Abstract
Background
A prescribing cascade is the treatment of an adverse drug reaction (ADR) with another drug. In this review, we discuss (a) the different types of prescribing cascade and (b) the measures that can be taken so that they will be recognized and dealt with appropriately, both in the hospital and in the outpatient setting.
Method
This review is based on pertinent publications retrieved by a selective literature search.
Results
The literature distinguishes intentional from unintentional prescribing cascades, and appropriate from inappropriate ones. We further distinguish prophylactic from therapeutic prescribing cascades and draw a line between those that are necessary and those that are merely appropriate. The following main questions are essential for dealing with prescribing cascades appropriately: (1) Did the precipitating drug cause a clinically relevant ADR or risk of an ADR? (2) Is the precipitating drug still indicated? (3) Can an ADR be avoided by altering the treatment with the precipitating drug, or by (4) switching to another drug instead? (5) Can the drug used to treat the ADR actually affect it beneficially? (6) Do the benefits of the prescribing cascade outweigh its risks?
Conclusion
Prescribing cascades are not problematic in themselves; on the contrary, they are sometimes a necessary part of good prescribing practice. There is still a lack of practically implementable instruments to help physicians detect prescribing cascades reliably, assess them properly, and put them to appropriate use.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.arzteblatt.de. The deadline for submission is 03 November 2023.
The prescription of drugs is by far the most common medical intervention. However, in addition to positive effects, drugs also cause adverse drug reactions (ADRs). In Germany, they are responsible, at least in part, for approximately 6.5% of all emergency hospital admissions (1). The main risk factor for ADR-related hospitalizations is polypharmacy, that is, the use of five or more drugs (2, 3, e1). Polypharmacy affects approximately 20% of all individuals covered by statutory health insurance and around 40% of over 65-year-olds (e2, e3).
Polypharmacy and ADRs also harbor the risk of what is referred to as prescribing cascades. These occur when a prescribed drug (the precipitating drug) causes an ADR, for the treatment of which a second, subsequent drug is prescribed (4). In turn, the second drug can itself become a precipitating drug and lead to further ADRs. A typical example is the use of diuretics for the treatment of peripheral edema caused by dihydropyridine calcium channel blockers (such as amlodipine) (5). Diuretics, for their part, can interact with other active substances (6) or induce hypokalemia, which is then treated in some cases with a potassium-sparing diuretic. A US study based on data from 2014 estimates that one in 22 patients treated with dihydropyridine calcium channel blockers also received diuretics to treat peripheral edema (e4). In Canada in 2016, this figure was one in 71 patients in the first 90 days (5). Thus, it is possible that prescribing cascades are also an underestimated problem in Germany. The first prescribing cascades were described as early as around 20 years ago (4, 7). Since then, numerous further reports have followed (8– 12), including from Germany (13– 15).
The aim of this review article is to answer two questions:
Which types of prescribing cascades should be distinguished?
Which analysis strategies and instruments are helpful in recognizing and appropriately dealing with prescribing cascades in clinical practice?
Methods
We carried out a selective literature search with the search terms “prescribing cascade,” “medication cascade,” “(prescription) sequence symmetry analysis,” and “inappropriate prescribing” in Medline (last update, 13 February 2022) and, once publications had been narrowed down to those after 1990, obtained a total of 3990 hits (etable). In a first step, we compiled prescribing cascades described in the literature (review articles, commentaries, primary references). From these, and using an iterative approach, we developed a differentiated system to classify prescribing cascades as well as main questions on their management. To illustrate the subtypes of prescribing cascades, we selected what were in our view the most relevant examples for the outpatient treatment setting, conducting further targeted literature searches where necessary to explain these in more detail. Identified instruments that we considered significant in the recognition or prevention of prescribing cascades in both the inpatient and the outpatient setting were compiled in tabular form.
eTable. Instruments for the identification of potentially inappropriate medication.
| Name/last update | Characteristics, structure, and presentation | Comments | Evidence from interventional studies to improve drug therapy safety or clinical endpoints |
| STOPP/START England/Ireland 2015 (25) |
STOPP: Screening tool (80 PIM) by organ and functional system to identify potentially inappropriate medications START: Screening tool (34 recommendations) by organ and functional system to identify potentially necessary medications |
Provides the rationale for classification as STOPP and START criterion, complemented by information from NICE guidelines; in a future version, STOPP criteria relevant to falls (STOPP Fall) to be integrated. | Manual screening on hospital admission leads to a reduction in ADRs and length of hospital stay. Computer-generated alerts based on STOPP/START were not effective in the SENATOR trial (e19). |
| FORTA Germany 2022 (29) |
List of the most common pharmaceuticals in long-term use, presented according to areas of indication (e.g., CHD or oncological diseases/solid tumors) | Graded as positive/negative based on four classes (A–D); classes A and B identify potentially necessary medications. Classes C and D identify potentially inadequate medications. | A randomized trial in two German hospitals found significant improvements in adherence to FORTA recommendations through training and weekly meetings with the FORTA team (e16). |
| PRISCUS Germany 2010 (30) |
Negative list (83 PIMs), presented according to medication classes | Information on concerns, alternatives, and measures if use of drugs to be continued. The criteria updated in 2021 will be published shortly (e17). | A cluster-randomized trial (RIME) in 137 German primary care practices found no relevant reduction in PRISCUS-PIM prescriptions through one-off training for primary care physicians or practice teams (e18). |
| Beers USA 2019 (31) |
PIM list (individual medications in 35 drug groups), presented according to organ system and therapeutic category; important interactions with other drugs (n = 17) or underlying diseases or syndromes (n = 10); drugs that are problematic in kidney failure (n = 23); drugs with strong anticholinergic properties (n = 55) | Provides the rationale for classification as a PIM; quality of evidence and strength of recommendation | The D-Prescribe cluster randomized trial (e19) in 69 Canadian pharmacies foundsignificantly more frequent discontinuation of treatment with sedatives/hypnotics, sulfonylureas, and NSAIDs. |
| STOPP Fall EU/Finland 2021 (32) |
Screening tool to identify fall risk increasing drugs (FRIDs), i.e., medication classes that increase the risk of falls (14 medication classes) | Recommendations on the situations in which an attempt at discontinuation should be undertaken, how this should be done where necessary (e.g., tapering), as well as monitoring criteria after discontinuation | To date, there is no explicit evaluation of this tool. However, in a placebo-controlled trial, discontinuation of psychotropic drugs significantly reduced the risk of falls (e20). Nevertheless, according to a recent meta-analysis, the currently available evidence is insufficient to recommend discontinuation of FRIDs alone as a fall prevention strategy (e21). |
| STOPP Frail 2017 (33) |
PIM list (n = 27) to identify PIMs in older persons in whom: – Symptom control is prioritized over prevention or avoidance of disease progression – There is a low 1-year probability of survival – There is irreversible end-stage disease – There is severe functional or cognitive impairment or both categorized according to physiological system |
Rationale for categorization as PIM given | Interventional study pending |
| ACB Score Germany 2018 (34) |
Classification of medications available in Germany according to their anticholinergic strength: 29 drugs with strong, 18 withmoderate, and 104 with weak anticholinergic properties | General algorithm for the reduction of anticholinergic burden | In a patient-randomized US trial of 50 patients, a collaboration between physicians and pharmacists significantly reducedanticholinergic load (e23). |
Table modified from Moßhammer D, Haumann H, Mörike K, Joos S: Polypharmacy—an upward trend with unpredictable effects. Dtsch Arztebl Int 2016; 113: 627–33.
