Abstract
Objectives
Endo Peripheral Artery Tonometry (EndoPAT-2000) is a non-invasive technology for measuring endothelial dysfunction (ED). The reactive hyperaemia index (RHI) is resulted and is low when ED is present. We aim to synthesise the literature on paediatric ED that used Endo-PAT analysis.
Design
A comprehensive systematic review was conducted from January 2015 to March 2021. The databases included Cochrane, MEDLINE EBSCO, EMBASE (Ovid), PUBMED and CINAHL EBSCO. Exclusion criteria were: (1) If a study used a different device, for example, (2) If the study had no results. Inclusion criteria were: (1) Published in the English, (2) more than 50% of study subjects were in the paediatric age range, (3) data relevant to paediatric age range children could be extrapolated from all data, where not all study subjects were children.
Results
Following the removal of duplicates, 156 articles were initially identified. Following exclusion, 50 articles were included for review. We have subdivided these papers into different systems for ease of reference and have reported our findings in six tables: patients with type 1/2 diabetes, obesity, cardiovascular, respiratory, psychiatric conditions and miscellaneous diseases. For each, the study design, population, control group (if available), RHI results and conclusions were reported.
Conclusions
A number of papers using Endo-PAT for children with various chronic diseases have evidence of ED. However, in many cases, there has only been a single cohort study using Endo-PAT. Further studies are required to validate these findings and to help characterise the cardiovascular risk profile of children with chronic disease. Further studies are also required that will characterise more completely the cardiovascular risk profile of these children.
Consensus on other vascular risk markers that could be included in future studies is ideal and if accomplished, this would facilitate meta-analyses of studies of relatively rare conditions.
Keywords: Paediatrics, Community child health, Paediatric endocrinology, Education & training (see Medical Education & Training), Change management, Organisational development
Strengths and limitations of this study.
Comprehensive systematic review to synthesise the literature on endothelial dysfunction using Endo Peripheral Artery Tonometry (Endo-PAT) in paediatric patients.
All study types were reviewed and even the studies without results but were relevant were included in our discussion.
In many cases, there has only been a single cohort study using Endo-PAT for a particular disease.
Separate paediatric results were obtained where possible from studies with combined adult and paediatric data; however, some papers were of poor quality and had limited results available.
Only papers from January 2015 to March 2021 were included in our review.
Introduction
Endothelial dysfunction (ED) is an early predictor of cardiovascular disease.1 Negative alterations in endothelial physiology, also known as ED, cause the endothelium to lose its ability to promote vasodilation, fibrinolysis and antiaggregation.2 It is the beginning of atherosclerosis formation, which can lead to plaque progression and luminal narrowing.3 There is an imbalance between vasodilation and vasoconstriction, abnormal reactive oxygen species and nitric oxide (NO) bioavailability.2 ED is a complication of cardiovascular risk factors such as smoking, hypercholesterolemia, hypertension, hyperglycaemia and family history of premature atherosclerosis. ED can be caused by oxidative stress with loss of vasoactive or inflammatory homeostasis within the body’s vascular system. It may be secondary to mechanical stimuli, for example, increased intraluminal pressure within the blood vessel or metabolic factors such as hormones (oestrogen’s vasodilation action).4
Damaged endothelium can release a cascade of substances which pose a risk of thrombosis, inflammation and ultimately atherosclerosis.5 ED in paediatric populations has been associated with several conditions including type 1 diabetes (T1D), type 2 diabetes (T2D), renal impairment, obesity and metabolic syndrome.6–9 In patients with T2D, obesity and metabolic syndrome, insulin resistance is one of the most important factors contributing to ED.9 Metabolic syndrome is a proinflammatory state where dyslipidaemia, hyperuricemia and hypertension occur and can predispose to ED.10
ED can progress to atherosclerosis which is a chronic condition that poses severe risk of certain diseases, including coronary artery disease, stroke and peripheral arterial disease. If detected early and specific patient modifications are made, the progression to permanent vessel damage may be halted. ED can be detected by invasive techniques assessing the coronary vessels or by non-invasive techniques via the peripheral circulation. The gold-standard test would use coronary angiography and assess response to vasodilators. However, this is not feasible in practice as a screening tool, especially in paediatrics.
This review highlights the variety of conditions that Endothelial Peripheral Artery Tonometry (Endo-PAT) can be useful in paediatric patients. This systematic review will add to other reviews of endothelial function assessments in paediatric populations as it includes further studies and an increasing variety of paediatric conditions as well.11
Endo-PAT 2000
Endo-PAT 2000 is a non-invasive technology for measuring ED developed by Itamar Ltd. Non-invasive pneumatic probes which are placed on the both index fingers, which continuously records pulse wave amplitude. A blood pressure cuff is inflated to occlude blood flow and response after deflation is recorded. The reactive hyperaemic index (RHI) is resulted following this mini-ischaemic stress to the vessel. The pulse wave amplitude (PWA) is measured and computes an RHI result automatically. RHI is calculated as the ratio of average PWA divided by the average amplitude during the equilibration period. To compensate for any systemic changes, this ratio is normalised to a concurrent signal from the contralateral finger.
Numerous studies in both adult and paediatric literature reveal Endo-PAT’s excellent reproducibility and reliability.12–14 However, RHI has limitations as a reliable method for defining ED, especially in paediatric patients due to the metabolic changes children go through throughout childhood, including growth and puberty. There is no RHI cut-off value in paediatric patients. In ED, the RHI is low and pulse amplitude is high. PAT also provides results on the peripheral augmentation index (PAT-AIx). Bonetti et al report a RHI of <1.35–1.49 as indicative of coronary ED in adults.14 15
Prior to Endo-PAT, ED had been assessed by flow-mediated vasodilation (FMD). FMD uses an ultrasound to assess the change in brachial artery diameter in response to increased flow after a period of vascular occlusion by a blood pressure cuff and is highly dependent on NO bioavailability. ED is identified by less vasodilatation (reduced FMD) of the brachial artery. FMD is technically challenging to perform, user dependent and requires training. FMD results macroblood vessel reactivity, whereas Endo-PAT results micro, which may account for the challenges in comparing the two techniques. Endo-PAT is easier to set up, is automated and less user dependent. It can be used at the patient’s bedside, without extensive training required of the operator. Wilk et al reported that RHI correlated with FMD (r=0.35, p<0.01); however, there are other studies which have not reported a correlation between the two techniques.16
Objective
A systematic review was conducted on the use of Endo-PAT 2000 in paediatric populations in assessing the risk of ED, with the aim of synthesising the literature, to determine a cohort of paediatric patients at high risk of ED and who may benefit from screening.
Methods
A comprehensive systematic review was conducted to identify publications that investigated Endo-PAT 2000. All papers published from January 2015 to March 2021 in paediatric populations age birth to 16 years of age were analysed.
The following scientific databases were searched: The Cochrane Database, MEDLINE EBSCO, EMBASE (Ovid), PUBMED and CINAHL EBSCO. The search was limited by to English studies. The search was limited by type of subjects (human), date (2015 to March 2021) and included all study types. Snowballing method was used. Authors of joint adult and paediatric papers were contacted by email to obtain separate paediatric data.
The database search was repeated several times using the combinations of keywords, Medical Subject Headings (MeSH) terms and filters (child: birth-16 years). The following MeSH terms or key words were used for searching: Peripheral arterial tonometry, PAT test, endopat, adolescent, ado*, child, paediatric, pediatric, preschool, schoolboy, schoolgirl, boy, girl, teen, toddler, infant, baby.
Exclusion criteria were: (1) if a study used a different device, for example ‘Watch-PAT’ and (2) if the study had no results. Inclusion criteria were: (1) published in the English; (2) more than 50% of study subjects were in the paediatric age range; (3) data relevant to paediatric age range children could be extrapolated from all data, where not all study subjects were children. A child was defined as up to 16 years, and this is consistent with PubMed’s definition of a child, where data relevant to children could not be extrapolated from the whole data set, the study authors were contacted for additional information prior to study inclusion or exclusion.
Patient and public involvement
No patient involved.
Data collection and analysis
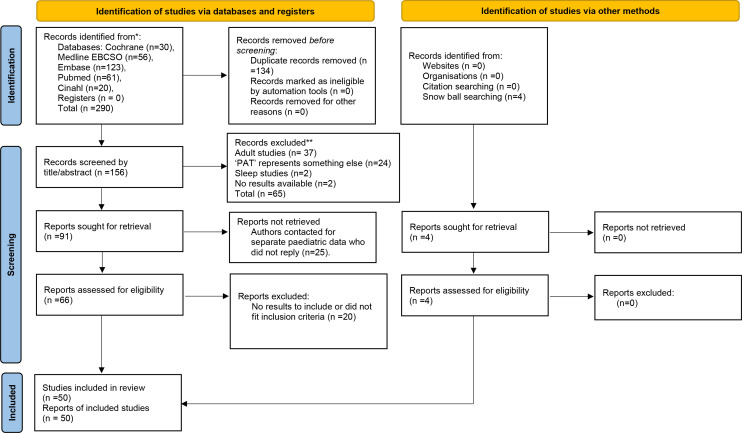
A total of 290 articles were obtained via the online database search (figure 1: flow diagram). Following removal of duplicates, 158 articles remained. The second screening was conducted by ‘Rayyan-systematic review software’. Two further duplicate articles were removed, with 156 remaining for review.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 flow diagram of systematic search for Endo-PAT 2000 in paediatric populations. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers).**If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From Page et al.86 EndoPAT-2000, Endo Peripheral Artery Tonometry.
Two independent authors separately performed a blind screen on the 156 abstracts. Sixty-five articles were initially excluded based on title or abstract: 37 adult studies, 18 ‘PAT’ did not represent peripheral arterial tonometry (eg, prism adaptation test, psychosocial assessment tool), 6 Watch-PAT, 2 sleep studies and 2 had no results available.
The remaining 91 articles were analysed viewing full-text articles for further information. A further 20 were excluded as they did not fit inclusion criteria or have results to report. Some of these articles that included Endo-PAT 2000 in paediatrics did not have results for the systematic review but had conclusions that were relevant to the paper were referenced in the Results section.
Twenty-eight authors of studies including both adults and paediatric patients were contacted two times by email to gather separate information on the paediatric participants. Twenty authors did not reply and were, thus, excluded. Eight authors replied: three providing results, four unable to give separate paediatric data and one author’s research was on adult patients so was excluded. Three of the articles whose authors replied with data were included in our review. Four studies were obtained via snow balling searching.
A total of 50 articles were included in our results and are represented in tables 1–6. For each eligible study, the following data were reported: author, year of publication, design of the study, population studied, control group (if available), RHI results.
