ABSTRACT
As COVID-19 vaccines became widely available, there have been reports of neurovascular complications. In this article, we aim to report a case of cerebral venous sinus thrombosis (CVST) induced by COVID-19 vaccination, with a literature review on similar cases as well as the potential pathophysiological mechanisms. Our case is a healthy male who developed headache, vomiting, photophobia and diplopia after receiving the Ad26.COV2.S vaccine. Fundus examination showed papilledema, and magnetic resonance imaging of the brain and cerebral veins showed CVST involving the superior sagittal sinus and right transverse sinus extending into the right jugular vein. Hypercoagulability workup was unremarkable, and the patient received immunotherapy and anticoagulation. Following this treatment, symptoms resolved, and he had no residual neurologic deficits. Developing neurologic manifestations, especially severe headaches with papilledema, after COVID-19 vaccination should warrant neuroimaging. Early recognition and management of CVST are essential for good clinical outcomes.
INTRODUCTION
Several vaccines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became available for use during the COVID-19 pandemic. The Ad26.COV2.S vaccine is a recombinant replication-incompetent human adenovirus type 26 vector encoding full-length SARS-CoV-2 spike protein in a prefusion-stabilized conformation [1]. The ChAdOx1 nCoV-19 vaccine is another adenovirus-based vaccine that consists of a replication-deficient chimpanzee adenoviral vector ChAdOx1, containing the SARS-CoV-2 structural surface glycoprotein antigen (spike protein; nCoV-19) gene [2]. Both vaccines have been shown to be safe and efficacious in protecting against COVID-19 infection and reducing the risk of critical illness [3].
The most common side effects of COVID-19 vaccination include fatigue, headache and local pain around the injection site [4]. However, there have been rare cases of cerebral venous sinus thrombosis (CVST) associated with either the Ad26.COV2.S vaccine or ChAdOx1 nCoV-19 vaccine [5, 6]. CVST can present with headache or seizures and may involve elevated intracranial pressure [7]. When CVST follows a COVID-19 vaccination, it may be referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT) [8]. The proposed mechanism of VITT is similar to that of heparin-induced thrombotic thrombocytopenia (HITT) in terms of developing high levels of antibodies against the complexes of platelet factor 4 (PF4) and heparin with associated thrombocytopenia [6]. However, the immune response is triggered by the vaccine and considered to be heparin independent [8]. Medical literature discussed many VITT cases to date. Our report also offers a VITT–related CVST case in a young man with no prior history of thrombosis. We also provide a literature review on CVST associated with adenovirus vector-based vaccines, with discussion of proposed mechanisms and recent treatment guidelines.
CASE REPORT
A 28-year-old previously healthy man presented to the emergency room with severe bifrontal headaches for 2 days. The headaches were acute, throbbing and associated with blurred vision, diplopia, photophobia, nausea and vomiting, which worsened with coughing, bending forward and straining. His headaches were refractory to over-the-counter medications. He had no prior headaches. His maternal grandmother had a brain aneurysm diagnosed at the age of 50 years. He otherwise had no family history of headaches or clotting disorders. He was not on any medications but received the Ad26.COV2.S vaccine 10 days prior to symptom onset. A fundus exam revealed papilledema, and the rest of his physical and neurologic examination was otherwise unremarkable.
