Abstract
Background
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality in patients with lung cancer. Systemic therapies, such as chemotherapy (chemo), are associated with increased risk of VTE. Immune checkpoint inhibitors (ICIs) are a new standard of care for the treatment of lung cancer, but their association with VTE is not fully understood. We evaluated the incidence of VTE and risk factors for patients with advanced non-small cell lung cancer (aNSCLC) treated with first-line ICI-based, chemo-based, or ICI+chemo regimens.
Methods
This retrospective cohort study used HealthCore Integrated Research Environment - Oncology data, an integrated database of administrative claims, coupled with clinical data from a cancer-care quality program. Patients with first-line treatment of stage IV non-small cell lung cancer from July 2014 to August 2020 were grouped based on three treatment types: ICI-based, chemo-based, or ICI+chemo. Patients with VTE before initiation of systemic treatment were excluded. Newly diagnosed VTE events were identified via inpatient and outpatient diagnosis codes. Cox proportional hazards models were used to investigate the factors associated with VTE risk.
Results
Among 2299 eligible patients (ICI-based, n=605; chemo-based, n=1092; ICI+chemo, n=602) with a median follow-up of 9.1 months, the VTE incidence rates (95% CI) per 100 person-years were 17.8 (95% CI 16.0 to 19.5) overall, 13.5 (95% CI 10.6 to 16.5) for ICI-based, 18.0 (95% CI 15.5 to 20.5) for chemo-based, and 22.4 (95% CI 20.2 to 24.5) for ICI+chemo. The 6-month cumulative incidence of VTE was 8.1% for ICI-based, 10.9% for chemo-based, and 12.8% for ICI+chemo. Pulmonary embolism was most common, accounting for 63% of the VTE events. After controlling for baseline patient characteristics, the risk of VTE was 26% lower for ICI-based regimens than for chemo-based regimens (HR 0.74, p=0.03). There was no meaningful difference in the risk between ICI+chemo and chemo-based regimens (HR 1.12, p=0.36). Previous radiation and severe obesity (body mass index ≥40) were associated with VTE.
Conclusions
VTE incidence rate per 100 person-years was common across regimens in patients with aNSCLC, but numerically lower for patients receiving ICI-based regimens compared with those receiving chemo-based and ICI+chemo regimens. VTE is a common complication of lung cancer, and there is a continued need for awareness of VTE as a comorbidity in this population.
Keywords: Immunotherapy, Lung Neoplasms, Programmed Cell Death 1 Receptor
WHAT IS ALREADY KNOWN ON THIS TOPIC
The risk of venous thromboembolism (VTE) development in patients with cancer—particularly those treated with systemic therapies and especially those with lung cancer—is well established. The evolution of the cancer treatment landscape to include newer, novel immunotherapies presents the need to evaluate the VTE risk associated with these therapies as well. To date, the risk of VTE and the contributory risk factors among patients treated with immunotherapy have not been adequately characterized.
WHAT THIS STUDY ADDS
We evaluated risk and assessed risk factors in patients with lung cancer who were treated with either chemotherapy-based treatment, immunotherapy-based treatment, or combination treatment. In this population of patients with lung cancer, the risk of developing a VTE was consistent with the range of risk previously reported in the literature. We also found that the risk of developing a VTE was numerically lower in patients treated with immunotherapy-based regimens compared with chemotherapy-based or immunotherapy plus chemotherapy combination regimens.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
With growing evidence to support the persistent risk of VTE, even in patients treated with immunotherapy, it is important to remain vigilant about the risk assessment and prophylactic measures in patients with lung cancer.
Introduction
Patients with cancer have an increased risk of thrombotic events compared with the general population, with reported incidence rates as high as 20%–30%.1–6 This association has been well established and has critical impacts on morbidity and mortality in patients with cancer.7 Thromboembolic complications including venous thromboembolism (VTE; ie, pulmonary embolism (PE) and deep vein thrombosis (DVT)) and arterial thromboembolism are the second-most common cause of death in patients with cancer, second only to disease progression.2 7–13
A cancer diagnosis independently increases the risk of thrombosis by approximately 4-fold to 12-fold, and with the addition of chemotherapy (chemo) or targeted therapy, upwards of 6.5-fold to 23-fold.5 6 8 12 14–18 Reported incidences and risks of VTE development show wide variation, which could be a consequence of differences in study designs, patient population selection, definitions of VTE, or the systematic exclusion of patients with a history of thrombosis from most clinical trials.3 12 19
The reasons for the increased risk of thrombosis in patients with cancer are multifactorial, including patient-related factors such as older age, history of VTE, obesity, or other comorbidities; tumor-related factors such as type and stage of malignancy; and treatment-related factors such as surgery, radiation, or systemic anticancer therapies.11 18–21
Despite the well-established association of thrombosis with cancer, it is important to note that the risk of VTE varies considerably according to the primary site of the malignancy, histology of the cancer, and extent of disease.11 14 20 Lung cancer is not only the leading cause of cancer-related death globally but is one of the cancers more commonly associated with thrombosis, with incidence rates reported to be as high as 14%–30%.1 10 14 22 23 Advanced cancer is associated with a greater risk of VTE compared with localized or early-stage disease.17
Chemotherapy—particularly platinum-based regimens—and radiation therapy both have been associated with an increased risk of VTE.3 14 16 19 22 The treatment-related risk of VTE development has persisted with the advent of newer anticancer therapies, and has been documented with antiangiogenic agents, multitargeted tyrosine kinase inhibitors, immunomodulatory drug combinations, and immunotherapy regimens.20 For novel immunotherapies, a possible underlying inflammatory mechanism and immune response has been theorized to promote the increased risk of thrombosis.8 15 24 25
Validated risk stratification tools, such as the Khorana score, allow clinicians to estimate VTE risk in patients with cancer and implement appropriate interventions.11 26 27 The Khorana score is a validated assessment tool that stratifies patients treated with chemo into VTE risk groups based on their individual risk according to clinical (cancer type, body mass index (BMI)) and laboratory parameters (hemoglobin, leukocytes, and platelet count).17 28
Efforts are ongoing to characterize VTE risk associated with the newer therapies, but the association between cancer immunotherapy and thrombosis has not been thoroughly investigated and existing studies have reported disparate results.1 4 6 8 10 12 14 16 22–24 29–31 Among these studies were one unplanned and two retrospective analyses in a prospective trial that were not designed to compare rates of VTE, but reported the VTE rates that were observed in patients with lung cancer who were treated with immune checkpoint inhibitor (ICI) therapy (8%–36%).1 10 23 When considering only studies that evaluated VTE risk in patients with lung cancer who were treated with ICI therapy, one reported a comparable risk of developing VTE compared with the risk associated with chemo,22 two reported a higher risk with ICI treatment compared with chemo,6 12 and one reported lower risk with ICI treatment.16 There also has been discrepancy in identifying baseline demographics and other clinical characteristics that may be risk factors for the development of VTE, or alternatively might confer protective effects. Thus, the goal of this study was to generate real-world evidence that describes the incidence and risk factors associated with VTE among patients with lung cancer who received ICIs compared with those who underwent chemo (for which the risk of VTE is better established). The study focuses on advanced non-small cell lung cancer (aNSCLC), which accounts for the largest portion of the ICI-eligible population. We used administrative claims data, supplemented by clinical oncology data, to study real-world insights from a large, commercially insured US population.
Methods
Study design and patients
This was an observational, retrospective cohort study of patients receiving ICI-based, chemotherapy-based (chemo-based), or ICI plus chemotherapy (ICI+chemo)-based regimens for the first-line treatment of aNSCLC between July 1, 2014, and August 31, 2020. Data were sourced from HealthCore Integrated Research Environment - Oncology (HIRE-O), an administrative claims database of medical, pharmacy, and health plan eligibility data from commercial and Medicare Advantage health plans in 14 US states, which was integrated with clinical data submitted from treating oncologists through a cancer care quality program (CCQP). The HealthCore Integrated Research Database represents claims information from the commercially insured and Medicare Advantage population in the USA and includes health maintenance organizations, point-of-service providers, preferred provider organizations, and indemnity plans. CCQP data include clinical information about patients undergoing cancer treatment.
