Abstract
We studied the safety and immunogenicity of a Shigella flexneri 2a vaccine comprising native S. flexneri 2a lipopolysaccharide (LPS) complexed to meningococcal outer membrane proteins—proteosomes—in normal, healthy adults. A two-dose series of immunizations was given by intranasal spray, and doses of 0.1, 0.4, 1.0, and 1.5 mg (based on protein) were studied in a dose-escalating design. The vaccine was generally well tolerated. The most common reactions included rhinorrhea and nasal stuffiness, which were clearly dose related (P ≤ 0.05). These reactions were self-limited and generally mild. The vaccine elicited S. flexneri 2a LPS-specific immunoglobulin A (IgA), IgG, and IgM antibody-secreting cells (ASCs) in a dose-responsive manner. At doses of 1.0 or 1.5 mg, highly significant (P < 0.001) increases in ASCs of all antibody isotypes occurred and 95% of subjects had an ASC response in at least one antibody isotype. Dose-related serum antibody responses were observed, with geometric mean two- to fivefold rises in specific serum IgA and IgG titers and two- to threefold rises in IgM in the 1.0- and 1.5-mg-dose groups (P < 0.0001 for each isotype). Elevated serum antibody levels persisted through day 70. Increases in fecal IgG and IgA and also in urinary IgA specific for S. flexneri 2a LPS were demonstrated. These were most consistent and approached statistical significance (P = 0.02 to 0.12 for various measures) on day 70 after the first dose. The magnitude of immune responses to intranasally administered proteosome-S. flexneri 2a LPS vaccine is similar to those reported for live vaccine candidates associated with protective efficacy in human challenge models, and further evaluation of this product is warranted.
Shigella flexneri is a major cause of endemic bloody diarrheal disease in the developing world and is also an important pathogen in travelers in some settings (20, 31). Epidemiologic (3, 5) data in humans and challenge data in primates (10) have shown that type-specific serum antibody recognizing the O-polysaccharide portion of the lipopolysaccharide (LPS) somatic antigen of shigellae is associated with protection against shigellosis. While serum antibodies obtained by natural or experimental exposure to virulent shigellae correlate with resistance to disease, parenteral vaccines have not consistently provided protection against shigellosis despite significant serum O-polysaccharide-specific antibody responses (2, 9, 14). In addition, some epidemiologic studies suggest that serum antibodies alone are an insufficient predictor of resistance to shigellosis (4). These observations, as well as the protective efficacy of oral hyperimmune bovine colostral immunoglobulins against Shigella challenge in humans, are compatible with the concept that mucosal immunity is a prime protective mechanism against enteric Shigella infections (12, 32). In this view, serum antibodies may be surrogate markers for multiple protective mechanisms operating at intestinal mucosal sites (12, 29, 33, 34). Measurement of specific antibody-secreting cells (ASCs), especially those producing immunoglobulin A (IgA) antibodies and transiting the peripheral blood to mucosal sites 6 to 10 days after infection or immunization, and/or measurement of antibodies in mucosal secretions has been proposed as a more predictive marker of mucosal vaccine-induced protection (15, 19).
Whereas parenteral vaccines are often ineffective in stimulating mucosal immune responses, such responses are most effectively elicited by application of antigens at mucosal surfaces (27). Further, immunization at one mucosal surface is capable of eliciting secretory antibodies at sites distant from the immunizing site, a phenomenon known as the “common mucosal immune system” (25). In addition, mucosal immunization can stimulate systemic antibody production. Live attenuated or recombinant organisms that express one or more Shigella antigens and are given orally have been the primary focus of mucosal Shigella vaccine development to date. The success of this approach has been limited, however, by the modest window between immunogenic doses and those associated with unacceptable reactogenicity (29). Accordingly, subunit mucosal vaccine delivery systems are being explored in an attempt to elicit both systemic and mucosal protective immune responses while avoiding the potential safety issues attending live attenuated vaccines. The product that is the subject of this report utilizes the proteosome system to deliver S. flexneri 2a LPS antigen. The term “proteosome” refers to purified preparations of meningococcal outer membrane proteins (OMPs) that form multimolecular vesicular structures with antigens noncovalently complexed to them, generally (but not exclusively) via hydrophobic interactions (21). The proteosome system has both biodelivery and immunostimulatory properties that enhance immunogenicity and may also significantly attentuate the toxicity of such antigens as LPS. Proteosome-based LPS vaccines for Shigella have been tolerated well by several animal species and have shown protective activity in the Séreny test and in a murine lethal pneumonia model when delivered via mucosal routes (21, 24, 28). In addition, proteosome-based mucosal vaccines have provided protection against respiratory challenge with staphylococcal enterotoxin B and have elicited neutralizing mucosal and systemic antibody responses to human immunodeficiency virus (22, 23). Here we report a phase I safety and immunogenicity evaluation of proteosome-S. flexneri 2a LPS vaccine delivered via the intranasal route in humans.
(Portions of this information were previously presented at the 36th Annual Meeting of the Infectious Diseases Society of America, 12 to 15 November, 1998.)
MATERIALS AND METHODS
Vaccine.
The vaccine used in this trial was manufactured in compliance with Good Manufacturing Practices at the Walter Reed Army Institute of Research Pilot BioProduction Facility. Group B Neisseria meningitidis serotype 2b, strain 8047, was fermented to stationary phase in modified Catlin's medium in a 300-liter vessel and was then inactivated with phenol and the cell paste collected by continuous-feed centrifugation. The OMPs were extracted as described previously with buffer containing 1 M CaCl2 and 6.0% Empigen (24). After purification by sequential ethanol and ammonium sulfate precipitation steps, the OMPs were concentrated and resolubilized in Tris-buffered saline, pH 8.0, with 0.1% Empigen and 10 mM EDTA. S. flexneri serotype 2a, strain BS103, was grown to stationary phase in Trypticase soy broth supplemented with glucose and magnesium sulfate and was harvested by continuous-feed centrifugation. The cell paste was dried with acetone and was subjected to extraction with 90% phenol at 68°C (35). Phenol was removed from the aqueous fractions, and the LPS was concentrated by an ultrafiltration/diafiltration step, followed by ethanol precipitation and resolubilization in water. Vaccine complexes were formed by combining OMPs and LPS in the desired proportions in the presence of Empigen and then removing the detergent by diafiltration. The final product contained 2.56 mg of OMP protein/ml by Lowry assay and 2.29 mg of LPS/ml by 2-keto-3-deoxy octonate assay (weight ratio of protein/LPS = 1.12) in Tris-buffered normal saline, pH 8.0 (TNS). More than 95% of the LPS in the final vaccine product was derived from S. flexneri 2a. Vaccine was stored frozen at −20°C until use. When required by the clinical protocol, stock vaccine was diluted in sterile TNS.