Table 1.
Total of 11 studies included
| Title, lead author | Year | Study design | Population: n=sample size, age; mean±SD or median (range), (F/M) | Control group: n=sample size, age; mean±SD or median (range), (F/M) | Results: RHI reported. if RHI not specified, we reported p/r values | Outcomes |
| Adolescents and young adults with type 1 diabetes display a high prevalence of endothelial dysfunction. Scaramuzza et al17 | 2015 | Cohort prospective observational study. Results at baseline and after a 1-year follow-up | n=73 T1D adolescents, diagnosed>1 year, 16.2±3.5 years,(F/M 25/48) | No controls. | 56 (76.7%) had ED, with lower mean RHI scores (1.26±0.22 vs 2.24±0.48, p<0.0001). More with ED had abnormal cardiac autonomic tests (p=0.02) and were more sedentary. After 1-year follow-up in 64/73 patients, 81.8% had ED, despite some improvement in HbA1c. | T1D adolescents had evidence of ED. Good metabolic control (HbA1c ≤7.5%) and regular physical activity might be protective. ED progression despite some improvement to HbA1c. |
| Alpha-lipoic acid and antioxidant diet help to improve endothelial dysfunction in adolescents with type 1 diabetes: a pilot trial. Scaramuzza et al19 | 2015 | Double- blind, randomised controlled trial—snow balling. Results at baseline and after follow-up | n=71 T1D patients, followed for at least 1 year, age 16.3±3.4 years,(F/M 29/42). (a) antioxidant diet 10.000 ORAC+alpha-lipoic acid; (b) antioxidant diet 10.000 ORAC+placebo; | (c)controls | Three double-blind study arms: (a) antioxidant diet 10 000 ORAC+lipoic acid: RHI 1.40±0.68 vs 1.72±0.66 (p<0.05) (baseline vs after 6 months). (b) Antioxidant diet 10 000 ORAC+placebo: RHI 1.39±0.41 vs 1.58±0.40 (p>0.05). (c) Controls: RHI 1.58±0.64 vs 1.54±0.42 (p>0.05). | Improved RHI with alpha-lipoic acid in T1D patients. |
| Effect of metformin on endothelial function in overweight adolescents with type 1 diabetes (T1D). Nadeau et al20 | 2016 | Conference abstract. Endo-PAT scores at baseline and 13 weeks. | Total n=70 overweight T1D patients. n=41 on metformin (up to 2000 mg/day), 12–19 years (mean 15.8) | n=29 placebo group. | Mean baseline RHI 1.8±0.6 in metformin group and 1.7±0.6 placebo group. At 13 weeks, no significant change from baseline RHI (+0.1 in metformin vs −0.0 in placebo, p=0.08). Some improvement in endothelial function in men. | No significant RHI change with metformin overall but some improvement in overweight T1D males. |
| Assessment of biomarkers of inflammation and premature atherosclerosis in adolescents with type-1 diabetes mellitus. Babar et al18 | 2019 | Cross-sectional study | T1D adolescents≥12 years. Two groups based on different HbA1c ranges. (a) HbA1c ≥9.5% (n=25) | (b) HbA1c ≤8.5% (n=27). | PAT results were not significantly different between the groups. Pearson correlation showed a significant direct relationship between rising HbA1c and PAT (p=0.03, r=0.31). | Suboptimal glycaemic control (rising HbA1c) causes early atherosclerosis. |
| Improvements in peripheral vascular function with vitamin D treatment in deficient adolescents with type 1 diabetes. Deda et al21 | 2018 | Research article—snow balling. Tested at two different time points. | n=21 T1D patients followed for~2 years. 25-OH-Vit. D levels<37.5 nmol/L. Age 15.7±1.4 years,(F/M 19/12) | Controls: matched age, sex and T1D. | After 4.8±1.3 months of Vit. D supplementation RHI improved: 1.83±0.42 vs 2.02±0.68 (p<0.05). | Vit. D supplementation associated with improvement to endothelial function and reduced urinary inflammatory markers. |
| Non-alcoholic fatty liver disease in hispanic youth with dysglycemia: risk for subclinical atherosclerosis? Bacha et al23 | 2017 | Cross-sectional study | n=23 overweight/ obese with NAFLD, age 15.2±0.5 years. n=12 pre-diabetes, n=11 T2D,(F/M 13/10) |
n=13 overweight/ obese without NAFLD, age 15.7±0.4 years. n=8 pre-diabetes, n=5 T2D,(F/M 3/10) | NAFLD group had lower RHI (1.4±0.05 vs 1.7±0.09, p=0.002). Hepatic fat is inversely related to RHI (r=−0.49, p=0.002). | Hepatic fat and AST/ALT levels inversely related to RHI. If dysglycemia, NAFLD is associated with worse ED. |
| Endothelial function in youth: A Biomarker modulated by adiposity-related insulin resistance. Tomsa et al22 | 2016 | Cross-sectional study | Total n=60. n=25 obese without DM, n=19 obese with impaired glucose tolerance, n=16 obese T2D but HB1Ac <8%. Age 15.5 (0.2),(F/M 37/23) |
n=21 normal weight, age 15.5 (0.2),(F/M 9/12) | RHI inversely related to % body fat (r = −0.29, p = 0.008), total (r = −0.37, p = 0.004), subcutaneous (r = −0.39, p = 0.003), and visceral abdominal fat (r = −0.26, p = 0.04). | Childhood obesity is associated with ED (lower RHI). RHI lower in obese and T2D. RHI negatively related with percentage body fat, WC, Leptin, TNF-alpha, blood glucose. |
| Circulating fibroblast growth factor-21 (FGF-21): A biomarker of subclinical atherosclerosis in obese youth with non-alcoholic fatty liver disease (NAFLD)? Bacha et al24 | 2017 | Conference abstract | Obese adolescents with NAFLD, 15.4±0.3 years. n=13 normal glucose tolerance, n=19 pre-diabetes, n=16 T2D patients | Control group: no NAFLD. No difference in age/gender between groups. |
Lower RHI in NAFLD group. High FGF-21 concentrations related to RHI (r=−0.33, p=0.03). | Increased FGF-21 in obese adolescents with NAFLD associated with insulin sensitivity and ED. FGF-21 may constitute a biomarker ED. |
| Assessment of Microvascular Function in Children and Adolescents with Diabetes and Obesity. Kochummen et al26 | 2019 | Cross-sectional study | DM group. n=33 T1D with normal weight. n=8 obese T2D, age 12.7 (3.8) years,(F/M 25/16) |
n=17 obese, non-DM children (normal BGL, BP and lipid profile), 12.8 (2.7) years,(F/M 9/8) | For every 1% increase in HbA1C, RHI decreased by 0.097 (p=0.01). RHI of DM group with HbA1C<10% (1.70±0.58) vs those with≥10% (1.21±0.19) (p=0.02). | Poorly-controlled DM (HbA1C ≥10%) had lower RHI. RHI negatively related with HbA1C. RHI similar between obese and normal weight with T1D. Similar between T1D and T2D. |
| Free Vitamin D: Relationship to Insulin Sensitivity and Vascular Health in Youth. Bacha et al27 | 2019 | Cross-sectional study. Comparison across tertiles of free 25(OH)D concentrations | n=79, age 15.4±0.2 years,(F/M 45/34). n=30 overweight. n=31 overweight with pre-diabetes |
n=18 normal weight and normal glucose tolerance. | The lowest tertile group had lower RHI (1.42±0.06, 1.54±0.06, and 1.77±0.09, p=0.002), compared with the second and third tertiles. | Youth with low free 25(OH)D or BioD concentrations have lower insulin sensitivity and worse endothelial function. |
| Urine Albumin-to-Creatinine Ratio (UACR): A Marker of Early Endothelial Dysfunction in Youth. Bartz et al25 | 2015 | Control study. Fasting UACR analysed. | n=25 overweight (OW) with normal glucose tolerance, 15.6±0.2 years,(F/M 17/8). n=20 OW with pre-diabetes,(F/M 11/9). |
n=13 normal weight, 16.3±0.4,(F/M 7/6). | Normal weight group RHI 1.84±0.1. OW with normal glucose tolerance 1.56±0.1. OW with pre-diabetes 1.56±0.1 (p=0.04). UACR was related to RHI (r=−0.33, p=0.01). | UACR is an early marker of endothelial dysfunction in youth, independent of glycaemia. |
Endo-PAT 2000 in paediatric type 1 diabetes mellitus (T1D) patients (five studies), type 2 diabetes and pre-diabetes (six studies).
ED, endothelial dysfunction; NAFLD, non-alcoholic fatty liver disease; ORAC, oxygen radical absorbance capacity units; OW, overweight; RHI, reactive hyperemia index; T1D, type 1 diabetes mellitus; T2D, type 2 diabetes mellitus; UACR, urine albumin-to-creatinine ratio.
Table 2.
