Abstract
Neurocysticercosis (NCC) is a common central nervous system (CNS) infection caused by Taenia solium metacestodes. Despite the well-documented importance of the granulomatous response in the pathogenesis of this infection, there is limited information about the types of cells and cytokines involved. In fact, there has been limited characterization of human brain granulomas with any infectious agent. In the present study a detailed histological and immunohistochemical analysis of the immune response was performed on eight craniotomy specimens where a granuloma surrounded each T. solium metacestode. The results indicated that in all the specimens there was a dying parasite surrounded by a mature granuloma with associated fibrosis, angiogenesis, and an inflammatory infiltrate. The most abundant cell types were plasma cells, B and T lymphocytes, macrophages, and mast cells. Th1 cytokines were prevalent and included gamma interferon, interleukin-18 (IL-18), and the immunosuppressive, fibrosis-promoting cytokine transforming growth factor β. The Th2 cytokines IL-4, IL-13, and IL-10 were also present. These observations indicate that a chronic immune response is elicited in the CNS environment with multiple cell types that together secrete inflammatory and anti-inflammatory cytokines. In addition, both collagen type I and type III deposits were evident and could contribute to irreversible nervous tissue damage in NCC patients.
Neurocysticercosis (NCC) is a common parasitic infection of the central nervous system (CNS) (7, 32, 42). Humans acquire it when they ingest food or water contaminated with eggs from the tapeworm, Taenia solium. NCC occurs when the oncosphere within the egg penetrates the gut and migrates to the CNS, where the metacestode or cyst stage of the worm develops. Interestingly, between 13 and 44% of the NCC infections are asymptomatic (11, 18). In the other cases an estimated 4- to 5-year incubation period precedes the presentation of symptoms (42). The most common manifestations are seizures that are usually associated with parasites in brain parenchyma (42). Hydrocephalus, another common symptom, is usually due to cysts located in ventricular or subarachnoid spaces. The severity of the symptoms is strongly associated with the intensity of the local immune response (11, 26). Meningeal parasites often induce a strong inflammation, perhaps due to their higher exposure to the immune system (3, 29). In addition, parasite viability plays an important role in disease progression. Vesicular or viable parasites can avoid immune system detection by masking their surface structures with host molecules and secreting immunomodulatory molecules (2, 14, 29, 43). In contrast, dying cysts lose these immunomodulatory capacities and, in addition, release a number of antigens that are readily detected by the immune system, causing inflammation. Therefore this stage is most frequently associated with an intense immune response and concomitant life-threatening symptoms. Finally, calcified cysts usually present a discrete residual immune reaction (11, 26).
In a preliminary study immunohistochemical analysis was used on four brain specimens from patients with severe NCC symptoms (29). In these cases granulomas were not present and there were differences in the types of cells and cytokines present (29). Given the absence of granulomas, it is likely that these patients represent earlier stages of the immune response.
In the present study we report for the first time the results from a detailed immunohistochemical analysis to determine the types of cells and cytokines that were present in eight human craniotomy specimens where granulomas were surrounding brain cysticerci. In all these cases we found that dying T. solium metacestodes induce a classical chronic, delayed-type hypersensitivity reaction that consists of a granuloma with associated immune infiltrate and fibrosis. This response was associated with a predominant Th1 cytokine pattern. However, in contrast to the previous immunohistochemical study of patients without granulomas, we also found evidence of cells and cytokines that are typical of a Th2 response.
MATERIALS AND METHODS
Patient and control nervous tissue specimens.
The nervous tissues from patients with histologically confirmed NCC were identified from the archives of the Departments of Pathology of the Hospital Universitario San Vicente de Paul (HUSVP) in Medellín (13 cases), Hospital San Miguel (2 cases) in San Juan de Pasto, and Hospital San Juan de Dios (3 cases) in Bogota, Colombia. Brain specimens from eight cases (A, D, E, J, P, L, M, and N) were chosen for this study based on their derivation from a craniotomy procedure and the presence of inflammatory and/or nervous tissue surrounding the parasite. By contrast, the specimens obtained by stereotaxic biopsy or those limited to the parasite itself were excluded because they did not contain enough adjacent tissue to determine the arrangement of inflammatory cells with respect to the parasite. There was limited medical information available that explained why these patients underwent a craniotomy. For the available cases, the main symptoms were seizures, increased intracranial pressure, and altered mental status. Likewise, there were no data available regarding corticosteroid treatment schedules prior to the surgery.
Two normal, uninfected human brain specimens with representative sections from CNS tissue were obtained from autopsies of patients who died from non-CNS pathologies at the HUSVP. Any changes in the immune response elements between the uninfected and infected brain specimens were attributed to the NCC infection. Research using patient specimens has complied with all relevant federal guidelines and institutional policies.
Tissue processing and histological staining.
The nervous tissue from patients and controls was fixed with neutral buffered formalin (10% [vol/vol] formaldehyde, 29 mM NaH2PO4, 45 mM Na2HPO4) for 12 to 24 h and then paraffin embedded using routine procedures (15). Serial 5-μm-thick sections were mounted on silane preparation slides (Sigma, St. Louis, Mo.) and used for histological and immunohistochemical procedures. Hematoxylin-eosin was used to determine the CNS location and stage of viability of the parasite, as well as the intensity and type of infiltrate. The presence of fibrous tissue was determined with both Masson's trichrome and Gomori's reticulum stainings to distinguish collagen types I and III, respectively (15). The collagen type I fibers were blue by Masson's trichrome, and collagen type III fibers were brown with Gomori's reticulum staining.
