Abstract
Anthocyanins, the red-orange to blue-violet colorants present in fruits, vegetables, and tubers, have antidiabetic properties expressed via modulating energy metabolism, inflammation, and gut microbiota. Acylation of the glycosyl moieties of anthocyanins alters the physicochemical properties of anthocyanins and improves their stability. Thus, acylated anthocyanins with probiotic-like property and lower bioavailability are likely to have different biological effects from nonacylated anthocyanins on diabetes. This work highlights recent findings on the antidiabetic effects of acylated anthocyanins from the perspectives of energy metabolism, inflammation, and gut microbiota compared to the nonacylated anthocyanins and particularly emphasizes the cellular and molecular mechanisms associated with the beneficial effects of these bioactive molecules, providing a new perspective to explore the different biological effects induced by structurally different anthocyanins. Acylated anthocyanins may have greater modulating effects on energy metabolism, inflammation, and gut microbiota in type 2 diabetes compared to nonacylated anthocyanins.
Keywords: acylated anthocyanins, diabetes, energy metabolism, gut microbiota, inflammation
1. Introduction
Type 2 diabetes (T2D) is a disorder characterized by chronic hyperglycemia that results from impairments in insulin resistance and/or secretion. In T2D, impaired insulin function increases plasma glucose, nonesterified fatty acids, and branched-chain amino acids, which causes the defected energy metabolism and shifts energy metabolism from carbohydrate catabolism to fatty acid oxidation due to impaired insulin-stimulated glucose disposal.1 Insulin resistance is often accompanied by low-grade and chronic inflammation.2 In T2D, high-calorie intake, such as a high-fat diet, can trigger dysbiosis of gut microbiota and damage in the gut barrier, and subsequently induced endotoxemia, further aggravating insulin resistance and inflammation.3 Besides genetic predisposition, also an unhealthy lifestyle can contribute to the development of T2D.4 Healthy food can be a choice for the prevention and management of T2D.4
Anthocyanins, as a class of polyphenols giving red-orange to blue-violet colors in plants, have antioxidant and anti-inflammatory properties and can also positively affect energy homeostasis and gut health.5 These properties originate from radical scavenging properties, inhibition of carbohydrate digestion enzymes, and complex anthocyanin-gut microbiota interactions.5
Acylation of the sugar moiety of anthocyanins alters their physicochemical properties. Compared to nonacylated anthocyanins, acylated anthocyanins are more stable. For example, acylated anthocyanins have shown more resistance to changes in heat, pH, and light, stronger potential to inhibit lipid peroxidation, and higher antioxidant activity.6 Berries are a major dietary source of nonacylated anthocyanins,7 while acylated anthocyanins are naturally present in dark-colored vegetables and tubers. Earlier reviews have concluded that anthocyanins modulate energy homeostasis, inflammation, and gut microbiota in T2D.4,8−15 However, these reviews have only focused on the nonacylated anthocyanins. Although there are also reviews comparing the postprandial carbohydrate metabolism between acylated and nonacylated anthocyanins,16 there is limited information on how structurally different anthocyanins vary in their antidiabetic effects. This review summarizes the effects of acylated anthocyanins on energy homeostasis, inflammation, and gut microbiota in T2D and discusses the differences in the antidiabetic metabolism between acylated and nonacylated anthocyanins.
2. Nonacylated vs Acylated Anthocyanins: Structure, Stability, and Bioavailability
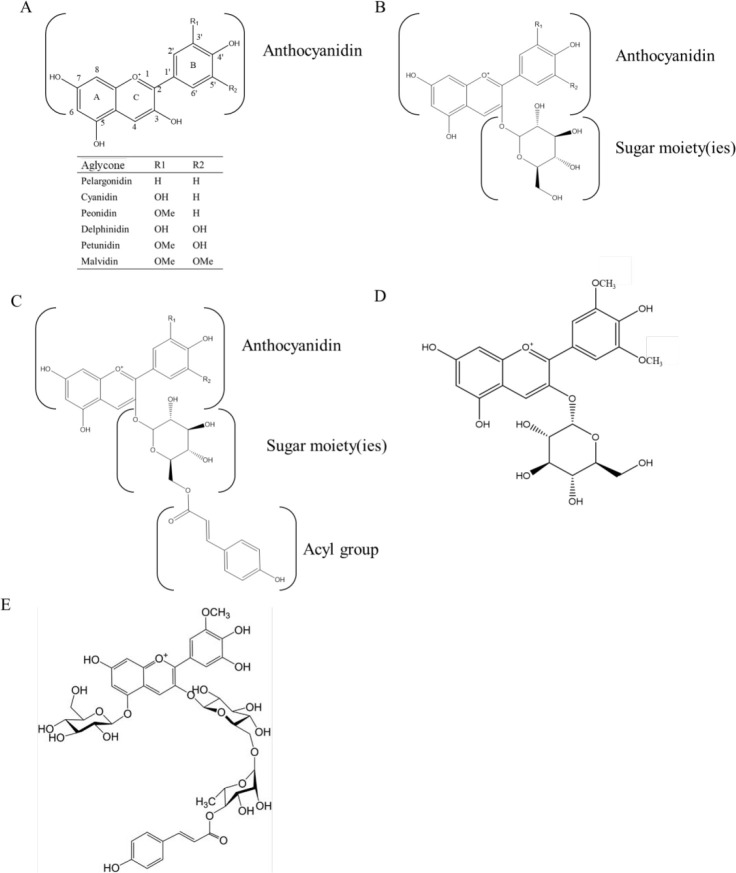
Hundreds of different anthocyanins are known to occur naturally. Their structural diversity arises from the number and the position of the sugar moieties in the aglycones and acyl groups in the sugar moieties and the hydroxylation of aglycones. Cyanidin, petunidin, pelargonidin, delphinidin, peonidin, and malvidin are the six most common aglycones (anthocyanidins) (Figure 1A).
Figure 1.
Structures of common anthocyanins. An example of nonacylated and acylated anthocyanins. Glucose is attached to the C3 position of anthocyanidin (A) to form nonacylated anthocyanin (B). Based on nonacylated anthocyanin, p-coumaric acid is acylated with a glucose residue at the C6 position to form acylated anthocyanin (C). Malvidin-3-O-glucoside (D). Petunidin-3-O-[6-O-(4-O-E-p-coumaroyl-O-α-l-rhamnopyranosyl)-β-d-glucopyranoside]-5-O-β-d-glucopyranoside (E), adapted with permission from ref (105). Copyright 2003 Elsevier.
The sugar moieties attached to aglycones of anthocyanin can be acylated with organic acids by plant enzymes (Figure 1B,C). Examples of specific nonacylated anthocyanins in bilberry (Vaccinium myrtillus) (malvidin-3-O-glucoside) and acylated anthocyanins (petunidin-3-O-[6-O-(4-O-E-p-coumaroyl-O-α-l-rhamnopyranosyl)-β-d-glucopyranoside]-5-O-β-d-glucopyranoside) in purple potato are shown in Figure 1D,E, respectively. Common acyl groups include hydroxycinnamic acids and aliphatic acids. Multiple acyl groups can be found in the same molecules, such as in poly acylated anthocyanins. Acylated anthocyanins are abundant in purple-fleshed potato (Solanum tuberosum), purple sweet potato (Ipomoea batatas), red radish (Raphanus sativus), purple carrot (Daucus carota), and red cabbage (Brassica oleracea) as reviewed elsewhere.16
Acylation of plant secondary metabolites plays an important role in their physicochemical properties and biological activity.17 In plants, anthocyanin acyltransferases (ACT) are responsible for the acylation process of anthocyanins.17 The two main types of ACTs have been classified based on the acyl group donors: acyl-activated sugar and acyl-CoA as acyl group donors.17
The polarity of anthocyanins is decreased by glycosyl acylation, and the vulnerability of the flavylium cation to a nucleophilic attack by water is also decreased by stacking the acyl groups with the pyrylium ring.16 The hydrophilic initiation of the interaction between anthocyanins and their membrane carriers (bilitranslocase) during absorption is inhibited by the steric hindrance effect of acylated anthocyanins.18
The conjugated carbon–carbon double bonds in aromatic acyl groups can donate electrons and absorb light energy, which contribute to the stability of anthocyanins under light irradiation.19 Acylation of anthocyanin can substantially improve the resistance of acylated anthocyanins to a variety of physicochemical and biochemical factors (e.g., pH, heat, light, oxidation, and gastrointestinal digestion).20 Anthocyanins in colored plant organs can be used as alternatives for synthetic colorants, in which acylated anthocyanins primarily contribute the stable colorations.21 The acylated anthocyanins from red cabbage have shown more stability to heating at 80 °C and changes in pH than the nonacylated anthocyanins in grape skin, black currant, and elderberry extracts.22 Acylated anthocyanins have shown to be more stable under direct exposure to sunlight than the nonacylated anthocyanins in fruit juices,23 and the acylated anthocyanins synthesized by lipase-catalyzed transesterification have also shown to be more stable under illumination with white fluorescent light compared to the corresponding nonacylated glucosides.24 A recent study has shown that enzymatic acylation of delphinidin-3-O-glucoside, delphinidin-3-O-rutinoside, cyanidin-3-O-glucoside, and cyanidin-3-O-rutinoside with lauric acid (12:0) significantly improved the anthocyanins’ thermostability, antioxidant activity, and capacity to inhibit lipid peroxidation.25 Acylated anthocyanins from purple yam (Dioscorea alata) have exhibited a higher level of antioxidant activity than the corresponding nonacylated anthocyanins from the same source, for example, acylated anthocyanin cyanidin-3-O-(6-O-(6-O-(E)-sinapoyl-β-d-glucopyranosyl)-β-d-glucopyranosyl)-7-O-(6-O-(E)-sinapoyl-β-d-glucopyranosyl)-2′-O-(β-d-glucopyranosyl) has shown five times higher antioxidant activity compared to nonacylated anthocyanin cyanidin-3-O-(6-O-β-d-glucopyranosyl)-β-d-glucopyranosyl) in vitro.6
When discussing the biological impacts of anthocyanins, it is important to address their bioavailability.16 Dietary anthocyanins are metabolized by phase I and phase II enzymes to produce phenolic acids and their conjugates after being absorbed into enterocytes as intact glycosides or aglycones.26 Up to 65% of dietary anthocyanins are not absorbed in the stomach and upper intestine,1 so they pass through to the colon and are degraded extensively by gut microorganisms. This process shapes the gut microbiota profile and gut metabolic profile.27
As systematically reviewed by Jokioja et al.,16 acylated anthocyanins have lower transport efficiency, higher resistance to digestion, and higher susceptibility to fermentation by gut microbiota than nonacylated anthocyanins. In a bioavailability study, acylated anthocyanins from purple-fleshed sweet potato have shown lower bioavailability compared to nonacylated anthocyanins from red wine under simulated digestion conditions in vitro, with a degradation percentage of about 30% and 45% at the intestinal level, respectively.28 A clinical bioavailability study has shown an 11–14-fold lower recovery of acylated anthocyanins in urine and an 8–14-fold lower concentration in plasma compared to their nonacylated forms, using purple carrots as the source of nonacylated and acylated anthocyanins.29 A similar finding of acylated anthocyanins having lower transport efficiency than nonacylated anthocyanins has also been observed in a transepithelial transport experiment based on the intestinal Caco-2 cell line model.30 In addition, a recent research study has shown that the absorption efficiency (in gastric epithelial cells) of two acylated anthocyanins (peonidin-3-(6′-hydroxybenzoyl)-sophoroside-5-glucoside and peonidin-3-(6′-hydroxybenzoyl-6″-caffeoyl)-sophoroside-5-glucoside) purified from purple sweet potato was 20–30% lower than nonacylated malvidin-3-O-glucoside.31
Lower absorption efficiency of acylated and nonacylated anthocyanins has been seen when the glucose transporters GLUT1 and GLUT3 were blocked, suggesting that GLUT1 and GLUT3 were involved in anthocyanin absorption.31 A computational study comparing the affinity of acylated anthocyanin malvidin 3-O-(6-O-coumaroyl)-glucoside-5-O-glucoside with nonacylated anthocyanin malvidin 3,5-O-diglucoside to GLUT1 and GLUT3 showed acylated anthocyanin had stronger affinity to GLUT3 and the nonacylated anthocyanin had stronger affinity to GLUT1.32
Acylated anthocyanins may be more readily degraded by the gut microbiota in the gut. The fecal total anthocyanin content has been shown to increase by more than 10-fold after antibiotic treatment to knock down the gut microbiota in obese rats fed with a Concord grape supplement rich in acylated anthocyanins for 8 weeks.33 This change was greater than that observed in obese rats fed with an equivalent dose of nonacylated anthocyanins in berries.33 Acylated anthocyanin cyanidin-3-(6′′-malonyl)-glucoside is likely to be more readily available for degradation by gut microbiota than its nonacylated form since the ratio between these two anthocyanins in the cecal contents in rats has been observed to shift to favor the nonacylated anthocyanin when compared to the ratio in the original food, red orange juice.34
3. Nonacylated vs Acylated Anthocyanins: Effects on Type 2 Diabetes
The antidiabetic properties of anthocyanins in nonacylated form have been reviewed extensively.4,8,9,11,12,15,27 The effects on energy metabolism, inflammation, and gut microbiota include (1) inhibition of digestive enzymes; (2) modulation of adenosine monophosphate-activated protein kinase (AMPK) activation and AMPK-mediated GLUT4 expression and translocation; (3) suppression of peroxisome proliferator-activated receptor gamma (PPARγ) and activation of phosphoinositide 3 kinase/protein kinase B (PI3K/AKT)-mediated energy metabolism; (4) suppression of nuclear factor κB (NF-κB) activation and downstream of inflammatory cytokines expression such as interleukins IL-1β and IL-6; and (5) activation of nuclear factor erythroid 2-related factor 2 (Nrf2). Recent studies on the antidiabetic effects of acylated anthocyanins and nonacylated anthocyanins are summarized in Tables 1 and 2 and reviewed in detail in the subsequent sections.
