Abstract
Salmonella enterica serovar Typhimurium causes cell death in bovine monocyte-derived and murine macrophages in vitro by a sipB-dependent mechanism. During this process, SipB binds and activates caspase-1, which in turn activates the proinflammatory cytokine interleukin-1β through cleavage. We used bovine ileal ligated loops to address the role of serovar Typhimurium-induced cell death in induction of fluid accumulation and inflammation in this diarrhea model. Twelve perinatal calves had 6- to 9-cm loops prepared in the terminal ileum. They were divided into three groups: one group received an intralumen injection of Luria-Bertani broth as a control in 12 loops. The other two groups (four calves each) were inoculated with 0.75 × 109 CFU of either wild-type serovar Typhimurium (strain IR715) or a sopB mutant per loop in 12 loops. Hematoxylin and eosin-stained sections were scored for inflammation, and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL)-positive cells were detected in situ. Fluid accumulation began at 3 h postinfection (PI). Inflammation was detected in all infected loops at 1 h PI. The area of TUNEL-labeled cells in the wild-type infected loops was significantly higher than that of the controls at 12 h PI, when a severe inflammatory response and tissue damage had already developed. The sopB mutant induced the same amount of TUNEL-positive cells as the wild type, but it was attenuated for induction of fluid secretion and inflammation. Our results indicate that serovar Typhimurium-induced cell death is not required to trigger an early inflammatory response and fluid accumulation in the ileum.
Salmonella enterica serovar Typhimurium is a common cause of enteritis in humans. The disease is typically localized to the intestine and mesenteric lymph nodes and characterized by acute diarrhea, vomiting, and abdominal pain. Rectal biopsies of patients reveal mucosal edema and acute inflammation with neutrophils (6, 22). Clinical features closely resemble those observed during infection of cattle but are different from those observed during infection of mice, an animal in which serovar Typhimurium does not cause diarrhea (reviewed in reference 33). Recent studies have thus focused on the calf model to study the pathogenesis of serovar Typhimurium induced diarrhea.
A study comparing the importance of major serovar Typhimurium virulence determinants, including Salmonella pathogenicity island (SPI)-1, SPI-2, SPI-5, and the spv operon, for diarrheal disease in calves revealed that SPI-1 is the only determinant essential for both diarrhea and inflammation in the intestinal mucosa (32). The main function of the type III secretion system encoded by SPI-1 is to translocate bacterial effector proteins into the cytosol of the host cell (10). SPI-1-dependent protein translocation elicits the release of proinflammatory cytokines in intestinal epithelial cells in vitro (15). Furthermore, in vitro assays have implicated a second cell type, namely the macrophage, in the SPI-1-dependent release of proinflammatory cytokines that may lead to neutrophil influx into the intestinal mucosa during serovar Typhimurium infection. serovar Typhimurium induces cell death in macrophages in vitro with features of apoptosis, including positive terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining (4, 19, 20, 24, 28). However, recent reports suggest that Salmonella-induced cell death of macrophages may represent necrosis rather than apoptosis (3, 35). Nonetheless, Salmonella-induced cell death in murine macrophages is dependent on the expression and translocation of the SPI-1-encoded protein SipB into the macrophage. It has also been demonstrated that SipB binds and activates caspase-1, which cleaves and activates the proinfammatory cytokine interleukin-1β (IL-1β) (13). Caspase-1 is required for cytotoxicity induced by SipB, since macrophages of caspase-1 knockout mice are resistant to Salmonella-induced cell death (13). In addition, serovar Typhimurium colonization of Peyer's patches of caspase-1 knockout mice is significantly lower after oral challenge (23).
These findings suggest that Salmonella-induced cell death may trigger an inflammatory response due to release of activated IL-1β, thereby contributing to the pathogenesis of diarrheal disease. A similar mechanism was previously proposed for Shigella flexneri, which induces apoptosis of murine macrophages in vitro, resulting in the release of IL-1β (14). In vivo studies using the rabbit ileal ligated loop model suggest that apoptosis may play a role in the outcome of S. flexneri infection (37). Salmonella-induced cell death defined by positive TUNEL reaction has been described in macrophages in the liver in a model for systemic infection in the mouse (27). However, there is currently no information available regarding the role of Salmonella-induced cell death on the enteric disease caused in calves and humans. The goal of this study was to determine the possible role of Salmonella-induced cell death in the pathogenesis of the diarrhea and to correlate Salmonella-induced cell death with inflammation and intestinal fluid accumulation induced by serovar Typhimurium infection in perinatal calves.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
serovar Typhimurium strain IR715 (31) is a spontaneous nalidixic acid-resistant derivative of the strain ATCC 14028 (American Type Culture Collection). A sopB (also known as sigD) mutant of serovar Typhimurium strain IR715 was generated by transducing the sopB::mudJ insertion from strain BA1567 (1) into strain IR715 using phage P22, as described previously (21). The resulting mutant was designated ZA15. The mudJ insertion was confirmed by Southern hybridization.
For both in vitro and in vivo studies, bacteria were grown in Luria-Bertani (LB) broth for 20 h at 37°C under agitation (230 rpm). A 50-μl aliquot of the culture was inoculated into 5 ml of fresh LB broth and cultured for an additional 6 h before inoculation.