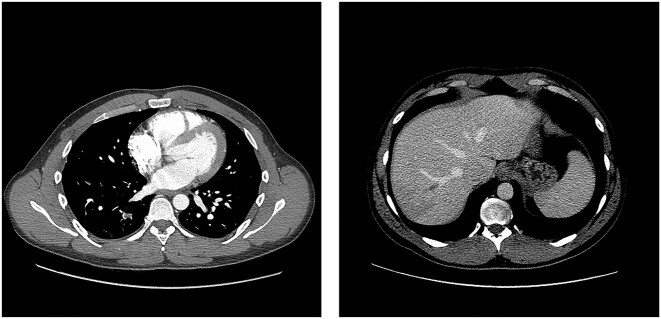
Magnetic resonance venography (MRV) of the brain showed filling defects within the superior sagittal sinus as well as the right transverse sinus extending into the right jugular vein (Fig. 1), thereby giving him the diagnosis of CVST. The initial laboratory workup was remarkable for platelet count 63 000/μL, international normalized ratio (INR) 1.5, partial thromboplastin time (PTT) 36.3 s and D-dimer 22 546 ng/mL. Computed tomography angiography (CTA) of the chest and abdomen showed multiple segmental and subsegmental pulmonary emboli throughout the right lung and occluded right hepatic vein with wedge-shaped hepatic segment hypodensity (Fig. 2). Venous duplex ultrasound of the bilateral lower extremities was unremarkable. An extensive workup for hypercoagulability was performed. Serum SARS-CoV-2 and PF4 antibodies were checked because of his recent COVID-19 vaccination and were positive. Along with ruling out other causes of hypercoagulability, patient's labs confirmed the suspicion for VITT. Noted that the routine COVID-19 PCR test for admission was negative.
Figure 1.
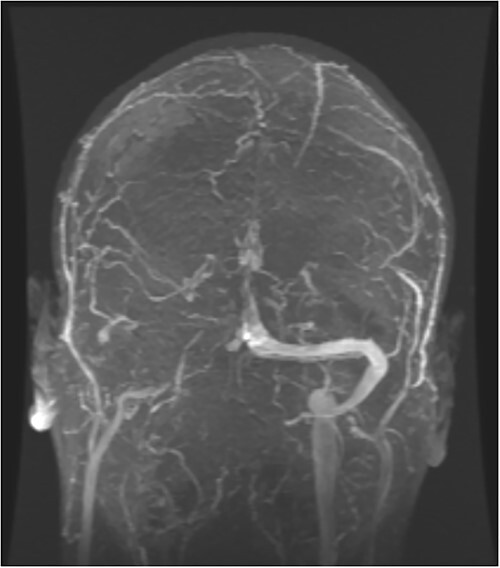
MRV filling defect within the superior sagittal sinus, as well as the right transverse sinus extending into the right jugular vein.
Figure 2.
Shows multiple segmental and subsegmental pulmonary emboli throughout the right lung and occluded right hepatic vein with wedge-shaped hepatic segment hypodensity.
Per the VITT protocol [9], the patient received intravenous immunoglobulins (IVIG) (1 g/kg/day) for 2 days and a continuous infusion of argatroban for 6 days, followed by oral dabigatran 150 mg two times daily. His headaches and nausea improved with supportive measures, and his diplopia soon resolved. He was discharged 2 days after being switched to oral anticoagulation. He did not have any residual neurologic deficits at the time of discharge, and normalization of platelet count was achieved prior to discharge.
DISCUSSION
CVST has to date been reported in a small number of cases after receiving the SARS-CoV2 adenoviral vector vaccines (Table 1). In these cases, the typical onset of symptoms was 1–2 weeks after vaccination. The reported clinical features included new-onset severe persistent headache, focal neurologic symptoms, visual changes and seizures [10]. In most of the cases, other clinical findings including severe abdominal pain, fever and shortness of breath have accompanied the neurological symptoms, which was indicative of systemic thrombosis [11].
Table 1.
