Abstract
To analyze the role of interleukin-10 (IL-10) in bacterial cerebral infections, we studied cerebral listeriosis in IL-10-deficient (IL-10−/−) and wild-type (WT) mice, the latter of which express high levels of IL-10 in both primary and secondary cerebral listeriosis. IL-10−/− mice succumbed to primary as well as secondary listeriosis, whereas WT mice were significantly protected from secondary listeriosis by prior intraperitoneal immunization with Listeria monocytogenes. Meningoencephalitis developed in both strains; however, in IL-10−/− mice the inflammation was more severe and associated with increased brain edema and multiple intracerebral hemorrhages. IL-10−/− mice recruited significantly increased numbers of leukocytes, in particular granulocytes, to the brain, and the intracerebral cytokine (tumor necrosis factor, IL-1, IL-12, gamma interferon, and inducible nitric oxide synthase) and chemokine (crg2/IP-10, RANTES, MuMig, macrophage inflammatory protein 1α [MIP-1α], and MIP-1β) transcription was enhanced compared to that in WT mice. Despite this prominent hyperinflammation, the frequencies of intracerebral L. monocytogenes-specific CD8+ T cells were reduced and the intracerebral bacterial load was not reduced in IL-10−/− mice compared to WT mice. Following intraperitoneal infection, IL-10−/− mice exhibited hepatic hyperinflammation without better bacterial clearance; however, in contrast to the mice with cerebral listeriosis, they did not succumb, illustrating that intrinsic factors of the target organ have a strong impact on the course and outcome of the infection.
Bacterial infections of the central nervous system (CNS) are associated with a high mortality. The poor prognosis of cerebral bacterial infections is explained by several factors including the limited capacity of the brain for regeneration and the impaired protective immune responses in the subarachnoid space, to which low levels of complement contribute. In addition, intracerebral (i.e.) immune responses to offending pathogens may also damage brain tissue.
One bacterium capable of inducing meningitis, encephalitis, and brain abscess is Listeria monocytogenes. In murine cerebral listeriosis the bacterium has a striking affinity for the highly vulnerable structures of the brain stem (45). In the brain, choroid plexus epithelial cells, ependymal cells, neurons, and macrophages are the target cells of L. monocytogenes (45). Inevitably, infected mice succumb to a progressive meningoencephalitis despite mounting a strong i.c. immune reaction. Active systemic immunization with L. monocytogenes prior to i.c. infection improves the clinical course of CNS listeriosis and significantly reduces mortality (48). This protection is mediated by CD4+ and CD8+ T cells and is associated with augmented i.c. tumor necrosis factor (TNF), gamma interferon (IFN-γ), and interleukin-1β (IL-1β) production. In addition, increased amounts of IL-10 mRNA are produced in immunized mice compared to nonimmunized mice (48).
IL-10, which is produced by a variety of cells including macrophages, B cells, subsets of CD4+ and CD8+ T cells, and resident brain cell populations including microglia, choroid plexus epithelial cells, and even neurons, has a strong immunosuppressive capacity (7, 10, 12, 32, 47). IL-10 synthesis is not confined to murine cerebral listeriosis but also occurs in more than 95% of all human bacterial CNS infections (12, 51). Functionally important, IL-10 released into the cerebrospinal fluid during cerebral listeriosis interferes with killing of L. monocytogens by IFN-γ-stimulated peritoneal macrophages in vitro (12). Whereas these data suggest a potentially deleterious role of IL-10 in cerebral listeriosis, other models of viral, protozoal, and bacterial infections have pointed out that the regulatory immunosuppressive properties of IL-10 are required to prevent pathological immune responses (13, 18, 29). Thus, one might speculate that the regulatory activity of IL-10 may be necessary to avoid overshooting of the immune response in cerebral listeriosis.
The aim of the present study was to clarify the functional role of IL-10 in murine cerebral listeriosis. Here we show that endogenous IL-10 is required to prevent immunopathology characterized by hyperinflammation and brain tissue destruction in murine cerebral listeriosis. In the absence of IL-10 (IL-10−/− mice) the protective effect of active systemic immunization on the survival of cerebral listeriosis was completely abrogated although control of bacteria was unimpaired. In systemic listeriosis, hepatic disease was characterized by the same findings; however, IL-10−/− mice did not succumb to the infection, illustrating that intrinsic features of the infected organ strongly influence the outcome.
MATERIALS AND METHODS
Animals.
Breeding pairs of C57BL/6 IL-10−/− mice (five backcrosses to C57BL/6 mice) were obtained from H. Mossmann (Freiburg, Germany). The colony was maintained by intercrossing male IL-10−/− and female heterozygous offspring. Offspring were tested by PCR of tail DNA for the presence of an intact IL-10 gene. C57BL/6 wild-type (WT) mice were obtained from Harlan-Winkelmann (Borchen, Germany). Mice aged 6 to 10 weeks were used for the experiments.
Infectious organisms.
L. monocytogenes (serovar 1/2a, EGD, SLCC 5835) was grown in tryptose soy broth, and aliquots of log-phase cultures were stored at −80°C. For each experiment, L. monocytogenes was thawed from the stock solution and diluted appropriately in sterile pyrogen-free phosphate-buffered saline (PBS) (pH 7.4). The percentage of viable L. monocytogenes cells recovered from the frozen stocks always exceeded 95%. For each experiment, the bacterial dose used for infection was controlled by plating an inoculum on tryptic soy agar.
Experimental procedures.
For i.c. infection, 102 L. monocytogenes cells in 30 μl of PBS were injected into the right caudate nucleus of mice deeply anesthetized with methoxyflurane. For primary systemic infection as well as for immunization of mice 14 or 28 days prior to either i.c. or intraperitoneal (i.p.) challenge, mice were injected i.p. with 104 L. monocytogenes cells in 200 μl of PBS. For systemic challenge, mice received 106 L. monocytogenes i.p. At the indicated days postinoculation (p.i.), mice were perfused intracardially with 0.9% saline in methoxyflurane anethesia. For flow cytometry analysis of brain-derived and liver-derived leukocytes, organs were passed through a 100-mesh stainless steel sieve and leukocytes were separated by Percoll gradient centrifugation (Amersham-Pharmacia, Freiburg, Germany) as described previously (47). For determination of the bacterial load in the liver and brain, tissues were homogenized with tissue grinders. Tenfold serial dilutions of the homogenate were plated on tryptose soy agar. Bacterial colonies were counted after incubation at 37°C for 24 h. For immunohistochemistry on frozen sections, reverse transcription-PCR (RT-PCR) analysis and RNase protection assays (RPA), brains and livers of three to six animals per group were dissected and blocks were mounted on thick filter paper with Tissue-Tek O.T.C. Compound (Miles Scientific, Naperville, Ill.), snap-frozen in isopentane precooled on dry ice, and stored at −80°C. For histology on paraffin sections, mice were anesthetized and perfused with 4% paraformaldehyde in PBS. The brains and livers were removed and fixed with 4% paraformaldehyde for 24 h.
Histopathology.
