Abstract
The vast majority of urinary tract infections are caused by strains of uropathogenic Escherichia coli that encode filamentous adhesive organelles called type 1 pili. These structures mediate both bacterial attachment to and invasion of bladder epithelial cells. However, the mechanism by which type 1 pilus-mediated bacterial invasion contributes to the pathogenesis of a urinary tract infection is unknown. Here we show that type 1-piliated uropathogens can invade the superficial epithelial cells that line the lumenal surface of the bladder and subsequently replicate, forming massive foci of intracellular E. coli termed bacterial factories. In response to infection, superficial bladder cells exfoliate and are removed with the flow of urine. To avoid clearance by exfoliation, intracellular uropathogens can reemerge and eventually establish a persistent, quiescent bacterial reservoir within the bladder mucosa that may serve as a source for recurrent acute infections. These observations suggest that urinary tract infections are more chronic and invasive than generally assumed.
Uropathogenic Escherichia coli (UPEC), the primary cause of urinary tract infections (UTIs) (16, 35), is not generally regarded as an invasive pathogen. Infections caused by UPEC are typically self-limiting and UPEC rarely spreads beyond the urinary tract (17, 35). Adherence of UPEC to host epithelial cells within the bladder and other tissues within the urinary tract is considered critical to the ability of UPEC to cause disease (2, 12, 33). Filamentous adhesive organelles called type 1 pili, which are encoded by virtually all UPEC isolates (25), can mediate bacterial attachment to host bladder cells and have been shown to be significant virulence factors associated with UTIs (6, 24, 25, 29, 37). These structures contain an adhesin molecule, FimH, that binds mannose-containing glycoprotein receptors expressed on the lumenal surface of the bladder (23, 38). In addition to mediating bacterial attachment, recent work has shown that the FimH adhesin can also directly stimulate host cell signaling cascades that lead to the induction of cytoskeletal rearrangements and the envelopment and internalization of adherent UPEC (27). These findings have suggested that the invasion of bladder epithelial cells by type 1-piliated UPEC may have an as-yet-appreciated role in the pathogenesis of UTIs.
Data from various experimental systems indicate that invasion of eukaryotic cells can provide bacterial pathogens refuge from both innate and adaptive host defenses and may also facilitate the dissemination of microbes within and across tissue barriers (8). Within the urinary tract, the bladder epithelium functions as a formidable physical barrier, preventing the diffusion of urine and other substances from within the bladder lumen (15, 26). The bladder epithelium, which is composed of a single layer of large, highly differentiated superficial cells overlying two or three layers of small, relatively undifferentiated basal and intermediate epithelial cells, also serves as an active component of the innate immune system. Interactions between type 1-piliated UPEC and bladder epithelial cells can stimulate cytokine and chemokine production and can also trigger the exfoliation and clearance of superficial epithelial cells (29, 34, 36). Exfoliation of infected bladder cells occurs via an apoptosis-like mechanism and appears to be an effective host defense strategy (29). To colonize the bladder successfully, UPEC must have a means of counteracting or circumventing bladder cell exfoliation and other innate host defenses within the urinary tract.
Here we report that following invasion of superficial bladder epithelial cells, UPEC can replicate intracellularly and eventually reemerge from the infected host cells in a manner reminiscent of a lytic virus cycle. Upon exiting the superficial cells, UPEC can interact with and invade surrounding and underlying epithelial cells, leading to the establishment of a quiescent bacterial reservoir within the bladder tissue. These findings suggest a means by which invasion, rather than promoting bacterial spread across mucosal layers and into other tissues, can facilitate the localized persistence of a bacterial pathogen.
MATERIALS AND METHODS
Bacterial strains.
The clinical cystitis isolate NU14 has been described previously (19, 25). Other cystitis isolates, including UTI89, were kindly provided by S. Langermann. The K-12 strains MG1655 and AAEC185 (Δ type 1 gene cluster), the latter of which was complemented with plasmid pSH2 (31), which encodes the type 1 pilus gene cluster, have been described previously (3, 4, 29). All bacterial strains were grown in static Luria-Bertani (LB) broth at 37°C for 48 h to induce expression of type 1 pili. Expression was verified by mannose-sensitive agglutination of a 3% solution of guinea pig erythrocytes (A640 of ≈1.9) or a 1% solution of baker's yeast in phosphate-buffered saline (PBS).
Inoculations of mice and microscopy.
Eight- to ten-week-old female C57BL/6 mice (Jackson Laboratories) were anesthetized with methoxyflurane and inoculated via transurethral catheterization with 50 μl of a bacterial suspension (≈108 CFU) in PBS as previously described (29). At the indicated times, mice were killed by cervical dislocation under anesthesia and their bladders were aseptically removed, weighed, and homogenized in 1 ml of 0.025% Triton X-100–PBS. For the results of the experiment shown in Fig. 1B, when possible, urine released at the time of death was collected from infected mice in Eppendorf tubes. Bacterial titers were determined by plating serial dilutions of homogenates or urine on LB agar plates.
