Abstract
Background
Acute kidney injury (AKI) is the most common major complication of cardiac surgery field. The purpose of this study is to investigate the association between acute kidney injury and the prognoses of cardiac surgery patients in the Medical Information Mart for Intensive Care III (MIMIC-III) database.
Methods
Clinical data were extracted from the MIMIC-III database. Adult (≥18 years) cardiac surgery patients in the database were enrolled. Multivariable logistic regression analyses were employed to assess the associations between acute kidney injury (AKI) comorbidity and 30-day mortality, 90-day mortality and hospital mortality. Different adjusting models were used to adjust for potential confounders.
Results
A total of 6,002 patients were involved, among which 485 patients (8.08%) had comorbid AKI. Patients with AKI were at higher risks of prolonged ICU stay, hospital mortality, 90-day mortality (all P < 0.001), and 30-day mortality (P = 0.008). AKI was a risk factor for hospital mortality [Model 1, OR (95% CI) = 2.50 (1.45–4.33); Model 2, OR (95% CI) = 2.44 (1.48–4.02)], 30-day mortality [Model 1, OR (95% CI) = 1.84 (1.05–3.24); Model 2, OR (95% CI) = 1.96 (1.13–3.22)] and 90-day mortality [Model 1, OR (95% CI) = 2.05 (1.37–3.01); Model 2, OR (95% CI) = 2.76 (1.93–3.94)]. Higher hospital mortality, 30-day mortality and 90-day mortality was observed in higher KDIGO grade for cardiac surgery patients with AKI (all P < 0.05).
Conclusion
Comorbid AKI increased the risk of hospital mortality, 30-day mortality, and 90-day mortality of cardiac surgery patients in the MIMIC-III database.
Keywords: acute kidney injury, risk factor, prognosis, MIMIC-III database, cardiac surgery
Introduction
Acute kidney injury (AKI) is the most common major complication of cardiac surgery field (1). More than two million cardiac surgeries are performed throughout the world each year, and the incidence of cardiac surgery-associated AKI is between 5.0% and 42.0% (2, 3). Cardiac surgery-associated AKI (CSA-AKI) is independently associated with increased morbidity and mortality, and is the second most common cause of AKI in the intensive care unit (ICU) setting (post-sepsis) (4). Severe CSA-AKI is correlated with 3–8-fold higher perioperative mortality, as well as prolonged lengths of stay in the ICU and hospital (5). The risk of death associated with AKI remains high for 10 years after cardiac surgery regardless of other risk factors, even for patients with complete renal recovery (3). However, most of the current studies on AKI in patients undergoing cardiac surgery took all patients undergoing cardiac surgery as research objects, while few studies take patients undergoing cardiac surgery in intensive care unit as research objects. Cardiac surgery patients in the intensive care unit may be more affected by AKI because their condition is more severe.
The Medical Information Mart for Intensive Care (MIMIC)-III v 1.4 is an openly available US-based critical care database (6). The MIMIC-III is a large, integrated, deidentified, comprehensive clinical dataset. The database contains data on all patients treated in the ICUs of the Beth Israel Deaconess Medical Center (BIDMC) in Boston, MA, from June 1, 2001 to October 31, 2012. The database includes 380 laboratory measurements and hourly records, as well as basic information, laboratory results, imaging examinations, diagnoses, and other clinical information. The International Classification of Diseases, 9th revision (ICD-9) was used to describe precise diseases.
In the current study, we aimed to evaluate the association of AKI with hospital mortality, 30-day mortality, and 90-day mortality in patients from the MIMIC-III database who underwent on-pumped cardiac surgery.
Methods
Data source
We used a retrospective cohort study design from MIMIC-III database (6) and selected the clinical data of patients who stayed in the ICU of the BIDMC between 2001 and 2012. The institutional review boards of both the BIDMC and the Massachusetts Institute of Technology Affiliates approved the access to the database. No informed consent was required because all of the data were deidentified.
Patient selection
Clinical data of eligible patients in the MIMIC-III database will be selected for entry into this study: (1) patients who underwent pump heart surgery; (2) patients with ages older than 18 years; and (3) patients with routine preoperative blood examinations within the first 24 h of admission.