Clinical trial.
The clinical trial was performed at the Clinical Trials Unit of the Walter Reed Army Institute of Research. The protocol, amendments to the protocol, and informed consent documents were reviewed and approved by all institutional review boards having jurisdiction, and all subjects gave written informed consent. Healthy adults of both sexes and between the ages of 18 and 50 years, inclusive, were evaluated for enrollment. After giving consent, subjects underwent baseline physical examination and clinical laboratory testing to ensure good general health. Individuals with chronic medication use for complaints relative to the nose, throat, or sinuses or with a history of reactive airway disease or other pulmonary disease were excluded. Female subjects underwent serum β-HCG testing within 48 h prior to each dose of the investigational vaccine to ensure that they were not pregnant.
Vaccine doses were delivered via nasal spray on days 0 and 14 of the protocol, as described below. After each dose, subjects were observed for 20 min and then had vital signs repeated. They completed a questionnaire eliciting such symptoms as nasal burning or stinging, sore throat, a sense of posterior nasal drainage or a medicinal taste, lightheadedness, or shortness of breath. Subjects were then asked to return for measurement of vital signs, interview, and examination as necessary at 1 and 4 h and 1, 2, and 7 days after each vaccine dose. Subjects were provided with digital thermometers and were instructed in taking and recording their oral temperature every evening for 7 days. In addition, each subject maintained a diary of potential reactogenicity complaints, including headache, fever, myalgia, fatigue, rhinorrhea and/or nasal congestion, cough, sore throat, nasopharyngeal burning, nausea, vomiting, anorexia, abdominal pain, and diarrhea. Subjects graded these events as grade 0 (“none”), 1 (“barely noticeable”), 2 (“noticeable but not interfering with daily activities”), or 3 (“interfering with daily activities to any extent”). Any events in the above spectrum that occurred within 7 days after either vaccine dose were assumed to be vaccine related. Adverse events were additionally elicited at each visit through day 70, and their severity and relationship to the vaccine were assessed by the clinical investigators (A.D.M. and D.N.T.). Adverse events outside the immediate postvaccinal period were graded as mild (“present but no impact on daily activities”), moderate (“partial impairment of daily activities”), or severe (“subject can do <50% of normal daily activities”).
Samples of serum, stool, saliva, and urine were collected on days 0, 14, 23, 42, and 70 for determinations of S. flexneri 2a LPS-specific antibodies as described below. Fresh stool (collected ≤24 h prior to processing and refrigerated) was suspended at a ratio of approximately 1:10 in phosphate-buffered normal saline (PBS), pH 7.4, supplemented with 5% bovine serum albumin and protease inhibitors (Complete [Boehringer Mannheim, Indianapolis, Ind.]; bestatin [CalBiochem, La Jolla, Calif.]) and vigorously mixed. Afterwards, solids were removed by centrifugation and the extract supernatant was aliquoted and frozen at −70°C until assay. Specimens of heparinized whole venous blood were collected on days 0, 6, 9, 20, and 23 for enumeration of S. flexneri 2a LPS-specific ASCs and were analyzed fresh.
All vaccine doses were delivered as an intranasal spray using a multidose pump device (VP3; Valois of America, Greenwich, Conn.). A nonrandomized, open-label, dose-escalating design was utilized, with all doses based on OMP protein content. The first five subjects received 0.1-mg doses comprising stock vaccine diluted with TNS so as to provide the desired dose in 400 μl (delivered as two 100-μl doses sprayed into each nostril.) These subjects were observed for 1 week, and then the next 10 subjects received 0.4 mg in a total of 400 μl. Following 2 weeks of observation, 10 additional subjects received 1.0-mg doses delivered as 400 μl of undiluted vaccine. Safety data from 1.0-mg recipients were presented to the institutional review board, and permission was granted to study a final 10 subjects at a dosage of 1.5 mg (three 100-μl sprays of undiluted vaccine into each nostril.)
Immunologic assays.
Specific antibody titers in serum and mucosal fluids were measured by enzyme-linked immunosorbent assays (ELISA). Briefly, 96-well round-bottom plates (Immulon II; Dynex, Chantilly, Va.) were coated with a predetermined optimum concentration of whole LPS prepared from S. flexneri 2a, strain BS103, and blocked with bovine serum albumin-casein buffer. Twelve serial dilutions of each fluid of interest were incubated in duplicate for 2 h at 37°C in the coated plates, which were then extensively washed and further incubated for 1 h at 37°C with optimal dilutions of peroxidase-conjugated, affinity-purified second antibody (goat anti-human γ chain [Kirkegaard & Perry Laboratories, Gaithersburg, Md.]; goat anti-human α or μ chain [Southern Biotechnology Associates, Birmingham, Ala.]). After further washing, a colorimetric signal was developed by incubation at room temperature with 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) (for IgG) or TMB (for IgA and IgM) substrates (both from Kirkegaard & Perry.) Color development was stopped by adding 0.5% sodium dodecyl sulfate after 45 min of incubation (for ABTS) or by adding 0.5 M phosphoric acid after 1 h of incubation (for TMB). Optical density (OD) at 405 nm (for ABTS) or 450 nm (for TMB) was read using a Vmax ELISA reader (Molecular Devices, Richmond, Calif.). SoftMax software provided with the ELISA reader was utilized to perform a four-parameter fit of mean OD versus reciprocal dilution for each specimen. The resulting equation was used to interpolate that reciprocal dilution yielding an OD of 1.0, and this value was arbitrarily designated the titer for that specimen. Competitive inhibition studies using fluid-phase LPS from related species revealed that the signal in this assay was ≥95% specific for the O-polysaccharide of S. flexneri 2a, and repeated assays on standard specimens yielded a day-to-day coefficient of variation of <10%. Specific IgA titers in urine were normalized based on urine creatinine concentration determined by a licensed clinical laboratory; specific antibody titers in stool extracts and saliva were normalized based on total IgA content. Total IgA was determined by coating ELISA plates with an IgG fraction of goat anti-human IgA (Jackson ImmunoResearch Laboratories, West Grove, Pa.) IgA from a series of dilutions of the specimen of interest was captured by incubation in the coated plates in parallel with a standard curve comprising known concentrations of human secretory IgA (ICN Biomedicals, Costa Mesa, Calif.) Following detection of bound IgA and generation of a colorimetric signal essentially as above, the SoftMax software was used to develop a standard curve. No less than two dilutions of each specimen yielding ODs within the linear portion of the standard curve were then used to calculate the total IgA content of the specimen by comparison with the standards. Control specimens were included in each run and again demonstrated a coefficient of variation in calculated IgA concentration of <10%.