Antibodies.
The identification of cell surface markers was done by immunohistochemical analysis with a panel of anti-human antibodies. The following mouse monoclonal antibodies were purchased from Dako (Carpinteria, Calif.): anti-CD8 for cytotoxic T cells, anti-CD16 (FcγRIII) for granulocytes and NK cells, anti-CD20 for B cells, anti-CD68 for macrophages, epithelioid cells, giant cells, or microglia, antitryptase for mast cells, anti-HLA-DR for major histocompatibility complex class II (MHC-II), and an anti-prolyl-4-hydroxylase for fibroblasts. The polyclonal rabbit anti-CD3 for T cells was also from Dako. The distinction between NK cells and granulocytes was done by staining of the former with the monoclonal antibody B199.2.1, which is human NK cell specific (4). Gamma delta (γδ) T cells were tested with anti-γδ T-cell receptor monoclonal antibody (Pharmingen, San Diego, Calif.). Cytokine expression was assessed with the goat anti-human interleukin-4 (IL-4), IL-18, and IL-10 purchased from R&D Systems (Minneapolis, Minn.). The polyclonal antibodies were antigen affinity purified. Transforming growth factor β (TGF-β) was detected with a rabbit anti-human polyclonal antibody (R&D Systems). Gamma interferon (IFN-γ) expression was detected with a goat anti-human IFN-γ (Santa Cruz Biotechnology, Santa Cruz, Calif.), and IL-13 expression was detected with a rat anti-human antibody (Pharmingen). The secondary antibodies were biotinylated and cross-absorbed with human serum proteins. They included goat anti-mouse immunoglobulin G (IgG), goat anti-rabbit IgG (Southern Biotechnology, Birmingham, Ala.), and rabbit anti-goat IgG (Sigma). The standardization of optimal conditions and establishment of positive controls for each antibody were done using human tonsils removed from patients with chronic tonsillitis. These control specimens were also formalin fixed and paraffin embedded.
Immunohistochemistry.
Tissue sections were deparaffinized with an overnight incubation at 58°C and subsequent immersion in xylene and then rehydrated in solutions of decreasing ethanol. Endogenous peroxidase was blocked by incubating the tissues for 10 min in 6% hydrogen peroxide at room temperature. Depending on the surface marker, the tissues were submitted to two alternative unmasking protocols. For the first method the slides were submerged in a 10 mM citrate buffer at pH 6.0, placed in a deGalicia microwave pressure cooker (Vigo, Spain), and incubated in an 830-W microwave oven for 15 min. The second was proteolytic digestion with 0.01% porcine tryptase (wt/vol; ICN, Aurora, Ohio)–0.01% (wt/vol) CaCl2 (Sigma) diluted in a Tris-HCl buffer (9.7 mM Tris–249 mM NaCl) at pH 7.8 for 10 to 15 min at 37°C. Epitopes for CD4 or tumor necrosis factor alpha (TNF-α) could not be unmasked with either protocol. To eliminate nonspecific staining, Fc receptors were blocked for 30 min at room temperature in a humid chamber with a 1:10 dilution of serum from the host species in which the biotinylated antibody was generated. Sections were then incubated for 1 h at 37°C with the primary antibody diluted in phosphate-buffered saline with magnesium chloride (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, 1 mM MgCl2). The sections were then washed with 0.05% Tween 20–Tris-HCl buffer (100 mM Tris–150 mM NaCl) three times for 3 min each and then incubated with the secondary antibody for 40 min at 37°C. After washing, the slides were incubated for 30 min at room temperature with an avidin-biotin-peroxidase complex (ABC Elite kit; Vector, Burlingame, Calif.). The presence of peroxidase was detected with the chromogen 3,3′-diaminobenzidine (Dako), which results in a brown color. Sections were counterstained with Harris hematoxylin (Sigma Diagnostics, St. Louis, Mo.), dehydrated, and mounted with Cytoseal 60 mounting medium (Stephens Scientific, Riverdale, N.J.). The optimal conditions for detecting each marker were established by using chronically infected tonsils from humans.
The detection of IFN-γ, TGF-β, CD8, and γδ T cells required additional enhancement. After the avidin-biotin-peroxidase complex was washed away, the sections were incubated for 10 min at room temperature with biotinylated Tyramide (NEN Life Science Products, Boston, Mass.). Then the sections were washed and reincubated for 30 min at room temperature with the avidin-biotin-peroxidase complex. The reaction was then developed with 3,3′-diaminobenzidine and processed as described above.
Semiquantitative analyses of cellular infiltrates were done by determining the number of each positive cell type or cytokine using an ocular reticulum in the microscope at ×400 magnification. The area defined by the reticulum was calculated to be 6.25 × 10−2 mm2, and 10 such areas containing infiltrate were counted per tissue section. The data were recorded as the average number of cells in 10 areas ± the standard error of the mean (SEM).
RESULTS
Immunohistochemical analysis of the uninfected brain specimens.
To determine the activation changes induced in the cyst-infected nervous tissue, two normal, “uninfected” brain specimens were analyzed. There was no evidence of either an inflammatory reaction or fibrosis, and most of the cell surface markers for immune cells were negative (Table 1). The only exception was the presence of CD68- and MHC-II-positive perivascular microglia. Regarding cytokines, there was a detectable expression of TGF-β in both tissues (Table 2).