Animals, surgical procedure, and sampling.
Twelve male Holstein calves 4 to 5 weeks of age and weighing 45 to 55 kg were used. They were fed milk replacer twice a day and water ad libitum. The calves were clinically healthy before the experiment and were culture negative for fecal excretion of Salmonella. Detection of Salmonella serotypes in fecal swabs was performed by enrichment in tetrathionate broth (Difco) and streaking on brilliant green agar (BBL).
The calves were fasted for 24 h prior to the surgery. Anesthesia was induced with propofol (Propoflo; Abbott Laboratories, Chicago, Ill.) followed by placement of an endotracheal tube and maintenance with isofluorane (Isoflo; Abbott Laboratories) for the duration of the experiment. A laparotomy was performed, the ileum was exposed, and 13 loops with length ranging from 6 to 9 cm were ligated leaving 1-cm loops between them. The loops were infected by intralumenal injection of 3 ml of a suspension of either wild-type or the sopB mutant of serovar Typhimurium in LB broth containing approximately 0.75 × 109 CFU/ml. Sterile LB broth was injected into the control loops. The loops were replaced into the abdominal cavity. Samples for bacteriologic culture, histopathology, and ultrastructural studies were collected at 5, 15, and 30 min and 1, 2, 3, 4, 5, 6, 8, 10, and 12 h. Tissue samples from Peyer's patches were weighed, homogenized in phosphate-buffered saline (PBS), serially diluted, and plated onto LB agar plates containing nalidixic acid (50 μg/ml) for counting CFU.
Macrophage isolation, culture, and infection.
The protocol used for monocyte isolation was described previously (26). Briefly, venous blood was collected into anticoagulant (acid citrate-dextrose), diluted 1:2 in PBS-citrate (pH 7.4), layered over a Percoll (Amersham Pharmacia Biotech AB, Uppsala, Sweden) solution with a specific density of 1.0770 (mixture of the following solutions: 10:1 percoll and 1.5 M NaCl in 1.2% NaH2PO4; 130 mM trisodium citrate; 5% bovine serum albumin; PBS, adjusted for a final refractive index of 1.3460), and centrifuged at 1,000 × g for 30 min. The fraction containing white blood cells was collected, washed in PBS-citrate, resuspended in supplemented RPMI (Gibco BRL Life Technologies, Inc., Grand Island, N.Y.) with 4% autologous serum, and incubated at 37°C with 5% CO2 overnight in Teflon flasks. Medium containing the nonadherent cells was removed and replaced by supplemented RPMI with 12.5% autologous serum. The medium was changed every 3 days. Monocytes differentiate into macrophages after 7 to 10 days in culture. All the experiments were conducted with macrophages kept in culture for 10 to 11 days.
For inoculation, the bacterial suspension was diluted in supplemented RPMI. The macrophages were inoculated in Teflon flasks with a multiplicity of infection of 50:1. Inoculation was followed by centrifugation (1,500 rpm, 5 min) and incubation at 37°C in 5% CO2 for 30 min. Subsequently, gentamycin (Gibco BRL Life Technologies, Inc.) was added to the medium to a final concentration of 25 μg/ml in order to kill extracellular bacteria.
Morphologic evaluation.
Fragments from the Peyer's patches were fixed in formalin, processed according to the standard procedures for paraffin embedding, sectioned at 5-μm thickness, and stained with hematoxylin and eosin.
Inflammatory changes were scored from 1 to 5 according to the following criteria: 1, no inflammation; 2, margination and perivascular infiltration of neutrophils and/or mild diffuse infiltration of neutrophils at the tips of absorptive villi; 3, moderate diffuse infiltration of neutrophils in the mucosa and perivascular multifocal infiltration in the submucosa; 4, severe diffuse infiltration of neutrophils in the mucosa and mild to moderate infiltration in the submucosa; and 5, severe diffuse infiltration of neutrophils throughout the mucosa and submucosa associated with edema and necrosis of the mucosa.
Small fragments from Peyer's patches were fixed overnight at 4°C in a solution of 5% glutaraldehyde and 4% paraformaldehyde in 0.1 M sodium cacodylate buffer. Tissues were then washed three times with 0.1 M sodium cacodylate buffer and postfixed for 2 h at 4°C in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer. For transmission electron microscopy, the samples were stained overnight at 4°C in a saturated uranyl acetate solution. Tissues were dehydrated in a graduated series of ethanol solutions and propylene oxide and embedded in Epon Araldite. Then, 0.5-μm sections were stained with toluidine blue and examined under light microscopy for selection of the microscopic fields. The blocks were trimmed and thin sections (60 to 90 nm) were cut, mounted onto copper grids, stained with uranyl acetate and lead citrate, and examined with a Zeiss 10C transmission electron microscope. For scanning electron microscopy, the samples were dehydrated, critical point dried, coated with a thin layer (approximately 500 Å) of AuPd, and examined with a JEOL JSM-6400 scanning electron microscope at an accelerating voltage of 15 kV.
TUNEL.