Table 1. An Overview of Studies Involved in Antidiabetic Effects of Nonacylated Anthocyaninsa,b.
| Source of anthocyanins | Main anthocyanin(s) | Model | Effects |
|---|---|---|---|
| Pure nonacylated anthocyanins106,107 | 18–20 varieties of nonacylated anthocyanins | Enzyme inhibition study | Inhibited α-amylase and α-glucosidase. Structure-dependent enzymes inhibition property was observed |
| Purified nonacylated anthocyanin87 | Cya-3-glc | 3T3-L1 adipocytes and human omental adipocytes | Enhanced glucose transport, GLUT4 membrane translocation, and insulin sensitivity |
| Purified anthocyanin59 | Cya-3-glc | KKAy diabetic mice; Diet containing 100 mg/kg; 12 weeks | Ameliorated hepatic steatosis by decreasing hepatic mtGPAT1 activity. Anthocyanin inhibited high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through protein kinase C |
| Purified anthocyanin108 | Cya-3-glc | db/db diabetic mice; Diet containing 100 mg/kg; 8 weeks | Increased the GSH synthesis through protein kinase A/cAMP-response element binding protein-dependent induction of Gclc expression |
| Attenuated lipid peroxidation, neutrophil infiltration, and hepatic steatosis | |||
| Purified anthocyanins from Cornus fruits (C. officinalis and C. mas)109 | Cya-3-glc, del-3-glc, cya-3-gal, and pg-3-gal, and anthocyanidins | Rodent pancreatic β-cells (INS-1832/13) | Enhanced insulin secretion and prevent insulin resistance |
| Anthocyanins extract from mulberry (Morus alba)44 | Cya-3-glc, cya-3-rut, pg-3-glc | db/db mice; 50 and 125 mg/kg body weight; 8 weeks | Modulated AKT, GSK3β and FOXO1 in liver, muscle, and adipose tissues. Decreased triglycerides, LDL, insulin, blood glucose, leptin, β cell protection. Reversed insulin resistance by regulating AMPK/ACC/mTOR pathway |
| Anthocyanin extract from mulberry (Morus alba) fruits110 | Que-3-glc and other phenolic acids | HFD- induced obese Syrian golden hamster; Diet containing 2% (w/w); 12 weeks | Reduced serum triacylglycerol, cholesterol, free fatty acid, and the LDL/HDL ratio |
| Decreased hepatic lipids and hepatic PPAR-γ, fatty acid synthase, and HMG-CoA reductase | |||
| Anthocyanins extract from mulberry (Morus alba)52 | Cya-3-glc, cya-3-rut | db/db diabetic mice; diet containing 0.5%, w/w; 6 weeks | Decreased blood levels of glucose and HbA1c |
| Increased insulin sensitivity | |||
| Activated pAMPK and p-AKT substrate of 160 kDa (pAS160) and enhanced GLUT4 in skeletal muscles | |||
| Increased pAMPK and decreased the levels of G6pase and PEPCK in the liver | |||
| Anthocyanins extract from mulberry (Morus alba)111 | Cya-3-glc, cya-3-rut, pg-3-glc, pg-3-rut | Zucker diabetic fatty rats; 125 and 250 mg/kg body weight; 5 weeks | Decreased glucose level |
| Maintained insulin level and β cell histology | |||
| Anthocyanin extract from mulberry (Morus Australis Poir)112 | Cya-3-glc, cya-3-rut, pg-3-glc | HFD-induced obese C57b1/6J mice; Diet containing 4% (w/w); 90 days | Decreased body weight, food intake, cholesterol, triglycerides, glucose, and leptin |
| Anthocyanin extrat from bilberry (Vaccinium myrtillus)113 | Del-, cya-, pet-, peo-, and mal- derived nonacylated anthocyanin | High-sucrose diet induced insulin-resistant mice; 0.2 mg/mL anthocyanin in drink water;15 weeks | Showed antioxidant property and changed genes expression in metabolic pathway (ACC1, Bcl2, Akt, mTOR, GPDH1, HK2, GLUT1, and GLUT4) |
| Commercial bilberry (Vaccinium myrtillus) anthocyanins extract capsule114 | Del-3-gal, del-3-glc, del-3-ara, cya-3-gal, cya-3-glc | 16 overweight volunteers; 0.47 g bilberry extract (36% (w/w) anthocyanins) for one dose | Improved oral glucose tolerance but not for plasma insulin level and anti-inflammatory markers |
| Commercial bilberry anthocyanin extract (Vaccinium myrtillus)49 | Not available | KK-Ay diabetic mice; Diet containing 2.7% (w/w); 5 weeks | Decreased blood glucose and triglycerides and cholesterol. Improved insulin sensitivity |
| Inactivated acetyl-CoA carboxylase and activated PPARa, acyl-CoA oxidase, and carnitine palmitoyltransferase-1A in liver | |||
| Decreased PEPCK and G6pase in liver | |||
| Activated AMPK in white adipose tissue, skeletal muscle, and the liver | |||
| Upregulated GLUT4 in white adipose tissue and skeletal muscle | |||
| Freeze-dried highbush blueberries (50/50 blend of Vaccinium virgatum and V. corymbosum)115 | Not available | 52 men with T2D, 22 g freeze-dried blueberry twice a day for 8 weeks | Lowered hemoglobin A1c, triglycerides, AST, and ALT |
| Fasting plasma glucose, serum insulin, total cholesterol, LDL, HDL, C-reactive protein concentrations, blood pressure, and body weight were not changed | |||
| Anthocyanins powder from two blueberries (Tifblue and Rubel cultivars, 1:1)116 | Del-3-gal, peo-3-glc, mal-3-gal, cya-3-gal, pet-3-gal | Zucker diabetic fatty rats; Diet containing 2% (w/w); 90 days | Reduced triglycerides, fasting insulin. Improved insulin sensitivity |
| Reduced abdominal fat mass, increased adipose and skeletal muscle PPAR activity, and affected PPAR transcripts involved in fat oxidation and glucose uptake/oxidation (Fas, Irs1, Pfk, Glut4) | |||
| Anthocyanins extract from honeyberry (Lonicera caerulea)51 | Nonacylated anthocyanin dominant by cya-3-glc | HFD-induced obese ICR mice; diet containing 0.5%-1%, w/w; 6 weeks | Decreased the expressions of adipogenic genes (SREBP-1c, C/EBPα, PPARγ, FAS) in liver |
| Increased mRNA and protein levels of CPT-1 and PPARα and ncreased the phosphorylation of AMPK and ACC in liver | |||
| Anthocyanin extract from purple corn (Zea mays)46 | Nonacylated anthocyanins dominant by cya-3-glc | db/db diabetic mice; 10 or 50 mg/kg body weight; 8 weeks | Increased C-peptide and adiponectin and decreased HbA1c and glucose levels in plasma |
| Prevented pancreatic β-cell damage and increased insulin content | |||
| Increased the phosphorylation of AMPK and decreased PEPCK, G6pase genes in liver, and increased GLUT4 expression in skeletal muscle | |||
| Anthocyanin extracts from 20 purple maize genotypes117 | Cya-3-glc, pg-3-glc, peo-3-glc and corresponding acylated forms | 3T3-L1 adipocytes | Regulated TNF-α, IL-6 and MCP-1 and improved insulin sensitivity |
| Anthocyanin extract from purple rice (Oryza sativa)71 | Cya-3-glc | STZ-induced diabetes SD rat; 250 mg/kg body weight; 4 weeks | Decreased blood glucose and gene expression of COX-2 and IL-6 inflammatory marker in heart |
| Decreased TLR4 protein and p65-NF-κB levels in heart | |||
| Activated p-Ikkα/βin heart |
Note: Anthocyanidins: cya, cyanidin; del, delphinidin; mv, malvidin; pg, pelargonidin; peo, peonidin; pet, petunidin. Sugar moieties: glc, glucopyranoside; gal, galactoside; rut, rutinoside; sam, sambubioside.
Abbreviations: ACC, acetyl-CoA carboxylase; AKT, protein kinase B; AMPK, AMP-activating protein kinase; CPT, carnitine palmitoyltransferase; C/EBPα, CCAAT enhancer binding protein α; FAS, fatty acid synthase; FOXO1, forkhead box protein O1; Gclc, glutamate–cysteine ligase catalytic subunit; GSK3, Glycogen synthase kinase-3; GLUT, glucose transporter; G6pase, Glucose 6-phosphatase; HbA1c, hemoglobin A1c; HFD, high-fat diet; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; IL, interleukin; IRS-1, Insulin receptor substrate 1; mtGPAT1, mitochondria glycerol-3-phosphate acyltransferase 1; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; SREBP-2, sterol regulatory element-binding protein 2; TNF, tumor necrosis factor; PEPCK, Phosphoenolpyruvate carboxykinase; PPARγ, peroxisome proliferator-activated receptor γ; VCAM-1, vascular cell adhesion protein 1.
Table 2. An Overview of Studies Involved in Antidiabetic Effect of Acylated Anthocyanins and Comparison of Potential Antidiabetic Effects between Nonacylated and Acylated Anthocyaninsa,b.
| Source of anthocyanins | Main anthocyanin(s) | Model | Effects |
|---|---|---|---|
| The potential antidiabetic effects of acylated anthocyanins | |||
| Acylated anthocyanins extract from purple sweet potato (Ipomoea batatas)79 | Cya-3-(6″-caf-6′′′-hba-sop)-5-glc, peo-3-(6″,6′′′-dicaf-sop)-5-glc, peo-3-(6″-caf-6′′′-p-hba-sop)-5-glc, peo-3-(6″-caf-6′′′-fer-sop)-5-glc | Enzyme inhibition study; human liver cell line HepG2 cells | Inhibited α-amylase, α-glucosidase, and xanthine oxidase; phenolic compound but not anthocyanin faction induced the transcription factor Nrf2 and Nrf2 target gene Gclc |
| Acylated anthocyanins extract from purple sweet potato (Ipomoea batatas)118 | Not available | 3T3-L1 adipocytes | Suppressed leptin secretion. Exerted anti-inflammatory, lipolytic effects on adipocytes and radical scavenging and reducing activity |
| Diacylated anthocyanin extracts from purple sweet potatoes (Ipomoea batatas L. cultivar Eshu No. 8)39 | Peo-3-(6′-caf-6′′-hba-sop)-5-glc, peo-3-(6′-caffeoyl-6′′-fer-sop)-5-glc | SD rats. 80 and 160 mg/kg. One dose | Decreased postprandial blood glucose |
| Purple sweet potato (cultivar ‘NingZi No. 2’) anthocyanin extract119 | Peo-3-caf-sop-5-glc, peo-3- (6′′,6′′′-dicaf-sop)-5-glc, peo-3-caf-hba-sop-5-glc, peo-3-(6′′-caf-6′′′-fer-sop)-5-glc, cya3-(6′′-caf-6′′′-fersop)-5-glc | HFD-indued obese SD rat; 100–400 mg/kg bodyweight; 6 weeks | Decreased adipocyte number and size of adipose tissue |
| Decreased glucose, triglyceride, and total cholesterol levels | |||
| Reduced the level of ROS and inhibited the receptor of AGE products and thioredoxin interacting protein in the hypothalamus | |||
| Preserved the leptin signaling capability, decreased in hypothalamic AMPK activity | |||
| Anthocyanin extract from purple sweet potato (Ipomoea batatas)74 | Peo-3-(6-caf-glc-β-glc)-5-glc, peo-3 -(2-(6-caf-glc)-6-caf-glc)-5-glc, peo-3-(2-(6-fer-glc)-6-caf-glcp)-5-glc, cya-3-(6-cou)-glc | HFD-induced obese C57BL/6 mice; 700 mg/kg/day; 20 weeks | Ameliorated obesity and liver injuries. Blocked hepatic oxidative stress. Restored NAD+ level in liver |
| Suppressed the NF-κBp65 nuclear translocation, NOD1/2 signaling, the NLRP3 inflammasome activation and inflammation-related genes (TNF-α, MCP-1, and IL-1) in liver | |||
| Anthocyanin extract from purple sweet potato (Ipomoea batatas)120 | Peo-3-(6-caf-glc-β-glc)-5-glc, peo-3 -(2-(6-caf-glc)-6-caf-glc)-5-glc, peo-3-(2-(6-fer-glc)-6-caf-glc)-5-glc, cya-3-(6-cou)-glc | HFD-induced obese C57BL/6 mice; 500 mg/kg/day; 32 weeks | Alleviated the cognitive impairment |
| Decreased the expression of Iba1, TNF-α, IL-1β, SOCS3, galectin-3 in hippocampus | |||
| Increased insulin signaling molecules including the p-IRS1 (Tyr608), PI3K p110α and p-AKT (Ser473) | |||
| Increased Bcl-2 expression and diminished the Bak and the cleaved-caspase 3 expressions in hippocampus | |||
| Anthocyanin extract from purple sweet potato (Ipomoea batatas)50 | Peo-3-(6-caf-glc-β-glc)-5-glc, peo-3 -(2-(6-caf-glc-6-caf-glc)-5-glc, peo-3-(2-(6-fer-glc)-6-caf-glc)-5-glc, cya-3-(6-cou)-glc | HFD-induced obese ICR mice; 200 mg/kg per day;4 weeks | Reduced weight gain and hepatic triglyceride accumulation and improved serum lipid parameters |
| Increased the phosphorylation of AMPK and ACC in the liver | |||
| Anthocyanin extract from purple sweet potato (Ipomoea batatas)45 | Cya- and peo-derived acylated anthocyanins | HFD-induced obese ICR mice; 700 mg/kg/day; 20 weeks | Improved the fasting blood glucose level, glucose, insulin tolerance and oxidative-stress-mediated endoplasmic reticulum stress |
| Suppressed ROS production and GSH and antioxidant enzymes’ activities. Suppressed the JNK1and Ikb kinase β activation and NF-κB p65 nuclear translocation | |||
| Restored the impairment of the insulin receptor substrate-1/phosphoinositide 3 kinase/protein kinase B (AKT) insulin signaling in the livers | |||
| Anthocyanin extract from purple sweet potato (Ipomoea batatas)48 | Cya-3-sop-5-glc, peo-3-sop-5-glc, cya-3-hba-sop-5-glc, peo-3-hba-sop-5-glc, cya-3-(6″-fer- sop)-5-glc, peo-3-(6″-fer-sop)-5-glc, cya-3-caff-hba-sop-5-glc, cya-3-(6″-caff-sop)-5-glc, cya-3-(6″-caf-6″′-fer-sop)-5-glc, peo-3-caf-hba-sop-5-glc, peo-3-caf-sop-5-glc, peo-3-(6″-caf-6″′-fer-sop)-5-glc, Peo-3-(6″-hba-6″′-fer-sop)-5-glc | HFD and streptozotocin- induced obese ICR mice; 500 mg/kg body weight; 12 weeks | Activated AMPK in liver |
| Increased GLUT4, glucokinase, and insulin receptor α in liver | |||
| Upregulated glycolysis key genes (Pfk and Pkm) | |||
| Downregulated gluconeogenic genes (G6pase and Pepck). | |||
| Anthocyanin extract from Blue Congo potatoes121 | Acylated anthocyanins dominant by pet-3-cou-rut-5-glc | Streptozotocin- induced diabetic Wistar rats; 165 mg/kg bodyweight; 2 weeks | Lowered blood glucose, glycated hemoglobin, malondialdehyde levels in plasma |
| Restored antioxidant enzyme activities | |||
| Improved glucose tolerance | |||
| Inhibited oxidative modified proteins OMP, AGE, and advanced oxidation protein products formation in plasma | |||
| Acylated anthocyanin extract from purple potato (Solanum tuberosum L. var. “Synkeä Sakari”)122 | Acylated anthocyanins dominated by pet-cou-rut-glc and peo-cou-rut-glc | 17 healthy volunteers; postprandial study; a meal containing acylated anthocyanins (152 mg) | Acylated anthocyanin extract alleviates postprandial glycemia and insulinemia and affects postprandial inflammation |
| Anthocyanin extract from black goji berry (Lycium ruthenicum)75 | Pet-3-rut-cou-5-glc as the main anthocyanin | HFD and vitamin-D3- induced atherosclerosis rat, 50–200 mg/kg body weight; 8 weeks | Decreased total glyceride, total cholesterol, low density lipoprotein, TNF-α, IL-6 levels, and atherogenic index and increased HDL-C concentrations |
| Upregulated NF-κB, VCAM-1, and CYP7A1, and downregulated SREBP-2 | |||
| Anthocyanin extract from black goji berry (Lycium ruthenicum)53 | Del-3–6″-rha-glc-5-glc, pet-3-rut-cou-5-glc, pet-3-rut-caf-5-glc, pet-3–6-cou-rha-pyr)-glc-5-glc | HFD-induced insulin resistance C57BL/6J mice; 50–200 mg/kg body weight; 12 weeks | Decreased the weight gain, hepatic lipid, dyslipidemia, inflammation, and oxidative stress |
| Inactivated TLR4/NF-κB/JNK in the liver tissues and ameliorated oxidative stress and insulin resistance by activating the Nrf2/HO-1/NQO1 and IRS-1/AKT pathways | |||
| Comparison of potential antidiabetic effects between nonacylated and acylated anthocyanins | |||
