Abstract
Using in situ hybridization histochemistry, we studied the distribution of neurons that express preproopiomelanocortin (pre-POMC), preprodynorphin (pre-PDYN), and preproenkephalin (pre-PENK) gene transcripts within the human hypothalamus and surrounding structures. Of the three opioid systems, pre-POMC neurons have the most restricted distribution. Pre-POMC cells are most numerous in the infundibular nucleus and retrochiasmatic area of the mediobasal hypothalamus; a few labeled cells are present within the boundaries of the ventromedial nucleus and infundibular stalk. Pre-POMC message was not found in the limited samples of structures adjacent to the hypothalamus.
In contrast to neurons that express pre-POMC, neurons expressing pre-PDYN and pre-PENK are more widely represented throughout the hypothalamus and extrahypothalamic structures. However, pre-PDYN and pre-PENK cells differ from one another in distribution. Pre-PDYN message is especially abundant in neurons of the tuberal and mammillary regions, with a distinct population of labeled cells in the premammillary nucleus and dorsal posterior hypothalamus. Pre-PDYN gene expression also is found in neurons of the dorsomedial nucleus, ventromedial nucleus, caudal magnocellular portion of the paraventricular nucleus, dorsolateral supraoptic nucleus, tuberomammillary nucleus, caudal lateral hypothalamus, and retrochiasmatic area. In structures immediately adjacent to the hypothalamus, pre-PDYN neurons were observed in the caudate nucleus, putamen, cortical nucleus of the amygdala, and bed nucleus of the stria terminalis.
Pre-PENK neurons occur in varying numbers in all hypothalamic nuclei except the mammillary bodies. The chiasmatic region is particularly rich in pre-PENK neurons, with the highest packing density in the intermediate nucleus {the intermediate nucleus (Braak and Braak [1987] Anat. Embryol. 176:315-330) has also been termed the sexually dimorphic nucleus of the preoptic area (SDA-POA, Swaab and Fliers [1985] Science 228:1112-1115) or the interstitial nucleus of the anterior hypothalamus 1 (Allen et al. [1989] J. Neurosci. 9:497-506)}, dorsal suprachiasmatic nucleus, medial preoptic area, and rostral lateral hypothalamic area. Pre-PENK neurons are numerous in the infundibular nucleus, ventromedial nucleus, dorsomedial nucleus, caudal parvicellular portion of the paraventricular nucleus, tuberomammillary nucleus, lateral hypothalamus, and retrochiasmatic area. Only a few lightly labeled cells were found in the periphery of the supraoptic nucleus and lateral tuberal nucleus. In areas adjacent to the hypothalamus, cells that contain pre-PENK message occur in the nucleus basalis of Meynert, central nucleus of amygdala, bed nucleus of the stria terminalis, caudate nucleus, and putamen. The differential distribution of pre-POMC, pre-PDYN, and pre-PENK neurons in the human hypothalamus suggests that these three opioid systems influence hypothalamic functions in quite different ways.
Keywords: basal forebrain, dynorphin, enkephalin, intermediate nucleus, proopiomelanocortin
INTRODUCTION
In mammals, cerebral opioid systems are anatomically heterogeneous and consist of three different classes of peptides, β-endorphins, enkephalins, and dynorphins (Dores et al., 1990; Brownstein, 1993). Each of these putative neurotransmitters is synthesized by a different gene with its own precursor molecule (Höllt, 1993). Proopiomelanocortin (POMC) is a 267-amino-acid precursor peptide that is coded for by the pre-POMC gene and yields a group of opioid peptides, the β-endorphins, as well as the nonopioid hormones adrenocorticotrophic hormone, and three melanocyte- stimulating hormone-like molecules (Mains et al., 1977; Young et al., 1993). The maturation and cleavage of the precursor molecule into its various products appear to be tissue-specific phenomena, and posttranslational processing plays a crucial role in determining the biological activities of POMC derivatives in the brain (Smith and Funder, 1988; Benjannet et al., 1991; Roberts et al., 1993). For example, different regions of the brain preferentially produce different POMC cleavage products that are processed into smaller forms and exhibit neurotransmitter/neuromodulatory functions (Akil et al., 1984; Smith and Funder, 1988; Young et al., 1993).
The most recently discovered class of endogenous opioid peptides consists of derivatives of the 256-amino-acid precursor proenkephalin (PENK B), or prodynorphin (PDYN), coded for by the pre-PDYN gene (Kakidani et al., 1982). The cleavage of PDYN yields three main opioid peptides (i.e., neoendorphin, dynorphin A, and dynorphin B), all of which contain the sequence of leu-enkephalin (Day and Akil, 1989). Neoendorphin can exhibit two different forms, ⍺-neoendorphin and β-neoendorphin, that differ by one amino acid (Minamino et al., 1980, 1981; Watson et al., 1982; Nakao et al., 1983; Akil et al., 1984). Currently, the best-known cleavage products of dynorphin A are two smaller fragments, DYN A1-8 and DYN A1-17 (Minamino et al., 1980; Seizinger et al., 1981). Processing of the dynorphin B domain can produce the 29-amino-acid peptide leumorphin or dynorphin B1-13 (Day and Akil, 1989). Mechanisms of complex PDYN tissue-specific processing are being investigated in a number of systems at the cellular, integrative, and behavioral levels (Day et al., 1993).
Enkephalins are produced by the 267-amino-acid peptide PENK, coded for by the pre-PENK gene (Gubler et al., 1982; Noda et al., 1982). All cleavage products of PENK, including four met-enkephalins, two carboxyl-extended met-enkephalins, and one leu-enkephalin, exhibit opioid-like activity. Larger fragments isolated from the adrenals, peptides E and F, contain met- and leu-enkephalin sequences (Schultzberg et al., 1978; Costa et al., 1979; Viveros et al., 1979; Lewis et al., 1980), but it is undetermined whether these adrenally derived peptides are processed into active fragments in the brain (Rossier, 1993). It is also possible that complex PENK tissue-specific processing may generate several nonopioid peptides with physiological activity (Rossier, 1993).
The opioid peptides are involved in a variety of behavioral and physiological processes such as eating, drinking, reproduction, stress, pain, emotions, learning, and homeostasis (Morley et al., 1983; Ferin and Vande Wiele, 1984; Olson et al., 1990; Dondi et al., 1991; Laatikainen, 1991; Neumann et al., 1992; Russell et al., 1992), and opioid dysfunction has been implicated in several neurological/psychiatric disorders (Berger et al., 1981; Franceschi et al., 1986; Seizinger et al., 1986; Berger and Nemeroff, 1987; Cavagnini et al., 1987; Terenius et al., 1987; Franceschi et al., 1988; Chamberlain and Herman, 1990; Zis and Garland, 1991). A number of immunocytochemical and biochemical studies have shown that the peptides are concentrated heavily in the hypothalamus of various species, including humans (Bloch et al., 1978; Gramsch et al., 1979, 1982; Kubek and Wilber, 1980; Maysinger et al., 1982; Pittius et al., 1983, 1984; Suda et al., 1985). Because the hypothalamus is a key modulator and integrator of numerous behavioral and physiological functions, it is important to determine the distribution of opioid peptides within hypothalamic nuclei.
Previous studies have used immunocytochemistry to study human cerebral opioid systems (Haber and Watson, 1985; Abe et al., 1988; Haber et al., 1990). However, the interpretation of immunocytochemical results can be compromised by the potential cross-reactivity of antibodies with certain members of the three opioid peptide families (Simerly et al., 1988; Haber et al., 1990) and by the loss of epitopes resulting from extended postmortem intervals common in obtaining human material. In situ hybridization histochemistry is particularly useful for localizing transmitter-specific substances in human postmortem tissues (Mengod et al., 1992). In this study, we exploited the postmortem stability of mRNA (Johnson et al., 1986) and the high specificity and sensitivity of oligonucleotide probes to map opioid peptide precursor gene expression in neurons of the human hypothalamus.
MATERIALS AND METHODS
Tissue blocks were taken at autopsy from four 16–61-year-old human males with a mean postmortem interval of 9 hours (Walker et al., 1991; Sukhov et al., 1993). All subjects died acutely of non-neurological conditions, and complete autopsies did not disclose disorders that would be expected to compromise our analysis (Table 1). Autopsy specimens were collected in accordance with guidelines set forth in Federal Register 46 and with the institutional guidelines of The Johns Hopkins University School of Medicine and The University of Arizona College of Medicine. Each tissue sample included the hypothalamus and all or part of the parolfactory/paraterminal cortex, septum, substantia innominata, dorsal amygdala, caudate nucleus, putamen, globus pallidus, and thalamus. Blocks of tissue were placed on foil-coated glass slides, frozen at −30°C in isopentane, and stored at −80°C.
TABLE 1.
Perimortem Histories of Human Subjects
| Case no. | Sex | Age (years) | Cause of death | Postmortem interval (hours) |
|---|---|---|---|---|
|
| ||||
| 426 | Male | 61 | Acute myocardial infarction | 13 |
| 810 | Male | 16 | Lacerated lung | 12 |
| 814 | Male | 52 | Asphyxiation | 4 |
| 871 | Male | 23 | Gunshot wound | 7 |
Hybridization histochemistry
Sections (20 microns thick) were cut coronally (cases 814, 426) or sagittally (cases 814, 871, 810) on a cryostat-microtome at −20°C and thaw-mounted onto gelatin-coated slides. Every twentieth section was processed for hybridization histochemistry using a specific probe as described previously (Young, 1989; Walker et al., 1991; Sukhov et al., 1993; Rance et al., 1994). Briefly, sections were brought to room temperature, postfixed in 4% formaldehyde in phosphate-buffered saline for 5 minutes, treated with 0.25% acetic anhydride for 10 minutes, and delipidated in a graded series of ethanols and chloroform. After drying, sections were incubated for 20 hours at 37°C in a buffer consisting of 600 mM NaCl, 80 mM Tris-HC1, pH 7.5, 4 mM ethylenediaminetetraacetic acid, 0.1% sodium pyrophosphate, 0.2% sodium dodecyl sulfate, 10% dextran sulfate, 0.2 mg/ml heparin sulfate, 100 mM dithiothreitol, and ~106 dpm of 35S-labeled probe per 50 microliters (see below). Sections were then washed in a solution of 0.3 M NaCl/30 mM sodium citrate buffer (2x SSC) and 50% formamide at 45°C. After drying, sections were dipped in Kodak NTB-3 nuclear emulsion and exposed at 4°C for pre-POMC (7–14 days), pre-PDYN (60–120 days), and pre-PENK (30–60 days). Oligonucleotide probes were targeted toward bases of the human pre-POMC (7,106–7,153; Takahashi et al., 1983), rat pre-PDYN [862–909; Civelli et al., 1985; 94% homology with human (Horikawa et al., 1983)], and human pre-PENK (963–1,010; Comb et al., 1982) mRNA sequences. Oligonucleotide probes were labeled on the 3’ end using terminal deoxynucleotidyl transferase (Boehringer-Mannheim, Indianapolis, IN) and [35S]deoxyadenosine triphosphate ( > 1,000 Ci/mmol; New England Nuclear, Boston, MA). Probes used in these experiments were designed to preclude cross-hybridization with other transcripts. The specificity of probes was determined through a series of controls. First, probes were used for Northern analysis under similar conditions, which identified the appropriate size of transcripts (Rance and Young, 1991). Second, probes in this study gave distributions different from each other, as did a number of other 48mers in our previous studies (Rance and Young, 1991, 1994; Sukhov et al., 1993). Third, a 48-base vasopressin sense probe gave no signal above background (Young, unpublished observations). After hybridization histochemistry, sections were stained with toluidine blue.
Analysis of tissue
Sections were mapped with a computerized microscopic mapping system (Sukhov et al., 1993). Computer-assisted mapping was carried out using a combination of brightfield and darkfield illumination. The boundaries of various hypothalamic nuclei and extrahypothalamic structures were first drawn under brightfield illumination using a 2.5x Plan-Neofluar (Zeiss Axiophot) objective. Sections were then systematically scanned under darkfield illumination for labeled pre-POMC, pre-PDYN, and pre-PENK neurons using a 20x Plan-Neofluar objective. Neuronal perikarya were considered “labeled” (i.e., containing pre-POMC, pre-PDYN, or pre-PENK mRNA) only if silver grains were localized over the cell soma (verified at 40x under brightfield illumination) and if the density of superimposed silver grains exceeded three times that of the surrounding neuropil. (Hereafter, we will refer to pre-POMC, pre-PDYN, and pre-PENK mRNA-containing cells as POMC, PDYN, and PENK neurons, respectively.) Based on labeling intensity, positive neurons were subdivided into lightly, moderately, and heavily labeled groups (for illustration, see Fig. 1B in Sukhov et al., 1993). Lightly labeled neurons were mapped only if grains within the neuronal somata exceeded three times background and the nucleus of each cell was present. In some cases, heavily labeled neurons were completely covered with superimposed silver grains, obscuring cellular details. Several sources were consulted to assist in the identification of basal forebrain structures (Le Gros Clark, 1936, 1938; Nauta and Haymaker, 1969; Veazey et al., 1982; Bleier, 1984; Braak and Braak, 1987, 1992; Gai et al., 1990; Saper, 1990).
RESULTS
POMC
The distribution of POMC mRNA in human hypothalamus is restricted to neurons of the mediobasal hypothalamus (Table 2). Most POMC neurons are confined to two major neuronal groups, the infundibular nucleus and the retrochiasmatic area. A small number of POMC neurons also are found in the ventromedial nucleus and infundibular stalk (Figs. 1,2).
TABLE 2.