ASCs were enumerated essentially as described previously (8). Peripheral blood mononuclear cells (PBMCs) were purified from heparinized venous blood diluted in RPMI 1640 medium without Ca2+ or Mg2+ (Life Technologies, Gaithersburg, Md.). Diluted blood specimens were layered over Lymphoprep (Life Technologies) cushions and centrifuged at 400 × g for 30 min at room temperature. Interface bands were washed and resuspended at 2.5 × 106 cells/ml in RPMI 1640 with 2 mM l-glutamine, 50 μg of gentamicin/ml, and 10% fetal bovine serum. For each antibody isotype to be studied, quadruplicate wells on each of two duplicate 96-well flat-bottomed plates (MaxiSorp; Nunc, Naperville, Ill.) previously coated with S. flexneri 2a LPS and blocked with 5% fetal bovine serum were inoculated with 100 μl of cell suspension and incubated for 3.5 h at 37°C in a 5% CO2 atmosphere. The plates were then washed with PBS containing 0.05% Tween 20 (Sigma, St. Louis, Mo.) and were incubated overnight at 4°C with alkaline phosphatase-conjugated affinity-purified goat antibodies to human γ, μ, or α chains (Kirkegaard & Perry) at a predetermined optimal dilution. After further washing with PBS–0.05% Tween, each well was coated with 100 μl of 0.7% agarose in barbital buffer, pH 9.6, containing 5-bromo-4-chloro-3-indolylphosphate (BCIP) substrate (Sigma) and 4 mM MgCl2. After overnight incubation at room temperature, the plates were inverted and spots were enumerated using a stereomicroscope. ASC counts for each antibody isotype were based on means of all wells on the duplicate plates and were expressed as ASC per 106 PBMC. ASC “responders” were defined by fitting the day 0 values (i.e., preimmunization values) for all subjects to a log-normal model and calculating the 99th percentile of the implied distribution. After immunization, values exceeding this level were deemed to indicate a specific ASC response to the vaccine. The baseline IgA ASC distribution yielded a good fit to the model, but baseline IgG and IgM ASC counts were dominated by zero values. The IgA distribution was used to define the response criterion for all three antibody isotypes, but this should be viewed as a conservative criterion for IgG and IgM results (i.e., actual IgG and IgM ASC response rates are probably underestimated by this approach.)
RESULTS
Safety.
The vaccine was well tolerated. Subjects were prompted in order to elicit a series of complaints (listed in Materials and Methods) in the 7-day period immediately following each vaccine dose, and a defined grading scale was applied. Gastrointestinal complaints, such as nausea, vomiting, belly cramps, and anorexia, were minimal. The complaint of grade 1 diarrhea did occur but on further questioning appeared to represent reports of one or more “softer than usual” stools without either increased volume or frequency. Twenty to 30% of each treatment group had subjective complaints of grade 1 “feverishness” after one or more doses, but no subject in the trial had objectively documented fever (oral temperature of >100.4°F) at any time, either when monitored in the clinic 1 and 4 h and 2 and 7 days after dosing or as recorded by their 7-day evening temperature logs. Constitutional symptoms of malaise, myalgia, and headache were reported by 46, 26, and 37% of subjects, respectively. The frequencies of these complaints were unrelated to dose, as illustrated by Table 1. These complaints were predominantly mild and persisted two days or less. Rhinorrhea, generally scanty and clear, was the most consistent complaint in the 7 days after each dose. The frequency, severity, and duration of this local complaint in the various dose groups are summarized in Table 2. Table 2 also includes the “severity index,” the product of the greatest severity and the greatest duration of rhinorrhea reported by each subject. An exact linear-by-linear test for association yields a two-tailed P < 0.05 for dose-related trend in maximum severity, and there is a similar trend for the severity index (P ≈ 0.05). Maximum duration of rhinorrhea also tends to increase with dose, with a P of <0.1 by the exact linear-by-linear test for association. These results notwithstanding, only 2 of 35 subjects (5.7%) reported grade 3 rhinorrhea, i.e., rhinorrhea that interfered with normal daily activities to any extent, and these reports occurred after only 2 of 69 vaccine administrations (2.9%). Notably, rhinorrhea (and most other reactogenicity complaints) did not escalate after the second vaccine dose and may in fact have lessened (mean severity index of 3.03 ± 3.94 after dose 1 versus 2.18 ± 3.05 after dose 2). The complaint of mild to moderate nasal congestion closely paralleled rhinorrhea in frequency and duration. Overall, grade 3 vaccine reactogenicity complaints (those interfering with normal daily activities to any extent) comprised only 8.4% of all positive responses and were restricted to three subjects, two in the 0.4-mg-dose group and one in the 1.0-mg-dose group. No subject in the highest dose group had any grade 3 reactogenicity complaint. None of the volunteers with grade 3 complaints was sufficiently symptomatic to decline a second dose, and none had repeated grade 3 complaints after the second dose. A single 0.4-mg-dose recipient (who contributed the numerical majority of all grade 3 complaints in the entire trial) was diagnosed as having an intercurrent streptococcal pharyngitis 9 days after the first vaccine dose, which may have contributed to his symptoms. After oral amoxicillin treatment, the subject received the second vaccine dose, which he tolerated well.
TABLE 1.
Frequency and severity of headache, malaise, and myalgia after either dose, tabulated by treatment groups
| Size of dose (mg) | Headache results
|
Malaise results
|
Myalgia results
|
|||
|---|---|---|---|---|---|---|
| No. of subjects with any headachea | Median severity gradeb | No. of subjects with any malaisea | Median severity gradeb | No. of subjects with any myalgiaa | Median severity gradeb | |
| 0.1 | 4/5 | 1 | 3/5 | 1 | 2/5 | 1 |
| 0.4c | 3/10 | 1 | 4/10 | 1.5 | 4/10 | 2.5 |
| 1.0 | 2/10 | 2 | 4/10 | 1 | 1/10 | 2 |
| 1.5 | 4/10 | 1.5 | 5/10 | 1 | 2/10 | 1 |
Subjects reporting the complaint at any nonzero grade after either immunization. Total number of subjects is also given.
Results are based on highest severity grade reported by all subjects with nonzero reports after either immunization. Grades: 0, none; 1, barely noticeable; 2, noticeable but not interfering with any daily activity; 3, interfering with daily activities to any extent.
Includes subject with documented streptococcal pharyngitis.
TABLE 2.