TABLE 1.
Types of cells present in the infiltrate associated with T. solium metacestode-induced granulomas
| Tissue identification | Number of each cell typea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| T cells | CD8 T cell | NK cell | B cell | Plasma cell | Mast cell | PMNb | Eosinophil | Macrophage or microgliac | |
| Controls | —d | NDe | — | — | — | — | — | — | 2–3 |
| P | 107 ± 13.8 | 54 ± 2.5 | 2 | 137 ± 15.5 | >150 | 10 ± 0.4 | 13 ± 2.2 | 0.4 ± 0.2 | 36 ± 2 |
| A | 105 ± 5.9 | 55 ± 3.5 | 134 ± 13.4 | >150 | 10 ± 0.7 | 15 ± 2.8 | 0.4 ± 0.2 | 59 ± 5.2 | |
| D | 130 ± 10.3 | 67 ± 5.7 | >150d | >150 | 9 ± 1 | 13 ± 1.9 | 0.2 ± 0.1 | 33 ± 2.2 | |
| E | 99 ± 8.3 | 56 ± 3.7 | >150 | >150 | 8 ± 0.8 | 14 ± 1.4 | 0.3 ± 0.2 | 51 ± 3.4 | |
| J | >150 | 38 ± 5.3 | 1 | >150 | >150 | 12 ± 0.6 | 13 ± 1.3 | 0.5 ± 0.2 | 119 ± 7.2 |
| L | >150 | 91 ± 4.5 | ND | 122 ± 11.5 | >150 | 8 ± 0.4 | 11 ± 1.9 | 37 ± 3.8 | ND |
| M | >150 | 94 ± 9.6 | ND | 122 ± 11.8 | >150 | 10 ± 1 | 12 ± 1.9 | 0.3 ± 0.2 | ND |
| N | >150 | 93 ± 5.9 | ND | >150 | >150 | 11 ± 1 | 17 ± 2.1 | 0.7 ± 0.2 | ND |
Cyst-infected human brain tissues P, A, D, E, J, L, M, and N and two noninfected controls were subjected to immunohistochemical analysis to determine the presence of immune cells in the granuloma surrounding T. solium cysts. The control tissues did not contain granulomas. Cells were counted as described in Materials and Methods, and the data are presented as the average number of cells in 10 areas (6.25 × 10−2 mm2) of immune cell-infiltrated tissue ± SEM.
Neutrophils (PMNs) were mainly present in the lumen of blood vessels from the infiltrate.
The CD68-positive staining in the uninfected brain specimens resembled microglia.
—, not detectable.
ND, not determined.
TABLE 2.
Cytokines associated with T. solium metacestode-induced granulomas
| Tissue identification | Number of cells positive for each cytokinea
|
|||
|---|---|---|---|---|
| IFN-γ | IL-18 | IL-4 | TGF-β | |
| Controls | —b | — | — | 1 |
| P | >150 | 37 ± 2.9 | 30.1 ± 7.6 | >150 |
| A | >150 | 39 ± 2.9 | NDc | >150 |
| D | >150 | 54 ± 3.3 | ND | >150 |
| E | >150 | ND | ND | >150 |
| J | >150 | ND | ND | >150 |
| L | >150 | 41 ± 2.3 | 34.7 ± 3.6 | ND |
| M | >150 | ND | 31.2 ± 6.9 | ND |
| N | >150 | ND | 26.3 ± 4.2 | ND |
Cyst-infected human brain tissues P, A, D, E, J, and L and two noninfected controls were subjected to immunohistochemical analysis to determine the presence of cytokines in the granulomas surrounding T. solium cysts. The control tissues did not contain granulomas. Cells were counted as described in Materials and Methods and the data are presented as the average number of cells per 6.25 × 10−2 mm2 of infiltrated tissue ± SEM.
—, not detectable.
ND, not determined.
Characteristics of the parasites and surrounding nervous tissue.
The parasites were in different stages of disintegration as judged by the hematoxylin-eosin and immunohistochemical stainings of the craniotomy specimens. Cases P, A, D, E, and M had colloidal cysticerci, with intact regions of vesicular membrane interspersed with amorphous material. In cases J, N, and L the parasites appeared to be in a granular-nodular stage because the center of the granuloma had immune cells and amorphous material and only a few regions with identifiable cyst remains. In each patient the parasite was lodged in different regions of the brain. In cases P and N the parasites were exposed to subarachnoid space, and the specimens contained meninges, cerebral cortex, and white matter. In cases A and M, a rim of white matter and a small region of cerebral cortex surrounded the metacestodes. The granuloma from cases J, E, and L was surrounded by white matter, and that from case D was adjacent to the cerebellum.
Immunological components of the granuloma from NCC patients.