A TUNEL assay (12) was used for in situ detection of apoptotic cells. Five-micrometer sections of the Peyer's patches were deparaffinized, hydrated, and treated with a proteinase K (Sigma, St. Louis, Mo.) solution (20 μg/ml) and 0.5% Triton X-100 (Sigma). In situ detection of apoptotic cells was performed using a commercial kit (Apoptag Plus kit; Intergen, Purchase, N.Y.) according to the manufacturer's instructions. Briefly, endogenous peroxidase was quenched in 3% hydrogen peroxide. Digoxigenin-deoxynucleoside triphosphate was catalytically incorporated to the 3′ ends by incubating with terminal deoxynucleotidyl transferase (TdT) in a humidified chamber at 37°C for 1.2 h. After washing in PBS (50 mM sodium phosphate (pH 7.4), 200 mM NaCl), the sections were incubated with antidigoxigenin-peroxidase antibody in a humidified chamber at room temperature. Color development was obtained with a diaminobenzidine substrate solution. Specimens were counterstained with methyl green, dehydrated, and mounted with coverslips. Sections of normal female rodent mammary gland, obtained 3 to 5 days after weaning of rat pups, were used as positive controls. Negative controls were obtained by replacing the TdT with buffer on the same tissues used as positive control.
To detect induction of macrophage apoptosis in vitro by the sopB mutant, TUNEL was performed using a commercial kit (Pharmingen, San Diego, Calif.) following the manufacturer's instructions, except for an additional incubation with purified mouse immunoglobulin G (Sigma). Macrophages were harvested by placing them on ice 1 h after inoculation and incubation for 30 min as described above. Then, the cells were fixed in 1% paraformaldehyde in PBS for 15 min on ice, washed, and stored in 70% ethanol at −20°C for 2 days. The cells were then incubated with a labeling solution containing TdT and Br-dUTP, followed by washes and incubation with purified mouse immunoglobulin G, fluorescein-labeled anti-BrdUTP antibody, and finally propidium iodide. The cells were then analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, Calif.). Flow cytometric data were analyzed in Flow Jo (Tree Star, Inc., Palo Alto, Calif.).
Image analysis.
TUNEL-labeled sections were examined with a light microscope. The images from 20 microscopic fields (36,500 μm2 each), including 10 from the mucosa (epithelium and lamina propria) plus 10 from the lymphoid nodules, were captured from each slide by a microcamera and analyzed using the NIH image 1.60 software. The images were processed in order to measure the labeled areas, which corresponds to the number of apoptotic cells.
Statistical analysis.
The ileal ligated loop experiment was a split plot design. The data from fluid accumulation were submitted to analysis of variance, and the averages were compared by Student's t test (29). The image analysis data were evaluated by the nonparametric Kruskal-Wallis test (5).
RESULTS
Fluid secretion and inflammatory response.
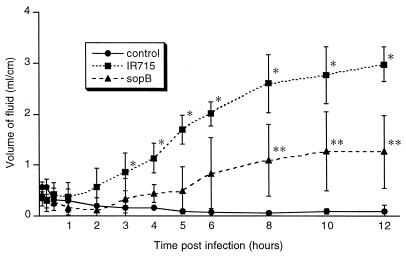
Salmonella-induced cell death in murine macrophages in vitro is associated with release of the proinflammatory cytokine IL-1β (13). Considering that a similar mechanism of cell death, which is dependent on caspase-1 (IL-1β-converting enzyme) also occurs in cattle (28, 35), this may be a key event in the pathogenesis of diarrhea. In order to determine the association of cell death in the Peyer's patches with diarrhea, we first evaluated the dynamics of fluid accumulation into the lumen of the ileal ligated loops infected with serovar Typhimurium. In addition, the development of inflammatory response was assessed by histopathology. Fluid accumulation began at 3 h postinfection (Fig. 1), when there was a significant difference in the fluid content between wild-type infected and control loops (P < 0.05). From 3 to 12 h postinfection, there was a consistent increase in the volume of fluid in the lumen of the wild-type infected loops (Fig. 1).
FIG. 1.
Time course of fluid accumulation into the ileal lumen during serovar Typhimurium infection from 5 min through 12 h postinfection. Each data point represents the average (± standard deviation) of four independent experiments. ∗, Values corresponding to wild-type infected loops (IR715) are significantly higher than those for the uninfected controls and loops infected with sopB mutant from 3 to 12 h postinfection (P < 0.05). ∗∗, Values corresponding to sopB-infected loops are significantly higher than those for uninfected controls and significantly lower than wild-type infected loops (IR715) from 8 to 12 h postinfection (P < 0.05).
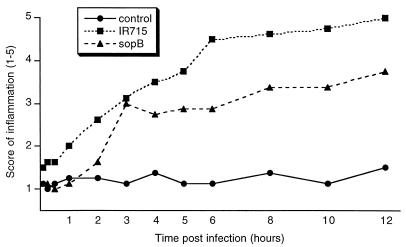
Hematoxylin and eosin-stained sections were examined by light microscopy, and a scoring system was used for evaluation of the inflammatory changes. The scores ranged from 1 (absence of inflammation) to 5 (severe inflammatory reaction associated with necrosis of the mucosa). Early inflammatory changes such as intravascular margination and mild perivascular infiltration of neutrophils or a few neutrophils scattered throughout the lamina propria were present in the mucosa of all loops infected with wild-type serovar Typhimurium at 1 h postinfection (Fig. 2). These changes rapidly progressed from 1 to 12 h (Fig. 2). The average scores for inflammatory changes had a continuous increase from 1 to 12 h postinfection (Fig. 3).
