and N1-3 stage (HR, 2.45; 95% CI, 1.14-5.30; P=0.022) were independent risk factors. For TC, univariate analysis of CSS determined that age (HR, 1.06; 95% CI, 1.04-1.09; P<0.001), male sex (HR, 1.86; 95% CI, 1.06-3.26; P=0.028), T2-4 stage (HR, 1.70; 95% CI, 0.94-3.09; P=0.077), N1-3 stage (HR, 2.23; 95% CI, 1.15-4.35; P=0.015), M1 stage (HR, 9.99; 95% CI, 4.42-22.66; P<0.001), upper lobe location (HR, 1.25; 95% CI, 0.68-2.30; P=0.086), cigarette smoking [former smoker (HR, 2.13; 95% CI, 1.15-3.95); current smoker (HR, 1.86; 95% CI, 0.81-4.27); P=0.044] and inadequate N2 lymphadenectomy (HR, 3.14; 95% CI, 1.74-5.66; P<0.001) were all P<0.10 so were included in the multivariate analysis. For the multivariate analysis, age (HR, 1.07; 95% CI, 1.04-1.10; P<0.001), male sex (HR, 2.03; 95% CI, 1.10-3.75; P=0.026), M1 stage (HR, 4.63; 95% CI, 1.73-12.38; P=0.005), cigarette smoking [former smoker (HR, 2.14; 95% CI, 1.12-4.11); current smoker (HR, 2.50; 95% CI, 1.03-6.08); P=0.032] and inadequate N2 lymphadenectomy (HR, 3.45; 95% CI, 1.85-6.43; P<0.001) were significant risk factors.
Figure 2.
Survival of patients with pulmonary carcinoid tumors. (A) The 10-year carcinoid-specific survival Kaplan-Meier curve (AC vs. TC, 49.1 vs. 86.8%, respectively; P<0.001). (B) The 10-year disease-free survival Kaplan-Meier curve (AC vs. TC, 55.2 vs. 92.6%, respectively; P<0.001). AC, atypical carcinoid; TC, typical carcinoid.
Table II.
Survival analysis of 404 patients with pulmonary carcinoid tumors using univariate and multivariate Cox models.
| Atypical carcinoid (n=97) | Typical carcinoid (n=307) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
|
|
|
|
|
|||||
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | 1.03 | 0.002 | 1.04 | 0.001 | 1.06 | <0.001 | 1.07 | <0.001 |
| (1.00-1.05) | (1.01-1.07) | (1.04-1.09) | (1.04-1.10) | |||||
| Sex | 0.946 | 0.028 | 0.026 | |||||
| Female | - | - | - | |||||
| Male | 0.98 | 1.86 | 2.03 | |||||
| (0.51-1.86) | (1.06-3.26) | (1.10-3.75) | ||||||
| BMIa | 0.419 | 0.353 | ||||||
| Underweight/normal | - | - | ||||||
| Overweight/ | 0.68 | 0.73 | ||||||
| obese | (0.37-1.25) | (0.38-1.42) | ||||||
| T stage | 0.159 | 0.483 | 0.077 | 0.154 | ||||
| T1 | - | - | - | - | ||||
| T2-4 | 1.51 | 1.33 | 1.70 | 1.62 | ||||
| (0.85-2.67) | (0.60-2.91) | (0.94-3.09) | (0.85-3.11) | |||||
| N stage | 0.002 | 0.022 | 0.015 | 0.092 | ||||
| N0 | - | - | - | - | ||||
| N1-3 | 2.39 | 2.45 | 2.23 | 2.06 | ||||
| (1.34-4.26) | (1.14-5.30) | (1.15-4.35) | (0.92-4.60) | |||||
| M stage | 0.025 | 0.604 | <0.001 | 0.005 | ||||
| M0 | - | - | - | - | ||||
| M1 | 2.59 | 1.33 | 9.99 | 4.63 | ||||
| (1.09-6.15) | (0.46-3.82) | (4.42-22.66) | (1.73-12.38) | |||||
| Tumor side | 0.852 | 0.167 | ||||||
| Left | 0.95 | 1.48 | ||||||
| (0.52-1.70) | (0.84-2.60) | |||||||
| Right | - | - | ||||||