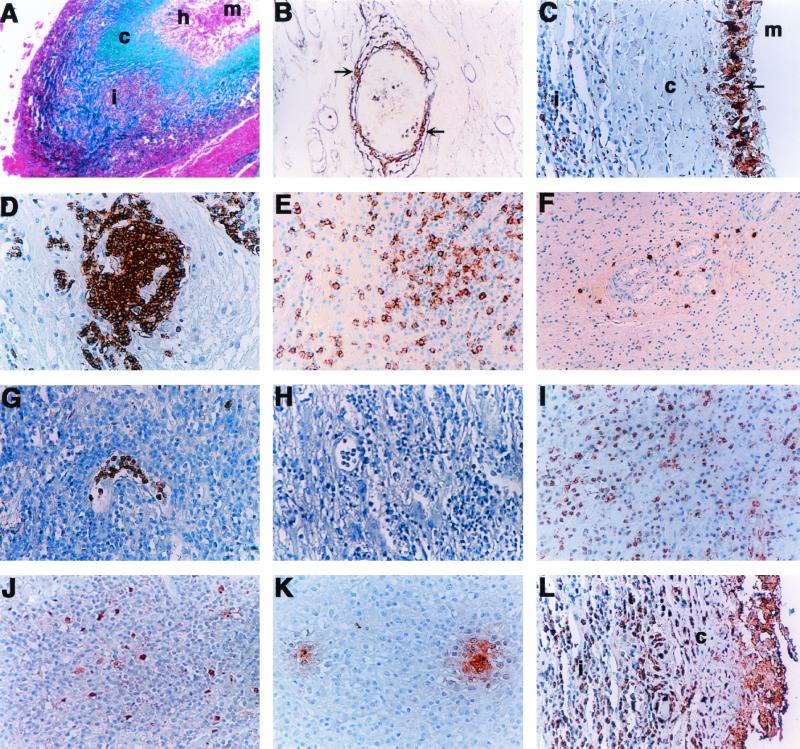
In the eight specimens obtained by craniotomy, a mature immunological granuloma was observed around the parasite remains. The general architecture of this granuloma was very similar in all cases and is shown as a low-power view in Fig. 1A. The distribution of collagen types I and III is shown in Fig. 1A and B, and the characteristic distribution of some cells is shown in Fig. 1C to G. Namely, at the center of the lesion was the parasite in different stages of disintegration (region m in Fig. 1A). In the more necrotic parasites the remains were associated with an amorphous material that resembled cells undergoing cariorexis. A layer of CD68-positive and MHC-II-negative epithelioid histiocytes was arranged in a typical “palisade” formation surrounding the parasite (region h in Fig. 1C). In one case some of these cells fused to form typical multinucleated giant cells (not shown). The epithelioid cells were surrounded by a thick and dense fibrous layer (region c in Fig. 1A). The main component was collagen type I (Fig. 1A) and, to a lesser extent, fibrin and collagen type III (not shown). This collagenous capsule became less dense as it extended towards the outermost region where an inflammatory infiltrate rich in blood vessels formed another layer (region i in Fig. 1A). In this region the fibrous tissue appeared to be mostly collagen type III arranged around the blood vessels (Fig. 1B). Few to moderate amounts of fibroblast-like cells were associated with collagen type I and III fibers. Finally, the inflammatory infiltrate was adjacent to the nervous tissue that had not been reabsorbed by the granulomatous response.
FIG. 1.
Histological staining of collagen fibers (A and B) and immunohistochemical analysis of the cells (C to G) and cytokines (I to L) present in the inflammatory infiltrate of brain granulomas. (A) Low-power view (×40) of a granuloma stained with Masson's trichrome. The metacestode remnants (m) were in the center of the lesion, surrounded by epithelioid histiocytes (h) and then by the thick layer of collagen type I fibers (c) that stain blue. Within the adjacent infiltrate (i) are also clusters of collagen type I fibers. (B) High-power magnification (×200) of the collagen type III fibers (brown; see arrow) that surround blood vessels when stained with Gomori's reticulum. (C) View (×200) of CD68-positive epithelioid histiocytes and multinucleated giant cells in “palisade” formation (arrow) adjacent to the space occupied by the metacestode (m). The histiocytes were surrounded by a layer of collagen (c) and then followed by an infiltrate with CD68-positive macrophages (i). (D) Perivascular infiltrates were predominated by B cells (×200). (E) CD8-positive cells were abundant in the infiltrate (×200). (F) Neutrophils were mainly present in the lumen of blood vessels (×200). (G) Mast cells were adjacent to blood vessels (×100). (H) Negative control. (I) Infiltrate with mononuclear cells positive for IFN-γ. (J) IL-18-positive cells resembled macrophages or microglia. (K) IL-10-positive gradients within the inflammatory infiltrates. (L) TGF-β staining in cells from the inflammatory infiltrate (i); c indicates the location of the fibrous layer. Magnification of pictures H to L is ×200.
Within the leukocyte-rich area a particular distribution was noted for each cell type (Fig. 1). The cells closer to the fibrous layer were usually CD68-positive macrophages (region i in Fig. 1C). There was a strong humoral response reflected by abundant B cells concentrated around the blood vessels (Fig. 1D) and plasma cells distributed throughout the infiltrate (not shown). There were moderate amounts of CD3+ T cells dispersed throughout the infiltrate (not shown). About 50% of these cells were CD8 positive (Fig. 1E and Table 1), and all were negative for γδ T-cell receptor (not shown). The unmasking of the CD4 epitope was not possible in these formalin-fixed tissues. Neutrophils were predominantly located in the lumen of the blood vessels (Fig. 1F), while mast cells were in a perivascular location (Fig. 1G). Eosinophils were relatively frequent in one specimen but scarce in the others. Scanty NK cells were detected in two specimens. Within these infiltrates, MHC-II expression was localized in the regions rich for B lymphocytes and macrophages. This was particularly noticeable in the perivascular spaces where B cells were abundant (Fig. 1D).
The cytokine profile in the granulomatous tissue.