Immunohistochemistry was performed on frozen sections as described previously (47). In brief, the sections were stained by an indirect immunoperoxidase method using rat anti-mouse CD45 (clone M1/9.3.4.HL.2), CD4 (clone G.K.1.5.), CD8 (clone 2.43), B220 (clone RA3-3A1/6.1), and Ly6-G (clone RB6-8C5) as primary antibodies and peroxidase-linked sheep anti-rat immunoglobulin G (IgG) F(ab')2 (Amersham-Pharmacia) as the secondary antibody. In addition, the avidin-biotin complex technique using rat anti-mouse F4/80 (clone F4/80) and major histocompatibility complex (MHC) classes 1 (clone M1/42.3.9.8HLK) and II (I-Ab,d,q, clone M5/114.15.2) as primary antibodies, biotinylated mouse serum-preadsorbed mouse anti-rat IgG F(ab')2 (Dianova, Hamburg, Germany) as the secondary antibody, and streptavidin-biotin complex (Dakopatts, Hamburg, Germany) was employed. Paraffin sections (4 μm) were stained with hematoxylin and eosin (H&E), cresyl violet, luxol fast blue, and periodic acid-Schiff stain (PAS). L. monocytogenes was demonstrated immunohistochemically by incubating deparaffinized sections with a polyclonal rabbit anti-L. monocytogenes antiserum (Difco, Freiburg, Germany) followed by peroxidase-labeled goat anti-rabbit IgG F(ab')2 fragments (Dianova). Peroxidase reaction products were visualized using 3,3′-diaminobenzidine and H2O2 as cosubstrates. Sections were lightly counterstained with hemalum.
Quantitative assessment and phenotypic characterization of cerebral and hepatic leukocytes.
Brain- and liver-derived leukocytes were analyzed by double immunofluorescence staining followed by flow cytometry as described previously (47). Murine microglia and macrophages were identified by staining with anti-CD45 (LAC)–biotin (Becton Dickinson, Heidelberg, Germany) and anti-F4/80–fluorescein isothiocyanate FITC (Alexis Biochemicals, Grünberg, Germany) followed by avidin-PE/Cy5 (Biozol, Freising, Germany). Inflammatory leukocytes recruited to the brain are CD45high F4/80−, macrophages are CD45high F4/80+, and microglia are CD45low F4/80+. CD4+ and CD8+ T lymphocytes were stained with rat anti-mouse-CD4 followed by goat anti-rat–phycoerythrin (Biozol) and anti-CD8-FITC (Becton-Dickinson). B lymphocytes were detected by staining with anti-CD45R (B220)–FITC (Becton Dickinson) and anti-CD45 (LCA)-biotin (Becton-Dickinson) followed by avidin-phycoerythrin/Cy5. Granulocytes were stained with anti-Ly6-G (GR-1) followed by goat anti-rat–PE and F4/80-FITC. Granulocytes were defined as Ly6-G+ F4/80−. Control staining included incubation of brain-derived leukocytes with unlabeled or fluorochrome-labeled isotype-matched control antibodies. Flow cytometry was performed on a FACScan instrument (Becton-Dickinson, Heidelberg, Germany), and the data were analyzed with Cell Quest Software (Becton-Dickinson).
Detection of cytokine mRNA by RT-PCR.
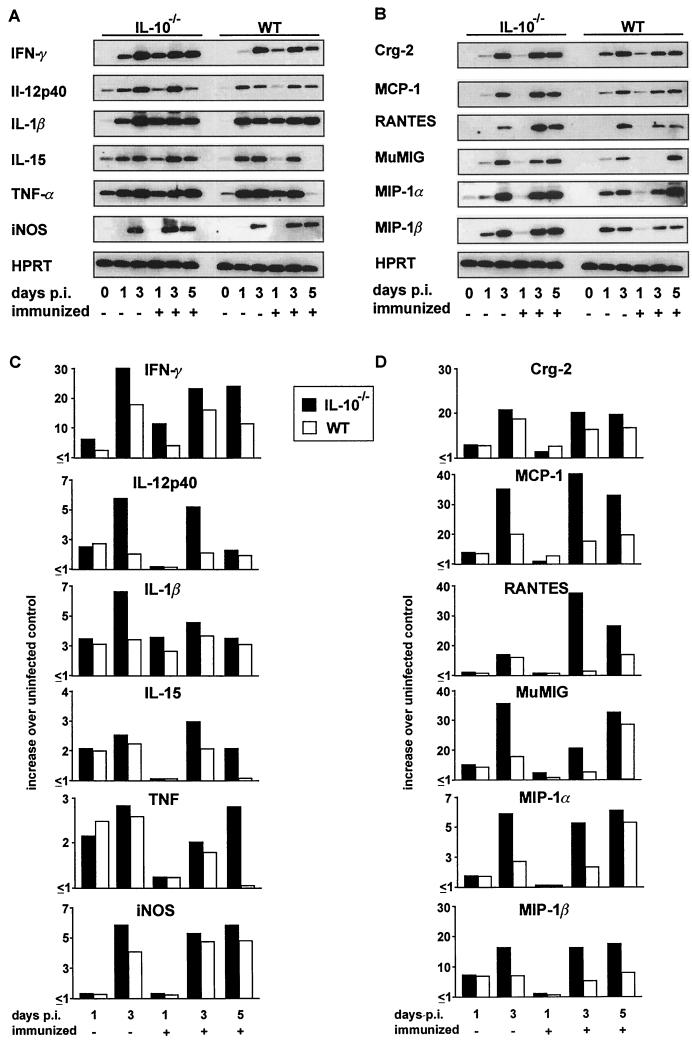
IL-10, IFN-γ, TNF, IL-1β, IL-12p40, IL-15, inducible nitric oxide synthase (iNOS), crg-2/IP-10, MuMig, monocyte chemotactic protein 1 (MCP-1), RANTES, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β mRNA transcript and hydroxyphosphoribosyltransferase (HPRT) mRNA expression was analyzed in brain and liver tissue homogenates by a protocol described in detail previously (6). Primer and probe sequences for cytokines and HPRT were as published previously (6, 47); those for chemokines were as follows: crg-2/IP-10, 5′-CCACGTGTTGAGATCATTGC-3′ (sense), 5′-GCTTACAGTACAGAGCT AGG-3′ (antisense), and 5′-TGTGATGGACAGCAGAGAGC-3′ (probe); MuMig, 5′-GAGGAACCCTAGTGATAAGG-3′ (sense), 5′-GTAGTCTTCCT TGAACGACG-3′ (antisense), and 5′-CCTGCCTAGATCCGGACTCG-3′ (probe); MCP-1, 5′-AGAGAGCCAGACGGAGGAAG-3′ (sense), 5′-GTC ACACTGGTCACTCCTAC-3′ (antisense), and 5′-GAGAGAGGTCTGTG CTGACC-3′ (probe); RANTES, 5′-GGTACCATGAAGATCTCTGC-3′ (sense), 5′-GGGTCAGAATCAAGAAACCC-3′ (antisense), and 5′-CTCTC CCTAGAGCTGCCTCG-3′ (probe); MIP-1α, 5′-CCTGCTCAACATCATGA AGG-3′ (sense), 5′-GAATTGGCGTGGAATCTTCC-3′ (antisense), and 5′-TCTGTACCATGACACTCTGC-3′ (probe); and MIP-1β, 5′-GCAGCTTCACA GAAGCTTTG-3′ (sense), 5′-TCTCAGTGAGAAGCATCAGG-3′ (antisense), and 5′-CAGACAGATCTGTGCTAACC-3′ (probe). PCR amplifications were performed in the linear range of amplification. Quantitation of RNA was performed with a densitometer (Biometra, Göttingen, Germany). The relative intensity of the autoradiographic bands for each cytokine and chemokine was related to the intensity of the autoradiographic band obtained for the internal control, HPRT. The results were expressed as x-fold increases over mRNA levels for the various cytokines and chemokines in the noninfected control animals of the same strain.