Patients meeting criteria for AKI was according to the Kidney Disease: Improving Global Outcomes (KDIGO), which was also used in the MIMIC III database analysis reported previously (7). Briefly, AKI stages were defined by both serum creatinine and the volume of urine output during the first 48 h after ICU admission. Stage 1 was defined by increase in serum creatinine by ≥0.3 mg/dl (≥26.5 µmol/L) within 48 h after ICU admission or after cardiac surgery, or increase in serum creatinine of 1.5–1.9-fold of the baseline level, or urine output <0.5 ml/kg/h for 6–12 h. Stage 2 was defined by increase in serum creatinine of 2.0–2.9-fold of the baseline level, or urine output <0.5 ml/kg/h for ≥12 h. Stage 3 was defined by Increase in serum creatinine of 3.0-fold of the baseline level or increase in serum creatinine to ≥4.0 mg/dl (≥353.6 µmol/L), or urine output <0.3 ml/kg/h for ≥24 h or anuria for ≥12 h. Minimum of the serum creatinine values available within the 7 days before admission was used as the baseline serum creatinine. When the pre-admission serum creatinine was not available, the first serum creatinine measured at admission was used as the baseline serum creatinine.
Data extraction
All of the data were obtained and extracted by using the Structured Query Language (SQL), and pgAdmin4 for PostgreSQL was used as the administrative platform. The extracted data mainly included demographics (age and sex), vital signs (diastolic blood pressure (DBP), heart rate (HR), respiratory rate (RR), systolic blood pressure (SBP), percutaneous oxygen saturation (SpO2), and temperature), comorbidities (AKI, cardiac arrhythmias, chronic pulmonary disease, congestive heart failure, hypertension, liver disease, peripheral vascular disorder, pulmonary circulation disorder, uncomplicated or complicated diabetes, and valvular disease), laboratory events (peripheral white blood cell count, platelet count, serum creatinine, serum glucose, serum potassium, and serum sodium), SAPS II and SOFA score, and coronary bypass artery grafting (CABG). When considering that the proportion of missing data for each variable was <1.5%, we directly omitted these data in further analyses.
Outcome variables
The following outcome variables were extracted: 30-day and 90-day mortality (post-ICU-admission), hospital mortality, and ICU-stay length. Because the patient may have received more than one ICU hospitalization during a single hospitalization, the length of ICU stay is entirely determined by the first ICU hospitalization.
Statistical analysis
The continuous variables are presented as the mean ± standard deviation or median (interquartile range), and were compared via t-test or Mann-Whitney U tests. The categorical data are presented as numbers with proportions and were analyzed via the χ2-test. Logistic regression with the uni-/multi-variate analyses was used for identifying independent prognostic factors of mortality (hospital, 30-day, and 90-day) after cardiac surgery. Two different models were designed for adjusting potential confounders. Model 1 was adjusted for cardiac arrhythmias, congestive heart failure, hypertension liver disease, SBP, and valvular disease. Moreover, Model 2 was adjusted for age, height, and weight. P-values of less than 0.05 were considered to indicate statistical significance. Further, we constructed the receiver operating characteristic (ROC) curves and calculated and analyzed the area under the curve (AUC), sensitivity, and specificity. All statistical analyses were performed using STATA, version 14.0 (StataCorp, College Station, TX).
Results
Baseline characteristics of the study population
In total, 6,002 patients who met the selection criteria participated in this study, among which 485 patients (8.08%) had AKI comorbidities. Table 1 briefly summarized the baseline characteristics of patients, including comorbidities, demographics, laboratory events, scores, and vital signs.
Table 1.
Comparison of baseline characteristics between cardiac surgery patients with or without AKI.
| Without AKI (n = 5,517) | With AKI (n = 485) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 66.38 ± 12.26 | 66.69 ± 12.28 | <0.001 |
| Male, n (%) | 3,795 (68.8%) | 358 (73.8%) | 0.022 |
| Weight (kg) | 81.8 (70.5–94.5) | 81.1 (68–96.6) | 0.973 |
| Height (cm) | 172.72 (162.56–177.8) | 170.18 (162.56–177.8) | 0.278 |
| Vital signs | |||
| HR, beats/minute | 84.28 (78.49–90.63) | 82.38 (77.36–87.38) | <0.001 |