Frequency, severity, and duration of rhinorrhea by proteosome-S. flexneri 2a LPS vaccine dose
| Dose size (mg) | No. of subjects with any rhinorrheaa | Median severity gradeb | Mean ± SD duration (days)c | Median durationc | Mean ± SD severity indexd | Median severity index |
|---|---|---|---|---|---|---|
| 0.1 | 2/5 | 0 | 0.8 ± 1.3 | 0 | 0.8 ± 1.3 | 0 |
| 0.4 | 7/10 | 1 | 1.9 ± 1.7 | 2 | 3.6 ± 4.2 | 2.5 |
| 1.0 | 7/10 | 1 | 2.0 ± 2.1 | 1 | 4.1 ± 5.0 | 1 |
| 1.5 | 9/10 | 2 | 2.8 ± 2.0 | 3 | 4.8 ± 3.9 | 4 |
Subjects reporting rhinorrhea at any nonzero grade after either immunization. Total number of subjects is also given.
Results are based on highest severity grade reported by each subject after either immunization. Grades: 0, none; 1, barely noticeable; 2, noticeable but not interfering with any daily activity; 3, interfering with daily activities to any extent.
Results are based on greatest duration of rhinorrhea reported by each subject after either immunization.
Severity index = greatest duration × greatest severity grade reported by each subject after either immunization.
There were no serious adverse events, and no adverse events were attributed to the vaccine outside the immediate postimmunization period. There was no significant association of more frequent adverse events outside the immediate postdosing period with any treatment group or significant dose-related trend in the frequency of such adverse events.
ASC responses.
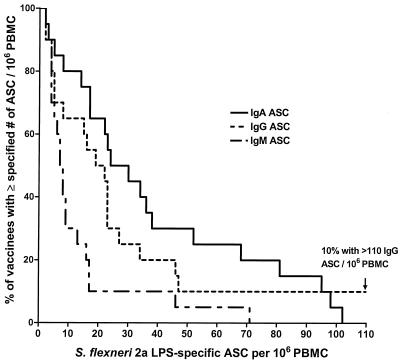
ASC responses specific for S. flexneri 2a LPS were found in all dose groups and in each antibody isotype tested. Table 3 summarizes the peak S. flexneri 2a LPS-specific ASC responses and frequency of responders achieved by each dose group. All dose groups demonstrated a significant ASC response in all antibody isotypes when compared to the baseline (with the sole exception of IgM ASCs in the 0.4-mg-dose group). A test for linear trend in geometric mean peak ASC count with increasing dose was significant for each antibody isotype (Table 2); this was confirmed using the Jonckheere-Terpstra nonparametric test for trend. Peak counts were positively correlated across the various antibody isotypes; i.e., subjects with high peak ASC counts for one isotype tended to be strong responders in the other isotypes as well. The frequency of individual responders was also significantly associated with dose for all three antibody isotypes (P < 0.02 for IgA, P < 0.04 for IgG, and P < 0.004 for IgM; χ2 test for trend.) Of 20 subjects who received 1.0- or 1.5-mg doses, 19 (95%) had a specific ASC response in at least one antibody isotype. The distributions of individual peak ASC responses, based on pooled data from the very similar 1.0- and 1.5-mg-dose groups, are shown in Fig. 1. Twenty-five of 34 evaluable subjects (i.e., subjects receiving two doses) had their peak ASC responses in all antibody isotypes after the first dose, while nine (26.5%) had a peak, and sometimes substantial, response in at least one isotype after the second vaccine dose.
TABLE 3.
Peak S. flexneri 2a LPS-specific ASC responses as a function of proteosome S. flexneri 2a LPS vaccine dose
| Size of dose (mg) | Peak IgA ASC countsa
|
Peak IgG ASC countsa
|
Peak IgM ASC countsa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric meanb | 95% CIe | Median | % Responsed | Geometric mean | 95% CI | Median | % Response | Geometric mean | 95% CI | Median | % Response | |
| Predose | 0.4 | 0.2–0.6 | 0 | 0 | 0.1 | 0–0.2 | 0 | 0 | 0.2 | 0.1–0.3 | 0 | 0 |
| 0.1 | 4.8c | 1.4–24.9 | 3.0 | 40 | 2.2c | 0.1–8.4 | 2.0 | 40 | 2.1c | 1.2–3.4 | 2.0 | 0 |
| 0.4 | 7.6c | 2.8–18.9 | 6.0 | 60 | 4.3c | 0.7–16.1 | 2.0 | 40 | 2.7 | 0.1–8.3 | 1.0 | 30 |
| 1.0 | 20.2c | 8.0–46.2 | 22.5 | 90 | 13.7c | 4.7–37.2 | 16.5 | 80 | 6.8c | 4.2–10.7 | 1.0 | 70 |
| 1.5 | 26.4c | 11.0–61.5 | 31.0 | 90 | 16.2c | 5.8–42.8 | 22.0 | 80 | 8.2c | 3.0–20.8 | 7.0 | 70 |
All counts are based on peak values at any time postimmunization for each individual in each dose group and are expressed as specific ASCs per 106 PBMCs.
Zero values were accommodated by adding 1 to all data, calculating geometric means and 95% confidence interval, and then subtracting 1 from results. A linear ascending trend of geometric mean with vaccine dose is detected for each antibody isotype: P < 0.01 for IgA: P < 0.03 for IgG and IgM.
Significantly different from preimmunization values by paired sample t test (P < 0.05; P < 0.001 for all isotypes in the 1.0- and 1.5-mg-dose groups).
Percentage of subjects attaining ASC levels exceeding four specific ASCs per 106 PBMCs, the 99th percentile of the day 0 distribution (see text).
CI, confidence interval.
FIG. 1.
Distribution of individual peak S. flexneri 2a LPS-specific ASCs in the pooled 1.0- and 1.5-mg-dose groups. The reverse-cumulative-frequency graph displays the frequency of subjects (expressed as percentage of the population of 20 on the vertical axis) with peak circulating specific ASC numbers equal to or greater than the values specified on the horizontal axis. Data are displayed for IgA-, IgG-, and IgM-secreting cells producing antibodies specific for S. flexneri 2a LPS. Median values exceed 20 specific IgA- and IgG-secreting cells per 106 PBMCs.
Serum antibody responses.