RPA.
Total RNA was isolated from the brain and liver by a commercially available RNA isolation kit (Pharmingen). For RPA, a chemokine multiprobe RPA kit (mCK-5b; Pharmingen) was used as specified by the manufacturer. [α-32P]UTP was obtained from ICN (Meckenheim, Germany). Reaction products were detected with an imaging plate (Raytest, Straubenhardt, Germany) and a phosporimager.
ELISPOT assay.
The frequency of L. monocytogenes p60-specific CD4+ T cells and L. monocytogenes-specific CD8+ T cells was determined in an IFN-γ-specific enzyme-linked immunospot (ELISPOT) assay as described in detail previously (14). On day 3 after challenge, leukocytes were isolated from the brain or liver. Freshly isolated leukocytes (105/well) were cocultured with different types of antigen-presenting cell (APC) for the selective detection of L. monocytogenes-specific CD4+ and CD8+ T cells in nitrocellulose-backed 96-well microtiter plates coated with rat anti-mouse IFN-γ monoclonal antibody (MAb). CD4+ T cells specific for L. monocytogenes p60 were detected in the presence of nonimmune syngeneic spleen cells (4 × 105/well), which had been preloaded with 5 μg of purified L. monocytogenes p60 per ml for 4 h (14). CD8+ T cells specific for L. monocytogenes were measured in the presence of L. monocytogenes-infected IC21 cells (H-2b). IC21 cells were infected with L. monocytogenes at a multiplicity of infection of 10 for 60 min. Thereafter, extracellular bacteria were killed by addition of gentamicin (5 μg/ml). To control for the specificity of lymphocytes activated by CD4 and CD8 target cells, 1 × 105 CD4+ T cells or 5 × 104 CD8+ T cells purified from the spleens of WT mice, which had been infected with L. monocytogenes 14 days earlier, were added to either 105 L. monocytogenes-infected IC21 cells or 105 p60-loaded splenic APC. CD4+ and CD8+ T cells were purified by magnetic activated cell sorting (MACS; Milteny, Bergisch Gladbach, Germany). Spleen cells were stained with either FITC-labeled rat anti-mouse CD4 or FITC-conjugated rat anti-mouse CD8 antibodies and separated after secondary labeling with paramagnetic microbeads coupled with mouse anti-FITC isomer 1 antibody (Milteny), applying the standard positive selection protocol provided by the manufacturer. The purity of separated T-cell populations was always between 80 and 90% as controlled by flow cytometry. All ELISPOT assay plates were incubated overnight and developed with biotin-labeled rat anti-mouse IFN-γ, peroxidase-conjugated streptavidin, and aminoethylcarbazole dye solution. The frequency of antigen-specific cells was calculated as the number of spots per leukocytes seeded.
Statistics.
Survival rate, bacterial load, number of inflammatory leukocytes in the brain and liver, and frequencies of L. monocytogenes-specific CD4+ and CD8+ T cells were compared between IL-10−/− and IL-10+/+ mice. For statistical evaluation of these parameters, the χ2 test and Student's t test were used. P < 0.05 was accepted as indicating significance.
RESULTS
Bacterial replication and survival rates.
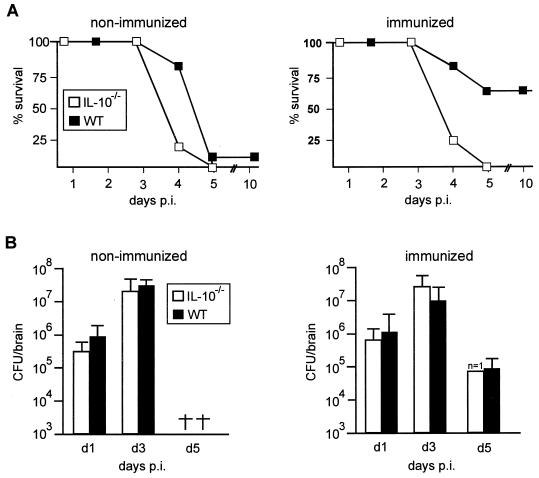
Following i.c. application of 102 L. monocytogenes, all IL-10−/− and WT mice succumbed to the disease before day 5 p.i. (Fig. 1A). The survival of WT mice after systemic immunization was significantly increased compared to that of nonimmunized WT mice (P < 0.05), whereas immunization did not increase the survival rate of IL-10−/− mice ( P < 0.05).
FIG. 1.
(A) Survival rates of nonimmunized and immunized IL-10−/− and WT mice. Ten mice per experimental group were infected i.c. with 102 L. monocytogenes. Immunized animals were infected i.p. with 104 L. monocytogenes 28 days prior to i.c. challenge. Similar results were obtained in four repeat experiments and for mice immunized 14 days prior to cerebral challenge. (B) Bacterial load of nonimmunized and immunized IL-10−/− and WT mice after i.c. infection. At each time point after infection, four to six mice per experimental group were analyzed, except for the group of i.c.-infected immunized IL-10−/− mice, of which only one had survived by day 5 p.i. Data represent the mean and standard deviation of each group. †, deceased. The results of one of three experiments, which yielded similar results, are shown.
To analyze whether the more severe course of cerebral listeriosis of IL-10−/− mice was due to an impaired control of L. monocytogenes, the i.c. bacterial load was determined (Fig. 1B). In nonimmunized IL-10−/− and WT mice, the i.c. bacterial load increased from days 1 to 3 p.i. (P < 0.01 for both groups of mice) and the bacterial titers did not differ significantly between IL-10−/− and WT mice (P > 0.05). In both groups of immunized mice, bacterial titers also increased from days 1 to 3 p.i. From days 3 to 5 p.i., immunized WT mice showed a significantly reduced i.c. bacterial load (P < 0.05), and the bacterial titers in the single surviving IL-10−/− mouse were also reduced on day 5 p.i. Thus, these experiments did not reveal significant differences for the kinetics and numbers of L. monocytogenes cells between IL-10−/− and WT mice; therefore, the death of immunized IL-10−/− mice cannot be attributed to an impaired control of i.c. L. monocytogenes.
Histopathology.