| SBP, mmHg | 111.43 (105.80–118.07) | 113.81 (107.41–121.14) | <0.001 |
| DBP, mmHg | 56.75 (52.81–61.16) | 56.03 (50.86–60.00) | <0.001 |
| RR, times/minute | 16.86 (15.23–18.83) | 16.89 (15.50–18.71) | 0.398 |
| Temperature, °C | 36.84 (36.54–37.18) | 36.72 (36.37–37.00) | <0.001 |
| SpO2, % | 98.13 (97.20–98.93) | 98.34 (97.20–99.16) | 0.124 |
| Laboratory events | |||
| WBC, 109/L | 12.1 (9.4–15.5) | 11.8 (8.6–15.6) | 0.100 |
| Platelets, 109/L | 154 (122–196) | 154 (121–201) | 0.550 |
| Glucose, mg/dl | 126.9 (118.14–139.56) | 124.33 (115.06–135.3) | 0.018 |
| Serum sodium, mmol/L | 137 (135–138) | 136 (134–138) | <0.001 |
| Serum potassium, mmol/L | 4.4 (3.9–5.1) | 4.7 (4.2–5.3) | <0.001 |
| Serum creatinine, mg/dl | 0.8 (0.7–1) | 1.4 (1.1–2.1) | <0.001 |
| Comorbidities | |||
| Congestive heart failure | 1,316 (23.9%) | 224 (46.2%) | <0.001 |
| Cardiac arrhythmias | 2,734 (49.6%) | 280 (57.7%) | 0.001 |
| Valvular disease | 1,190 (21.6%) | 131 (27.0%) | 0.006* |
| Pulmonary circulation disorder | 388 (7.0%) | 56 (11.5%) | <0.001 |
| Peripheral vascular disorder | 686 (12.4%) | 103 (21.2%) | <0.001 |
| Hypertension | 3,782 (68.6%) | 437 (90.1%) | <0.001 |
| Chronic pulmonary | 1,017 (18.4%) | 121 (24.9%) | <0.001 |
| Uncomplicated diabetes | 1,414 (25.6%) | 148 (30.5%) | 0.019 |
| Complicated diabetes | 225 (4.1%) | 115 (23.7%) | <0.001 |
| Liver disease | 140 (2.5%) | 28 (5.7%) | <0.001 |
| Scores | |||
| SAPS II | 32 (26–40) | 41 (34–50) | <0.001 |
| SOFA | 4 (3–6) | 6 (5–8) | <0.001 |
| Surgical type | |||
| CABG | 3,825 (69.3%) | 379 (78.1%) | <0.001 |
Values are presented as the mean ± standard deviation, median (interquartile range), or number of patients (%). AKI, acute kidney injury; CABG, coronary artery bypass grafting; DBP, diastolic blood pressure; HR, heart rate; RR, respiratory rate; SAPS II, Simplified Acute Physiology Score II; SBP, systolic blood pressure; SOFA, Sequential Organ Failure Assessment; SpO2, percutaneous oxygen saturation; WBC, white blood cell.
There are significant differences of baseline characteristics between the two groups. Cardiac surgery patients with AKI had a higher average age than patients without AKI (66.38 ± 12.26 vs. 66.69 ± 12.28, P < 0.001). More cardiac surgery patients with AKI were male, whereas the difference between the groups was little (68.8% vs. 73.8%, P = 0.022). Patients with AKI tended to have lower HR, DBP, temperature, glucose, and serum sodium, as well as higher SBP, RR, serum potassium, serum creatinine, SAPS II and SOFA scores, in addition to histories of cardiac arrhythmias, chronic pulmonary disease, congestive heart failure, hypertension, liver disease, peripheral vascular disorder, pulmonary circulation disorder, uncomplicated and complicated diabetes, and valvular disease (Table 1).
Clinical outcomes of the study population
When compared with patients without AKI, patients with AKI were at higher risks of prolonged ICU stay, hospital mortality, 30-day mortality, and 90-day mortality (2.1 vs. 3.2 days, P < 0.001; 1.6% vs. 4.1%, P < 0.001; 1.8% vs. 3.5%, P = 0.008; 3.0% vs. 8.7%, P < 0.001, respectively) (Table 2).
Table 2.
Outcomes of cardiac surgery patients with or without AKI.
| Without AKI (n = 5,517) | With AKI (n = 485) | P value | |
|---|---|---|---|
| ICU stay, days | 2.1 (1.2–3.7) | 3.2 (1.9–5.9) | <0.001 |
| Hospital mortality (%) | 88 (1.6%) | 20 (4.1%) | <0.001 |
| 30-day mortality (%) | 98 (1.8%) | 17 (3.5%) | 0.008 |
| 90-day mortality (%) | 165 (3.0%) | 42 (8.7%) | <0.001 |
Values are presented as the median (interquartile range) or number of patients (%). AKI, acute kidney injury; ICU, intensive care unit.
A univariate-logistic-regression analysis was shown in Table 3, AKI, age, DBP, height, cardiac arrhythmias, congestive heart failure, hypertension, liver disease, peripheral vascular disorder, SBP, and valvular disease were associated with hospital mortality (all P < 0.01). Moreover, AKI, age, height, cardiac arrhythmias, congestive heart failure, hypertension, liver disease, peripheral vascular disorder, SBP, and valvular disease (all P < 0.01) were associated with 30-day mortality. Furthermore, AKI, age, DBP, cardiac arrhythmias, chronic pulmonary disease, complicated diabetes, congestive heart failure, height, hypertension, liver disease, peripheral vascular disorder, pulmonary circulation disorder, SBP, weight, and valvular disease (all P < 0.01) were associated with 90-day mortality.