Demographics, clinical presentation, treatment and outcomes in patients with CVST post-vector-based SARS-CoV2 vaccines administration
| Author | Age and gender | Presenting symptoms | Duration between vaccination and symptom onset | Administered vaccine | Lowest platelet count (109/L) | Clotting screen on admission | Peak D-Dimer levels | PF4 antibody ELISA | Neuroimaging findings | Extracranial thrombosis | Treatment | Residual neurologic deficits |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Our case report | 28 M | Headache Blurry vision Diplopia Photophobia | 10 days | Ad26.COV2.S | 63 | PT: 18.3 INR: 1.5 APTT: 36.3 | 22 546 | Positive | Thrombosis of superior sagittal and right transverse sinuses, and right IJV | Right segmental and subsegmental PE, Right hepatic vein thrombosis | Argatroban, dabigatran, and IVIG | Recovery with no residual neurologic deficits |
| Graf | 29 M | Headache Aphasia Apraxia | 9 days | ChAdOx1 nCoV-19 | 32 | NA | 65.7 | NA | Left transverse, sigmoid sinuses and IJV thrombosis, Temporo-parietal ICH | Mesenteric and portal vein thrombosis | Argatroban and IVIG | Aphasia improved |
| Soleimani | 34 M | Headache Photophobia Hemiparesis | 13 days | ChAdOx1 nCoV-19 | 23 | PT: 14.8 INR: 1.5 APTT: 23.9 | 37 293 | Positive | Superior sagittal, bilateral transverse sinuses, and left vein of Trolard thrombosis, Right sided midline shit and uncal herniation | Right lower lobe segmental PE | PLEX, IVIG, argatroban, decompressive hemicraniectomy | Right hemiparesis and aphasia |
| Soleimani | 59 F | Headache Hemiparesis Sensory loss Neglect Seizure | 14 days | ChAdOx1 nCoV-19 | 18 | PT: 14.5 INR: 1.2 APTT: 24.4 | 38 588 | Positive | Superior sagittal and right transverse sinus thrombosis, Frontal hematoma and midline shift. | Thrombosed right hepatic vein and right lower lobe pulmonary emboli | IVIG, IVSM, argatroban, and decompressive hemicraniectomy | Left hemiparesis |
| Soleimani | 39 F | Headache Photophobia | 10 days | ChAdOx1 nCoV-19 | 63 | PT: 15.1 INR: 1.1 APTT: 33.2 | 20 289 | Negative | Left transverse, sigmoid, and sagittal sinuses and jugular venous thrombosis | None | IVSM, PLEX and argatroban | None |
| Zanferrari | 40 F | Headache Aphasia Hemiparesis | 8 days | ChAdOx1 nCoV-19 | 27 | APTT: 24.9 | 27 546 | Positive | Left sigmoid, transverse rectus and inferior longitudinal sinuses thrombosis | None | Fondaparinux and IVIG | None |
| Castelli | 50 M | Headache Vision loss Hemiparesis | 11 days | ChAdOx1 nCoV-19 | 20 | NA | >10 000 | Negative | Left hemisphere ICH, and lack of opacification of the left transverse and sigmoid sinuses, Uncal herniation | None | Bilateral decompressive craniectomy | Death |
| De Michele | 57 W | Hemiparesis Gaze deviation Dysarthria Neglect | 9 days | ChAdOx1 nCoV-19 | 23 | NA | elevated | Positive | Right MCA infarct | None | IV steroids IVIG Decompressive craniectomy | Critical condition |
| De Michele | 55 W | Aphasia Hemiparesis Seizures Coma Abdominal pain | 10 days | ChAdOx1 nCoV-19 | 59 | NA | elevated | Positive | Right ICA and bilateral MCA occlusion, Uncal herniation | Portal vein thrombosis, Left lower lobe subsegmental PE | IVIG, and IV steroid | Death |
(Continued)
Table 1.