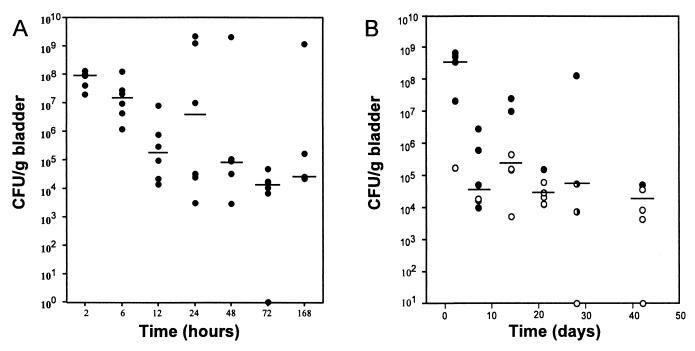
FIG. 1.
Kinetics of bacterial clearance from the bladder following infection with type 1-piliated UPEC. (A) The majority of bacteria were cleared within the first 48 h after infection of C57BL/6 mice with UTI89. (B) Significant numbers of bacteria, however, persist within the bladder tissue up to 6 weeks after infection. In addition, the bacteriologic status of urine samples collected from each mouse at the time of death is also indicated in panel B. ○, urine titer of >103 CFU/ml; ●, urine titer of <103 CFU/ml; ◑, no urine collected. Horizontal lines indicate the median titer at each time (n = 5 or 6).
Hematoxylin and eosin staining and immunofluorescence microscopy were performed as described previously (34). Electron and confocal microscopy analyses of mouse bladders have been previously described (27, 29).
Intracellular growth assays.
The 5637 bladder epithelial cells (ATCC HTB-9) were seeded into 24-well plates and grown to confluency in RPMI 1640 medium supplemented with 10% fetal bovine serum (Sigma), 2 g of sodium bicarbonate per liter, and 0.3 g of l-glutamine per liter. In two sets of triplicate wells, bladder cells were infected with a multiplicity of infection of 5 to10 bacteria per host cell (20 μl of a bacterial solution diluted in LB broth [A600 of ∼0.5]). Bacterial contact with host cells was expedited by centrifugation of plates at 600 × g for 5 min. After 2 h of incubation at 37°C, medium was replaced with 1.5 ml of fresh medium containing 100 μg of gentamicin (Sigma)/ml to kill any extracellular bacteria. In duplicate sets of wells, medium containing gentamicin and trimethoprim-sulfamethoxazole (TMP-SMZ) (54 and 270 μg/ml, respectively) was added to the host cells to inhibit intracellular bacterial growth and to kill any extracellular bacteria. After an additional 2-hour incubation, cells were washed once with PBS (with Mg2+/Ca2+) and fresh medium containing 15 μg of gentamicin/ml (with or without TMP-SMZ) was added to the cells. This submaximal concentration of gentamicin prevented extracellular bacterial growth and reduced the chances of gentamicin leaching into the host cells during longer incubation times. At the indicated times, host cells were washed three times in PBS and lysed in 1 ml of 0.1% Triton X-100–double-distilled H2O (ddH2O). Lysates were plated on LB agar plates to determine numbers of surviving intracellular bacteria.
Bacterial fluxing assays.
The 5637 cells in 24-well plates were infected and incubated in the presence of gentamicin as described for the intracellular growth assays. At 24 h after the addition of gentamicin, host cells were washed five times with PBS (with Mg2+/Ca2+). One set of triplicate wells was lysed to determine the number of intracellular bacteria surviving the 24-hour incubation in the presence of gentamicin. Fresh medium (980 μl) with or without 15 μg of gentamicin/ml was added to the remaining triplicate sets of wells, and these mixtures were incubated for an additional 7 h at 37°C. Twenty microliters of 5% Triton X-100–ddH2O was added directly to the wells lacking gentamicin, and lysates were plated to determine the total number of intra- and extracellular bacteria. Wells containing gentamicin were washed three times with PBS prior to lysis in 1 ml of 0.1% Triton X-100–ddH2O.