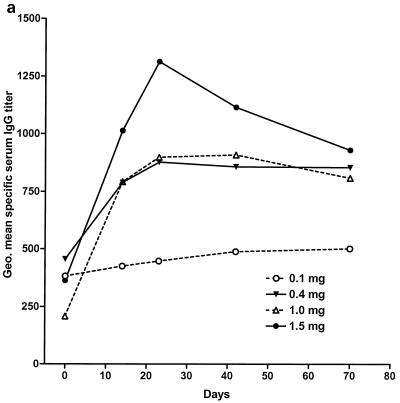
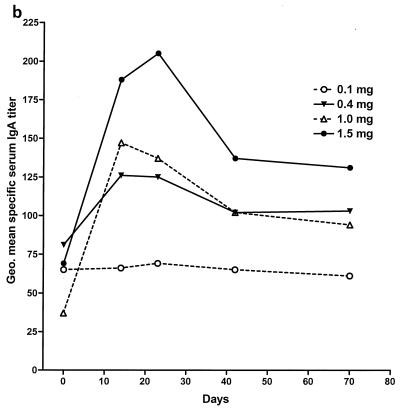
Figure 2 illustrates the time course of changes in geometric mean serum IgA, IgG, and IgM titers specific for S. flexneri 2a LPS. The 0.1-mg dose evoked little or no response in specific IgA, although both IgG and IgM showed a slight but significant rising trend over time (Fig. 2 and its legend). S. flexneri 2a LPS-specific serum IgG and IgA rose sharply between days 0 and 14 in the recipients of 0.4-mg or greater doses (Fig. 2a and b). Peak values were noted on day 14 with the 0.4- and 1.0-mg doses, while 1.5-mg-dose recipients demonstrated a modest further increase on day 23. Changes in IgM were smaller (Fig. 2c). Only the rise in specific IgG titers was significant in the 0.4-mg-dose group, but all three isotypes showed significant increases by repeated-measure analysis of variance (ANOVA) in the 1.0- and 1.5-mg-dose groups (Fig. 2 and its legend). Table 4 summarizes the peak responses in each serum antibody isotype for the various dose groups in terms of fold increases. As was observed with ASC counts, a significant or near-significant dose-responsive linear trend was present for all antibody isotypes when the log-transformed peak response data were analyzed (P < 0.04 for IgA, P < 0.02 for IgG, and P < 0.06 for IgM). Median and geometric mean values for the magnitudes of serum antibody responses to the 1.5-mg dose were slightly lower than for the 1.0-mg dose but with broadly overlapping 95% confidence intervals, suggesting a plateau above 1.0-mg doses. A direct, pairwise comparison of the serum antibody responses in the 1.0- and 1.5-mg-dose groups showed no significant differences.
FIG. 2.
Kinetics of serum antibody responses to intranasal proteosome-S. flexneri 2a LPS vaccine. (a) IgG. (b) IgA. (c) IgM. Data shown are the geometric mean serum titers specific for S. flexneri 2a LPS in the 0.1-, 0.4-, 1.0-, and 1.5-mg-dose groups. Baseline titers in the 1.5-mg-dose group were higher than those in the 1.0-mg-dose group. Thus, while peak absolute values were lower in the 1.0-mg-dose group, increases in antibody titer expressed as a multiple of baseline values for the 1.0-mg-dose group were actually equal to or greater than those for the 1.5-mg-dose recipients (Table 3). In the 1.0-mg- and 1.5-mg-dose groups, all three antibody isotypes show significant rises in response to immunization (P ≤ 0.0001 in each case, repeated-measure ANOVA) and every postimmunization time point demonstrates a significant rise relative to baseline (P < 0.01 in each case, Dunnett's multiple-comparison test). IgG responses were significant in the 0.1- and 0.4-mg-dose groups (P < 0.008 in both cases, repeated-measure ANOVA), but IgA responses were not. The rise in specific IgM was not significant in the 0.4-mg-dose group but was significant among the 0.1-mg-dose recipients (P = 0.02, repeated-measure ANOVA.) Geo., geometric.
TABLE 4.
Peak S. flexneri 2a LPS-specific serum antibody responses as a function of proteosome S. flexneri 2a LPS vaccine dose
| Dose size (mg) | IgA titer fold increases
|
IgG titer fold increases
|
IgM titer fold increases
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric meana | 95% CIb | Median | % >4-foldc | Geometric mean | 95% CI | Median | % >4-fold | Geometric mean | 95% CI | Median | % >4-fold | |
| 0.1 | 1.13 | 1.0–1.3 | 1.19 | 0 | 1.36 | 1.1–1.7 | 1.25 | 0 | 1.18 | 0.9–1.5 | 1.09 | 0 |
| 0.4 | 1.84 | 0.8–4.0 | 1.24 | 20 | 2.12 | 1.1–4.1 | 1.53 | 10 | 1.57 | 0.9–2.6 | 1.22 | 10 |
| 1.0 | 4.63 | 1.1–0.1 | 3.32 | 30 | 4.92 | 2.7–8.9 | 4.52 | 40 | 2.90 | 1.7–5.1 | 2.58 | 30 |
| 1.5 | 2.71 | 1.7–8.8 | 2.93 | 30 | 3.20 | 1.9–5.4 | 3.00 | 20 | 1.97 | 1.5–2.6 | 1.59 | 10 |
A linear ascending trend of geometric mean with vaccine dose is detected for each antibody isotype: P < 0.03 for IgA, P < 0.02 for IgG, and P < 0.05 for IgM.
CI, confidence interval.
Percentage by which result exceeds fourfold increase is given.
Antibody responses in stool and other mucosal specimens.
Tables 5 and 6 summarize geometric mean changes from baseline values in normalized titers of S. flexneri LPS-specific IgG and IgA in stool extracts in the various dose groups over time. Because antibody titers in stool are highly derivative values and because the consistency in the kinetics of these responses from subject to subject is unknown, the tables also summarize the peak responses within each group. Day 23 data for the 1.5-mg-dose group cannot be interpreted because 4 of the 10 subjects, all of them responders at other time points, failed to provide specimens on that day. Despite the considerable variability in these data and small sample sizes, the geometric mean fold rises in specific stool antibodies show increases over time in the higher-dose groups. As was noted in serum, the 1.5-mg-dose group is not superior to the 1.0-mg-dose group. Interestingly, S. flexneri-specific stool antibodies appear to increase at 14 and 23 days after initiation of the immunization series, subside toward the baseline at day 42, and then rebound to higher levels at day 70. This pattern is apparent to some extent in all dose groups and in both the IgA and IgG antibody classes. Although some earlier sampling times yielded greater mean increases than day 70, there was large variability. At day 70, a substantial proportion of the population showed consistent increases over baseline levels, resulting in a concentration of significant or near-significant findings at this time point. Unfortunately, later time points were not examined. In comparison to serum antibodies and urinary IgA responses (see below), the influence of dose on peak specific IgG and IgA responses in stool was less clear, but in keeping with the other compartments, the 1.5-mg dose appeared to confer no advantage over the 1.0-mg dose.
TABLE 5.