Continued
| Author | Age and gender | Presenting symptoms | Duration between vaccination and symptom onset | Administered vaccine | Lowest platelet count (109/L) | Clotting screen on admission | Peak D-Dimer levels | PF4 antibody ELISA | Neuroimaging findings | Extracranial thrombosis | Treatment | Residual neurologic deficits |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayas | 55 W | Chemosis Orbital pain Diplopia Hemiparesis Aphasia Seizure | 10 days | ChAdOx1 nCoV-19 | 30 | NA | NA | Positive | Superior ophthalmic vein thrombosis. Ischemic stroke in the MCA territory | None | IV steroids | NA |
| D’Agostino | 54 W | Confusion | 12 days | ChAdOx1 nCoV-19 | NA | PT: 33.2 APPT: 41 | elevated | NA | Right frontal and temporal lobes ICH, Right superior longitudinal sinus, vein of Galen and superior sagittal thrombosis | Floating thrombus within the aortic arch. | Balloon angioplasty of the right coronary artery | Death |
| Blauenfeldt | 60 W | Headache Abdominal pain Confusion | 7 days | ChAdOx1 nCoV-19 | 5 | INR: 1 APPT: 28 | 106 200 | Negative | Infarction in the right MCA territory | None | Platelet concentrate | Death |
| Wolf | 22 W | Headache Seizure | 4 days | ChAdOx1 nCoV-19 | 75 | NA | 2590 | Positive | SAH, superior sagittal, left transverse and sigmoid sinuses thrombosis | None | Enoxaparin, followed by dabigatran | None |
| Wolf | 46 F | Headache Aphasia Homonymous hemianopia | 8 days | ChAdOx1 nCoV-19 | 60 | NA | 22 800 | Positive | Superior sagittal, transverse and sigmoid sinuses thrombosis, acute left occipital lobe ICH. | None | Rheolysis via balloon angioplasty, Enoxaparin followed by danaparoid, followed by dabigatran | Mild residual deficits (mRS 1) |
| Wolf | 36 F | Headache Aphasia Confusion | 7 days | ChAdOx1 nCoV-19 | 92 | NA | 2120 | Positive | Thrombosis of the straight and superior sagittal sinuses, Bilateral thalamic edema | None | Danaparoid, followed by Enoxaparin, and dabigatran | None |
| Mehta | 32 M | Headache Hemiparesis Ataxia | 9 days | ChAdOx1 nCoV-19 | 30 | NA | NA | NA | Superior sagittal sinus and cortical vein thrombosis and significant cortical oedema with small areas of parenchymal and SAH | None | None | Death |
| Mehta | 25 M | Headache Photophobia Vomiting Rash Gum bleeding Hemiparesis Ataxia | 6 days | ChAdOx1 nCoV-19 | 19 | NA | NA | Positive | Superior sagittal sinus thrombosis extending into the cortical veins, SAH and ICH | None | IV unfractionated heparin, platelet transfusions, IV dexamethasone, IVIG | Death |
| Suresh | 27 M | Headaches Vomiting Homonymous hemianopia | 2 days | ChAdOx1 nCoV-19 | 73 | PT: 12.9 APTT: 27.5 | 34 071 | Positive | Right transverse venous sinus thrombosis, ICH in the right parietal lobe | None | IVIG Dabigatran Prednisone | Death |
(Continued)
Table 1.
Continued
| Author | Age and gender | Presenting symptoms | Duration between vaccination and symptom onset | Administered vaccine | Lowest platelet count (109/L) | Clotting screen on admission | Peak D-Dimer levels | PF4 antibody ELISA | Neuroimaging findings | Extracranial thrombosis | Treatment | Residual neurologic deficits |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| George | 40 W | Headache | 7 days | Ad26.COV2.S | 20 | APTT: 26.4 | 27 150 | Negative | Left transverse and sigmoid sinuses thrombosis | Right PE | Bivalirudin IVIG | Recovery with no residual neurologic deficits |
| Muir | 48 W | Malaise Abdominal pain | 14 days | Ad26.COV2.S | 13 | APTT: 41 | 117 500 | Positive | Right transverse and straight sinuses thrombosis | Splanchnic, right hepatic and splenic venous thrombosis | Heparin switched to argatroban and IVIG | Patient remained in critical condition |
| See | ≥40 F | Headaches Hemiparesis | 6 | Ad26.COV2.S | 43 | INR: 1.4 APTT: 31 | >20 | NA | Right transverse, sigmoid and Left IJV thrombosis, Right temporoparietal ICH | None | NA | NA |