Eligible patients were ≥18 years of age at the time of their diagnosis of stage IV lung cancer with non-small cell lung cancer (NSCLC) pathology (clinical CCQP data). Patients had at least one claim for administration of ICIs or chemo as first-line systemic anticancer therapy on or after their stage IV diagnosis date and before February 29, 2020. The index date was the start date of their first systemic anticancer therapy. Patients were required to have continuous health plan enrollment (medical and pharmacy benefits) for at least 12 months before (ie, baseline period) and at least 1 month after the index date. Exclusion criteria, assessed during the 12-month baseline period, included record of prior stage IV diagnosis (to limit the study to newly diagnosed patients with aNSCLC), prior treatment for stage IV cancer or metastatic disease, two or more claims for other primary cancers, or one or more claims for acute or chronic VTE.
A Khorana risk score for VTE was calculated for the subset of patients whose BMI and laboratory data (platelet count, hemoglobin level, leukocyte count) were available during the baseline period. This score uses multiple independent predictors, including the primary site of the tumor, pre-chemo platelet count ≥350×109/L, hemoglobin levels <10 g/dL (or use of red blood cell growth factors), pre-chemo leukocyte count >11×109/L, and BMI ≥35 kg/m2. Each component is assigned a score of 1 point, except for site of primary cancer (high-risk cancers are assigned a value of 2 points). In this study, we assigned a score of ‘1’ to all patients in the study for having lung cancer. The classification identifies patients as low (score=0), intermediate (1–2), or high risk (≥3).11 17 28
Patients were assigned to one of the three mutually exclusive study cohorts based on the regimens received within 30 days after the first claim for systemic therapy (the index date) following an NSCLC stage IV diagnostic record in the database: (1) an ICI-based cohort (ICI monotherapy, ICI+ICI combination, or ICI+targeted therapy); (2) a chemo-based cohort (chemo alone, chemo+chemo combination, or chemo+targeted therapy), or (3) an ICI+chemo cohort (ICI+chemo or ICI+chemo+targeted therapy).
The primary objective was to compare the incidence of VTE after initiation across these three systemic anticancer treatments. In addition to a crude estimate, an adjusted comparison incorporated potential risk factors for developing VTE, including baseline comorbidities, and history of previous treatments.
Endpoints and assessments
The primary endpoint was newly diagnosed VTE, including DVT and PE, identified in the inpatient or outpatient settings using International Classification of Diseases-9th revision (ICD-9)//10th revision Clinical Modification (10-CM) diagnosis codes, Current Procedural Terminology-4th edition (CPT-4) codes for ultrasound procedures, and filled anticoagulant prescriptions.32 We used ICD-9-CM codes for acute VTE identified by a previous study,32 and mapped these codes to their corresponding ICD-10-CM diagnosis codes. Acute VTE events during the follow-up period were identified by either ≥1 claims for VTE diagnosis in inpatient/emergency department settings, ≥2 claims for VTE on distinct dates in outpatient setting, or ≥1 claims for VTE diagnosis in outpatient settings plus anticoagulant prescription dispensed within 31 days of diagnosis. The additional requirements of ≥2 claims on distinct dates or anticoagulant use are used to increase the validity of VTE diagnosis found in outpatient settings.
The secondary endpoint was anticoagulant use, identified from pharmacy and medical claims using national drug codes (or generic product identifier codes) and Healthcare Common Procedure Coding System codes. Anticoagulant use was reported by drug class and duration of therapy during three periods: (1) the pre-index baseline period, (2) a follow-up period from initiation of systemic anticancer therapy to first post-index VTE event, and (3) from the occurrence of the first VTE event until the end of study follow-up. Duration of anticoagulant use (as measured by medication persistence) was recorded as time from initiation to discontinuation of therapy within a specific period. Whether anticoagulant use was for prophylaxis or for treatment of VTE, or for other indications, could not be determined from the data source. Switching between different anticoagulants was not evaluated in this study.
Statistical analyses
Demographics, baseline characteristics, cancer treatment history, and treatment patterns were described using univariate statistics for each of the three study cohorts. Frequencies and percentages are reported for categorical variables. Relevant measures of centrality and variance, such as mean, median, standard deviation (SD), and interquartile range (IQR), are presented for continuous variables. Statistical comparisons of VTE incidence rates across cohorts (incorporating baseline characteristics) were conducted using one-way analysis of variance or Kruskal-Wallis test for continuous variables. Χ2 or Fisher’s exact tests were used for categorical variables.
VTE outcomes are reported as cumulative incidences (percent with event) at 6 and 12 months after the index date and as incidence rates during the entire follow-up period (number of cases per 100 patient-years). Patients who did not experience a VTE event were censored at the end of their health plan enrollment, the end of the study period, or date of death. Kaplan-Meier curves and log-rank test were used to compare time to event across cohorts. Cox proportional hazards models were used to compare the risks of VTE across the three study cohorts, while controlling for baseline characteristics and time-dependent factors such as systemic anticancer therapy. The duration of index treatment, time to initiation of second-line therapy, and duration of second-line therapy was adjusted for in the Cox proportional hazards model as time-varying covariates or were further examined using logistic regression models in a sensitivity analysis of first-line and second-line therapy, as appropriate. Cox proportional hazards models or logistic regression models were also used to identify risk factors potentially associated with VTE.
In the Cox proportional hazards models, VTE was the event of interest, and for those patients who died without having had a VTE, it was assumed that there was not enough time to observe the event; therefore, death was treated as a competing risk. The cumulative incidence was unadjusted and was reported from the data without adjusting for patient demographics or other competing risks.
Study power was calculated corresponding to predicted sample sizes for detecting a difference between VTE rates for ICI-based versus chemo-based cohorts, assuming a VTE rate of 0.108 events per person-year for the chemo group and Poisson distribution with a two-sided significance-level of 0.05. An expected sample size of 604 patients in the ICI group and 1092 patients in the chemo group was estimated to have 80% power to detect a difference of 0.041 between the two groups, corresponding to an event rate of 0.067 in the ICI group.
Results
Patients
The study included 2299 patients: 605 treated with ICI-based regimens, 1092 with chemo-based, and 602 with ICI+chemo. Baseline patient characteristics are shown in table 1.33 34 There were several notable differences among cohorts. The ICI-based cohort was older (64 vs 62 years in the chemo cohort and 61.5 years in the ICI+chemo cohort). The ICI-based cohort also had a higher percentage of patients treated with radiation therapy during the 12-month baseline period (46.8% vs 42.9% for chemo-based and 34.7% for the ICI+chemo) and a higher percentage of patients treated with chemo during the baseline period (26.6% vs 13.6% in the chemo cohort and 7.1% in the ICI+chemo cohort). There was a lower percentage of patients in the ICI cohort who had a central venous catheter or peripherally inserted central catheter during the baseline period (23.0%) compared with the chemo cohort (39.7%) and the ICI+chemo cohort (42.2%). Though ALK and ROS1 mutations did exist in this patient population, only a minority of the patients were positive for either mutation, and few patients were treated with targeted therapy.
Table 1.