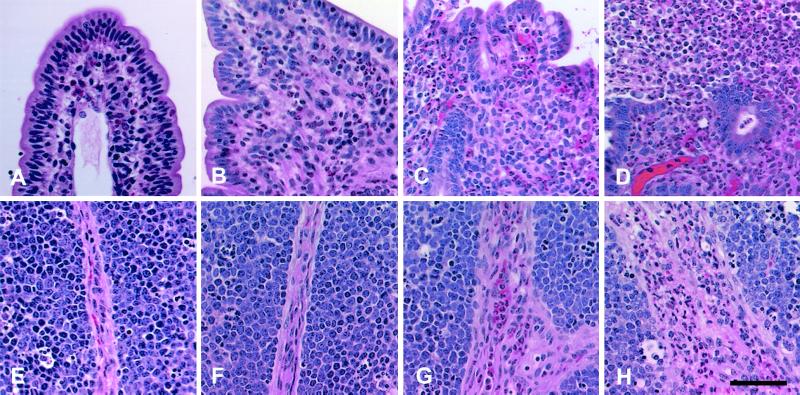
FIG. 2.
Micrographs of bovine Peyer's patches infected with wild-type serovar Typhimurium. (A to D) Sections of the mucosa. (A) Uninfected control, no significant histological changes; (B) at 1 h postinfection, mild focal infiltration of neutrophils; (C) mucosa at 6 h postinfection, diffuse infiltration of neutrophils and marked blunting of the villi; (D) at 12 h postinfection, severe diffuse infiltration of neutrophils with loss of superficial epithelium and extensive exudation into the lumen. (E to H) Sections of lymphoid nodules. (E) uninfected control, no significant histological changes; (F) at 1 h postinfection, with no significant histological changes; (G) at 6 h post infection, perivascular infiltration of neutrophils in the interstitial connective tissue; (H) at 12 h postinfection, severe diffuse infiltration of neutrophils in the interstitial connective tissue. Stain is hematoxylin and eosin. Bar = 50 μm.
FIG. 3.
Inflammatory changes in the Peyer's patches after serovar Typhimurium infection. Magnitude of inflammation was scored (1 to 5) according to the criteria described in Materials and Methods. Each data point represents the average of four independent experiments.
The averages of fluid accumulation per loop length unit and the inflammatory scores in loops infected with wild-type serovar Typhimurium had the same time course profile (Fig. 1 and 3) and a positive and significant correlation (r = 0.957, P < 0.05). Early inflammatory changes were clearly present in infected loops at 1 h postinfection, therefore preceding the accumulation of fluid into the lumen, which began at 3 h postinfection. Although this does not necessarily prove a causal relation, it suggests that inflammation may be an important mechanism contributing to fluid accumulation, since it is associated with an increase in vascular permeability and leakage of intravascular fluids.
In situ detection of TUNEL-positive cells.
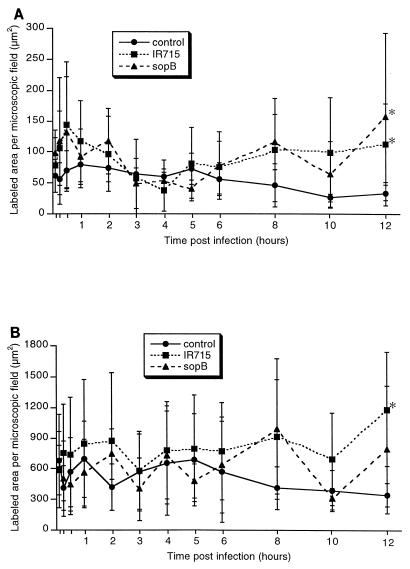
Considering the possible role of Salmonella-induced cell death in triggering the inflammatory response through the release of IL-1 (13), it would be expected that an increase in the number of TUNEL-positive cells in the Peyer's patches would coincide with or precede inflammatory changes. In order to test this notion, sections of the Peyer's patches were processed for TUNEL staining, and positive labeling was measured by computer morphometric analysis. Visual counting of TUNEL-positive cells had a high positive correlation with the measurement of the area of positive staining in the section obtained by computer analysis (data not shown). In both wild-type infected and control groups, most of the TUNEL-positive cells were detected in the lymphoid component of the Peyer's patches either at lymphoid nodules or at the domed villi (Fig. 4). Virtually no TUNEL-positive epithelial cells were detected in the control loops and very few were detected in infected loops at late time points. The area of TUNEL-positive cells per microscopic field was determined separately for both mucosa including absorptive epithelium, M cells, lamina propria (Fig. 5A), and lymphoid nodules (Fig. 5B). No significant differences were observed in the area of TUNEL-positive staining between the uninfected controls and loops infected with wild-type serovar Typhimurium, except at 12 h postinfection when a significant increase in positive TUNEL staining was detected in infected loops in both mucosa and lymphoid nodules (P < 0.05). Although a higher number of TUNEL-positive cells was detected at early time points such as 15 and 30 min in the mucosa of infected loops (Fig. 5A), the differences when compared to the uninfected control loops were not statistically significant (P > 0.05). Many apoptotic cells were detected by ultrastructural examination, most of which were lymphoid cells, and in most cases only apoptotic bodies within the cytoplasm of phagocytic cells were observed. The same distribution of apoptotic cells in the mucosa and lymphoid nodules was observed in both controls and wild-type infected loops. Although a large number of bacteria was observed within epithelial and phagocytic cells of infected tissues, no bacteria were detected in association with or within the cytoplasm of cells with morphologic features of apoptosis.