RESULTS
Establishment and persistence of a UTI
A mouse cystitis model was used to examine the capacity of UPEC to resist clearance and to persist within the bladder. Female C57BL/6 mice were inoculated via transurethral catheterization with the type 1-piliated clinical cystitis isolate, UTI89, and bacterial titers were determined at various times. Between 2 and 12 h after inoculation, the number of bacteria within the bladder decreased substantially (an average of 3 log units) (Fig. 1A). The reduction of titers during the first 12 h of infection correlates with massive exfoliation of the superficial cells in C57BL/6 mice and with the influx of neutrophils into the bladder tissue in response to infection (29, 30). Despite these and other innate host defenses, however, considerable numbers of bacteria were able to avoid rapid clearance from the bladder during the first 2 days of the infection. From 2 to 7 days after infection, bacteria persisted within the bladder at fairly constant levels (Fig. 1A), and bacterial titers often remained substantially high and stable for at least 6 weeks (Fig. 1B). Interestingly, after 2 days of infection, bacteria were undetectable in 58% of the urine samples collected at the time of death from infected mice, even though bacteria persisted in the bladder tissue. These data are in agreement with those of a previous study suggesting that urine titers do not necessarily reflect the bacteriologic status of the bladder tissue (18). Similar infections comparing the cystitis strain NU14 with the fimH isogenic mutant strain, NU14-1, were also performed. At 14 days after infection, 75% of the bladders from NU14-infected mice remained colonized (mean titer, 160 CFU/bladder), whereas the bladders of all four mice infected with NU14-1 remained sterile.
Invasion of the bladder epithelium.
The ability of UPEC to resist rapid clearance from the bladder is potentially linked to the capacity of UPEC to invade the bladder epithelium. Within 48 h after infection with UPEC, the majority of bacteria that persist within infected mouse bladders are protected from the bactericidal effects of gentamicin treatment ex vivo (29). Furthermore, the in vivo treatment of infected mice with gentamicin, cefuroxime, or the bacteriostatic drug combination TMP-SMZ fails to substantially reduce bacterial titers within the bladder tissue, although these antibiotics are able to effectively sterilize the urine (20, 30). Such observations are consistent with the possibility that UPEC might enter a protective niche within the bladder tissue during a UTI.
Histological examination of C57BL/6 mouse bladders recovered 2 h after inoculation with UTI89 demonstrated that UPEC could penetrate the superficial epithelial cells lining the lumenal surface of the bladder (Fig. 2A and B). Multiple bacteria could invade a single superficial cell, although the bacteria did not enter the host cells en masse. No large groups of intracellular bacteria were observed at this time, and bacteria were not detected within the small, underlying intermediate and basal epithelial cells. These observations support previous transmission and scanning electron microscopy studies which found that UPEC can enter superficial bladder cells in vivo (11, 28, 29).
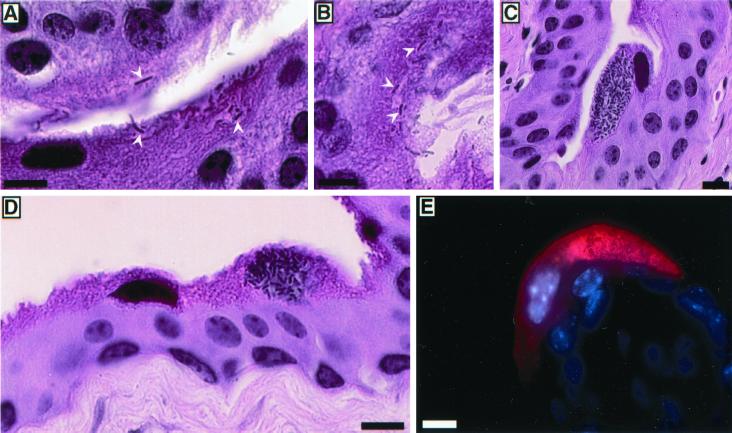
FIG. 2.
Replication of UPEC within superficial bladder cells. (A and B).Two hours after infection of C57BL/6 mice with UTI89, bacteria were detected in hematoxylin-and-eosin-stained sections entering or already within the superficial epithelial cells lining the lumenal surface of the bladder (arrowheads). (C to E) By 6 h after inoculation, large foci of intracellular E. coli were apparent within many of the superficial bladder cells. (E) Bacteria (red) were stained using an anti-E. coli primary antibody and Cy3-labeled secondary antibody. Host cell nuclei were visualized using Hoechst dye. Bars, 10 μm.
By 6 h after inoculation of C57BL/6 mice with UTI89, most of the superficial cells had exfoliated in response to the infection (11). Within many of the remaining superficial cells, however, large inclusions of intracellular bacteria were present (Fig. 2C and D). Immunohistological staining confirmed that the intracellular organisms were E. coli (Fig. 2E). Bacteria were not detected in bladder sections from mock-infected mice. No large accumulations of bacteria were seen within the underlying intermediate and basal epithelial cells, although individual bacteria were occasionally observed within these bladder cells at this and later times. These observations suggest that UTI89 can replicate effectively within the superficial cells but may have a diminished capacity to multiply within the less differentiated underlying bladder epithelium. In contrast to UTI89 and several other clinical UTI E. coli isolates examined, the laboratory K-12 strains AAEC185/pSH2 and MG1655, which express type 1 pili and are able to invade superficial cells in vivo (11; M. A. Mulvey, J. D. Schilling, and S. J. Hultgren, unpublished observations), did not appear to replicate within the host cells and did not form large intracellular inclusions. These data indicate that UPEC strains may encode specific virulence factors, which are missing in laboratory K-12 strains such as AAEC185/pSH2 and MG1655, that enable UPEC to multiply within host superficial bladder cells, essentially converting the host cells into bacterial factories.