| See | 18–39 F | Headaches Aphasia | 9 | Ad26.COV2.S | 78 | INR: 1.2 APTT: 22.3 | 1.1 | Positive | Left transverse, sigmoid sinus, confluence of sinuses, and straight sinus thrombosis, Left temporal lobe ICH | None | NA | NA |
| See | 18–39 F | Headaches Hemiparesis Gaze deviation Neglect Seizure | 8 | Ad26.COV2.S | 18 | INR: 1.5 APTT: 31.1 | 8.46 | Positive | Superior and inferior sagittal, straight sinuses and cortical venous thrombosis, Bilateral frontal ICH, right SAH and IVH | None | NA | NA |
| See | 18–39 F | Headaches | 8 | Ad26.COV2.S | 127 | INR: 1.1 APTT:31.2 | 5.45 | Positive | Right transverse and sigmoid sinuses thrombosis | Portal vein thrombosis and right pulmonary embolus | NA | NA |
| See | 18–39 F | Headaches | 6 | Ad26.COV2.S | 10 | INR: 1.1 APTT: 18.1 | 7.05 | Positive | Right transverse and sigmoid sinuses thrombosis | Bilateral lower extremity DVT | NA | NA |
| See | ≥40 F | Headache | 13 | Ad26.COV2.S | 13 | INR: 1.2 | 112.07 | Positive | Right transverse and straight sinuses thrombosis, Right IVJ thrombosis, Right occipital ICH | Portal, splenic, right hepatic, distal superior mesenteric venous thrombosis, Right posterior tibial and peroneal DVT | NA | NA |
| See | 18–39 F | Headache Photophobia | 15 | Ad26.COV2.S | 64 | INR: 0.9 APTT: 28 | 7.84 | Positive | Superior sagittal, transverse, straight and sigmoid sinuses thrombosis, Right IJV thrombosis | None | NA | NA |
(Continued)
Table 1.
Continued
| Author | Age and gender | Presenting symptoms | Duration between vaccination and symptom onset | Administered vaccine | Lowest platelet count (109/L) | Clotting screen on admission | Peak D-Dimer levels | PF4 antibody ELISA | Neuroimaging findings | Extracranial thrombosis | Treatment | Residual neurologic deficits |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| See | 18–39 F | Headache | 10 | Ad26.COV2.S | 90 | INR: 1.1 APTT: 26.9 | 6.7 | Positive | Right transverse, sigmoid sinuses thrombosis, Right IJV thrombosis | Lower extremity DVT and PE | NA | NA |
| See | ≥40 F | Headache Confusion Hemiparesis Aphasia Seizure | 7 | Ad26.COV2.S | 15 | INR: 1.2 APTT: 24.1 | >4 | Positive | Superior sagittal sinus, bilateral cortical venous thrombosis | None | NA | NA |
| See | 18–39 F | Headaches Photophobia Comma Seizure | 7 | Ad26.COV2.S | 9 | INR: 1.2 APTT: 30.2 | 13.47 | Positive | Superior sagittal, right transverse and sigmoid sinuses thrombosis, Right temporal lobe and left cerebellar hemisphere ICH, SAH | None | NA | NA |
| See | 18–39 F | Headaches Neck stiffness Blurry vision | 11 | Ad26.COV2.S | 102 | APTT: 26.4 | 41.71 | Positive | Torcula, bilateral transverse, right sigmoid sinuses thrombosis, Bilateral IJV thromboses, Right posterior temporal ICH | None | NA | NA |
| See | ≥40 F | Headaches Neck pain Photophobia | 6 | Ad26.COV2.S | 20 | 45.57 | Positive | Left transverse and sigmoid sinuses thrombosis, Left IJV thromboses | Right PE | NA | NA |
Based on these reports, initial recommended investigations included complete blood count, platelet count, coagulation studies (Prothrombin time (PT), INR and PTT), D-dimer, fibrinogen and peripheral blood smear to rule out pseudo-thrombocytopenia and other causes of thrombocytopenia [12]. Other differential diagnoses to evaluate for include: DIC, sepsis, malignancy, thrombotic microangiopathy, systemic lupus erythematosus, antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria and sickle cell anemia, for which investigations were performed in our case [13]. Similar to the 4Ts score used in HITT evaluation, there has been a proposed 4Ts score for VITT evaluation to improve the diagnostic certainty [14]. The 4Ts score in VITT depends on the degree of thrombocytopenia, the timeline of symptom onset, the history of thrombosis and the presence of alternative diagnosis of thrombosis [15]. Our case had a 4Ts score of 8, which indicated a high probability of VITT.