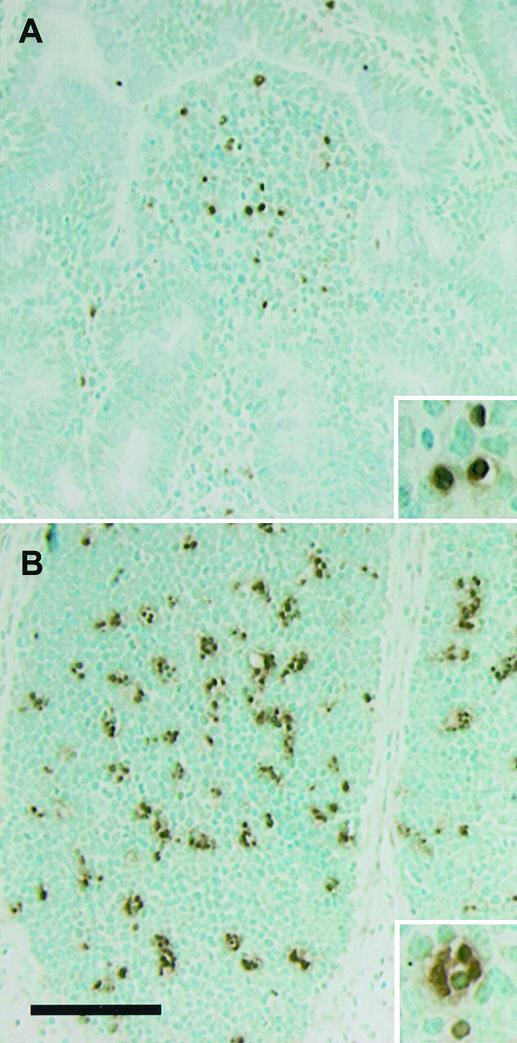
FIG. 4.
Localization of TUNEL-positive cells. In sections of Peyer's patches at 2 h after infection with wild-type serovar Typhimurium, TUNEL-stained cells were more concentrated in the domed villi (A) and lymphoid nodules (B). Methyl green counterstaining was used. Bar = 100 μm.
FIG. 5.
Area of in situ TUNEL labeling per microscopic field in the Peyer's patches during serovar Typhimurium infection. The area of labeling per 36,500-μm2 microscopic field was measured using the NIH Image software as described in Materials and Methods. Each data point represents the average ± standard deviation of four independent experiments. (A) Mucosa; ∗ indicates differences between control and loops infected with either the wild type (IR715) or sopB mutant at 12 h postinfection are statistically significant (P < 0.05). (B) Lymphoid nodules; ∗ indicates the difference between control and wild-type (IR715) infected loops at 12 h postinfection is statistically significant (P < 0.05).
These data document a significant increase in the number of TUNEL-positive cells during serovar Typhimurium infection, which was observed exclusively at 12 h postinfection, that occurs only after a severe inflammatory response is established. This suggests that the increase in cell death may be a consequence rather than a cause of inflammation. Considering the lack of a significant difference in the TUNEL staining between infected and control loops at early stages of infection and that no bacteria were detected in or associated with TUNEL-positive cells, serovar Typhimurium induced cell death may not be required for triggering the inflammatory response. However, the data described above do not exclude the possibility that serovar Typhimurium-induced cell death may play a role in diarrhea at a later stage postinfection.
sopB mutant of serovar Typhimurium induces the same level of TUNEL staining as wild-type in spite of attenuation in fluid secretion and inflammatory response.