Endo-PAT 2000 in paediatric patients who are overweight (OW)/obese (14 studies)
| Title, lead author | Year | Study design | Population: n=sample size, age; mean±SD or median (range),(F/M) | Control group: n=sample size, age; mean±SD or median (range),(F/M) | Results: RHI reported. if RHI not specified, we reported p/r values | Outcomes |
| Effects of a dietary strawberry powder on parameters of vascular health in adolescent males. Djurica et al28 | 2016 | Randomised, double-blind, cross-over study | n=15 OW/obese males, 14–18 years (mean 16). 1-week daily 50 g freeze-dried strawberry powder (FDSP) Before/after nitrate/nitrite levels measured. | n=10 control powder, 14–18 years (mean 16). | Acute plasma nitrate/nitrite levels increased 1 hour after consuming the FDSP (p<0.001). When nitrate levels increased after FDSP intake compared with controls, had an increase in RHI (p=0.014). | Strawberries can provide vascular health benefits to OW/obese adolescent males. |
| Flow-mediated dilation in obese adolescents: Correlation with waist circumference (WC) and systolic blood pressure (SBP). Hussid et al29 | 2018 | Case–control study | n=20 obese patients, median age 14 years | n=10 normal weight, median age 15 years, paired for gender | No RHI difference between groups. 35% obese group had metabolic syndrome, none in control group. OSA in 86.6% obese and 50% of normal weight group. | Obese group had evidence of ED and metabolic syndrome. Increased WC and SBP seem to be related to this finding. |
| Improvement of microvascular endothelial dysfunction induced by exercise and diet is associated with microRNA-126 in obese adolescents. Donghui et al30 | 2019 | Quasi-randomised study | n=57 obese male adolescents, 12–18 (15.38±2.82) years,(F/M=0/57), 6-week exercise programme with dietary intervention. | n=10 normal weight adolescents, 15.38±2.82 years,(F/M 0/10), maintained sedentary | Obese group RHI 1.43 (0.35) vs controls 1.67 (0.36) (p<0.05). After 6 weeks RHI increased (p<0.01) and microRNA-126 decreased (p<0.01). miRNA-126 positively correlated with ΔRHI (r=0.69, p<0.05). | RHI improved in obese group after exercise and diet interventions. Findings might be related to changes in serum miRNA-126. |
| Distribution of peripheral arterial stiffness and endothelial function as well as their correlations with cardiovascular risk factors in children and adolescents. Mu et al31 | 2016 | Cross-sectional population-based study, conference abstract | n=94 obese, 7–17 years, used automatic waveform analyser (BP-203RPE-I) and Endo-PAT 2000. | n=452 normal weight | In normal weight group, RHI increased with age (r=0.33, p<0.01; r=0.36, p<0.01). RHI positively correlated with BMI (r=0.10, p=0.018) but negatively with DBP (r=−0.10, p=0.016). | RHI increased along with age. Arterial stiffness and endothelial function continued to develop in the normal weight group. |
| Urinary biomarkers as indicator of chronic inflammation and endothelial dysfunction in obese adolescents. Singh et al87 | 2017 | Control study, research article | n=63 total. n=14 overweight (OW), n=29 obese, age 13.8 (2.4),(F/M 23/20) | n=20 normal weight (NW), age 13.9,2(F/M 8/12) | There were no differences in RHI levels: NW 1.6 (0.1), OW 1.66 (0.1) and obese 1.67 (0.1). NW girls RHI 1.9 vs NW boys 1.25. | No significant correlation between RHI and urinary markers. RHI higher in NW female adolescents. |
| Prevalence of Type D personality in obese adolescents and associated cardiovascular risk. Bruyndonckx et al88 | 2018 | Control study, conference abstract | Obese adolescents-no definite numbers | Healthy normal weight children | Positive correlation in obese adolescents between negative affectivity and vascular stiffness (r=0.28; p=0.04) | Obese adolescents have worse cardiovascular risk profile with ED. |
| Endothelial function and arterial stiffness in obese adolescents - A relation to barorefex function. Czippelova et al36 | 2017 | Conference abstract | n=22 obese, 15.28±2.8 years,(F/M 10/12) | n=22 non-obese, 15.98±2.46 years,(F/M 10/12) | No significant difference in RHI (p=0.473). Baro-reflex sensitivity was also calculated. | No difference in RHI between groups. Findings require further study. |
| Obesity in children and adolescents: A relation to endothelial function and arterial stiffness. Czippelova et al37 | 2016 | Conference abstract | n=16 obese adolescents,15.22±2.2 years, (F/M 7/9) | n=16 non-obese, 16.22±1.5 years, (F/M 7/9) | Significant difference in RHI (p=0.018) with RHI higher in obese group (1.66±0.28 vs 1.4±0.25). | Less early atherosclerotic changes in obese group; in contrast to expectations. Findings require further study. |
| Preclinical vascular alterations in obese adolescents detected by Laser-Doppler Flowmetry technique. Fusco et al39 | 2020 | Research article | n=22 obese adolescents, 14.11 ±2.53, (F/M 13/9) |
n=24 normal weight, 15.2±1.56, (F/M 11/13) | Similar RHI between obese and non-obese groups (1.80±0.62 and 1.86±0.51). | RHI did not differ between groups. RHI did not correlate with LDF. |
| Impaired endothelial function in adolescents with overweight or obesity measured by peripheral artery tonometry. Pareyn et al89 | 2015 | Cross-sectional study | n=27 overweight (OW)/obesity, 14.7 (13.0–16.4) years, (F/M 11/16) | n=25 normal weight controls, 15.5 (13.9–16.2) years,(F/M 13/12) | RHI normal weight 1.88 (1.7–2.4) vs OW/ obese 1.5 (1.3–1.9) (p<0.05). Lower RHI if OW/obese (p=0.027). RHI positively correlated with age and tanner stage (p<0.05). |
ED and higher baseline pulse amplitude in OW group. |
| C-type natriuretic peptide (CNP) plasma levels and whole blood mRNA expression show different trends in adolescents with different degree of endothelial dysfunction. Del Ry et al32 | 2020 | Research article—snow balling | n=16 primary obesity, not DM, age 13.3 (0.5) years, (F/M 8/8). | n=24 normal weight, age 14.3 (0.4) years, (F/M 14/10). | RHI normal weight 2.1 (0) vs obese 1.4 (0) (p<0.005). RHI negatively associated with CNP and diastolic BP (p<0.005). | RHI significantly lower in obese group. RHI negatively related with CNP, DBP, fat mass and HbA1C. |
| C-type natriuretic peptide (CNP) is closely associated to obesity in Caucasian adolescents. Del Ry et al33 | 2016 | Research article—snow balling | n=10 overweight, age 12.8 (1.6) years, (F/M 5/5). n=45 obese, 12.8 (1.6) years, (F/M 19/26) | n=27 normal weight, age 12.8 (1.4) years, (F/M 14/13) | Normal weight group RHI 2.1 (0.2) vs OW 1.6 (0.4) (p<0.05). Normal weight vs obese group RHI 1.4 (0.3) (p<0.005). RHI negatively associated with CNP (p<0.005). | RHI lower in overweight/ obese groups. CNP negatively related with RHI. |
| Arterial Stiffness and Endothelial Function in Young Obese Patients - Vascular Resistance Matters. Czippelova et al7 | 2019 | Research article | Author contacted for separate paediatric data. n=16 obese group, age<16 years, (F/M 7/9) | n=15 controls, age<16 years, (F/M 7/8) | RHI control vs obese groups: 1.320±0.427 and 1.457±0.280. RHI obese girls and boys: 1.410±0.253 and 1.494±0.308. RHI control girls and boys: 1.171±0.210 and 1.436±0.524 | RHI is influenced by vascular tone and resistance. RHI in obese positively related with SVR. |
| Cardiovascular adaptations after 10 months of intense school-based physical training for 8- to 10-year-old children. Larsen et al35 | 2018 | Randomised control study | n=93 small-sided games group, 9.3±0.4 years. n=83 circuit strength training group, 9.3±0.3 years (10–16 years) | n=115 controls, 9.3±0.3 years | No significant differences in RHI. Pubertal status is a main predictor of RHI; positive correlation between Tanner stages and RHI. | 10 months of regular exercise per week decreased DBP and had effects on cardiovascular health. |
CNP, C-type natriuretic peptide; ED, endothelial dysfunction; FDSP, freeze-dried strawberry powder; LDF, laser-Doppler flowmetry; NW, normal weight; OW, overweight; RHI, reactive hyperemia index; WC, waist circumference.
Table 3.
Endo-PAT 2000 in paediatric patients with cardiac and vascular conditions (seven studies)
| Title, lead author | Year | Study design | Population: n=sample size, age; mean±SD or median (range),(F/M) | Control group: n=sample size, age; mean±SD or median (range),(F/M) | Results: RHI reported. if RHI not specified, we reported p/r values | Outcomes |
| Nocturnal blood pressure dipping as a marker of endothelial and cardiac function in pediatric-onset systemic lupus erythematosus (SLE). Chang et al8 | 2020 | Cross-sectional study—author contacted for separate paeds data | n=20, 9–19 years (mean 16.5), (7 were age 16 or under). Average disease duration 3.2 years (± 2.1).(F/M 17/3) | Separated into two groups based on nocturnal BP dipping status. | Mean RHI for n=7 (aged 16/under): 0.529. 22% had ED. Reduced diastolic BP dipping was associated with poorer endothelial function (r 0.5, p = 0.04). |
Isolated nocturnal BP non-dipping is associated with ED and atherosclerotic changes. Potential role for routine ABPM for youth with SLE. |
| Physiological changes in blood pressure (BP) impact peripheral endothelial function during adolescence. Deda et al90 | 2015 | Control study. Assessing association between RHI and known cardiovascular risk factors. |
n=90 healthy adolescents to assess normal RHI response, 14.2±1.91 years,(F/M 46/44). |
No controls | Mean arterial pressure significantly associated with RHI (p=0.01). Positive correlation RHI and age in women (r=0.33, p<0.02). RHI correlated with pubertal status: men (r=0.411, p=0.03), women (r=0.36, p=0.03). |
Physiological changes in BP significantly impact RHI results. |
| Endothelial function and arterial stiffness relate to functional outcomes in adolescent and young adult fontan survivors. Goldstein et al91 | 2016 | Cross-sectional prospective observational study | n=60, 8–25 years (mean 13.9±4.1),(F/M 29/31) | No controls | PAT derived baseline pulse amplitude (p<0.05) negatively associated with minute ventilation to C02 ratio. RHI 1.2 (0.2–4.8). | Worse vascular measures associated with worse functional measures. Increased arterial stiffness and decreased endothelial function are associated with lower aerobic capacity, physical activity, and QOL in Fontan survivors. |
| Natural history of vascular function in adolescent and young adult Fontan survivors: A longitudinal assessment of endothelial function and arterial stiffness. Goldstein et al92 | 2017 | Prospective single-centre longitudinal study, conference abstract. Paired testing at a mean interval of 2.0±0.2 years of Fontan survivors. | n=50, mean 13.7±4.2 years,(F/M 23/27) | No controls | Decreases in RHI (0.002±0.01/yr) were not significant. BMI was a predictor for RHI (R 0.17, p=0.007). | Vascular function does not change uniformly in Fontan survivors. Changes in vascular function do not relate to changes in aerobic capacity but are associated with changes in anthropometric measures and O2 saturation. |
| Vascular function long term after Kawasaki disease: another piece of the puzzle? Pinto et al77 | 2017 | Single-centre prospective study | n=43 Kawasaki patients, age>11 years, diagnosed>5 years ago, with no coronary lesions or any other risk factors for cardiovascular disease. | n=43 control group of individuals without cardiovascular risk factors. | Kawasaki patients had decreased RHI compared with controls (1.59±0.45 vs 1.98±0.41; p<0.001). | Children with Kawasaki disease may have long-term sequelae, even when there is no detectable coronary artery involvement in the acute stage of disease. |
| Endothelial function in children with a history of Henoch Schonlein purpura (HSP). Butbul Aviel et al41 | 2017 | Observational prospective study | n=19 with HSP, 13.5±3.9 years,(F/M 8/11) | n=23 healthy children, 12.8±4.5 years,(F/M 7/16) | Mean RHI 1.81 study group and 1.87 control group (p = 0.18). RHI higher in patients who had endothelial function measured >6 years since HSP diagnosis compared with <6 years (1.98 + 0.74 vs 1.38 ± 0.43 p = 0.037). | This study suggests that HSP causes short-term endothelial dysfunction that improves with time. |
| Reactive hyperaemia index and detection of endothelial dysfunction in children with familial hypercholesterolaemia (FH). Jehlicka et al40 | 2015 | Conference abstract | n=24 with FH, 13.9±2 years. Biochemical markers of endothelial function were assessed. | n=17 healthy controls, 15.2±2.2 years | Significantly lower RHI in FH group (1.63±0.50 and 2.03±0.54; p<0.05). Lower RHI and elevated E-selectin in children with FH. | Possible relationship of ED in children with FH, highlighting the importance of early detection of ED when the atherosclerotic process is still reversible. |
ABPM, ambulatory blood pressure monitoring; BP, systolic blood pressure; FH, familial hypercholesterolaemia; HSP, Henoch Schonlein purpura; peak VO, peak O2 consumption; QOL, quality of life; RHI, reactive hyperemia index; SLE, systemic lupus erythematosus; WC, waist circumference.