| Pure nonacylated anthocyanins and diacylated anthocyanins38 | Cya-3-(2-(6-fer-glc)-6-caf-glc)-5-glc, cya and peo-3-(2-(6-fer-glc)-6-caf-glc)-5-glc, pg-3-(2-(6–3-glc-caf)-glc)-6-caf-glc)-5-glc, pg-3-(2-(6-caf-glc)-6-caf-glc)-5-glc, pg, cya, and peo-3-(2-glc-glc)-5-glc-3-sop-5-glc | Enzyme inhibition study | Diacylated anthocyanin showed the best ability to inhibit α-glucosidase |
| Nonacylated anthocyanin extracts from (V. corymbosum L. × V. angustifolium Aiton.; V. ashei Reade) berries; Monoacylated and diacylated extracts from purple sweet potatoes (Ipomoea batatas cultivar Eshu No. 8)39 | Del-, cya-, pet-, peo-, and mal-3-gal, glc, ara; cy-, pet-, and peo-3-hba-sop-5-glc; cy and peo-3-(6′′-caf-sop)-5-glc; cya and peo-fer-sop-5-glc; cya-3-caf-sop-5-glc; peo-3-(6′-caf-6′′-hba-sop)-5-glc; peo-3-(6′-caffeoyl-6′′-fer-sop)-5-glc | Enzyme inhibition study | Diacylated anthocyanin extracts showed the highest inhibition ability of α-amylase and α-glucosidase than monoacylated anthocyanin extracts and deacylated anthocyanin extract |
| Acylated anthocyanin extract from purple carrot and pure cya-3-glc and del-3-rut54 | Cya-3-(2″-xyl-6-glc-gal), cya-3-(2′′-xyl-gal), cya-3-(2′′-xylose-6′′-sin-glc-gal, cya-3-(2′′-xyl-6′′-fer-glc-gal, cya-3-(2′′-xyl-6′′(4-cou)-glc-gal | Wistar rats; Single intragastric doses | Acylated anthocyanin induced highest level of AKT phosphorylation in aorta than cya-3-glc and del-3-rut |
| Nonacylated anthocyanin extract from bilberry (Vaccinium myrtillus) and acylated anthocyanin extract from purple potato (Solanum tuberosum var. “Synkeä Sakari”)58 | Nonacylated anthocyanins dominated by del-3-gal, del-3-glc, cya-3-gal, del-3-ara, cya-3-glc; | Zucker diabetic fatty rats; daily doses of 25–50 mg/kg body weight; 8 weeks | Both anthocyanin extracts decreased the levels of plasma glucose, branched-chain amino acids, and improved lipid profiles. Acylated anthocyanin extract increased the glutamine/glutamate ratio and decreased the levels of glycerol and metabolites involved in glycolysis. Acylated anthocyanin extract decreased the hepatic TBC1D1 and G6PC mRNA levels |
| Acylated anthocyanins dominated by pet-cou-rut-glc and peo-cou-rut-glc | |||
| Nonacylated anthocyanin extract from bilberry (Vaccinium myrtillus) and acylated anthocyanin extract from purple potato (Solanum tuberosum var. “Synkeä Sakari”)57 | Nonacylated anthocyanins dominated by del-3-gal, del-3-glc, cya-3-gal, del-3-ara, cya-3-glc; | Zucker diabetic fatty rats; daily doses of 25–50 mg/kg body weight; 8 weeks | Both anthocyanin extracts restored the levels of metabolites (glucose, lactate, alanine, and pyruvate) and expression of genes (G6pac, Pck1, Pklr, and Gck) involved in glycolysis and gluconeogenesis. Acylated anthocyanin extract decreased the hepatic glutamine level. Nonacylated anthocyanin extract regulated the expression of Mgat4a, Gstm6, and Lpl, whereas acylated anthocyanin extract modified the expression of Mgat4a, Jun, Fos, and Egr1 |
| Acylated anthocyanins dominated by pet-cou-rut-glc and peo-cou-rut-glc | |||
Note: Anthocyanidins: cya, cyanidin; del, delphinidin; mv, malvidin; pg, pelargonidin; peo, peonidin; pet, petunidin. Acyl moieties: ace, acetyl; caf, caffeoyl; cou, coumaroyl; hba, hydroxybenzoyl; mal, malonyl; oxa, oxaloyl; sin, sinapoyl; suc, succinyl; pyr, pyranosyl. Sugar moieties: glc, glucopyranoside; gal, galactoside; rut, rutinoside; sop, sophoroside; xyl, xyloside.
Abbreviations: ACC, acetyl-CoA carboxylase; AKT, protein kinase B; AMPK, AMP-activating protein kinase; Bcl-2, B-cell lymphoma 2; CPT, carnitine palmitoyltransferase; CYP7A1, cytochrome P450 family 7 subfamily A member 1; C/EBPα, CCAAT enhancer binding protein α; FAS, fatty acid synthase; FOXO1, forkhead box protein O1; Gclc, glutamate–cysteine ligase catalytic subunit; GSK3, Glycogen synthase kinase-3; GLUT, glucose transporter; G6pase, Glucose 6-phosphatase; HbA1c, hemoglobin A1c; HFD, high-fat diet; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; HO-1, Heme oxygenase 1; IL, interleukin; IRS-1, Insulin receptor substrate 1; mtGPAT1, mitochondria glycerol-3-phosphate acyltransferase 1; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; Nrf2, nuclear factor-erythroid factor 2-related factor 2; NOD, nucleotide-binding oligomerization domain; NQO1, NAD(P)H quinone dehydrogenase 1; SOCS3, suppressor of cytokine signaling 3; SREBP-2, sterol regulatory element-binding protein 2; TNF, tumor necrosis factor; PEPCK, Phosphoenolpyruvate carboxykinase; PPARγ, peroxisome proliferator-activated receptor γ; VCAM-1, vascular cell adhesion protein 1.
3.1. Inhibition of Carbohydrate Digestion Enzymes
Anthocyanins from plants are known to inhibit α-glucosidase and α-amylase, the key enzymes responsible for the digestion of dietary carbohydrates to glucose.16 Recent developments in structure-based design and computational techniques have shown that, out of 665 monomeric anthocyanins (nonacylated anthocyanins), pelargonidin-3-O-rutinoside and malvidin-3-O-arabinoside have the lowest binding energies with human pancreatic amylase and to interact with its amino acid residues (Asp197 and Glu233) through hydrogen bonds, thereby limiting its catalytic mechanism.35 Also, acylated anthocyanin extracts of purple potatoes and purple carrots have been reported to decrease the intestinal glucose uptake in a gastrointestinal model.36
A recent metabolomic study has shown cecal sugar levels (glucose, arabinose, galactose, xylose) were higher in rats fed with acylated anthocyanin extract (from purple potato, S. tuberosum var. “Synkeä Sakari”) and nonacylated anthocyanin extracts (from bilberry), with the acylated anthocyanin extract fed group showing the highest levels of those sugars, and the inhibition of digestive enzymes by anthocyanins might contribute to this result.37 Acylated anthocyanins have been observed to have more potent inhibitory effects on α-glucosidase and α-amylase than nonacylated ones.38,39 An in vitro study comparing the inhibitory effect of nonacylated anthocyanins fraction (547.94 ± 0.88 mg/g) from berries, monoacylated anthocyanins fraction (322.64 ± 4.84 mg/g) from purple sweet potatoes, and diacylated anthocyanins fraction (541.00 ± 7.06 mg/g) from purple sweet potatoes against α-glucosidase and α-amylase has shown that diacylated anthocyanins had the most potent inhibitory effect on these two enzymes.39 The inhibitory activity of the diacylated anthocyanins fraction on α-glucosidase is comparable to that of acarbose, one of the antidiabetic medications to decrease the digestion and absorption of carbohydrates.39 Feeding this diacylated anthocyanins fraction to SD rats has significantly decreased blood glucose level by 20.5% after a standard meal administration containing starch.39 The most potent inhibitory activity of diacylated anthocyanins to α-glucosidase might be due to the higher affinity to certain key enzyme binding sites.39 Moreover, protein-anthocyanin binding can prevent acylated anthocyanins from degrading, increasing their antioxidant activity.40 The inhibitory effect of monoacylated anthocyanins to α-glucosidase was, however, not significantly different from nonacylated anthocyanins, possibly because of a lower concentration.39
3.2. Modulation of Energy Metabolism
The energy metabolism is regulated by a number of kinases systems and related pathways, such as AMPK and PI3K/AKT pathways, which are the primary effectors in response to metabolic stress and crucial to energy metabolism and have been considered as therapeutic targets in metabolic syndrome, especially diabetes.41 PI3K/AKT, which controls energy metabolism and cell differentiation, proliferation, motility, and survival, can be activated by both exogenous and endogenous stimuli such as environmental stresses, insulin, growth hormones, and cytokines.41,42 Adipocytokines and several physiological factors, such as oxidative stress, hypoxia, glucose deprivation, and muscle contraction, can activate AMPK pathway.41,43
AKT and AMPK have been reported to activated by both types of anthocyanins in T2D (Tables 1 and 2). The PI3K/AKT pathway is necessary for insulin-dependent regulation of glucose and lipid metabolisms by regulating gluconeogenesis (FOXO1, Forkhead box protein O1), glycogen synthesis (GSK3β, glycogen synthase kinase 3β), and glucose uptake (TBC1D4, TBC1 domain family member 4).41,42 The AMPK pathway also participates in glucose and lipid metabolisms such as inhibiting glycogen synthesis (GYS1, muscle glycogen synthase) and lipogenesis (ACC, acetyl-CoA carboxylase α), activating glucose uptake (AS160, a substrate for the protein kinase AKT that links insulin signaling and GLUT4 trafficking), and enhancing β-oxidation (MCD, malonyl-CoA decarboxylase,).41
Since the liver and muscle are the main energy expenditure organs, the energy metabolism-regulatory effects of the two types of anthocyanins on the liver and muscle are presented in this review. Similar to nonacylated anthocyanins, we found feeding acylated anthocyanin extracts for 12–32 weeks (at daily doses of 200–700 mg/kg body weight) to streptozotocin- or high-fat diet-induced diabetic animal models have shown hypoglycemic effects by modulating hepatic AKT44,45 and hepatic AMPK.46−49 Activation of ACC mediated by AMPK contributing to lipid synthesis and suppression of glucose 6-phosphatase (G6pase) and phosphoenolpyruvate carboxykinase (PEPCK) mediated by PI3K/AKT leading to gluconeogenesis were observed by the intervention of nonacylated anthocyanin extract from berries or acylated anthocyanin extract from purple sweet potatoes (Tables 1 and 2).46−49 Mice fed with a high-fat diet and purple sweet potato acylated anthocyanin extract for 4 weeks at a daily dose of 200 mg/kg body weight have shown a decreased serum glucose by ca. 30% and induced hepatic phosphorylation of AMPK and ACC compared to the obese mice.50 Similarly, feeding mice with high-fat diet containing nonacylated anthocyanin extract from honeyberry (Lonicera caerulea) predominant in cyanidin-3-O-glucoside has induced hepatic phosphorylation of ACC and AMPK.51 The db/db diabetic mice fed with mulberry (Morus alba) nonacylated anthocyanin extract for 2 weeks had decreased blood glucose levels by ca. 35% and induced hepatic activation of AKT as well as upregulated G6pase and PEPCK levels.52 A study found that intervention of purple sweet potato acylated anthocyanin extract fed to high-fat diet-induced obese mice for 5 weeks has activated IRS-1 (Insulin receptor substrate 1)/PI3K/AKT pathway and contributed to the hypoglycemic effect (ca. 30% decrease in blood glucose).45 Feeding acylated anthocyanin extract from purple sweet potato for 4 weeks at a daily dose of 500 mg/kg body weight to diabetic mice induced by high-fat diet and STZ induced the AKT pathway and increased hepatic levels of PEPCK and G6pase, which led to ca. 35% blood glucose decrease in the treatment group.48 Acylated anthocyanins from black goji berry (Lycium ruthenicum) upregulated hepatic activation of AKT and gene expression of GLUT4, G6pase, and PEPCK in obese mice, which reversed glucose levels in normal healthy mice.53
Compared to the liver, the regulating influence of anthocyanins on energy metabolism in muscles has received less attention. Feeding a diet containing 2.7% (w/w) nonacylated anthocyanin extract from bilberry to KK-Ay diabetic mice for 5 weeks decreased blood glucose levels ca. 30% and activated AMPK in skeletal muscle, the liver, and adipose tissue.49 Purple corn (Zea mays) anthocyanin extract containing both nonacylated and acylated anthocyanins fed to db/db diabetic mice at a daily dose of 10 or 50 mg/kg body weight for 8 weeks reversed the blood glucose to normal levels and increased muscle GLUT4 gene expressions.46 Mulberry nonacylated anthocyanin extract fed to db/db diabetic mice for 2 weeks has shown both activated AKT and AMPK and increased GLUT4 level in skeletal muscle.52
However, nonacylated and acylated anthocyanins might not always activate AMPK and AKT to the same extent or activate the same downstream effectors. A single oral dosage of an acylated anthocyanin extract from purple carrot and an equivalent dose of two nonacylated anthocyanins (delphinidin-3-O-rutinoside and cyanidin-3-O-glycoside, 1 mg/kg body weight) have been used to assess their capacities to phosphorylate AKT phosphorylation in the aorta.54 As compared to the other two nonacylated anthocyanins, acylated anthocyanin extract has exhibited a higher level of AKT phosphorylation.54 This result suggests acylated anthocyanins might play a better role in regulating AKT phosphorylation in T2D (Figure 2). A common downstream target of AKT and AMPK is TBC1D1 which regulates the translocation of GLUT4, lipogenesis, and insulin resistance in T2D.55,56 Feeding acylated anthocyanin extract from purple potato (var. “Synkeä Sakari”) at a daily dose of 50 mg/kg body weight for 8 weeks to Zucker diabetic fatty (ZDF) rats decreased hepatic gene expression of the TBC1D1 and G6pc as well as plasma gluconeogenic substrate glycerol; however, nonacylated anthocyanin extract from bilberry did not show similar results.57 The purple potato acylated anthocyanins extract, but not the bilberry nonacylated anthocyanins extract, reversed the increased plasma (systemic) glycolysis fluxes in ZDF rats,58 while both types of anthocyanins extracts decreased hepatic glycolysis fluxes.57 Although a decrease in hepatic glycolysis fluxes by both types of anthocyanin extracts has been observed, the impact of the two types of anthocyanins might have been due to different metabolism pathways since nonacylated anthocyanin extract increased gene expression of hepatic glucokinase, indicating its potential role as a glucokinase activator, while acylated anthocyanin extract decreased the hepatic expression of pyruvate kinase gene (Pklr type).57 However, contrary results have been reported showing that purple sweet potato acylated anthocyanin extract increased hepatic gene expression of pyruvate kinase (Pkm type) and phosphofructokinase (Pfk type) in a high-fat diet and STZ-induced diabetic mice.48 This difference might be due to the different animal models used in these two studies and/or the different acylated anthocyanin composition in purple potato (var. “Synkeä Sakari”, mainly monoacylated anthocyanins) and purple sweet potato (mainly diacylated anthocyanins).