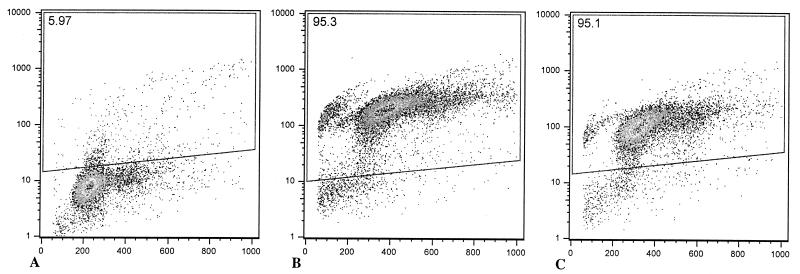
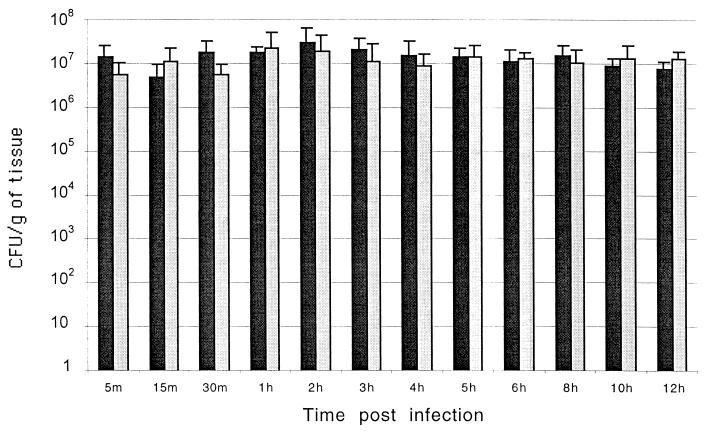
To further characterize the occurrence of cell death in vivo, we infected ileal ligated loops with a mutant of serovar Typhimurium lacking sopB. It has been previously demonstrated that a sopB mutant of Salmonella enterica serovar Dublin causes a delayed host inflammatory response in cattle (11, 25). Although Salmonella-induced cell death is mediated by SipB, a sipB mutant is not suitable for in vivo studies since such a mutant is unable to enter epithelial cells (17, 18) and has a strongly reduced ability to invade and colonize the intestinal mucosa in calves (32). A sopB mutant of serovar Typhimurium was used in this study not because this gene is directly related to sipB but because a sopB mutant is fully capable of invading the intestinal mucosa in spite of its attenuation for eliciting an inflammatory response (11). Thus, the induction of cell death in macrophages in vitro and in the Peyer's patches in vivo by the sopB mutant of serovar Typhimurium as well as the time course of the inflammatory response elicited by this mutant was compared to that in the wild type. Bovine macrophages infected with either the sopB mutant or the wild type had the same level of DNA fragmentation, as measured by flow cytometric analysis of TUNEL-stained cells (Fig. 6). Furthermore, ileal loops infected with the sopB mutant had the same profile of TUNEL-positive cells as the wild-type infected loops, both in the mucosa (Fig. 5A) and in the lymphoid nodules (Fig. 5B). Although no statistically significant differences in the area of in situ TUNEL staining were detected between sopB mutant and wild type, the sopB mutant induced a significantly lower level of fluid accumulation as compared to wild type (Fig. 1) and also had lower scores of inflammation (Fig. 3). No difference in the level of invasion of the Peyer's patches was detected between the sopB mutant and wild type (Fig. 7). The fact that the sopB mutant is fully able to induce macrophage cell death in vitro while inducing the same profile of TUNEL-positive cells in vivo, in spite of the attenuation in fluid secretion and inflammatory response, corroborates the results described above and supports the concept that Salmonella-induced cell death is not critical for triggering the inflammatory response.
FIG. 6.
Flow cytometric analysis of bovine macrophages infected with wild-type serovar Typhimurium or of sopB mutant grown to the logarithmic phase. Macrophages were infected in Teflon flasks with multiplicity of infection of 50:1, harvested 1 h after infection, processed for TUNEL staining (see Materials and Methods), and analyzed by flow cytometry. In each dot plot, the x axis corresponds to propidium iodide staining and the y axis corresponds to Br-dUTP incorporation. TUNEL-positive cells are within the area indicated by the quadrilateral, and the percentage of these cells is indicated at the left top corner of each panel. These data are from a representative experiment showing uninfected macrophages with a low background of TUNEL-positive cells (A) or macrophages infected with the wild type (B) or sopB mutant (C) containing a high percentage of apoptotic cells.
FIG. 7.
Invasion of bovine Peyer's patches by the wild type (black bars) or a sopB mutant (gray bars) of serovar Typhimurium. Tissue samples from Peyer's patches were weighed, homogenized in PBS, serially diluted, and plated onto LB agar plates containing nalidixic acid (50 μg/ml) for counting CFU. Each bar represents the mean and standard deviation of a time point from four independent experiments.
DISCUSSION
serovar Typhimurium is able to induce cell death in murine (4, 19, 20, 24, 34) and bovine (28, 35) macrophages in vitro. In murine macrophages, the secreted bacterial protein SipB binds to and activates caspase-1, which once activated cleaves and activates the potent proinflammatory cytokine IL-1β (13). An intact sipB gene is also required for serovar Typhimurium-induced cell death in bovine monocyte-derived macrophages (28) and for eliciting diarrhea and mortality in calves (32). The importance of inflammatory cytokines for disease in calves is demonstrated by a massive infiltration with neutrophils observed in the intestinal mucosa of infected animals (32). This neutrophil infiltration occurs rapidly (Fig. 3) and precedes fluid secretion (Fig. 1). Thus, activation of IL-1β during the SipB-mediated cell death suggests that this mechanism may play a role in the recruitment of neutrophils and the pathogenesis of diarrhea. S. flexneri induces macrophage cell death by a mechanism similar to the one proposed for serovar Typhimurium (13, 14), and experimental infection with S. flexneri in rabbit ileal ligated loops resulted in a 10- to 20-fold increase in the number of apoptotic cells in the Peyer's patches at 4 h postinfection, compared to that in uninfected controls and avirulent strains (37). A previous study showed that apoptosis of phagocytic cells in the liver of mice is induced during systemic infection with serovar Typhimurium (27). However, diarrhea does not develop during the systemic infection caused by serovar Typhimurium in mice and these data may thus not be representative of the diarrheal disease and pathology in the intestine that occurs in man and cattle infected with this pathogen (32). The purpose of this study was therefore to investigate the occurrence and possible role of serovar Typhimurium-induced cell death in vivo using a diarrhea model.