Baseline demographic and clinical characteristics
| Overall (N=2299) |
ICI (N=605) |
Chemo (N=1092) |
ICI+chemo (N=602) |
|
| Demographic characteristics | ||||
| Median age at index (IQR), years | 62 (58–69) | 64 (58–73) | 62 (58–68) | 62 (57–68) |
| Male, n (%) | 1274 (55.4) | 326 (53.9) | 633 (58.0) | 315 (52.3) |
| Baseline BMI, median | 25.7 | 25.7 | 25.8 | 25.7 |
| Insurance type, % | ||||
| Commercial | 1627 (70.8) | 380 (62.8) | 802 (73.4) | 445 (73.9) |
| Medicare Advantage | 672 (29.2) | 225 (37.2) | 290 (26.6) | 157 (26.1) |
| Cancer treatment history during the 12-month baseline period, n (%) | ||||
| Surgery | 321 (14.0) | 78 (12.9) | 166 (15.2) | 77 (12.8) |
| Radiation therapy | 960 (41.8) | 283 (46.8) | 468 (42.9) | 209 (34.7) |
| ICIs | 80 (3.5) | 36 (6.0) | 28 (2.6) | 16 (2.7) |
| Chemotherapy | 353 (15.4) | 161 (26.6) | 149 (13.6) | 43 (7.1) |
| Targeted therapy | 77 (3.3) | 31 (5.1) | 38 (3.5) | ≤10 (NA) |
| Clinical characteristics | ||||
| Modified DCCI* at baseline, median (range) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 1 (1–3) |
| Baseline individual DCCI comorbidities,† n (%) | ||||
| CHF | 267 (11.6) | 72 (11.9) | 135 (12.4) | 60 (10.0) |
| Diabetes with chronic complication | 162 (7.0) | 45 (7.4) | 78 (7.1) | 39 (6.5) |
| MI | 183 (8.0) | 55 (9.1) | 87 (8.0) | 41 (6.8) |
| Renal disease | 188 (8.2) | 65 (10.7) | 82 (7.5) | 41 (6.8) |
| Cerebrovascular disease | 358 (15.6) | 105 (17.4) | 168 (15.4) | 85 (14.1) |
| Chronic pulmonary disease | 1498 (65.2) | 393 (65.0) | 710 (65.0) | 395 (65.6) |
| Atrial fibrillation | 239 (10.4) | 79 (13.1) | 109 (10.0) | 51 (8.5) |
| Baseline other comorbidities, n (%) | ||||
| Hypertension | 1434 (62.4) | 388 (64.1) | 675 (61.8) | 371 (61.6) |
| CVC or PICC | 826 (35.9) | 139 (23.0) | 433 (39.7) | 254 (42.2) |
| Obesity | 319 (13.9) | 78 (12.9) | 150 (13.7) | 91 (15.1) |
| Bleeding | 320 (13.9) | 88 (14.5) | 145 (13.3) | 87 (14.5) |
| Baseline ECOG PS at baseline, n (%) | ||||
| 0 | 739 (32.1) | 192 (31.7) | 342 (31.3) | 205 (34.1) |
| 1 | 1189 (51.7) | 311 (51.4) | 556 (50.9) | 322 (53.5) |
| 2 | 186 (8.1) | 70 (11.6) | 74 (6.8) | 42 (7.0) |
| 3 | 16 (0.7) | ≤10 (NA) | 13 (1.2) | ≤10 (NA) |
| Khorana risk score | ||||
| Patients with baseline Khorana risk score, n (%) | 472 (21) | 112 (19) | 227 (21) | 133 (22) |
| Patients’ baseline characteristics for Khorana risk score calculation, n (%) | ||||
| Platelet count ≥350×10⁹/L | 166 (35.2) | 46 (41.1) | 59 (26.0) | 61 (45.9) |
| Hemoglobin level <10 g/dL | 36 (7.6) | ≤10 (NA) | 19 (8.4) | ≤10 (NA) |
| Leukocyte count >11×10⁹/L | 106 (22.5) | 26 (23.2) | 50 (22.0) | 30 (22.6) |
| BMI ≥35 kg/m² | 181 (38.3) | 54 (48.2) | 77 (33.9) | 50 (37.6) |
| Baseline Khorana risk score, n (%) | ||||
| 1, low risk for VTE | 260 (55.1) | 58 (51.8) | 132 (58.1) | 70 (52.6) |
| ≥2, high risk for VTE | 212 (44.9) | 54 (48.2) | 95 (41.9) | 63 (47.4) |
*An index that assigns a score to various chronic medical conditions and uses the sum to predict long-term mortality.33 34
†These are select DCCI comorbidities. The full complement of DCCI comorbidities are AIDS, any malignancy, cerebrovascular disease, chronic pulmonary disease, CHF, dementia, diabetes without complications, diabetes without chronic complications, hemiplegia or paraplegia, metastatic solid tumor, mild liver disease, moderate/severe liver disease, MI, peptic ulcer disease, peripheral vascular disease, renal disease, rheumatoid disease.33 34
AIDS, acquired immune deficiency syndrome; BMI, body mass index; CHF, congestive heart failure; CVC, central venous catheter; DCCI, Deyo-Charlson Comorbidity Index; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; IQR, interquartile range; MI, myocardial infarction; NA, not applicable; PICC, peripherally inserted central catheter; VTE, venous thromboembolism.
VTE cumulative incidence and incidence rates
Cumulative incidence
VTE events occurred in 387 of 2299 patients (16.8%) during the entire follow-up period (median, 9.1 months). In the ICI-based cohort, cumulative incidences (percent with event) were 8.1% at 6 months, 11.6% at 12 months, and 13.4% (81 of 605) overall (table 2). Cumulative incidences were higher in the chemo-based cohort: 10.9% at 6 months, 14.0% at 12 months, and 18.0% (197 of 1092) overall. In the ICI+chemo cohort, cumulative incidences were 12.8% at 6 months, 16.1% at 12 months, and 18.1% (109 of 602) overall.
Table 2.