Table 3.
Univariate logistic regression analyses for prognosis in cardiac surgery patients.
| Variable | Hospital mortality | 30-day mortality | 90-day mortality | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| AKI | 2.65 (1.62–4.35) | <0.001 | 2.01 (1.19–3.39) | 0.009 | 3.07 (2.16–4.37) | <0.001 |
| Age | 1.03 (1.02–1.05) | <0.001 | 1.02 (1.00–1.04) | 0.018 | 1.04 (1.03–1.05) | <0.001 |
| Weight | 1.00 (0.99–1.01) | 0.650 | 1.00 (0.99–1.01) | 0.780 | 0.99 (0.98–1.00) | 0.014 |
| Height | 0.96 (0.95–0.98) | <0.001 | 0.97 (0.95–0.99) | 0.001 | 0.97 (0.96–0.98) | <0.001 |
| Congestive heart failure | 2.97 (2.03–4.34) | <0.001 | 2.72 (1.88–3.94) | <0.001 | 2.61 (1.97–3.45) | <0.001 |
| Cardiac arrhythmias | 2.09 (1.40–3.14) | <0.001 | 1.88 (1.28–2.77) | <0.001 | 2.38 (1.76–3.23) | <0.001 |
| Valvular disease | 1.72 (1.14–2.58) | 0.009 | 1.99 (1.35–2.94) | <0.001 | 2.17 (1.62–2.98) | <0.001 |
| Pulmonary circulation disorder | 1.73 (0.96–3.12) | 0.066 | 1.61 (0.90–2.90) | 0.109 | 1.60 (1.02–2.49) | 0.039 |
| Peripheral vascular disorder | 2.13 (1.36–3.34) | 0.001 | 1.96 (1.26–3.06) | 0.003 | 2.12 (1.52–2.95) | <0.001 |
| Hypertension | 0.42 (0.28–0.61) | <0.001 | 0.47 (0.32–0.68) | <0.001 | 0.56 (0.42–0.74) | <0.001 |
| Chronic pulmonary | 1.36 (0.87–2.13) | 0.173 | 1.52 (1.00–2.32) | 0.051 | 1.49 (1.09–2.06) | 0.014 |
| Uncomplicated diabetes | 0.68 (0.42–1.10) | 0.118 | 0.83 (0.53–1.29) | 0.400 | 0.81 (0.58–1.12) | 0.205 |
| Complicated diabetes | 1.72 (0.89–3.33) | 0.107 | 1.43 (0.72–2.84) | 0.314 | 1.72 (1.06–2.80) | 0.028 |
| Liver disease | 12.84 (8.02–20.58) | <0.001 | 8.16 (4.90–13.58) | <0.001 | 8.66 (5.79–12.95) | <0.001 |
| DBP | 0.96 (0.93–0.99) | 0.009 | 0.98 (0.95–1.01) | 0.139 | 0.96 (0.94–0.98) | <0.001 |
| SBP | 0.95 (0.93–0.97) | <0.001 | 0.95 (0.93–0.97) | <0.001 | 0.98 (0.96–1.00) | 0.015 |
AKI, acute kidney injury; CI, confidence interval; DBP, diastolic blood pressure; OR, odds ratio; SBP, systolic blood pressure.
Table 4 displayed the results of the multivariate analyses for Models 1 and 2. AKI was a risk factor for hospital mortality (Model 1: OR = 2.50, 95% CI: 1.45–4.33, P < 0.001; Model 2: OR = 2.44, 95% CI: 1.48–4.02, P < 0.001), 30-day mortality (Model 1: OR = 1.84, 95% CI: 1.05–3.24, P = 0.034; Model 2: OR = 1.96, 95% CI: 1.13–3.22, P = 0.016), and 90-day mortality (Model 1: OR = 2.05, 95% CI: 1.37–3.01, P < 0.001; Model 2: OR = 2.76, 95% CI: 1.93–3.94, P < 0.001). Furthermore, the Kaplan-Meier survival curves showed that cardiac surgery patients with AKI had a significantly lower 90-day survival rate compared with the patients without AKI (Figure 1).
Table 4.
Multivariate logistic regression analyses for prognosis in cardiac surgery patients.