Cytokines exert an essential influence on the type of immune response and the development of granulomas and fibrosis in different host-parasite systems. Despite the previously documented importance of IL-12 in the frozen cyst-infected brain tissues, the detection of this cytokine was not possible in the formalin-fixed specimens (29). All the cyst-infected brain specimens had the simultaneous presence of Th1 and Th2 cytokines (Table 2) consistent with the immune cell types present. The positive staining for cytokines predominated in the mononuclear-cell-rich infiltrate. IFN-γ, TGF-β, and IL-18 were the most abundant and were present in all the tissues (Fig. 1 H to J). IFN-γ staining was localized in regions rich in T lymphocytes and macrophages (Fig. 1H). The cells associated with IL-18 staining resembled macrophages (Fig. 1I). TGF-β was abundant among the inflammatory cells (Fig. 1J). The frequency of IL-4-producing cells was roughly comparable to the frequency of cells producing IL-18 (Table 2), whereas the frequency of IL-10- and IL-13-producing cells was relatively low (data not shown).
DISCUSSION
Considering the strong correlation between the pathogenesis of NCC and the intensity of the immune response surrounding CNS cysticerci, it was important to perform a detailed analysis of the type of localized immunity in brain specimens obtained from patients with severe clinical symptoms. This study focused on specimens that had a granuloma surrounding brain cysticerci. Despite the previously documented heterogeneity of cellular and humoral immune responses among patients with NCC, in the present study a consistent in situ response was observed in eight cases where granulomas surrounded brain cysticerci (21, 28, 29). Essentially, each specimen contained a dead and disintegrating parasite that was surrounded by an immune granuloma with intense fibrosis, inflammation, and angiogenesis. This response was accompanied by cells and cytokines typical of both a Th1 and a Th2 response.
In a previous immunohistochemical analysis done by our group on four brain specimens of patients with NCC, there was a predominant Th1-like response with NK cells, macrophages, variable numbers of T cells, and abundant IL-12 (29). In those cases there was no evidence of granuloma or fibrosis and very few or undetectable B cells, plasma cells, mast cells, eosinophils, or IL-4-producing cells. In the present study these findings were extended by analyzing specimens that contained granulomas around the cysts. Altogether, the combined results from both analyses suggest that in NCC there is initially a predominant Th1 response that eventually evolves into a chronic Th1 and Th2 phenotype associated with a mature granuloma formation, fibrosis, and angiogenesis. The data from the 12 patients suggest that the evolving immune response could be influenced by the parasite's involutionary process from viable to a final calcified stage. Most of the specimens we have analyzed by immunohistochemistry are in the colloidal or granular-nodular stage. The immune response elicited by the release of dying parasite antigens appears to be the most frequent cause of severe clinical symptoms. In contrast, determining the immune events that precede parasite death will be possible mainly from extrapolations with animal studies. In a mouse model for NCC, the initial stages of the infection are also predominated by a Th1 response that appears to be orchestrated by the CNS influx of γδ T cells (5). The influence of this cell type in the initial stages of human NCC is yet to be determined.
In the present study both Th1 and Th2 cytokines were present, including IFN-γ, IL-18, and IL-4. IL-13 and IL-10 were found to a lesser extent (data not shown). The probable influence of these cytokines on the formation of immune granulomas may be inferred from other infection models of animals. An initial Th1 response that evolves into a mixed Th1-Th2 phenotype associated with mature granuloma formation has been observed in other helminthic infections. An example includes the peritoneal infection of mice with Taenia crassiceps (30). It should be noted, however, that when spleen cells, ascites, and regional lymph nodes were studied after infection with T. crassiceps, an interesting result was found in terms of parasite burden (33, 35, 40). Thus an initial, transient Th1 response limited parasite replication, and the subsequent Th2 response was associated with increased parasite burden. However, it is not surprising that the immunological response involved in controlling parasite burden differs from that of granuloma formation. Consistent with this, another helminth, Schistosoma mansoni, also induces an initial Th1 phenotype followed by a mixed Th1-Th2 response with accompanying granuloma formation (45). In this model, the combined role of IL-4 and IL-13 was shown to be essential for the induction of mature granulomas (6). Another regulatory cytokine in terms of granuloma formation is IL-10 as it appears to diminish the size of granulomas in schistosomiasis in both humans and mice (12, 44). Finally, in other nonhelminthic infections like tuberculosis, TNF-α seems to be essential for granuloma formation (8, 13, 24). In the present study the epitopes for TNF-α could not be unmasked following formalin fixation and paraffin embedding.
Cytokines are also known for their influence on the irreversible replacement of functional tissue with collagen. In the present study the upregulation of the fibrinogenic cytokine TGF-β was consistent with the fibrosis observed in the NCC granuloma (41). In contrast, the role of IL-13 in the process of collagen deposition is still not clear in NCC despite its recent association with fibrinogenesis in S. mansoni infection (6). IL-13 was minimal or undetectable in the specimens analyzed, but the possibility that it may play an active role at an earlier stage of the evolving immune response cannot be ruled out. Considering the deleterious consequences of fibrotic scars, a key aspect of an immune therapy would be to identify and antagonize the cytokines involved in collagen deposition.
The formation of fibrous granulomas within the brain is a process that remains poorly understood compared to the advances achieved with other human organs like the lungs and liver. This may be explained by the brain's innate resistance against delayed hypersensitivity reactions (10, 19). In our experience the intraperitoneal or intracranial infections in mice with another cestode, Mesocestoides corti, have indicated that the kinetics of granuloma formation is delayed by several months in the brain versus the periphery (A. E. Cardona and J. M. Teale, unpublished results). In the present study the presence of mature granulomas in the brain suggested that the natural resistance of the brain microenvironment towards delayed hypersensitivity reactions was overcome by a chronic immunogenic stimulus and/or the presence of parasite antigens that are highly granulomatogenic.