Table 4.
Endo-PAT 2000 in paediatric patients with respiratory conditions (four studies)
| Title, lead author | Year | Study design | Population: n=sample size, age; mean±SD or median (range),(F/M) | Control group: n=sample size, age; mean±SD or median (range),(F/M) | Results: RHI reported. if RHI not specified, we reported p/r values | Outcomes |
| Vascular function in asthmatic children and adolescents. Augusto et al45 | 2017 | Cross-sectional controlled study | n=19 asthmatic patients, age 13.6 ± 0.6 years. (F/M 0/19) |
n=18 controls. 14.9 ± 0.7 years.(F/M 0/18) | RHI were similar between groups (p = 0.23). Asthmatic group RHI did not correlate with the different variables. | The increased AIx@75 without changes in RHI in asthmatic patients could mean that an early detection of vascular impairment may precede ED. |
| The effect of weight loss on endothelial function and sleep disordered breathing (SDB) in obese children. Ysebaert et al46 | 2018 | Conference abstract. Baseline and reassessed after 6-month weight loss programme. | n=62 obese, age 11–19 (mean 15.8) years,(F/M 20/42) | No controls. | Baseline: 39% had SDB. After 6 months: 86% had resolution of earlier diagnosed SDB. All had significant improvement of endothelial function after programme (p<0.001). No correlations between SDB and improvement in endothelial function found. | Endothelial function significantly improves after weight loss. |
| Polysomnographic correlates of endothelial function in children with obstructive sleep apnoea (OSA). Zhang et al47 | 2018 | Cross-sectional study | n=121 mild OSA, 6.2±1.6 years,(F/M 37/84). n=127 moderate-severe OSA, 6.0±1.6 years,(F/M 31/96) | n=107 primary snorers (PS), age 6.4±1.8 years,(F/M 37/70) | OSA groups lower RHI than PS (p<0.001, p=0.001). RHI positively correlated with age (r=0.17, p=0.002), BMI z score (r=0.14, p=0.008) and oxygen saturation (r=0.15, p=0.006). | Children with OSA are at increased risk for abnormal endothelial function than habitually snoring children. |
| Endothelial dysfunction in children with obstructive sleep apnoea syndrome (OSAS). Xu et al48 | 2020 | Cross-sectional study | n=248 OSAS, age 3–11 years | n=107 primary snorers (PS). No significant differences in age/gender. | OSAS had lower RHI 1.1±0.1 vs 1.2±0.2 (p<0.01). RHI independently correlated with age, gender, obstructive apnoea hypopnea index, oxygen desaturation index (p<0.01). | OSAS have significant ED compared with PS. Frequent arousals due to obstructive respiratory events during sleep may be a candidate risk factor for ED. |
AIx@75, heart rate-corrected augmentation index; ED, endothelial dysfunction; OSA, obstructive sleep apnoea; OSAS, obstructive sleep apnoea syndrome; PS, primary snorers; RHI, reactive hyperaemia index.
Table 5.
Endo-PAT 2000 in paediatric patients with psychiatric conditions (four studies)
| Title, lead author | Year | Study design | Population: n=sample size, age; mean±SD or median (range),(F/M) | Control group: n=sample size, age; mean±SD or median (range),(F/M) | Results: RHI reported. if RHI not specified, we reported p/r values | Outcomes |
| Do self-reported stress and depressive symptoms effect endothelial function in healthy youth? The LOOK longitudinal study. Olive et al49 | 2018 | Longitudinal cohort study. LOOK longitudinal study, who were followed through to adolescence (16 years). | n=203, 7.6±0.3 years, (F/M 111/92). | No controls. | All relationships occurred in the hypothesised direction, but no cross-sectional or prospective evidence of early psychological stress or depression was associated with ED (all p>0.05). | Contrast to previous findings in adolescents, little evidence between current or previous psychosocial stress or depression and endothelial function in 16-year-old adolescents. |
| Cerebrovascular reactivity is associated with peripheral endothelial function (EF) among adolescents. Urback et al93 | 2016 | Conference abstract | n=11 with bipolar disorder. EF measured by PAT and cerebrovascular reactivity (CVR) by blood oxygen-level dependent fMRI. | n=35 healthy controls | EF was positively correlated with CVR in grey matter (r=0.41, p=0.012), and a peak voxel in the left medial-frontal gyrus (r=0.35, p=0.036). | Breath-hold CVR and peripheral EF are linked, suggesting that vascular function may be a multi-systemic phenotype. EF may be a potential proxy for cerebral blood vessel function with greater accessibility and lower cost than fMRI. |
| Retinal-vascular photography as a window into the cardiovascular and neurocognitive burden of adolescent bipolar disorder (BD). Naiberg et al50 | 2017 | Cross-sectional study, author emailed for separate paediatric data—most were teenagers | n=30 with bipolar disorder, 17.97±1.86 years | n=32 healthy controls, 16.00±1.62 years | In BD group, higher endothelial function associated with higher arterio-venular ratio (r=0.375, p=0.041). | Retinal photography may help assessing cardiovascular and neurocognitive burden of BD. |
| Impact of psychological health on peripheral endothelial function and the HPA-axis activity in healthy adolescents. Chen et al94 | 2017 | Longitudinal 3-year follow-up study. Baseline and 3 year follow-up. | n=162, 14.5±1 years. (F/M 94/68). | No controls. | Lower peripheral endothelial function was associated with high level of anger (β = −0.332, p=0.018) and disruptive behaviour (β = −0.390, p=0.006) over 3 years in men, but not in women, adjusted for covariates. | High amounts of negative emotions may have adverse effects on peripheral endothelial function and regulation of the HPA-axis activity. High level of self-concept might be protective. |
BD, bipolar disorder; CVR, cerebrovascular reactivity; ED, endothelial dysfunction; EF, endothelial function; fMRI, functional magnetic resonance imaging; HPA, hypothalamic–pituitary–adrenal.
Table 6.
Endo-PAT 2000 in paediatric patients with other miscellaneous paediatric conditions (10 studies)
| Title, lead author | Year | Study design | Population: n=sample size, age; mean±SD or median (range), (F/M) | Control group: n=sample size, age; mean±SD or median (range), (F/M) | Results: RHI reported. if RHI not specified, we reported p/r values | Outcomes |
| Vascular endothelial function in inflammatory bowel disease (IBD). Winderman et al60 | 2018 | Case–control study | n=16 with IBD (all in clinical remission), age 16.7±2.6 years,(F/M 8/7) | n=16, age 15.1±2.8 years,(F/M 7/8) | RHI IBD vs controls 1.66 vs 2.02 (p=0.036). IBD group had a mean RHI within the range associated with VD risk in adults (1.67). | IBD group lower RHI compared with controls. IBD patients may need to be monitored for thromboembolic phenomena. |
| Endothelial health in childhood acute lymphoid leukaemia (ALL) survivors: pilot evaluation with peripheral artery tonometry. Ruble et al52 | 2015 | Case–control study | n=16 ALL survivors, age 8–20 years (12.9±0.9),(F/M 8/8). | n=16 healthy sibling pairs 13.8 (0.9),(F/M 10/6). | Both groups similar in cardiovascular risk measures but survivors had lower RHI (1.54 vs sibling 1.77; p=0.0474). | Evidence of poorer vascular health in cancer survivors. |
| Microvascular endothelial function in Japanese early adolescents. Odanaka et al95 | 2017 | Control study | n=157 healthy adolescents divided by gender. Womwen n=82, median age 14, (1) 13.7±0.9 years | Males n=75, median age 14 (2) years | No difference in RHI according to sex: boys and girls 1.85 ±0.6, 1.82 ±0.66 and 1.87±0.54. RHI was significantly associated with systolic and diastolic BP, and had no correlation with anthropometric parameters and arterial stiffness markers. |
RHI among adolescents were similar to those reported in previous studies on children and early adolescents. |
| Endothelial dysfunction and the effect of arginine and citrulline supplementation in children and adolescents with mitochondrial diseases. Al Jasmi, et al59 | 2020 | Case–control study | Nine participants, age 6–17 years (mean 9.6). | 3–15 years (mean 9.4). Baseline endothelial dysfunction was assessed in controls. | Lower RHI with mitochondrial diseases. RHI increased with arginine or citrulline supplementation | Supplementation with NO precursors may improve ED by enhancing NO production. First study to use Endo-PAT methodology in mitochondrial diseases. |
| Assessment of traditional and non-traditional risk factors for premature atherosclerosis in children with juvenile dermatomysoitis (JDM) and paediatric controls. Wahezi et al96 | 2020 | Retrospective controlled study | n=40 JDM, age 6–22 (mean 12.4±4.1) years,(F/M 28/12) | n=20 controls, age 12.7±3.9 years, (F/M 14/8) | RHI controls 1.43(1.2, 1.7) and JDM 1.57(1.2,1.9). If controlled for lipoprotein A (atherogenic confounder), JDM patients had 41% RHI increase, thus indicating less ED compared with controls. | Rheumatological childhood disorders may be at increased risk of developing ED, but sociodemographic factors may have a greater role in developing cardiovascular disease. |
| Vascular health of children conceived via in vitro fertilization (IVF). Zhang et al97 | 2019 | Cross-sectional pilot study | n=17 IVF children, 10–14 years. Also used carotid ultrasound and pulse wave velocity measurements. | Compared to published norms or to historical Stanford controls |
Mean Endo-PAT index in the IVF cohort was 1.66±0.52, 71% had abnormal values (<1.9). Mean RHI was not significantly different between IVF and controls. | Children conceived by IVF seem to have evidence of abnormal vascular health. |
| Endothelial dysfunction in South African youth living with perinatally acquired human immunodeficiency virus (PHIV) on antiretroviral therapy. Mahtab et al98 | 2020 | Case–control study | n=431 PHIV, median 14.1 (12.8, 15.5) years,(F/M 213/218) | n=93 without HIV, median 13.9 (12.1, 15.3) years,(F/M 53/40) | PHIV had higher rates of ED (50% vs 34%; p=0.01); relationship persisted after adjusting for age, sex, BMI, high BP, high cholesterol (RR, 1.43; p=0.02). PHIV, CD4 count, viral load and current ART class were not associated with ED after adjustment. | PHIV appear to have increased risk of ED. These findings have important implications as HIV has increased risk of premature CVD and complications. |
| Soluble CD14 (sCD14) is associated with endothelial dysfunction in South African youth on ART. Dirajlal-Fargo et al62 | 2020 | Case–control study | n=283 perinatally acquired HIV (PHIV), 9–14 years. | n=69 age-matched without HIV | PHIVs had lower RHI despite viral suppression (RHI=1.36 vs 1.52, p<0.01). sCD14 at 24 months correlated with ED (p≤0.04). PHIV with ED, sCD14 was associated with lower RHI (β−0.05, p=0.01). | Higher sCD14 is independently associated with ED in PHIVs. |
| Role of insulin resistance and hyperandrogenemia in early vascular dysfunction in adolescents with PCOS. Bartz et al99 | 2015 | Conference abstract | n=14 PCOS adolescents PCOS (on no treatment). | n=7 non-PCOS. Both groups had similar age, tanner stage, race, glucose tolerance status. | Despite higher peripheral and hepatic insulin resistance with PCOS, RHI is not significantly lower when compared with controls of similar total body and abdominal adiposity. | PCOS has evidence of increased vascular inflammation. Hyperandrogenemia and insulin resistance may play an important role in vascular inflammation. |
| Endothelial function in children and adolescents is mainly influenced by age, sex and physical activity-an analysis of reactive hyperemic peripheral artery tonometry. Mueller et al85 | 2017 | Randomised controlled study, Leipzig School Project followed over 5-year period. | n=931 RHI measurements in 445 students, age 10–17 years (baseline 11.66±0.93). n=247: 60 min physical exercise (PE) daily (intervention group). |
n=181: 2 units of 45 min PE weekly (control group). | Higher RHI in the intervention group: 0.09 (−0.05, 0.23). Increase RHI from 1.53±0.42 in the youngest to 1.96±0.59 in the oldest students. This increase adjusted by age and sex was estimated as 0.11 (0.08, 0.14)per year. | If Endo-PAT is used for research in adolescents, age and sex must to be taken in account when reporting RHI results. |
ALL, acute lymphoid leukaemia; ED, endothelial dysfunction; IBD, inflammatory bowel disease; IVF, in vitro fertilisation; NO, nitric oxide; PCOS, polycystic ovarian syndrome; PE, physical exercise; PHIV, perinatally acquired human immunodeficiency virus; RHI, reactive hyperemia index; sCD14, soluble CD 14.