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Variable | OR (95% CI) | P value | Variable | OR (95% CI) | P value |
| Hospital mortality | |||||
| AKI | 2.50 (1.45–4.33) | <0.001 | AKI | 2.44 (1.48–4.02) | <0.001 |
| Congestive heart failure | 2.05 (1.36–3.08) | 0.001 | Age | 1.02 (1.01–1.04) | 0.003 |
| Cardiac arrhythmias | 1.76 (1.15–2.69) | 0.009 | Height | 0.97 (0.94–0.99) | <0.001 |
| Valvular disease | 1.11 (0.72–1.71) | 0.628 | |||
| Liver disease | 10.01 (6.10–16.42) | <0.001 | |||
| SBP | 0.96 (0.94–0.98) | <0.001 | |||
| Hypertension | 0.45 (0.30–0.67) | <0.001 | |||
| 30-day mortality | |||||
| AKI | 1.84 (1.05–3.24) | 0.034 | AKI | 1.96 (1.13–3.22) | 0.016 |
| Congestive heart failure | 1.96 (1.33–2.90) | 0.001 | Age | 1.01 (1.00–1.03) | 0.124 |
| Cardiac arrhythmias | 1.55 (1.04–2.32) | 0.031 | Height | 0.97 (0.96–0.99) | 0.003 |
| Valvular disease | 1.38 (0.93–2.01) | 0.112 | |||
| Liver disease | 6.24 (3.67–10.61) | <0.001 | |||
| SBP | 0.96 (0.94–0.98) | <0.001 | |||
| Hypertension | 0.54 (0.36–0.80) | 0.002 | |||
| 90-day mortality | |||||
| AKI | 2.05 (1.37–3.01) | <0.001 | AKI | 2.76 (1.93–3.94) | <0.001 |
| Congestive heart failure | 1.48 (1.10–2.01) | 0.011 | Age | 1.03 (1.02–1.04) | <0.001 |
| Cardiac arrhythmias | 1.82 (1.33–2.50) | <0.001 | Height | 0.98 (0.96–0.99) | 0.001 |
| Valvular disease | 1.45 (1.07–2.00) | 0.018 | |||
| Liver disease | 5.28 (3.39–8.23) | <0.001 | |||
| SBP | 0.99 (0.98–1.01) | 0.455 | |||
| Hypertension | 0.54 (0.40–0.73) | <0.001 | |||
Model 1 was adjusted for cardiac arrhythmias, congestive heart failure, hypertension, liver disease, SBP, valvular disease. Model 2 was adjusted for age, height, weight. AKI, acute kidney injury; CI, confidence interval; OR, odds ratio; SBP, systolic blood pressure.
Figure 1.
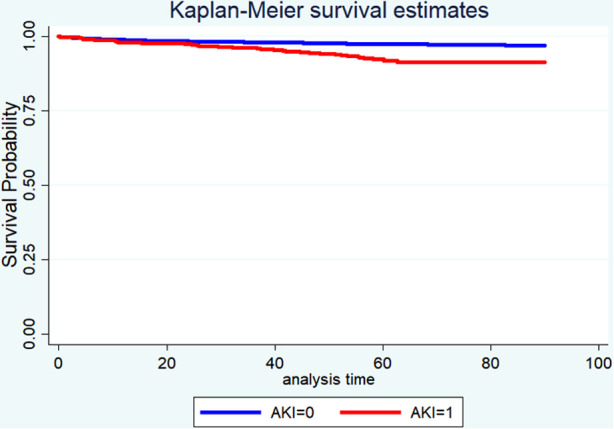
Kaplan-Meier survival analysis for 90-day overall survival.
Table 5 showed that higher hospital mortality, 30-day mortality and 90-day mortality was observed in higher KDIGO grade for cardiac surgery patients with AKI (all P < 0.05).
Table 5.
The relationship between KDIGO grade with mortality for cardiac surgery patients with AKI.
| KDIGO grade | Hospital mortality | 30-day mortality | 90-day mortality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Survivors | Nonsurvivors | P value | Survivors | Nonsurvivors | P value | Survivors | Nonsurvivors | P value | |
| 1 | 168 (34.64%) | 2 (0.04%) | 0 . 04 | 170 (35.05%) | 2 (0.04%) | 0 . 023 | 169 (34.85%) | 4 (0.82%) | 0 . 001 |
| 2 | 154 (31.75%) | 5 (1.03%) | 161 (33.20%) | 4 (0.82%) | 142 (29.28%) | 6 (1.24%) | |||
| 3 | 143 (29.48%) | 13 (2.68%) | 137 (28.25%) | 11 (2.27%) | 132 (27.22%) | 32 (6.60%) | |||
Values are presented as the number of patients (%). AKI, acute kidney injury; KDIGO, kidney disease: improving global outcomes.