The identity and nature of the granulomatogenic factors from T. solium metacestodes remain to be elucidated. In other granuloma-inducing pathogens much importance has been given to polysaccharides. In S. mansoni infections the carbohydrate portion from the soluble egg antigen induces the shift to a Th2 response that is associated with granuloma formation (38). The polysaccharides from the pathogenic fungus Paracoccidioides brasiliensis are also thought to induce granuloma formation (34). Thus, candidate granulomatogenic molecules for T. solium cysts may include the highly antigenic glycoproteins that can induce high antibody titers in NCC patients (17, 27).
The discrete distribution of B cells around the blood vessel and the abundance of plasma cells throughout the infiltrate may suggest that B cells enter the CNS parenchyma and then differentiate into plasma cells. The abundance of plasma cells in this study differs from previous immunohistochemical analysis, where B cells were scanty or absent (21, 29, 37). These contrasting observations are consistent with an evolving immune response that ranges from Th1-like in cases with few B cells to Th2 with a predominance of plasma cells. Accordingly, this evolving immune response supports the marked variations of anti-cysticercus antibody titers between patients in the diagnostic serology for NCC (21, 27). In addition to their role in promoting a humoral response, B cells may also be participating in antigen presentation, given the colocalization of B cells and high levels of MHC-II expression in the perivascular infiltrates.
The present study also revealed for the first time the presence of mast cells in the vicinity of the blood vessels within the infiltrates surrounding brain cysticerci. Mastocytosis has been noted previously in helminthic responses (22, 39). The role of mast cell tryptase is not clear in the human brain but has been documented in the CSF, perivascular infiltrates, and parenchyma of multiple sclerosis patients (20, 31). In addition, mast cells secrete a number of other proinflammatory products that were not tested in this study. Histamine and compound 48/80 are vasoactive substances that increase the permeability of the blood-brain barrier, and basic fibroblast growth factor also increases vascular permeability in addition to promoting fibrogenesis (23, 36, 46). Therefore, it will be important to determine the expression of these products in NCC brain specimens to assess the role of mast cells in the pathogenesis of this infection. The combined presence of mast cells, B lymphocytes, and plasma cells further substantiates the eventual development of a Th2 response resulting in a chronic Th1-Th2 mixed response in NCC patients exhibiting granulomas.
Despite the apparent increase in blood-brain barrier permeability that allowed the influx of a number of inflammatory cells, granulocytes were present in minor amounts. The sporadic presence of eosinophils in high numbers has been noted in previous studies of NCC patients (11). It contrasts with the predominance of this cell type in pigs that are naturally infected with T. solium metacestodes or with what is observed in other peripheral helminthic infections like schistosomiasis and filariasis in humans (1, 9, 25). Neutrophils were mostly located in the lumen of the vessels. This underrepresentation of granulocytes is likely influenced by the local expression of chemokines and adhesins induced during the chronic stages of NCC infections (16).
The components of the T. solium cyst-induced granuloma appear to reflect the combined participation of innate, cell-mediated, and humoral immunities. The granuloma associated with intense fibrosis seems to be a double-edged sword for the NCC patient. It protects the adjacent CNS tissue from an overt injury due to the bystander inflammatory response but damages irreversibly the nervous tissue that surrounds the cysticercus. The specimens analyzed belonged to patients who were subjected to craniotomy because they presented life-threatening symptoms that included seizures, mental alterations, and increased intracranial pressure. Unfortunately, in this study we did not have access to NCC infections that were asymptomatic. Such cases would be ideal to determine if the development of fibrous brain granulomas is associated with patients exhibiting the more severe symptoms of the disease.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants NS35974, AI19896, and TW00953, the Directión General de Investigaciones from Universidad Pontificia Bolivariana, and the Comité para el Desarrollo de la Investigación from the Universidad de Antioquia. Jorge I. Alvarez was partially supported by the Young Investigator program from COLCIENCIAS.
We thank Fernando Sanzón for providing the specimens from San Juan de Pasto, Oscar Cardona for identification of the cyst-infected tissues, Beatriz Vieco for technical support, and Christine Vasquez for secretarial support.