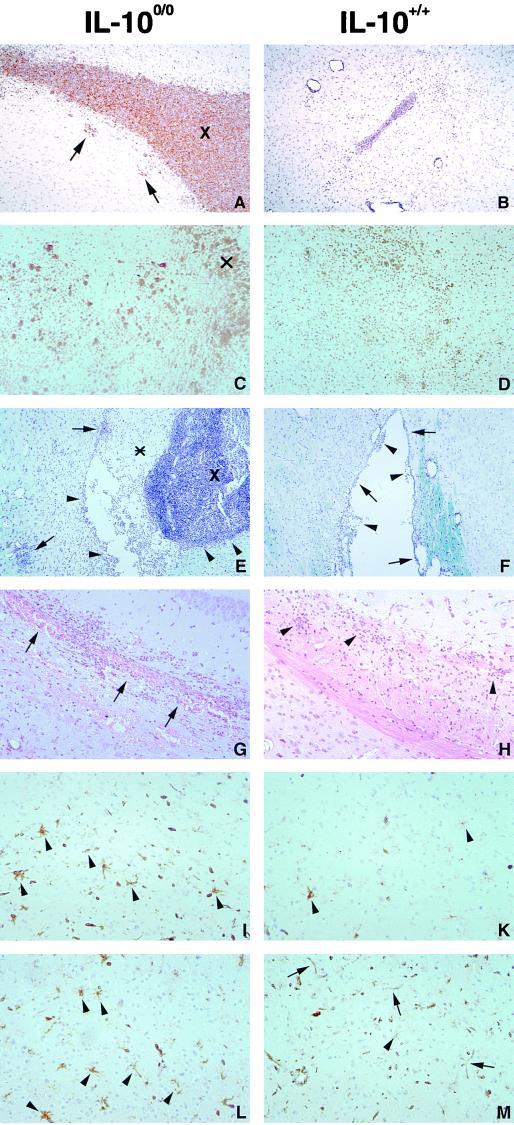
To assess the cause of death in immunized IL-10−/− mice, a detailed histopathological analysis was performed. Nonimmunized IL-10−/− and WT mice developed an ultimately fatal encephalitis and ventriculitis, which was even more severe in IL-10−/− mice (Fig. 2A, C, E, and G). Whereas bacterium-associated infiltrates were circumscribed in WT mice and peaked on day 3 p.i. (Fig. 2B, D, F, and H), inflammation was much more severe in IL-10−/− mice, with leukocytes diffusely flooding the brain parenchyma (Fig. 2C and E). The number of inflammatory leukocytes, in particular granulocytes, was increased in immunized IL-10−/− mice. Furthermore, microglial activation was more prominent and more widespread in IL-10−/− mice, as evidenced by a generalized MHC class II antigen induction (Fig. 2I and L). In contrast, microglial activation was largely confined to the periventricular parenchyma in the vicinity of bacterium-associated inflammatory infiltrates in WT mice (Fig. 2K and M). Interestingly, IL-10−/− mice developed multiple petechial hemorrhages, predominantly in the periventricular parenchyma, including the periaqueductal grey matter of midbrain and pons, as well as within large fiber tracts (Fig. 2G), which showed marked vacuolation, a hallmark of brain edema, and, interestingly, were also heavily infiltrated by inflammatory cells, in particular granulocytes and macrophages (Fig. 2G). In addition, cortical neurons were damaged. Together with prominent ventricular hemorrhages, these changes were severe enough to cause the death of IL-10−/− mice. In contrast, i.c. hemorrhage was not observed in immunized WT mice and edema with neuronal damage was also much less severe in these animals (Fig. 2H). However, some WT mice also developed a necrotizing brain stem encephalitis, which is consistent with a survival rate of 65% in WT mice.
FIG. 2.
Histopathological findings in the L. monocytogenes-infected CNS of nonimmunized and immunized IL-10−/− and WT mice. (A) Numerous bacteria in the grossly enlarged fourth ventricle (X) of an immunized IL-10−/− mouse on day 3 p.i. The ventricular wall has been largely destroyed, and many bacteria have invaded the periventricular brain stem parenchyma (arrows). Anti-L. monocytogenes immunostaining; magnification, ×80. (B) Brain of an immunized WT mouse on day 3 p.i. Bacteria are confined to the lumen of the ventricular system, and the third ventricle is of normal size. Anti-L. monocytogenes immunostaining; magnification, ×80. (C) Large numbers of CD45+ leukocytes are scattered throughout the brain parenchyma in an immunized IL-10−/− mouse on day 3 p.i. X indicates the tissue adjacent to the lateral ventricle. Anti-CD45 (LCA) immunostaining; magnification, ×42. (D) On day 3 p.i., which corresponds to maximal disease activity, leukocytic infiltrates are less prominent in the brain of an immunized WT mouse than in the brains of IL-10−/− mice (C). Anti-CD45 immunostaining; magnification, ×42. (E) Purulent exudate (∗) and numerous leukocytes in the enlarged fourth ventricle (X) of an immunized IL-10−/− mouse on day 5 p.i. Destruction of the ependyma which is infiltrated by leukocytes is evident (arrowheads). Arrows point to parenchymatous infiltrates. Cresyl violet and luxol fast blue staining; magnification, ×42. (F) In contrast to the situation in IL-10−/− mice (E), disease activity has declined in immunized WT mice on day 5 p.i. Residual small infiltrates in the fourth ventricle are shown (arrowheads). The ependyma is largely intact (arrows), and infiltrates are largely absent from the periventricular tissue, except for small perivascular cuffs. Cresyl violet and luxol fast blue staining; magnification, ×42. (G) Multiple hemorrhages (arrows) in the corpus callosum in close association with numerous leukocytes in an immunized IL-10−/− mouse on day 5 p.i. H&E staining; magnification, ×83. (H) In contrast to the situation in IL-10−/− mice (G), there are only small infiltrates (arrowheads) in the corpus callosum of an immunized WT mouse on day 5 p.i. Note the absence of hemorrhages. H&E staining; magnification, ×83. (I) Marked activation of microglia (arrowheads) in the frontal cortex of a nonimmunized IL-10−/− mouse on day 3 p.i. Anti-I-A immunostaining; magnification, ×83. (K) Weak activation of single microglial cells in the brain of a nonimmunized WT mouse, as evidenced by induction of MHC class II antigens (arrowheads), on day 3 p.i. Anti-I-A immunostaining; magnification, ×83. (L) Prominent expression of MHC class II antigens on microglia in the frontal cortex of an immunized IL-10−/− mouse on day 3 p.i. Anti-I-A immunostaining; magnification, ×83. (M) In contrast to the situation in IL-10−/− mice (L), MHC class II antigens are largely confined to blood vessel endothelial cells (arrows) and are only rarely induced on cortical microglia (arrowhead) in an immunized WT mouse on day 3 p.i. Anti-I-A immunostaining; magnification, ×83. Panels A to D and I to M had slight counterstaining with hemalum.
Quantitation and characterization of inflammatory infiltrates.
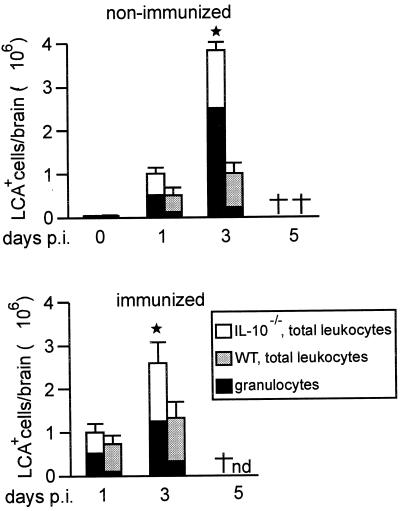
To quantitatively assess the hyperinflammatory reaction in the L. monocytogenes-infected brains of IL-10−/− mice, the number of i.c. leukocytes was determined. On day 3 p.i., both nonimmunized and immunized IL-10−/− mice showed significantly increased numbers of i.c. CD45+ leukocytes compared to WT mice (Fig. 3, P < 0.05). Further phenotyping of i.c. leukocytes by flow cytometry revealed that granulocyte levels were particularly increased in nonimmunized and immunized IL-10−/− mice compared to WT mice (Fig. 3). In contrast, other cell populations including macrophages, B cells, and CD4+ and CD8+ T cells did not differ significantly between IL-10−/− and WT mice (data not shown).
FIG. 3.