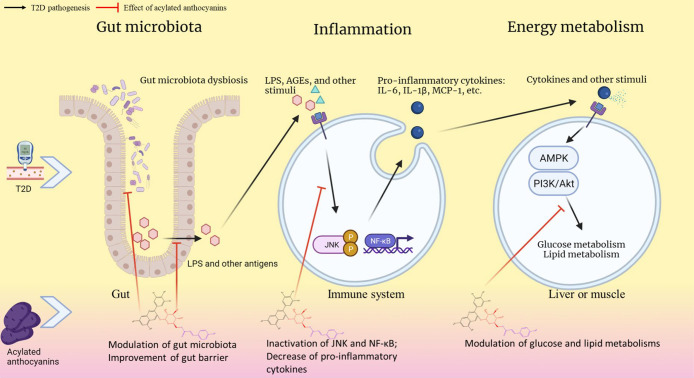
Figure 2.
Potential antidiabetic effect of acylated anthocyanins. AGEs, advanced glycation end products; AMPK, AMP-activating protein kinase; IL, interleukin; JNKs, JUN N-terminal kinases; LPS, lipopolysaccharides; NF-κB, nuclear factor-κB; PI3K/AKT, phosphoinositide 3 kinase/protein kinase B; T2D, type 2 diabetes; TNF, tumor necrosis factor.
Other potential different antidiabetic targets between acylated and nonacylated anthocyanins might also play a role in the observed antidiabetic effects due to the structural difference between the two types of anthocyanins. Glycerol-sn-3-phosphate acyltransferase (GPAT) can produce phosphatidic acid which is the precursor of triglyceride and glycerophospholipids from acyl-CoA and glycerol-3-phosphate. Suppression of GPAT1 by nonacylated anthocyanins has been frequently observed, for which the potential mechanism might be PPARγ/PKC-mediated.59,60 Although both f types of anthocyanins have demonstrated cholesterol- and TAG-lowering effects in animal models and human, acylated anthocyanins have not been proven to work via this route.4,16,61,62
3.3. Modulation of Inflammation
Insulin resistance in T2D has been attributed to low-grade and chronic inflammation in the liver, skeletal muscles, and adipose tissue.63 Thus, preventing inflammation can be an important strategy to manage insulin resistance in diabetes.
This low-grade and chronic inflammation is referred as “para-inflammation”, describing the immune responses where sustained tissue malfunction and stress are induced by a variety of factors, such as reactive oxygen species (ROS), advanced glycation end products (AGEs), and oxidized lipoproteins.64 ROS produced by phagocytes can oxidize and degrade lipoproteins to pro-inflammatory products.65 AGEs can accumulate under pro-oxidative status (such as aging) and hyperglycemia (such as diabetes).65 Para-inflammation may result in maladaptive chronic nonresolving immune activation, inhibition of insulin signaling pathways, and the development of insulin resistance.65 Moreover, para-inflammation has been associated with overfeeding or consumption of high-energy food. When caloric intake exceeds the energy expenditure, overloaded tricarboxylic acid cycle intermediates cause an excessive amount of mitochondrial NADH (mtNADH) and ROS. When the excess of mtNADH cannot be handled by oxidative phosphorylation, the mitochondrial proton gradient increases, and electrons are transferred to oxygen, resulting in the formation of free radicals, which might induce inflammation and insulin resistance.65 Oxidative stress induced by high caloric intake can activate NF-κB and release of its downstream pro-inflammatory cytokines.66 Consumption of a high-fat diet has been reported to lead to the activation of circulating and adipose immune cells via Toll-like receptor 4 (TLR4) signaling, causing the subsequent release of pro-inflammatory cytokines.63 The inflammation involved in adipose tissue recruitment of pro-inflammatory M1 macrophages, which partly contributes to insulin resistance.63
According to a pharmacological review, NF-κB is the main cause of inflammatory-induced insulin resistance in diabetes.67 NF-κB, a nuclear transcription factor, is a crucial regulator of inflammatory and immunological responses that could be triggered by a variety of proinflammatory stimuli and oxidative stress, such as cytokines, free radicals, AGEs, and bacterial or viral antigens. The downstream effects include the expression of proinflammatory cytokines (IL-6, TNF-α, IL-1, IL-2, etc.), chemokines (MCP1, IL-8, etc.), immunoreceptors, acute phase proteins, cell adhesion molecules (VCAM-l, vascular cell adhesion molecule-l), growth factors, and inducible enzymes including inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2).67 The function of NF-κB is controlled by inhibitors of NF-κB (IκB). The deactivated form of NF-κB interacts with IκB proteins in cytoplasm.67 Pro-inflammatory signals can activate IκB kinase (IKK) to degrade IκB proteins and release NF-κB p65/p50 heterodimer for nuclear translocation.68 Ikk±ob/ob mice with lower IKKβ expression have been shown to be protected from the development of insulin resistance.69 JNKs (JUN N-terminal kinases) are also important to insulin resistance due to their pro-inflammatory role, the JNK pathway is overactive in the muscle, liver, and adipose tissue in T2D, while blocking the JNK pathway has been reported to protect against insulin resistance.70
Both acylated anthocyanins and nonacylated anthocyanins have been shown to decrease activation of NF-κB, JNKs and reduce their downstream pro-inflammatory effects in T2D.71 Amelioration of inflammation by anthocyanins has suggested improved insulin resistance and glucose metabolism in diabetes.71 Oral administration of anthocyanin extracts from purple rice (predominantly cyanidin-3-glucoside) at a daily dose of 700 mg/kg body weight for 4 weeks to STZ-induced diabetic mice has shown decreased levels of blood glucose (ca. 13%), phosphorylated IKKβ, and COX-2 and IL-6 in the heart.71 Feeding diets containing 8% wild blueberry (Vaccinium angustifolium) powder to ZDF rats for 8 weeks downregulated NF-κB p50, TNF-α, and IL-6 expression in abdominal adipose tissue and liver.72 Supplementation of a diet containing 0.2% nonacylated anthocyanin cyanidin-3-O-glucoside to high-fat diet-induced and db/db diabetic mice for 5 weeks has shown a decreased blood glucose level by 20% and mitigated pro-inflammatory cytokines and insulin resistance, by regulating the JNK pathway.73
Supplementation of a daily dose of 700 mg/kg body weight acylated anthocyanin extract from purple sweet potato for 20 weeks has shown improvement of hepatic IRS-1/PI3k/AKT insulin signaling pathway and less activation of JNK1 and IKKβ in high-fat diet-induced obese mice.45 Supplementation of daily dose of 700 mg/kg body weight acylated anthocyanin extract from purple sweet potato for 20 weeks decreased blood glucose to the normal level and inhibited the NF-κB p65 nuclear translocation and nucleotide oligomerization domain protein1/2 (NOD1/2) signaling as well as the downstream inflammatory cytokines expression (IL-1β and IL-6, etc.) in high-fat diet-induced diabetic mice.74 Feeding acylated anthocyanin extract from black goji berry to mice with atherosclerosis has shown a lower arterial NF-κB p65 and VCAM-1 expression, plasma IL-1β, and TNF-α level, and hepatic CYP7A1 and SREBP-2 level.75 Both acylated and nonacylated anthocyanins have been reported to alleviate NF-κB through downregulating TLR4 (Figure 2).53,71
Although studies comparing acylated and nonacylated anthocyanins in terms of their ability to inhibit the NF-κB pathway have not been published, one study compared the effects of five structurally different anthocyanins on AGEs formation which can induce the NF-κB pro-inflammatory pathway.76 Petunidin-3-rutinoside-(coumaroyl)-5-glucoside, an acylated anthocyanin, was found to have the strongest anti-AGEs formation effects by slowing the glycation progression, followed by diglycosides of anthocyanidins (delphinidin-3-sambubioside and cyanidin-3-sophoroside) and then monoglycoside of anthocyanidins delphinidin-3-glucoside and (pelargonidin-3-glucoside),76 demonstrating that acylated anthocyanins may be more effective at suppressing NF-κB activation than nonacylated anthocyanins.
The impact of acylated anthocyanins from different dietary sources on inflammatory pathways may also differ. An in vitro study has shown acylated anthocyanin-rich extracts from purple carrots and purple potatoes suppressed lipopolysaccharide (LPS)-induced phosphorylation of JNK and IkBα in mucosal innate immune cells with purple potato anthocyanins extract showing more efficiency.77 Another study has compared the effect of acylated anthocyanin extract from purple potato (var. “Synkeä Sakari”) and nonacylated anthocyanin extract from bilberry on hepatic transcriptomic profile in high-fat diet-induced ZDF rats.57 Only the potato anthocyanin extract decreased the AP-1 (a transcriptional regulator, activator protein 1) level and IL-1β production, which are downstream effectors of JNK.57
Nrf2 is a transcription factor that regulates antioxidant protein production and protects against oxidative damage caused by inflammation or injury.61 Nonacylated anthocyanins have been shown to activate Nrf2 in HepG2 cell line and different organs of obese and diabetic animals.12,61,78 Acylated anthocyanin fraction of purple sweet potato has not affected Nrf2 activation in Huh-7 cells,79 and this effect has not been accessed in vivo.