Opioid Peptide Precursor Gene Expression in Neurons of the Hypothalamic Nuclei and Some Extrahypothalamic Structures1
| Region | Structure | Pre-POMC | Pre-PDYN | Pre-PENK |
|---|---|---|---|---|
|
| ||||
| Chiasmatic | Supraoptic nucleus, dorsolateral | − | + | + |
| Supraoptic nucleus, ventromedial | − | + | + | |
| Medial preoptic area | − | − | +++ | |
| Intermediate nucleus | − | − | +++ | |
| Suprachiasmatic nucleus | − | − | +++ | |
| Paraventricular nucleus | − | + | +++ | |
| Retrochiasmatic area | +++ | ++ | ++ | |
| Tuberal | Paraventricular nucleus | − | ++ | ++ |
| Dorsomedial nucleus | − | +++ | + | |
| Ventromedial nucleus | + | ++ | +++ | |
| Premammillary nucleus | − | +++ | + | |
| Lateral tuberal nucleus | − | − | + | |
| Tuberomammillary nucleus | − | + | + | |
| Infundibular nucleus | +++ | − | +++ | |
| Mammillary | Mammillary body | − | − | − |
| Posterior hypothalamus | − | +++ | ++ | |
| Paraventricular nucleus | − | ++ | ++ | |
| Extrahypothalamic | Amygdala, central nucleus | − | − | +++ |
| Amygdala, cortical nucleus | − | +++ | − | |
| Bed nucleus of the stria terminalis | − | ++ | +++ | |
| Nucleus basalis of Meynert | − | − | + | |
| Nucleus accumbens | − | +++ | + | |
| Putamen | − | + | +++ | |
| Caudate nucleus | − | ++ | + | |
| Globus pallidus | − | − | ++ | |
| Parolfactory gyrus | − | + | + | |
| Zona incerta | − | ++ | ++ | |
−, Opioid peptide precursor mRNA-containing cells were absent (0% of the whole neuronal population); +, only a few cells that express the opioid peptide precursor gene were present in the structure (1–10%); ++, cells that express the opioid peptide precursor gene were present in moderate numbers in the nucleus (10–60%); +++, cells that express the opioid peptide precursor gene were very abundant in the nucleus (60–100%).
Figure 1.
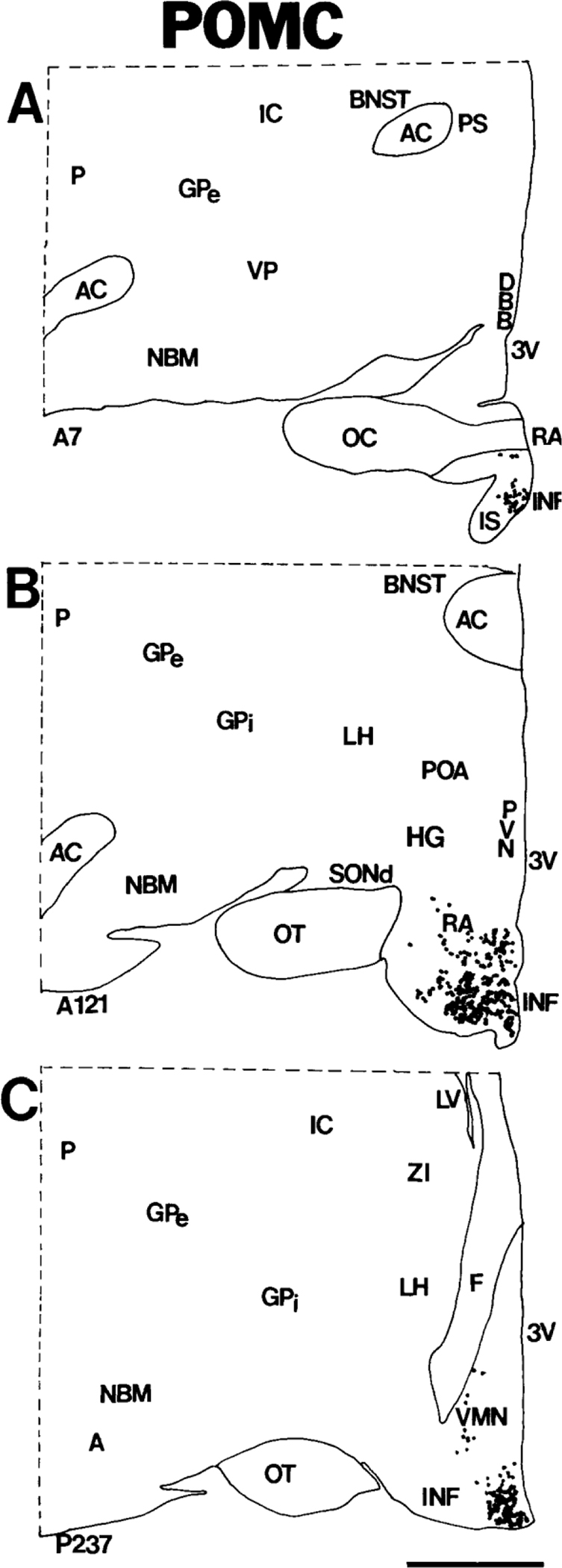
A-C: Computer-assisted maps of the distribution of POMC cells in coronal sections of human hypothalamus arranged rostrocaudally from A to C. Each dot represents a single neuron. Numbers at the lower left correspond to sequential locations of sections from anterior to posterior. Each section is 20 microns thick; hence, the distance between A and C is ~4.6 mm. Scale bar = 5 mm.
Figure 2.
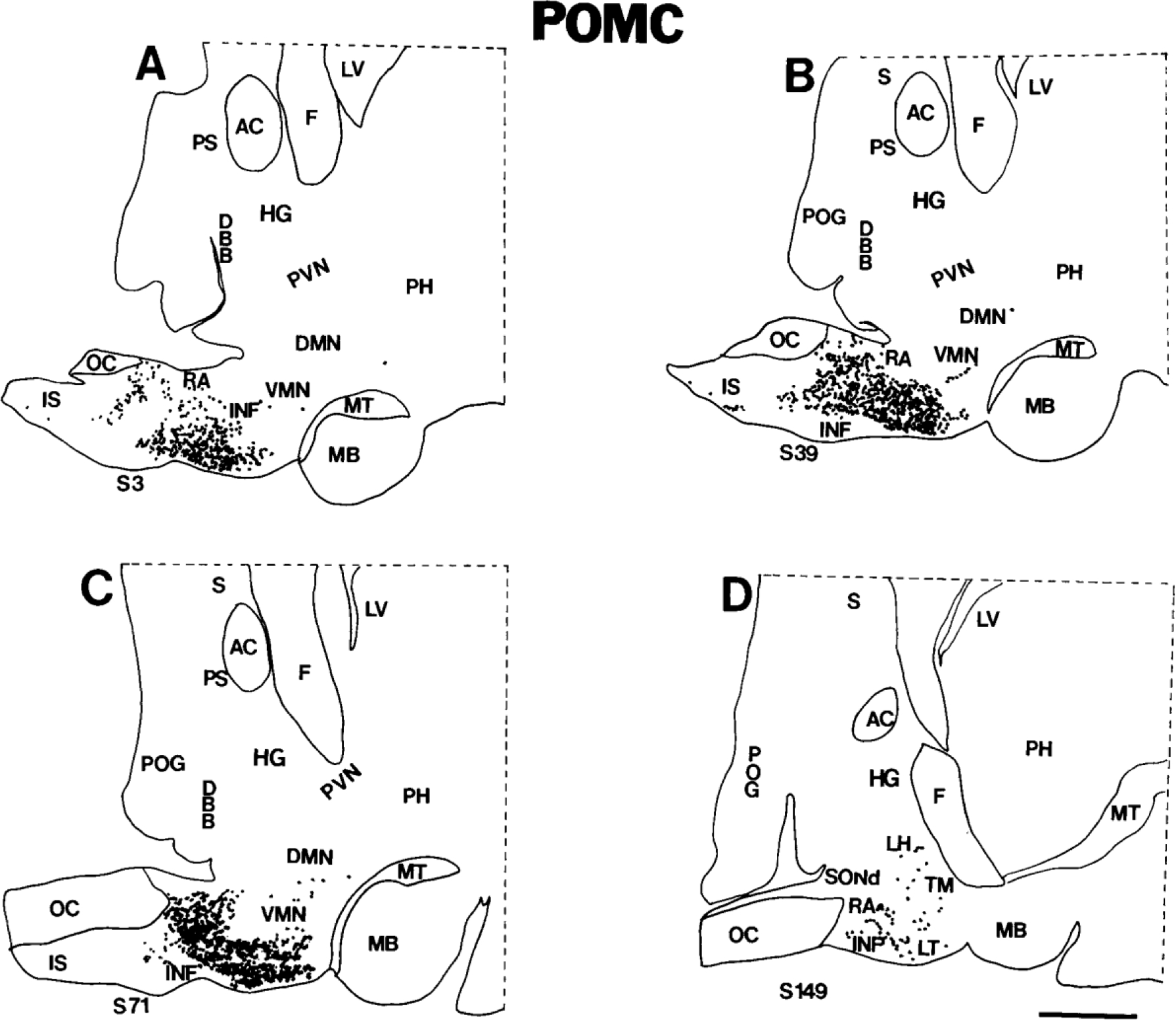
A-D: Computer-assisted maps of the distribution of POMC cells in sagittal sections of the human hypothalamus. The most medial section is A, and the most lateral section is D. Sagittal sections are numbered serially from medial to lateral; each section is 20 microns thick; hence, the distance between A and D is ~1.9 mm. Each dot represents a single neuron. Scale bar = 5 mm.
POMC in the infundibular nucleus.
The infundibular nucleus, also known as the arcuate nucleus (Nauta and Haymaker, 1969; Saper, 1990), occupies a considerable portion of the mediobasal hypothalamus, extending rostrocaudally from the infundibular stalk to the origin of the mammillothalamic tract (Bleier, 1984; Saper, 1990; Swaab et al., 1993). Significant populations of neurons express POMC throughout the entire length of the infundibular nucleus (Figs. 1A–C, 2A–D). Most neurons that contain POMC transcripts are small and round, with moderate-to-heavy labeling over the cytoplasm (Fig. 3A). At the most rostral part of the infundibular nucleus that borders the retrochiasmatic area, infundibular POMC neurons overlap with oblong, medium-sized, labeled neurons of the retrochiasmatic area.
Figure 3.
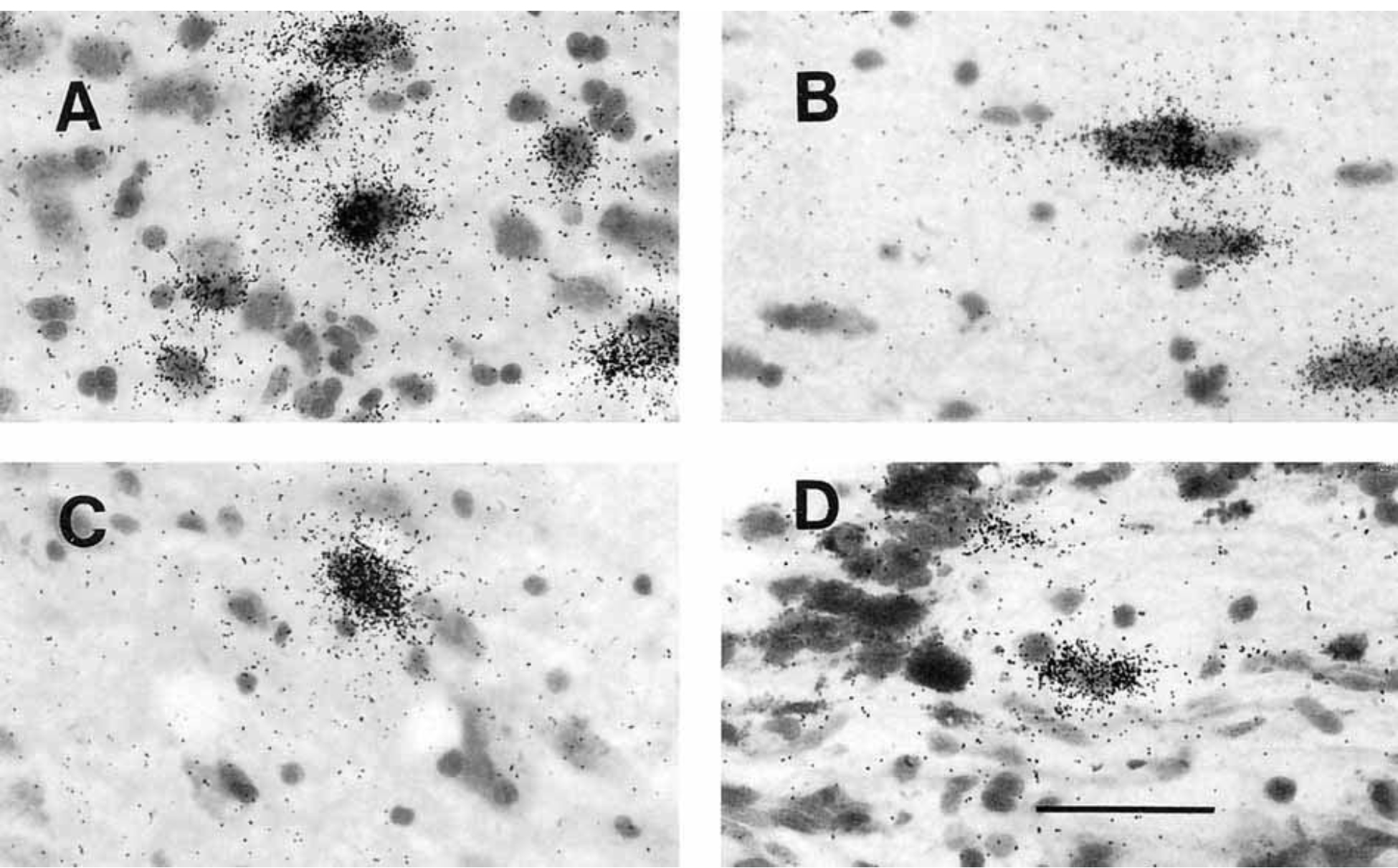
Photomicrographs of POMC cells in the human hypothalamus. A: Robustly labeled neurons of the infundibular nucleus. B: Typical oblong neurons of the retrochiasmatic area. The long axis is oriented toward infundibular stalk. C: One of the rare POMC neurons in the territory of the ventromedial nucleus. D: Cell that contains POMC mRNA in the infundibular stalk. Scale bar = 50 microns.
POMC in the retrochiasmatic area.
Numerous POMC neurons were identified in the retrochiasmatic area. The human retrochiasmatic area is a poorly delineated area that lies in the anterior hypothalamus, caudal to the optic chiasm (Figs. 1, 2). The caudoventral retrochiasmatic region merges with the infundibular nucleus. Most POMC neurons in the retrochiasmatic area are oriented with their long axes toward the infundibular stalk (Fig. 3B).
POMC in the ventromedial nucleus.
We detected a few labeled POMC neurons in the ventrolateral part of the ventromedial nucleus (Fig. 3C). Some moderately and heavily labeled POMC cells appear to have strayed rostrocaudally from the infundibular nucleus into the ventromedial nucleus in the tuberal hypothalamus (Fig. 2C). Other cells seem to extend from the ventromedial nucleus into the lateral hypothalamic area and dorsomedial nucleus (Fig. 3C).
POMC in the infundibular stalk.
Labeled POMC cells were found in the infundibular stalk adjacent to the optic chiasm (Figs. 1A, 2A–C).