Results
Endothelial dysfunction in paediatric diabetes mellitus patients
Five studies involve only T1D patients (table 1). 2-fifth studies reported lower RHI results in the T1D group.17 18 One study which included only adolescent patients reported RHI negatively correlates with impaired metabolic control and subclinical signs of autonomic neuropathy.17 They concluded that good metabolic control (haemoglobin A1c (HbA1c) ≤7.5%) and regular physical activity might be protective against ED. One study reports an improved RHI result with an alpha-lipoic acid and antioxidant diet.19 Nadeau et al reported no significant RHI change with metformin overall but some improvement in overweight T1D men.20 Barber et al report suboptimal glycaemic control causes early atherosclerosis.18 One study noted an improvement in RHI post-vitamin D supplementation in T1D patients with vitamin D deficiency.21
Six studies focused on type 2 diabetes (T2D) and impaired glucose tolerance or ‘pre-diabetes.’ Tomsa et al note a link between insulin resistance and obesity by utilising Endo-PAT.22 They also noted that RHI is higher if HbA1c is less than 5.5%.22 Two studies compare on non-alcoholic fatty liver disease (NAFLD), T2D and pre-diabetes patients.23 24 If dysglycemia, NAFLD is associated with worse endothelial function. Circulating FGF-21 levels are elevated in obese youth with NAFLD and are associated ED and, therefore, may be a biomarker for ED.24 Bartz et al report urine albumin creatinine ratio may be an early marker of ED independent of glycaemia.25 Endothelial dysfunction (EDF) may mediate the link between obesity-related insulin resistance and early microalbuminuria.25 Kochummen et al reported a mean RHI in obese adolescents without diabetes was similar to T1D and T2D patients.26 One study noted an improvement in RHI-post vitamin D supplementation in T1D patients with vitamin D deficiency.21 Another study noted that lower vitamin D concentrations are associated with lower insulin sensitivity and worse endothelial function.27
EDF and obesity
Fourteen studies describe the use of Endo-PAT 2000 in overweight or obese patients (table 2). Studies included measurement of the following parameters: body mass index (BMI), T1D, T2D, gender, pubertal stage, age, blood pressure values, NAFLD, obstructive sleep apnoea (OSA), insulin, plasma glucose levels, inflammatory markers (urinary markers, CNP, micro-RNA-126, E-Selectin). In numerous studies, RHI was significantly lower in obese groups.7 26 28–34 ED may mediate the link between obesity-related insulin resistance and early microalbuminuria.25 Exercise and diet control improve glycolipid metabolism.35 Two studies by Czippelova et al did not find a lower RHI in obese groups, but recommended further studies.36 37 Noma et al38 report the beneficial effects of exercise in paediatric patients and is an important message in reducing future endothelial complications.38 Fusco et al noted preclinical microvascular changes in obese patients compared with controls using LDF but noted no RHI change.39
EDF in cardiac and vascular conditions
Seven studies report the use of Endo-PAT and cardiovascular conditions (table 3). Lower RHI is seen with patients with familial hypercholesterolaemia.40 Studies assess ED in patients with systemic lupus erythematosus (SLE) and Henoch Schonlein purpura (HSP).8 41 42 Negishi et al43 used Endo-PAT to compare Fontan survivors and healthy controls. The Fontan patients were aged 15–2 years. Mean RHI 0.56±0.26 in Fontan patients and 0.78±0.31 in controls (p=0.09). RHI in Fontan patients was associated with diastolic blood pressure, heart rate and HbA1c level.43 Endothelial function in Fontan patients was associated with abnormal glucose tolerance and arterial stiffness and, therefore, concluded that glucose regulation might be a potential target to improve ED in this cohort. Nozaki et al44 assessed ED in conduit and resistance arteries and used FMD and Endo-PAT in paediatric patients with repaired coarctation of aorta.44
EDF in respiratory conditions
Four studies used Endo-PAT in respiratory conditions (table 4). Augusto et al noted an increased augmentation index (AIx) without changes in RHI in asthmatic patients.45 One study reported an improvement in sleep-disordered breathing post weight loss and also, endothelial function significantly improved after weight loss.46 Two studies report children with OSA compared with habitual snorers are at increased risk for ED.47 48 Frequent wakening due to obstructive respiratory events may be a risk factor for ED in OSA.
EDF and psychological conditions
Four studies report the use of Endo-PAT in psychiatric conditions (table 5). Potential limitations in this area are self-reported methods for detecting psychological distress of children, for example, in the LOOK longitudinal study.49 Naiberg et al50 used retinal vascular photography as a proxy for cerebral microvasculature, and Endo-PAT to assess cardiovascular and neurocognitive burden in adolescents with bipolar disorder (BD).50 In the BD group, better endothelial function was associated with higher arteriovenular ratio (r=0.375, p=0.041). Olive 51 published ‘The emerging field of paediatric psycho-cardiology’ highlighting the importance of the childhood origins of adult cardiovascular disease (CVD).51 This article highlights that psychological distress can influence CVD risk, directly by physiological change that can negatively impact the integrity of the cardiovascular system.
EDF and other paediatric conditions
Childhood cancer survivors
There is evidence of ED in cancer survivors (table 6).52 Chemotherapy causes cardiomyocyte damage and also negatively affects endothelial function. Broberg et al53 utilised Endo-PAT in childhood cancer survivors and noted a lower RHI in this cohort compared with controls.53 Broberg et al identified one-third of cancer survivors (31.2%) compared with 8% of controls (p=0.02) had ED in their study.54 They concluded this may be a useful screening tool of cardiovascular disease in asymptomatic cancer survivor patients. Pao et al55 assessed the relationship between blood pressure and ED using Endo-PAT in haematopoietic stem cell transplant recipients. Hypertension on ambulatory blood pressure monitoring (p= 0.045) and blunted nocturnal dipping (p= 0.04) were associated with a lower Endo-PAT scores.55
Autoimmune conditions
Children with autoimmune diseases may have a high tendency to develop ED which was highlighted in a study using a novel technique.56 Atherosclerosis is an emerging cause of morbidity and mortality in patients with rheumatological conditions such as juvenile idiopathic arthritis, SLE and dermatomyositis. Borenstein-Levin et al assessed a cohort with autoimmune conditions compared with controls: 29% in the study group had ED compared with 6% (p<0.05).56 Chang et al noted nocturnal blood pressure (BP) non-dipping is associated with ED in SLE patients highlighting a potential role for ambulatory BP monitoring in these patients(table 3).8
Metabolic diseases
Yano et al research in Fabry disease patients demonstrated that early diagnosis of ED can help determine the timing of initiating enzyme replacement therapy.57 Utilising RH-PAT as a screening tool for early renal involvement may be helpful as it may detect abnormalities even prior to microalbuminuria.58 This can provide guidance on enzyme replacement therapy which is required to prevent irreversible progressive renal failure. Al Jasmi et al research in mitochondrial diseases reported that arginine or citrulline supplementation may improve ED, which provides evidence that these amino acids may be therapeutic (table 6).59
Inflammatory bowel disease:
One study (table 6) highlights that IBD patients had lower RHI compared with controls.60 Petr et al61 provided evidence of increased ED in children with Crohn’s disease compared with healthy controls.61 RHI values were significantly lower in the patients with Crohn’s than controls (p<0.05).
Infectious diseases
Dirajlal-Fargo et al used Endo-PAT to assess ED in HIV patients (table 6).62 63 Perinatally acquired HIV patients appear to have higher levels of ED (RHI 1.34 (1.20, 1.42) compared with controls (1.52 (1.27, 1.80) (p< 0.01)).63 The pathogenesis of severe Plasmodium vivax malaria is poorly understood. ED and reduced NO bioavailability characterise severe falciparum malaria. Barber et al64 identified that endothelial function was impaired in proportion to disease severity. Those with severe vivax malaria, mild-moderate infection and healthy controls: median RHI 1.49, 1.73, and 1.97 respectively (p=0.018).64 ED in this cohort was associated with reduced L-arginine bioavailability, which may contribute to microvascular pathogenesis.