We demonstrate that a significant increase in the number of TUNEL-positive cells in the Peyer's patches is detected only at 12 h postinfection, when a pronounced inflammatory response and extensive tissue damage have already developed (Fig. 1, 3, and 6). Furthermore, at 12 h postinfection we detected a two- to fourfold increase in TUNEL-positive cells in the mucosa and lymphoid nodules of infected loops, which is a modest increase compared to the more than 20-fold increase in TUNEL-positive cells over the control background reported after Shigella infection in rabbit ileal loops (37). This result also contrasts with the report of a high number of TUNEL-positive cells in murine ileal loops at 1 h postinfection with serovar Typhimurium (23). Ultrastructural evaluation failed to detect bacteria associated with apoptotic cells. Furthermore, a sopB mutant of serovar Typhimurium induced the same level of cell death as the wild type in spite of its attenuation for induction of fluid secretion and induction of inflammation (Fig. 5). The number of TUNEL-positive cells was determined regardless of the cell type undergoing cell death. This could be considered a limitation of this study, however, as no significant differences between infected and control loops were detected at the early time points and the sopB mutant induced the same levels of cell death as the wild type; identification of the cell types undergoing cell death was considered beyond the scope of this study.
The sopB mutant presented marked attenuation in induction of both fluid secretion and inflammatory response, which has been previously demonstrated with a sopB mutant of serovar Dublin (11). SopB is an inositol phosphate phosphatase (25) secreted by the SPI-1-encoded type III secretion system (16), and it has been hypothesized that this protein could induce fluid accumulation by increasing secretion of Cl− by intestinal epithelial cells (25). However, the SopB interferes with other intracellular signaling pathways that may be involved in regulation of cytokine expression (9), such as activation of the serine-threonine kinase Akt (30), which may provide a better explanation for the attenuation of the sopB mutant (Fig. 1 and 3). Although Cl− secretion may be involved in serovar Typhimurium-induced diarrhea, it possibly has a secondary role since there is a massive infiltration of neutrophils during the first few hours postinfection, which suggests an exudative inflammatory mechanism for accumulation of fluid. Furthermore, the intestinal epithelium loses its integrity very early after infection (data not shown), and as a consequence the active transport of ions would not be expected to be effective.
Our data demonstrated no correlation between cell death and inflammatory changes at the onset of fluid accumulation. These data support the concept that inflammatory changes, which occur independently of Salmonella-induced cell death, contribute more significantly to the pathogenesis of diarrhea. Cell death has also been described in macrophages infected with bacteria such as Staphylococcus aureus and Escherichia coli, which are not able to survive intracellularly in macrophages (2). Instead of a role in eliciting inflammatory cytokine release, bacterium-induced macrophage cell death may be a more general response that plays a role during induction of an adaptive immune response. In support of this idea, a recent study demonstrated that Salmonella-induced macrophage apoptosis may be required for antigen presentation by macrophages (36).
Our data indicate that serovar Typhimurium-induced cell death, as measured by TUNEL staining, is neither required nor sufficient for triggering an early inflammatory response in cattle. The role of sopB in inducing inflammation and fluid secretion indicates that serovar Typhimurium has other mechanisms that modulate host inflammatory responses.
ACKNOWLEDGMENTS
This work was supported by grant DHHS/PHS/NIH-1 RO1 A144170 from the National Institutes of Health. R.L.S. was supported by CAPES, Brasília, Brazil, and Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
We thank Roberta Pugh, Linda McCallum, John Roths, Thomas Stephens, Miles Frey, Rosemary Vollmar, Melissa Kahl, and Denise Santos for technical assistance, Colin Tanksley and Alan Patranella for care of animals, and Ivan Sampaio for statistical analysis.