Increases in normalized stool IgG titers specific for S. flexneri 2a LPS
| Time (day or peak) | Results for different doses (mg)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.1
|
0.4
|
1.0
|
1.5
|
|||||
| Geometric mean fold rise | % >2-fold riseg | Geometric mean fold rise | % >2-fold rise | Geometric mean fold rise | % >2-fold rise | Geometric mean fold rise | % >2-fold rise | |
| 14 | 1.91 | 40 | 1.93a | 67a | 1.87 | 40 | 0.90 | 40 |
| 23 | 1.58 | 60 | 1.49a | 33a | 1.89 | 40 | 0.30b | 33b |
| 42 | 0.88 | 20 | 0.66a | 0a | 1.14 | 40 | 1.33 | 40 |
| 70 | 0.72 | 40 | 1.71c | 50 | 2.71d | 40 | 2.47e | 50 |
| Peakf | 3.48 | 60 | 4.37 | 88 | 5.65 | 80 | 3.73 | 50 |
Nine subjects were evaluable.
Only six subjects were evaluable.
P < 0.05 for the comparison of the geometric mean fold rise to 1, i.e., no change.
P < 0.02 for the comparison of the geometric mean fold rise to 1, i.e., no change.
P = 0.12 for the comparison of the geometric mean fold rise to 1, i.e., no change.
Includes the strongest response for each subject with at least three postimmunization values.
Percentage by which result exceeds a twofold increase is given.
TABLE 6.
Increases in normalized stool IgA titers specific for S. flexneri 2a LPS
| Time (day or peak) | Results for different doses (mg)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.1
|
0.4
|
1.0
|
1.5
|
|||||
| Geometric mean fold rise | % >2-fold risef | Geometric mean fold rise | % >2-fold rise | Geometric mean fold rise | % >2-fold rise | Geometric mean fold rise | % >2-fold rise | |
| 14 | 1.07 | 20 | 1.19a | 11a | 1.42 | 50 | 1.48 | 40 |
| 23 | 0.80 | 20 | 1.62a | 33a | 2.11 | 60 | 0.71b | 17b |
| 42 | 0.70 | 0 | 0.85a | 10a | 1.03 | 20 | 1.13 | 30 |
| 70 | 0.65 | 0 | 1.10 | 10 | 1.59c | 50 | 1.48d | 40 |
| Peake | 1.35 | 20 | 2.07 | 33 | 3.36 | 70 | 2.37 | 70 |
Subjects were evaluable.
Only six subjects were evaluable.
P = 0.10 for the comparison of the geometric mean fold rise to 1, i.e., no change.
P < 0.06 for the comparison of the geometric mean fold rise to 1, i.e., no change.
Includes the strongest response for each subject with at least three postimmunization values.
Percentage by which result exceeds twofold increase is given.
S. flexneri 2a LPS-specific IgA titers in urine showed kinetics different from those manifested by stool antibody, peaking at 14 or 23 days after the start of the immunization series and generally decreasing thereafter (data not shown). The magnitude of urine specific IgA response was positively correlated with the magnitude of the specific IgA response in serum, but the magnitudes of urine and stool specific IgA responses showed only a weakly positive and not significant correlation. Specific IgA responses in saliva followed a time course similar to that of specific IgA in urine but were smaller (data not shown). Neither urine nor saliva specific IgA responses appeared to be a useful surrogate for specific IgA responses measured in stool.
DISCUSSION
We have evaluated the capacity of a proteosome-based vaccine, incorporating native S. flexneri 2a LPS, to be administered safely by the intranasal route and to elicit immune responses potentially capable of protecting against shigellosis. The vaccine was generally well tolerated. Constitutional symptoms of vaccine reactogenicity were modest, self-limited, and not clearly dose related. Objective fever was not observed. Local vaccine reactions, including rhinorrhea and nasal congestion, were noted, and these were dose related in severity and duration. However, nasal discharge was generally scanty and clear, nasal complaints that limited normal daily activities to any extent were uncommon, and local complaints were short-lived and resolved spontaneously.
Overall, vaccine immunogenicity demonstrated dose-responsive behavior. The major increases in immunogenicity occurred between the 0.1- and 1.0-mg doses; only modest, if any, immunologic advantage was accrued when dose was increased from 1.0 to 1.5 mg. Coupled to the safety data, this suggests that the 1.0-mg dose is optimal for further exploration.
Specific ASC responses were greatest and most frequent 7 to 10 days after the first vaccine dose. These kinetics are similar to those reported after oral immunization strategies for enteric pathogens, even when multiple doses are given, and are also similar to ASC kinetics after intranasal immunizations with live, attenuated influenza vaccines (15–19, 26). Reports of ASC responses to sequential mucosal doses of nonliving antigens are infrequent, but multiple oral doses of killed Salmonella enterica serovar Typhi Ty21a also appear to yield only a single wave of ASCs (17). In contrast, two parenteral doses of killed Ty21a resulted in two distinct and roughly equal ASC peaks. The establishment of protective mucosal responses has been invoked to explain the reduced ASC response to later doses of live, attentuated mucosal vaccines (16), and a similar mechanism could conceivably limit the effect of a second dose of nonliving antigen on a mucosal surface. In this study we did not measure development of nasal antibodies to S. flexneri 2a LPS, but the suggestive trend to milder local reactogenicity after the second dose might reflect the presence of an active nasal immune response binding and eliminating our proinflammatory antigen, LPS. A minority of subjects (approximately 15%) had their greatest, or only, ASC response after the second dose, suggesting that the second dose may be important in establishing the broadest protection in a vaccinee population. This heterogeneity is also consistent with the individual subject ASC kinetics seen after administration of oral live or killed serovar Typhi Ty21a (17). Future studies in larger numbers will be needed to assess possible immunologic predictors of the need for a second dose.
Serum antibody titers rose sharply after the first vaccine dose, most often with a further small increase after the second dose. Serum antibody levels in the 1.0- and 1.5-mg-dose groups remained significantly elevated through the 70-day time point. Although both stool and urine specific antibody titers were derivative values dependent on normalization, the appearance of repeating kinetic patterns across the various treatment groups lends credence to the kinetic patterns of antibody responses observed in the stool and urine. The time course of specific antibody responses in stool shows an acute rise followed by a return toward the baseline and a later, consistent rising phase exemplified by multiple, statistically significant increases over the baseline at day 70. While we presently have no later data points in humans, it is interesting that this temporal pattern is similar to that seen in the stool pellets of mice immunized intranasally with proteosome-LPS vaccines. In that model, large increases in specific antibodies in stool are observed shortly after immunization, followed by a transient decline toward the baseline and then a rising phase which persists through at least 180 days (D. Burt, unpublished data). Thus, it is possible that later samples from humans may also reveal the establishment of a lasting increase in stool antibodies to the LPS antigen. Like Cohen et al. (6), we found the behavior of urinary specific IgA antibody to be correlated with that of serum antibody. Unfortunately, urinary specific IgA responses were only weakly correlated with specific antibodies measured in stool and were thus a poor surrogate marker of intestinal responses.