ACB, anticholinergic burden; FRIDs, fall risk increasing drugs; CHD, coronary heart disease; PIM, potentially inappropriate medication; ADR, adverse drug reaction
Results
Spectrum and classification of prescribing cascades
Previous definitions of prescribing cascades differentiated between intentional and unintentional as well as appropriate and inappropriate prescribing cascades (16, 17). We propose additional distinctions between prophylactic versus therapeutic prescribing cascades, and between necessary and merely appropriate prescribing cascades. We discuss the reasons for this in the following sections.
Intentional versus unintentional prescribing cascades
In the case of intentional prescribing cascades, an ADR is recognized and the second drug is intentionally used to treat this ADR. In unintentional prescribing cascades, on the other hand, the ADR is interpreted as a new medical condition and the second drug is prescribed without first considering the relevance of the precipitating drug (17).
Appropriate versus inappropriate prescribing cascades
A prescribing cascade is appropriate if the prescription of a precipitating drug and a second drug, when combined, has a positive benefit–risk balance. It is inappropriate if the benefit–risk balance is negative (17). A prescribing cascade is potentially inappropriate if theoretically more suitable treatment alternatives are available (for example, given that switching the precipitating drug could in principle prevent the ADR), but a patient-specific assessment of the benefit–risk balance is still pending.
Necessary versus appropriate prescribing cascades
This distinction is intended to emphasize the fact that prescribing cascades can be not only appropriate but also even necessary. Prescribing cascades are classified as appropriate if their benefit merely outweighs the risks. Prescribing cascades are necessary if the relative benefit is so great that non-prescription would be inconsistent with appropriate treatment (18). The classification into appropriate versus necessary prescribing cascades ultimately depends on to the extent to which their benefits outweigh their risks. However, this has practical implications. Whereas the non-use of necessary prescription cascades represents undertreatment (and thus their use should be actively recommended), this is not necessarily true for the non-use of merely appropriate prescribing cascades.
Prophylactic versus therapeutic prescribing cascades
Up until now, prescribing cascades have been seen primarily as a response to ADRs. However, second drugs can also be used preventively. For example, proton pump inhibitors (PPI) are prescribed to prevent gastrointestinal ADRs from non-steroidal anti-inflammatory drugs (NSAIDs). The term “prophylactic prescribing cascades” is intended to widen the spectrum of prescribing cascades and, for the first time, give a name to these often necessary prescribing practices.
Recognition and appropriate use of prescribing cascades in the hospital and outpatient setting
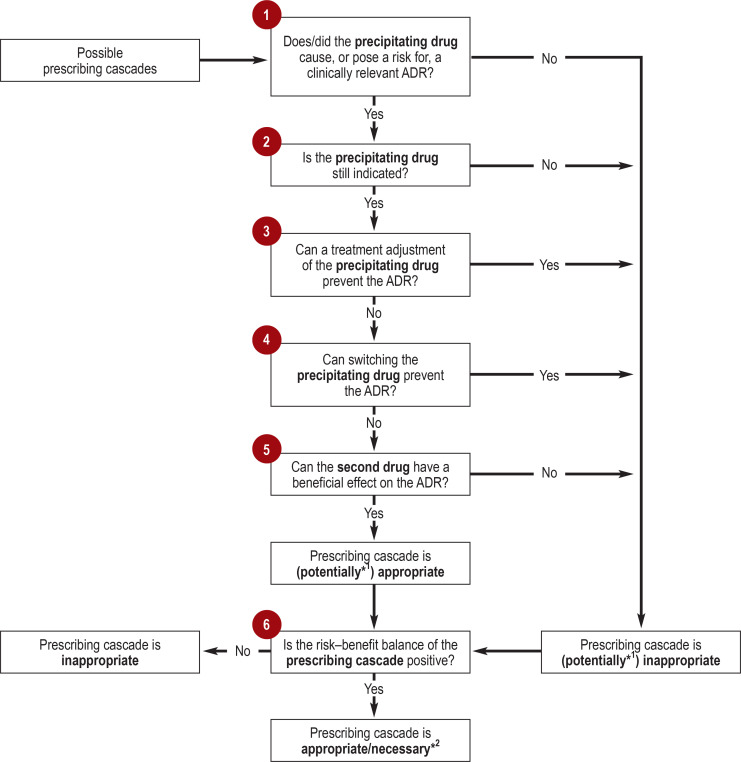
One can assume that many prescribing cascades have not yet been described and that many known per se remain unrecognized in everyday routine. The Figure shows six key questions that are relevant in the assessment of prescribing cascades. In the following sections, these questions will be discussed in more details with reference to this schematic representation. Table 1 lists illustrative examples of prescribing cascades.
Figure.
Six main questions for the assessment of prescribing cascades
*1 If questions 1–5 cannot be unequivocally answered, one is dealing with a potentially appropriate/inappropriate prescribing cascade.
*2 The distinction between an appropriate and necessary prescribing cascade depends on the extent to which the benefits outweigh the risks.