3.4. Effect on Gut Microbiota
The human gastrointestinal tract has the largest interface (250–400 m2) between the environmental factors and the host. The human gastrointestinal tract harbors at least 1014 bacterial cells belonging to over 1000 bacterial species.80 The genome of those bacteria has been estimated to include over 3.3 million genes, which is 150 times more compared to the human genome.80 The gut microbiota has been reported to have impacts on the digestion, regulation of metabolic pathways, and innate and adaptive immunity.80
The dynamics of the gut microbiota profile is influenced by genetic factors, lifestyle, drugs (especially antibiotics), and dietary habits.81 Host-symbiotic interactions of the microbiota play important roles in the host physiology. For example, the gut epithelial cells and antimicrobial peptides produced by the gut microbiota contribute to the gut barrier, the first frontline against exogenous molecules and pathogens in the gastrointestinal tract. Gut microbiota influence the gut epithelial morphology, mucus production, secretion of immune factors, and gut permeability when interacting with the epithelial and mucosal immune cells.82 Exogenous molecules in the lumen can pass through the damaged gut barrier to the endothelium and even systemic circulation and induce the inflammatory response (Figure 2).83 For example, metabolic endotoxemia is linked with the development of obesity and T2D.83 In particular, dysregulated gut microbiota composition in obesity and T2D may damage the gut barrier and expose LPS to systemic circulation (para-inflammation), which renders chronic low-grade inflammation linked to insulin resistance, adiposity, and de novo synthesis of triglycerides.83 LPS can induce the innate immune system response by binding to TLR4 and its coreceptors.82 TLR4 belongs to the TLR family, one of the pattern-recognition receptors. When these receptors are activated, myeloid differentiation primary response protein 88 (MyD88, adaptor proteins of Toll-like receptors) is recruited and pro-inflammatory signaling cascades are activated, for example, the aforementioned NF-κB pathway.84 Moreover, deletion of MyD88 has been shown to protect against obesity and insulin resistance in mice.85
In humans, there are no endogenous esterases to release phenolic acids from the acylated anthocyanins; however, the esterases present in gut microbiota is able to do so.86 As aforementioned, due to the lower bioavailability of acylated anthocyanins than nonacylated anthocyanins, more acylated anthocyanins would be exposed to gut microbiota compared to nonacylated anthocyanins.77
Gut metabolism of anthocyanins including the absorption, distribution, and excretion were extensively reviewed elsewhere.16,26 Briefly, gut bacterial metabolism of anthocyanins involves the cleavage of glycosidic linkages and breakdown of anthocyanidin heterocycle (from C-ring), degradation into phloroglucinol derivatives (from A-ring) and benzoic acids (from B-ring), and O-demethylation, forming simple phenolics and phenolic-sulfated, phenolic-glucuronidated, phenolic-methylated metabolites.16,26
The more bioavailable phenolic metabolites may contribute to the biological benefits of anthocyanins mentioned above. For example, protocatechuic acid has been reported to stimulate the insulin signaling pathway and upregulate PPARγ activity, adiponectin release, GLUT4 translocation, and glucose uptake in human adipocytes cell lines87,88 and human primary adipocytes cells89 as well as have an antihyperglycemic in diabetic rats.90
So far, there are no studies investigating the difference in gut metabolites between acylated and nonacylated anthocyanins. However, our previous study has detected novel metabolites of monoacylated anthocyanins from purple potatoes in human urine for the first time, such as hydroxybenzoic and hydroxycinnamic acids as well as glucuronyl and sulfonyl conjugates of protocatechuic acid, of which some might be related to the gut metabolism of acylated anthocyanins.88,91 Our results have shown that the absorbed potato acylated anthocyanins are likely to be resistant to deglycosylation since 91% of nonacylated monoglycosylated anthocyanins are deglycosylated after ingestion and form conjugates of aglycones in urine.91,92
Despite nonacylated anthocyanins have been frequently reported in reviews12 to affect the abundance of fecal Lactobacillus spp., Bifidobacterium spp., and Clostridium spp., contradictory results have also been observed. For example, supplementation of nonacylated anthocyanin extract from blackcurrant (Ribes nigrum) has enriched the abundance of Bifidobacterium spp. in humans, while anthocyanins from black raspberry (Rubus racemosus) has depleted the abundance of Bifidobacterium ssp. in rats.26 This could be due to different metabolic states, interspecies differences, and structures of anthocyanins. In this review, we examine regulatory effects of anthocyanins on the gut microbiota in the condition of obesity and diabetes for the first time. Table 3 presents a summary of the studies on the effects of anthocyanins on gut microbiota.
Table 3. Gut Microbiota Regulation Effects of Anthocyanin in the Conditions of Obesity and Diabetes and the Different Prebiotic Effects between Acylated and Nonacylated Anthocyaninsa.
| Source of anthocyanins | Main anthocyanin(s) | Methods | Effect |
|---|---|---|---|
| Anthocyanin extract from black rice (Oryza sativa Japonica)93 | Cya-3,5-diglc, cya-3-glc, cya-3-rut, peo-3-glc | HFD- induced obese C57BL/6J mice; 150 mg/kg bodyweight; 14 weeks | Decreased body weight gain, triglycerides, total cholesterol, steatosis scores and insulin resistance index |
| Improved the gene expression profiles involved in lipid metabolism | |||
| Increased the abundances of Bacteroides, Akkermansia, Lactobacillus, Ruminococcaceae_UCG014 and Alloprevotella at genus level; at the species level, decreased the proportions of Dorea_sp._5–2Blautia coccoides, Lactobacillus gasseri, Mucispirillum schaedleri, Akkermansia muciniphila and Parabacteroides merdae | |||
| Diversity and richness of microbiota were not changed | |||
| Freeze-dried strawberry95 | Not available | db/db diabetic mice; diet containing 2.35% freeze-dried strawberry; 10 weeks | α-Diversity indices and β-diversity were different at the phylum and genus levels |
| At the phylum level, decreased the abundance of Verrucomicrobia, at the genus level, increased Bifidobacterium | |||
| PICRUSt revealed significant differences in 45 predicted metabolic functions | |||
| Purified anthocyanins from purple sweet potato (Ipomoea batatas Lam.)99 | Peo-3-sop-5-glc; peo-3-fer- sop-5-glc; peo-3-caf-sop-5-glc; peo-3-caf-hba-sop-5-glc; peo-3-caf-fer-sop-5-glc | Antioxidant activities, proliferative effects on probiotics, and their inhibition on harmful bacteria in vitro were tested | Diacylated anthocyanin had the best antioxidant ability and inhibition ability of harmful bacteria (Staphylococcus aureus and Salmonella typhimurium) followed by monoacylated anthocyanin and then nonacylated anthocyanin |
| Purified anthocyanin96 | Cya-3-glc | High fat-high sucrose diet-induced insulin-resistant C57 BL/6J mice; 7.2 mg/kg body weight; 11 weeks | Decreased Lachnospiraceae and Erysipelotrichaceae and increased the Bacteroidetes/Firmicutes and family Muriculaceae |
| Purified anthocyanin97 | Pg-3-glc | db/db diabetic mice; 150 mg/kg body weight; 8weeks | Decreased plasma glucose and promoted glucose uptake |
| Induced autophagy by modulating Transcriptional factor EB | |||
| Increased abundance of Prevotella, Bacteroidetes/Firmicutes ratio, and intestinal barrier integrity | |||
| Concord grape freeze-dried powder33 | Del- and pet- derived acylated anthocyanins | polygenic obese C57 BL/6J mice; dose of grape powder normalized to 400 μg/g total anthocyanin content; 12 weeks | Increased abundance of Actinobacteria |
| Anthocyanins extract from purple sweet potato (Ipomoea batatas Lam)98 | Cya-3-caf-cou-diglc-5-glc, cya-3-fer-sop-5-glc, cya-3-sin-diglc-5-xyl, pg-3-ace-diglc-5-glc, cya-3-hba-oxa-diglc-5-glc, cya-3-diglc-5-glc, cya-3-difer-sop-5-glc, cya-3-caf-fer-sop-5-glc | In vitro stimulation of gut microbiota | Induced the growth of Bifidocterium spp. and Lactobacillus spp. and inhibited the growth of Prevotella and Clostridium histolyticum |
| Increased SCFAs | |||
| Anthocyanin extract from black goji berry (Lycium ruthenicum)75 | Pet-3-rut-cou-5-glc as the main anthocyanin | HFD and vitamin-D3- induced atherosclerosis rat, 50–200 mg/kg body weight; 8 weeks | Increased abundance of Bifidobacterium, Lactobacillus, Roseburia, Akkermansia, and Lachnospiraceae_NK4A136_group and decreased Prevotellaceae_NK3B31_group |
| Increased gut barrier | |||
| Nonacylated anthocyanin extract from bilberry (Vaccinium myrtillus) and acylated anthocyanin extract from purple potato (Solanum tuberosum var. “Synkeä Sakari”)37 | Nonacylated anthocyanins dominated by del-3-gal, del-3-glc, cya-3-gal, del-3-ara, cya-3-glc | Zucker diabetic fatty rats; daily doses of 25–50 mg/kg body weight; 8 weeks | Both anthocyanin extracts increased abundance of Peptostreptococcaceae sp. and decreased abundance of Parabacteroides spp. Acylated anthocyanins decreased Ruminococcus torques and Lachnospiraceae bacterium 4_1_37FAA abundances |
| Acylated anthocyanins dominated by pet-cou-rut-glc and peo-cou-rut-glc |
Note: Anthocyanidins: cya, cyanidin; del, delphinidin; mv, malvidin; pg, pelargonidin; peo, peonidin; pet, petunidin. Acyl moieties: ace, acetyl; caf, caffeoyl; cou, coumaroyl; fer, feruloyl; hba, hydroxybenzoyl; mal, malonyl; oxa, oxaloyl; sin, sinapoyl; suc, succinyl; pyr, pyranosyl. Sugar moieties: gal, galactoside; rut, rutinoside; sop, sophoroside; xyl, xyloside; glc; glucopyranoside.
Nonacylated anthocyanins extracted from black rice fed to high-fat diet-induced obese mice at a daily dose of 150 mg/kg body weight for 14 weeks did not affect the overall diversity and richness of microbiota but did enrich the abundance of genera Ruminococcaceae, Alloprevotella, Lactobacillus, Bacteroides, and Akkermansia and species Blautia coccoides, Dorea sp. 5–2, Akkermansia muciniphila, Lactobacillus gasseri, Mucispirillum schaedleri, and Parabacteroides merdae.93 The decreased abundance of A. muciniphila has been reported in T2D. Feeding A. muciniphila to T2D mice has been shown to improve mucus layer thickness, metabolic function, glucose tolerance, and systemic inflammation.82 Pasteurization of A. muciniphila also reduced fat mass development, insulin resistance, and dyslipidemia in mice, and the mechanism might be due to a specific protein from the outer membrane of A. muciniphila interacting with Toll-like receptor 2 which shapes the host metabolism by regulating bacterial recognition and intestinal homeostasis.94 Feeding a diet contaning 2.35% freeze-dried strawberry to db/db mice for 10 weeks has been reported to enhance gut microbiota diversity and lower the abundance of Verrucomicrobia.95 Supplementation of purified cyanidin-3-glucoside at a daily dose of 7.2 mg/kg body weight for 11 weeks enriched Erysipelotrichaceae and Lachnospiraceae and increased the Bacteroidetes/Firmicutes ratio and the family Muriculacea in high-fat and high-sucrose diet-induced insulin-resistant C57 BL/6J mice.96 Feeding a daily dose of 50 mg/kg body weight pelargonidin-3-O-glucoside for 8 weeks to db/db diabetic mice increased the Prevotella abundance and Bacteroidetes/Firmicutes ratio and strengthened gut barrier integrity.97 Incubation of potato acylated anthocyanins extract from purple sweet with human gut microbiota enriched the abundance of Lactobacillus spp. and Bifidobacterium, depleted the abundance of Clostridium histolyticum and Prevotella, and induced the production of short-chain fatty acids (SCFAs).98
Although the prebiotic role of acylated anthocyanins has not been extensively studied, acylation status of anthocyanins might influence the effect of anthocyanins on gut microbiota homeostasis in diabetes. An in vitro study assessed the prebiotic activity and antioxidant ability of purified diacylated anthocyanin, monoacylated anthocyanin, and nonacylated anthocyanin, showing diacylated anthocyanin has the most potent antioxidant ability and inhibition on the growth of harmful bacteria (Salmonella typhimurium and Staphylococcus aureus) followed by monoacylated anthocyanin and then nonacylated anthocyanin.99 Obese mice fed with Concord grape rich in acylated anthocyanin for 12 weeks showed the highest level of Actinobacteria compared to supplementation of berries rich in nonacylated anthocyanin, followed by obese models.33Peptostreptococcaceae has been shown to be significantly lower in T2D patients,100 and both nonacylated anthocyanin extract from bilberry and acylated anthocyanin extract from purple potato increased the abundance of Peptostreptococcaceae in ZDF ras.37 In addition, only the acylated anthocyanin extract from purple potato enriched the abundance of Ruminococcus torques, Parabacteroides disdasonis, and Lanchnospiraceae bacterium 4_1_37FAA in ZDF rats.37P. distasonis is a dominant species producing propionate in human gut flora, contributing to the increased level of propionate in the rats fed with acylated anthocyanin extract.37Parabacteroides has been shown to have a positive correlation with serum proinflammatory cytokine IL-17 and splenic CD4+ Th17 cells in arthritic mice101 and to be more enriched in patients with nonalcoholic steatohepatitis.102R. torques is able to decrease the gut barrier integrity and has been shown to have a positive correlation with insulin resistance in obesity.103 As for gut metabolites, only acylated anthocyanin extract in contrast to nonacylated anthocyanin increased cecal SCFAs and succinate levels in diabetes,37 and a study showed that cecal succinate is a substrate for intestinal gluconeogenesis and benefit glycemic responses and hepatic glucose production.104 However, the gut microbiota-modulating effect of acylated anthocyanins deserves further study in different models as well as different physiological and pathological conditions (Figure 2).
4. Conclusion and Future Perspectives
Anthocyanins have been reported to affect energy metabolism, inflammation, and gut microbiota in T2D. The physicochemical properties, bioavailability, and metabolism differ between acylated and nonacylated anthocyanins. Acylated anthocyanins with higher stability can pass through the upper gastrointestinal tract and reach the colon, where they are extensively metabolized by gut microbiota. Glucose transporters are involved in anthocyanin absorption, and different glucose transporters are responsible for the absorption of acylated and nonacylated anthocyanins. There are indications that acylated and nonacylated anthocyanins might have different effects on T2D. Acylated anthocyanins have a greater inhibitory effect on α-glucosidase and α-amylase compared to nonacylated anthocyanins. Acylated and nonacylated anthocyanins modulate key enzymes or metabolites involved in energy metabolism and inflammation to different degrees; for example, acylated anthocyanins induce a higher level of AKT phosphorylation and AP-1 activation. Acylated anthocyanin has a better antioxidant ability and inhibition on the AGEs formation and growth of harmful bacteria compared to nonacylated anthocyanins. Acylated anthocyanins can improve the gut barrier and microbiota composition, suppress the pro-inflammatory pathways, and modulate glucose and lipid metabolisms. Drawing clear conclusions about the different biological activity in diabetes between acylated and nonacylated anthocyanins based on the current literature is challenging, as the studies differ in design and analytical methods, and most importantly the insufficient data from comparative in vivo study between two types of anthocyanins. Further studies are needed to compare the effects of structurally different anthocyanins. The currently available evidence suggests that acylated anthocyanins may have greater potential modulation effects on energy metabolism, inflammation, and gut microbiota in T2D compared to nonacylated anthocyanins.
Acknowledgments
The study was supported by the China Scholarship Council (grant no. 201706790015), the Graduate School of University of Turku, the Finnish Food Research Foundation, and the Finland-China Network in Food and Health as a pilot of the global program for research and innovation funded by the Ministry of Education and Culture of Finland.