REFERENCES
- 1.Aluja A S, Martinez M J, Villalobos A N. Taenia solium cysticercosis in young pigs: age at first infection and histological characteristics. Vet Parasitol. 1998;76:71–79. doi: 10.1016/s0304-4017(97)00059-9. [DOI] [PubMed] [Google Scholar]
- 2.Arechavaleta F, Molinari J L, Tato P. A Taenia solium metacestode factor nonspecifically inhibits cytokine production. Parasitol Res. 1998;84:117–122. doi: 10.1007/s004360050367. [DOI] [PubMed] [Google Scholar]
- 3.Bandres J, White A C, Jr, Samo T, Murphy E, Harris R. Extraparenchymal neurocysticercosis: report of five cases and review of the literature on management. Clin Infect Dis. 1992;15:799–822. doi: 10.1093/clind/15.5.799. [DOI] [PubMed] [Google Scholar]
- 4.Bennett I M, Zatsepina O, Zamai L, Azzoni L, Mikheeva T, Perussia B. Definition of a natural killer NKR-PIA+/CD56-/CD16- functionally immature human NK cell subset that differentiates in vitro in the presence of interleukin 12. J Exp Med. 1996;184:1845–1856. doi: 10.1084/jem.184.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardona A E, Restrepo B I, Jaramillo J M, Teale J M. Development of an animal model for neurocysticercosis: immune response in the central nervous systerm is characterized by a predominance of γδ T cells. J Immunol. 1999;162:995–1002. [PubMed] [Google Scholar]
- 6.Chiaramonte G, Donaldson D, Cheever A, Wynn T. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Investig. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook B. Neurocysticercosis: parasitology, clinical presentation, diagnosis and recent advances in management. Q J Med. 1988;256:575–583. [PubMed] [Google Scholar]
- 8.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper P J, Beck L A, Espinel I, Deyampert N M, Hartnell A, Jose P J, Paredes W, Guderian R H, Nutman T B. Eotaxin and RANTES expression by the dermal endothelium is associated with eosinophil infiltration after ivermectin treatment of onchocerciasis. Clin Immunol. 2000;95:51–61. doi: 10.1006/clim.1999.4829. [DOI] [PubMed] [Google Scholar]
- 10.Cserr H F, Knopf P. Cervical lymphatics, the blood-brain barrier, and the immunoreactivity of the brain. In: Keene R, Hickey W, editors. Immunology of the nervous system. Oxford, England: Oxford University Press; 1997. pp. 134–152. [Google Scholar]
- 11.Escobar A. The pathology of neurocysticercosis. In: Palacios E, Rodríguez-Carvajal J, Taveras J M, editors. Cysticercosis of the central nervous system. Springfield, Ill: Charles C Thomas, Publisher; 1983. pp. 27–54. [Google Scholar]
- 12.Falcao P, Malaquias L, Martins-Filho O, Silveira A, Passos V, Prata A, Gazzinelli G, Coffman R, Correa-Oliveira R. Human schistosomiasis mansoni: IL-10 modulates the in vitro granuloma formation. Parasite Immunol. 1998;20:447–454. doi: 10.1046/j.1365-3024.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- 13.Fenhalls G, Wong A, Bezuidenhout J, van Helden P, Bardin P, Lukey P. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infect Immun. 2000;68:2827–2836. doi: 10.1128/iai.68.5.2827-2836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flisser A, Espinoza B, Tovar A, Plancarte A, Correa D. Host-parasite relationship in cysticercosis: immunological study in different compartments of the host. Vet Parasitol. 1986;20:95–102. doi: 10.1016/0304-4017(86)90094-4. [DOI] [PubMed] [Google Scholar]
- 15.Garcia del Moral R. Laboratorio de Anatomía Patológica. 1st ed. Madrid, Spain: McGraw-Hill Interamericana; 1993. [Google Scholar]
- 16.Glabinski A R, Tani M, Aras S, Stoler M H, Tuohy V K, Ransohoff R M. Regulation and function of central nervous system chemokines. Int J Dev Neurosci. 1995;13:153–165. doi: 10.1016/0736-5748(95)00017-b. [DOI] [PubMed] [Google Scholar]
- 17.Greene R, Wilkins P, Tsang V W. Diagnostic glycoproteins of Taenia solium share homologous 14 and 18 kD subunits. Mol Biochem Parasitol. 1999;99:257–261. doi: 10.1016/s0166-6851(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 18.Grisolia J S, Wiederholt W C. CNS cysticercosis. Arch Neurol. 1982;39:540–544. doi: 10.1001/archneur.1982.00510210010003. [DOI] [PubMed] [Google Scholar]
- 19.Harling-Berg C, Park J, Knopf P. Role of the cervical lymphatics in the Th2-type hierachy of CNS immune regulation. J Neuroimmunol. 1999;101:111–127. doi: 10.1016/s0165-5728(99)00130-7. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim M, Teder A, Lawand R, Takash W, Sallouh-Khatib S. The mast cells of the multiple sclerosis brain. J Neuroimmunol. 1996;70:131–138. doi: 10.1016/s0165-5728(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 21.Jaramillo J M, Restrepo B I, Llaguno P, Enciso J A, Teale J M. 47th Annual Meeting of the American Society of Tropical Medicine and Hygiene. Atlanta, Ga: American Society of Tropical Medicine and Hygiene; 1998. Characterization of the peripherical and in situ humoral response in patients with neurocysticercosis; pp. 358–359. [Google Scholar]
- 22.King C L, Nutman T B. Cytokines and immediate hypersensitivity in protective immunity to helminth infections. Infect Agents Dis. 1993;2:103–108. [PubMed] [Google Scholar]
- 23.Liu H M, Yang H B, Chen R M. Expression of basic fibroblast growth factor, nerve growth factor, platelet-derived growth factor and transforming growth factor-beta in human brain abscess. Acta Neuropathol. 1994;88:143–150. doi: 10.1007/BF00294507. [DOI] [PubMed] [Google Scholar]