ADR, adverse drug reaction
Table 1. Illustrative examples of prescribing cascades.
| Types/examples of prescribing cascades | Explanatory notes | |
| 1. Less established or difficult to detect | Statins → myasthenia gravis → pyridostigmine (21) | Statins have repeatedly been linked to symptoms of myastheniagravis (21). |
| Various blood–brain barrier-crossing drugs → depression → antidepressants (24) | Blood–brain barrier-crossing drugs can modulate neurotransmitters, which can lead to depressive symptoms (e6). | |
| 2. Frequently necessary … | ||
| … for prevention | Opioids → high risk of constipation → laxatives (12, 25) | In chronic opioid use, laxatives should be prescribed on a regular basis (25, e8). |
| Platelet aggregation inhibitors → high risk ofgastrointestinal bleeding → PPI (26) | Prophylactic administration of PPI is usually appropriate in patients with additional risk factors (e10) | |
| Methotrexate → high risk of hepatotoxicity/gastrointestinal/ hematological complications → folic acid (27) | Folic acid effectively substitutes folic acid synthesis reduced bymethotrexate and lowers the risk of hepatotoxicity, hematotoxicity, gastric ulcers, and bleeding (e11, e12). | |
| … for treatment | Gabapentin → atrial fibrillation → betablocker/anticoagulant (16) | In atrial fibrillation of longer duration, treatment is generallynecessary irrespective of the cause (e13, e14). |
| Antibiotics/PPI → pseudomembranousenterocolitis → metronidazole/vancomycin (26) | All cases of pseudomembranous enterocolitis require treatment (e9); if PPI is indicated for prophylaxis: continuation at half the maximum therapeutic dose (25). | |
| AChEI → seizure → antiepileptic drugs (29) | In acute cases, seizures must be treated irrespective of their cause (e15). | |
| 3. Frequently preventable: precipitating medication is potentially inappropriate | NSAID → hypertension → antihypertensive drugs (8, 11, 12, 15) | Question NSAID therapy due to potentially severe ADRs andinteractions (e.g., with acetylsalicylic acid) (25, 29– 31). |
| Amitriptyline → dementia → antidementia drugs (11, 12, 15) | Use amitriptyline with caution in older patients (risk of falls) (25, 29– 32). | |
| Gabapentin → edema → diuretics (11, 12) | Use gabapentinoids with caution in older persons (tolerance,habituation, addiction potential, falls) (25, 29) | |
| 4. Frequently preventable: effective ADR prevention strategies | Gliflozin antidiabetic drugs (SGLT2 inhibitors) → genital infections → antifungal drugs, antibiotics (23) | Glucosuria promotes genital mycotic infections (fungi, bacteria), which are often multicausal. The risk of these ADRs can be reduced through intensified genital hygiene (e24). |
| AChEI → nausea/diarrhea → antiemetics/antidiarrheal drugs (35) | The risk of these ADRs can be reduced through gradual up-titration of the dose (e25). | |
| Steroid inhalers → oral thrush → antifungal drugs (36) | The risk of these ADRs can be reduced through oral hygiene and using spacers (e26). | |
| 5. Frequently preventable: safe treatment alternatives for precipitating drugs | Metoclopramide → extrapyramidal movementdisorders → anti-Parkinson’s drugs (8, 11, 12, 15) | Extrapyramidal movement disorders can be prevented by using the non-blood–brain barrier-crossing domperidone (30). |
| Antipsychotic drugs → extrapyramidal movement disorders → anti-Parkinson’s drugs (8, 11, 12, 15) | Consider dose reduction and antipsychotic drug switching: towards drugs with lower potential to cause Parkinson’s-like symptoms (e27). | |
| Antipsychotic drugs → metabolic syndrome → antidiabetic drugs (12) | Metabolic ADRs are are less severe with certain antipsychotic drugs (e.g., aripiprazole (e28). | |
| ACE inhibitors → cough → antitussive drugs (8, 11, 12, 15). | ARBs are mostly therapeutically equivalent to ACE inhibitors and rarely cause dry cough (e29). | |
| 6. Frequently preventable: unsuitable second drug | Dihydropyridine calcium channelblockers → edema → diuretics (11, 12, 15) | Diuretics are barely effective, but combination with ACE inhibitors or ARBs can reduce edema (37, e30, e31). |
| AChEI → incontinence → anticholinergics (8, 11, 12, 15, 35, e32) | The use of blood–brain barrier-crossing anticholinergics (e.g.,oxybutynin) antagonises the effects of AChEI (e33). | |
| Statins → myopathy → NSAIDs (e34) | Chronic NSAIDs increase the risk of gastrointestinal, renal, andcardiovascular events (e35). | |
| 7. Frequently preventable: often complex benefit–risk assessment | NSAIDs → gastrointestinal bleeding → PPI (12, 38) | Other analgesics often inadequately effective against joint or lower back pain (e36). |
Examples of prescribing cascades: precipitating drug → adverse drug reaction → second drug(s)
AChEI, acetylcholinesterase inhibitors; ARB, angiotensin receptor blocker; NSAIDs, non-steroidal anti-inflammatory drugs; PPI, proton pump inhibitors
Does/did the precipitating drug cause or pose a risk for a clinically relevant adverse drug reaction?
A prerequisite for avoiding prescription cascades is to identify a possible association between the precipitating drug and a presenting symptom or finding. The Naranjo score (19) can be helpful to this end. It consists of 10 questions, the answers to which are each assigned a score (table 2). A score of ≤ 0 suggests the absence of an ADR, and a score of > 4 its presence. No reliable statement can be made in the gray area between 1 and 4.
Table 2. Naranjo score for the identification of adverse drug reactions.