VTE incidence
| Overall (N=2299) |
ICI (N=605) |
Chemo (N=1092) |
ICI+chemo (N=602) |
|
| Cumulative incidence | ||||
| Median time to event (IQR), months | 3.4 (1.4–9.2) | 3.3 (1.3–8.9) | 3.9 (1.5–10.3) | 2.9 (1.2–7.4) |
| Overall VTE cumulative incidence during 6-month follow-up period (95% CI), cases per 100 patients | 10.7 (9.3 to 12.0) | 8.1 (5.8 to 10.4) | 10.9 (8.9 to 12.9) | 12.8 (11.3 to 14.3) |
| PE | 6.6 (5.6 to 7.7) | 5.1 (3.3 to 6.9) | 6.5 (5.0 to 8.0) | 8.3 (7.1 to 9.5) |
| DVT, lower extremity | 4.9 (4.0 to 5.8) | 4.5 (2.8 to 6.2) | 5.0 (3.7 to 6.4) | 5.0 (4.1 to 5.9) |
| DVT, upper extremity | 1.7 (1.2 to 2.2) | 1.5 (NA) | 1.7 (0.9 to 2.4) | 2.0 (1.4 to 2.6) |
| Other | 0.8 (0.5 to 1.2) | 0.5 (NA) | 1.0 (0.4 to 1.6) | 0.8 (NA) |
| Overall VTE cumulative incidence during 12-month follow-up period (95% CI), cases per 100 patients | 13.9 (12.4 to 15.4) | 11.6 (8.9 to 14.3) | 14.0 (11.8 to 16.2) | 16.1 (14.5 to 17.8) |
| PE | 8.7 (7.5 to 9.9) | 7.1 (5.0 to 9.2) | 8.6 (6.9 to 10.4) | 10.3 (9.0 to 11.6) |
| DVT, lower extremity | 6.4 (5.3 to 7.4) | 6.1 (4.2 to 8.1) | 6.4 (4.9 to 7.9) | 6.5 (5.4 to 7.5) |
| DVT, upper extremity | 2.2 (1.6 to 2.8) | 2.0 (0.9 to 3.1) | 2.1 (1.3 to 3.0) | 2.5 (1.9 to 3.1) |
| Other | 1.1 (0.7 to 1.6) | 0.7 (NA) | 1.3 (0.6 to 2.0) | 1.3 (NA) |
| Overall VTE cumulative incidence during complete follow-up period (95% CI), cases per 100 patients | 16.8 (15.2 to 18.5) | 13.4 (10.5 to 16.3) | 18.0 (15.5 to 20.6) | 18.1 (16.4 to 19.8) |
| PE | 10.6 (9.3 to 12.0) | 8.8 (6.4 to 11.1) | 11.0 (9.0 to 13.0) | 11.8 (10.4 to 13.2) |
| DVT, lower extremity | 7.6 (6.5 to 8.7) | 6.9 (4.8 to 9.0) | 8.0 (6.3 to 9.6) | 7.6 (6.5 to 8.8) |
| DVT, upper extremity | 2.8 (2.1 to 3.5) | 2.0 (0.9 to 3.1) | 3.3 (2.2 to 4.4) | 2.8 (2.1 to 3.5) |
| Other | 1.5 (1.0 to 2.0) | 0.7 (NA) | 1.7 (1.0 to 2.5) | 1.8 (1.3 to 2.4) |
| Incidence rates | ||||
| Overall VTE incidence rate during entire follow-up period (95% CI), cases per 100 PY | 17.8 (16.0 to 19.5) | 13.5 (10.6 to 16.5) | 18.0 (15.5 to 20.5) | 22.4 (20.2 to 24.5) |
| PE | 10.7 (9.4 to 12.1) | 8.6 (6.3 to 10.9) | 10.4 (8.6 to 12.3) | 14.0 (12.4 to 15.7) |
| DVT, lower extremity | 7.6 (6.4 to 8.7) | 6.7 (4.7 to 8.8) | 7.4 (5.9 to 9.0) | 8.8 (7.5 to 10.1) |
| DVT, upper extremity | 2.8 (2.1 to 3.4) | 1.9 (0.8 to 2.9) | 3.0 (2.0 to 4.0) | 3.2 (2.4 to 4.0) |
| Other | 1.4 (0.9 to 1.9) | 0.6 (NA) | 1.6 (0.9 to 2.3) | 2.1 (1.4 to 2.7) |
| Overall VTE incidence rate or anticoagulant within 3 days of venous ultrasound (95% CI), cases per 100 PY | 18.7 (16.8 to 20.5) | 13.8 (10.8 to 16.8) | 19.0 (16.4 to 21.5) | 24.0 (21.8 to 26.3) |
| Overall VTE incidence rate (first event of PE, DVT, or other) by baseline use of oral anticoagulant (95% CI), cases per 100 PY | ||||
| Yes | 15.3 (9.6 to 20.9) | 16.2 (NA) | 11.7 (5.1 to 18.4) | 25.1 (NA) |
| No | 18.0 (16.1 to 19.8) | 13.3 (10.3 to 16.4) | 18.6 (16.0 to 21.3) | 22.2 (20.0 to 24.4) |
DVT, deep vein thrombosis; ICI, immune checkpoint inhibitor; NA, not available; PE, pulmonary embolism; PY, patient-years; VTE, venous thromboembolism.
In the overall cohort, the most common VTE event was PE, accounting for 63% (n=244) of events over the entire follow-up period. The next most common events were lower-extremity DVT (n=175 events; 45.2%), followed by upper-extremity DVT (n=65 events; 16.8%), and then other VTE events (n=34 events; 8.8%). A similar pattern was observed in each individual cohort.
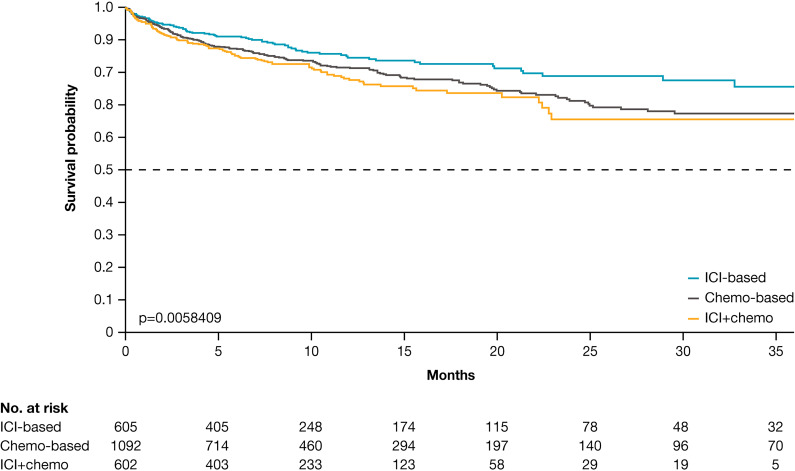
The Kaplan-Meier curves (figure 1) show time from index date to the first VTE event by cohort. Among those with VTE, the median time from index date to the first VTE event was shortest for the ICI+chemo cohort (2.9 months) and longest for the chemo-based cohort (3.9 months), and was 3.3 months in the ICI-based cohort.
Figure 1.
Kaplan-Meier curves examining the time from index date to venous thromboembolism events by study cohort. chemo, chemotherapy; ICI, immune checkpoint inhibitor.
Crude incidence rate
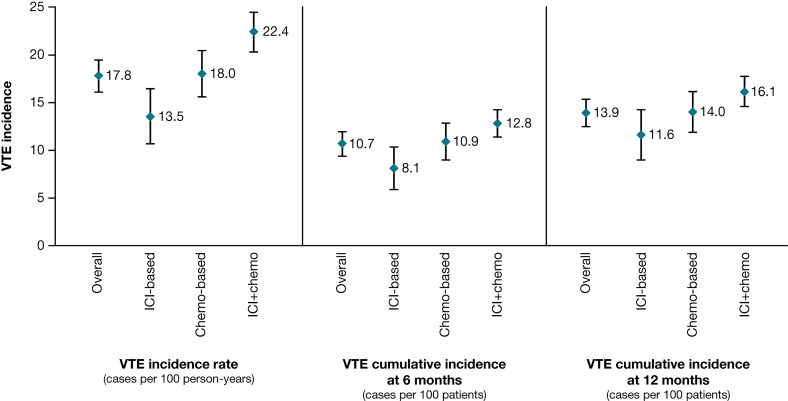
The crude VTE incidence rate, expressed as cases per 100 patient-years, over the entire period was 17.8 (95% CI 16.0 to 19.5) for the entire population, 13.5 (95% CI 10.6 to 16.5) for the ICI-based cohort, 18.0 (95% CI 15.5 to 20.5) for the chemo-based cohort, and 22.4 (95% CI 20.2 to 24.5) for the ICI+chemo cohort (figure 2).
Figure 2.
VTE incidence. chemo, chemotherapy; ICI, immune checkpoint inhibitor; VTE, venous thromboembolism.
Adjusted incidence rate
The Cox proportional hazards model (table 3) identified several baseline demographic and clinical characteristics that were associated with increased or decreased risk of VTE in the overall population. These factors included treatment with ICI, receiving radiation therapy, and severe obesity.
Table 3.