PDYN
PDYN neurons are more widely distributed in hypothalamic structures than are POMC cells (Table 2). We detected labeled PDYN cells in the supraoptic, paraventricular, dorsomedial, ventromedial, premammillary, and tuberomammillary nuclei, in the retrochiasmatic area, and in lateral and posterior hypothalamic areas. However, the packing density of PDYN neurons varied among hypothalamic cell groups; in the caudal aspect of the paraventricular nucleus, the premammillary nucleus, and the posterior hypothalamic area, a significant number of neurons express pre-PDYN. The packing density of PDYN cells was lower in other PDYN-containing hypothalamic nuclei (Figs. 4A–F, 5A–D). In general, PDYN neurons predominate in the tuberal and dorsal posterior regions, with only rare labeled neurons in more rostral parts of the hypothalamus.
Figure 4.
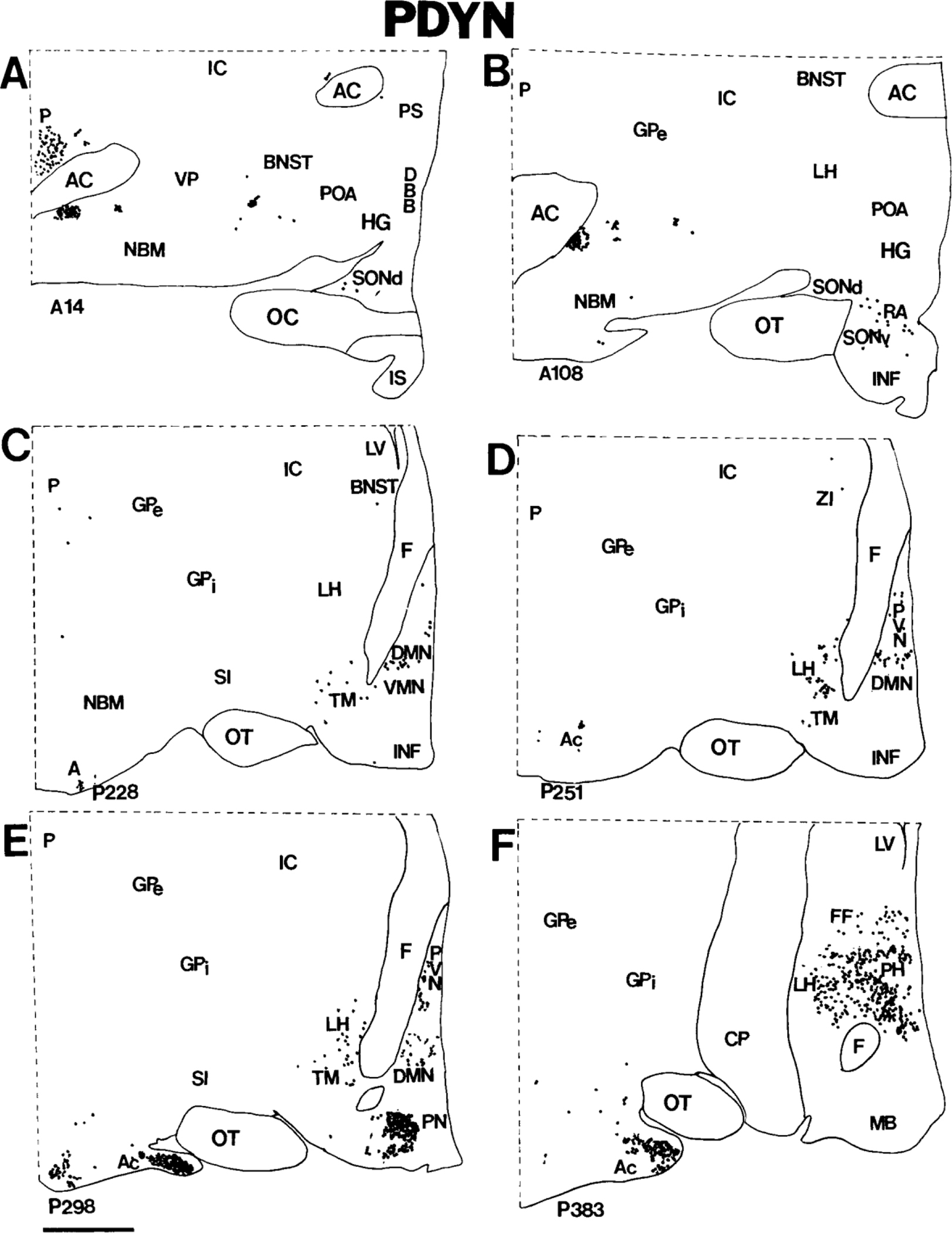
A-F: Computer-assisted maps of the distribution of PDYN cells in coronal sections of the human hypothalamus arranged rostrocaudally from A to F. The most anterior section is A, and the most posterior section is F. Numbers at the lower left correspond to sequential locations of sections from anterior to posterior. Each section is 20 microns thick. Each dot represents a single neuron. Scale bar = 5 mm.
Figure 5.
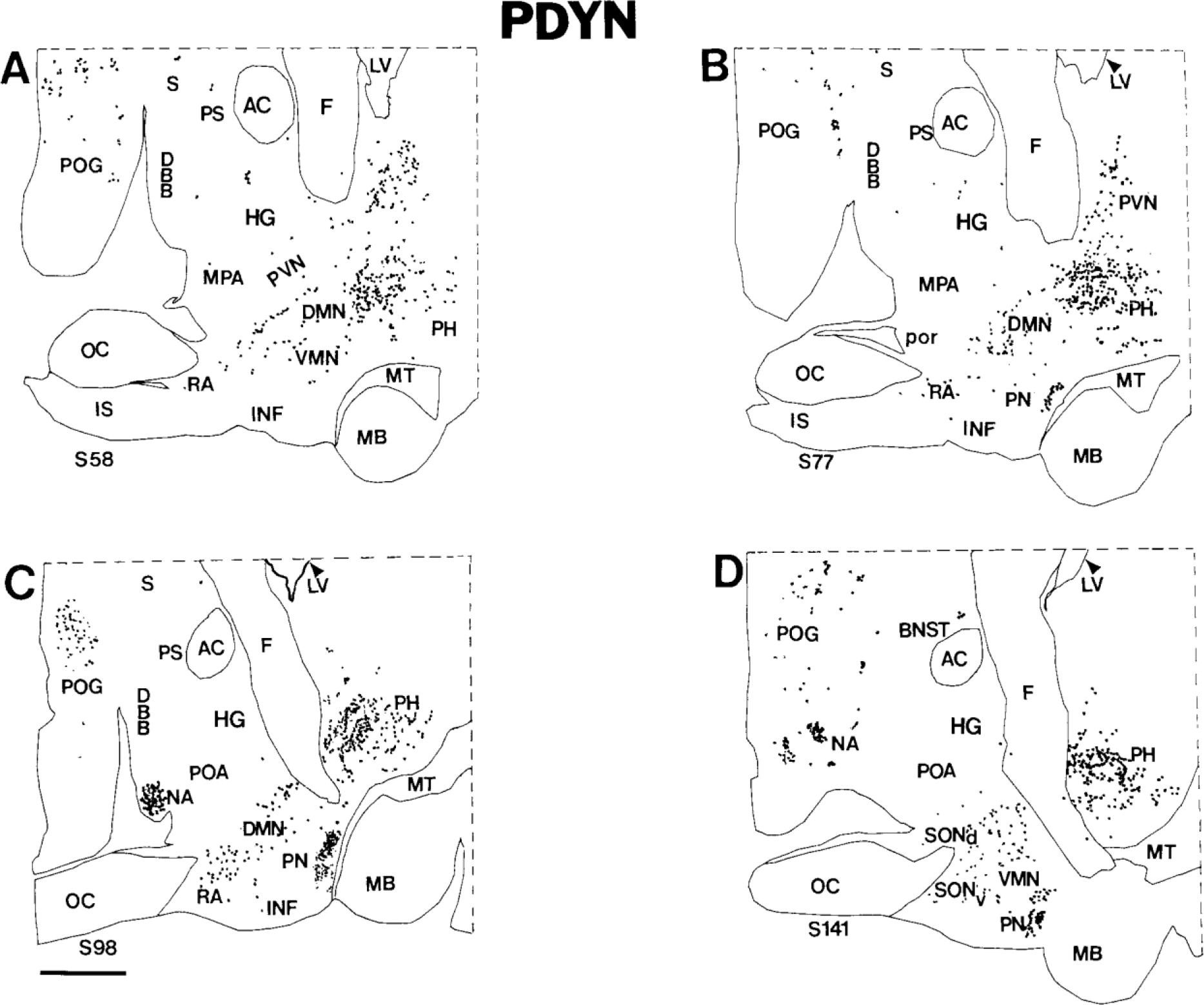
A-D: Computer-assisted maps of the distribution of PDYN cells in sagittal sections of the human hypothalamus. The most medial section is A, and the most lateral section is D. Each dot represents a single neuron. Scale bar = 5 mm.
PDYN in the premammillary nucleus.
The premammillary nucleus is located in the tuberal region just rostral to the medial mammillary nucleus (Figs. 4E, 5B,C). Many neurons of the premammillary nucleus express pre-PDYN. In coronal sections, these cells are round, with moderately labeled cytoplasm (Fig. 6F); in sagittal sections, neurons of the premammillary nucleus appear more elongated. Because of the high packing density of PDYN neurons, the premammillary nucleus is highlighted in pre-PDYN hybridization histochemical preparations (Figs. 4E, 5B,C). Some PDYN neurons in the periphery of the nucleus are medium-sized to small, round or pear-shaped neurons that resemble cells of the ventrolateral division of the ventromedial nucleus.
Figure 6.
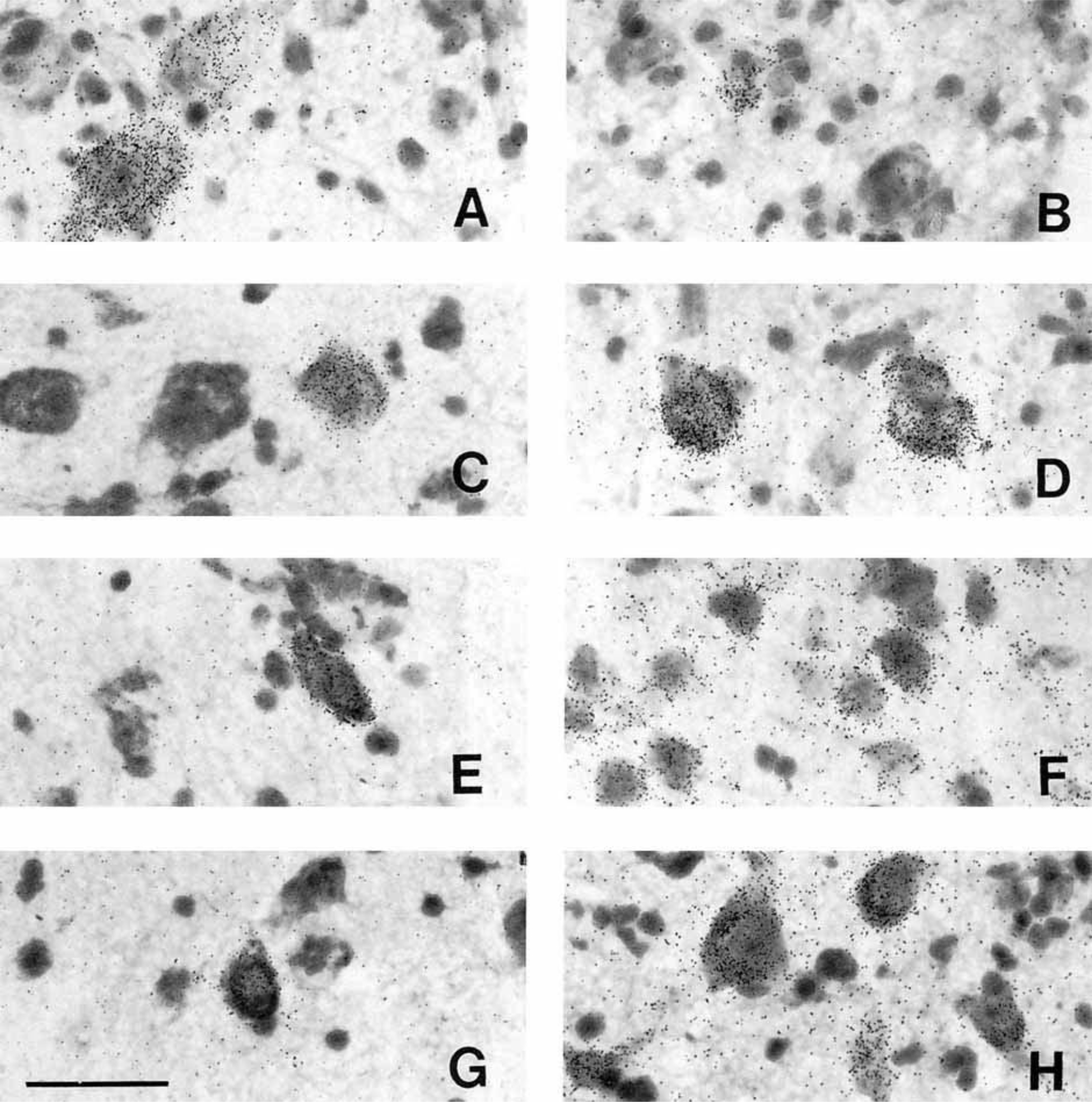
Photomicrographs of PDYN neurons in the human hypothalamus. A: Moderately and lightly labeled magnocellular neurons of the caudal paraventricular nucleus. B: Lightly labeled, small, peripherally located neuron in the dorsolateral supraoptic nucleus. C: Typical PDYN neuron of the tuberomammillary nucleus. D: Large, heavily labeled neurons of the posterior hypothalamic nucleus (coronal view). E: One of the few PDYN neurons of the retrochiasmatic area. F: PDYN neurons of the premammillary nucleus. G: Lightly labeled neuron of the ventromedial nucleus. H: PDYN neurons of the dorsomedial nucleus. Scale bar = 50 microns.
PDYN in the paraventricular nucleus.
Viewed in sagittal sections, the human paraventricular nucleus extends along the medial surface of the hypothalamus as a rostroventral-to-caudodorsal continuum (Sukhov et al., 1993). PDYN cells are scattered throughout this nucleus (Figs. 4D,E, 5A,B) but are most abundant caudally. Only a few labeled neurons were detected in the rostroventral paraventricular nucleus. The majority of PDYN neurons are magnocellular with lightly to moderately labeled perikarya (Fig. 6A), but some medium-sized neurons also express pre-PDYN.