| Question (score depends on answer) | Patient history is usually sufficient to answer the question |
|
1. Is the adverse event (AE) a known adverse drug reaction (ADR)? (yes: +1; no: 0; unknown: 0) |
Yes |
|
2. Did the AE occur following administration of the suspected drug? (yes: +2; no: –1; unknown: 0) |
Yes |
|
3. Did the AE improve following discontinuation of the suspected precipitating drug or administration of a specific antagonist? (yes: +1; no: 0; unknown: 0) |
No |
|
4. Did the AE reoccur following resumption of the suspected precipitating drug? (yes: +2; no: –1; unknown: 0) |
No |
|
5. Are there alternative causes for the AE? (yes: –1; no: +2; unknown: 0) |
Yes |
|
6. In the case that a placebo was given: did the AE reoccur? (yes: –1; no: +1; unknown: 0) |
No |
|
7. Was there a toxic concentration in body fluids? (yes: +1; no: 0; unknown: 0) |
No |
|
8. Did a dose escalation exacerbate, or a dose reduction improve, symptoms? (yes: +1; no: 0; unknown: 0) |
No |
|
9. Have similar drugs caused the patient to experience similar AEs in the past? (yes: +1; no: 0; unknown: 0) |
Yes |
|
10. Can the AE be objectively confirmed? (yes: +1; no: 0; unknown: 0) |
Yes |
| Probability that the AE is an ADR … | Point score |
| … Definite | ≥ 9 |
| … Probable | 5–8 |
| … Possible | 1–4 |
| … Doubtful | ≤ 0 |
| Maximum achievable score, assuming that… | Point score |
| … all information is available | 13 |
| … only patient history is available | 6 |
| … only patient history is available and question 5 was answered with “yes,” question 7 with “unknown,” and question 9 with “no” or “unknown” | 3 |
Accordingly, the probability of an ADR depends, for example, on possible alternative causes, known similar reactions in the patient history, drug levels in the blood, as well as response to discontinuation, resumption, and dose changes of the suspected precipitating drug. However, in individuals with multimorbidity, additional causes are often present, only in exceptional cases are drug levels determined, and similar reactions to similar active substances are often not known. Therefore, determining whether the problem is an ADR often requires discontinuing the suspected precipitating drug or reducing the dose and monitoring the effects. This is particularly the case for adverse events that often have non-pharmacological causes, such as depression or dementia. Furthermore, a consultation with the patient in person is often required in order to obtain important contextual information, for example regarding temporal course and symptom severity (20). Table 1 shows examples of typical, albeit potentially less established or difficult-to-recognize prescribing cascades (21– 24, e6).
Treatment with a second drug should only be considered if an ADR is causing, or has the potential to cause, a relevant impairment to an individual. Otherwise, the prescribing cascade should be classified as at least potentially inappropriate. However, in the case of a severe ADR (e7), or if there is a high risk for one, treatment with second drugs is often necessary. An example of an unequivocally necessary prophylactic prescribing cascade is the prescription of laxatives to prevent constipation due to opioid treatment (12, 25, e8). An example of an unequivocally necessary therapeutic prescribing cascade is the prescription of metronidazole or oral vancomycin for pseudomembranous enterocolitis (26, e9), which can be induced by, for example, broad-spectrum antibiotics. Further examples can be found in Table 1 (12, 15, 25– 28, e10– e15).
Is the precipitating drug still indicated?
Some precipitating drugs are unquestionably indicated, such as analgesics for cancer pain or diuretics in advanced heart failure. Other precipitating drugs should be classified as potentially inappropriate medications (PIMs) since they often have an unfavorable benefit–risk balance in older patients. In the last 10 years, a number of lists identifying missing drugs and PIMs have been created to assist clinicians in their evaluation of the indication for the precipitating drug. A selection and description of these lists can be found in the eTable 3 (25, 29– 34, e16– e22). PIMs primarily include certain psychotropic drugs (such as benzodiazepines due to the increased risk of falls, and tricyclic antidepressants due to their anticholinergic side effects). However, PIMs also include other drugs with sedative and/or anticholinergic effects, substances that can cause orthostatic dysregulation or movement disorders, as well as NSAIDs. Examples of prescribing cascades in which the precipitating drug is a PIM can be found in Table 1 (8, 11, 12, 15, 25, 29– 32).
Can a treatment adjustment of the precipitating drug prevent adverse drug reactions?
If it is not possible to avoid a precipitating drug, one should determine in a first step whether, if necessary, ADRs can be prevented or mitigated by reducing the dose or changing the mode of use. An example would be the use of corticosteroid inhalersbefore food, rinsing out the oral cavity after use, or using spacers to prevent oral thrush. Further examples can be found in Table 1 (23, 35, 36, e24– e26).
Can switching the precipitating drug prevent adverse drug reactions?
In some cases, ADRs can be resolved by switching the precipitating drug for similarly effective active substances with the same indication. Examples include the use of domperidone instead of metoclopramide to prevent extrapyramidal movement disorders (11, 12, 15, 30) and switching ACE inhibitors for angiotensin receptor blockers to prevent dry cough (8, 11, 12, 15, e29).
Can the second drug have a beneficial effect on adverse drug reactions?
If the use of a precipitating drug is essential and ADRs cannot be avoided or the risk of serious ADRs is high, the last resort is permanent treatment or prophylaxis, where appropriate. However, there are also non-pharmacological measures to be considered. For example, lifestyle interventions may be introduced to avoid the use of antidiabetic drugs while taking antipsychotics (e28). Added to this is the fact that not all symptoms can be effectively treated with drugs. For example, diuretics are not suitable for the treatment of peripheral edema caused by dihydropyridine-type calcium channel blockers (5, e30, e31). Further examples can be found in Table 1 (8, 11, 12, 15, 36, e30– e35).
If the prescription of effective second drugs is indicated to treat acute but transient symptoms (for example, antiemetics at the beginning of opioid treatment), care must be taken to ensure that these drugs are also discontinued when the precipitating drug is discontinued. However, in some cases, longer-term administration of a second drug is necessary, such as PPI for the prevention of gastrointestinal complications in individuals with gastrointestinal risk profiles who require permanent antiplatelet therapy. Here, the minimum effective dose should be prescribed (for PPIs, the semi-therapeutic dose) to minimize ADRs from the second drug (such as the development of osteoporosis) (25).
Is the benefit–risk balance of the prescribing cascade positive?
It is often not possible in clinical practice to unequivocally answer main questions 1–5 (figure). For example, the distinction between pharmacological/non-pharmacological causes of an adverse event is not always straightforward (see main question 1). Therefore, whether in such cases a prescription cascade is to be classified as appropriate/necessary or inappropriate requires a patient-specific consideration of the benefits and risks.
The individual benefit–risk balance depends not only on the indication and evidence of effectiveness but also on patient-specific factors such as age, comorbidity, life expectancy, and personal preferences. An example here would be prescribing cascades caused by NSAIDs (8, 11, 12, 15, 38). Despite the risks associated with the use of NSAIDs, many patients with osteoarthritis or lower back pain complain of the inadequate efficacy of alternative analgesics (e34). In a physician–patient discussion, one must then jointly consider whether these drugs and the ADRs, or risk thereof, caused by second drugs (PPI, antihypertensive drugs) can and should be accepted in order to maintain quality of life. Although prescription cascades precipitated by NSAIDs (and other PIMs) should therefore be classified as potentially inappropriate, they may nevertheless be appropriate or even necessary in light of a patient-specific benefit–risk assessment.