Cox proportional hazards models examining time to VTE events
| Full cohort (N=2299) |
Patients with a Khorana risk score (n=472)* |
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Cohort (LOT1) (ref: chemo) | ||||
| ICI | 0.74 (0.56 to 0.97) | 0.03 | 0.52 (0.27 to 1.01) | 0.05 |
| ICI+chemotherapy | 1.12 (0.88 to 1.42) | 0.36 | 0.94 (0.55 to 1.61) | 0.81 |
| Age category (ref: 18–55 years) | ||||
| 55–65 years | 0.86 (0.65 to 1.15) | 0.31 | 1.12 (0.55 to 2.27) | 0.76 |
| 65–75 years | 0.87 (0.61 to 1.24) | 0.43 | 1.47 (0.62 to 3.49) | 0.38 |
| ≥75 years | 0.78 (0.50 to 1.22) | 0.28 | 1.60 (0.50 to 5.05) | 0.43 |
| Baseline Khorana risk score | ||||
| 1: low risk for VTE | NA | – | 1.00 | – |
| ≥2: high risk for VTE | NA | 1.17 (0.71 to 1.92) | 0.54 | |
| Cancer pathology (ref: squamous carcinoma) | ||||
| Adenocarcinoma | 1.24 (0.96 to 1.60) | 0.11 | 1.29 (0.75 to 2.23) | 0.36 |
| Other/undetermined/missing | 0.74 (0.36 to 1.53) | 0.42 | 0.45 (0.05 to 4.10) | 0.48 |
| Cancer treatment history during the 12-month baseline period |
||||
| Surgery | 0.88 (0.64 to 1.19) | 0.40 | 0.42 (0.18 to 0.95) | 0.04 |
| Radiation therapy | 1.25 (1.02 to 1.54) | 0.03 | 0.95 (0.57 to 1.57) | 0.84 |
| Baseline BMI category | ||||
| Overweight: 25 to <30 | 1.19 (0.93 to 1.53) | 0.17 | 1.00 (0.57 to 1.78) | 0.99 |
| Class 1 obesity: 30 to <35 | 1.21 (0.89 to 1.64) | 0.22 | 0.73 (0.36 to 1.49) | 0.39 |
| Class 2 obesity: 35 to <40 | 1.26 (0.81 to 1.97) | 0.31 | 0.73 (0.19 to 2.79) | 0.64 |
| Class 3 obesity: ≥40 | 1.77 (0.98 to 3.20) | 0.06 | 2.40 (0.81 to 7.15) | 0.12 |
| Unknown BMI | 1.21 (0.66 to 2.22) | 0.55 | – | – |
| Other baseline comorbidities or procedures | ||||
| Atrial fibrillation | 1.02 (0.69 to 1.52) | 0.92 | 1.26 (0.60 to 2.65) | 0.54 |
| Stroke | 0.87 (0.50 to 1.50) | 0.61 | 1.25 (0.44 to 3.49) | 0.68 |
| Hypertension | 0.94 (0.75 to 1.18) | 0.61 | 1.20 (0.71 to 2.02) | 0.49 |
| Bleeding | 0.97 (0.71 to 1.31) | 0.82 | 1.31 (0.71 to 2.43) | 0.38 |
| Fracture | 1.01 (0.65 to 1.57) | 0.96 | 0.54 (0.14 to 2.10) | 0.37 |
| Transfusions | 1.63 (0.84 to 3.15) | 0.15 | 3.61 (0.68 to 19.21) | 0.13 |
| CVC or PICC | 1.08 (0.87 to 1.34) | 0.48 | 2.13 (1.35 to 3.36) | 0.001 |
| ECOG PS (ref: ECOG PS=0) | ||||
| 1 | 1.04 (0.83 to 1.31) | 0.73 | 1.03 (0.62 to 1.69) | 0.92 |
| 2 and 3 | 1.18 (0.79 to 1.76) | 0.43 | 1.58 (0.64 to 3.89) | 0.32 |
| Unknown | 1.00 (0.58 to 1.71) | 0.99 | 0.32 (0.04 to 2.71) | 0.29 |
| Medication use | ||||
| NSAIDs | 0.91 (0.64 to 1.31) | 0.62 | 0.73 (0.31 to 1.73) | 0.48 |
| Anticoagulants | 0.80 (0.51 to 1.23) | 0.30 | 0.71 (0.29 to 1.75) | 0.45 |
| Antiplatelets | 0.96 (0.65 to 1.42) | 0.83 | 1.03 (0.41 to 2.55) | 0.96 |
| Oral glucocorticoids | 0.90 (0.71 to 1.15) | 0.40 | 0.84 (0.51 to 1.39) | 0.50 |
*For patients with a Khorana risk score, targeted therapy and other combinations were grouped together.
BMI, body mass index; CVC, central venous catheter; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; LOT, line of therapy; NSAIDs, non-steroidal anti-inflammatory drugs; PICC, peripherally inserted central catheter; ref, referent; VTE, venous thromboembolism.
After adjustment, the risk of VTE was 26% lower for patients treated with ICI-based therapy versus chemo-based (HR, 0.74; p=0.03). No statistically significant difference was observed between ICI+chemo versus chemo alone (HR, 1.12; p=0.36).
Receiving radiation therapy during the baseline period was associated with an increased risk of VTE (HR, 1.25; p=0.03). Severe obesity (BMI ≥40) was also marginally associated with a higher risk of VTE (HR, 1.77; p=0.06). An analysis with a time-dependent variable for treatment pattern yielded similar results. No other variables, including baseline comorbidities and medication use, significantly affected the risk of VTE event in this analysis.
Sensitivity analysis
A sensitivity analysis used a broader case definition for VTE. Patients who did not have a diagnostic code for a VTE were still counted as a case if there was evidence of new anticoagulant use within 3 days of a venous ultrasound. Although by definition the incidence rates were slightly higher than in the primary analysis, the results were similar.
VTE incidence rates in the sensitivity analysis were 18.7 (95% CI 16.8 to 20.5) in the overall population, 13.8 (95% CI 10.8 to 16.8) for the ICI-based cohort, 19.0 (95% CI 16.4 to 21.5) for the chemo-based cohort, and 24.0 (95% CI 21.8 to 26.3) for the ICI+chemo cohort (table 2).
By Khorana risk score
In patients with a known Khorana risk score (n=472), the risk of VTE was 48% lower for ICI-based versus chemo-based therapy (HR, 0.52; table 3). No significant difference was observed between ICI+chemo versus chemo alone. Central venous catheter or peripherally inserted central catheter line use during baseline was associated with a significantly higher risk of VTE (HR, 2.13; p=0.001). Surgery during the baseline period was associated with lower VTE risk (HR, 0.42; p=0.04) in this population. A relationship between time to VTE and other baseline or clinical characteristics, including other baseline comorbidities or medication use, was not found to be significant in this analysis. In patients with a Khorana risk score ≥2, the risk of developing VTE was non-significantly elevated (HR 1.17; 95% CI 0.71 to 1.92; p=0.54).
Overall VTE incidence was lower in patients with a Khorana risk score of 1 versus ≥2 (16.0 vs 22.4, table 4). The most common VTE event for both risk levels was PE, followed by lower-extremity DVT and upper-extremity DVT. The sensitivity analysis, using the broader case definition outlined above, yielded results by risk level consistent with the primary analysis.
Table 4.
VTE incidence by Khorana score
| 1, low risk (n=260) |
≥2, high risk (n=212) |
|
| Overall VTE incidence rate during entire follow-up period, cases per 100 PY | 16.0 | 22.4 |
| PE | 10.5 | 14.2 |
| DVT, lower extremity | 7.0 | 9.8 |
| DVT, upper extremity | 1.4 | 4.3 |
| Other | 1.4 | 1.4 |
| Overall VTE incidence rate or anticoagulant within 3 days of venous ultrasound, cases per 100 PY |
16.7 | 25.4 |
| Overall VTE incidence rate (first event of PE, DVT, or other) by baseline use of oral anticoagulant), cases per 100 PY | ||
| Yes | 23.1 | 17.4 |
| No | 15.3 | 22.9 |
| Overall VTE cumulative incidence during 6-month follow-up period, cases per 100 patients | 10.8 | 15.1 |
| PE | 6.5 | 9.4 |
| DVT, lower extremity | 6.2 | 7.6 |
| DVT, upper extremity | 1.2 | 3.8 |
| Other | 1.2 | 1.4 |
DVT, deep vein thrombosis; ICI, immune checkpoint inhibitor; PE, pulmonary embolism; PY, patient-years; VTE, venous thromboembolism.