PDYN in the supraoptic nucleus.
We detected only a few PDYN neurons in the dorsolateral part of the human supraoptic nucleus. We did not observe magnocellular neurons that express the pre-PDYN gene in the nucleus. Usually, cells that contain pre-PDYN mRNA are medium-sized or small, with moderate labeling intensity (Fig. 6B).
PDYN in the dorsal posterior hypothalamus.
Two major groups of PDYN neurons were found in the posterior hypothalamus. First, there is a prominent subpopulation of posterior hypothalamic neurons, sometimes called the posterior hypothalamic nucleus in primates (Veazey et al., 1982; Bleier, 1984). Large, elongated neurons of the posterior hypothalamic nucleus span the posterior hypothalamic area rostrocaudally at the level of the medial mammillary nucleus (Figs. 4F, 5C,D; Braak and Braak, 1992). Numerous PDYN neurons in this group are heavily labeled (Fig. 6D). Second, posterior hypothalamic PDYN neurons are widely dispersed throughout the region and intermingle laterally with unlabeled neurons of the zona incerta and fields of Forel. Labeled neurons in this diffuse collection of cells are morphologically heterogeneous, reflecting, in general, the complex cytology of the dorsal posterior hypothalamic area (Veazey et al., 1982; Bleier, 1984).
PDYN in the dorsomedial nucleus.
A large subpopulation of PDYN neurons occupies a relatively cell-sparse region within the conventional boundaries of the dorsomedial nucleus. Although the columns of the fornix serve as landmarks that divide the human hypothalamus into medial and lateral zones, PDYN neurons of the more medial dorsomedial nucleus extend into the lateral hypothalamic area at the rostral perifornical area (Figs. 4C–E, 5A–C). Predominantly medium-sized, round and pyramidal PDYN neurons of the dorsomedial nucleus (Fig. 6H) intermingle with the more oblong and pear-shaped PDYN neurons of the lateral hypothalamic area near the descending columns of the fornix.
PDYN in the ventromedial nucleus.
The human ventromedial nucleus occupies a relatively large area in the tuberal region (Braak and Braak, 1992). PDYN neurons are dispersed sporadically throughout the ventromedial nucleus, and most are lightly labeled (Fig. 6G).
PDYN in the tuberomammillary nucleus.
The tuberomammillary nucleus consists of three subdivisions that extend from the basolateral surface of the hypothalamus in three directions, ventromedial, dorsal, and dorsolateral. The tuberomammillary nucleus contains only a few large, lightly labeled PDYN neurons, mostly in the dorsolateral subdivision (Figs. 4C–E, 6C).
PDYN in the retrochiasmatic area.
In the rostral hypothalamus, PDYN neurons can be found in the retrochiasmatic area, where oblong neurons with moderately labeled cytoplasm occupy the territory caudoventral to the optic chiasm (Figs. 4B, 6E).
PENK
Neurons that express pre-PENK are widely distributed in the human hypothalamus and basal forebrain (Table 2). We observed PENK neurons in virtually all hypothalamic nuclei except the mammillary complex. The packing density of PENK neurons is greatest in anterior hypothalamic structures, especially in the chiasmatic region. Numerous heavily labeled PENK neurons are located in the medial preoptic area, intermediate nucleus, dorsal suprachiasmatic nucleus, and rostral part of the lateral hypothalamus (Figs. 7A–D, 8B–D). Other subsets of PENK neurons were dispersed throughout the retrochiasmatic area, the dorsolateral supraoptic nucleus, and the area called the hypothalamic gray by Braak and Braak (1992; Figs. 7, 8). In the tuberal region, PENK neurons are present in the infundibular nucleus, ventromedial nucleus, dorsomedial nucleus, paraventricular nucleus, tuberomammillary nucleus, lateral tuberal nucleus, and lateral hypothalamic area. The posterior hypothalamic area dorsal to the mammillary complex contains a sparse population of PENK neurons (Fig. 8C,D).
Figure 7.
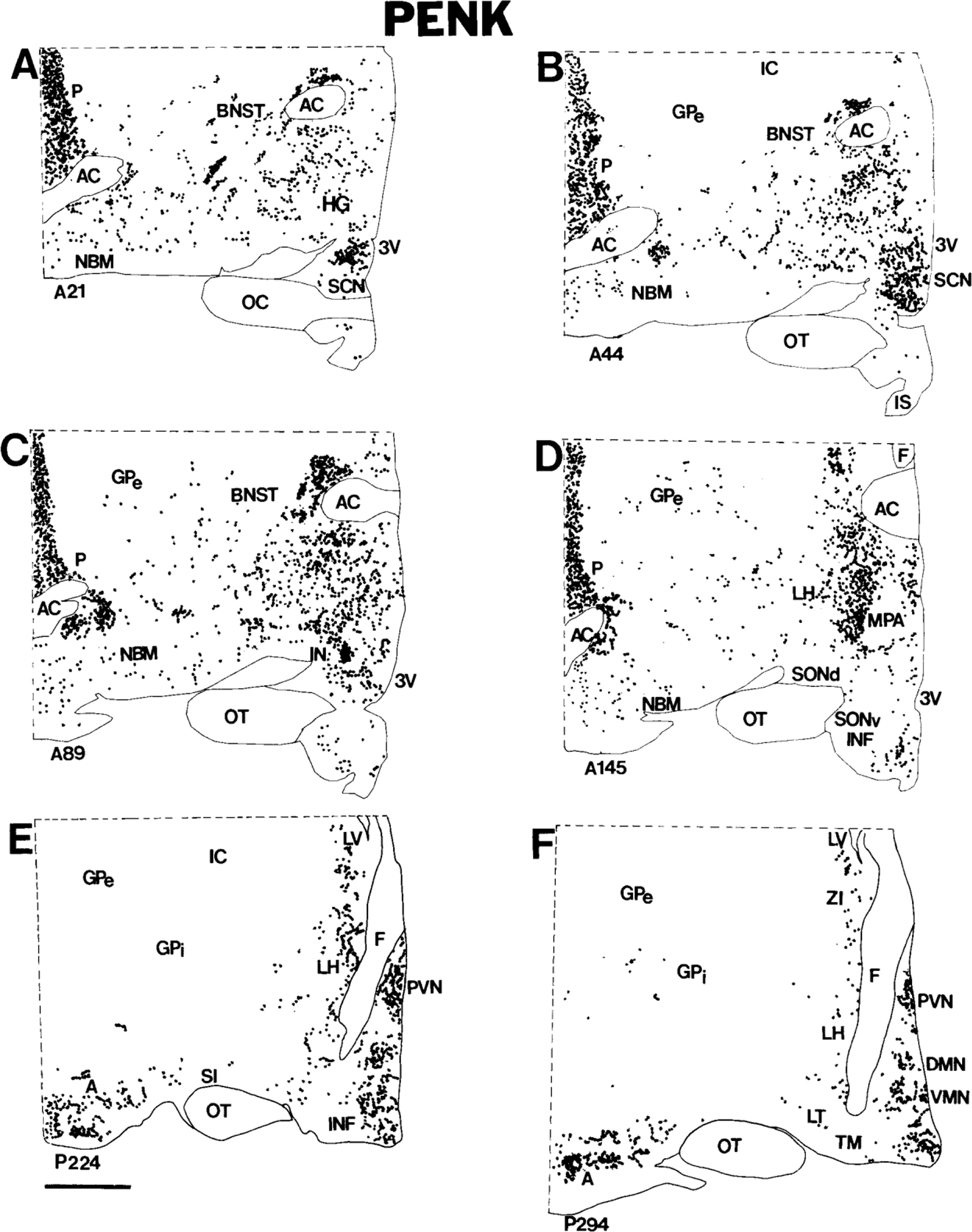
A-F: Computer-assisted maps of the distribution of PENK cells in coronal sections of the human hypothalamus. The most anterior section is A, and the most posterior section is F. Each dot represents a single neuron. Numbers at the lower left correspond to sequential locations of sections from anterior to posterior. Each section is 20 microns thick. Scale bar = 5 mm.
Figure 8.
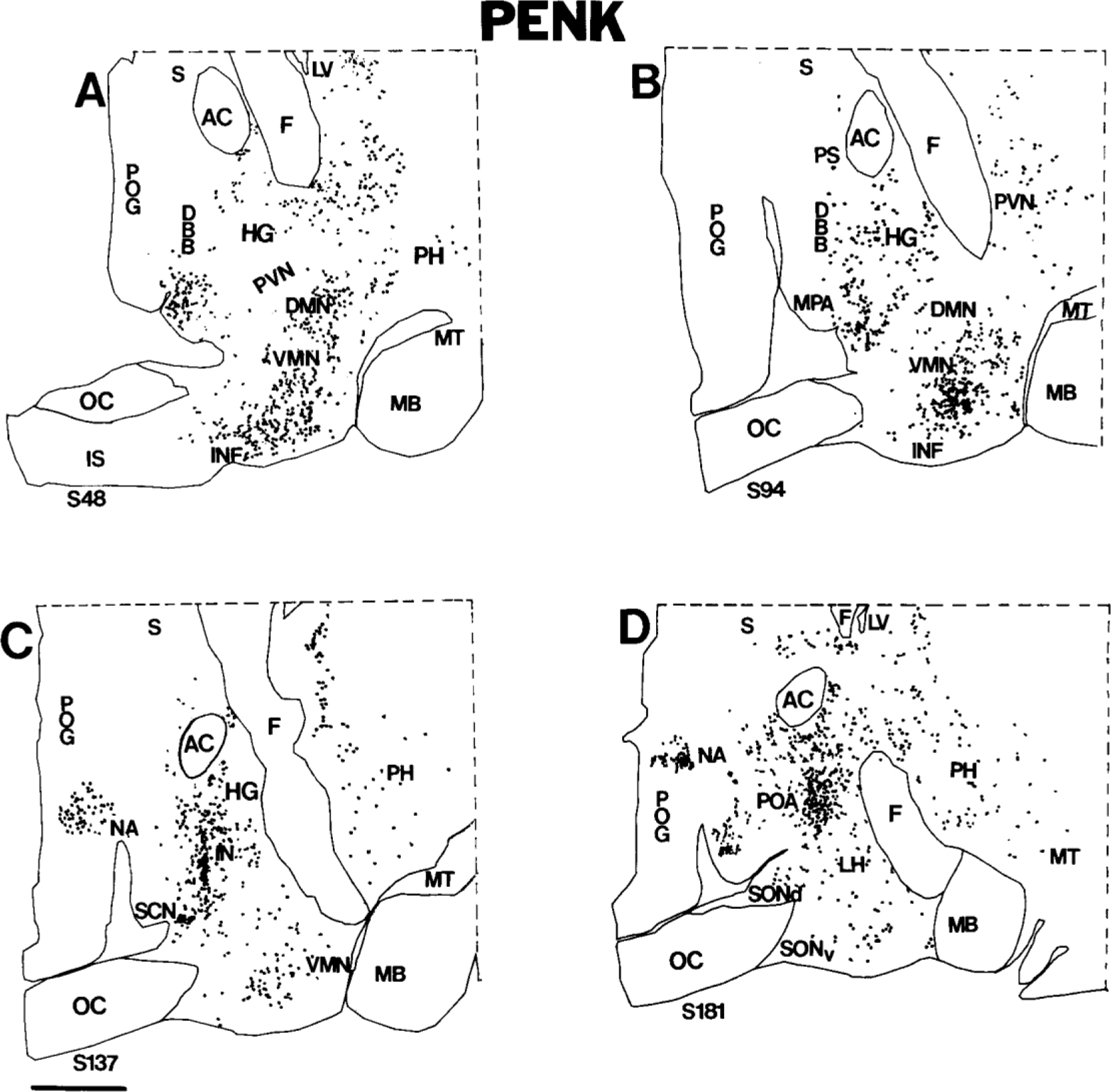
A-D: Computer-assisted maps of the distribution of PENK cells in 20-micron-thick sagittal sections of the human hypothalamus. The most medial section is A, and the most lateral section is D. Each dot represents a single neuron. Scale bar = 5 mm.
PENK in the chiasmatic region.
The medial preoptic area contains many PENK neurons (Figs. 7A–D, 8A–D). Labeling in the intermediate nucleus is particularly prominent; almost all cells in this nucleus, which lies midway between the rostroventral tip of the paraventricular nucleus and the dorsomedial supraoptic nucleus, express pre-PENK (Figs. 7C, 8C, 9). Neurons of the intermediate nucleus are medium-sized and slightly elongated (Fig. 10D). Another nucleus in the chiasmatic area containing densely packed PENK neurons is the suprachiasmatic nucleus (Figs. 7A,B, 8B,C). Recently, five major subdivisions of the suprachiasmatic nucleus were described in humans: dorsal, central, ventral, medial, and external (Mai et al., 1991). Numerous small, round or oval neurons in the dorsal subdivision of the suprachiasmatic nucleus are lightly labeled (Fig. 10C). In contrast, only sparse cells are labeled in other parts of the suprachiasmatic nucleus (data not shown). We detected dispersed PENK neurons in the rostroventral portion of the paraventricular nucleus just medial and dorsal to the suprachiasmatic nucleus (Fig. 7A,B): Most paraventricular PENK neurons are parvicellular and resemble the more superiorly located PENK cells of the periventricular region. Only a few small, lightly labeled neurons expressing pre- PENK were detected in the periphery of the dorsolateral and ventromedial parts of the supraoptic nucleus (Fig. 10B); the core of the supraoptic nucleus is devoid of PENK cells. In the rest of the chiasmatic region, PENK neurons are dispersed throughout the anterior hypothalamus in the hypothalamic gray.
Figure 9.