Flow cytometric analysis of leukocytes recruited to the brain. In each experiment, the brains of three mice per experimental group were pooled. Leukocytes were isolated by density centrifugation and counted, and the percentage of LCAhigh leukocytes was determined by flow cytometry. The data show the mean number of LCAhigh leukocytes per brain and the standard deviation of two independent experiments. LCAlow brain resident microglial cells were not included. The asterisks indicate that IL-10−/− mice had significantly increased numbers of inflammatory leukocytes compared to the respective group of WT mice; the dagger indicate deceased animals; nd, not done.
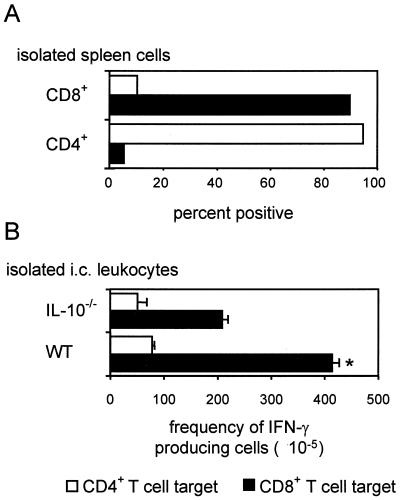
To analyze whether the number of i.c. L. monocytogenes-specific CD4+ and CD8+ T cells was regulated by IL-10, an ELISPOT assay was developed which allows the selective ex vitro quantification of the frequencies of L. monocytogenes-specific CD4+ and CD8+ T cells. These experiments, which were performed with WT mice, revealed that MACS-isolated splenic CD8+ T cells of L. monocytogenes-infected mice reacted with L. monocytogenes-infected IC21 cells but not with p60-loaded syngenic splenic APC (Fig. 4A). Conversely, purified L. monocytogenes-immune CD4+ T cells reacted selectively with p60-loaded spleen cells. Thus, L. monocytogenes-infected IC21 cells served as a target for CD8+ T cells while p60-loaded splenic APC are a target for CD4+ T cells.
FIG. 4.
Specificity of inflammatory T cells in L. monocytogenes-infected organs. (A) Antigen presentation by CD4 (syngenic spleen cells plus p60 protein) and CD8 (L. monocytogenes-infected IC21 cells) target cells was controlled with purified CD4+ and CD8+ T cells isolated from L. monocytogenes-infected WT mice 14 days p.i. Results are shown as the percentage of reactive CD4+ (open bars) and CD8+ (black bars) T cells. (B) IL-10−/− and WT mice were immunized with L. monocytogenes and subsequently boosted by i.e. infection. Three days postchallenge, leukocytes were isolated from the brain; the frequency of IFN-γ-secreting p60-specific CD4+ T cells and L. monocytogenes-specific CD8+ T cells is shown as the number of spots per ×105 leukocytes (open bars; L. monocytogenes p60-specific CD4+ T cells; black bars, L. monocytogenes-specific CD8+ T cells). The indicates statistical significant differences in the numbers of the respective L. monocytogenes-specific T-cell population between IL-10−/− and WT mice. The data represent means and standard deviation of triplicates.
To compare the frequencies of L. monocytogenes-specific CD4+ and CD8+ T cells in IL-10−/− and WT mice, we focused on immunized mice on day 3 p.i., since a strong L. monocytogenes-specific T-cell response can be expected at this stage of infection in the absence of significant mortality. These experiments revealed that the frequency of L. monocytogenes-specific CD8+ T cells was significantly reduced in IL-10−/− mice compared to WT mice (P < 0.05) whereas the frequency of L. monocytogenes-specific CD4+ T cells did not differ significantly between IL-10−/− and WT mice (Fig. 4B).
Taken together, these data show that endogenous IL-10 significantly influences the number of leukocytes recruited to the brain, the composition of i.c. infiltrates, and the frequencies of i.c. L. monocytogenes-specific CD8+ T cells.
Cytokine and chemokine mRNA expression in IL-10−/− mice.
To determine the influence of endogenous IL-10 on the i.c. cytokine response and to analyze whether the increased recruitment of leukocytes to the brains of L. monocytogenes-infected IL-10−/− mice was correlated with an increased i.c. chemokine expression, the mRNA expression of a panel of cytokines and chemokines was studied. In general, IL-10−/− and WT mice produced the same cytokines but the cytokines were induced earlier and reached increased levels in IL-10−/− mice compared to WT animals (Fig. 5A and C). In the brains of uninfected IL-10−/− and WT mice, faint signals of TNF, IL-12p40 and IL-15 mRNA were occasionally detected (Fig. 5A). In cerebral listeriosis, both nonimmunized and immunized IL-10−/− mice and WT mice upregulated these cytokines and showed a de novo expression of IFN-γ, IL-1β, and iNOS mRNA.
FIG. 5.
RT-PCR analysis of cytokine (A) and chemokine (B) mRNA production in the L. monocytogenes-infected brain. At the indicated time points, three mice per experimental group were analyzed, and representative autoradiograms are shown. The relative levels of cytokine (C) and chemokine (D) mRNA expression in the brain are shown. Data are expressed as relative increase over cytokine and chemokine mRNA levels in uninfected mice from the respective mouse strain. Values represent data from one of three independent experiments, which yielded similar results.
In the normal brains of IL-10−/− and WT mice, chemokine mRNAs were not detected by RT-PCR (Fig. 5B). In nonimmunized and immunized IL-10−/− mice, crg-2/IP-10, MCP-1, MuMIG, MIP-1α, MIP-1β, and RANTES mRNAs were induced on infection. Both RT-PCR and RPA demonstrated slightly stronger chemokine mRNA signals in both nonimmunized and immunized IL-10−/− mice than in WT animals (Fig. 5B and D; only the RT-PCR is shown).
Systemic listeriosis in IL-10−/− and WT mice.