In second-line therapy
VTE incidence was also reported for the 986 patients receiving second-line therapy who had not experienced a VTE with first-line therapy (online supplemental table 1). In this subset of patients, the overall incidence rate for the overall population was 20.7 per 100 patient-years during the entire follow-up period. VTE incidence rate was lowest for patients treated with an ICI as second-line therapy (17.6), followed by ICI+chemo (20.2), and was highest for patients treated with chemo as second-line therapy (26.3). The sensitivity analysis yielded the lowest per 100 patient-years incidence rate for the ICI-based group (18.4), followed by the ICI+chemo group (20.2), and the highest incidence rate in the chemo-based group (26.8).
jitc-2022-006072supp001.pdf (83.9KB, pdf)
Anticoagulant use
Of the 17.6% of patients in the overall population who had a VTE event during the follow-up period (404 of 2299), 121 (30.0%) had received an anticoagulant during the time between their index date and their first post-index VTE event (table 5). The specific indication (treatment or prophylaxis) and duration of use for anticoagulants was not available.
Table 5.
Anticoagulant use from index date to first post-index VTE event
| Overall (N=2299) |
ICI (N=605) |
Chemo (N=1092) |
ICI+chemo (N=602) |
|
| Among patients with VTE events during follow-up, n (%) | 404 (17.6) | 82 (13.6) | 206 (18.9) | 116 (19.3) |
| Patients with anticoagulant use, n (%) | 121 (30.0) | 21 (25.6) | 70 (34.0) | 30 (25.9) |
|
Treatment duration of medications from index date to first post-index VTE event, mean days |
48.3 | 69.9 | 51.4 | 27.6 |
| Low-molecular-weight heparins, n (%) | 66 (16.3) | 10 (12.2) | 43 (20.9) | 13 (11.2) |
| Warfarin, n (%) | 12 (3.0) | ≤10 (3.7) | ≤10 (3.9) | ≤10 (0.9) |
| Direct-acting oral anticoagulants, n (%) | 59 (14.6) | 10 (12.2) | 33 (16.0) | 16 (13.8) |
| Fondaparinux, n (%) | ≤10 (0.7) | 0 | ≤10 (1.5) | 0 |
ICI, immune checkpoint inhibitor; VTE, venous thromboembolism.
Of the 82 patients in the ICI-based cohort who had a VTE event in the follow-up (post-index) period (13.6%), 21 (25.6%) received prior anticoagulation between the index date and first VTE event (pre-index). In the chemo-based cohort, in which 18.9% (206 of 1092) of the patients experienced a VTE, 70 (34.0%) received an anticoagulant. In the ICI+chemo cohort, in which 19.3% (116 of 602) of the patients experienced a VTE, 30 (25.9%) received an anticoagulant. In the overall population and within each cohort, the most commonly used anticoagulant classes were low-molecular-weight heparins and direct-acting oral anticoagulants.
Limitations
Administrative claims data are collected primarily for billing and reimbursement purposes and are subject to potential coding biases, inconsistencies, and missing data. Despite diligent efforts, claims data for the identification of newly developed VTE events, which rely on ICD-9/10-CM coding and anticoagulant use, can be prone to misclassification.
Clinical data from HIRE-O are collected at the time of the treatment authorization request and not necessarily at the time of diagnosis. Additionally, use of over-the-counter drugs is not captured by prescription claims data.
Duration of anticoagulant use (as measured by medication persistence) was recorded as time from initiation to discontinuation of therapy within a specific period. Using prescription claims data to estimate medication persistence can be prone to inaccurate estimates of use. Whether anticoagulant use was for prophylaxis or for treatment of VTE, or for other indications, could not be determined from the data source. Switching between different anticoagulants was not evaluated in this study.
Another limitation is the use of the Social Security Administration’s (SSA) Death Master File to ascertain the death status of members of the study population. Since 2011, the SSA eliminated the requirement for US states to provide death records; thus, a number of records of deaths were expunged from the publicly available master database. To mitigate the loss of records, the Death Master File was supplemented with mortality data captured in the claims-based inpatient discharge status, reasons for health plan disenrollment, and third-party obituary data.
The population in this analysis was derived from US commercially insured and Medicare Advantage enrollees and those with available clinical data from HIRE-O, which may limit the generalizability of these results to other population segments, such as traditional fee-for-service Medicare and the uninsured.
Although the analyses adjusted for baseline differences between the cohorts, the potential unrecognized confounding should still be considered a limitation, though the adjusted analysis should account for most of the differences.
The rates of VTE were also not reported by index year, which may have allowed for additional exploration of potential reasons for fluctuations in VTE incidence over time. Of note, the percentages of patients in the chemo-only cohort were highest in years 2016–2018, while the usage of ICI-based therapy or ICI+chemo increased during most of the course of the study period. As a result, there may have been changes in VTE rates over time that were not detected. In addition, increased use of prophylactic anticoagulation over the course of the study period may have been driven by shifts in clinical practice that preceded guideline recommendations for prophylaxis.
Lastly, though the Cox proportional hazards model adjusted for a multitude of confounders (treatment, age, cancer histology, comorbidities, ECOG performance status, medication use, BMI, prior cancer treatment, DCCI), development of VTE may be influenced by factors that were not prospectively accounted for in the modeling.
Discussion
This observational, retrospective cohort study was designed to generate real-world evidence that describes the incidence of and risk factors for developing VTE among patients with aNSCLC after initiating systemic anticancer therapy with ICI-based therapy, chemo-based therapy, or ICI+chemo. Overall, results were consistent across analyses regardless of outcome variable: cumulative incidence, crude incidence rates, adjusted incidence rates, and sensitivity analyses (case definition and including Khorana risk score in patients for whom data were available). VTE risk was lowest in the ICI-based cohort, followed by the chemo-based cohort, and highest in the ICI+chemo cohort.