Approaches to identifying new prescribing cascades
The prevention and detection of prescribing cascades can be supported by systematically identifying and reliably communicating new prescribing cascades that are relevant in practice. Three methods have been used for this to date (9): case reports, retrospective observational studies based on administrative prescription databases, and an approach that uses social media.
Case reports
Case reports are spontaneously published by interested clinicians. Case reports have the advantage that a causal relationship between precipitating drug, ADR, and second drug can be established with a high degree of probability by intensively studying clinical circumstances and alternative causes in the individual case. However a disadvantage is that their publication is voluntary. Case reports can therefore only yield an incomplete picture of new prescribing cascades and do not allow any statement regarding their incidence.
Retrospective observational studies in administrative prescription databases
Compared to case reports, retrospective observational studies based on administrative prescription data represent a systematic approach to identifying new prescribing cascades. In addition to traditional cohorts and case control studies, more and more so-called prescription symmetry sequence analyses (PSSA) have been carried out in the last 10 years (10). The principle of PSSA is based on testing the hypothesis that in a given study population, the new prescription of a precipitating drug followed by a second drug (for example, acetylcholinesterase inhibitors → antiepileptic drugs [29]) occurs more frequently in this order than in the reverse order (for example, antiepileptic drugs → acetylcholinesterase inhibitors). Using PSSA, signals of possible prescribing cascades can be efficiently generated. However, since the administrative data sources used to this end typically contain only limited information on other confounding factors, there is a high risk of incorrect signals. Therefore, further studies are often needed to verify prescribing cascades detected in this way.
Social media data
In an original and novel approach, Twitter and other Internet platforms were used to generate signals of new prescribing cascades (39). This data pool also contains reports on experiences with non-prescription drugs that are typically not included in administrative databases. In addition, signals can be generated more quickly than in administrative databases. In a feasibility study, this data mining concept was able, firstly, to detect two known prescribing cascades: NSAID → hypertension → antihypertensive drugs and ACE inhibitors → dry cough → antitussive agents; and secondly, it was able to identify previously unknown prescribing cascades that seemed plausible to the authors since they confirmed previously identified associations between precipitating drugs and ADRs, for example, trazodone → hypertension → prazosin.
Conclusions
Implications for clinical practice
Numerous prescribing cascades have already been described in the literature. A differentiated consideration of these shows, on the one hand, that unintentional and avoidable prescription cascades must be prevented more effectively in order to reduce unnecessary polypharmacy and its associated risks. On the other hand, prescribing cascades may be part of good prescribing practice and necessary for a positive benefit–risk balance in the overall treatment approach. One can also assume that many prescribing cascades have yet to be detected and that with the use of novel drugs, new ADR profiles will emerge (for example, checkpoint inhibitors) that lead to new prescribing cascades.
Implications for research
It has been shown that it is important to further develop current approaches for the systematic identification of previously undetected prescribing cascades and enable a better distinction between clinically relevant prescription cascades and spurious signals in which the prescription of a second drug has no causal relationship to the prescription of the precipitating drug. A systematic review compiles currently known prescribing cascades (40).
The extended classification system for prescribing cascades proposed here can provide a theoretical framework to classify the identified prescribing cascades into appropriate, necessary, and potentially inappropriate prescribing cascades. This can be used to develop practically implementable, potentially electronic instruments, aiming to alert physicians to both potentially inappropriate and potentially omitted prescribing cascades.
Questions on the article in issue 44/2022:
Prescribing Cascades: How to Detect Them, Prevent Them, and Use Them Appropriately
The submission deadline is 3 November 2023. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
If a patient is taking multiple medications, from what number of medications does one refer to this as polypharmacy?
Three
Five
Seven
Ten
Twelve
Question 2
Approximately what percentage of individuals covered by statutory health insurance are affected by polypharmacy?
5%
10%
20%
40%
80%
Question 3
Which prescribing cascade is often necessary and recommended for prevention?
NSAID – antihypertensive drugs
Gabapentin – diuretics
Amitriptyline – antidementia drugs
Opioids – laxatives
NSAID – PPI
Question 4
Which instrument can be used to identify adverse drug reactions?
VAS
Naranjo score
MAI score
PRISCUS list
PASI score
Question 5
Which drugs have been repeatedly linked to symptoms of myasthenia gravis?
Statins
Opioids
Antibiotics
Antidiabetic drugs
NSAIDs
Question 6
How should the risk of oral thrush be prevented when using steroid inhalers?
Through concomitant administration of an antifungal agent
Through prior administration of an antifungal agent
Through a weekly switch of the type of steroid
Through mouth hygiene and the use of spacers
Through the consumption of sugary foods
Question 7
What does the abbreviation PIM stand for in the text?
Potentially irritating medication
Potentially inappropriate medication
Primary interacting medication
Prevention of inappropriate medication
Post-interventional medication
Question 8
ACE inhibitors sometimes need to be switched for other medications to prevent dry cough. Which alternative is mentioned in the text?
Beta-blockers
Calcium channel blockers
Statins
Paracodin
Angiotensin-receptor blockers
Question 9
Which of the following combinations represents a typical prescribing cascade?
Antipsychotic drugs – antidiabetic drugs
ACE inhibitors – antifungal agents
Amitriptyline – antiepileptic drugs
NSAIDs – antiemetic drugs
Statins – antibiotics
Question 10
PPIs are often used long term to prevent gastrointestinal complications due to side effects of antiplatelet drugs. Which dose should be used if possible?
The maximum approved dose
Twice the therapeutic dose
Half the therapeutic dose
The therapeutic dose
Three times the therapeutic dose
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interest statement
Prof. Dreischulte received funding from the Techniker Krankenkasse to compile information materials on drug therapy. He received research funds from the German Research Foundation, the German Ministry of Education and Research (BMBF), and the German Innovation Fund. He received fees for expert opinions from the German Innovation Fund.
Prof. Muth receives royalties as co-editor of the book „Praxishandbuch Multimorbidität.“ She received funding from the BMBF and the German Innovation Fund for a research project of her own initiation.