Predictive ability of creatinine for AKI
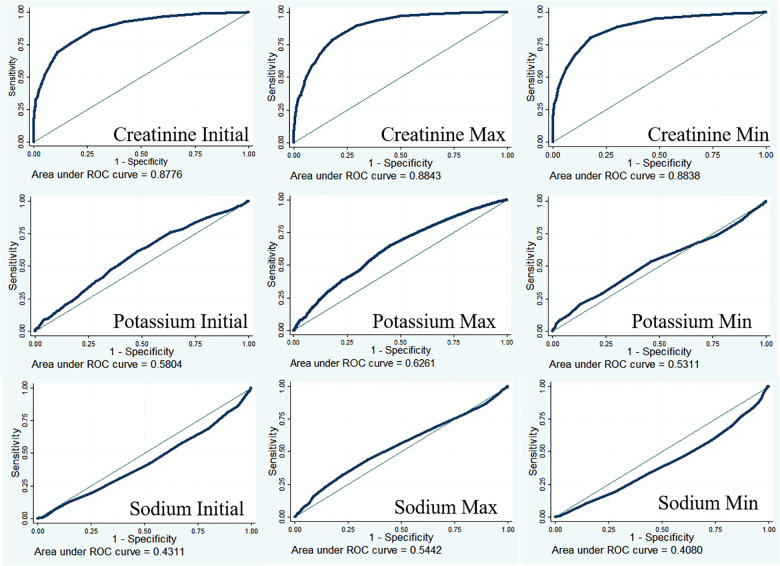
Creatinine is an important diagnostic indicator for AKI (8). The diagnostic value of creatinine was examined using receiver operating characteristic curves (Figure 2). The results showed that the diagnostic performance of creatinine was moderately good (AUC for creatinine initial = 0.8776; AUC for creatinine max = 0.8843; AUC for creatinine min = 0.8838), and a low AUC was found in potassium (AUC for potassium initial = 0.5804; AUC for potassium max = 0.6261; AUC for potassium min = 0.5311) and sodium (AUC for sodium initial = 0.4311; AUC for sodium max = 0.5442; AUC for sodium min = 0.4080) detection.
Figure 2.
Receiver operating characteristic curves for creatinine, potassium, and sodium in AKI.
Discussion
In the present study, we found that the incidence of AKI in cardiac surgery patients was 8.08%. Patients with AKI had a higher average age than patients without AKI, and more patients with AKI were male. When compared with patients without AKI, patients with AKI were at higher risks of prolonged ICU stay, hospital mortality, 30-day mortality, and 90-day mortality. Higher hospital mortality, 30-day mortality and 90-day mortality was observed in higher KDIGO grade for cardiac surgery patients with AKI. In the multivariate analysis, AKI was a risk factor for hospital mortality, 30-day mortality, and 90-day mortality. The diagnostic performance of creatinine was moderately good for AKI.
According to the literature, the mortality rate of all patients undergoing cardiac surgery is as high as 8%, and that of patients with postoperative complications of AKI can be as high as 60% or more (9). Patients undergoing cardiac surgery will increase mortality from 0.4–4.4% to 1.3–22.3% once complicated AKI; when these same patients need clinical hemodialysis, the mortality rate rises from 25% to 88.9%, thus indicating that severe postoperative complications AKI an independent risk factor for mortality, resulting in an eightfold increase in the risk of death (10, 11). Therefore, the postoperative AKI of cardiac surgery is closely related to other complications, ICU hospital stay, and worse quality of life. These also indicate an increase in the early and late mortality rates of such populations and in health care financial expenditures (5, 12, 13). Our data is consistent with previous studies. In the present study, patients with AKI had longer ICU stays (2.1 vs. 3.2 days), as well as higher hospital mortality (1.6% vs. 4.1%), 30-day mortality (1.8% vs. 3.5%), and 90-day mortality (3.0% vs. 8.7%) than patients without AKI. Moreover, higher hospital mortality, 30-day mortality and 90-day mortality was observed in higher KDIGO grade for cardiac surgery patients with AKI (all P < 0.05, Table 5). The development of AKI in cardiac surgery might be connected with several major injury pathways, including hypoperfusion, ischemia-reperfusion injury, inflammation, oxidative stress, nephrotoxins, neurohumoral activation and mechanical factor, all of which could occur preoperatively, intraoperatively and postoperatively. Although these factors have been associated with AKI in cardiac surgery, the current evidence was associative rather than causal (1). Furthermore, our understanding of the pathophysiology of AKI and cardiac surgery remained rudimentary due to the huge logistic challenges and costs, as well as the lacking of animal models of cardiac surgery. Therefore, the lack of understanding of the pathogenesis of AKI greatly limits the development of prevention and management measures for AKI in cardiac surgery. So far, AKI is still one of the important factors affecting the mortality and ICU stay in cardiac surgery patients.