Although it has been well established that standard anticancer therapies such as surgery, radiation, and some systemic chemotherapeutic drugs increase the risk of VTE in patients with cancer,3 14 16 17 19 20 22 until recently there has been very little published data that adequately and consistently quantified this risk with respect to immuno-oncology therapy or to identified definitive risk factors.1 4 6 8 10 12 14 16 22–24 29–31
In our retrospective real-world study of patients with aNSCLC, the observed overall VTE crude incidence rate of 17.8% and the cumulative overall incidence rate of 16.8% were consistent with previous reporting in the published scientific literature.1 3 4 6 8 10 12 14 16 18 22–24 29–31 35 36 The incidence rate was highest for patients treated with combination ICI+chemo (22.4%) and lowest for ICI-treated patients (13.5%). Incidence rates reported in the literature are variable, with some studies reporting the higher rates in dual immuno-oncology combination therapies1 4 or chemo-treated patients.12 16 30 Also, in alignment with the other published studies, the most common VTE event in this study was PE regardless of cohort or risk classification.1 23 30 This may be due to increased incidental PE diagnosis in patients with lung cancer, as they are likely to have routine thoracic imaging.37 38
The median time from index date to first VTE was shortest for the ICI+chemo cohort (2.9 months) and longest for the chemo-based cohort (3.9 months), and was 3.3 months in the ICI-based cohort. This aligns with other recently published studies that suggest that the development of VTE tends to occur relatively early after treatment initiation.6 22 24
Recent studies have evaluated the risk of VTE development in patients with cancer who were treated with currently available systemic therapy options. The study designs and selection criteria varied greatly with respect to tumor types, patient characteristics, and treatment arms. Some studies evaluated lung cancer alone, NSCLC specifically, or a range of unselected tumor types.4 6 8 10 12 14 16 22–24 29–31 When considering only studies that focused on lung cancer and included an ICI treatment arm, one report suggests that the incidence of VTE in patients with cancer who are treated with ICIs is comparable with that reported in other cohorts of patients treated with chemo.22 This was a retrospective multicentric cohort study that included 593 patients with NSCLC who were treated with ICIs. The cumulative incidence of VTE in the cohort was 14.8% (95% CI 7.4% to 22.2%), with most thromboses occurring rapidly after treatment initiation. Of note, patients with previous VTE, receiving anticoagulation or treated with antiplatelets were not excluded. Though there was no chemo comparison arm, the observed VTE incidence was comparable to what has been reported in the literature, as the authors concluded.22 A single-institution retrospective study of 1587 adults with NSCLC who received first-line ICI, chemo, or targeted therapy reported that the 6-month cumulative VTE incidence was highest in patients who received targeted therapies (11.1%) or ICI+chemo (9.9%).12 VTE rates for patients treated with chemo alone (5.0%) and ICI alone (7.6%) were lower, though notably the observed risk was higher for single-agent ICI compared with chemo.12 Another retrospective population-based cohort study in 95,466 patients with lung cancer who were treated with chemo, ICI, or targeted therapy, or combinations thereof, reported statistically significantly higher rates of VTE in patients treated with ICI alone compared with chemo alone (9.1% vs 6.9%; p<0.02).6 Patients who received ICI alone were not only most likely to experience a VTE, but also experience an event sooner after the start of treatment compared with patients receiving chemo alone, combined chemotherapies and immunotherapies, or neither of these therapies; this finding was unchanged when death was considered as a competing risk. The study also reported that patients who received prophylactic anticoagulation or aspirin experienced VTE at both a higher rate and a shorter interval than patients who did not.6 Lastly, a retrospective cohort study in 345 patients with NSCLC who were treated with either ICI or platinum-based chemo reported a 6-month cumulative incidence of VTE of 7.1% in the chemo cohort and 4.5% in the ICI cohort (HR for chemo, 1.6; 95% CI 0.66 to 3.9).16 The heterogeneity in design and patient selection among these studies and our study explain the heterogeneity in findings, underscoring the ongoing need to adequately identify and treat VTE events in patients with lung cancer.
Though overall our study demonstrated high rates of VTE in patients with lung cancer, they were not higher with ICI treatment, and were potentially lower with ICI treatment compared with the treatments in the other cohorts. The risk of VTE was 26% lower for patients treated with ICI-based therapy versus chemo-based therapy in this study, and no significant difference was observed between ICI+chemo versus chemo alone. Receiving radiation therapy during the baseline period was associated with an increased risk of VTE, and severe obesity was also marginally and independently associated with a higher risk of VTE. In patients with a known Khorana risk score, the risk of VTE was 48% lower for ICI-based therapy versus chemo-based therapy, and no significant difference was observed between ICI+chemo versus chemo alone. These risks have been reported previously in the literature.3 11 14 16 18–22 In our study, we did not observe a relationship between higher Khorana risk score and the development of VTE. The association between Khorana risk score and development of thrombosis was inconsistent among previously published studies, with most reporting a lack of association in their NSCLC population.6 12 16 22–24 31 A potential explanation for the inconsistency in findings is that, like most VTE risk prediction models, the Khorana risk score was developed for patients with solid tumors or lymphoma who were treated with chemo, and not designed specifically for lung cancer.23 28 The Vienna CATS, PROTECHT, and CONKO scores were developed to improve the VTE risk discrimination capabilities of the Khorana score with additional factors such as biomarkers (eg, D-dimer concentration) or type of chemo (platinum-based or gemcitabine-based), or removal and replacement of existing variables (BMI for WHO performance status).17 Despite these modifications, the Khorana score is currently the most widely used and validated predictive model for the development of VTE in patients with cancer, and remains the only risk assessment recommended by multiple guidelines.15 39 The limitations of the Khorana risk score are well known, and an unmet need remains for a risk assessment tool that can consistently predict risk of VTE in patients treated with newer systemic therapies. Future research opportunities may lie in the development of a tool that could predict VTE risk in patients across a range of malignancies, and those treated with therapy other than chemo.
While this study evaluated the risk of VTE in patients treated for aNSCLC and did not identify an increased risk associated with ICI treatment, the advent of ICIs has changed the treatment paradigm for NSCLC and for many other tumors. ICIs now span a breadth of US Food and Drug Administration–approved indications, including but not limited to NSCLC, small-cell lung cancer, melanoma, hepatocellular carcinoma, gastric cancer, esophageal and/or gastroesophageal junction cancer, urothelial carcinoma, renal cell carcinoma, colorectal cancer, classical Hodgkin’s lymphoma, and primary mediastinal large B-cell lymphoma.40–45 The risk of VTE associated with the treatment of these tumors may be an area of interest for future research. Approximately 40% of patients with cancer in the USA are eligible for ICI therapy and with additional studies in the metastatic, adjuvant, neoadjuvant, and perioperative settings underway, the number of ICI treated patients can potentially expand further.46
This retrospective real-world study of patients with aNSCLC adds to the growing body of evidence that suggests that patients with aNSCLC are at an increased risk of VTE. However, we found that the VTE incidence was lower for patients receiving ICI-based therapy compared with chemo-based regimens. Additional baseline and clinical characteristics that were associated with or were positively associated with an increased risk of VTE included history of radiation therapy and severe obesity. Given the frequency of VTE in this population and the trend toward long-term treatment with ICI therapy and improved survival, there is a continued need for awareness of VTE as a comorbidity in the NSCLC population, and appropriate patient management to optimize outcomes.
Acknowledgments
All authors contributed to and approved the manuscript; writing and editorial assistance was provided by Jenny Reinhold, PharmD, of Parexel, funded by Bristol Myers Squibb.
Footnotes
Contributors: AAK was an active participant in the protocol development process and study design conceptualization and had access to the data throughout. The guarantor (AAK) accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.AAK: Conception of design, data analysis, data interpretation. JP: Data analysis, data interpretation. LR: Conception of design, data analysis, data interpretation. RP: Conception of design, data analysis, data interpretation. NH: Conception of design, data analysis, data interpretation. CN: Conception of design, data acquisition, data analysis, data interpretation. JB: Conception of design, data interpretation. KG: Data acquisition, data analysis. TCB: Conception of design, data analysis, data interpretation.
Funding: This work was supported by Bristol Myers Squibb.