Prof. Haefeli received financial support from ABF Pharmaceutical Services, Amgen GmbH, Acerta Pharma, Bayer AG, BioNTech AG, Bristol-Myers Squibb, Celgene GmbH, Genentech Inc, Gilead Sciences, GlaxoSmithKline, Heidelberg; ImmunoTherapeutics (HDIT), Immunocore Ltd, INCYTE Inc, Janssen Germany, Molecular Partners, MorphoSys AG, MYR GmbH, Neurimmune AG, Novartis AG, Roche, AG, Sumaya Biotech GmbH, Vaccibody A.S., and 4SC AG. He received fees for consulting services from Dr. Wilmar Schwabe, Chiesi GmbH, and Daiichi Sankyo.
Prof. Schmidl receives consultancy fees from Biocon Limited, Bangalore, India. He received funding for the preparation of continuing medical education events from Daiichi Sankyo.
Faiza Shahid declares that no conflict of interest exists.
References
- 1.Schurig AM, Böhme M, Just KS, et al. Adverse drug reactions (ADR) and emergencies—the prevalence of suspected ADR in four emergency departments in Germany. Dtsch Arztebl Int. 2018;115:251–258. doi: 10.3238/arztebl.2018.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumbreck S, Flynn A, Nairn M, et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015;350 doi: 10.1136/bmj.h949. h949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Medicine. 2015;13 doi: 10.1186/s12916-015-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096–1099. doi: 10.1136/bmj.315.7115.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage RD, Visentin JD, Bronskill SE, et al. Evaluation of a common prescribing cascade of calcium channel blockers and diuretics in older adults with hypertension. JAMA Intern Med. 2020;180:643–651. doi: 10.1001/jamainternmed.2019.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreischulte T, Morales DR, Bell S, Guthrie B. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int. 2015;88:396–403. doi: 10.1038/ki.2015.101. [DOI] [PubMed] [Google Scholar]
- 7.Rochon PA, Gurwitz JH. Drug therapy. Lancet. 1995;346:32–36. doi: 10.1016/s0140-6736(95)92656-9. [DOI] [PubMed] [Google Scholar]
- 8.Kalisch LM, Caughey GE, Roughead EE, Gilbert AL. The prescribing cascade. Aust Prescriber. 2011;34:162–166. [Google Scholar]
- 9.Brath H, Mehta N, Savage RD, et al. What is known about preventing, detecting, and reversing prescribing cascades: a scoping review. J Am Geriatr Soc. 2018;66:2079–2085. doi: 10.1111/jgs.15543. [DOI] [PubMed] [Google Scholar]
- 10.Morris EJ, Hollmann J, Hofer AK, et al. Evaluating the use of prescription sequence symmetry analysis as a pharmacovigilance tool: a scoping review. Res Social Adm Pharm. 2022;18:3079–3093. doi: 10.1016/j.sapharm.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Rochon PA, Gurwitz JH. The prescribing cascade revisited. Lancet. 2017;389:1778–1780. doi: 10.1016/S0140-6736(17)31188-1. [DOI] [PubMed] [Google Scholar]
- 12.Kwan D, Farrell B. Polypharmacy: Optimizing medication use in elderly patients. CGS Journal of CME. 2014;4:21–27. [Google Scholar]
- 13.Leitliniengruppe Hessen. Hausärztliche Leitlinie Multimedikation 2021. Version 2.00 vom 05.05.2021. AWMF. online. www.awmf.org/uploads/tx_szleitlinien/053-043l_S3_Multimedikation_2021-08.pdf (last accessed on 23 April 2022) [Google Scholar]
- 14.Moßhammer D, Haumann H, Mörike K, Joos S. Polypharmacy—an upward trend with unpredictable effects. Dtsch Arztebl Int. 2016;113:627–633. doi: 10.3238/arztebl.2016.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heppner HJ. Aus eins mach vier Verordnungskaskaden aufdecken. MMW Fortschr Med. 2019;161:20–22. doi: 10.1007/s15006-019-1218-9. [DOI] [PubMed] [Google Scholar]
- 16.Ponte ML, Wachs L, Wachs A, Serra HA. Prescribing cascade A proposed new way to evaluate it. Medicina. 2017;77:13–16. [PubMed] [Google Scholar]
- 17.McCarthy LM, Visentin JD, Rochon PA. Assessing the scope and appropriateness of prescribing cascades. J Am Geriatr Soc. 2019;67:1023–1026. doi: 10.1111/jgs.15800. [DOI] [PubMed] [Google Scholar]
- 18.Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA appropriateness method user‘s manual Santa Monica, CA: RAND Corporation; www.rand.org/content/dam/rand/pubs/monograph_reports/2011/MR1269.pdf (last accessed on 23 April 2022) 2001 [Google Scholar]
- 19.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 20.Patel I, Trinh S, Phan T, Johnson M. Prescription cascading in developmentally disabled individuals. Indian J Pharmacol. 2016;48:334–335. doi: 10.4103/0253-7613.182893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalid R, Ibad A, Thompson PD. Statins and myasthenia gravis. Muscle Nerve. 2016;54 doi: 10.1002/mus.25155. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Choi E, Park E, et al. Risk of genital and urinary tract infections associated with SGLT-2 inhibitors as an add-on therapy to metformin in patients with type 2 diabetes mellitus: a retrospective cohort study in Korea. Pharmacol Res Perspect. 2022;10 doi: 10.1002/prp2.910. e00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adimadhyam S, Schumock GT, Calip GS, Smith Marsh DE, Layden BT, Lee TA. Increased risk of mycotic infections associated with sodium-glucose co-transporter 2 inhibitors: a prescription sequence symmetry analysis. Br J Clin Pharmacol. 2019;85:160–168. doi: 10.1111/bcp.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt-Christensen M, Kvist K, Nilsson FM, Andersen PK, Kessing LV. Treatment with antiparkinson and antidepressant drugs: a register-based, pharmaco-epidemiological study. Mov Disord. 2007;2:2037–2042. doi: 10.1002/mds.21472. [DOI] [PubMed] [Google Scholar]
- 25.O‘Mahony D, O‘Sullivan D, Byrne S, O‘Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age ageing. 2014;44:213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roughead EE, Chan EW, Choi NK, et al. Proton pump inhibitors and risk of clostridium difficile infection: a multi-country study using sequence symmetry analysis. Expert Opin Drug Saf. 2016;15:1589–1595. doi: 10.1080/14740338.2016.1238071. [DOI] [PubMed] [Google Scholar]