Glossary
Abbreviations
- ACC
acetyl-coenzyme A carboxylase
- AGEs
advanced glycation end products
- AKT
protein kinase B
- AMPK
adenosine monophosphate-activated protein kinase
- AP-1
activate protein 1
- G6pase
glucose 6-phosphatase
- GLUT
glucose transporter
- GPAT
glycerol-sn-3-phosphate acyltransferase
- HbA1c
glycated hemoglobin
- HFD
high-fat diet
- IL
interleukin
- IRS-1
insulin receptor substrate 1
- LPS
lipopolysaccharide
- PEPCK
phosphoenolpyruvate carboxykinase
- PI3K
phosphoinositide 3 kinase
- MCP-1
monocyte chemoattractant protein-1
- NF-κB
nuclear factor κB
- Nrf2
nuclear factor erythroid 2-related factor 2
- PPAR
peroxisome-proliferator-activated receptor
- ROS
reactive oxygen species
- SGLT1
sodium-glucose cotransporter 1
- SOD
superoxide dismutase
- SCFAs
short-chain fatty acids
- TNF-α
tumor necrosis factor-α
Author Contributions
Kang Chen: Conceptualization, investigation, methodology, writing the original draft, and funding acquisition. Maaria Katariina Kortesniemi and Kaisa Marjut Linderborg: Supervision and writing - reviewing and editing. Baoru Yang: Conceptualization, supervision, funding acquisition, and writing - reviewing and editing. All authors agreed to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Jones J. G. Hepatic Glucose and Lipid Metabolism. Diabetologia 2016, 59 (6), 1098–1103. 10.1007/s00125-016-3940-5. [DOI] [PubMed] [Google Scholar]
- Scherer P. E. The Many Secret Lives of Adipocytes: Implications for Diabetes. Diabetologia 2019, 62 (2), 223–232. 10.1007/s00125-018-4777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden M.; Shulman G. I. The Integrative Biology of Type 2 Diabetes. Nature 2019, 576 (7785), 51–60. 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- Gowd V.; Jia Z.; Chen W. Anthocyanins as Promising Molecules and Dietary Bioactive Components against Diabetes - A Review of Recent Advances. Trends Food Sci. Technol. 2017, 68, 1–13. 10.1016/j.tifs.2017.07.015. [DOI] [Google Scholar]
- Belwal T.; Nabavi S. F.; Nabavi S. M.; Habtemariam S. Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients 2017, 9, 1111. 10.3390/nu9101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya C.; Hosoya T.; Agawa S.; Sugiyama Y.; Shin-ya K.; Terahara N.; Kumazawa S. New Acylated Anthocyanins from Purple Yam and Their Antioxidant Activity. Biosci Biotechnol Biochem 2015, 79, 1484–1492. 10.1080/09168451.2015.1027652. [DOI] [PubMed] [Google Scholar]
- Jordheim M.; Måge F.; Andersen Ø. M. Anthocyanins in Berries of Ribes Including Gooseberry Cultivars with a High Content of Acylated Pigments. J. Agric. Food Chem. 2007, 55 (14), 5529–5535. 10.1021/jf0709000. [DOI] [PubMed] [Google Scholar]
- Blesso C. N. Dietary Anthocyanins and Human Health. Nutrients 2019, 11 (9), 2107. 10.3390/nu11092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanska D.; Regulska-Ilow B. The Significance of Anthocyanins in the Prevention and Treatment of Type 2 Diabetes. Adv. Clin Exp Med. 2018, 27, 135. 10.17219/acem/64983. [DOI] [PubMed] [Google Scholar]
- Faria A.; Fernandes I.; Norberto S.; Mateus N.; Calhau C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62 (29), 6898–6902. 10.1021/jf501808a. [DOI] [PubMed] [Google Scholar]
- Hidalgo M.; Oruna-Concha M. J.; Kolida S.; Walton G. E.; Kallithraka S.; Spencer J. P. E.; Gibson G. R.; de Pascual-Teresa S. Metabolism of Anthocyanins by Human Gut Microflora and Their Influence on Gut Bacterial Growth. J. Agric. Food Chem. 2012, 60 (15), 3882–3890. 10.1021/jf3002153. [DOI] [PubMed] [Google Scholar]
- Jayarathne S.; Stull A. J.; Park O. H.; Kim J. H.; Thompson L.; Moustaid-Moussa N. Protective Effects of Anthocyanins in Obesity-Associated Inflammation and Changes in Gut Microbiome. Mol. Nutr Food Res. 2019, 63 (20), 1900149. 10.1002/mnfr.201900149. [DOI] [PubMed] [Google Scholar]
- Morais C.; de Rosso V. V.; Estadella D.; Pisani L. Anthocyanins as Inflammatory Modulators and the Role of the Gut Microbiota. Journal of Nutritional Biochemistry 2016, 33, 1–7. 10.1016/j.jnutbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Shields M.; Tremblay M. S. Dietary Flavonoids and Insulin Signaling in Diabetes and Obesity. Statistics (Ber) 2008, 19 (82), 138–146. [Google Scholar]
- Jiang X.; Li X.; Zhu C.; Sun J.; Tian L.; Chen W.; Bai W. The Target Cells of Anthocyanins in Metabolic Syndrome. Crit Rev. Food Sci. Nutr 2019, 59 (6), 921–946. 10.1080/10408398.2018.1491022. [DOI] [PubMed] [Google Scholar]
- Jokioja J.; Yang B.; Linderborg M. K. Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Comprehensive Reviews in Food Science and Food Safety 2021, 20 (6), 5570. 10.1111/1541-4337.12836. [DOI] [PubMed] [Google Scholar]
- He F.; Mu L.; Yan G. L.; Liang N. N.; Pan Q. H.; Wang J.; Reeves M. J.; Duan C. Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15 (12), 9057–9091. 10.3390/molecules15129057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti S.; Vrhovsek U.; Mattivi F. The Interaction of Anthocyanins with Bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296 (3), 631–636. 10.1016/S0006-291X(02)00927-0. [DOI] [PubMed] [Google Scholar]
- Xu J.; Su X.; Lim S.; Griffin J.; Carey E.; Katz B.; Tomich J.; Smith J. S.; Wang W. Characterisation and Stability of Anthocyanins in Purple-Fleshed Sweet Potato P40. Food Chem. 2015, 186, 90–96. 10.1016/j.foodchem.2014.08.123. [DOI] [PubMed] [Google Scholar]
- Zhao C. L.; Yu Y. Q.; Chen Z. J.; Wen G. S.; Wei F. G.; Zheng Q.; Wang C. de.; Xiao X. L. Stability-Increasing Effects of Anthocyanin Glycosyl Acylation. Food Chem. 2017, 214, 119–128. 10.1016/j.foodchem.2016.07.073. [DOI] [PubMed] [Google Scholar]
- Bakowska-Barczak A. Acylated Anthocyanins as Stable, Natural Food Colorants-a Review. Pol J. Food Nutr Sci. 2005, 14 (2), 107–115. [Google Scholar]
- Dyrby M.; Westergaard N.; Stapelfeldt H. Light and Heat Sensitivity of Red Cabbage Extract in Soft Drink Model Systems. Food Chem. 2001, 72 (4), 431–437. 10.1016/S0308-8146(00)00251-X. [DOI] [Google Scholar]
- Inami O.; Tamura I.; Kikuzaki H.; Nakatani N. Stability of Anthocyanins of Sambucus Canadensis and Sambucus Nigra. J. Agric. Food Chem. 1996, 44 (10), 3090–3096. 10.1021/jf9507809. [DOI] [Google Scholar]
- Nakajima N.; Sugimoto M.; Yokoi H.; Tsuji H.; Ishihara K. Comparison of Acylated Plant Pigments: Light-Resistance and Radical-Scavenging Ability. Biosci Biotechnol Biochem 2003, 67 (8), 1828–1831. 10.1271/bbb.67.1828. [DOI] [PubMed] [Google Scholar]
- Yang W.; Kortesniemi M.; Ma X.; Zheng J.; Yang B. Enzymatic Acylation of Blackcurrant (Ribes Nigrum) Anthocyanins and Evaluation of Lipophilic Properties and Antioxidant Capacity of Derivatives. Food Chem. 2019, 281, 189–196. 10.1016/j.foodchem.2018.12.111. [DOI] [PubMed] [Google Scholar]
- Tian; et al. Metabolism of Anthocyanins and Consequent Effects on the Gut Microbiota. Crit Rev. Food Sci. Nutr 2019, 59 (6), 982–991. 10.1080/10408398.2018.1533517. [DOI] [PubMed] [Google Scholar]
- Faria A.; Fernandes I.; Norberto S.; Mateus N.; Calhau C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62 (29), 6898–6902. 10.1021/jf501808a. [DOI] [PubMed] [Google Scholar]
- Oliveira H.; Perez-Gregorio R.; de Freitas V.; Mateus N.; Fernandes I. Comparison of the in Vitro Gastrointestinal Bioavailability of Acylated and Non-Acylated Anthocyanins : Purple- Fl Eshed Sweet Potato vs Red Wine. Food Chem. 2019, 276 (15), 410–418. 10.1016/j.foodchem.2018.09.159. [DOI] [PubMed] [Google Scholar]
- Kurilich A. C.; Clevidence B. A.; Britz S. J.; Simon P. W.; Novotny J. A. Plasma and Urine Responses Are Lower for Acylated vs Nonacylated Anthocyanins from Raw and Cooked Purple Carrots. J. Agric. Food Chem. 2005, 53, 6537–6542. 10.1021/jf050570o. [DOI] [PubMed] [Google Scholar]
- Olejnik A.; Kowalska K.; Kidoń M.; Czapski J.; Rychlik J.; Olkowicz M.; Dembczyński R. Purple Carrot Anthocyanins Suppress Lipopolysaccharide-Induced Inflammation in the Co-Culture of Intestinal Caco-2 and Macrophage RAW264.7 Cells. Food Funct 2016, 7 (1), 557–564. 10.1039/C5FO00890E. [DOI] [PubMed] [Google Scholar]
- Oliveira H.; Roma-Rodrigues C.; Santos A.; Veigas B.; Brás N.; Faria A.; Calhau C.; de Freitas V.; Baptista P. v.; Mateus N.; Fernandes A. R.; Fernandes I. GLUT1 and GLUT3 Involvement in Anthocyanin Gastric Transport- Nanobased Targeted Approach. Sci. Rep 2019, 9 (1), 789. 10.1038/s41598-018-37283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F.; Oliveira H.; Brás N. F.; Fernandes I.; Cruz L.; de Freitas V.; Mateus N. In Vitro Gastrointestinal Absorption of Red Wine Anthocyanins - Impact of Structural Complexity and Phase II Metabolization. Food Chem. 2020, 317, 126398. 10.1016/j.foodchem.2020.126398. [DOI] [PubMed] [Google Scholar]
- Overall J.; Bonney S. A.; Wilson M.; Beermann A.; Grace M. H.; Esposito D.; Lila M. A.; Komarnytsky S. Metabolic Effects of Berries with Structurally Diverse Anthocyanins. Int. J. Mol. Sci. 2017, 18 (2), 422. 10.3390/ijms18020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgines C.; Talavéra S.; Texier O.; Besson C.; Fogliano V.; Lamaison J.-L.; Fauci L. la; Galvano G.; Rémésy C.; Galvano F. Absorption and Metabolism of Red Orange Juice Anthocyanins in Rats. Br. J. Nutr. 2006, 95 (5), 898–904. 10.1079/BJN20061728. [DOI] [PubMed] [Google Scholar]
- Xie L.; Mo J.; Ni J.; Xu Y.; Su H.; Xie J.; Chen W. Structure-Based Design of Human Pancreatic Amylase Inhibitors from the Natural Anthocyanin Database for Type 2 Diabetes. Food Funct 2020, 11 (4), 2910–2923. 10.1039/C9FO02885D. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Hassan Y. I.; Renaud J.; Liu R.; Yang C.; Sun Y.; Tsao R. Bioaccessibility, Bioavailability, and Anti-Inflammatory Effects of Anthocyanins from Purple Root Vegetables Using Mono- and Co-Culture Cell Models. Mol. Nutr Food Res. 2017, 61 (10), 1600928. 10.1002/mnfr.201600928. [DOI] [PubMed] [Google Scholar]
- Chen K.; Wei X.; Kortesniemi M.; Pariyani R.; Zhang Y.; Yang B. Effects of Acylated and Nonacylated Anthocyanins Extracts on Gut Metabolites and Microbiota in Diabetic Zucker Rats: A Metabolomic and Metagenomic Study. Food Research International 2022, 153, 110978. 10.1016/j.foodres.2022.110978. [DOI] [PubMed] [Google Scholar]
- Matsui T.; Ueda T.; Oki T.; Sugita K.; Terahara N.; Matsumoto K. α-Glucosidase Inhibitory Action of Natural Acylated Anthocyanins. 2. α-Glucosidase Inhibition by Isolated Acylated Anthocyanins. J. Agric. Food Chem. 2001, 49 (4), 1952–1956. 10.1021/jf0012502. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhang J.-l.; Shen L.-h.; Feng L.-j.; Zhou Q. Inhibition Mechanism of Diacylated Anthocyanins from Purple Sweet Potato (Ipomoea Batatas L.) against α-Amylase and α-Glucosidase. Food Chem. 2021, 359 (1), 129934. 10.1016/j.foodchem.2021.129934. [DOI] [PubMed] [Google Scholar]
- Gong S.; Yang C.; Zhang J.; Yu Y.; Gu X.; Li W.; Wang Z. Study on the Interaction Mechanism of Purple Potato Anthocyanins with Casein and Whey Protein. Food Hydrocoll 2021, 111, 106223. 10.1016/j.foodhyd.2020.106223. [DOI] [Google Scholar]
- Schultze S. M.; Hemmings B. A.; Niessen M.; Tschopp O. PI3K/AKT, MAPK and AMPK Signalling: Protein Kinases in Glucose Homeostasis. Expert Rev. Mol. Med. 2012, 14, 1–21. 10.1017/S1462399411002109. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Hu X.; Liu Y.; Dong S.; Wen Z.; He W.; Zhang S.; Huang Q.; Shi M. ROS Signaling under Metabolic Stress: Cross-Talk between AMPK and AKT Pathway. Molecular Cancer 2017, 16 (1), 79. 10.1186/s12943-017-0648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day E. A.; Ford R. J.; Steinberg G. R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends in Endocrinology and Metabolism 2017, 28 (8), 545–560. 10.1016/j.tem.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Yan F.; Zheng X. Anthocyanin-Rich Mulberry Fruit Improves Insulin Resistance and Protects Hepatocytes against Oxidative Stress during Hyperglycemia by Regulating AMPK/ACC/MTOR Pathway. J. Funct Foods 2017, 30, 270–281. 10.1016/j.jff.2017.01.027. [DOI] [Google Scholar]