- 24.Munk M E, Emoto M. Functions of T-cell subsets and cytokines in mycobacterial infections. Eur Respir J Suppl. 1995;20:668s–675s. [PubMed] [Google Scholar]
- 25.Nutten S, Trottein F, Gounni A S, Papin J P, Capron A, Capron M. From allergy to schistosomes: role of Fc receptors and adhesion molecules in eosinophil effector function. Mem Inst Oswaldo Cruz. 1997;92:9–14. doi: 10.1590/s0074-02761997000800003. [DOI] [PubMed] [Google Scholar]
- 26.Rabiela-Cervantes M T, Rivas-Hernandez A, Rodriguez-Ibarra J, Castillo-Medina S, Canción F M. Anatomopathological aspects of human brain cysticercosis. In: Flisser A, Willms K, Laclette J P, Larralde C, Ridaura C, Beltran F, editors. Cysticercosis: present stage of the knowledge and perspectives. New York, N.Y: Academic Press; 1982. pp. 179–200. [Google Scholar]
- 27.Restrepo B, Obregón A, Mesa M, Gil D, Ortiz B, Mejía J, Villota G, Sanzón A, Teale J. Characterization of the carbohydrate components of Taenia solium metacestode glycoprotein antigens. Int J Parasitol. 2000;30:689–696. doi: 10.1016/s0020-7519(00)00057-6. [DOI] [PubMed] [Google Scholar]
- 28.Restrepo, B. I., M. I. Aguilar, P. C. Melby, and J. M. Teale. Analysis of the peripheral immune response in patients with neurocysticercosis: evidence for T cell reactivity to parasite glycoprotein and vesicular fluid antigens. Am. J. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 29.Restrepo B I, Llaguno P, Sandoval M A, Enciso J A, Teale J M. Analysis of immune lesions in neurocysticercosis patients: central nervous system response to helminth appears Th1-like instead of Th2. J Neuroimmunol. 1998;89:64–72. doi: 10.1016/s0165-5728(98)00112-x. [DOI] [PubMed] [Google Scholar]
- 30.Robinson P, Atmar R L, Lewis D E, White A C., Jr Granuloma cytokines in murine cysticercosis. Infect Immun. 1997;65:2925–2931. doi: 10.1128/iai.65.7.2925-2931.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozniecki J J, Hauser S L, Stein M, Lincoln R, Theoharides T C. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 32.Schantz P. Taenia solium taeniasis/cisticercosis is a potentially eradicable disease: developing a strategy for action and obstacles to overcome. In: García H H, Martinez S M, editors. Taeniasis/cisticercosis por Taenia solium. 2nd ed. Lima, Peru: Editorial Universo; 1999. p. 346. [Google Scholar]
- 33.Sciutto E, Fragoso G, Baca M, De la Cruz V, Lemus L, Lamoyi E. Depressed T-cell proliferation associated with susceptibility to experimental Taenia crassiceps infection. Infect Immun. 1995;63:2277–2281. doi: 10.1128/iai.63.6.2277-2281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva C, Fazioli R. A Paracoccidioides brasiliensis polysaccharide having granuloma-inducing toxic and macrophage-stimulating activity. J Gen Microbiol. 1985;131:1497–1501. doi: 10.1099/00221287-131-6-1497. [DOI] [PubMed] [Google Scholar]
- 35.Terrazas L I, Bojalil R, Govezensky T, Larralde C. Shift from an early protective Th1-type immune response to a late permissive Th2-type response in murine cysticercosis (Taenia crassiceps) J Parasitol. 1998;84:74–81. [PubMed] [Google Scholar]
- 36.Theoharides T C. Mast cells: the immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. [DOI] [PubMed] [Google Scholar]
- 37.Thomas J A, Knoth R, Schwechheimer K, Volk B. Disseminated human neurocysticercosis: a morphologic analysis of two cases. Acta Neuropathol. 1989;78:594–604. doi: 10.1007/BF00691286. [DOI] [PubMed] [Google Scholar]
- 38.Velupillai P, Harn D A. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T-cell subsets. Proc Natl Acad Sci USA. 1994;91:18–22. doi: 10.1073/pnas.91.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vercelli D, De Monte L, Monticelli S, Di Bartolo C, Agresti A. To E or not to E? Can an IL-4-induced B cell choose between IgE and IgG4? Int Arch Allergy Immunol. 1998;116:1–4. doi: 10.1159/000023918. [DOI] [PubMed] [Google Scholar]
- 40.Villa O, Kuhn R. Mice infected with Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology. 1996;112:561–570. doi: 10.1017/s0031182000066142. [DOI] [PubMed] [Google Scholar]
- 41.Wahl S, Frazier-Jessen M, Jin W, Kopp J, Sher A, Cheever A. Cytokine regulation of schistosoma-induced granuloma and fibrosis. Kidney Int. 1997;51:1370–1375. doi: 10.1038/ki.1997.187. [DOI] [PubMed] [Google Scholar]
- 42.White A C. Neurocysticercosis: updates on epidemiology, pathogenesis, diagnosis and management. Annu Rev Med. 2000;51:187–206. doi: 10.1146/annurev.med.51.1.187. [DOI] [PubMed] [Google Scholar]
- 43.White A C, Jr, Tato P, Molinari J L. Host-parasite interactions in Taenia solium cysticercosis. Infect Agents Dis. 1992;1:185–193. [PubMed] [Google Scholar]
- 44.Wynn T A, Cheever A W, Williams M E, Hieny S, Caspar P, Kuhn R, Muller W, Sher A. IL-10 regulates liver pathology in acute murine schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J Immunol. 1998;160:4473–4480. [PubMed] [Google Scholar]
- 45.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 46.Zhuang X, Silverman A J, Silver R. Brain mast cell degranulation regulates blood-brain barrier. J Neurobiol. 1996;31:393–403. doi: 10.1002/(SICI)1097-4695(199612)31:4<393::AID-NEU1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]