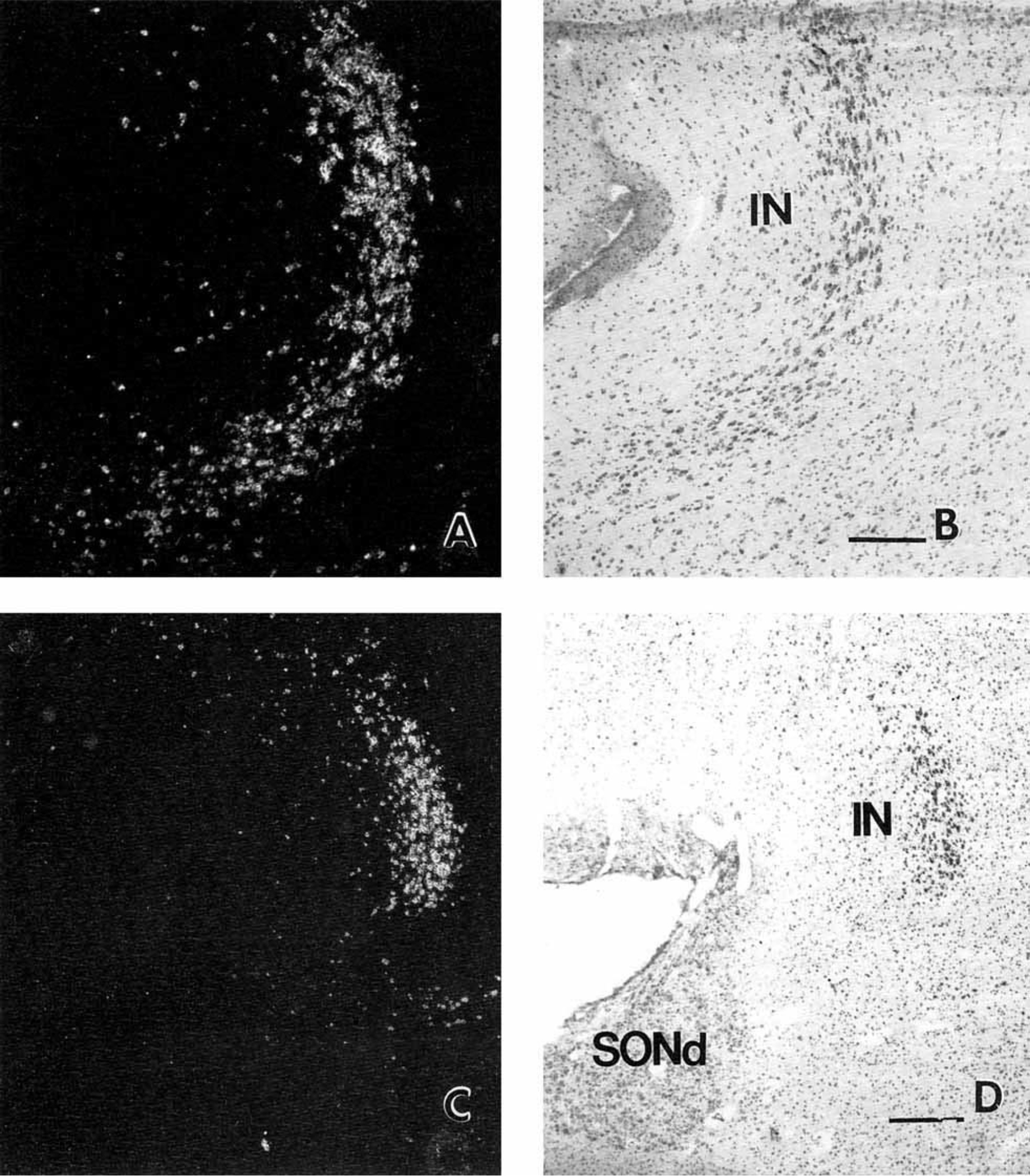
Pre-PENK gene expression in neurons of the intermediate nucleus of the human hypothalamus. Darkfield (A) and brightfield (B) photomicrographs of the intermediate nucleus in a sagittal section; rostral is to the left, and ventral is toward the bottom. Lower magnification darkfield (C) and brightfield (D) photomicrographs of the human intermediate nucleus in a coronal section. Scale bars = 740 microns in A,B, and 1,480 microns in C,D.
Figure 10.
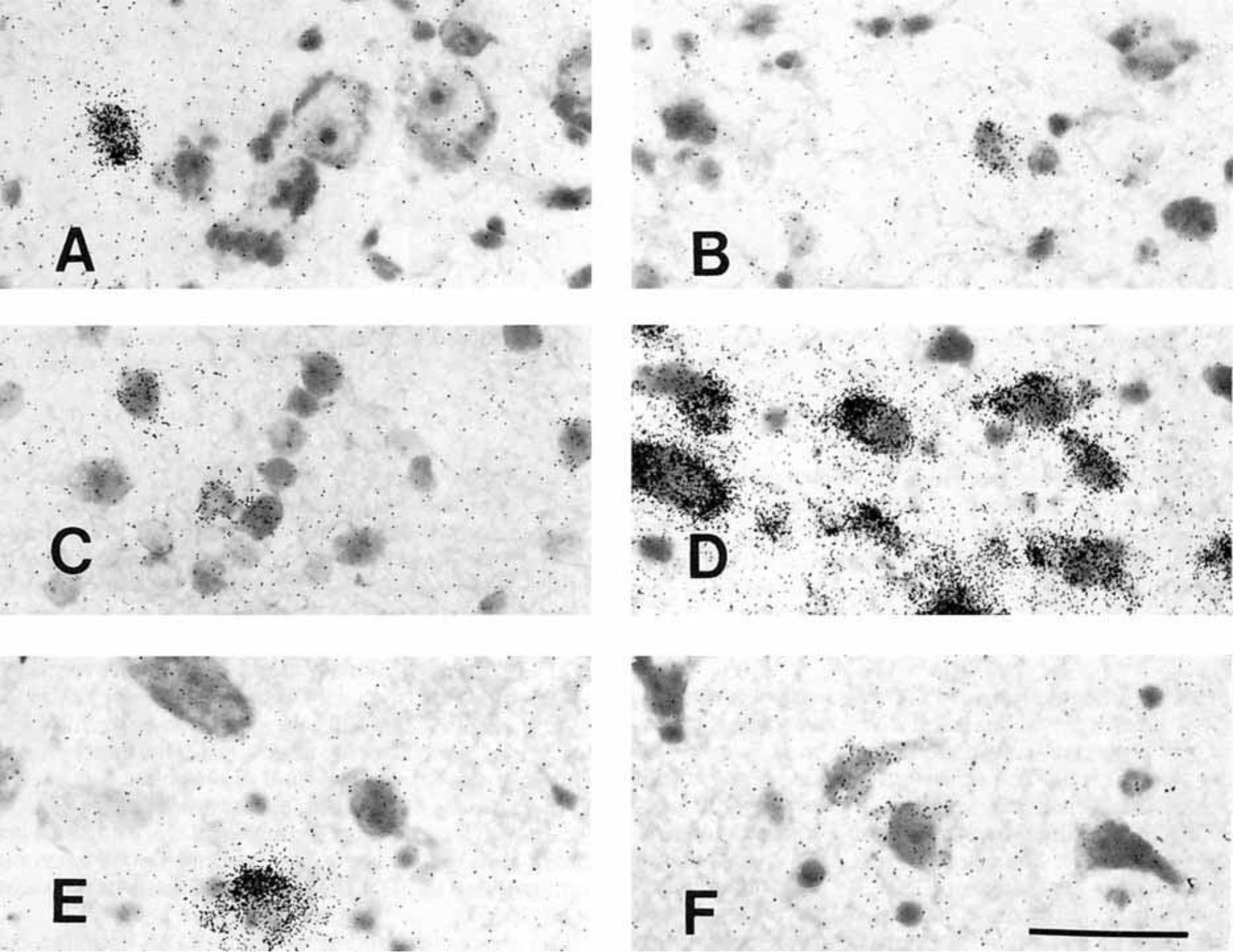
Photomicrographs of PENK cells in the human hypothalamus. A: Typical parvicellular PENK neuron in the caudal paraventricular nucleus. B: Small, lightly labeled neuron of the dorsolateral supraoptic nucleus. C: PENK neurons of the dorsal suprachiasmatic nucleus; cells are typically small and lightly labeled. D: Robustly labeled, medium-sized, elongated neurons of the intermediate nucleus. E: PENK neurons of the tuberomammillary nucleus. F: Lightly labeled neurons of the lateral tuberal nucleus. Scale bar = 50 microns.
In the mediobasal anterior hypothalamus, neurons of the retrochiasmatic area express pre-PENK, and the pattern of labeling is similar to that described above for POMC and PDYN cells. More caudally, these cells intermingle with round, medium-sized, and small PENK cells of the infundibular nucleus, which are less abundant than POMC neurons but show similar robust labeling.
PENK in the tuberal and mammillary regions.
As was mentioned above, PENK neurons are present in all nuclei of the tuberal and mammillary regions except for the mammillary complex itself. The greatest packing density of PENK neurons is in the ventromedial nucleus and paraventricular nucleus (Figs. 7E,F, 8B,C). Significant numbers of labeled neurons also are present in the infundibular nucleus, lateral hypothalamic area, and dorsomedial nucleus (Figs. 7, 8). A few labeled neurons are also present in the tuberomammillary nucleus, premammillary nucleus, and posterior hypothalamic area (Fig. 7, 8). Most PENK neurons of the tuberomammillary nucleus are found in the dorsolateral subdivision (Fig. 10E). Most neurons of the lateral tuberal nucleus and posterior hypothalamic nucleus are devoid of pre-PENK; only a few neurons of the lateral tuberal nucleus express pre-PENK (very weakly; Fig. 10F).
Extrahypothalamic opioid precursor gene expression
Our tissue samples also included all or part of the amygdala, caudate nucleus, putamen, parolfactory gyrus, globus pallidus, nucleus basalis of Meynert, bed nucleus of the stria terminalis, septum, and infundibular stalk. In several of these regions, we detected PDYN and PENK neurons; extrahypothalamic POMC cells were noted only in the infundibular stalk. Although a detailed description of extrahypothalamic opioids is beyond the scope of this study, we will briefly summarize some of our observations. The caudate nucleus and putamen contain robustly labeled cells expressing both pre-PDYN and pre-PENK. In the caudate nucleus, PDYN cells form islands, whereas PENK cells are distributed more uniformly. We detected numerous robustly labeled PENK neurons but relatively few PDYN neurons in the putamen. PDYN and PENK cells have similar distributions in the anteriormost part of the lateral hypothalamus, where peripheral islands of small neurons of the bed nucleus of the stria terminalis accompany the main core of the nucleus as it traverses the rostral lateral hypothalamic area toward the amygdala (Martin et al., 1991). We observed, at the level of the suprachiasmatic nucleus, only a few PDYN cells in the bed nucleus of the stria terminalis, whereas PENK neurons were more abundant (Figs. 4A,B, 7A,B). We observed, in parts of the amygdala, a differential distribution of PDYN and PENK neurons. The central nucleus of the amygdala contains PENK cells, whereas PDYN neurons are present in the cortical nucleus (Figs. 4C–F, 7E,F). We observed morphologically distinct types of PENK neurons in the nucleus basalis of Meynert (including the nucleus of the diagonal band of Broca; Fig. 11) but did not identify PDYN neurons in this part of the basal forebrain. Clusters of densely packed, very small PDYN and PENK neurons in the nucleus accumbens appeared to invade the territory of the septum and parolfactory gyrus (Figs. 5D; 8C,D).
Figure 11.
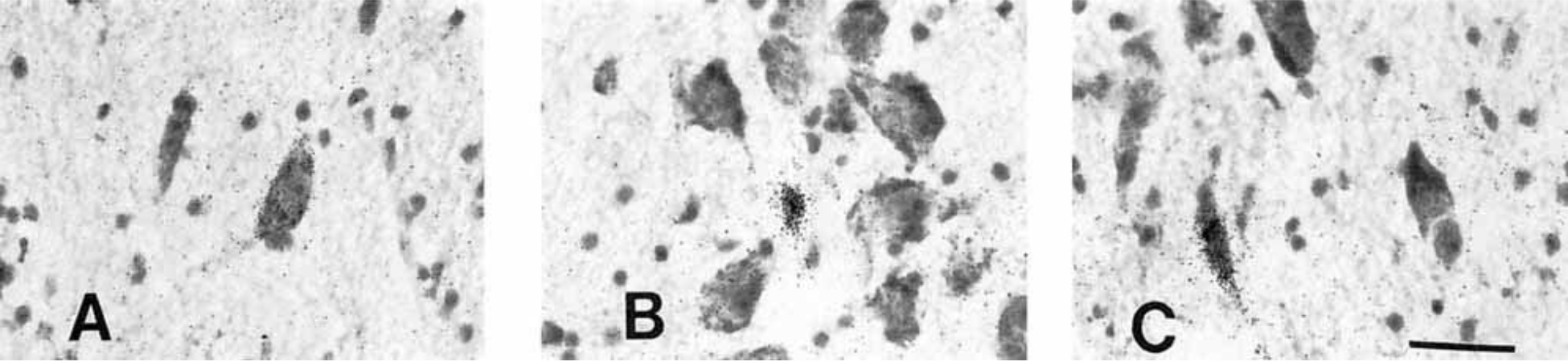
Examples of the three types of PENK neurons in the basal forebrain magnocellular complex. A: Magnocellular neuron of the nucleus of the diagonal band of Broca. B: Small neuron of the nucleus basalis of Meynert. C: Typical elongated neurons of the nucleus basalis of Meynert; oblong cells usually abut the anterior commissure. Scale bar = 40 microns.
DISCUSSION
Our knowledge of opioid systems in the brain is based mainly on immunocytochemical and hybridization histochemical studies in rodents and in nonhuman primates (for review, see Khachaturian et al., 1993). Only a few immunocytochemical reports have been devoted to human cerebral opioid systems (Bloch et al., 1978; Gramsch et al., 1979; Maysinger et al., 1982; Haber and Watson, 1985; Abe et al., 1988; Haber et al., 1990; Martin et al., 1991). The present study provides the first detailed description of opioid peptide precursor gene expression in the human hypothalamus and basal forebrain.
The differential distribution of separate classes of opioid peptide-containing neurons has been described previously in rodents and in nonhuman primates (Khachaturian et al., 1985b; Haber et al., 1990). We observed a distinct anatomical segregation of the three different opioid peptide precursor mRNA in the human hypothalamus and adjacent structures. Specifically, pre-POMC gene expression is largely restricted to neurons of the mediobasal hypothalamus. PDYN and PENK neurons are more widely represented in the human basal forebrain, although they differ in distribution. PDYN neurons are located primarily in the tuberal and dorsal posterior hypothalamus, whereas PENK neurons predominate in the chiasmatic region. The expression of PDYN and PENK genes in several different extrahypothalamic cell populations contrasts sharply with the absence, except in the infundibular stalk, of POMC neurons outside the hypothalamus.