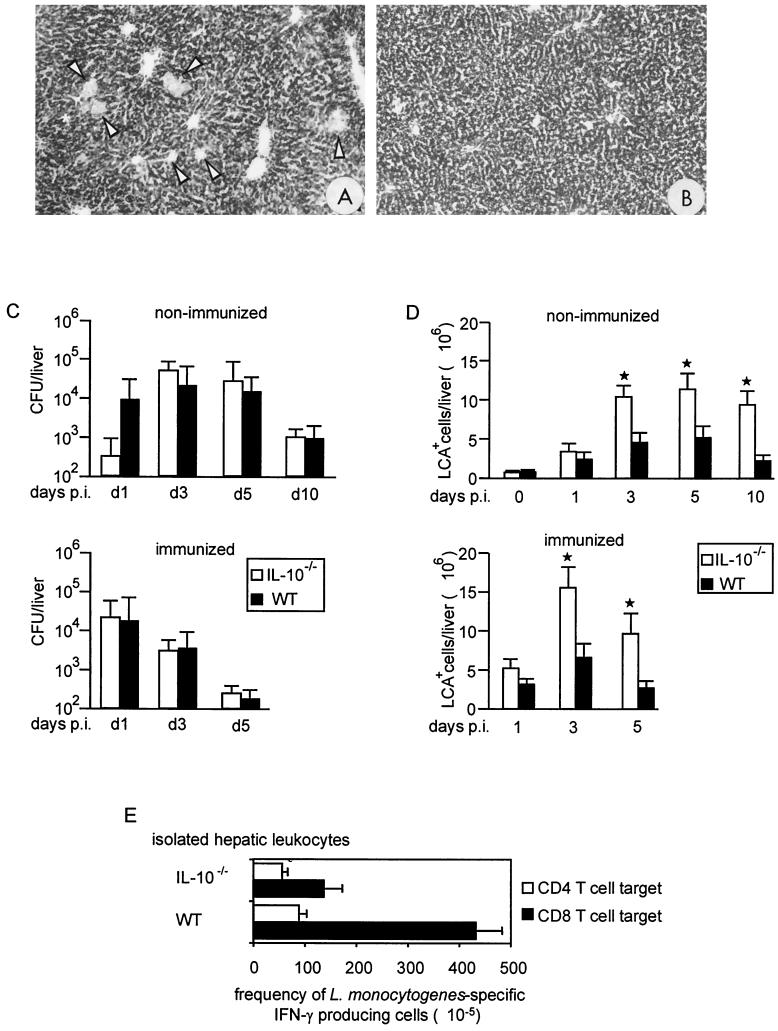
To evaluate whether the negative effect of IL-10 deficiency on the course of listeriosis is unique to the CNS, we also analyzed systemic listeriosis in IL-10−/− and WT mice. All IL-10−/− and WT mice survived primary and secondary i.p. infection with 104 or 106 L. monocytogenes cells, respectively (data not shown). Histopathology revealed that animals of both strains developed small bacterium-associated granulomas containing CD45+ leukocytes. However, nonimmunized and immunized IL-10−/− mice exhibited a larger number of inflammatory hepatic infiltrates than did WT mice, and the infiltrates in the IL-10−/− mice were less circumscribed. This resulted in more severe hepatic damage in IL-10−/− mice, as evidenced by a more pronounced glycogen loss by hepatocytes (Fig. 6A and B). The hepatic bacterial load did not differ significantly between IL-10−/− and WT mice, except for day 1 p.i., when nonimmunized IL-10−/− mice had a significantly lower bacterial load than WT mice did (Fig. 6C). A quantitative analysis of the hepatic leukocytes by flow cytometry showed that after day 1 p.i., both nonimmunized and immunized IL-10−/− mice had significantly increased numbers of LCA+ inflammatory leukocytes compared to WT mice (Fig. 6D). The frequencies of L. monocytogenes-specific CD8+ T cells were also significantly reduced in the livers of i.p.-infected IL-10−/− mice compared to WT mice (P < 0.01), thereby paralleling cerebral listeriosis (Fig. 6E). As in a previous study (5), both nonimmunized and immunized IL-10−/− mice transcribed increased hepatic levels of IFN-γ, TNF, IL-1β, IL-12p40, and IL-15 mRNA on days 1, 3, and 5 p.i. (data not shown).
FIG. 6.
Systemic listeriosis in IL-10−/− and WT mice. (A and B) There are markedly more inflammatory infiltrates (arrowheads) in the liver of a nonimmunized IL-10−/− mouse (A) than in the liver of a nonimmunized WT mouse (B) on day 5 p.i. PAS staining; magnification, ×50. (C) Bacterial loads of nonimmunized and immunized IL-10−/− and WT mice after i.p. infection. At each time point after infection, four to six mice per experimental group were analyzed. The mean and standard deviation of each group is shown. The results of one of three experiments, which yielded similar results, are shown. (D) Flow cytometric analysis of leukocytes recruited to the liver. The percentage of LCAhigh leukocytes was determined by flow cytometry. The data show the mean number of LCAhigh leukocytes per liver and the standard deviation of two independent experiments. The asterisks indicate that IL-10−/− mice had significantly increased numbers of inflammatory leukocytes compared to the respective group of WT mice. (E) Specificity of inflammatory T cells in the L. monocytogenes-infected liver. L. monocytogenes-immune mice were boosted i.p. with L. monocytogenes. On day 3 after the challenge infection, hepatic leukocytes were isolated. The frequency of IFN-γ secreting p60-specific CD4+ T cells and L. monocytogenes-specific CD8+ T cells was determined by use of an ELISPOT assay. The frequency of reactive cells is shown as the number of spots per 105 leukocytes. The data represent the mean ± standard deviation of experiments performed in triplicate.
DISCUSSION
Cerebral listeriosis of IL-10−/− mice was characterized by immunopathology with severe hyperinflammation and multiple i.c. hemorrhages, which were completely absent from the brains of WT mice. These i.c. hemorrhages, in association with a pronounced brain edema, significantly damaged neurons in IL-10−/− mice and, in association with the strong and ubiquitous encephalitis, caused their death. A recent autopsy study illustrated that i.c. hemorrhages and a severe brain edema are also characteristic findings in fatal human Staphylococcus aureus meningitis (28), which underscores the pathogenetic relevance of these neurological complications.
The development of i.c. hemorrhages and an aggravated brain edema in IL-10−/− mice may be influenced by several, non-mutually exclusive factors including hyperinflammation, increased production of cytokines and chemokines, and stronger activation of microglial cells, which are all associated with the pathogenesis of bacterial meningitis and, in particular, with the development of brain edema (17, 38, 39, 44).
Hyperinflammation in cerebral listeriosis of IL-10−/− animals was characterized by significantly increased numbers of i.c. leukocytes, in particular granulocytes. The prominent increase in the number of granulocytes indicates that IL-10 may inhibit their recruitment, an assumption supported by the negative influence of macrophage-derived IL-10 on the recruitment of granulocytes in a murine model of peritoneal inflammation (2). An anti-inflammatory activity of IL-10 has also been observed in other infectious diseases including viral and protozoal cerebral infections (7, 29). In contrast, intrathecal application of exogenous IL-10 augmented the recruitment of leukocytes into the cerebrospinal fluid in rat pneumococcal meningitis as early as 6 h p.i.; later time points were, however, not studied (23). Thus, in bacterial meningitis, the additional i.c. application of IL-10 may yield other effects than endogenously produced IL-10. Interestingly, in rat pneumococcal meningitis, the i.p. application of IL-10 did not affect the recruitment of leukocytes to the brain but reduced the extent of brain edema, indicating that the functional activities of systemically and intrathecally applied IL-10 may differ (23).
The hyperinflammatory i.c. immune response of both nonimmunized and immunized IL-10−/− mice was characterized by an increased transcription of crg2/IP-10, RANTES, MuMIG, MIP-1α, and MIP-1β mRNAs, which is in agreement with a direct regulation of lipopolysaccharide-inducible chemokines including crg2/IP-10, MIP-1α, MIP-1β, and RANTES by IL-10 (25, 34). Interestingly, chemokine levels increased in parallel with the number of i.c. inflammatory leukocytes and did not peak before a significant number of leukocytes had been recruited to the brain. These findings indicate that leukocytes were either an important source of chemokines or critical inducers of these leukocyte-attracting molecules, which is in accordance with a previous study on the induction of MIP-1α, MIP-1β, and MIP-2 in murine L. monocytogenes meningoencephalitis (49).