Evasion of the exfoliation response.
The exfoliation and clearance of infected superficial cells pose a significant challenge to the persistence of UPEC within the bladder (1, 7, 11, 29). Without the ability of UPEC to escape from dying and exfoliating host epithelial cells, UPEC invasion of the superficial bladder cells could be considered a dead-end process as far as bacterial survival within the bladder is concerned. To understand how UPEC might resist clearance by exfoliation, we used scanning electron microscopy to examine C57BL/6 mouse bladders recovered 6 h after inoculation with UTI89 and other type 1-piliated UPEC isolates. At this time, exfoliation of the superficial cells was apparent and many of the underlying, smaller and less differentiated intermediate epithelial cells were exposed. The superficial cells remaining had lost their normal, distinctive pentagonal and hexagonal outlines, appeared shrunken, and frequently displayed membrane blebbing characteristic of apoptotic cells (Fig. 3A to C). Virtually all of the remnants of the superficial cells had at least a few surface-localized bacteria. Occasionally, the host superficial cells appeared bloated in regions, possibly as a result of large inclusions of intracellular E. coli as can be observed in Fig. 2C to E. Large numbers of UPEC organisms were often observed on the surfaces of the dying superficial cells and, in many cases, appeared to be erupting from within the host cells (Fig. 3B and C).
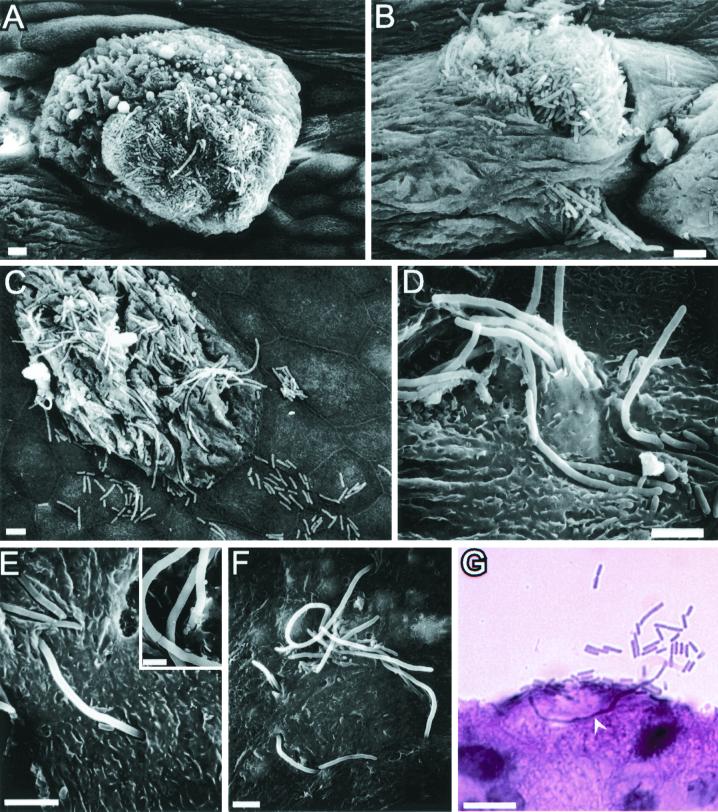
FIG. 3.
Efflux of UPEC from superficial bladder epithelial cells. (A and B) Six hours after infection with type 1-piliated UPEC, the superficial bladder cell layer was in the process of undergoing exfoliation. The remaining superficial cells often appeared swollen with evidence of membrane blebbing. (B) Bacteria frequently appeared to be spilling out from within the superficial cells. (C to G) Elongated forms of bacteria, along with their normal-sized counterparts, were observed seemingly emerging from within superficial cells and spilling onto underlying and surrounding epithelial cells. (E) Filamentous bacteria were sometimes seen bridging ss host cell sutures and interacting with two adjoining superficial cells simultaneously. (Inset) The elongated bacteria contained at least partial septa at variable distances along their lengths. (G) Examination of hematoxylin-and-eosin-stained bladder sections indicated that the filamentous forms of bacteria could extend a significant distance through the interior of the superficial bladder cells. Bars, 5 μm (A to F) and 10 μm (G).