For treating our patient, we followed the latest guidelines which recommend anticoagulation with direct oral anticoagulants (rivaroxaban, apixaban or dabigatran) or fondaparinux, and treatment with IVIG (1 g/kg/day for 2 days) [6]. Anticoagulation with heparin or warfarin should be avoided [16]. Platelet count should be monitored for recovery, and recovery itself is identified as platelet count of > 150 × 109/mm3 [17]. Even in some cases of intracranial hemorrhage, anticoagulation should be considered to prevent progressive thrombosis. Our patient tolerated the treatment with no further complications and achieved complete normalization of his platelet count and neurological symptoms.
There are a few proposed pathophysiologic mechanisms implicated in CVST development. One of these is VITT which is similar to HITT in its pathophysiology. HITT results from the formation of immune complexes consisting of autoantibodies against PF4 and heparin. These immune complexes bind to the surface of platelets and monocytes, provoking their activation by cross-linking Fc γIIA receptors [18]. In the case of VITT, it is believed that the leakage of DNA from the adenovirus infected cells binds to PF4 and triggers the production of autoantibodies [19]. Our case tested positive for PF4 autoantibodies as in previously reported CVST cases associated with Ad26.COV2.S vaccine, which might indicate a high probability of VITT being the underlying pathophysiology of CVST in this patient. Another proposed mechanism of VITT may be independent of PF4 autoantibodies. Viral vector-based COVID-19 vaccines contain high amounts of viral particles, which may be distributed across different body tissues including the brain. The COVID-19 adenoviral vectors might trigger an immune response in the brain, leading to localized vascular thrombosis [20]. According to the reported cases in Table 1, this mechanism could explain CVST in patients who tested negative for PF4 autoantibodies. Interestingly, those patients did not have any associated systemic thrombosis and were reported to have CVST secondary to ChAdOx1 nCoV-19 vaccine.
Despite these recent reports of COVID-19 vaccine-associated CVST, it should be noted that COVID-19 infection itself has been suggested to be a more significant risk factor for CVST. A retrospective study showed that the incidence of CVST after COVID-19 was 39.0 per million (95% CI, 25.2–60.2) compared with any 2-week period in the pre-COVID-19 [21]. There have been a few epidemiologic studies to assess the incidence of CVST during postvaccination period, which has shown conflicting evidence regarding the presence of increased incidence of CVST postvaccination. Epidemiologic studies in Europe regarding the ChAdOx1 nCoV-19 vaccine showed that the incidence of CVST has significantly increased after COVID-19, and greater than what was observed with COVID-19 mRNA vaccines and ChAdOx1 nCoV-19 vaccine [21]. However, when comparing the incidence rate between mRNA-based vaccines and vector-based vaccines, specifically ChAdOx1 nCoV-19 vaccine, the incidence rate was higher in individuals who received ChAdOx1 nCoV-19 [22]. In another epidemiologic study in the United States, there was no increased risk of CVST in the 30 days prior to COVID-19 vaccination (Pfizer-BioNTech, Moderna and Johnson & Johnson) compared with the 30 days after vaccination [23].
CONCLUSION
Although headaches may be a common side effect of COVID-19 vaccinations, a headache with increased severity should warrant further neuroimaging, and a fundus exam must be performed to assess for intracranial hypertension, a common feature of CVST. Following a thorough workup for hypercoagulability, we were able to intervene in a timely manner, which resulted in an excellent outcome with no residual symptoms or neurological deficits. Our case demonstrates the impact of early recognition of symptoms and signs of CVST in the setting of headache following COVID-19 vaccination.
Supplementary Material
ACKNOWLEDGEMENTS
None.
Contributor Information
Mohamed Elfil, Department of Neurological Sciences, University of Nebraska Medical Center, Omaha, NE, USA.
Mohammad Aladawi, Department of Neurological Sciences, University of Nebraska Medical Center, Omaha, NE, USA.
Dmitry Balian, Department of Neurological Sciences, University of Nebraska Medical Center, Omaha, NE, USA.