- Zhang Z. F.; Lu J.; Zheng Y. L.; Wu D. M.; Hu B.; Shan Q.; Cheng W.; Li M. Q.; Sun Y. Y. Purple Sweet Potato Color Attenuates Hepatic Insulin Resistance via Blocking Oxidative Stress and Endoplasmic Reticulum Stress in High-Fat-Diet-Treated Mice. Journal of Nutritional Biochemistry 2013, 24 (6), 1008–1018. 10.1016/j.jnutbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Huang B.; Wang Z.; Park J. H.; Ryu O. H.; Choi M. K.; Lee J. Y.; Kang Y. H.; Lim S. S. Anti-Diabetic Effect of Purple Corn Extract on C57BL/KsJ Db/Db Mice. Nutr Res. Pract 2015, 9 (1), 22–29. 10.4162/nrp.2015.9.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. P.; Choi J. H.; Han E. H.; Kim H. G.; Wee J. H.; Jung K. O.; Jung K. H.; Kwon K. il; Jeong T. C.; Chung Y. C.; Jeong H. G. Purple Sweet Potato Anthocyanins Attenuate Hepatic Lipid Accumulation through Activating Adenosine Monophosphate-Activated Protein Kinase in Human HepG2 Cells and Obese Mice. Nutr. Res. (N.Y.) 2011, 31 (12), 896–906. 10.1016/j.nutres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Jiang T.; Shuai X.; Li J.; Yang N.; Deng L.; Li S.; He Y.; Guo H.; Li Y.; He J. Protein-Bound Anthocyanin Compounds of Purple Sweet Potato Ameliorate Hyperglycemia by Regulating Hepatic Glucose Metabolism in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. J. Agric. Food Chem. 2020, 68 (6), 1596–1608. 10.1021/acs.jafc.9b06916. [DOI] [PubMed] [Google Scholar]
- Takikawa M.; Inoue S.; Horio F.; Tsuda T. Dietary Anthocyanin-Rich Bilberry Extract Ameliorates Hyperglycemia and Insulin Sensitivity via Activation of AMP-Acin Diabetic Mice. J. Nutr. 2010, 140 (3), 527–533. 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- Hwang Y. P.; Choi J. H.; Han E. H.; Kim H. G.; Wee J. H.; Jung K. O.; Jung K. H.; Kwon K. il; Jeong T. C.; Chung Y. C.; Jeong H. G. Purple Sweet Potato Anthocyanins Attenuate Hepatic Lipid Accumulation through Activating Adenosine Monophosphate-Activated Protein Kinase in Human HepG2 Cells and Obese Mice. Nutr. Res. (N.Y.) 2011, 31 (12), 896–906. 10.1016/j.nutres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Park M.; Yoo J. H.; Lee Y. S.; Lee H. J. Lonicera Caerulea Extract Attenuates Non-Alcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Hepatocytes and in High Fat Diet-Fed Mice. Nutrients 2019, 11 (3), 494. 10.3390/nu11030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. H.; Lee H. A.; Park M. H.; Han J.-S. Mulberry (Morus Alba L.) Fruit Extract Containing Anthocyanins Improves Glycemic Control and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic C57BL/Ksj-Db/Db Mice. J. Med. Food 2016, 19 (8), 737–745. 10.1089/jmf.2016.3665. [DOI] [PubMed] [Google Scholar]
- Tian B.; Zhao J.; Xie X.; Chen T.; Yin Y.; Zhai R.; Wang X.; An W.; Li J. Anthocyanins from the Fruits of: Lycium Ruthenicum Murray Improve High-Fat Diet-Induced Insulin Resistance by Ameliorating Inflammation and Oxidative Stress in Mice. Food Funct 2021, 12 (9), 3855–3871. 10.1039/D0FO02936J. [DOI] [PubMed] [Google Scholar]
- Tsutsumi A.; Horikoshi Y.; Fushimi T.; Saito A.; Koizumi R.; Fujii Y.; Hu Q. Q.; Hirota Y.; Aizawa K.; Osakabe N. Acylated Anthocyanins Derived from Purple Carrot (: Daucus Carota L.) Induce Elevation of Blood Flow in Rat Cremaster Arteriole. Food Funct 2019, 10 (3), 1726–1735. 10.1039/C8FO02125B. [DOI] [PubMed] [Google Scholar]
- Chen L.; Chen Q.; Xie B.; Quan C.; Sheng Y.; Zhu S.; Rong P.; Zhou S.; Sakamoto K.; MacKintosh C.; Wang H. Y.; Chen S. Disruption of the AMPK-TBC1D1 Nexus Increases Lipogenic Gene Expression and Causes Obesity in Mice via Promoting IGF1 Secretion. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (26), 7219–7224. 10.1073/pnas.1600581113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; Liu G.; Guo J.; Su Z. Q. The PI3K/AKT Pathway in Obesity and Type 2 Diabetes. Int. J. Biol. Sci. 2018, 14 (11), 1483–1496. 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.; Wei X.; Pariyani R.; Kortesniemi M.; Zhang Y.; Yang B. H NMR Metabolomics and Full-Length RNA-Seq Reveal Effects of Acylated and Nonacylated Anthocyanins on Hepatic Metabolites and Gene Expression in Zucker Diabetic Fatty Rats. J. Agric. Food Chem. 2021, 69, 4423–4437. 10.1021/acs.jafc.1c00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.; Wei X.; Zhang J.; Pariyani R.; Jokioja J.; Kortesniemi M.; Linderborg K. M.; Heinonen J.; Sainio T.; Zhang Y.; Yang B. Effects of Anthocyanin Extracts from Bilberry (Vaccinium Myrtillus L.) and Purple Potato (Solanum Tuberosum L. Var. ‘Synkeä Sakari’) on the Plasma Metabolomic Profile of Zucker Diabetic Fatty Rats. J. Agric. Food Chem. 2020, 68 (35), 9436–9450. 10.1021/acs.jafc.0c04125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H.; Li D.; Ling W.; Feng X.; Xia M. Anthocyanin Inhibits High Glucose-Induced Hepatic MtGPAT1 Activation and Prevents Fatty Acid Synthesis through PKCζ. J. Lipid Res. 2011, 52 (5), 908–922. 10.1194/jlr.M013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. J.; Hsu M. J.; Huang H. P.; Chung D. J.; Chang Y. C.; Wang C. J. Mulberry Anthocyanins Inhibit Oleic Acid Induced Lipid Accumulation by Reduction of Lipogenesis and Promotion of Hepatic Lipid Clearance. J. Agric. Food Chem. 2013, 61 (25), 6069–6076. 10.1021/jf401171k. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhang J.-l.; Zhou Q. Targets and Mechanisms of Dietary Anthocyanins to Combat Hyperglycemia and Hyperuricemia: A Comprehensive Review. Crit Rev. Food Sci. Nutr 2022, 62, 1119. 10.1080/10408398.2020.1835819. [DOI] [PubMed] [Google Scholar]
- Les F.; Cásedas G.; Gómez C.; Moliner C.; Valero M. S.; López V. The Role of Anthocyanins as Antidiabetic Agents: From Molecular Mechanisms to in Vivo and Human Studies. J. Physiol Biochem 2021, 77 (1), 109–131. 10.1007/s13105-020-00739-z. [DOI] [PubMed] [Google Scholar]
- Zmora N.; Bashiardes S.; Levy M.; Elinav E. The Role of the Immune System in Metabolic Health and Disease. Cell Metab 2017, 25 (3), 506–521. 10.1016/j.cmet.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Ahmadi A.; Panahi Y.; Johnston T. P.; Sahebkar A. Antidiabetic Drugs and Oxidized Low-Density Lipoprotein: A Review of Anti-Atherosclerotic Mechanisms. Pharmacol. Res. 2021, 172, 105819. 10.1016/j.phrs.2021.105819. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and Physiological Roles of Inflammation. Nature 2008, 454 (7203), 428–435. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Gregersen S.; Samocha-Bonet D.; Heilbronn L. K.; Campbell L. v. Inflammatory and Oxidative Stress Responses to High-Carbohydrate and High-Fat Meals in Healthy Humans. J. Nutr Metab 2012, 2012, 1. 10.1155/2012/238056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.; Santani D. Role of NF-ΚB in the Pathogenesis of Diabetes and Its Associated Complications. Pharmacological Reports 2009, 61 (4), 595–603. 10.1016/S1734-1140(09)70111-2. [DOI] [PubMed] [Google Scholar]
- Giridharan S.; Srinivasan M. Mechanisms of NF-ΚB P65 and Strategies for Therapeutic Manipulation. J. Inflamm Res. 2018, 11, 407. 10.2147/JIR.S140188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M.; Konstantopoulos N.; Lee J.; Hansen L.; Li Z.-W.; Karin M.; Shoelson S. E. Reversal of Obesity- and Diet-Induced Insulin Resistance with Salicylates or Targeted Disruption of Ikkβ. Science 2001, 293 (5535), 1673–1677. 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Yung J. H. M.; Giacca A. Role of C-Jun N-Terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9 (3), 706. 10.3390/cells9030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. F.; Shibu M. A.; Fan M. J.; Chen M. C.; Viswanadha V. P.; Lin Y. L.; Lai C. H.; Lin K. H.; Ho T. J.; Kuo W. W.; Huang C. Y. Purple Rice Anthocyanin Extract Protects Cardiac Function in STZ-Induced Diabetes Rat Hearts by Inhibiting Cardiac Hypertrophy and Fibrosis. Journal of Nutritional Biochemistry 2016, 31, 98–105. 10.1016/j.jnutbio.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Vendrame S.; Daugherty A.; Kristo A. S.; Riso P.; Klimis-Zacas D. Wild Blueberry (Vaccinium Angustifolium) Consumption Improves Inflammatory Status in the Obese Zucker Rat Model of the Metabolic Syndrome. Journal of Nutritional Biochemistry 2013, 24 (8), 1508–1512. 10.1016/j.jnutbio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Guo H. H.; Xia M.; Zou T. B.; Ling W. H.; Zhong R. M.; Zhang W. G. Cyanidin 3-Glucoside Attenuates Obesity-Associated Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed and Db/Db Mice via the Transcription Factor FoxO1. Journal of Nutritional Biochemistry 2012, 23 (4), 349–360. 10.1016/j.jnutbio.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Wang X.; Zhang Z. F.; Zheng G. H.; Wang A. M.; Sun C. H.; Qin S. P.; Zhuang J.; Lu J.; Ma D. F.; Zheng Y. L. The Inhibitory Effects of Purple Sweet Potato Color on Hepatic Inflammation Is Associated with Restoration of Nad+ Levels and Attenuation of Nlrp3 Inflammasome Activation in High-Fat-Diet-Treated Mice. Molecules 2017, 22 (8), 1315. 10.3390/molecules22081315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.; Fang J. L.; Yuan K.; Jin S. H.; Guo Y. Ameliorative Effect of Purified Anthocyanin from Lycium Ruthenicum on Atherosclerosis in Rats through Synergistic Modulation of the Gut Microbiota and NF-ΚB/SREBP-2 Pathways. J. Funct Foods 2019, 59, 223–233. 10.1016/j.jff.2019.05.038. [DOI] [Google Scholar]
- Wang R.; Khalifa I.; Du X.; Li K.; Xu Y.; Li C. Effects of Anthocyanins on β-Lactoglobulin Glycoxidation: A Study of Mechanisms and Structure-Activity Relationship. Food Funct 2021, 12, 10550. 10.1039/D1FO01665B. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Hassan Y. I.; Renaud J.; Liu R.; Yang C.; Sun Y.; Tsao R. Bioaccessibility, Bioavailability, and Anti-Inflammatory Effects of Anthocyanins from Purple Root Vegetables Using Mono- and Co-Culture Cell Models. Mol. Nutr Food Res. 2017, 61 (10), 1600928. 10.1002/mnfr.201600928. [DOI] [PubMed] [Google Scholar]
- Krga I.; Milenkovic D. Anthocyanins : From Sources and Bioavailability to Cardiovascular- Health Bene Fi Ts and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. 10.1021/acs.jafc.8b06737. [DOI] [PubMed] [Google Scholar]
- Esatbeyoglu T.; Rodríguez-Werner M.; Schlösser A.; Winterhalter P.; Rimbach G. Fractionation, Enzyme Inhibitory and Cellular Antioxidant Activity of Bioactives from Purple Sweet Potato (Ipomoea Batatas). Food Chem. 2017, 221, 447–456. 10.1016/j.foodchem.2016.10.077. [DOI] [PubMed] [Google Scholar]
- Zhu B.; Wang X.; Li L. Human Gut Microbiome: The Second Genome of Human Body. Protein Cell 2010, 1 (8), 718–725. 10.1007/s13238-010-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon M. A.; Bird A. R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7 (1), 17–44. 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino G.; Inturri R.; Lazzara F.; di Rosa M.; Malaguarnera L. Impact of Gut Microbiota on Diabetes Mellitus. Diabetes Metab 2016, 42 (5), 303–315. 10.1016/j.diabet.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Moya-Pérez A.; Neef A.; Sanz Y. Bifidobacterium Pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PLoS One 2015, 10 (7), e0126976. 10.1371/journal.pone.0126976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N.; Gaboriau-Routhiau V. The Immune System and the Gut Microbiota: Friends or Foes?. Nat. Rev. Immunol 2010, 10 (10), 735–744. 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- Mahmassani Z. S.; Reidy P. T.; McKenzie A. I.; Petrocelli J. J.; Matthews O’C.; Hart N. M.; Ferrara P. J.; O’Connell R. M.; Funai K.; Drummond M. J. Absence of MyD88 from Skeletal Muscle Protects Female Mice from Inactivity-Induced Adiposity and Insulin Resistance. Obesity 2020, 28 (4), 772–782. 10.1002/oby.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalbert A.; Morand C.; Manach C.; Rémésy C. Absorption and Metabolism of Polyphenols in the Gut and Impact on Health. Biomedicine & Pharmacotherapy 2002, 56 (6), 276–282. 10.1016/S0753-3322(02)00205-6. [DOI] [PubMed] [Google Scholar]