The bacteria associated with the dying superficial cells at 6 h after inoculations were frequently elongated, sometimes reaching lengths of greater than 50 μm (Fig. 3C to G). The elongated bacteria possessed partial septa at variable distances along their lengths (Fig. 3E, inset). In many instances, bacteria appeared to be spilling out of infected superficial cells and colonizing adjacent and underlying host cells (Fig. 3C and D). The filamentous bacteria were observed exiting, and/or entering, superficial cells through tight openings in the host cell membrane (Fig. 3E and F) and were occasionally seen looping within and between adjacent superficial cells (Fig. 3E and F). Histological examination of bladder sections from infected mice also revealed filamentous bacteria protruding into the lumen from within superficial cells (Fig. 3G). These various observations suggest that UPEC has the capacity to replicate within superficial bladder cells and subsequently to escape before the host cells completely exfoliate and are cleared from the urinary tract by micturition.
Intracellular persistence and reemergence of UPEC.
The ability of UPEC to persist within and reemerge from infected bladder epithelial cells was confirmed by in vitro assays. Previous work demonstrated that E. coli strains expressing type 1 pili can invade 5637 cells and other bladder epithelial cell lines (27). In a modified gentamicin protection assay, in which extracellular bacteria are selectively killed, the intracellular titers of the cystitis isolates UTI89 and NU14 remained fairly constant within 5637 cells over the course of a 48-hour infection (Fig. 4A). In contrast, the intracellular titers of the laboratory K-12 strains AAEC185/pSH2 (which expresses type 1 pili encoded by the pSH2 plasmid) and MG1655 (which expresses type 1 pili encoded on the chromosome) decreased continuously throughout the assay (Fig. 4A). The coaddition of the membrane-permeating, bacteriostatic antibiotic TMP-SMZ blocked bacterial survival of the clinical strains but had no effect on the K-12 strains, suggesting that intracellular persistence is in fact a dynamic process that requires intracellular replication (Fig. 4A).
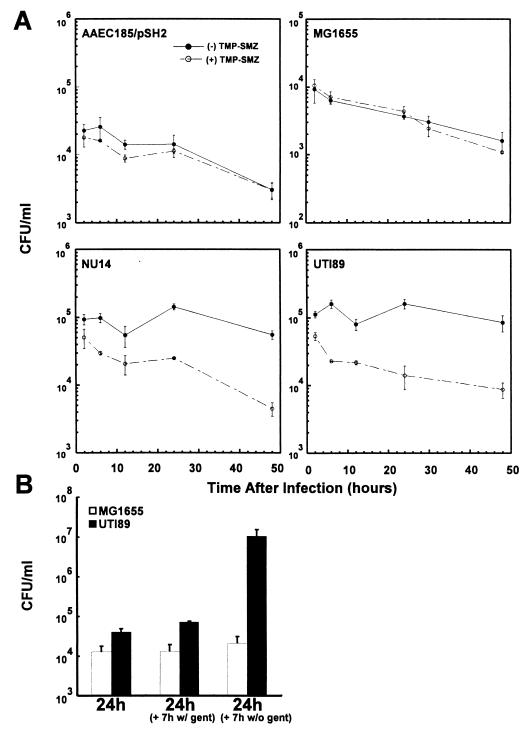
FIG. 4.
In vitro intracellular persistence and reemergence of UPEC. 5637 bladder epithelial cells were infected with uropathogenic isolates (UTI 89 or NU14) or with a laboratory K-12 strain (AAEC185/pSH2 or MG1655) that expresses type 1 pili, and intracellular growth assays were performed in the presence of gentamicin. (A) Intracellular levels of UTI89 and NU14 remained constant for 48 h in the presence of gentamicin alone. In contrast, intracellular titers of the K-12 strains decreased significantly during the same time interval. Inhibition of bacterial replication using the bacteriostatic antibiotics TMP-SMZ greatly reduced the ability of the clinical isolates to survive intracellularly. TMP-SMZ had no effect on the persistence profile of the K-12 strains. (B) Bacterial fluxing assays indicate that intracellular UPEC isolates can exit host 5637 bladder cells. Following a 24-hour incubation of infected host cells in the presence of gentamicin, the cell culture medium was replaced with fresh medium with and without gentamicin. Within 7 h after removal of gentamicin (to allow extracellular bacterial growth), the titers of total extra- and intracellular UTI89 cells greatly increased. In contrast, the titers of MG1655 remained nearly unchanged regardless of the absence or continued presence of gentamicin.