| Tumor lobe | 0.506 | 0.086 | 0.062 | |||||
| Upper | 0.69 | 1.25 | 2.13 | |||||
| (0.36-1.30) | (0.68-2.30) | (1.10-4.13) | ||||||
| Middle/main | 0.78 | 0.42 | 0.80 | |||||
| bronchus | (0.34-1.76) | (0.16-1.10) | (0.28-2.24) | |||||
| Lower | - | - | - | |||||
| Smoking status | 0.740 | 0.044 | 0.032 | |||||
| Never smoker | - | - | - | |||||
| Former smoker | 1.27 | 2.13 | 2.14 | |||||
| (0.67-2.42) | (1.15-3.95) | (1.12-4.11) | ||||||
| Current smoker | 1.22 | 1.86 | 2.50 | |||||
| (0.56-2.67) | (0.81-4.27) | (1.03-6.08) | ||||||
| Family history of lung cancer | 0.042 | 0.065 | 0.921 | |||||
| No | - | - | - | |||||
| Yes | 0.16 | 0.21 | 1.05 | |||||
| (0.02-1.20) | (0.03-1.66) | (0.42-2.65) | ||||||
| Family history of other cancer | 0.756 | 0.314 | ||||||
| No | - | - | ||||||
| Yes | 1.10 | 0.74 | ||||||
| (0.60-2.00) | (0.41-1.33) | |||||||
| Surgery type | 0.086 | 0.148 | 0.151 | |||||
| Pneumo/bi- | 2.18 | 2.15 | 2.01 | |||||
| lobectomy | (1.08-4.42) | (0.88-5.27) | (0.77-5.25) | |||||
| Lobectomy/sleeve | - | - | - | |||||
| Sub-lobectomy | 1.20 | 1.50 | 1.64 | |||||
| (0.56-2.55) | (0.61-3.71) | (0.88-3.04) | ||||||
| N2 Lymphadenectomy | 0.049 | 0.335 | <0.001 | <0.001 | ||||
| Inadequate | 1.78 | 1.44 | 3.14 | 3.45 | ||||
| (0.99-3.22) | (0.69-3.00) | (1.74-5.66) | (1.85-6.43) | |||||
| Adequate | - | - | - | - | ||||
| Comorbidity | 0.925 | 0.436 | ||||||
| No | 1.04 | 0.57 | ||||||
| (0.44-2.47) | (0.14-2.37) | |||||||
| Yes | - | - | ||||||
15 missing BMI were not analyzed using the Cox model. CI, confidence interval; HR, hazard ratio.
Tumor recurrence analysis
The DFS of AC was inferior to that of TC; the 10-year DFS rates were 55.2 vs. 92.6% (P<0.001), respectively (Fig. 2B). Univariate and multivariate analyses are found in Table III. For AC, the univariate analysis revealed that N1-3 stage (HR, 3.61; 95% CI, 1.86-7.01; P<0.001), M1 stage (HR, 2.51; 95% CI, 0.97-6.46; P=0.049), pneumonectomy/bi-lobectomy (HR, 2.42; 95% CI, 1.15-5.12; P=0.030) and inadequate N2 lymphadenectomy (HR, 1.95; 95% CI, 1.02-3.73; P=0.039) were all P<0.10 so were included in the multivariate analysis. For the multivariate analysis, N1-3 stage (HR, 2.62; 95% CI, 1.16-5.95; P=0.018) and inadequate N2 lymphadenectomy (HR, 2.13; 95% CI, 1.04-4.39; P=0.041) were independent risk factors for recurrence. For TC, the univariate analysis showed that male sex (HR, 4.19; 95% CI, 1.59-11.03; P=0.002), overweight/obese BMI (HR, 0.45; 95% CI, 0.17-1.16; P=0.090), N1-3 stage (HR, 2.42; 95% CI, 0.92-6.36; P=0.065) and M1 stage (HR, 31.22; 95% CI, 10. 95–88.99; P<0.001) were all P<0.10 so were included in the multivariate analysis. For the multivariate analysis, male sex (HR, 3.72; 95% CI, 1.33-10.42; P=0.010) and M1 stage (HR, 14.93; 95% CI, 4.77-46.77; P<0.001) were independent risk factors for recurrence.
Table III.