- 27.Hachiken H, Murai A, Wada K, Kuwahara T, Hosomi K, Takada M. Difference between the frequencies of antisecretory drug prescriptions in users of buffered vs enteric-coated low-dose aspirin therapies. Int J Clin Pharmacol Ther. 2013;51:807–815. doi: 10.5414/CP201914. [DOI] [PubMed] [Google Scholar]
- 28.Venäläinen O, Bell JS, Kirkpatrick CM, Nishtala PS, Liew D, Ilomäki J. Adverse drug reactions associated with cholinesterase inhibitors-sequence symmetry analyses using prescription claims data. J Am Med Dir Assoc. 2017;18:186–189. doi: 10.1016/j.jamda.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Pazan F, Weiss C, Wehling M. Forta: The FORTA (Fit fOR The Aged) List 2021: Fourth version of a validated clinical aid for improved pharmacotherapy in older adults. Drugs Aging. 2022;39:245–247. doi: 10.1007/s40266-022-00922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: The PRISCUS List. Dtsch Arztebl Int. 2010;107:543–551. doi: 10.3238/arztebl.2010.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American geriatrics society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 32.Seppala LJ, Petrovic M, Ryg J, et al. STOPPFall (screening tool of older persons prescriptions in older adults with high fall risk): a Delphi study by the EuGMS task force and finish group on fall-risk-increasing drugs. Age ageing. 2020;50:1189–1199. doi: 10.1093/ageing/afaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtin D, Gallagher P, O‘Mahony D. Deprescribing in older people approaching end-of-life: development and validation of STOPPFrail version 2. Age Ageing. 2021;50:465–471. doi: 10.1093/ageing/afaa159. [DOI] [PubMed] [Google Scholar]
- 34.Kiesel EK, Hopf YM, Drey M. An anticholinergic burden score for German prescribers: score development. BMC Geriatr. 2018;18 doi: 10.1186/s12877-018-0929-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venäläinen O, Bell JS, Kirkpatrick CM, Nishtala PS, Liew D, Ilomäki J. Adverse drug reactions associated with cholinesterase inhibitors-sequence symmetry analyses using prescription claims data. J Am Med Dir Assoc. 2017;18:186–189. doi: 10.1016/j.jamda.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Henriksen DP, Davidsen JR, Christiansen A, Laursen CB, Damkier P, Hallas J. Inhaled corticosteroids and systemic or topical antifungal therapy: a symmetry analysis. Ann Am Thorac Soc. 2017;14:1045–1047. doi: 10.1513/AnnalsATS.201612-1043LE. [DOI] [PubMed] [Google Scholar]
- 37.Woodford HJ. Calcium channel blockers co-prescribed with loop diuretics: a potential marker of poor prescribing? Drugs Aging. 2020;37:77–81. doi: 10.1007/s40266-019-00730-4. [DOI] [PubMed] [Google Scholar]
- 38.Bytzer P, Hallas J. Drug-induced symptoms of functional dyspepsia and nausea A symmetry analysis of one million prescriptions. Aliment Pharmacol Ther. 2000;14:1479–1484. doi: 10.1046/j.1365-2036.2000.00862.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoang T, Liu J, Pratt N, et al. Detecting signals of detrimental prescribing cascades from social media. Artif Intell Med. 2016;71:43–56. doi: 10.1016/j.artmed.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Doherty A, Moriarty F, Boland F, et al. Prescribing cascades in community-dwelling adults: protocol for a systematic review. HRB Open Res. 2021;4 doi: 10.12688/hrbopenres.13345.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Duerden MAA, Payne R. Polypharmacy and medicines optimisation making it safe and sound. The Kings Fund 2013. wwwkingsfundorguk/sites/default/files/field/field_publication_file/polypharmacy-and-medicines-optimisation-kingsfund-nov13pdf. (last accessed on 23 April 2022) 2018 [Google Scholar]
- E2.Grandt DLV, Schubert I. Arzneimittelreport 2018 Schriftenreihe zur Gesundheitsanalyse. Barmer Ersatzkasse. www.barmer.de/blob/159166/b9999fb6ca0a7b98f523c70dbc29c251/data/dl-report-komplett.pdf Last accessed on 12 October 2022) [Google Scholar]
- E3.Güster C, Klose J. Versorgungs-Report 2012. Schwerpunkt: Gesundheit im Alter. In: Schmacke N, editor. Schattauer. Stuttgart: 2012. pp. 111–130. [Google Scholar]
- E4.Vouri SM, van Tuyl JS, Olsen MA, Xian H, Schootman M. An evaluation of a potential calcium channel blocker-lower-extremity edema-loop diuretic prescribing cascade. J Am Pharm Assoc. 2018;58:534–539e4. doi: 10.1016/j.japh.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Read SH, Giannakeas V, Pop P, et al. Evidence of a gabapentinoid and diuretic prescribing cascade among older adults with lower back pain. J Am Geriatr Soc. 2021;69:2842–2850. doi: 10.1111/jgs.17312. [DOI] [PubMed] [Google Scholar]
- E6.Dagytė G, Den Boer JA, Trentani A. The cholinergic system and depression. Behav Brain Res. 2011;221:574–582. doi: 10.1016/j.bbr.2010.02.023. [DOI] [PubMed] [Google Scholar]
- E7.European Medicines Agency (EMA) Clinical safety data management: Definitions and standards for expedited reporting. www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-15.pdf (last accessed on 23 April 2022) [Google Scholar]
- E8.Saha S, Nathani P, Gupta A. Preventing opioid-induced constipation: A teachable moment. JAMA Intern Med. 2020;180:1371–1372. doi: 10.1001/jamainternmed.2020.3285. [DOI] [PubMed] [Google Scholar]
- E9.Lübbert C, John E, von Müller L. Clostridium difficile infection—guideline-based diagnosis and treatment. Dtsch Arztebl Int. 2014;111:723–731. doi: 10.3238/arztebl.2014.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Burkhardt R. CME Fortbildung Pharmakotherapie (Aktualisierte Version 2020): Protonenpumpenhemmer für alle Fälle. www.cme.medlearning.de/medlearning/protonenpumpenhemmer_rez2/pdf/cme.pdf (last accessed on 23 April 2022) [Google Scholar]
- E11.Liu L, Liu S, Wang C, et al. Folate supplementation for methotrexate therapy in patients with rheumatoid arthritis: a systematic review. J Clin Rheumatol. 2019;25:197–202. doi: 10.1097/RHU.0000000000000810. [DOI] [PubMed] [Google Scholar]