The in vivo images of UPEC during an acute infection suggest that the ability of E. coli to reemerge from infected cells may be a critical event in UTI pathogenesis. To examine the capacity of UTI89 and other type 1-piliated strains to exit host bladder epithelial cells, bacteria were allowed to invade 5637 bladder cells and the cells were then incubated in the presence of gentamicin for 24 h. Next, the cell culture medium was replaced with fresh medium with and without gentamicin, and bacterial titers were determined after an additional 7 h of incubation. During this latter incubation in the absence of gentamicin, total UTI89 titers increased significantly, while little increase was observed in the continued presence of gentamicin (Fig. 4B). NU14 behaved similarly (Mulvey et al., unpublished observations). These data indicate that intracellular UPEC can emerge from host bladder cells and subsequently multiply in the cell culture medium when gentamicin is removed. In contrast, MG1655 titers remained nearly constant during the 7-hour incubation with and without gentamicin (Fig. 4B), despite the fact that these bacteria can multiply as efficiently as UTI89 when delivered into cell culture medium lacking antibiotics. Thus, relative to the UPEC strains, the intracellular type 1-piliated K-12 strains have a decreased capacity to exit host bladder epithelial cells.
Microscopic examination of 5637 cells maintained in gentamicin-containing medium for 1 to 3 days after infection with either UTI89 or NU14 (Fig. 5) showed that these pathogens could form large intracellular inclusions similar to the bacterial factories seen in in vivo studies (Fig. 2C to E). The intracellular bacteria observed within 5637 cells by transmission electron microscopy (Fig. 5A) or by confocal microscopy (Fig. 5B and C) crowded the host cell cytoplasm, and some bacteria were filamentous, reminiscent of the elongated microbes observed in infected mouse bladders (Fig. 3). The addition of TMP-SMZ blocked the formation of these intracellular bacterial factories (Mulvey et al., unpublished observations). Most of the infected 5637 bladder cells within a monolayer, rather than being inundated with E. coli, harbored only one or a few bacteria, suggesting that intracellular environmental factors may influence whether UPEC begins to multiply within an individual host cell. In contrast to the UPEC strains, the K-12 strains did not form large intracellular inclusions, consistent with in vitro growth assays (Fig. 4A) and in vivo microscopic data indicating that these organisms do not multiply efficiently within bladder epithelial cells.
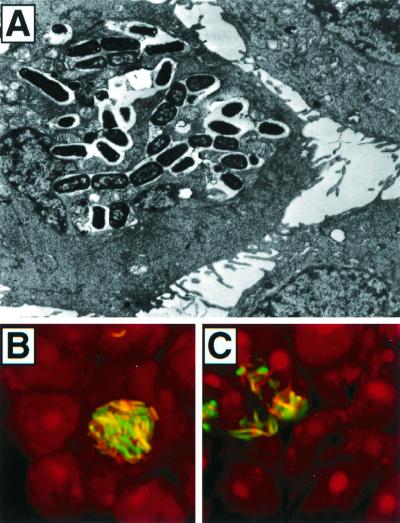
FIG. 5.
In vitro formation of intracellular bacterial inclusions. (A) One day after infection of 5637 cells with UTI89, large foci of intracellular bacteria were detected within 5637 cells by transmission electron microscopy. (B and C) Similar inclusions of intracellular bacteria were visualized by confocal microscopic examination of 5637 cells 72 h after infection with NU14 constitutively expressing green fluorescent protein. Host cells were counterstained with propidium iodide. Gentamicin prevented extracellular bacterial growth during these assays.
DISCUSSION
According to prevailing assumptions, UPEC strains are strictly extracellular pathogens. In this study, however, we provide evidence that host cell invasion enhances the ability of UPEC to successfully infect the bladder epithelium. Upon making contact with superficial epithelial cells in the bladder lumen, type 1-piliated UPEC induces a cascade of signaling events leading to bacterial internalization (27, 29). Following internalization UPEC is able to replicate, resulting in the formation of large collections of intracellular bacteria, which due to their appearance we have termed bacterial factories. UPEC strains were also shown to have the capacity to flux out of bladder epithelial cells, a process that may allow bacteria to escape from host superficial cells that are induced to exfoliate in response to infection. Bacteria exiting dying host cells are often filamentous, enabling them to maintain contact with the bladder epithelium as they leave one host cell and interact with neighboring and underlying epithelial cells. In comparison with the UPEC isolates, type 1-piliated K-12 E. coli strains MG1655 and AAEC185/pSH2, although able to invade bladder epithelial cells, were unable to persist, multiply intracellularly, or effectively exit the host cells. Furthermore, relative to UPEC, the K-12 strains are cleared more rapidly from the bladder during an acute infection (Mulvey et al., unpublished observations), suggesting that intracellular replication and the fluxing activity of UPEC enhance bacterial survival in vivo.