Discussion
To our knowledge, this study is the first to conduct a rigorous systematic review of published and presented literature on the results of RHI as measured by Endo-PAT 2000 as a measure of EDF in children and adolescents. One of the benefits of RHI as a measure of ED is that it is an easy test to conduct, is well-tolerated by children and adolescents and it can be performed at the point of care.
Weaknesses of the paper include the quality of the papers are limited and varied; 11 are conference abstracts that had little information available on methods or results and have limited analysis. Observational studies are also limited in research value. Many are case-control studies which are not as valuable as randomised controlled trials (RCT). Only four studies are RCTs. The studies cannot be compared for a meta-analysis as most are not RCT level research of high enough quality. Therefore, the conclusions drawn from many of these studies are limited. There are also limitations of RHI as reliable method for defining ED. There is no defined RHI cut-off value in paediatric populations. Moreover, there may be significant findings in studies in the grey literature or in conference presentations that were not included, for example, in the studies where 25 authors did not respond to emails. Only papers from 2015 to March 2021 were included. Many of the papers did not include other factors that would be important in a cardiovascular assessment of children, for example, family history, cholesterol and blood pressure parameters and BMI and standardised BMI measurements. So, in many studies, it cannot be excluded that there were confounding variables affecting the ED score. Regardless, this study indicates that there are a significant number of published paediatric papers that indicate the presence of ED in children as young as 8 years old.
Strengths of the paper include a comprehensive literature search including contacting authors by email for separate paediatric results in studies with combined adult and paediatric data. All study types were reviewed and even the studies without results but had interesting points were included in our discussion. Also, we do not think that this paediatric Endo-PAT review has been done before. Our results highlight that Endo-PAT has benefits including point-of-care and ease of conduct of test for assessor.
The potential future role of Endo-PAT for paediatric patients may be an adjunct tool in screening for cardiovascular risk factors. If atherosclerosis is identified early, it can be halted in its process in certain conditions. There is huge potential for its use in diabetic patients. Improving glucose control can protect endothelial function. Persistent high sugars can impair endothelial function via oxidative stress and production of free radicals.2 Lower insulin sensitivity poses a risk of diabetic nephropathy.9 Microangiopathic renal damage increases oxygen consumption and increases resistance in the afferent arterioles. Shah et al report T2D patients have greater vascular thickness and stiffness and worse endothelial function compared with obese and lean children.65 This is raising concern that adolescents with T2D are already at risk of developing early-onset cardiovascular disease.
Diabetic microangiopathy can result in retinopathy, neuropathy and peripheral vascular neuropathy. Subclinical evidence of these complications can be seen in paediatric patients, especially in those with poor glycaemic control. Unfortunately, there have been reports of T2D paediatric patients diagnosed with microangiopathic complications, particularly nephropathy.66 This early endothelial damage can be linked with increased morbidity and mortality.67 Moreover, new onset diabetes after transplantation is characterised by insulin resistance and T2D.68 Endo-PAT has multiple benefits in obesity as it can identify if early ED is present and, therefore, strategies to reverse or halt this process can be made (table 2).7 30 37
In recent decades, the number of childhood cancer survivors is increasing.69 Treatments used such as haematopoietic stem cell transplantation have increased risk of cardiovascular disease.70 71 Following chemotherapy, radiotherapy, immunosuppressive treatments, the risk of insulin resistance has been noted.72 With advances in treating malignant paediatric conditions there are long term complications emerging in survivors. High-dose chemotherapy including anthracyclines, alkylating agents and vinca alkaloids may disrupt the substances on the surface of the endothelium and impair its ability to dilate and constrict. Moreover, total body radiation poses a risk by damaging the elastic matrix. Heart disease in long-term cancer survivors is 5–10 times higher than their siblings.72 Brouwer et al73 studied cancer survivor patients after potential cardiovascular toxic treatment (eg, anthracyclines, platinum) and/or radiotherapy and noted a higher risk of ED compared with sibling controls.73 Jehlicka et al74 used Endo-PAT and noted acute lymphoblastic leukaemia patients had lower RHI compared with controls (1.57±0.50, 1.96±0.63; p≤0.05).74
Turner syndrome (TS) patients have increased cardiovascular risk factors, which predispose to cardiac and cerebrovascular complications.75 A case–control study on TS patients noted a statistically significant increase in RHI in growth hormone-treated girls.76 There are countless other paediatric syndromes with risk of ED that could benefit from screening.
Furthermore, in cardiac diseases and postcardiac surgery, Endo-PAT has been proven useful in multiple studies (table 3).44 75 77 Dietz et al’s78 systematic review and metanalysis on peripheral ED in Kawasaki disease, report coronary arterial aneurysms had higher surrogate markers for cardiovascular disease risk.78 This may indicate that these patients should be monitored for CVD in adulthood; however, significant heterogeneity was noted. Endo-PAT has been shown to be beneficial postoperatively in Fontan survivors and comparing surgical techniques like in the ‘The LOVE-COARCT study’ (Long-term Outcomes and Vascular Evaluation After Successful Coarctation of the Aorta Treatment).79–81
With the rising premature population, Endo-PAT may prove useful in this cohort. Harris et al82 assessed cardiovascular outcomes for those born with very low birth weights (VLBW) <1500 g. The VLBW cohort (n=229; 71% of survivors) and term-born controls (n=100) were assessed at age 26–30 years. The VLBW cohort had lower RHI compared with controls.82 Endo-PAT is also used in haematological conditions. Sivamurthy et al83 reported lower RHI in the majority sickle cell disease in a paediatric population (1.53 and 1.71; p value 0.032). RHI was not normal in children with chronic transfusions or hydroxyurea.83
The psychological studies in our paper raise an interesting link between the vascular system and the psychiatric diagnoses. Retinal vascular calibre was shown to be associated with endothelial function in BD patients and it has been suggested that it may be used as an assessment tool in this cohort.
Finally, many paediatric autoimmune conditions are linked with ED.8 56 In patients with SLE, ED may occur from impaired clearance of apoptotic cells, oxidative stress or B cell activation with different circulating autoantibodies.42 Regular ED assessment in SLE patients has been recommended due to risk of subclinical atherosclerosis.42 Moreover, several factors may impact microvascular function in children, for example, puberty, which is of particular interest in our paediatric review. Bhangoo et al report improved RHI in correlation with an increase in Tanner stages and postulated that this may be due to sex steroids.84 If Endo-PAT is used in research in adolescents, age, sex and tanner staging must be taken in account when reporting RHI results.85
Conclusion
There are a number of papers in the paediatric literature describing ED at young ages using Endo-PAT. However, in many cases, there has only been a single cohort study using Endo-PAT. Further studies are required to validate these findings. Additionally, longitudinal studies are required to evaluate how this ED may change as the child ages and their chronic conditions change. Further studies are also required that will characterise more completely the cardiovascular risk profile of these children with chronic disease. Consensus on other vascular risk markers that could be included in future studies is ideal and if accomplished, this would facilitate meta-analyses of studies of conditions with relatively rare conditions.
The establishment of a threshold RHI for normal or abnormal would be helpful if it correlated well with clinical outcomes. This might be achieved in the future either by meta-analysis of the literature, if outcomes are measured and reported in a standardised manner, or by the conduct of a prospective longitudinal study that follows RHI in childhood to adulthood along with identification of cardiac outcomes. The latter would by its nature require to be a long-term study and would require a repeated iterative process to establish the threshold of normal for RHI, as a continuous variable. Therefore, a meta-analysis may be preferable. In the short term, a systematic approach to cardiovascular risk assessments should be promoted.