Common preoperative risk factors for the development of AKI after cardiac surgery include age, obesity, the female sex, the presence of multiple comorbidities (for example, chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, hypertension, hypercholesterolaemia, left ventricular ejection fraction of <35%, pre-existing chronic kidney disease, and previous cardiac surgery), and obesity (14–16). In our investigation, patients with AKI tended to have lower HR, DBP, temperature, glucose, and serum sodium, as well as higher SBP, RR, creatinine and potassium of serum, SAPS II and SOFA score, in addition to a history of cardiac arrhythmias, chronic pulmonary disease, congestive heart failure, hypertension, liver disease, peripheral vascular disorder, pulmonary circulation disorder, uncomplicated and complicated diabetes, and valvular disease. Hence, it is important to identify risk factors for concurrent AKI in patients undergoing cardiac surgery, which can facilitate clinical prognostic assessment and develop new and more effective clinical strategies to prevent and reduce this complication, thereby reducing associated morbidity and mortality.
Serum creatinine is an important diagnostic indicator for AKI (8). Under normal physiological conditions, serum levels of creatinine increase by 0.1–0.2 mg/dl after cardiac surgery. When creatinine levels increase by more than 0.3 mg/dl, within 2 days, patients are considered stage 1 AKI according to KDIGO criteria (17). In the present study, serum creatinine showed a moderately good diagnostic performance for AKI (AUC for creatinine initial = 0.8776; AUC for creatinine max = 0.8843; AUC for creatinine min = 0.8838). In addition to creatinine, a series of new biomarkers for the early diagnosis and prognostication of AIK have been proposed, such as NGAL (18–20), cystatin C (19), and IL-18 (21, 22). However, these biomarkers showed poor predictive performances in these patients, with a heterogeneous timing of the onset of injury (23).
Limitation
Our investigation has some limitations. Firstly, the definition of AKI changed in 2012, while the MIMIC-III database only contains data on critically ill patients admitted between 2001 and 2012. Although we tried to identify the AKI patients according to KDIGO, our cohort may not fully comply with the newly defined AKI. What's more, transient and persistent AKI, as well as severity degree of AKI were not been obtained. Secondly, this was a single center study. The results need to be verified by multi-center trials. Moreover, the disease definition of the MIMIC-III database was based on the ICD-9-CM code, which may lead to some important information lacking in the database. Then, it was a retrospective design and it is difficult to completely eliminate residual confounding. Due to the retrospective nature the temporal relationship is frequently difficult to assess. Furthermore, the findings in this study may not be generalizable to the up-to-date ICD-10 codes or other data sources or hospitals as MIMIC-III data comes from a single hospital system. Moreover, the present results could only show the correlation between AKI and mortality, but not the causal relationship. To solve this problem, causal inference could be used in the future as the previous study (24).
Conclusion
In summary, we found that AKI can lead to prolonged ICU stay, hospital mortality, 30-day mortality, and 90-day mortality. AKI was a risk factor for hospital mortality, 30-day mortality, and 90-day mortality. The diagnostic performance of creatinine was moderately good for AKI.
Acknowledgments
The authors greatly appreciate the editors and peer reviewers for their critical reading and insightful comments, which are helpful to improve our manuscript substantially. We apologize to all those re-searchers whose work could not be cited due to space limitations.
Funding
This work was financially supported by the Zhejiang Provincial Basic-Public-Welfare Planning Project, China (grants no. LQ22H230001, LY23H020008 and LGF19H050003) and the Major Transverse Research Projects of Jiaxing University, China (grants no. 00619006 and 00522195). The sponsors of the study were not involved in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The institutional review boards of the MIT (Cambridge, Massachusetts) and BIDMC (Boston, Massachusetts) reviewed and approved studies involving human participants (approval No. 41268972, completion date 02-Mar-2021). According to national laws and institutional requirements, this study does not require written informed consent.