POMC:
Our study shows that the distribution of POMC neurons in humans is similar to that observed in rodents and in nonhuman primates (Khachaturian et al., 1984; Lewis et al., 1984; Haber et al., 1990). In particular, the human retrochiasmatic area and infundibular nucleus contain significant populations of POMC cells. In rodents and monkeys, these POMC-enriched areas have extensive projections throughout the brain and innervate several diencephalic, limbic, mesencephalic, and lower brainstem structures (Khachaturian et al., 1985a; Haber et al., 1990). Because POMC efferents are comparable in rodents and monkeys, it is conceivable that similar connections are present in humans.
Numerous lines of evidence indicate that opioid peptides have an inhibitory influence in the regulation of gonadotropin secretion (Ferin et al., 1984; Howlett and Rees, 1986; Kalra and Kalra, 1986; Gindoff and Ferin, 1987). This role is best documented for the POMC system. POMC neurons are located within the medial basal hypothalamus, the primary control center for reproduction (Krey et al., 1975; Knobil, 1980) and the location of the gonadotropin-releasing hormone pulse generator (Wilson et al., 1984). POMC efferents terminate on capillaries in the median eminence, and β-endorphin is secreted into the hypophyseal portal circulation of rats (Wehrenberg et al., 1982) and primates (Wardlaw et al., 1980; Gindoff and Ferin, 1987). In addition, levels of β-endorphin in portal blood are modified by ovarian steroids and vary with the menstrual cycle (Wardlaw et al., 1982). Numerous studies have shown that POMC gene expression in the medial basal hypothalamus is modified by gonadal steroids (Wilcox and Roberts, 1985; Chowen-Breed et al., 1989; Tong et al., 1990; Wise et al., 1990; Adams et al., 1991; Rasmussen et al., 1992; Treiser and Wardlaw, 1992; Pelletier, 1993). Only a small population of β-endorphin-immunoreactive neurons in the medial basal hypothalamus of rats and mice concentrates estrogen (Morrell et al., 1985; Jirikowski et al., 1986). However, there are increased numbers of arcuate β-endorphin-immunoreactive neurons after estrogen treatment in the guinea pig (Thornton et al., 1994), and estrogen receptors are colocalized in 15–20% of β-endorphin cells in the medial basal hypothalamus of sheep (Lehman and Karsch, 1993).
PDYN:
Information on the distribution of PDYN is limited to immunocytochemical studies in rodents, rhesus monkeys (Khachaturian et al., 1982, 1985a), and humans (Abe et al., 1988). We confirmed a previous description of PDYN immunoreactivity in magnocellular neurons of the human paraventricular nucleus (Abe et al., 1988), especially its caudal part. Whether these neurons coexpress PDYN and vasopressin (Watson et al., 1981) remains to be assessed in double-labeling studies. We found in the SON only a few PDYN neurons, in contrast to immunocytochemical data that indicate many dynorphin-like immunoreactive neurons in this area (Abe et al., 1988). In agreement with the demonstration of dynorphin-like immunoreactive perikarya in the perifornical area of rats (Khachaturian et al., 1985a; Fallon and Leslie, 1986) and humans (Abe et al., 1988), we observed relatively large numbers of PDYN neurons in the dorsomedial nucleus and lateral hypothalamic area surrounding the fornix from the medial and lateral sides, respectively. In rats, the perifornical area is implicated in the control of cardiovascular function (Allen and Cechetto, 1992, 1993; Oppenheimer et al., 1992) and eating (Stanley et al., 1993a,b).
The most striking finding in the human hypothalamic PDYN system is the presence of densely packed PDYN neurons in the premammillary nucleus. Knowledge of premammillary nuclear function and connectivity is extremely limited. Unlike the case in rodents, where the premammillary nucleus has been subdivided into dorsal and ventral components (Krieg, 1932; Saper et al., 1979), in nonhuman primates (Veazey et al., 1982) and humans this nucleus is relatively meager and is composed of small and medium-sized neurons.
Recently, anterograde and retrograde tracing studies have described connections of the dorsal and ventral parts of the premammillary nucleus in rodents (Canteras and Swanson, 1992; Canteras et al., 1992). Current evidence suggests that the premammillary nucleus may play an important role in goal-oriented behavior associated with hunger, thirst, and reproduction (Canteras and Swanson, 1992). The connectivity of the ventral premammillary nucleus implicates this structure in neuroendocrine and sexually dimorphic circuitry (Canteras et al., 1992). Our hybridization histochemical preparations show that the majority of neurons in the human premammillary nucleus express the pre-PDYN gene, readily distinguishing this nucleus from adjacent hypothalamic nuclei. Thus, PDYN may be a key peptide in the function of the premammillary nucleus.
Another significant group of PDYN neurons occurs in the dorsal posterior hypothalamic area. The neuronal composition of the posterior hypothalamic area is heterogeneous (Veazey et al., 1982). The posterior hypothalamic nucleus consists of large neurons, some of which stray into the posterior hypothalamic area in ventrodorsal and mediolateral directions. The majority of posterior hypothalamic nucleus neurons express pre-PDYN. The remainder of the posterior hypothalamic area consists of small and medium-sized neurons that make up the posterior hypothalamic gray; some of these neurons contain pre-PDYN. There is no information on PDYN in posterior hypothalamic structures of rodents or monkeys.
PENK:
Of the three classes of opioid cells, PENK neurons are the most abundant in the human hypothalamus. All structures except the mammillary bodies contain PENK neurons. The massive numbers of PENK neurons throughout hypothalamic structures suggest that PENK neurons participate in numerous homeostatic functions. However, the chiasmatic region has the greatest number of neurons, especially in the intermediate nucleus, but also in the suprachiasmatic nucleus and scattered throughout the medial and lateral preoptic regions. There was also robust labeling of neurons by the pre-PENK probe in the bed nucleus of the stria terminalis and in the central nucleus of the amygdala. Several of these structures have been reported to be sexually dimorphic in humans (Swaab and Fliers, 1985; Allen et al., 1989; Hofman and Swaab, 1989; Allen and Gorski, 1990; LeVay, 1991) and rats (Gorski, 1968; Simerly et al., 1984, 1988; Turkenburg et al., 1988; De Jonge et al., 1989; Herbison and Dye, 1993).
The present study provides the first identification of PENK mRNA in the intermediate nucleus of the hypothalamus. Nearly all neurons in the intermediate nucleus were labeled with the pre-PENK probe. The intermediate nucleus is a well-defined collection of darkly staining neurons that lies within the preoptic area between the supraoptic nucleus and paraventricular nuclei and rostral to the accessory magnocellular nuclei (Brockhaus, 1942; Braak and Braak, 1987; Saper, 1990). In 1985, Swaab and Fliers reported that the volume of the intermediate nucleus is more than twice as large in men as in women. These authors referred to the intermediate nucleus as the “sexually dimorphic nucleus of the preoptic (SDN-POA),” implying that this nucleus is homologous to the SDN-POA described in rat brain (Gorski et al., 1978, 1980; Simerly et al., 1984); however, this issue has become controversial (Allen et al., 1989; LeVay, 1991).
Additional studies will be necessary to determine whether and where PENK neurons are sexually dimorphic in the human hypothalamus. Sexual dimorphism has been found in the number of enkephalin-immunoreactive cells within an anteroventral periventricular nucleus of the rat (Simerly et al., 1988). However, enkephalin-immunoreactive neurons were not identified in SDN-POA of the rat preoptic area (Simerly et al., 1988).
For the hypothalamic magnocellular complex, we observed PENK cells mainly in the parvicellular caudal paraventricular nucleus and in the periphery of dorsolateral and ventromedial portions of the supraoptic nucleus. A hybridization histochemical study in rats showed labeling in both parvicellular and magnocellular neurons (Harlan et al., 1987). Immunocytochemical studies have been inconsistent, showing labeling in parvicellular neurons only (Wamsley et al., 1980; Hokfelt et al., 1983; Khachaturian et al., 1983), in both parvicellular and magnocellular neurons (Sar et al., 1978; Sawchenko et al., 1982) of rodents or, in bovine brain, in magnocellular neurons only (Vanderhaeghen et al., 1983). The location of PENK neurons in the periphery of the supraoptic nucleus and the small size of PENK neurons in both the paraventricular nucleus and the supraoptic nucleus indicate that PENK could colocalize with oxytocin in some neurons (Meister et al., 1990; Sukhov et al., 1993). Recently, it has been suggested that the expression of enkephalin in both vasopressin and oxytocin neurons in rats may increase in response to chronic stress, thus supplementing parvicellular neurons as a source of enkephalin (Young and Lightman, 1992). It remains to be determined whether similar processes occur in nonhuman primates and humans.
The presence of a significant population of PENK neurons in the ventromedial nucleus corresponds well with results obtained in rats (Shivers et al., 1986; Harlan et al., 1987). One suggested function of the ventromedial nucleus in rats is the regulation of steroid-dependent, motivated behavior through progesterone, estrogen, and androgen receptors (Pfaff, 1980; Simerly et al., 1990). In addition, the ventromedial nucleus exhibits sexually dimorphic features (Matsumoto and Arai, 1983), and pre-PENK gene expression is regulated by estrogens (Romano et al., 1990). Because we found the greatest expression of pre-PENK in structures such as the medial preoptic area, ventromedial nucleus, amygdala, and bed nucleus of the stria terminalis, which are thought to mediate the hormonal control of copulatory behavior, it may be informative to relate the expression of androgen and estrogen receptors to that of pre-PENK in these areas.
ACKNOWLEDGMENTS
The authors thank Drs. Stephen D. Ginsberg, Brenda D. Shivers, and Miklos Palkovits for helpful discussions as well as Ms. Emily Shepard for expert technical assistance. Part of this paper was presented at the 23rd annual meeting of the Society for Neuroscience in Washington, DC, November, 1993. This work was supported by grants from the U.S. Public Health Service (NS AG 05146, NS 20471, NS 07179, AG 09214) as well as the Metropolitan Life Foundation. D.L.P. is the recipient of a Leadership and Excellence in Alzheimer’s Disease (LEAD) award (AG 07914) and a Javits Neuroscience Investigator Award (NS 10580).
Abbreviations
- 3v
third ventricle
- A
amygdala, central nucleus
- AC
anterior commissure
- Ac
amygdala, cortical nucleus
- BNST
bed nucleus of the stria terminalis
- DBB
diagonal band of Broca
- DMN
dorsomedial nucleus
- F
fornix
- FF
fields of Fore1
- GPe
globus pallidus pars externa
- GPi
globus pallidus pars interna
- HG
hypothalamic gray
- IC
internal capsule
- IN
intermediate nucleus
- INAH-1
intermediate nucleus of anterior hypothalamus-1
- INF
infundibular nucleus
- IS
infundibular stalk
- LH
lateral hypothalamus
- LT
lateral tuberal nucleus
- LV
lateral ventricle
- MB
mammillary body
- MPA
medial preoptic area
- MT
mammillothalamic tract
- NA
nucleus accumbens
- NBM oc
nucleus basalis of Meynert
- OT
optic tract
- P
putamen
- PDYN
prodynorphin
- PENK
proenkephalin
- PH
posterior hypothalamus
- PN
premammillary nucleus
- POA
preoptic area
- POG
parolfactory gyrus
- POMC
proopiomelanocortin
- por
preoptic recess
- pre-PDYN
preprodynorphin
- pre-PENK
preproenkephalin
- pre-POMC
preproopiomelanocortin
- PS
precommissural septum
- PVN
paraventricular nucleus
- RA
retrochiasmatic area
- S
septum
- SCN
suprachiasmatic nucleus
- SDN-POA
sexually dimorphic nucleus of preoptic area
- SI
substantia innominata
- SONd
supraoptic nucleus, dorsolateral
- SONv
supraoptic nucleus, ventromedial
- TM
tuberomammillary nucleus
- VMN
ventromedial nucleus
- VP
ventral pallidum
- ZI
zona incerta
Contributor Information
RENAT R. SUKHOV, Department of Neuropathology Laboratory, The Johns Hopkins University, School of Medicine, Baltimore, Maryland 21205-2196
LARY C. WALKER, Department of Pathology, The Johns Hopkins University, School of Medicine, Baltimore, Maryland 21205-2196 Department of Neuropathology Laboratory, The Johns Hopkins University, School of Medicine, Baltimore, Maryland 21205-2196; Department of Psychology, The Johns Hopkins University, Baltimore, Maryland 21218.
NAOMI E. RANCE, Departments of Pathology, Neurology, and Anatomy, The University of Arizona College of Medicine, Tucson, Arizona, 85724
DONALD L. PRICE, Department of Pathology, The Johns Hopkins University, School of Medicine, Baltimore, Maryland 21205-2196 Department of Neurology, The Johns Hopkins University, School of Medicine, Baltimore, Maryland 21205-2196; Department of Neuroscience, The Johns Hopkins University, School of Medicine, Baltimore, Maryland 21205-2196; Department of Neuropathology Laboratory, The Johns Hopkins University, School of Medicine, Baltimore, Maryland 21205-2196.