Supplementary Material
Footnotes
Contributors: All authors contributed to the initial search strategy. Id performed the online database search. JH and CO'G are responsible overall for the content of the paper and JH is the guarantor of the work. JH and GO'D separately performed a blind screen of the abstracts and analysed the papers. GO'D contacted the authors of joint adult and paediatric papers to obtain separate paediatric data. JH wrote the initial manuscript that was revised by CO’G. All authors reviewed the manuscript prior to submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Mudau M, Genis A, Lochner A, et al. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr 2012;23:222–31. 10.5830/CVJA-2011-068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avogaro A, Albiero M, Menegazzo L, et al. Endothelial dysfunction in diabetes. Diabetes Care 2011;34:S285–90. 10.2337/dc11-s239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadi HAR, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 2005;1:183–98. [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor MS, Choi C-S, Bayazid L, et al. Changes in vascular reactivity and endothelial Ca2+ dynamics with chronic low flow. Microcirculation 2017;24. 10.1111/micc.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leite AR, Borges-Canha M, Cardoso R, et al. Novel biomarkers for evaluation of endothelial dysfunction. Angiology 2020;71:397–410. 10.1177/0003319720903586 [DOI] [PubMed] [Google Scholar]
- 6.Haller MJ, Samyn M, Nichols WW, et al. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care 2004;27:2911. 10.2337/diacare.27.12.2911 [DOI] [PubMed] [Google Scholar]
- 7.Czippelova B, Turianikova Z, Krohova J, et al. Arterial stiffness and endothelial function in young obese patients - vascular resistance matters. J Atheroscler Thromb 2019;26:1015–25. 10.5551/jat.47530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JC, Xiao R, Meyers KE, et al. Nocturnal blood pressure dipping as a marker of endothelial function and subclinical atherosclerosis in pediatric-onset systemic lupus erythematosus. Arthritis Res Ther 2020;22:129. 10.1186/s13075-020-02224-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornstad P, Nehus E, El Ghormli L, et al. Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: an observational analysis of data from the today clinical trial. Am J Kidney Dis 2018;71:65–74. 10.1053/j.ajkd.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 2010;362:485–93. 10.1056/NEJMoa0904130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Valle A, Crocco M, Chiarenza DS, et al. Endothelial impairment evaluation by peripheral arterial tonometry in pediatric endocrinopathies: a narrative review. World J Diabetes 2021;12:810–26. 10.4239/wjd.v12.i6.810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selamet Tierney ES, Newburger JW, Gauvreau K, et al. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr 2009;154:901–5. 10.1016/j.jpeds.2008.12.028 [DOI] [PubMed] [Google Scholar]
- 13.Moerland M, Kales AJ, Schrier L, et al. Evaluation of the EndoPAT as a tool to assess endothelial function. Int J Vasc Med 2012;2012:904141. 10.1155/2012/904141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatihoglu SG, Jam F, Okutucu S, et al. Noninvasive investigation of the presence and extent of coronary artery disease by the evaluation of fingertip-reactive hyperemia. Med Princ Pract 2022;31:262–8. 10.1159/000522098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004;44:2137–41. 10.1016/j.jacc.2004.08.062 [DOI] [PubMed] [Google Scholar]
- 16.Wilk G, Osmenda G, Matusik P, et al. Endothelial function assessment in atherosclerosis: comparison of brachial artery flow‑mediated vasodilation and peripheral arterial tonometry. Pol Arch Med Wewn 2013;123:443–52. 10.20452/pamw.1879 [DOI] [PubMed] [Google Scholar]
- 17.Scaramuzza AE, Redaelli F, Giani E, et al. Adolescents and young adults with type 1 diabetes display a high prevalence of endothelial dysfunction. Acta Paediatr 2015;104:192–7. 10.1111/apa.12877 [DOI] [PubMed] [Google Scholar]
- 18.Babar G, Clements M, Dai H, et al. Assessment of biomarkers of inflammation and premature atherosclerosis in adolescents with type-1 diabetes mellitus. J Pediatr Endocrinol Metab 2019;32:109–13. 10.1515/jpem-2018-0192 [DOI] [PubMed] [Google Scholar]
- 19.Scaramuzza A, Giani E, Redaelli F, et al. Alpha-Lipoic acid and antioxidant diet help to improve endothelial dysfunction in adolescents with type 1 diabetes: a pilot trial. J Diabetes Res 2015;2015:474561. 10.1155/2015/474561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadeau KM, Nathan K, Bacha B, et al. Effect of metformin on endothelial function in overweight adolescents with type 1 diabetes (T1D). Pediatric Diabetes 2016;17:152–3. [Google Scholar]
- 21.Deda L, Yeshayahu Y, Sud S, et al. Improvements in peripheral vascular function with vitamin D treatment in deficient adolescents with type 1 diabetes. Pediatr Diabetes 2018;19:457–63. 10.1111/pedi.12595 [DOI] [PubMed] [Google Scholar]
- 22.Tomsa A, Klinepeter Bartz S, Krishnamurthy R, et al. Endothelial function in youth: a biomarker modulated by adiposity-related insulin resistance. J Pediatr 2016;178:171–7. 10.1016/j.jpeds.2016.07.025 [DOI] [PubMed] [Google Scholar]
- 23.Bacha F, Tomsa A, Bartz SK, et al. Nonalcoholic fatty liver disease in Hispanic youth with dysglycemia: risk for subclinical atherosclerosis? J Endocr Soc 2017;1:1029–40. 10.1210/js.2017-00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacha . Circulating fibroblast growth factor-21 (FGF-21): a biomarker of subclinical atherosclerosis in obese youth with non-alcoholic fatty liver disease (NAFLD)? 88, 2017. [Google Scholar]
- 25.Bartz SK, Caldas MC, Tomsa A, et al. Urine Albumin-to-Creatinine ratio: a marker of early endothelial dysfunction in youth. J Clin Endocrinol Metab 2015;100:3393–9. 10.1210/JC.2015-2230 [DOI] [PubMed] [Google Scholar]
- 26.Kochummen E, Umpaichitra V, Marwa A, et al. Assessment of microvascular function in children and adolescents with diabetes and obesity. Int J Endocrinol Metab 2020;18:e90094. 10.5812/ijem.90094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacha F, Bartz SK, Tomsa A, et al. Free vitamin D: relationship to insulin sensitivity and vascular health in youth. J Pediatr 2019;212:28–34. 10.1016/j.jpeds.2019.04.057 [DOI] [PubMed] [Google Scholar]
- 28.Djurica D, Holt RR, Ren J, et al. Effects of a dietary strawberry powder on parameters of vascular health in adolescent males. Br J Nutr 2016;116:639–47. 10.1017/S0007114516002348 [DOI] [PubMed] [Google Scholar]
- 29.Hussid MF, Jordão CP, Lopes‐Vicente WR, et al. Flow‐ediated dilation in obese adolescents: correlation with waist circumference and systolic blood pressure. Faseb J 2018;32:713.7–7. 10.1096/fasebj.2018.32.1_supplement.713.7 [DOI] [Google Scholar]
- 30.Donghui T, Shuang B, Xulong L, et al. Improvement of microvascular endothelial dysfunction induced by exercise and diet is associated with microRNA-126 in obese adolescents. Microvasc Res 2019;123:86–91. 10.1016/j.mvr.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 31.Mu K, Zhang Y, Niu DY, et al. [Distribution of peripheral arterial stiffness and endothelial function as well as their correlations with cardiovascular risk factors in children and adolescents]. Zhonghua Liu Xing Bing Xue Za Zhi 2016;37:805–9. 10.3760/cma.j.issn.0254-6450.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 32.Del Ry S, Cabiati M, Bianchi V, et al. C-type natriuretic peptide plasma levels and whole blood mRNA expression show different trends in adolescents with different degree of endothelial dysfunction. Peptides 2020;124:170218. 10.1016/j.peptides.2019.170218 [DOI] [PubMed] [Google Scholar]
- 33.Del Ry S, Cabiati M, Bianchi V, et al. C-type natriuretic peptide is closely associated to obesity in Caucasian adolescents. Clin Chim Acta 2016;460:172–7. 10.1016/j.cca.2016.06.045 [DOI] [PubMed] [Google Scholar]
- 34.Bacha F, Tomsa A, Bartz SK, et al. Nonalcoholic fatty liver disease in Hispanic youth with dysglycemia: risk for subclinical atherosclerosis? J Endocr Soc 2017;1:1029–40. 10.1210/js.2017-00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen MN, Nielsen CM, Madsen M, et al. Cardiovascular adaptations after 10 months of intense school-based physical training for 8- to 10-year-old children. Scand J Med Sci Sports 2018;28 Suppl 1:33–41. 10.1111/sms.13253 [DOI] [PubMed] [Google Scholar]
- 36.Czippelova BTZ, Krohova J, Lazarova Z. Endothelial function and arterial stifness in obese adolescents - A relation to barorefex function. Obes Facts 2017;10 10.5551/jat.47530 [DOI] [Google Scholar]
- 37.Czippelova BTZ, Lazarova Z, Krohova J, et al. Obesity in children and adolescents: a relation to endothelial function and arterial stiffness. Acta Physiologica 2016;217. [Google Scholar]
- 38.Noma K, Kihara Y, Higashi Y. Outstanding effect of physical exercise on endothelial function even in children and adolescents. Circ J 2017;81:637–9. 10.1253/circj.CJ-17-0291 [DOI] [PubMed] [Google Scholar]
- 39.Fusco E, Pesce M, Bianchi V, et al. Preclinical vascular alterations in obese adolescents detected by laser-Doppler flowmetry technique. Nutr Metab Cardiovasc Dis 2020;30:306–12. 10.1016/j.numecd.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Jehlicka PHM, Masopustova A, Trefil L, et al. Reactive hyperaemia index and detection of endothelial dysfunction in children with familial hypercholesterolaemia. European Heart Journal 2015;36:262.25763440 [Google Scholar]
- 41.Butbul Aviel Y, Dafna L, Pilar G, et al. Endothelial function in children with a history of Henoch Schonlein purpura. Pediatr Rheumatol Online J 2017;15:3. 10.1186/s12969-016-0135-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sciatti E, Cavazzana I, Vizzardi E, et al. Systemic lupus erythematosus and endothelial dysfunction: a close relationship. Curr Rheumatol Rev 2019;15:177–88. 10.2174/1573397115666181126105318 [DOI] [PubMed] [Google Scholar]
- 43.Negishi JOH, Hayama Y, Noritake K. Digital assessment of endothelial function and its association with clinical variable in patients with fontan operation. Cardiol Young 2016;26:S134. 10.1159/000489691 [DOI] [Google Scholar]
- 44.Nozaki Y, Nakayama-Inaba K, Ishizu T, et al. Endothelial dysfunction of conduit arteries in patients with repaired coarctation of the aorta. Int Heart J 2018;59:1340–5. 10.1536/ihj.17-564 [DOI] [PubMed] [Google Scholar]
- 45.Augusto LS, Silva GC, Pinho JF, et al. Vascular function in asthmatic children and adolescents. Respir Res 2017;18:17. 10.1186/s12931-016-0488-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ysebaert M, Bruyndonckx L, De Winter B. The effect of weight loss on endothelial function and sleepdisordered breathing in obese children. Obes Facts 2018;11:225–6. 10.1159/000489691 [DOI] [Google Scholar]
- 47.Zhang F, Wu Y, Feng G, et al. Polysomnographic correlates of endothelial function in children with obstructive sleep apnea. Sleep Med 2018;52:45–50. 10.1016/j.sleep.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 48.Xu ZF, Zhang FJ, Ge WT, et al. [Endothelial dysfunction in children with obstructive sleep apnea syndrome]. Zhonghua Er Ke Za Zhi 2020;58:13–18. 10.3760/cma.j.issn.0578-1310.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 49.Olive LS, Abhayaratna WP, Byrne D, et al. Do self-reported stress and depressive symptoms effect endothelial function in healthy youth? The look longitudinal study. PLoS One 2018;13:e0196137. 10.1371/journal.pone.0196137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naiberg MR, Hatch JK, Selkirk B, et al. Retinal photography: a window into the cardiovascular-brain link in adolescent bipolar disorder. J Affect Disord 2017;218:227–37. 10.1016/j.jad.2017.04.066 [DOI] [PubMed] [Google Scholar]