- E12.Shea B, Swinden MV, Ghogomu ET, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Journal Rheumatol. 2014;41:1049–1060. doi: 10.3899/jrheum.130738. [DOI] [PubMed] [Google Scholar]
- E13.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2020;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- E14.Tisdale JE, Chung MK, Campbell KB, et al. Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214–e233. doi: 10.1161/CIR.0000000000000905. [DOI] [PubMed] [Google Scholar]
- E15.Chen HY, Albertson TE, Olson KR. Treatment of drug-induced seizures. Br J Clin Pharmacol. 2016;81:412–419. doi: 10.1111/bcp.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Wehling M, Burkhardt H, Kuhn-Thiel A, et al. VALFORTA: a randomised trial to validate the FORTA (Fit fOR The Aged) classification. Age Ageing. 2016;45:262–267. doi: 10.1093/ageing/afv200. [DOI] [PubMed] [Google Scholar]
- E17.Mann NK, Mathes T, Sönnichsen A, Niepraschk-von Dollen K, Pieper D, Thürman PA. www.priscus2-0.de/publikationen.html (last accessed on 23 April 2022) Bonn, Germany: PRISCUS 2.0 - An update and expansion of the first German list of potentially inappropriate medications. 26th annual meeting of the German drug utilisation research group (GAA); 2019 Nov 21-22. [Google Scholar]
- E18.Rudolf H, Thiem U, Aust K, et al. Reduction of potentially inappropriate medication in the elderly—results of a cluster-randomized, controlled trial in German primary care practices (RIME) Dtsch Arztebl Int. 2021;118:875–882. doi: 10.3238/arztebl.m2021.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E19.Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: The D-PRESCRIBE randomized clinical trial. JAMA. 2018;320:1889–1898. doi: 10.1001/jama.2018.16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.O‘Mahony D, Gudmundsson A, Soiza RL, et al. Prevention of adverse drug reactions in hospitalized older patients with multi-morbidity and polypharmacy: the SENATOR* randomized controlled clinical trial. Age ageing. 2020;49:605–614. doi: 10.1093/ageing/afaa072. [DOI] [PubMed] [Google Scholar]
- E21.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home-based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47:850–853. doi: 10.1111/j.1532-5415.1999.tb03843.x. [DOI] [PubMed] [Google Scholar]
- E22.Lee J, Negm A, Peters R, Wong EKC, Holbrook A. Deprescribing fall-risk increasing drugs (FRIDs) for the prevention of falls and fall-related complications: a systematic review and meta-analysis. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2019-035978. e035978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E23.Moga DC, Abner EL, Rigsby DN, et al. Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden. Alzheimers Res Ther. 2017;9 doi: 10.1186/s13195-017-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Engelhardt K, Ferguson M, Rosselli JL. Prevention and management of genital mycotic infections in the setting of sodium-glucose cotransporter 2 Inhibitors. Ann Pharmacother. 2021;55:543–548. doi: 10.1177/1060028020951928. [DOI] [PubMed] [Google Scholar]
- E25.Ruangritchankul S, Chantharit P, Srisuma S, Gray LC. Adverse drug reactions of acetylcholinesterase inhibitors in older people living with dementia: a comprehensive literature review. Ther Clin Risk Manag. 2021;17:927–949. doi: 10.2147/TCRM.S323387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E26.Gani F, Caminati M, Bellavia F, et al. Oral health in asthmatic patients: a review: Asthma and its therapy may impact on oral health. Clin Mol Allergy. 2020;18 doi: 10.1186/s12948-020-00137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs tardive dyskinesia-key differences in pathophysiology and clinical management. Neurol Ther. 2018;7:233–248. doi: 10.1007/s40120-018-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E28.Ijaz S, Bolea B, Davies S, et al. Antipsychotic polypharmacy and metabolic syndrome in schizophrenia: a review of systematic reviews. BMC Psychiatry. 2018;18 doi: 10.1186/s12888-018-1848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.Pinto B, Jadhav U, Singhai P, Sadhanandham S, Shah N. ACEI-induced cough: a review of current evidence and its practical implications for optimal CV risk reduction. Indian Heart J. 2020;72:345–350. doi: 10.1016/j.ihj.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E30.Fogari R, Zoppi A, Derosa G, et al. Effect of valsartan addition to amlodipine on ankle oedema and subcutaneous tissue pressure in hypertensive patients. J Hum Hypertens. 2007;21:220–224. doi: 10.1038/sj.jhh.1002140. [DOI] [PubMed] [Google Scholar]
- E31.Weir MR, Rosenberger C, Fink JC. Pilot study to evaluate a water displacement technique to compare effects of diuretics and ACE inhibitors to alleviate lower extremity edema due to dihydropyridine calcium antagonists. Am J Hypertens. 2001;14:963–968. doi: 10.1016/s0895-7061(01)02167-7. [DOI] [PubMed] [Google Scholar]
- E32.Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165:808–813. doi: 10.1001/archinte.165.7.808. [DOI] [PubMed] [Google Scholar]
- E33.Welk B, Richardson K, Panicker JN. The cognitive effect of anticholinergics for patients with overactive bladder. Nat Rev Urol. 2021;18:686–700. doi: 10.1038/s41585-021-00504-x. [DOI] [PubMed] [Google Scholar]
- E34.Silwer L, Petzold M, Hallas J, Lundborg CS. Statins and nonsteroidal anti-inflammatory drugs-an analysis of prescription symmetry. Pharmacoepidemiol Drug Saf. 2006;15:510–511. doi: 10.1002/pds.1250. [DOI] [PubMed] [Google Scholar]
- E35.Varga Z, Sabzwari SRA, Vargova V. Cardiovascular risk of nonsteroidal anti-inflammatory drugs: an under-recognized public health issue. Cureus. 2017;9 doi: 10.7759/cureus.1144. e1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E36.Chenot JF, Greitemann B, Kladny B, Petzke F, Pfingsten M, Schorr SG. Clinical practice guideline: Non-specific low back pain. Dtsch Arztebl Int. 2017;114:883–890. doi: 10.3238/arztebl.2017.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]