Author contributions
RS, GX, and CS conceived and designed the study. DC, GX, and RS administratively supported this work. CS, DC, XJ, CT, XG, ZT, YB, and FW provided, selected, assembled, analyzed and interpretated data. All authors contributed toward data analysis, drafting and critically revising the paper, and agree to be accountable for all aspects of the work. All authors have read and confirmed that they meet ICMJE criteria for authorship. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. (2017) 13:697–711. 10.1038/nrneph.2017.119 [DOI] [PubMed] [Google Scholar]
- 2.Weisse AB. Cardiac surgery: a century of progress. Tex Heart Inst J. (2011) 38:486–90. PMID: [PMC free article] [PubMed] [Google Scholar]
- 3.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. (2009) 119:2444–53. 10.1161/CIRCULATIONAHA.108.800011 [DOI] [PubMed] [Google Scholar]
- 4.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adybelli Z, Giuliani A, et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. (2013) 3:178–99. 10.1159/000353134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortega-Loubon C, Fernandez-Molina M, Carrascal-Hinojal Y, Fulquet-Carreras E. Cardiac surgery-associated acute kidney injury. Ann Card Anaesth. (2016) 19:687–98. 10.4103/0971-9784.191578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. 10.1038/sdata.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao GJ, Xu C, Ying JC, Lu WB, Hong GL, Li MF, et al. Association between furosemide administration and outcomes in critically ill patients with acute kidney injury. Crit Care. (2020) 24:75. 10.1186/s13054-020-2798-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. (2013) 6:8–14. 10.1093/ckj/sfs160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta RL. Acute renal failure and cardiac surgery: marching in place or moving ahead? J Am Soc Nephrol. (2005) 16:12–4. 10.1681/ASN.2004110954 [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, et al. Preoperative renal risk stratification. Circulation. (1997) 95:878–84. 10.1161/01.CIR.95.4.878 [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Xu J, Shen B, Wang C, Teng J, Ding X. Validation of four prediction scores for cardiac surgery-associated acute kidney injury in Chinese patients. Braz J Cardiovasc Surg. (2017) 32:481–6. 10.21470/1678-9741-2017-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreiro A, Lombardi R. Acute kidney injury after cardiac surgery is associated with mid-term but not long-term mortality: a cohort-based study. PLoS One. (2017) 12:e0181158. 10.1371/journal.pone.0181158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Zhao Y, Lei M, Zhao G, Li D, Sun R, et al. Remote ischemic preconditioning to prevent acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Front Cardiovasc Med. (2021) 8:601470. 10.3389/fcvm.2021.601470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman MD, Shaefi S, Sladen RN. Preventing acute kidney injury after cardiac surgery. Curr Opin Anaesthesiol. (2011) 24:70–6. 10.1097/ACO.0b013e3283422ebc [DOI] [PubMed] [Google Scholar]
- 15.De Santo LS, Romano G, Mango E, Iorio F, Savarese L, Numis F, et al. Age and blood transfusion: relationship and prognostic implications in cardiac surgery. J Thorac Dis. (2017) 9:3719–27. 10.21037/jtd.2017.08.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jyrala A, Weiss RE, Jeffries RA, Kay GL. Effect of mild renal dysfunction (s-crea 1.2-2.2mg/dl) on presentation characteristics and short- and long-term outcomes of on-pump cardiac surgery patients. Interact Cardiovasc Thorac Surg. (2010) 10:777–82. 10.1510/icvts.2009.231068 [DOI] [PubMed] [Google Scholar]
- 17.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery–a prospective cohort study. Crit Care Med. (2009) 37:553–60. 10.1097/CCM.0b013e318195846e [DOI] [PubMed] [Google Scholar]
- 18.Antonucci E, Lippi G, Ticinesi A, Pigna F, Guida L, Morelli I, et al. Neutrophil gelatinase-associated lipocalin (NGAL): a promising biomarker for the early diagnosis of acute kidney injury (AKI). Acta Biomed. (2014) 85:289–94. PMID: [PubMed] [Google Scholar]
- 19.Yegenaga I, Kamis F, Baydemir C, Erdem E, Celebi K, Eren N, et al. Neutrophil gelatinase-associated lipocalin is a better biomarker than cystatin C for the prediction of imminent acute kidney injury in critically ill patients. Ann Clin Biochem. (2018) 55:190–7. 10.1177/0004563217694051 [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Yu XA, Zhang Y, Zhang R, Bai X, Lu M, et al. Rapid and sensitive detection of NGAL for the prediction of acute kidney injury via a polydopamine nanosphere/aptamer nanocomplex coupled with DNase I-assisted recycling amplification. Analyst. (2020) 145:3620–5. 10.1039/D0AN00474J [DOI] [PubMed] [Google Scholar]
- 21.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. (2006) 70:199–203. 10.1038/sj.ki.5001527 [DOI] [PubMed] [Google Scholar]
- 22.Zdziechowska M, Gluba-Brzozka A, Poliwczak AR, Franczyk B, Kidawa M, Zielinska M, et al. Serum NGAL, KIM-1, IL-18, L-FABP: new biomarkers in the diagnostics of acute kidney injury (AKI) following invasive cardiology procedures. Int Urol Nephrol. (2020) 52:2135–43. 10.1007/s11255-020-02530-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ, et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int. (2010) 77:1020–30. 10.1038/ki.2010.25 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Jin P, Feng M, Yang J, Huang J, Chen L, et al. Causal inference with marginal structural modeling for longitudinal data in laparoscopic surgery: a technical note. Laparosc Endosc Robot Surg. (2022) 5:146–52. 10.1016/j.lers.2022.10.002 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.