- 51.Olive LS. Youth psychological distress and intermediary markers of risk for CVD: the emerging field of pediatric psychocardiology. Atherosclerosis 2017;261:158–9. 10.1016/j.atherosclerosis.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 52.Ruble K, Davis CL, Han H-R. Endothelial health in childhood acute lymphoid leukemia survivors: pilot evaluation with peripheral artery tonometry. J Pediatr Hematol Oncol 2015;37:117–20. 10.1097/MPH.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broberg O, Maxedius A, Öra I, et al. Peripheral arterial structure and function in young survivors of childhood cancer. Cardiol Young 2018;28:118. 10.1017/S104795111800031828847337 [DOI] [Google Scholar]
- 54.Broberg OOI, Maxedius A, Wiebe T, et al. Peripheral vascular endothelial function is impaired in childhood cancer survivors. Cardiol Young 2016;26:36. 10.1017/S1047951116000500 [DOI] [Google Scholar]
- 55.Pao E, Gove NE, Flynn JT, et al. Ambulatory blood pressure and endothelial dysfunction in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant 2018;24:1678–84. 10.1016/j.bbmt.2018.04.024 [DOI] [PubMed] [Google Scholar]
- 56.Borenstein-Levin L, Brik R, Pillar G, et al. [Early detection of endothelial dysfunction in children with autoimmune diseases by a novel noninvasive technique]. Harefuah 2017;156:411–4. [PubMed] [Google Scholar]
- 57.Yano S, Moseley K, Azen C. Detection of early end-organ damage by endothelial dysfunction with reactive hyperemia-digital peripheral arterial tonometry in patients with Fabry disease. Mol Genet Metab 2017;120:S143. 10.1016/j.ymgme.2016.11.380 [DOI] [Google Scholar]
- 58.Yano SMK, Azen C. Evaluation of endothelial function with reactive hyperemia-digital peripheral arterial tonometry as a non-invasive biomarker to reflect vascular pathology in patients with Fabry disease. J Inborn Errors Metab Screen 2017;5:306–7. [Google Scholar]
- 59.Al Jasmi F, Al Zaabi N, Al-Thihli K, et al. Endothelial dysfunction and the effect of arginine and citrulline supplementation in children and adolescents with mitochondrial diseases. J Cent Nerv Syst Dis 2020;12:1–6. 10.1177/1179573520909377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winderman RRS, Vaidy K, Schwarz S. Vascular endothelial function in inflammatory bowel disease (IBD). J Pediatr Gastroenterol Nutr 2018;67:S79–80. [Google Scholar]
- 61.Petr J, Michal H, Jan S, et al. Reactive hyperaemia index as a marker of endothelial dysfunction in children with Crohn's disease is significantly lower than healthy controls. Acta Paediatr 2014;103:e55–60. 10.1111/apa.12467 [DOI] [PubMed] [Google Scholar]
- 62.Dirajlal-Fargo S, Yu J, Albar Z, et al. Monocyte activation and gut barrier dysfunction in South African youth on antiretroviral therapy and their associations with endothelial dysfunction. AIDS 2020;34:1615–23. 10.1097/QAD.0000000000002615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dirajlal-Fargo S, Sattar A, Kulkarni M, et al. HIV-positive youth who are perinatally infected have impaired endothelial function. AIDS 2017;31:1917–24. 10.1097/QAD.0000000000001556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barber BE, William T, Grigg MJ, et al. Nitric oxide-dependent endothelial dysfunction and reduced arginine bioavailability in Plasmodium vivax malaria but no greater increase in intravascular hemolysis in severe disease. J Infect Dis 2016;214:1557–64. 10.1093/infdis/jiw427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah AS, Urbina EM. Vascular and endothelial function in youth with type 2 diabetes mellitus. Curr Diab Rep 2017;17:1–7. 10.1007/s11892-017-0869-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naylor LH, Davis EA, Kalic RJ, et al. Exercise training improves vascular function in adolescents with type 2 diabetes. Physiol Rep 2016;4. 10.14814/phy2.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tryggestad JB, Willi SM. Complications and comorbidities of T2DM in adolescents: findings from the today clinical trial. J Diabetes Complications 2015;29:307–12. 10.1016/j.jdiacomp.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jodele S, Dandoy CE, Myers KC, et al. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci 2016;54:181–90. 10.1016/j.transci.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the childhood cancer Survivor study. J Clin Oncol 2009;27:2319–27. 10.1200/JCO.2008.21.1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajendran R, Abu E, Fadl A, et al. Late effects of childhood cancer treatment: severe hypertriglyceridaemia, central obesity, non alcoholic fatty liver disease and diabetes as complications of childhood total body irradiation. Diabet Med 2013;30:e239–42. 10.1111/dme.12234 [DOI] [PubMed] [Google Scholar]
- 71.Baker KS, Chow E, Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone Marrow Transplant 2012;47:619–25. 10.1038/bmt.2011.118 [DOI] [PubMed] [Google Scholar]
- 72.Friedman DN, Hilden P, Moskowitz CS, et al. Cardiovascular risk factors in survivors of childhood hematopoietic cell transplantation treated with total body irradiation: a longitudinal analysis. Biol Blood Marrow Transplant 2017;23:475–82. 10.1016/j.bbmt.2016.12.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brouwer CAJ, Postma A, Hooimeijer HLH, et al. Endothelial damage in long-term survivors of childhood cancer. J Clin Oncol 2013;31:3906–13. 10.1200/JCO.2012.46.6086 [DOI] [PubMed] [Google Scholar]
- 74.Jehlicka P, Huml M, Votava T, et al. P8.05 reactive hyperemia index and detection of endothelial dysfunction in paediatric hemato/oncology patients. Artery Res 2011;5:184. 10.1016/j.artres.2011.10.127 [DOI] [Google Scholar]
- 75.Mavinkurve M, O'Gorman CS. Cardiometabolic and vascular risks in young and adolescent girls with Turner syndrome. BBA Clin 2015;3:304–9. 10.1016/j.bbacli.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'Gorman CS, Syme C, Bradley T, et al. Impaired endothelial function in pediatric patients with turner syndrome and healthy controls: a case-control study. Int J Pediatr Endocrinol 2012;2012:5. 10.1186/1687-9856-2012-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinto FF, Gomes I, Loureiro P, et al. Vascular function long term after Kawasaki disease: another piece of the puzzle? Cardiol Young 2017;27:488–97. 10.1017/S1047951116000780 [DOI] [PubMed] [Google Scholar]
- 78.Dietz SM, Tacke CEA, Hutten BA, et al. Peripheral endothelial (Dys) function, arterial stiffness and carotid intima-media thickness in patients after Kawasaki Disease: a systematic review and meta-analyses. PLoS One 2015;10:e0130913. 10.1371/journal.pone.0130913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goldstein BH, Marino BS, Gao Z, et al. Abstract 13486: patient and procedural factors are associated with autonomic and endothelial dysfunction in adolescent and young adult Fontan survivors. Circulation 2016;134:A13486. 10.1161/circ.134.suppl_1.13486 [DOI] [Google Scholar]
- 80.Martins JD, Zachariah J, Selamet Tierney ES, et al. Impact of Treatment Modality on Vascular Function in Coarctation of the Aorta: The LOVE - COARCT Study. J Am Heart Assoc 2019;8:e011536. 10.1161/JAHA.118.011536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldstein BH, Urbina EM, Khoury PR, et al. Abstract 13536: vascular function is predictive of functional outcomes in adolescent and young adult Fontan survivors. Circulation 2015;132. 10.1161/circ.132.suppl_3.13536 [DOI] [Google Scholar]
- 82.Harris SL, Bray H, Troughton R, et al. Cardiovascular outcomes in young adulthood in a population-based very low birth weight cohort. J Pediatr 2020;225:74–9. 10.1016/j.jpeds.2020.06.023 [DOI] [PubMed] [Google Scholar]
- 83.Sivamurthy KM, Dampier C, MacDermott M, et al. Peripheral arterial tonometry in assessing endothelial dysfunction in pediatric sickle cell disease. Pediatr Hematol Oncol 2009;26:589–96. 10.3109/08880010903271689 [DOI] [PubMed] [Google Scholar]
- 84.Bhangoo A, Sinha S, Rosenbaum M, et al. Endothelial function as measured by peripheral arterial tonometry increases during pubertal advancement. Horm Res Paediatr 2011;76:226–33. 10.1159/000328455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mueller UM, Walther C, Adam J, et al. Endothelial function in children and adolescents is mainly influenced by age, sex and physical activity - an analysis of Reactive hyperemic peripheral artery tonometry. Circ J 2017;81:717–25. 10.1253/circj.CJ-16-0994 [DOI] [PubMed] [Google Scholar]
- 86.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh R, Verma A, Aljabari S, et al. Urinary biomarkers as indicator of chronic inflammation and endothelial dysfunction in obese adolescents. BMC Obes 2017;4:11. 10.1186/s40608-017-0148-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bruyndonckx LMF, Verhulst S, De Guchtenaere A, et al. Prevalence of type D personality in obese adolescents and associated cardiovascular risk. Cardiol Young 2018;28. [Google Scholar]
- 89.Pareyn A, Allegaert K, Verhamme P, et al. Impaired endothelial function in adolescents with overweight or obesity measured by peripheral artery tonometry. Pediatr Diabetes 2015;16:98–103. 10.1111/pedi.12139 [DOI] [PubMed] [Google Scholar]
- 90.Deda L, Sochett EB, Mahmud FH. Physiological changes in blood pressure impact peripheral endothelial function during adolescence. Cardiol Young 2015;25:777–9. 10.1017/S1047951114001024 [DOI] [PubMed] [Google Scholar]
- 91.Goldstein BH, Urbina EM, Khoury PR, et al. Endothelial function and arterial stiffness relate to functional outcomes in adolescent and young adult Fontan survivors. J Am Heart Assoc 2016;5:e004258. 10.1161/JAHA.116.004258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goldstein BH, Gao Z, Khoury PR, et al. Natural history of vascular function in adolescent and young adult Fontan survivors: a longitudinal assessment of endothelial function and arterial stiffness. Circulation 2017;136. 10.1161/circ.136.suppl_1.17719 [DOI] [Google Scholar]
- 93.Urback ALM AWS, Islam AH, Korczak DJ, et al. Cerebrovascular reactivity is associated with peripheral endothelial function among adolescents. Biological Psychiatry 2016;79:112S. [Google Scholar]
- 94.Chen Y, Osika W, Dangardt F, et al. Impact of psychological health on peripheral endothelial function and the HPA-axis activity in healthy adolescents. Atherosclerosis 2017;261:131–7. 10.1016/j.atherosclerosis.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 95.Odanaka Y, Takitani K, Katayama H, et al. Microvascular endothelial function in Japanese early adolescents. J Clin Biochem Nutr 2017;61:228–32. 10.3164/jcbn.17-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wahezi DM, Liebling EJ, Choi J, et al. Assessment of traditional and non-traditional risk factors for premature atherosclerosis in children with juvenile dermatomyositis and pediatric controls. Pediatr Rheumatol Online J 2020;18:25. 10.1186/s12969-020-0415-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang WY, Selamet Tierney ES, Chen AC, et al. Vascular health of children conceived via In Vitro fertilization. J Pediatr 2019;214:47–53. 10.1016/j.jpeds.2019.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mahtab S, Zar HJ, Ntusi NAB, et al. Endothelial dysfunction in South African youth living with perinatally acquired human immunodeficiency virus on antiretroviral therapy. Clin Infect Dis 2020;71:e672–9. 10.1093/cid/ciaa396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bartz SKCMC, Krishnamurthy R, Bacha FF. Role of insulin resistance and hyperandrogenemia in early vascular dysfunction in adolescents with PCOS. Endocrine Reviews 2015;36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.