Cerebral listeriosis of IL-10−/− mice was characterized by increased levels of TNF, IL-1β, IL-12, IL-15, iNOS, and IFN-γ mRNA. Although all these cytokines are protective in systemic listeriosis (3, 16, 19, 27, 33, 36, 40, 41, 50), their overproduction may be toxic for the brain. TNF, IL-1, and NO induce cerebral edema; TNF may additionally be involved in the apoptosis of cortical and hippocampal neurons (4, 24, 44), which has also been observed in L. monocytogenes meningoencephalitis (46). IFN-γ, IL-12, and IL-15 have a proinflammatory activity, augment the production of TNF, IL-1, and NO, and thus may also contribute to immune-mediated damage of the brain. A critical role of IL-10 in the expression of TNF has also been observed in experimental pneumococcal meningitis (35), in lipopolysaccharide-induced i.c. TNF production (1), and in protozoal and viral encephalitis (7, 22, 29).
A striking difference between IL-10−/− and WT mice was the prominent and widespread microglial MHC class II expression in IL-10−/− mice and a rather weak, locally restricted microglial MHC class II expression in WT mice. This increased activation of microglia in IL-10−/− mice may be directly caused by the absence of IL-10, since IL-10 suppresses microglial activation including MHC class II expression and their release of TNF-α in vitro (11). The pronounced microglial activation in IL-10−/− mice may be detrimental and may contribute to the fatal outcome of immunized IL-10−/− mice, since activated microglia produces several neurotoxic mediators and excitatory amino acids such as glutamate, which may also contribute to neuronal damage (37).
Interestingly, the hyperinflammatory i.c. immune response in IL-10−/− mice was not associated with an increased clearance of L. monocytogenes from the brain. Since both CD4+ and CD8+ T cells play a pivotal protective role in cerebral listeriosis (48), the frequencies of L. monocytogenes-specific CD4+ and CD8+ T cells in cerebral infiltrates were assessed. In our experiments, the failure of IL-10−/− mice to more effectively eliminate i.c. bacteria correlated with lower i.c. frequencies of L. monocytogenes-specific CD8+ T cells in their brains while the favorable prognosis of IL-10+/+ mice was associated with increased frequencies of these cells. These findings indicate that the regulatory activity of IL-10 is crucial for focusing the i.c. immune response on antigen-specific CD8+ T-cell responses, which is in agreement with recent studies illustrating that IL-10 favors the development of antigen-specific T cells, in particular CD8+ T cells (9, 15, 30, 31, 42, 43). Since IL-10−/− mice expressed increased levels of IFN-γ, a key factor for the clearance of L. monocytogenes, but lacked a more efficient clearance of the bacteria, we assume that the reduced frequency of L. monocytogenes-specific CD8+ T cells, which are able to eradicate L. monocytogenes by Fas- and perforin-dependent lysis of infected host cells (20, 21), accounts for this observation. In addition to Listeria-specific CD4+ and CD8+ T cells, NK cells (8) and bystander T cells may contribute to the increased IFN-γ expression of IL-10−/− mice. In fact, it has been demonstrated that T cells of IL-10−/− mice produce much higher levels of IFN-γ than do T cells of WT mice after CD3 ligation, i.e., after antigen-independent stimulation (26).
To analyze whether the hyperinflammatory immune reaction is unique to cerebral listeriosis, we compared meningoencephalitis with systemic listeriosis. We found that IL-10 deficiency did not alter hepatic clearance of the bacteria but resulted in an overshooting immune response with the same characteristics as depicted for cerebral listeriosis. Only on day 1 p.i. did the livers of nonimmunized IL-10−/− animals contain significantly reduced numbers of L. monocytogenes, which is in agreement with a previous study showing an increased early resistance of mice to L. monocytogenes after IL-10 neutralization (52). However, our findings are only partially in agreement with another study of systemic listeriosis in IL-10−/− mice (5). In these experiments, IL-10−/− mice had an increased hepatic cytokine production in association with a more effective clearance of the pathogen from the liver in both primary and secondary listeriosis, thereby leading to a less severe hepatic damage. At present, the reason for these divergent findings cannot be determined, but the reduced frequencies of L. monocytogenes-specific CD4+ and CD8+ T cells in the hepatic infiltrates may account for the lack of more effective bacterial clearance in our experiments.
In conclusion, the present study on bacterial meningoencephalitis demonstrates that IL-10-mediated immunosuppression is required to ensure the finely tuned balance between pro- and anti-inflammatory mediators and to overcome the high vulnerability of the brain to immune-mediated damage.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (Schl 392/3-1).
We thank W. Müller for providing IL-10−/− mice; E. Neumann, O. Schmidt, and N. Kaefer for expert technical assistance; and H. U. Klatt for photographical help.
REFERENCES
- 1.Agnello D, Villa P, Ghezzi P. Increased tumor necrosis factor and interleukin-6 production in the central nervous system of interleukin-10-deficient mice. Brain Res. 2000;869:241–243. doi: 10.1016/s0006-8993(00)02392-1. [DOI] [PubMed] [Google Scholar]
- 2.Ajuebor M N, Das A M, Virag L, Flower R J, Szabo C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;162:1685–1691. [PubMed] [Google Scholar]
- 3.Beckerman K P, Rogers H W, Corbett J A, Schreiber R D, McDaniel M L, Unanue E R. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993;150:888–895. [PubMed] [Google Scholar]
- 4.Bogdan I, Leib S L, Bergeron M, Chow L, Täuber M G. Tumor necrosis factor-alpha contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J Infect Dis. 1997;176:693–697. doi: 10.1086/514092. [DOI] [PubMed] [Google Scholar]
- 5.Dai W J, Köhler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 6.Deckert-Schlüter M, Albrecht S, Hof H, Wiestler O D, Schlüter D. Dynamics of the intracerebral and splenic cytokine mRNA production in Toxoplasma gondii-resistant and -susceptible congenic strains of mice. Immunology. 1995;85:408–418. [PMC free article] [PubMed] [Google Scholar]
- 7.Deckert-Schlüter M, Buck C, Weiner D, Kaefer N, Rang A, Hof H, Wiestler O D, Schlüter D. Interleukin-10 downregulates the intracerebral immune response in chronic Toxoplasma encephalitis. J Neuroimmunol. 1997;76:167–76. doi: 10.1016/s0165-5728(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 8.Dunn P L, North R J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebert E C. IL-10 enhances IL-2-induced proliferation and cytotoxicity by human intestinal lymphocytes. Clin Exp Immunol. 2000;119:426–432. doi: 10.1046/j.1365-2249.2000.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flesch I E, Kaufmann S H. Role of macrophages and alpha beta T lymphocytes in early interleukin 10 production during Listeria monocytogenes infection. Int Immunol. 1994;6:463–468. doi: 10.1093/intimm/6.3.463. [DOI] [PubMed] [Google Scholar]
- 11.Frei K, Lins H, Schwerdel C, Fontana A. Antigen presentation in the central nervous system. The inhibitory effect of IL-10 on MHC class II expression and production of cytokines depends on the inducing signals and the type of cell analyzed. J Immunol. 1994;152:2720–2728. [PubMed] [Google Scholar]