- Scazzocchio B.; Varì R.; Filesi C.; D’Archivio M.; Santangelo C.; Giovannini C.; Iacovelli A.; Silecchia G.; Volti G. L.; Galvano F.; Masella R. Cyanidin-3-O-β-Glucoside and Protocatechuic Acid Exert Insulin-like Effects by Upregulating PPARγ Activity in Human Omental Adipocytes. Diabetes 2011, 60 (9), 2234–2244. 10.2337/db10-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scazzocchio B.; Varì R.; Filesi C.; del Gaudio I.; D’Archivio M.; Santangelo C.; Iacovelli A.; Galvano F.; Pluchinotta F. R.; Giovannini C.; Masella R. Protocatechuic Acid Activates Key Components of Insulin Signaling Pathway Mimicking Insulin Activity. Mol. Nutr Food Res. 2015, 59 (8), 1472–1481. 10.1002/mnfr.201400816. [DOI] [PubMed] [Google Scholar]
- Ormazabal P.; Scazzocchio B.; Varì R.; Santangelo C.; D’Archivio M.; Silecchia G.; Iacovelli A.; Giovannini C.; Masella R. Effect of Protocatechuic Acid on Insulin Responsiveness and Inflammation in Visceral Adipose Tissue from Obese Individuals: Possible Role for PTP1B. International Journal of Obesity 2018 42:12 2018, 42 (12), 2012–2021. 10.1038/s41366-018-0075-4. [DOI] [PubMed] [Google Scholar]
- Harini R.; Pugalendi K. V. Antihyperglycemic Effect of Protocatechuic Acid on Streptozotocin-Diabetic Rats. J. Basic Clin Physiol Pharmacol 2010, 21 (1), 79–92. 10.1515/JBCPP.2010.21.1.79. [DOI] [PubMed] [Google Scholar]
- Jokioja J.; Percival J.; Philo M.; Yang B.; Kroon P. A.; Linderborg K. M. Phenolic Metabolites in the Urine and Plasma of Healthy Men After Acute Intake of Purple Potato Extract Rich in Methoxysubstituted Monoacylated Anthocyanins. Mol. Nutr Food Res. 2021, 65 (9), 2000898. 10.1002/mnfr.202000898. [DOI] [PubMed] [Google Scholar]
- Kalt W.; Liu Y.; McDonald J. E.; Vinqvist-Tymchuk M. R.; Fillmore S. A. E. Anthocyanin Metabolites Are Abundant and Persistent in Human Urine. J. Agric. Food Chem. 2014, 62 (18), 3926–3934. 10.1021/jf500107j. [DOI] [PubMed] [Google Scholar]
- Song H.; Shen X.; Zhou Y.; Zheng X. Black Rice Anthocyanins Alleviate Hyperlipidemia, Liver Steatosis and Insulin Resistance by Regulating Lipid Metabolism and Gut Microbiota in Obese Mice. Food Funct 2021, 12 (20), 10160–10170. 10.1039/D1FO01394G. [DOI] [PubMed] [Google Scholar]
- Plovier H.; Everard A.; Druart C.; Depommier C.; van Hul M.; Geurts L.; Chilloux J.; Ottman N.; Duparc T.; Lichtenstein L.; Myridakis A.; Delzenne N. M.; Klievink J.; Bhattacharjee A.; van der Ark K. C. H.; Aalvink S.; Martinez L. O.; Dumas M. E.; Maiter D.; Loumaye A.; Hermans M. P.; Thissen J. P.; Belzer C.; de Vos W. M.; Cani P. D. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nature Medicine 2017, 23 (1), 107–113. 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- Petersen C.; Wankhade U. D.; Bharat D.; Wong K.; Mueller J. E.; Chintapalli S. v.; Piccolo B. D.; Jalili T.; Jia Z.; Symons J. D.; Shankar K.; Anandh Babu P. V. Dietary Supplementation with Strawberry Induces Marked Changes in the Composition and Functional Potential of the Gut Microbiome in Diabetic Mice. Journal of Nutritional Biochemistry 2019, 66, 63–69. 10.1016/j.jnutbio.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F.; Zhao R.; Xia M.; Shen G. X. Impact of Cyanidin-3-Glucoside on Gut Microbiota and Relationship with Metabolism and Inflammation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice. Microorganisms 2020, 8 (8), 1238. 10.3390/microorganisms8081238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H.; Xie L.; Xu Y.; Ke H.; Bao T.; Li Y.; Chen W. Pelargonidin-3- O-Glucoside Derived from Wild Raspberry Exerts Antihyperglycemic Effect by Inducing Autophagy and Modulating Gut Microbiota. J. Agric. Food Chem. 2020, 68 (46), 13025–13037. 10.1021/acs.jafc.9b03338. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Yang Y.; Wu Z.; Weng P. The Modulatory Effect of Anthocyanins from Purple Sweet Potato on Human Intestinal Microbiota in Vitro. J. Agric. Food Chem. 2016, 64 (12), 2582–2590. 10.1021/acs.jafc.6b00586. [DOI] [PubMed] [Google Scholar]
- Sun H.; Zhang P.; Zhu Y.; Lou Q.; He S. Antioxidant and Prebiotic Activity of Five Peonidin-Based Anthocyanins Extracted from Purple Sweet Potato (Ipomoea Batatas (L.) Lam.). Sci. Rep 2018, 8 (1), 1–12. 10.1038/s41598-018-23397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund K.; Hildebrand F.; Nielsen T.; Falony G.; le Chatelier E.; Sunagawa S.; Prifti E.; Vieira-Silva S.; Gudmundsdottir V.; Krogh Pedersen H.; Arumugam M.; Kristiansen K.; Yvonne Voigt A.; Vestergaard H.; Hercog R.; Igor Costea P.; Roat Kultima J.; Li J.; Jørgensen T.; Levenez F.; Dore J.; Bjørn Nielsen H.; Brunak S.; Raes J.; Hansen T.; Wang J.; Dusko Ehrlich S.; Bork P.; Pedersen O. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature 2015, 528 (7581), 262–266. 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Zeng B.; Zhang J.; Li W.; Mou F.; Wang H.; Zou Q.; Zhong B.; Wu L.; Wei H.; Fang Y. Role of the Gut Microbiome in Modulating Arthritis Progression in Mice. Sci. Rep 2016, 6 (4), 30594. 10.1038/srep30594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V. W. S.; Tse C. H.; Lam T. T. Y.; Wong G. L. H.; Chim A. M. L.; Chu W. C. W.; Yeung D. K. W.; Law P. T. W.; Kwan H. S.; Yu J.; Sung J. J. Y.; Chan H. L. Y. Molecular Characterization of the Fecal Microbiota in Patients with Nonalcoholic Steatohepatitis - A Longitudinal Study. PLoS One 2013, 8 (4), e62885. 10.1371/journal.pone.0062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahe L. K.; le Chatelier E.; Prifti E.; Pons N.; Kennedy S.; Hansen T.; Pedersen O.; Astrup A.; Ehrlich S. D.; Larsen L. H. Specific Gut Microbiota Features and Metabolic Markers in Postmenopausal Women with Obesity. Nutr Diabetes 2015, 5, e159 10.1038/nutd.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vadder F.; Kovatcheva-Datchary P.; Zitoun C.; Duchampt A.; Bäckhed F.; Mithieux G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab 2016, 24 (1), 151–157. 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Fossen T.; Øvstedal D. O.; Slimestad R.; Andersen Ø. M. Anthocyanins from a Norwegian Potato Cultivar. Food Chem. 2003, 81 (3), 433–437. 10.1016/S0308-8146(02)00473-9. [DOI] [Google Scholar]
- Xie L.; Mo J.; Ni J.; Xu Y.; Su H.; Xie J.; Chen W. Structure-Based Design of Human Pancreatic Amylase Inhibitors from the Natural Anthocyanin Database for Type 2 Diabetes. Food Funct 2020, 11 (4), 2910–2923. 10.1039/C9FO02885D. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Xie L.; Xie J.; Liu Y.; Chen W. Pelargonidin-3-O-Rutinoside as a Novel α-Glucosidase Inhibitor for Improving Postprandial Hyperglycemia. Chem. Commun. 2019, 55 (1), 39–42. 10.1039/C8CC07985D. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Jia Q.; Wang Y.; Zhang Y.; Xia M. The Anthocyanin Cyanidin-3-O-β-Glucoside, a Flavonoid, Increases Hepatic Glutathione Synthesis and Protects Hepatocytes against Reactive Oxygen Species during Hyperglycemia: Involvement of a CAMP-PKA-Dependent Signaling Pathway. Free Radic Biol. Med. 2012, 52 (2), 314–327. 10.1016/j.freeradbiomed.2011.10.483. [DOI] [PubMed] [Google Scholar]
- Jayaprakasam B.; Vareed S. K.; Olson L. K.; Nair M. G. Insulin Secretion by Bioactive Anthocyanins and Anthocyanidins Present in Fruits. J. Agric. Food Chem. 2005, 53 (1), 28–31. 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- Peng C. H.; Liu L. K.; Chuang C. M.; Chyau C. C.; Huang C. N.; Wang C. J. Mulberry Water Extracts Possess an Anti-Obesity Effect and Ability to Inhibit Hepatic Lipogenesis and Promote Lipolysis. J. Agric. Food Chem. 2011, 59 (6), 2663–2671. 10.1021/jf1043508. [DOI] [PubMed] [Google Scholar]
- Sarikaphuti A.; Nararatwanchai T.; Hashiguchi T.; Ito T.; Thaworanunta S.; Kikuchi K.; Oyama Y.; Maruyama I.; Tancharoen S. Preventive Effects of Morus Alba L. Anthocyanins on Diabetes in Zucker Diabetic Fatty Rats. Exp Ther Med. 2013, 6 (3), 689–695. 10.3892/etm.2013.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.; Qi X.; Liu Y.; Guo J.; Zhu R.; Chen W.; Zheng X.; Yu T. Dietary Supplementation with Purified Mulberry (Morus Australis Poir) Anthocyanins Suppresses Body Weight Gain in High-Fat Diet Fed C57BL/6 Mice. Food Chem. 2013, 141 (1), 482–487. 10.1016/j.foodchem.2013.03.046. [DOI] [PubMed] [Google Scholar]
- Singh S.; Netticadan T.; Ramdath D. D. Expression of Cardiac Insulin Signalling Genes and Proteins in Rats Fed a High-Sucrose Diet: Effect of Bilberry Anthocyanin Extract. Genes Nutr 2016, 11 (1), 8. 10.1186/s12263-016-0516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnajjar M.; Kumar Barik S.; Bestwick C.; Campbell F.; Cruickshank M.; Farquharson F.; Holtrop G.; Horgan G.; Louis P.; Moar K. M.; Russell W. R.; Scobbie L.; Hoggard N. Anthocyanin-Enriched Bilberry Extract Attenuates Glycaemic Response in Overweight Volunteers without Changes in Insulin. J. Funct Foods 2020, 64, 103597. 10.1016/j.jff.2019.103597. [DOI] [Google Scholar]
- Stote K. S.; Wilson M. M.; Hallenbeck D.; Thomas K.; Rourke J. M.; Sweeney M. I.; Gottschall-Pass K. T.; Gosmanov A. R. Effect of Blueberry Consumption on Cardiometabolic Health Parameters in Men with Type 2 Diabetes: An 8-Week, Double-Blind, Randomized, Placebo-Controlled Trial. Curr. Dev Nutr 2020, 4 (3), 1–10. 10.1093/cdn/nzaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour E. M.; Tanone I. I.; Urcuyo-Llanes D. E.; Lewis S. K.; Kirakosyan A.; Kondoleon M. G.; Kaufman P. B.; Bolling S. F. Blueberry Intake Alters Skeletal Muscle and Adipose Tissue Peroxisome Proliferator-Activated Receptor Activity and Reduces Insulin Resistance in Obese Rats. J. Med. Food 2011, 14 (12), 1511–1518. 10.1089/jmf.2010.0292. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Gonzalez de Mejia E.; Luna-Vital D.; Tao T.; Chandrasekaran S.; Chatham L.; Juvik J.; Singh V.; Kumar D. Relationship of Phenolic Composition of Selected Purple Maize (Zea Mays L.) Genotypes with Their Anti-Inflammatory, Anti-Adipogenic and Anti-Diabetic Potential. Food Chem. 2019, 289, 739–750. 10.1016/j.foodchem.2019.03.116. [DOI] [PubMed] [Google Scholar]
- Ju J. H.; Yoon H. S.; Park H. J.; Kim M. Y.; Shin H. K.; Park K. Y.; Yang J. O.; Sohn M. S.; Do M. S. Anti-Obesity and Antioxidative Effects of Purple Sweet Potato Extract in 3T3-L1 Adipocytes in Vitro. J. Med. Food 2011, 14 (10), 1097–1106. 10.1089/jmf.2010.1450. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Niu F. X.; Sun J.; Xu F.; Yue R. X. Purple Sweet Potato (Ipomoea Batatas L.) Color Alleviates High-Fat-Diet-Induced Obesity in SD Rat by Mediating Leptin’s Effect and Attenuating Oxidative Stress. Food Sci. Biotechnol 2015, 24 (4), 1523–1532. 10.1007/s10068-015-0196-7. [DOI] [Google Scholar]
- Qin S.; Sun D.; Mu J.; Ma D.; Tang R.; Zheng Y. Purple Sweet Potato Color Improves Hippocampal Insulin Resistance via Down-Regulating SOCS3 and Galectin-3 in High-Fat Diet Mice. Behavioural Brain Research 2019, 359, 370–377. 10.1016/j.bbr.2018.11.025. [DOI] [PubMed] [Google Scholar]
- Strugała P.; Dzydzan O.; Brodyak I.; Kucharska A. Z.; Kuropka P.; Liuta M.; Kaleta-Kuratewicz K.; Przewodowska A.; Michałowska D.; Gabrielska J.; Sybirna N. Antidiabetic and Antioxidative Potential of the Blue Congo Variety of Purple Potato Extract in Streptozotocin-Induced Diabetic Rats. Molecules 2019, 24 (17), 3126. 10.3390/molecules24173126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokioja J.; Linderborg K. M.; Kortesniemi M.; Nuora A.; Heinonen J.; Sainio T.; Viitanen M.; Kallio H.; Yang B. Anthocyanin-Rich Extract from Purple Potatoes Decreases Postprandial Glycemic Response and Affects Inflammation Markers in Healthy Men. Food Chem. 2020, 310, 125797. 10.1016/j.foodchem.2019.125797. [DOI] [PubMed] [Google Scholar]