Appropriate immunologic markers of vaccine-induced resistance to shigellosis are not well established. In 1997, Cohen et al. reported efficacy of a parenteral Shigella sonnei O-polysaccharide conjugate vaccine in a field study of young adults in Israel (2). In that study, mean increases of serum O-polysaccharide-specific IgG and IgA of 15- to 40-fold were induced in the group of protected individuals shortly after immunization, but these levels had dropped to four- to sevenfold over the baseline by the time of the epidemic which yielded the majority of evidence for efficacy. Urine from subjects in this trial contained S. sonnei O-polysaccharide-specific IgA, apparently of secretory origin (6). While levels of urinary specific IgA correlated with serum specific antibody levels, a urinary titer associated with protection was not reported. In a recent study reported by Coster et al., subjects who had ingested a live, attenuated S. flexneri 2a vaccine candidate showed either complete protection or protection against severe symptoms when challenged orally with virulent S. flexneri 2a (7). Those subjects with complete protection against disease demonstrated a geometric mean of approximately 300 S. flexneri 2a O-polysaccharide-specific circulating IgA ASCs per 106 PBMCs. IgM ASC responses were similar, while IgG ASCs were fewer (geometric mean of 68 per 106 PBMCs in the completely protected subset.) Serum IgA responses in these subjects were similar in magnitude to those cited by Cohen, but IgG and IgM responses in serum were much more modest (two- to ninefold). Interestingly, a number of vaccinees showed significant attenuation of challenge-induced disease with more modest IgM ASC responses (geometric mean of 19 per 106 PBMC), little or no IgG or IgA ASC response, a geometric mean 1.6-fold rise in serum IgA, and essentially no serum IgG or IgM responses. These latter data, arising from a rather stringent challenge, suggest that useful protection may follow mucosal antigen exposure even in the presence of limited serum antibody or circulating ASC responses. This interpretation is consistent with earlier data, which suggest that at least partial protection against Shigella challenge can be associated with peak specific IgA ASC numbers of <50 and seroconversion rates (based on rises in anti-LPS titer of ≥4) of 20 to 40% (13, 18, 19). Data regarding fecal antibody responses to Shigella antigens as a marker of protection are extremely limited and inadequate to date to allow prediction of vaccine efficacy, not least because of the difficulty in standardizing such assays.
The proteosome mucosal vaccine delivery system has several advantages. It delivers subunit antigens without potential for transmission or reversion to pathogenicity. Manufacturing methods for meningococcal OMPs are simple, robust, and scalable, and these proteins have a good human safety record as covalent carriers for Hemophilus influenzae type b polysaccharide or as candidate group B meningococcal vaccines (1, 21). Complexing with any amphipathic antigen can occur under mild, nondenaturing conditions, and the resultant complexes are stable and of sufficient size to be handled as particles by mucosal M cells and/or antigen-presenting cells (21). Further, unlike some lipid or particulate delivery systems, neisserial OMPs are not immunologically inert but rather stimulate B cells to proliferate, increase surface expression of T-cell costimulatory ligand B7-2, and can also enhance T-cell-independent antipolysaccharide immunoglobulin responses (21, 30, 36). Antibody responses to the proteosomes themselves were not measured in this study but have been minimal in other human studies of intranasal LPS- and protein antigen-bearing proteosome vaccines (unpublished). In addition, preexisting antiproteosome antibody titers have not correlated with responses to vaccine antigens of interest. High-dose toxicity studies of intranasal proteosome vaccines in animals have revealed no local or central nervous system lesions, unlike the potent mucosal adjuvants cholera toxin and Escherichia coli heat-labile toxin, both of which are safe via the intranasal route only when used as trace additives to their much less active B subunits (11). The relative utility of proteosomes and adjuvants such as CpG motifs or other oligodeoxynucleotides remains to be defined, but only the former simultaneously combines adjuvant activity and particulate delivery.
Based on the data in hand, it is not possible to predict whether the proteosome-S. flexneri 2a LPS vaccine will have protective efficacy in humans. However, the success of proteosome-LPS vaccines in several in vivo models that have been used as indicators of potential clinical utility and the overlap of immunologic response markers shown here with those elicited by some prior vaccine candidates yielding at least partial protection suggest that further evaluation, including experimental challenge or field trials, is warranted. The present study has suggested an optimal dose for further exploration and provides evidence that this dose can safely induce significant systemic and mucosal immune responses in humans.
ACKNOWLEDGMENTS
We thank Roy Fuller, Mary Poth, and Quentin Rance for diligently performing immunologic assays and thank Jennifer Sun and Denise McKinney of WRAIR and Janine Linden of Intellivax for coordinating the clinical trial. Larry Muenz provided statistical assistance.
This work was partially supported by Cooperative Research and Development Agreement DAMD 17–96-0095 between Intellivax, Inc., and the Walter Reed Army Institute of Research.