These observations illustrate the dynamic complexity of host-pathogen interactions during the acute phase of an infection. While exfoliation of superficial bladder cells is an effective host defense (1, 7, 11, 29), the shedding of infected host cells with the flow of urine may also facilitate the spread of UPEC in the environment. In addition, the exfoliation of infected superficial cells provides an opportunity for UPEC to interact with and invade the underlying bladder epithelium. Thus, it appears that UPEC can utilize invasion at two distinct steps in the establishment of an infection. Initially, invasion of bladder superficial cells provides UPEC with a protective, but transient, environment in which the bacteria can replicate. Subsequently, bacteria that manage to avoid rapid clearance from the urinary tract can invade the underlying epithelium, where they can establish a more stable bacterial reservoir. This reservoir can persist for several weeks in a quiescent state, seemingly undetected by immune surveillance mechanisms and protected from antibiotics (20, 29, 30), possibly by virtue of the permeability barrier maintained by the bladder epithelium (15, 26). Potentially, signals from differentiating bladder epithelial cells or other environmental cues may trigger intracellular bacterial replication and the reemergence of the bacterial reservoir, leading to a recurrent acute infection.
Type 1 pili were shown to be critical for bacterial persistence in the bladder as strain NU14 establishes persistent long-lasting infections while the fimH mutant strain NU14-1 is rapidly cleared from the bladders of infected mice. In contrast, it has been shown that type 1 pili do not contribute to bacterial persistence in a mouse model of pyelonephritis (14). Therefore, the effect of type 1 pili appears to be localized to the bladder, whereas other adhesive factors such as Dr adhesins and P pili have been shown to be critical for establishing persistent kidney infections (13, 32). Epidemiologic studies have also correlated Dr family adhesins and class III PapG with chronic or recurrent UTIs (9); however, the importance of type 1 pili in bacterial persistence has likely been underappreciated in these clinical studies due to the ubiquitous nature of the fim gene cluster in all strains of E. coli.
UTIs, which affect at least 25% of women, have a strong propensity to recur (16). Within 6 months after an initial UTI, about one-fourth of women will experience a second infection and many individuals will endure multiple, recurrent UTIs throughout their lives (10, 16). Recurrent UTIs are usually attributed to the reinoculation of the urinary tract with uropathogens arising from intestinal or other environmental reservoirs. Intriguingly, the bacteria associated with a recurrent UTI often appear to be phenotypically or genotypically identical to the bacterial strain that caused the initial infection (5, 10, 21, 22). Based on such observations, it is feasible that many recurrent UTIs occur due to a resurgence of UPEC from quiescent reservoirs established within the bladder mucosa following an initial acute infection. Therefore, in many instances, the tendency of UTIs to recur may be directly linked to the ability of UPEC to replicate intracellularly and eventually reemerge from infected bladder epithelial cells. Other bacterial pathogens that have been traditionally considered to be noninvasive may use strategies similar to that of UPEC to establish long-term, quiescent bacterial reservoirs in other tissues. The existence of such reservoirs may help explain the recurrent nature of many infectious diseases.
ACKNOWLEDGMENTS
M.A.M. and J.D.S. contributed equally to this work.
We thank M. Veith for his excellent assistance with scanning electron microscopy, M. Levy for help with transmission electron microscopy, and J. J. Martinez for help with confocal microscopy. We are also grateful to K. Dodson, M. Chapman, and C. Vincent for helpful discussions and suggestions.
This work was supported by grants AI29549, DK51406, and AI48 from the National Institutes of Health (S.J.H.).
REFERENCES
- 1.Aronson M, Medalia O, Amichay D, Nativ O. Endotoxin-induced shedding of viable uroepithelial cells is an antimicrobial defense mechanism. Infect Immun. 1988;56:1615–1617. doi: 10.1128/iai.56.6.1615-1617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barza M. Urinary tract. In: Schaeter M, Engelberg N C, Eisenstein B I, Medoff G, editors. Mechanisms of microbial disease. Philadelphia, Pa: Williams & Wilkins; 1998. pp. 564–572. [Google Scholar]
- 3.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Blomfield I C, McClain M S, Eisenstein B I. Type 1 fimbriae mutants of Escherichia coli K12: characterization of recognized afimbriate strains and construction of new fim deletion mutants. Mol Microbiol. 1991;5:1439–1445. doi: 10.1111/j.1365-2958.1991.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 5.Brauner A, Jacobson S H, Kuhn I. Urinary Escherichia coli causing recurrent infections–a prospective follow-up of biochemical phenotypes. Clin Nephrol. 1992;38:318–323. [PubMed] [Google Scholar]
- 6.Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis C P, Uehling D T, Mizutani K, Balish E. Bladder surface alteration following infection with Escherichia coli. In: Becker R P, Johari O, editors. Scanning electron microscopy. II. O'Hare, Ill: AMF; 1978. pp. 315–320. [Google Scholar]
- 8.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. . (Erratum, 278:373.) [DOI] [PubMed] [Google Scholar]