Recurrence analysis of 404 patients with pulmonary carcinoid tumors using univariate and multivariate Cox models.
| Atypical carcinoid (n=97) | Typical carcinoid (n=307) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
|
|
|
|
|
|||||
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | 1.01 | 0.173 | 1.02 | 0.292 | ||||
| (0.98-1.03) | (0.98-1.05) | |||||||
| Sex | 0.842 | 0.002 | 0.010 | |||||
| Female | - | - | - | |||||
| Male | 0.93 | 4.19 | 3.72 | |||||
| (0.46-1.88) | (1.59-11.03) | (1.33-10.42) | ||||||
| BMIa | 0.116 | 0.090 | 0.163 | |||||
| Underweight/normal | - | - | - | |||||
| Overweight/ | 0.56 | 0.45 | 0.47 | |||||
| obese | (0.28-1.10) | (0.17-1.16) | (0.17-1.30) | |||||
| T stage | 0.422 | 0.661 | 0.414 | 0.663 | ||||
| T1 | - | - | - | - | ||||
| T2-4 | 1.30 | 0.83 | 1.47 | 1.26 | ||||
| (0.68-2.46) | (0.37-1.89) | (0.58-3.74) | (0.45-3.53) | |||||
| N stage | <0.001 | 0.018 | 0.065 | 0.167 | ||||
| N0 | - | - | - | - | ||||
| N1-3 | 3.61 | 2.62 | 2.42 | 2.14 | ||||
| (1.86-7.01) | (1.16-5.95) | (0.92-6.36) | (0.76-5.99) | |||||
| M stage | 0.049 | 0.275 | <0.001 | <0.001 | ||||
| M0 | - | - | - | - | ||||
| M1 | 2.51 | 1.91 | 31.22 | 14.93 | ||||
| (0.97-6.46) | (0.63-5.85) | (10.95-88.99) | (4.77-46.77) | |||||
| Tumor side | 0.726 | 0.583 | ||||||
| Left | 1.12 | 14.93 | ||||||
| (0.59-2.14) | (0.52-3.16) | |||||||
| Right | - | - | ||||||
| Tumor lobe | 0.198 | 0.618 | ||||||
| Upper | 0.51 | 0.74 | ||||||
| (0.24-1.07) | (0.26-2.13) | |||||||
| Middle/main | 0.78 | 0.55 | ||||||
| bronchus | (0.33-1.84) | (0.15-1.98) | ||||||
| Lower | - | - | ||||||
| Smoking status | 0.809 | 0.369 | ||||||
| Never smoker | - | - | ||||||
| Former smoker | 1.07 | 1.95 | ||||||
| (0.53-2.20) | (0.72-5.23) | |||||||
| Current smoker | 1.34 | 1.90 | ||||||
| (0.56-3.21) | (0.49-7.34) | |||||||
| Family history of lung cancer | 0.459 | 0.780 | ||||||
| No | - | - | ||||||
| Yes | 0.59 | 1.23 | ||||||
| (0.14-2.44) | (0.28-5.33) | |||||||
| Family history of other cancer | 0.631 | 0.934 | ||||||
| No | - | - | ||||||
| Yes | 1.18 | 1.04 | ||||||
| (0.60-2.31) | (0.41-2.65) | |||||||
| Surgery type | 0.030 | 0.355 | 0.295 | |||||
| Pneumo/bi- | 2.42 | 1.88 | 1.46 | |||||
| lobectomy | (1.15-5.12) | (0.80-4.41) | (0.33-6.37) | |||||
| Lobectomy/sleeve | - | - | - | |||||
| Sub-lobectomy | 0.77 | 0.83 | 0.37 | |||||
| (0.29-2.03) | (0.28-2.49) | (0.08-1.60) | ||||||
| N2 Lymphadenectomy | 0.039 | 0.041 | 0.002 | 0.138 | ||||
| Inadequate | 1.95 | 2.13 | 2.13 | 2.12 | ||||
| (1.02-3.73) | (1.04-4.39) | (1.54-9.98) | (0.78-5.79) | |||||
| Adequate | - | - | - | - | ||||
| Comorbidity | 0.485 | 0.807 | ||||||
| No | 1.40 | 0.78 | ||||||
| (0.54-3.58) | (0.10-5.83) | |||||||
| Yes | - | - | ||||||
15 missing BMI were not analyzed using the Cox model. CI, confidence interval; HR, hazard ratio.