W. SCOTT YOUNG, III, Laboratory of Cell Biology, National Institute of Mental Health, Bethesda, Maryland 20892.
LITERATURE CITED
- Abe J, Okamura H, Kitamura T, Ibata Y, Minamino N, Matsuo H, and Paull WK (1988) Immunocytochemical demonstration of dynorphin (PH-8P)-like immunoreactive elements in the human hypothalamus. J. Comp. Neurol. 276:508–513. [DOI] [PubMed] [Google Scholar]
- Adams LA, Vician L, Clifton DK, and Steiner RA (1991) Testosterone regulates pro- opiomelanocortin gene expression in the primate brain. Endocrinology 128:1881–1886. [DOI] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, and Walker JM (1984) Endogenous opioids: Biology and function. Annu. Rev. Neurosci. 7:223–255. [DOI] [PubMed] [Google Scholar]
- Allen GV, and Cechetto DF (1992) Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area: I. Descending projections. J. Comp. Neurol. 315:313–332. [DOI] [PubMed] [Google Scholar]
- Allen GV, and Cechetto DF (1993) Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area. II. Ascending projections. J. Comp. Neurol. 330:421–438. [DOI] [PubMed] [Google Scholar]
- Allen LS, and Gorski RA (1990) Sex difference in the bed nucleus of the stria terminalis of the human brain. J. Comp. Neurol. 302:697–706. [DOI] [PubMed] [Google Scholar]
- Allen LS, Hines M, Shryne JE, and Gorski RA (1989) Two sexually dimorphic cell groups in the human brain. J. Neurosci. 9:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S, Rondeau N, Day R, Chretien M, and Seidah NG (1991) PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc. Natl. Acad. Sci. USA 88:3564–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger PA, and Nemeroff CB (1987) Opioid peptides in affective disorders. In Meltzer HY (ed): Psychopharmacology: The Third Generation of Progress. New York Raven Press, pp. 637–646. [Google Scholar]
- Berger PA, Watson SJ, Akil H, and Barchas JD (1981) Clinical studies on the role of endorphins in schizophrenia. Mod. Probl. Pharmacopsychiatr. 17:226–235. [DOI] [PubMed] [Google Scholar]
- Bleier R (1984) The Hypothalamus of the Rhesus Monkey. A Cytoarchitectonic Atlas. Madison: University of Wisconsin Press. [Google Scholar]
- Bloch B, Bugnon C, Fellman D, and Lenys D (1978) Immunocytochemical evidence that the same neurons in the human infundibular nucleus are stained with anti-endorphins and antisera of other related peptides. Neurosci. Lett. 10:147–152. [DOI] [PubMed] [Google Scholar]
- Braak H, and Braak E (1987) The hypothalamus of the human adult: Chiasmatic region. Anat. Embryol. 176:315–330. [DOI] [PubMed] [Google Scholar]
- Braak H, and Braak E (1992) Anatomy of the human hypothalamus (chiasmatic and tuberal region). Progr. Brain Res. 93:3–16. [DOI] [PubMed] [Google Scholar]
- Brockhaus H (1942) Vergleichend-anatomische Untersuchungen uber den Basalkernkomplex. J. Psychol. Neurol. 51 :57–95. [Google Scholar]
- Brownstein MJ (1993) A brief history of opiates, opioid peptides, and opioid receptors. Proc. Natl. Acad. Sci. USA 90:5391–5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, and Swanson LW (1992) The dorsal premammillary nucleus: An unusual component of the mammillary body. Proc. Natl. Acad. Sci. USA 89:10089–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, and Swanson LW (1992) Projections of the ventral premammillary nucleus. J. Comp. Neurol. 324:195–212. [DOI] [PubMed] [Google Scholar]
- Cavagnini F, Invitti C, Dubini A, Danesi L, Silvestri G, Passamonti M, Magella A, and Morabito F (1987) Endogenous opioids and hypothalamic-pituitary-adrenal function in obesity and anorexia nervosa. In Nerozzi D, Goodwin FK, and Costa E (eds): Hypothalamic Dysfunction in Neuropsychiatric Disease. New York: Raven Press, pp. 273–281. [PubMed] [Google Scholar]
- Chamberlain RS, and Herman BH (1990) A novel biochemical model linking dysfunctions in brain melatonin, proopiomelanocortin peptides, and serotonin in autism. Biol. Psychiatr. 28:773–793. [DOI] [PubMed] [Google Scholar]
- Chowen-Breed J, Fraser HM, Vician L, Damassa DA, Clifton DK, and Steiner RA (1989) Testosterone regulation of proopiomelanocortin messenger ribonucleic acid in the arcuate nucleus of the male rat. Endocrinology 124:1697–1702. [DOI] [PubMed] [Google Scholar]
- Civelli O, Douglass J, Goldstein A, and Herbert E (1985) Sequence and expression of the rat prodynorphin gene. Proc. Natl. Acad. Sci. USA 82:4291–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comb M, Seeburg PH, Adelman J, Eiden L, and Herbert E (1982) Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature 295:663–666. [DOI] [PubMed] [Google Scholar]
- Costa E, Di Giulio AM, Kumakura K, and Yang H-YT (1979) Differences in the enkephalin-like immunoreactivity present in gland cells and nerve terminals of adrenal medulla (abstract). Fed. Proc. 38:1129. [Google Scholar]
- Day R, and Akil H (1989) The posttranslational processing of prodynorphin in the rat anterior pituitary. Endocrinology 124:2392–2405. [DOI] [PubMed] [Google Scholar]
- Day R, Trujillo KA, and Akil H (1993) Prodynorphin biosynthesis and posttranslational processing. In Akil H and Simon EJ (edsj: Handbook of Experimental Pharmacology. Berlin: Springer-Verlag, pp. 449–470. [Google Scholar]
- De Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, and Van De Poll NE (1989) Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res. Bull. 23:483–492. [DOI] [PubMed] [Google Scholar]
- Dondi D, Maggi R, Panerai AE, Piva F, and Limonta P (1991) Hypothalamic opiatergic tone during pregnancy, parturition and lactation in the rat. Neuroendocrinology 53:460–466. [DOI] [PubMed] [Google Scholar]
- Dores RM, McDonald LK, Steveson TC, and Sei CA (1990) The molecular evolution of neuropeptides: Prospects for the ‘90s. Brain Behav. Evol. 36:80–99. [DOI] [PubMed] [Google Scholar]
- Fallon JH, and Leslie FM (1986) Distribution of dynorphin and enkephalin peptides in the rat brain. J. Comp. Neurol. 249:293–336. [DOI] [PubMed] [Google Scholar]
- Ferin M, and Vande Wiele R (1984) Endogenous opioid peptides and the control of the menstrual cycle. Eur. J. Obstet. Gynecol. Reprod. Biol. 18:365–373. [DOI] [PubMed] [Google Scholar]
- Ferin M, Van Vugt D, and Wardlaw S (1984) The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Rec. Progr. Horm. Res. 40:441–485. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Cecchetto R, Panerai AE, Truci G, Smirne S, and Canal N (1986) Plasma β-endorphin and β-lipotropin in patients with Parkinson’s disease. Clin. Neuropharmacol. 9:549–555. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Perego L, Ferini-Strambi L, Smirne S, and Canal N (1988) Neuroendocrinological function in Alzheimer’s disease. Neuroendocrinology 48:367–370. [DOI] [PubMed] [Google Scholar]
- Gai WP, Geffen LB, and Blessing WW (1990) Galanin immunoreactive neurons in the human hypothalamus: Colocalization with vasopressin containing neurons. J. Comp. Neurol. 298:265–280. [DOI] [PubMed] [Google Scholar]
- Gindoff PR, and Ferin M (1987) Brain opioid peptides and menstrual cyclicity. Semin. Reprod. Endocrinol. 5:125–133. [Google Scholar]
- Gorski RA (1968) The neural control of ovulation. In Assali NS (ed): Biology of Gestation. New York: Academic Press, pp. 1–66. [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, and Southam AM (1978) Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 148:333–346. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne J, and Southam AM (1980) Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J. Comp. Neurol. 193:529–539. [DOI] [PubMed] [Google Scholar]
- Gramsch C, Höllt V, Mehraein P, Pasi A, and Herz A (1979) Regional distribution of methionine-enkephalin- and beta-endorphin-like immunoreactivity in human brain and pituitary. Brain Res. 171:261–270. [DOI] [PubMed] [Google Scholar]
- Gramsch C, Höllt V, Pasi A, Mehraein P, and Herz A (1982) Immunoreactive dynorphin in human brain and pituitary. Brain Res. 233:65–74. [DOI] [PubMed] [Google Scholar]
- Gubler U, Seeburg P, Hoffman BJ, Gage LP, and Udenfriend S (1982) Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature 295:206–208. [DOI] [PubMed] [Google Scholar]
- Haber SN, and Watson SJ (1985) The comparative distribution of enkephalin, dynorphin and substance P in the human globus pallidus and basal forebrain. Neuroscience 14:1011–1024. [DOI] [PubMed] [Google Scholar]
- Haber SN, Khachaturian H, and Watson SJ (1990) Endogenous opiate systems in the primate brain. In Riederer P, Kopp N, and Pearson J (eds): An Introduction to Neurotransmission in Health and Disease. Oxford: Oxford University Press, pp. 49–65. [Google Scholar]
- Harlan RE, Shivers BD, Romano GJ, Howells RD, and Pfaff DW (1987) Localization of preproenkephalin mRNA in the rat brain and spinal cord by in situ hybridization. J. Comp. Neurol. 258:159–184. [DOI] [PubMed] [Google Scholar]
- Herbison AE, and Dye S (1993) Perinatal and adult factors responsible for the sexually dimorphic calcitonin gene-related peptide-containing cell population in the rat preoptic area. Neuroscience 54:991–999. [DOI] [PubMed] [Google Scholar]
- Hofman MA, and Swaab DF (1989) The sexually dimorphic nucleus of the preoptic area in the human brain: A comparative morphometric study. J. Anat. 164:55–72. [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Skagerberg G, Skirboll L, and Bjorklund A (1983) Combination of retrograde tracing and neurotransmitter histochemistry. In Bjorklund A and Hokfelt T ieds): Methods in Chemical Neuroanatomy. Amsterdam: Elsevier Science Publishers, pp. 228–285. [Google Scholar]
- Höllt V (1993) Regulation of opioid peptide gene expression. In Akil H and Simon EJ (eds): Handbook of Experimental Pharmacology. Berlin: Springer-Verlag, pp. 307–336. [Google Scholar]
- Horikawa S, Takai T, Toyosato M, Takahashi H, Noda M, Kakidani H, Kubo T, Hirose T, Inayama S, Hayashida H, Miyata T, and Numa S (1983) Isolation and structural organization of the human preproenkephalin B gene. Nature 306:611–614. [DOI] [PubMed] [Google Scholar]
- Howlett TA, and Rees LH (1986) Endogenous opioid peptides and hypothalamo-pituitary function. Annu. Rev. Physiol. 48:527–536. [DOI] [PubMed] [Google Scholar]
- Jirikowski GF, Merchenthaler I, Rieger GE, and Stumpf WE (1986) Estradiol target sites immunoreactive for β-endorphin in the arcuate nucleus of rat and mouse hypothalamus. Neurosci. Lett. 65:121–126. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Morgan DG, and Finch CE (1986) Extensive postmortem stability of RNA from rat and human brain. J. Neurosci. Res. 16:267–280. [DOI] [PubMed] [Google Scholar]
- Kakidani H, Furutani Y, Takahashi H, Noda M, Morimoto Y, Hirose T, Asai M, Inayama S, Nakanishi S, and Numa S (1982) Cloning and sequence analysis of cDNA for porcine β-neo- endorphin/dynorphin precursor. Nature 298:245–249. [DOI] [PubMed] [Google Scholar]
- Kalra PS, and Kalra SP (1986) Steroidal modulation of the regulatory neuropeptides: Luteinizing hormone releasing hormone, neuropeptide Y and endogenous opioid peptides. J. Steroid Biochem. 25:733–740. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Watson SJ, Lewis ME, Coy D, Goldstein A, and Akil H (1982) Dynorphin immunocytochemistry in the rat central nervous system. Peptides 3:941–954. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, and Watson SJ (1983) Enkephalin systems in diencephalon and brainstem of the rat. J. Comp. Neurol. 220:310–320. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Haber SN, Akil H, and Watson SJ (1984) Proopiomelanocortin peptide immunocytochemistry in rhesus monkey brain. Brain Res. Bull. 13:785–800. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Haber SN, Houghten RA, Akil H, and Watson SJ (1985a) Prodynorphin peptide immunocytochemistry in rhesus monkey brain. Peptides (Suppl. 2) 6:155–166. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Schafer MK-H, and Watson SJ (1985b) Anatomy of the CNS opioid systems. Trends Neurosci. 8:111–119. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Schaefer MKH, and Lewis ME (1993) Anatomy and function of the endogenous opioid systems. In Akil H and Simon EJ (eds): Handbook of Experimental Pharmacology. Berlin: Springer-Verlag, pp. 471–497. [Google Scholar]
- Knobil E (1980) The neuroendocrine control of the menstrual cycle. Rec. Progr. Horm. Res. 36:53–88. [DOI] [PubMed] [Google Scholar]
- Krey LC, Butler WR, and Knobil E (1975) Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology 96:1073–1087. [DOI] [PubMed] [Google Scholar]
- Krieg WJS (1932) The hypothalamus of the albino rat. J. Comp. Neurol. 55:19–89. [Google Scholar]
- Kubek MJ, and Wilber JF (1980) Regional distribution of leucine-enkephalin in hypothalamic and extrahypothalamic loci of the human nervous system. Neurosci. Lett. 18:155–161. [DOI] [PubMed] [Google Scholar]
- Laatikainen TJ (1991) Corticotropin-releasing hormone and opioid peptides in reproduction and stress. Ann. Med. 23:489–496. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE (1936) The topography and homologies of the hypothalamic nuclei in man. J. Anat. 70:203–214. [PMC free article] [PubMed] [Google Scholar]
- Le Gros Clark WE, Beattie J, Riddoch G, and Dott NM (1938) The Hypothalamus. Morphological, Functional, Clinical and Surgical Aspects. London: Oliver and Boyd. [Google Scholar]
- Lehman MN, and Karsch FJ (1993) Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 133:887–895. [DOI] [PubMed] [Google Scholar]
- LeVay S (1991) A difference in hypothalamic structure between heterosexual and homosexual men. Science 253:1034–1037. [DOI] [PubMed] [Google Scholar]
- Lewis ME, Khachaturian H, Akil H, and Watson SJ (1984) Anatomical relationship between opioid peptides and receptors in rhesus monkey brain. Brain Res. Bull. 13:801–812. [DOI] [PubMed] [Google Scholar]
- Lewis RV, Stern AS, Kimura S, Rossier J, Stein S, and Udenfriend S (1980) An about 50,000-dalton protein in adrenal medulla: A common precursor of [Met]- and [Leu]-enkephalin. Science 208:1459–1461. [DOI] [PubMed] [Google Scholar]
- Mai JK, Kedziora 0, Teckhaus L, and Sofroniew MV (1991) Evidence for subdivisions in the human suprachiasmatic nucleus. J. Comp. Neurol. 305:508–525. [DOI] [PubMed] [Google Scholar]