Competing interests: AAK reports funding to conduct the study of current manuscript from Bristol Myers Squibb (BMS); consulting fees from Janssen, Bayer, BMS, Pfizer, Sanofi, and Anthos; honoraria from WebMD CME; travel support from Bayer, Pfizer, Sanofi, and Anthos; and an unpaid leadership role as chair from MASAB of NBCA. NH and KG received funding (institutional) to conduct the study of the current manuscript from BMS. KG was an employee of HealthCore when the paper was written. JB reports stock ownership in Anthem. JP, LR, RP, NH, and TCB are employed by and have stock ownership in BMS.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request. Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Roopkumar J, Kim AS, Bicky T, et al. Venous thromboembolism in cancer patients receiving immunotherapy. Blood 2018;132:2510. 10.1182/blood-2018-99-116439 [DOI] [Google Scholar]
- 2.Cohen AT, Katholing A, Rietbrock S, et al. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost 2017;117:57–65. 10.1160/TH15-08-0686 [DOI] [PubMed] [Google Scholar]
- 3.Grover SP, Hisada YM, Kasthuri RS, et al. Cancer therapy-associated thrombosis. Arterioscler Thromb Vasc Biol 2021;41:1291–305. 10.1161/ATVBAHA.120.314378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kartolo A, Yeung C, Moffat GT, et al. Venous thromboembolism events in patients with advanced cancer on immune checkpoint inhibitors. Immunotherapy 2022;14:23–30. 10.2217/imt-2021-0151 [DOI] [PubMed] [Google Scholar]
- 5.Khorana AA, Kuderer NM, McCrae K, et al. Cancer associated thrombosis and mortality in patients with cancer stratified by Khorana score risk levels. Cancer Med 2020;9:8062–73. 10.1002/cam4.3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madison CJ, Melson RA, Conlin MJ, et al. Thromboembolic risk in lung cancer patients receiving systemic therapy. Br J Haematol 2021;194:179–90. 10.1111/bjh.17476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res 2010;125:490–3. 10.1016/j.thromres.2009.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ando Y, Hayashi T, Sugimoto R, et al. Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Invest New Drugs 2020;38:1200–6. 10.1007/s10637-019-00881-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beyer-Westendorf J. Checkpoint inhibitors and thrombosis: what’s up? Blood 2021;137:1569–70. 10.1182/blood.2020009480 [DOI] [PubMed] [Google Scholar]
- 10.Bjørnhart B, Hansen KH, Jørgensen TL, et al. Incidence, risk factors and clinical outcome of venous thromboembolism in non-small cell lung cancer patients receiving immune checkpoint inhibition. Thrombosis Update 2021;4:100056. 10.1016/j.tru.2021.100056 [DOI] [Google Scholar]
- 11.Donnellan E, Khorana AA. Cancer and venous thromboembolic disease: a review. Oncologist 2017;22:199–207. 10.1634/theoncologist.2016-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill H, Robinson M, Lu L, et al. Venous thromboembolism incidence and risk factors in non-small cell lung cancer patients receiving first-line systemic therapy. Thromb Res 2021;208:71–8. 10.1016/j.thromres.2021.10.014 [DOI] [PubMed] [Google Scholar]
- 13.Xiong W. Current status of treatment of cancer-associated venous thromboembolism. Thromb J 2021;19:21. 10.1186/s12959-021-00274-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagchi A, Khan MS, Saraswat A, et al. Increased incidence of thrombotic complications with non-small cell lung cancer necessitates consideration of prophylactic anticoagulation in young individuals. Cureus 2021;13:e17769 10.7759/cureus.17769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gervaso L, Dave H, Khorana AA. Venous and arterial thromboembolism in patients with cancer: JACC: cardiooncology state-of-the-art review. JACC CardioOncol 2021;3:173–90. 10.1016/j.jaccao.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Icht O, Darzi N, Shimony S, et al. Venous thromboembolism incidence and risk assessment in lung cancer patients treated with immune checkpoint inhibitors. J Thromb Haemost 2021;19:1250–8. 10.1111/jth.15272 [DOI] [PubMed] [Google Scholar]
- 17.Khorana AA, Cohen AT, Carrier M, et al. Prevention of venous thromboembolism in ambulatory patients with cancer. ESMO Open 2020;5:e000948. 10.1136/esmoopen-2020-000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T-F, Khorana AA, Carrier M. Thrombotic complications associated with immune checkpoint inhibitors. Cancers 2021;13:4606. 10.3390/cancers13184606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khorana AA. Cancer and coagulation. Am J Hematol 2012;87:S82–7. 10.1002/ajh.23143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khorana AA, DeSancho MT, Liebman H, et al. Prediction and prevention of cancer-associated thromboembolism. Oncologist 2021;26:e2–7. 10.1002/onco.13569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khorana AA, McCrae KR. Risk stratification strategies for cancer-associated thrombosis: an update. Thromb Res 2014;133:S35–8. 10.1016/S0049-3848(14)50006-0 [DOI] [PubMed] [Google Scholar]
- 22.Deschênes-Simard X, Richard C, Galland L, et al. Venous thrombotic events in patients treated with immune checkpoint inhibitors for non-small cell lung cancer: a retrospective multicentric cohort study. Thromb Res 2021;205:29–39. 10.1016/j.thromres.2021.06.018 [DOI] [PubMed] [Google Scholar]
- 23.Nichetti F, Ligorio F, Zattarin E, et al. Is there an interplay between immune checkpoint inhibitors, thromboprophylactic treatments and thromboembolic events? Mechanisms and impact in non-small cell lung cancer patients. Cancers 2020;12:67. 10.3390/cancers12010067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong J, Drobni ZD, Alvi RM, et al. Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur J Cancer 2021;158:99–110. 10.1016/j.ejca.2021.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roopkumar J, Swaidani S, Kim AS, et al. Increased incidence of venous thromboembolism with cancer immunotherapy. Med 2021;2:423–34. 10.1016/j.medj.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Es N, Di Nisio M, Cesarman G, et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study. Haematologica 2017;102:1494–501. 10.3324/haematol.2017.169060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overvad TF, Skjøth F, Piazza G, et al. The Khorana score and venous and arterial thrombosis in patients with cancer treated with immune checkpoint inhibitors: a Danish cohort study. J Thromb Haemost 2022;20:2921–9. 10.1111/jth.15883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902–7. 10.1182/blood-2007-10-116327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attia D, Khorana AA. Evolving treatment options for cancer-related venous thromboembolism. JACC CardioOncol 2020;2:441–2. 10.1016/j.jaccao.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmona-Bayonas A, Gómez D, Martínez de Castro E, et al. A snapshot of cancer-associated thromboembolic disease in 2018-2019: first data from the TESEO prospective registry. Eur J Intern Med 2020;78:41–9. 10.1016/j.ejim.2020.05.031 [DOI] [PubMed] [Google Scholar]
- 31.Moik F, Chan W-SE, Wiedemann S, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood 2021;137:1669–78. 10.1182/blood.2020007878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang MC, Fan D, Sung SH, et al. Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTe study. Med Care 2017;55:e137–43. 10.1097/MLR.0000000000000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 34.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 35.Sheng IY, Gupta S, Reddy CA, et al. Thromboembolism in patients with metastatic urothelial cancer treated with immune checkpoint inhibitors. Target Oncol 2022;17:563–9. 10.1007/s11523-022-00905-x [DOI] [PubMed] [Google Scholar]
- 36.Sussman TA, Li H, Hobbs B, et al. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J Immunother Cancer 2021;9:e001719. 10.1136/jitc-2020-001719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delluc A, Wang T-F. How to treat incidental pulmonary embolism in cancer patients? Recent advances. Kardiol Pol 2021;79:1305–10. 10.33963/KP.a2021.0164 [DOI] [PubMed] [Google Scholar]
- 38.Shinagare AB, Okajima Y, Oxnard GR, et al. Unsuspected pulmonary embolism in lung cancer patients: comparison of clinical characteristics and outcome with suspected pulmonary embolism. Lung Cancer 2012;78:161–6. 10.1016/j.lungcan.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khorana AA, Barnard J, Wun T, et al. Biomarker signatures in cancer patients with and without venous thromboembolism events: a substudy of CASSINI. Blood Adv 2022;6:1212–21. 10.1182/bloodadvances.2021005710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regeneron Pharmaceuticals, Inc . Libtayo [prescribing information]. Tarrytown, NY, 2021. [Google Scholar]
- 41.Merck & Co., Inc . Keytruda [prescribing information]. Whitehouse Station, NJ, 2022. [Google Scholar]
- 42.AstraZeneca Pharmaceuticals LP . Imfinzi [prescribing information]. Wilmington, DE, 2021. [Google Scholar]
- 43.Bristol Myers Squibb . Opdivo [prescribing information]. Princeton, NJ, 2022. [Google Scholar]
- 44.Bristol Myers Squibb . Yervoy [prescribing information]. Princeton, NJ, 2022. [Google Scholar]
- 45.Genentech, Inc . Tecentriq [prescribing information]. South San Francisco, CA, 2022. [Google Scholar]
- 46.Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open 2020;3:e200423. 10.1001/jamanetworkopen.2020.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-006072supp001.pdf (83.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available on reasonable request. Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.