Discussion
This was a retrospective study, with the objective to evaluate additional risk factors associated with the survival of patients with resected PCT. To the best of our knowledge, these factors are not entirely clarified and are still a matter for discussion in multidisciplinary tumor boards. Using the HSR Study Data Management System, the patterns of these tumors were analyzed, which typically required a long-term follow-up to verify potential effects on the therapeutic strategies employed and the prognostic factors identified. It was found that the 10-year CSS and DFS rates for patients with TC were 86.8 and 92.6%, respectively. In patients with AC, however, the 10-year CSS and DFS rates were 49.1 and 55.2%, respectively. These findings corroborate the well-known body of evidence in the literature showing that AC carries a worse prognosis compared with TC (1,2).
In the present study, it was also found that age and lymph node involvement were associated with inferior CSS in AC, while age, male sex, distant metastasis, cigarette smoking and inadequate mediastinal lymphadenectomy were associated with worse CSS in TC. Regarding recurrence, lymph node involvement and inadequate mediastinal lymphadenectomy were associated with inferior DFS in AC, while male sex and distant metastasis were associated with inferior DFS in TC. The identified prognostic factors of the present study are similar to those of previous reports in the literature. The average age at the time of diagnosis is ~45 and 55 years for patients with TC and AC, respectively (8,9). Age (<45 years) is an important prognostic factor associated with a good prognosis in PCT (10). Moreover, some studies report a higher prevalence of smoking in patients with AC compared to TC. As previously published, most patients with TC are female and are never-smokers (11,12). Furthermore, it has been reported that the prognosis of female patients with PCT is worse than that of male patients (13).
TC is an indolent malignancy and excellent long-term outcomes can be achieved. The 10-year CSS of 86.8% observed in the present study is very similar to the results previously reported by other groups (14–16). In the present study, the median age of diagnosis was 57.0 years, and the majority of the patients presented with T1 (70.4%) and N0 (83.1%) stage disease. In the cohort, a 16.9% incidence of nodal involvement (8.8% N2-N3) was found, which is very similar to the findings reported by Kneuertz et al (17), where ≥10 lymph nodes were evaluated. The impact of nodal involvement in TC remains controversial and, in the present study, it was demonstrated that nodal involvement was an independent prognostic factor for TC prognosis. However, other studies have questioned the prognostic significance of nodal involvement in PCT (18,19). Martini et al (14) found no survival difference in patients with N1 or N2 disease. Kneuertz et al (17) found nodal involvement to be associated with worse overall survival only in tumors >2 cm. In a study by Lou et al (16), only 2% of patients with TC without nodal disease had recurrence, while there was increased recurrence in positive nodal disease following surgery. Regarding the surgical approach, the most common type of procedure in patients with TC in the present study was lobectomy (59.3%), followed by sublobectomy (24.4%). Despite a previous study demonstrating that sublobar resection does not seem to compromise oncological outcomes compared with lobar resection, assuming complete resection and adequate mediastinal staging is performed (15), no significant difference in the prognosis of patients with TC who underwent different lung resections (P>0.05) was found in the present study, which may be due to the small sample size. Mediastinal lymphadenectomy (≥3 groups) was performed in 67.4% of the patients with TC and was associated with a better prognosis compared with inadequate mediastinal lymphadenectomy. Given that lymphadenectomy has not been a routine practice in patients with TC, most previous studies did not address the role of lymphadenectomy in this subgroup of patients. Based on these results, it can be suggested that a complete lymphadenectomy should always be performed in order determine the staging and improve the prognosis of patients with TC.
In two small previous retrospective analyses of TC and AC, the 10-year survival rates for AC ranged from 59–69%, which were higher than the CSS rate of 49.1% reported in the present study, likely owing to the differences in sample size and the characteristics of the patients enrolled (20,21). The sample size was larger and there were more patients with lymph node involvement in the present study, which was also a factor affecting prognosis. In another larger study (n=507) utilizing the Surveillance, Epidemiology and End Results database, patients with AC had a 10-year survival rate of 59% after surgery, which may have been influenced by a lower proportion of nodal involvement (N1-3, 33.5%) compared with the nodal status of the cohort in the present study (N1-3, 41.2%) (22). Lymph node metastases have been shown to be associated with an inferior survival rate in patients with AC (19). In a recent national study of surgically managed AC (n=816), no significant difference in mortality was observed between lobectomy and sublobectomy (23). Similar to TC, it can be suggested that the extent of lymphadenectomy in AC seems to be more important than the extent of lung resection.