- Mains RE, Eipper BA, and Ling N (1977) Common precursor to corticotropins and endorphins. Proc. Natl. Acad. Sci. USA 74:3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Powers RE, Dellovade TL, and Price DL (1991) The bed nucleus-amygdala continuum in human and monkey. J. Comp. Neurol. 309:445–485. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, and Arai Y (1983) Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol. Jpn. 30:277–280. [DOI] [PubMed] [Google Scholar]
- Maysinger D, Höllt V, Seizinger BR, Mehraein P, Pasi A, and Herz A (1982) Parallel distribution of immunoreactive ⍺-neo-endorphin and dynorphin in rat and human tissue. Neuropeptides 2:211–225. [Google Scholar]
- Meister B, Villar MJ, Ceccatelli S, and Hokfelt T (1990) Localization of chemical messengers in magnocellular neurons of the hypothalamic supraoptic and paraventricular nuclei: An immunohistochemical study using experimental manipulations. Neuroscience 37:603–633. [DOI] [PubMed] [Google Scholar]
- Mengod G, Goudsmit E, Probst A, and Palacios JM (1992) In situ hybridization histochemistry in the human hypothalamus. Progr. Brain Res. 93:45–55. [DOI] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Fukuda A, and Matsuo H (1980) A new opioid octapeptide related to dynorphin from porcine hypothalamus. Biochem. Biophys. Res. Commun. 95:1475–1481. [DOI] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Chino N, Sakakibara S, and Matsuo H (1981) β-Neo-endorphin, a new hypothalamic “big” leu-enkephalin of porcine origin: Its purification and the complete amino acid sequence. Biochem. Biophys. Res. Commun. 99:864–870. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Yim GK, and Lowy MT (1983) Opioid modulation of appetite. Neurosci. Biobehav. Rev. 7:281–305. [DOI] [PubMed] [Google Scholar]
- Morrell JI, McGinty JF, and Pfaff DW (1985) A subset of β-endorphin or dynorphin-containing neurons in the medial basal hypothalamus accumulates estradiol. Neuroendocrinology 41:417–426. [DOI] [PubMed] [Google Scholar]
- Nakao K, Suda M, Sakamoto M, Yoshimasa T, Morii N, Ikeda Y, Yanaihara C, Yanaihara N, Numa S, and Imura H (1983) Leumorphin is a novel endogenous opioid peptide derived from preproenkephalin B. Biochem. Biophys. Res. Commun. 117:695–701. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, and Haymaker W (1969) Hypothalamic nuclei and fiber connections. In Haymaker W, Anderson E, and Nauta WJH (edsj: The Hypothalamus. Springfield, IL: Charles C. Thomas, pp. 136–209. [Google Scholar]
- Neumann I, Russell JA, and Landgraf R (1992) Endogenous opioids regulate intracerebral oxytocin release during parturition in a region-specific manner. Progr. Brain Res. 91:55–58. [DOI] [PubMed] [Google Scholar]
- Noda M, Teranishi Y, Takahashi H, Toyosato M, Notake M, Nakanishi S, and Numa S (1982) Isolation and structural organization of the human preproenkephalin gene. Nature 297:431–434. [DOI] [PubMed] [Google Scholar]
- Olson GA, Olson RD, and Kastin AJ (1990) Endogenous opiates: 1989. Peptides 11:1277–1304. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Saleh T, and Cechetto DF (1992) Lateral hypothalamic area neurotransmission and neuromodulation of the specific cardiac effects of insular cortex stimulation. Brain Res. 581:133–142. [DOI] [PubMed] [Google Scholar]
- Pelletier G (1993) Regulation of proopiomelanocortin gene expression in rat brain and pituitary as studied by in situ hybridization. Ann. NY Acad. Sci. 680:246–259. [DOI] [PubMed] [Google Scholar]
- Pfaff DW (1980) Estrogens and Brain Function. Neural Analysis of a Hormone-Controlled Mammalian Reproductive Behavior. New York: Springer-Verlag. [Google Scholar]
- Pittius CW, Seizinger BR, Mehraein P, Pasi A, and Herz A (1983) Proenkephalin-A-derived peptides are present in human brain. Life Sci Suppl.1 33:41–44. [DOI] [PubMed] [Google Scholar]
- Pittius CW, Seizinger BR, Pasi A, Mehraein P, and Herz A (1984) Distribution and characterization of opioid peptides derived from proenkephalin A in human and rat central nervous system. Brain Res. 304:127–136. [DOI] [PubMed] [Google Scholar]
- Rance NE, and Young WS I11 (1991) Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 128:2239–2247. [DOI] [PubMed] [Google Scholar]
- Rance NE, Young WS 111, and McMullen NT (1994) Topography of neurons expressing luteinizing hormone-releasing hormone gene transcripts in the human hypothalamus and basal forebrain. J. Comp. Neurol. 339:573–586. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Jakubowski M, Allen DL, and Roberts JL (1992) Positive correlation between proopiomelanocortin and tyrosine hydroxylase mRNA levels in the mediobasohypothalamus of ovariectomized rats: Response to estradiol replacement and withdrawal. Neuroendocrinology 56:285–294. [DOI] [PubMed] [Google Scholar]
- Roberts JL, Levin N, Lorang D, Lundblad JR, Dermer S, and Blum M (1993) Regulation of pituitary proopiomelanocortin gene expression. In Akil H and Simon EJ (eds): Handbook of Experimental Pharmacology. Berlin: Springer-Verlag, pp. 347–377. [Google Scholar]
- Romano GJ, Mobbs CV, Lauber A, Howells RD, and Pfaff DW (1990) Differential regulation of proenkephalin gene expression by estrogen in the ventromedial hypothalamus of male and female rats: Implications for the molecular basis of a sexually differentiated behavior. Brain Res. 536:63–68. [DOI] [PubMed] [Google Scholar]
- Rossier J (1993) Biosynthesis of enkephalins and proenkephalin-derived peptides. In Akil H and Simon EJ (eds): Handbook of Experimental Pharmacology. Berlin: Springer-Verlag, pp. 424–447. [Google Scholar]
- Russell JA, Douglas AJ, Bull PM, Pumford KM, Bicknell RJ, and Leng G (1992) Pregnancy and opioid interactions with the anterior peri-third ventricular input to magnocellular oxytocin neurones. Progr. Brain Res. 91:41–53. [DOI] [PubMed] [Google Scholar]
- Saper CB (1990) Hypothalamus. In Paxinos G (ed): The Human Nervous System. Sydney: Academic Press, pp. 389–412. [Google Scholar]
- Saper CB, Swanson LW, and Cowan WM (1979) Some efferent connections of the rostral hypothalamus in the squirrel monkey (Saimiri sciureus) and cat. J. Comp. Neurol. 184:205–242. [DOI] [PubMed] [Google Scholar]
- Sar M, Stumpf WE, Miller RJ, Chang K-J, and Cuatrecasas P (1978) Immunohistochemical localization of enkephalin in rat brain and spinal cord. J. Comp. Neurol. 182:17–38. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, and Joseph SA (1982) The distribution and cells of origin of ACTH(1–39)-stained varicosities in the paraventricular and supraoptic nuclei. Brain Res. 232:365–374. [DOI] [PubMed] [Google Scholar]
- Schultzberg M, Lundberg JM, Hokfelt T, Terenius L, Brandt J, Elde RP, and Goldstein M (1978) Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience 3:1169–1186. [DOI] [PubMed] [Google Scholar]
- Seizinger BR, Höllt V, and Herz A (1981) Evidence for the occurrence of the opioid octapeptide dynorphin-(1–8) in the neurointermediate pituitary of rats. Biochem. Biophys. Res. Commun. 102:197–205. [DOI] [PubMed] [Google Scholar]
- Seizinger BR, Liebisch DC, Kish SJ, Arendt RM, Hornykiewicz 0, and Herz A (1986) Opioid peptides in Huntington’s disease: Alterations in prodynorphin and proenkephalin system. Brain Res. 378:405–408. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Romano GJ, Howells RD, and Pfaff DW (1986) Cellular location and regulation of proenkephalin mRNA in rat brain. In Uhl GR (ed): In Situ Hybridization in Brain. New York: Plenum Press, pp. 3–20. [Google Scholar]
- Simerly RB, Swanson LW, and Gorski RA (1984) Demonstration of a sexual dimorphism in the distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus of the rat. J. Comp. Neurol. 225:151–166. [DOI] [PubMed] [Google Scholar]
- Simerly RB, McCall LD, and Watson SJ (1988) Distribution of opioid peptides in the preoptic region: Immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. J. Comp. Neurol. 276:442–459. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, and Swanson LW (1990) Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J. Comp. Neurol. 294:76–95. [DOI] [PubMed] [Google Scholar]
- Smith AI, and Funder JW (1988) Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocrine Rev. 9:159–179. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Ha LH, Spears LC, and Dee MG II (1993a) Lateral hypothalamic injections of glutamate, kainic acid, D,L-⍺-amino-3-hydroxyy-5-methyl-isoxazole propionic acid or N-methyl-D-aspartic acid rapidly elicit intense transient eating in rats. Brain Res. 613:88–95. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Willett VL III, Donias HW, Ha LH, and Spears LC (1993b) The lateral hypothalamus: A primary site mediating excitatory amino acid-elicited eating. Brain Res. 630:41–49. [DOI] [PubMed] [Google Scholar]
- Suda M, Nakao K, Sakamoto M, Yoshimasa T, Morii N, Yanaihara N, Numa S, and Imura H (1985) Leumorphin in human brain. Neuropeptides 5:461–464. [DOI] [PubMed] [Google Scholar]
- Sukhov RR, Walker LC, Rance NE, Price DL, and Young WS III (1993) Vasopressin and oxytocin gene expression in the human hypothalamus. J. Comp. Neurol. 337:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, and Fliers E (1985) A sexual dimorphic nucleus in the human brain. Science 228:1112–1115. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Hofman MA, Lucassen PJ, Purba JS, Raadsheer FC, and Van de Nes JAP (1993) Functional neuroanatomy and neuropathology of the human hypothalamus. Anat. Embryol. 187:317–330. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Hakamata Y, Watanabe Y, Kikuno R, Miyata T, and Numa S (1983) Complete nucleotide sequence of the human corticotropin- β-lipotropin precursor gene. Nucleic Acids Res. 11:6847–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius L, Lyrenas S, Lutsch H, Lindstrom L, Nyberg F, and Lindberg B (1987) Opioid peptides at term pregnancy in the early puerperium and in postpartum psychosis. In Nerozzi D, Goodwin FK, and Costa E (eds): Hypothalamic Dysfunction in Neuropsychiatric Disease. New York: Raven Press, pp. 201–209. [PubMed] [Google Scholar]
- Thornton JE, Loose MD, Kelly MJ, and Ronnekleiv OK (1994) Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J. Comp. Neurol. 341:68–77. [DOI] [PubMed] [Google Scholar]
- Tong Y, Zhao H, Labrie F, and Pelletier G (1990) Regulation of proopiomelanocortin messenger ribonucleic acid content by sex steroids in the arcuate nucleus of the female rat brain. Neurosci. Lett. 112:104–108. [DOI] [PubMed] [Google Scholar]
- Treiser SL, and Wardlaw SL (1992) Estradiol regulation of proopiomelanocortin gene expression and peptide content in the hypothalamus. Neuroendocrinology 55:167–173. [DOI] [PubMed] [Google Scholar]
- Turkenburg JL, Swaab DF, Endert E, Louwerse AL, and Van De Poll NE (1988) Effects of lesions of the sexually dimorphic nucleus on sexual behavior of testosterone-treated female Wistar rats. Brain Res. Bull. 21:215–224. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen JJ, Lotstra F, Liston DR, and Rossier J (1983) Proenkephalin, [Met]enkephalin, and oxytocin immunoreactivities are colocalized in bovine hypothalamic magnocellular neurons. Proc. Natl. Acad. Sci. USA 80:5139–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RB, Amaral DG, and Cowan WM (1982) The morphology and connections of the posterior hypothalamus in the cynomolgus monkey (Macaca fascicularis). I. Cytoarchitectonic organization. J. Comp. Neurol. 207:114–134. [DOI] [PubMed] [Google Scholar]
- Viveros OH, Diliberto EJ Jr., Hazum E, and Chang KJ (1979) Opiate-like materials in the adrenal medulla: Evidence for storage and secretion with catecholamines. Mol. Pharmacol. 16:1101–1108. [PubMed] [Google Scholar]
- Walker LC, Rance NE, Price DL, and Young WS III (1991) Galanin mRNA in the nucleus basalis of Meynert complex of baboons and humans. J. Comp. Neurol. 303:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley JK, Young WS III, and Kuhar MJ (1980) Immunohistochemical localization of enkephalin in rat forebrain. Brain Res. 190:153–174. [DOI] [PubMed] [Google Scholar]
- Wardlaw SL, Wehrenberg WB, Ferin M, Carmel PW, and Frantz AG (1980) High levels of β-endorphin in hypophyseal portal blood. Endocrinology 106:1323–1326. [DOI] [PubMed] [Google Scholar]
- Wardlaw SL, Wehrenberg WB, Ferin M, Antunes JL, and Frantz AG (1982) Effect of sex steroids on β-endorphin in hypophyseal portal blood. J. Clin. Endocrinol. Metab. 55:877–881. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Akil H, Ghazarossian VE, and Goldstein A (1981) Dynorphin immunocytochemical localization in brain and peripheral nervous system: Preliminary studies. Proc. Natl. Acad. Sci. USA 78:1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SJ, Akil H, and Khachaturian H (1982) Dynorphin neuronal systems: Distribution in brain and relationship to other opioid peptides. Soc. Neurosci. Abstr. 8:98. [Google Scholar]
- Wehrenberg WB, Wardlaw SL, Frantz AG, and Ferin M (1982) β-Endorphin in hypophyseal portal blood: Variations throughout the menstrual cycle. Endocrinology 111:879–881. [DOI] [PubMed] [Google Scholar]
- Wilcox JN, and Roberts JL (1985) Estrogen decreases rat hypothalamic proopiomelanocortin messenger ribonucleic acid levels. Endocrinology 117:2392–2396. [DOI] [PubMed] [Google Scholar]
- Wilson RC, Kesner JS, Kaufman J-M, Uemura T, Akema T, and Knobil E (1984) Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology 39:256–260. [DOI] [PubMed] [Google Scholar]
- Wise PM, Scarbrough K, Weiland NG, and Larson GH (1990) Diurnal pattern of proopiomelanocortin gene expression in the arcuate nucleus of proestrous, ovariectomized, and steroid-treated rats: A possible role in cyclic luteinizing hormone secretion. Mol. Endocrinol. 4:886–892. [DOI] [PubMed] [Google Scholar]
- Young E, Bronstein D, and Akil H (1993) Proopiomelanocortin biosynthesis, processing, and secretion: Functional implications. In Akil H and Simon EJ (eds): Handbook of Experimental Pharmacology. Berlin: Springer-Verlag, pp. 393–421. [Google Scholar]
- Young WS III (1989) In situ hybridization histochemical detection of neuropeptide mRNA using DNA and RNA probes. Methods Enzymol. 168:702–710. [DOI] [PubMed] [Google Scholar]
- Young WS III, and Lightman SL (1992) Chronic stress elevates enkephalin expression in the rat paraventricular and supraoptic nuclei. Mol. Brain Res. 13:111–117. [DOI] [PubMed] [Google Scholar]
- Zis AP, and Garland JE (1991) Opioid peptides and depression: The neuroendocrine approach. Bailliere’s Clin. Endocrinol. Metab. 5:97–117. [DOI] [PubMed] [Google Scholar]