REFERENCES
- 1.Aavitsland P, Bjune G, Aasen S, Halvorsen S. Adverse events following vaccine or placebo injection in an efficacy trial of an outer membrane vesicle vaccine against group B meningococcal disease in Norwegian secondary schools 1988–1991. NIPH (Natl Inst Public Health) Ann. 1991;14:133–137. [PubMed] [Google Scholar]
- 2.Cohen D, Ashkenazi S, Green M S, Gdalevich M, Robin G, Slepon R, Yazvori M, Orr N, Block C, Ashkenazi I, Shemer J, Taylor D N, Hale T L, Sadoff J C, Pavliakova D, Schneerson R, Robbins J B. Double-blind, vaccine-controlled randomized efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 3.Cohen D, Green M S, Block C, Rouach T, Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988;157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 4.Cohen D, Green M S, Block C, Slepon R, Lerman Y. Natural immunity to shigellosis in two groups with different previous risks of exposure to Shigella is only partly expressed by serum antibodies to lipopolysaccharide. J Infect Dis. 1992;165:785–787. doi: 10.1093/infdis/165.4.785. [DOI] [PubMed] [Google Scholar]
- 5.Cohen D, Green M S, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen D, Orr N, Robin G, Slepon R, Ashkenazi S, Ashkenazi I, Shemer J. Detection of antibodies to Shigella lipopolysaccharide in urine after natural Shigella infection or vaccination. Clin Diagn Lab Immunol. 1996;3:451–455. doi: 10.1128/cdli.3.4.451-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coster T S, Hoge C W, VanDeVerg L L, Hartman A B, Oaks E V, Venkatesan M M, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti P J, Hale T L. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czerinsky C C, Nilsson L A, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;57:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 9.Formal S B, Maenza R M, Austin S, Labrek E H. Failure of parenteral vaccines to protect monkeys against experimental shigellosis. Proc Soc Exp Biol Med. 1967;125:347–349. doi: 10.3181/00379727-125-32087. [DOI] [PubMed] [Google Scholar]
- 10.Formal S B, Oaks E V, Olsen R E, Wingfield-Eggleston M, Snoy P J, Cogan J P. The effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis. 1991;164:533–537. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- 11.Hagiwara Y, Iwasaki T, Asanuma H, Sato Y, Sata T, Aizawa C, Kurata T, Tamura S. Effects of intranasal administration of cholera toxin (or Escherichia coli heat-labile toxin) B subunits supplemented with a trace amount of the holotoxin on the brain. Vaccine. 2001;19:1652–1660. doi: 10.1016/s0264-410x(00)00412-6. [DOI] [PubMed] [Google Scholar]
- 12.Hale T L. Shigella vaccines. In: Ala'Aldeen D A A, Hormaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. New York, N.Y: John Wiley & Sons; 1995. pp. 179–204. [Google Scholar]
- 13.Herrington D A, Van De Berg L, Formal S B, Hale T L, Tal B D, Cryz S J, Tramont E C, Levine M M. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine. 1990;8:353–357. doi: 10.1016/0264-410x(90)90094-3. [DOI] [PubMed] [Google Scholar]
- 14.Higgins A R, Floyd T M, Kader M A. Studies in shigellosis. III. A controlled evaluation of a monovalent Shigella vaccine in a highly endemic environment. Am J Trop Med Hyg. 1955;4:281–288. [PubMed] [Google Scholar]
- 15.Kantele A. Peripheral blood antibody-secreting cells in the evaluation of the immune response to an oral vaccine. J Biotechnol. 1996;44:217–224. doi: 10.1016/0168-1656(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Kantele A, Kantele J M, Arvilommi H, Mäkelä P H. Active immunity is seen as a reduction in the cell response to oral live vaccine. Vaccine. 1991;9:428–431. doi: 10.1016/0264-410x(91)90130-x. [DOI] [PubMed] [Google Scholar]
- 17.Kantele A, Arvilommi H, Kantele J M, Rintala L, Mäkelä P H. Comparison of the human immune response to live oral, killed oral, or killed parenteral Salmonella typhi Ty21a vaccines. Microb Pathog. 1991;10:117–126. doi: 10.1016/0882-4010(91)90072-i. [DOI] [PubMed] [Google Scholar]
- 18.Kotloff K L, Herrington D A, Hale T L, Newland J W, Van De Verg L, Cogan J P, Snoy P P, Sadoff J C, Formal S B, Levine M M. Safety, immunogenicity and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1992;60:2218–2224. doi: 10.1128/iai.60.6.2218-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotloff K L, Losonsky G A, Nataro J P, Wasserman S S, Hale T L, Taylor D N, Newland J W, Sadoff J C, Formal S B, Levine M M. Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli-Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine. 1995;13:495–502. doi: 10.1016/0264-410x(94)00011-b. [DOI] [PubMed] [Google Scholar]
- 20.Lee L A, Shapiro C N, Hargrett-Bean N, Tauxe R V. Hyperendemic shigellosis in the United States: a review of surveillance data for 1967–1988. J Infect Dis. 1991;164:894–900. doi: 10.1093/infdis/164.5.894. [DOI] [PubMed] [Google Scholar]
- 21.Lowell G H. Proteosomes for improved nasal, oral or injectable vaccines. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker; 1997. pp. 193–206. [Google Scholar]
- 22.Lowell G H, Kaminski R W, Grate S, Hunt R E, Charney C, Zimmer S, Culleton C. Intranasal and intramuscular proteosome-staphylococcal enterotoxin B (SEB) toxoid vaccines: immunogenicity and efficacy against lethal SEB intoxication in mice. Infect Immun. 1996;64:1706–1713. doi: 10.1128/iai.64.5.1706-1713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowell G H, Kaminski R W, VanCott T C, Slike B, Kersey K, Zawoznik E, Loomis-Price L, Smith G, Redfield R R, Amselem S, Birx D. Proteosomes, emulsomes and cholera toxin B improve nasal immunogenicity of human immunodeficiency virus gp160 in mice: induction of serum, intestinal, vaginal and lung IgA and IgG. J Infect Dis. 1995;175:292–301. doi: 10.1093/infdis/175.2.292. [DOI] [PubMed] [Google Scholar]
- 24.Mallett C P, Hale T L, Kaminski R W, Larsen T, Orr N, Cohen D, Lowell G H. Intranasal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun. 1995;63:2382–2388. doi: 10.1128/iai.63.6.2382-2386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mestecky J. The common mucosal immune system and current strategies for induction of immune response in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 26.Moldoveanu Z, Clements M L, Prince S J, Murphy B R, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995;13:1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 27.Ogra P L. Mucosal immunoprophylaxis: an introductory overview. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press; 1996. pp. 5–14. [Google Scholar]
- 28.Orr N, Robin G, Cohen D, Arnon R, Lowell G H. Immunogenicity and efficacy of oral or intranasal Shigella flexneri 2a and Shigella sonnei proteosome-lipopolysaccharide vaccines in animal models. Infect Immun. 1993;61:2390–2394. doi: 10.1128/iai.61.6.2390-2395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pál T, Lindberg A A. Oral vaccines for Shigella. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press; 1996. pp. 213–228. [Google Scholar]
- 30.Snapper C M, Rosas F R, Kehry M R, Mond J J, Wetzler L M. Neisserial porins may provide critical second signals to polysaccharide-activated murine B cells for induction of immunoglobulin secretion. Infect Immun. 1997;65:3203–3208. doi: 10.1128/iai.65.8.3203-3208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoll B J, Glass R I, Huq M I, Khan M U, Banu H, Holt J. Epidemiologic and clinical features of patients infected with Shigella who attended a diarrheal disease hospital in Bangladesh. J Infect Dis. 1982;146:177–183. doi: 10.1093/infdis/146.2.177. [DOI] [PubMed] [Google Scholar]
- 32.Tacket C O, Binion S B, Bostwick E, Losonsky G, Roy M J, Edelman R. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg. 1992;47:276–283. doi: 10.4269/ajtmh.1992.47.276. [DOI] [PubMed] [Google Scholar]
- 33.Tagliabue A, Villa L, Nencioni L, Keren D F, Lowell G H, Boraschi D. Antibody-dependent cell-mediated antibacterial activity of intestinal lymphocytes with secretory IgA. Nature. 1983;306:184–185. doi: 10.1038/306184a0. [DOI] [PubMed] [Google Scholar]
- 34.Underedown B J, Smith D A. Immunoglobulin A. Strategic defence initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- 35.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;V:83–91. [Google Scholar]
- 36.Wetzler L M, Ho Y, Reiser H, Wetzler L W. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J Exp Med. 1996;183:1151–1159. doi: 10.1084/jem.183.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]