- 12.Frei K, Nadal D, Pfister H W, Fontana A. Listeria meningitis: identification of a cerebrospinal fluid inhibitor of macrophage listericidal function as interleukin 10. J Exp Med. 1993;178:1255–61. doi: 10.1084/jem.178.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 14.Geginat G, Nichterlein T, Kretschmar M, Schenk S, Hof H, Lalic-Multhaler M, Goebel W, Bubert A. Enhancement of the Listeria monocytogenes p60-specific CD4 and CD8 T cell memory by nonpathogenic Listeria innocua. J Immunol. 1999;162:4781–4789. [PubMed] [Google Scholar]
- 15.Groux H, Bigler M, de Vries J E, Roncarolo M G. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–3193. [PubMed] [Google Scholar]
- 16.Havell E A, Moldawer L L, Helfgott D, Kilian P L, Sehgal P B. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992;148:1486–1492. [PubMed] [Google Scholar]
- 17.Holmin S, Mathiesen T. Intracerebral administration of interleukin-Ibeta and induction of inflammation, apoptosis, and vasogenic edema. J Neurosurg. 2000;92:108–120. doi: 10.3171/jns.2000.92.1.0108. [DOI] [PubMed] [Google Scholar]
- 18.Holscher C, Mohrs M, Dai W J, Köhler G, Ryffel B, Schaub G A, Mossmann H, Brombacher F. Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect Immun. 2000;68:4075–4083. doi: 10.1128/iai.68.7.4075-4083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 20.Jensen E R, Glass A A, Clark W R, Wing E J, Miller J F, Gregory S H. Fas (CD95)-dependent cell-mediated immunity to Listeria monocytogenes. Infect Immun. 1998;66:4143–4150. doi: 10.1128/iai.66.9.4143-4150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kägi D, Ledermann B, Burki K, Hengartner H, Zinkernagel R M. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- 22.Knoblach S M, Faden A I. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Exp Neurol. 1998;153:143–151. doi: 10.1006/exnr.1998.6877. [DOI] [PubMed] [Google Scholar]
- 23.Koedel U, Bernatowicz A, Frei K, Fontana A, Pfister H W. Systemically (but not intrathecally) administered IL-10 attenuates pathophysiologic alterations in experimental pneumococcal meningitis. J Immunol. 1996;157:5185–5191. [PubMed] [Google Scholar]
- 24.Koedel U, Bernatowicz A, Paul R, Frei K, Fontana A, Pfister H W. Experimental pneumococcal meningitis: cerebrovascular alterations, brain edema, and meningeal inflammation are linked to the production of nitric oxide. Ann Neurol. 1995;37:313–323. doi: 10.1002/ana.410370307. [DOI] [PubMed] [Google Scholar]
- 25.Kopydlowski K M, Salkowski C A, Cody M J, van Rooijen N, Major J, Hamilton T A, Vogel S N. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163:1537–1544. [PubMed] [Google Scholar]
- 26.Kuhn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 27.Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan E B, Bartfai T, Solorzano C, Moldawer L L, Chizzonite R, McIntyre K W. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 28.Lerche A, Rasmussen N, Wandall J H, Bohr V A. Staphylococcus aureus meningitis: a review of 28 consecutive community-acquired cases. Scand J Infect Dis. 1995;27:569–573. doi: 10.3109/00365549509047069. [DOI] [PubMed] [Google Scholar]
- 29.Lin M T, Hinton D R, Parra B, Stohlman S A, van der Veen R C. The role of IL-10 in mouse hepatitis virus-induced demyelinating encephalomyelitis. Virology. 1998;245:270–280. doi: 10.1006/viro.1998.9170. [DOI] [PubMed] [Google Scholar]
- 30.Macatonia S E, Doherty T M, Knight S C, O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993;150:3755–3765. [PubMed] [Google Scholar]
- 31.MacNeil I A, Suda T, Moore K W, Mosmann T R, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167–4173. [PubMed] [Google Scholar]
- 32.Moore K W, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 33.Nakane A, Minagawa T, Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988;56:2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olszyna D P, Pajkrt D, Lauw F N, van Deventer S J, van Der Poll T. Interleukin 10 inhibits the release of CC chemokines during human endotoxemia. J Infect Dis. 2000;181:613–620. doi: 10.1086/315275. [DOI] [PubMed] [Google Scholar]
- 35.Paris M M, Hickey S M, Trujillo M, Ahmed A, Olsen K, McCracken G H., Jr The effect of interleukin-10 on meningeal inflammation in experimental bacterial meningitis. J Infect Dis. 1997;176:1239–1246. doi: 10.1086/514118. [DOI] [PubMed] [Google Scholar]
- 36.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 37.Piani D, Spranger M, Frei K, Schaffner A, Fontana A. Macrophage-induced cytotoxicity of N-methyl-d-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. Eur J Immunol. 1992;22:2429–2436. doi: 10.1002/eji.1830220936. [DOI] [PubMed] [Google Scholar]
- 38.Quagliarello V J, Wispelwey B, Long W J, Jr, Scheld W M. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J Clin Investig. 1991;87:1360–1366. doi: 10.1172/JCI115140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramilo O, Saez-Llorens X, Mertsola J, Jafari H, Olsen K D, Hansen E J, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken G H., Jr Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers H W, Sheehan K C, Brunt L M, Dower S K, Unanue E R, Schreiber R D. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci USA. 1992;89:1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 42.Rowbottom A W, Lepper M A, Garland R J, Cox C V, Corley E G. Interleukin-10-induced CD8 cell proliferation. Immunology. 1999;98:80–89. doi: 10.1046/j.1365-2567.1999.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santin A D, Hermonat P L, Ravaggi A, Bellone S, Pecorelli S, Roman J J, Parham G P, Cannon M J. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8(+) cytotoxic T lymphocytes. J Virol. 2000;74:4729–4737. doi: 10.1128/jvi.74.10.4729-4737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlüter D, Chahoud S, Lassmann H, Schumann A, Hof H, Deckert-Schlüter M. Intracerebral targets and immunomodulation of murine Listeria monocytogenes meningoencephalitis. J Neuropathol Exp Neurol. 1996;55:14–24. doi: 10.1097/00005072-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Schlüter D, Domann E, Buck C, Hain T, Hof H, Chakraborty T, Deckert-Schlüter M. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect Immun. 1998;66:5930–5938. doi: 10.1128/iai.66.12.5930-5938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlüter D, Kaefer N, Hof H, Wiestler O D, Deckert-Schlüter M. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am J Pathol. 1997;150:1021–1035. [PMC free article] [PubMed] [Google Scholar]
- 48.Schlüter D, Oprisiu S B, Chahoud S, Weiner D, Wiestler O D, Hof H, Deckert-Schlüter M. Systemic immunization induces protective CD4+ and CD8+ T cell-mediated immune responses in murine Listeria monocytogenes meningoencephalitis. Eur J Immunol. 1995;25:2384–2391. doi: 10.1002/eji.1830250839. [DOI] [PubMed] [Google Scholar]
- 49.Seebach J, Bartholdi D, Frei K, Spanaus K S, Ferrero E, Widmer U, Isenmann S, Strieter R M, Schwab M, Pfister H, et al. Experimental Listeria meningoencephalitis. Macrophage inflammatory protein-1 alpha and -2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J Immunol. 1995;155:4367–4375. [PubMed] [Google Scholar]
- 50.Tripp C S, Gately M K, Hakimi J, Ling P, Unanue E R. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- 51.van Furth A M, Seijmonsbergen E M, Langermans J A, Groeneveld P H, de Bel C E, van Furth R. High levels of interleukin 10 and tumor necrosis factor alpha in cerebrospinal fluid during the onset of bacterial meningitis. Clin Infect Dis. 1995;21:220–222. doi: 10.1093/clinids/21.1.220. [DOI] [PubMed] [Google Scholar]
- 52.Wagner R D, Maroushek N M, Brown J F, Czuprynski C J. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect Immun. 1994;62:2345–2353. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]