In a study examining disease recurrence in patients with TC and AC following surgery, there did not appear to be a significant difference in the recurrence rate of AC with positive nodes versus negative nodes (16). This is in contrast to the findings in the present study, which may be attributed to the inclusion of patients with higher nodal status, as well as data regarding inadequate N2 lymphadenectomy, which was shown to increase recurrence risk. In a retrospective Spanish study, a univariate analysis found that sublobar resections in AC were associated with increased rates of recurrence compared with lobar resections (24). These observed findings, in conjunction with increased recurrence risk with inadequate lymphadenectomy, reinforce the suggestion that mediastinal staging and mediastinal dissection are needed to improve the prognosis of patients with PCT.
Limitations of the present study largely derive from its retrospective nature. Additionally, being a tertiary referral center could result in more patients who are medically complex and/or with advanced stage PCT. Additional prospective studies are necessary to validate the findings as well as to determine the appropriate surgical approach for these patients. Furthermore, recurrence type may also be associated with prognosis, which is not the subject of the present study, but is of note. This will be investigated further in later work.
In conclusion, in the present study, patients with AC tumors had significantly worse CSS and DFS rates compared with patients with TC. The degree of nodal involvement in AC was a prognostic marker, in contrast to that in TC. Inadequate lymphadenectomy increased the risk of recurrence in AC and mortality in TC. However, the type of surgery did not have a significant impact. The present study emphasizes the importance of mediastinal nodal dissection in patients with PCT.
Acknowledgements
Not applicable.
Funding Statement
This study was supported by grants R01 CA80127 and R01 CA84354 from the National Institutes of Health and the Mayo Clinic Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LD, VE, AL, SES and DEJ designed the study. SDC, SEB, DW, YHL, JAW and PARS performed the acquisition of data, and DS, KRS and PY performed analysis and interpretation of data. LD, VE, AL, SES and DEJ prepared and wrote the original draft of the manuscript. SDC, SEB, DW, YHL, JAW and PARS reviewed and edited the manuscript. All authors read and approved the final version of the manuscript. DS, KRS and PY confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rekhtman N. Neuroendocrine tumors of the lung: An update. Arch Pathol Lab Med. 2010;134:1628–1638. doi: 10.5858/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 4.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, Thibodeau S, Adjei AA, Jett J, Deschamps C. Clinical features of 5,628 primary lung cancer patients: Experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services, corp-author. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The health consequences of smoking-50 years of progress: A report of the surgeon general. [Google Scholar]
- 7.Amin MB, Greene FL, Edge SB, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, et al. 8th edition. Springer International Publishing; New York, NY: 2017. AJCC staging manual; pp. 1–1024. [Google Scholar]
- 8.Cao C, Yan TD, Kennedy C, Hendel N, Bannon PG, McCaughan BC. Bronchopulmonary carcinoid tumors: Long-term outcomes after resection. Ann Thorac Surg. 2011;91:339–343. doi: 10.1016/j.athoracsur.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 9.Skuladottir H, Hirsch FR, Hansen HH, Olsen JH. Pulmonary neuroendocrine tumors: Incidence and prognosis of histological subtypes. A population-based study in Denmark. Lung Cancer. 2002;37:127–135. doi: 10.1016/S0169-5002(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 10.Stolz A, Harustiak T, Simonek J, Schutzner J, Polanecky O, Burkert J, Lischke R. Long-term outcomes and prognostic factors of patients with pulmonary carcinoid tumors. Neoplasma. 2015;62:478–483. doi: 10.4149/neo_2015_057. [DOI] [PubMed] [Google Scholar]