REFERENCES
- 1.Ahmer B M, van Reeuwijk J, Watson P R, Wallis T S, Heffron F. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol. 1999;31:971–982. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 2.Baran J, Guzik K, Hryniewicz W, Ernst M, Flad H D, Pryjma J. Apoptosis of monocytes and prolonged survival of granulocytes as a result of phagocytosis of bacteria. Infect Immun. 1996;64:4242–4248. doi: 10.1128/iai.64.10.4242-4248.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan M A, Cookson B T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen L M, Kaniga K, Galán J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 5.Conover W J. Practical nonparametric statistics. New York, N.Y: Wiley; 1980. [Google Scholar]
- 6.Day D W, Mandal B K, Morson B C. The rectal biopsy appearances in Salmonella colitis. Histopathology. 1978;2:117–131. doi: 10.1111/j.1365-2559.1978.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 7.Fields P I, Groisman E A, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- 8.Frost A J, Bland A P, Wallis T S. The early dynamic response of the calf ileal epithelium to Salmonella typhimurium. Vet Pathol. 1997;34:369–386. doi: 10.1177/030098589703400501. [DOI] [PubMed] [Google Scholar]
- 9.Galán J E, Zhou D. Striking a balance: modulation of the actin cystoskeleton by Salmonella. Proc Natl Acad Sci USA. 2000;97:8754–8761. doi: 10.1073/pnas.97.16.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galán J E. Interaction of Salmonella with host cells through the centrisome 63 type III secretion system. Curr Opin Microbiol. 1999;2:46–50. doi: 10.1016/s1369-5274(99)80008-3. [DOI] [PubMed] [Google Scholar]
- 11.Galyov E E, Wood M W, Rosqvist R, Mullan P B, Watson P R, Hedges S, Wallis T S. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25:903–912. doi: 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- 12.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilbi H, Moss J E, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell R A, Yuan J, Sansonetti P J, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IapB. J Biol Chem. 1988;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 15.Hobbie S, Chen L M, Galán J E. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 16.Hong K H, Miller V L. Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J Bacteriol. 1998;180:1793–1802. doi: 10.1128/jb.180.7.1793-1802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueck C J, Hantman M J, Bajaj V, Johnston C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaniga K, Tucker S, Trollinger D, Galán J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindgren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundberg U, Vinatzer U, Berdnik D, Gabain A, Baccarini M. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J Bacteriol. 1999;181:3433–3437. doi: 10.1128/jb.181.11.3433-3437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maloy R S, Stewart V J, Taylor R K. Genetic analysis of pathogenic bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 473–485. [Google Scholar]
- 22.McGovern V J, Slavutin L J. Pathology of Salmonella colitis. Am J Surg Pathol. 1979;3:483–490. doi: 10.1097/00000478-197912000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Monack D M, Hersh D, Ghori N, Bouley D, Zychlinsky A, Falkow S. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J Exp Med. 2000;192:249–258. doi: 10.1084/jem.192.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norris F A, Wilson M P, Wallis T S, Galyov E E, Majerus P W. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci USA. 1998;95:14057–14059. doi: 10.1073/pnas.95.24.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qureshi T, Templeton J W, Adams L G. Intracellular survival of Brucella abortus, Mycobacterium bovis BCG, Salmonella dublin, and Salmonella typhimurium in macrophages from cattle genetically resistant to Brucella abortus. Vet Immunol Immunopathol. 1995;50:1–10. doi: 10.1016/0165-2427(95)05492-8. [DOI] [PubMed] [Google Scholar]
- 27.Richter-Dahlfors A, Buchan A M J, Finlay B B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos R L, Tsolis R M, Bäumler A J, Smith III R, Adams L G. Salmonella enterica serovar Typhimurium induces cell death in bovine monocyte-derived macrophages by early sipB-dependent and delayed sipB-independent mechanisms. Infect Immun. 2001;69:2293–2301. doi: 10.1128/IAI.69.4.2293-2301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snedecor G W, Cochran W G. Statistical methods. Ames, Iowa: Iowa State University Press; 1994. [Google Scholar]
- 30.Steele-Mortimer O, Knodler L A, Marcus S L, Scheid M P, Goh B, Pfeifer C G, Duronio V, Finlay B B. Activation of Akt/protein kinase B in epithelial cells by the Salmonella typhimurium effector SigD. J Biol Chem. 2000;275:37718–37724. doi: 10.1074/jbc.M008187200. [DOI] [PubMed] [Google Scholar]
- 31.Stojiljkovic I, Bäumler A J, Heffron F. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J Bacteriol. 1995;177:1357–1366. doi: 10.1128/jb.177.5.1357-1366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsolis R M, Adams L G, Ficht T A, Bäumler A J. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect Immun. 1999;67:4879–4885. doi: 10.1128/iai.67.9.4879-4885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsolis R M, Kingsley R A, Townsend S M, Ficht T A, Adams L G, Bäumler A J. Of mice, calves, and man. Comparison of the mouse typhoid model with other Salmonella infections. Adv Exp Med Biol. 1999;473:261–274. [PubMed] [Google Scholar]
- 34.Van Der Velden A W M, Lindgren S W, Worley M J, Heffron F. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype Typhimurium. Infect Immun. 2000;68:5702–5709. doi: 10.1128/iai.68.10.5702-5709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson P R, Gautier A V, Paulin S M, Bland A P, Jones P W, Wallis T S. Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect Immun. 2000;68:3744–3747. doi: 10.1128/iai.68.6.3744-3747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yrlid U, Wick M J. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J Exp Med. 2000;191:613–623. doi: 10.1084/jem.191.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zychlinsky A, Thirumalai K, Arondel J, Cantey J R, Aliprantis A O, Sansonetti P J. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]