- 9.Foxman B, Gillespie B, Koopman J, Zhang L, Palin K, Tallman P, Marsh J V, Spear S, Sobel J D, Marty M J, Marrs C F. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194–1205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 10.Foxman B, Zhang L, Tallman P, Palin K, Rode C, Bloch C, Gillespie B, Marrs C F. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J Infect Dis. 1995;172:1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 11.Fukushi Y, Orikasa S, Kagayama M. An electron microscopic study of the interaction between vesical epithelium and E. coli. Investig Urol. 1979;17:61–68. [PubMed] [Google Scholar]
- 12.Funfstuck R, Smith J W, Tschape H, Stein G. Pathogenetic aspects of uncomplicated urinary tract infection: recent advances. Clin Nephrol. 1997;47:13–18. [PubMed] [Google Scholar]
- 13.Goluszko P, Moseley S L, Truong L D, Kaul A, Williford J R, Selvarangan R, Nowicki S, Nowicki B. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J Clin Investig. 1997;99:1662–1672. doi: 10.1172/JCI119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta R, Ganguly N K, Ahuja V, Joshi K, Sharma S. An ascending non-obstructive model for chronic pyelonephritis in BALB/c mice. J Med Microbiol. 1995;43:33–36. doi: 10.1099/00222615-43-1-33. [DOI] [PubMed] [Google Scholar]
- 15.Hicks R M. The mammalian bladder: an accommodating organ. Biol Rev (Cambridge) 1975;50:215–247. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 16.Hooton T M, Stamm W E. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin N Am. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins W J, Gendron-Fitzpatrick A, Balish E, Uehling D T. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect Immun. 1998;66:2798–2802. doi: 10.1128/iai.66.6.2798-2802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hultgren S J, Porter T N, Schaeffer A J, Duncan J L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985;50:370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hultgren S J, Schwan W R, Schaeffer A J, Duncan J L. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect Immun. 1986;54:613–620. doi: 10.1128/iai.54.3.613-620.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hvidberg H, Struve C, Krogfelt K A, Christensen N, Rasmussen S N, Frimodt-Moller N. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob Agents Chemother. 2000;44:156–163. doi: 10.1128/aac.44.1.156-163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikaheimo R, Siitonen A, Heiskanen T, Karkkainen U, Kuosmanen P, Lipponen P, Makela P H. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22:91–99. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson S H, Kuhn I, Brauner A. Biochemical fingerprinting of urinary Escherichia coli causing recurrent infections in women with pyelonephritic renal scarring. Scand J Urol Nephrol. 1992;26:373–377. doi: 10.3109/00365599209181229. [DOI] [PubMed] [Google Scholar]
- 23.Jones C H, Pinkner J S, Roth R, Heuser J, Nicholes A V, Abraham S N, Hultgren S J. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci USA. 1995;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langermann S, Mollby R, Burlein J E, Palaszynski S R, Auguste C G, DeFusco A, Strouse R, Schenerman M A, Hultgren S J, Pinkner J S, Winberg J, Guldevall L, Soderhall M, Ishikawa K, Normark S, Koenig S. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181:774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 25.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, Hultgren S J. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 26.Lewis S A. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- 27.Martinez J J, Mulvey M A, Schilling J D, Pinkner J S, Hultgren S J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McTaggart L A, Rigby R C, Elliott T S. The pathogenesis of urinary tract infections associated with Escherichia coli, Staphylococcus saprophyticus and S. epidermidis. J Med Microbiol. 1990;32:135–141. doi: 10.1099/00222615-32-2-135. [DOI] [PubMed] [Google Scholar]
- 29.Mulvey M A, Lopez-Boado Y S, Wilson C L, Roth R, Parks W C, Heuser J, Hultgren S J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. . (Erratum, 283:795, 1999.) [DOI] [PubMed] [Google Scholar]
- 30.Mulvey M A, Schilling J D, Martinez J J, Hultgren S J. From the cover: bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci USA. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orndorff P E, Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984;159:736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts J A, Marklund B I, Ilver D, Haslam D, Kaack M B, Baskin G, Louis M, Mollby R, Winberg J, Normark S. The Gal(alpha 1–4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci USA. 1994;91:11889–11893. doi: 10.1073/pnas.91.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaeffer A J. Potential role of phase variation of type 1 pili in urinary tract infection and bacterial prostatitis. Infection. 1991;19(Suppl. 3):S144–S149. doi: 10.1007/BF01643685. [DOI] [PubMed] [Google Scholar]
- 34.Schilling J D, Mulvey M A, Vincent C D, Lorenz R G, Hultgren S J. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166:1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 35.Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect Dis Clin N Am. 1997;11:513–529. doi: 10.1016/s0891-5520(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 36.Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr Opin Microbiol. 1999;2:99–105. doi: 10.1016/s1369-5274(99)80017-4. [DOI] [PubMed] [Google Scholar]
- 37.Thankavel K, Madison B, Ikeda T, Malaviya R, Shah A H, Arumugam P M, Abraham S N. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J Clin Investig. 1997;100:1123–1136. doi: 10.1172/JCI119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X R, Sun T T, Medina J J. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc Natl Acad Sci USA. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]