- 11.Froudarakis M, Fournel P, Burgard G, Bouros D, Boucheron S, Siafakas NM, Emonot A. Bronchial carcinoids. A review of 22 cases. Oncology. 1996;53:153–158. doi: 10.1159/000227552. [DOI] [PubMed] [Google Scholar]
- 12.Fink G, Krelbaum T, Yellin A, Bendayan D, Saute M, Glazer M, Kramer MR. Pulmonary carcinoid: Presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest. 2001;119:1647–1651. doi: 10.1378/chest.119.6.1647. [DOI] [PubMed] [Google Scholar]
- 13.Beasley MB, Thunnissen FB, Brambilla E, Hasleton P, Steele R, Hammar SP, Colby TV, Sheppard M, Shimosato Y, Koss MN, et al. Pulmonary atypical carcinoid: Predictors of survival in 106 cases. Hum Pathol. 2000;31:1255–1265. doi: 10.1053/hupa.2000.19294. [DOI] [PubMed] [Google Scholar]
- 14.Martini N, Zaman MB, Bains MS, Burt ME, McCormack PM, Rusch VW, Ginsberg RJ. Treatment and prognosis in bronchial carcinoids involving regional lymph nodes. J Thorac Cardiovasc Surg. 1994;107:1–7. doi: 10.1016/S0022-5223(94)70444-9. [DOI] [PubMed] [Google Scholar]
- 15.Yendamuri S, Gold D, Jayaprakash V, Dexter E, Nwogu C, Demmy T. Is sublobar resection sufficient for carcinoid tumors? Ann Thorac Surg. 2011;92:1774–1779. doi: 10.1016/j.athoracsur.2010.08.080. [DOI] [PubMed] [Google Scholar]
- 16.Lou F, Sarkaria I, Pietanza C, Travis W, Roh MS, Sica G, Healy D, Rusch V, Huang J. Recurrence of pulmonary carcinoid tumors after resection: Implications for postoperative surveillance. Ann Thorac Surg. 2013;96:1156–1162. doi: 10.1016/j.athoracsur.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Kneuertz PJ, Kamel MK, Stiles BM, Lee BE, Rahouma M, Harrison SW, Altorki NK, Port JL. Incidence and prognostic significance of carcinoid lymph node metastases. Ann Thorac Surg. 2018;106:981–988. doi: 10.1016/j.athoracsur.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 18.Johnson R, Trocha S, McLawhorn M, Worley M, Wheeler G, Thompson L, Schisler N, Schammel D, Schammel C, Stephenson J, Bolton W. Histology, not lymph node involvement, predicts long-term survival in bronchopulmonary carcinoids. Am Surg. 2011;77:1669–1674. doi: 10.1177/000313481107701241. [DOI] [PubMed] [Google Scholar]
- 19.Cardillo G, Sera F, Di Martino M, Graziano P, Giunti R, Carbone L, Facciolo F, Martelli M. Bronchial carcinoid tumors: Nodal status and long-term survival after resection. Ann Thorac Surg. 2004;77:1781–1785. doi: 10.1016/j.athoracsur.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 20.Okereke IC, Taber AM, Griffith RC, Ng TT. Outcomes after surgical resection of pulmonary carcinoid tumors. J Cardiothorac Surg. 2016;11:35. doi: 10.1186/s13019-016-0424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez RA, Beyer DT, Diebold AE, Voros BA, Chester MM, Wang YZ, Boudreaux JP, Woltering EA, Uhlhorn AP, Ryan P, et al. Prognostic factors in typical and atypical pulmonary carcinoids. Ochsner J. 2017;17:335–340. [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Pang Z, Wang Y, Bie F, Zeng Y, Wang G, Du J. The role of surgery for atypical bronchopulmonary carcinoid tumor: Development and validation of a model based on surveillance, epidemiology, and end results (SEER) database. Lung Cancer. 2020;139:94–102. doi: 10.1016/j.lungcan.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Walters SL, Canavan ME, Salazar MC, Resio BJ, Blasberg JD, Mase V, Boffa DJ. A national study of surgically managed atypical pulmonary carcinoid tumors. Ann Thorac Surg. 2021;112:921–927. doi: 10.1016/j.athoracsur.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Canizares MA, Matilla JM, Cueto A, Algar J, Muguruza I, Moreno-Mata N, Moreno-Balsalobre R, Guijarro R, Arrabal R, Garcia-Fontan E, et al. Atypical carcinoid tumours of the lung: Prognostic factors and patterns of recurrence. Thorax. 2014;69:648–653. doi: 10.1136/thoraxjnl-2013-204102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.