Ismail Fahad, Department of Neurological Sciences, University of Nebraska Medical Center, Omaha, NE, USA.
Daniel J Zhou, Department of Neurological Sciences, University of Nebraska Medical Center, Omaha, NE, USA.
Brian Villafuerte-Trisolini, Department of Neurological Sciences, University of Nebraska Medical Center, Omaha, NE, USA.
Thomas Scott Diesing, Department of Neurological Sciences, University of Nebraska Medical Center, Omaha, NE, USA.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This study has not received any funding.
ETHICAL APPROVAL
This research meets the ethical guidelines and adheres to local legal requirements.
INFORMED CONSENT/INSTITUTIONAL APPROVAL
The authors have obtained a signed informed consent from the patient.
GUARANTOR
Mohamed Elfil.
AUTHORS’ CONTRIBUTIONS
M.A.: contributed with drafting and revision of the manuscript, acquisition of data, study concept and design, organization and interpretation of data. M.E.: contributed with drafting and revision of the manuscript, acquisition of data, study concept and design, organization and interpretation of data. D.B.: contributed with the acquisition of data and revision of the manuscript. I.F.: contributed with the acquisition of data and revision of the manuscript. D.J.Z.: contributed with the acquisition of data and revision of the manuscript. B.V.-T.: contributed with the acquisition of data and revision of the manuscript. T.S.D.: contributed with drafting and revision of the manuscript, study concept and design, organization and interpretation of data, final revision and approval of the manuscript.
REFERENCES
- 1. Bos R, Rutten L, van der Lubbe JEM, Bakkers MJG, Hardenberg G, Wegmann F et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020;5:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396:1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 2021;21:939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. George G, Friedman KD, Curtis BR, Lind SE. Successful treatment of thrombotic thrombocytopenia with cerebral sinus venous thrombosis following Ad26.COV2.S vaccination. Am J Hematol 2021;96:E301–3. [DOI] [PubMed] [Google Scholar]
- 6. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo Y, Tian X, Wang X. Diagnosis and treatment of cerebral venous thrombosis: a review. Front Aging Neurosci 2018;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yocum A, Simon EL. Thrombotic thrombocytopenic purpura after Ad26.COV2-S vaccination. Am J Emerg Med 2021;49:e3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bourguignon A, Arnold DM, Warkentin TE, Smith JW, Pannu T, Shrum JM et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med 2021;385:720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F, Investigators I. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 2004;35:664–70. [DOI] [PubMed] [Google Scholar]
- 11. De Michele M, Iacobucci M, Nicolini E, Chistolini A, Pulcinelli F, Cerbelli B et al. Malignant cerebral infarction, systemic venous thrombosis and thrombocytopenia after ChAdOx1 nCov vaccination: a possible catastrophic variant of vaccine induced thrombotic thrombocytopenia. Res Sq 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv 2018;2:3360–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Warkentin TE. An overview of the heparin-induced thrombocytopenia syndrome. Semin Thromb Hemost 2004;30:273–83. [DOI] [PubMed] [Google Scholar]
- 14. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood 2012;120:4160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobson BF, Schapkaitz E, Mer M, Louw S, Haas S, Buller HR et al. Recommendations for the diagnosis and management of vaccine-induced immune thrombotic thrombocytopenia. S Afr Med J 2021;111:535–7. [PubMed] [Google Scholar]
- 16. Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J 2007;83:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pon TK, Mahajan A, Rosenberg A, Amin A, Shah D, Jenkins I et al. Platelet response to direct thrombin inhibitor or fondaparinux treatment in patients with suspected heparin-induced thrombocytopenia. J Thromb Thrombolysis 2018;45:536–42. [DOI] [PubMed] [Google Scholar]
- 18. Fathi M. Heparin-induced thrombocytopenia (HIT): identification and treatment pathways. Glob Cardiol Sci Pract 2018;2018:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Douxfils J, Favresse J, Dogne JM, Lecompte T, Susen S, Cordonnier C et al. Hypotheses behind the very rare cases of thrombosis with thrombocytopenia syndrome after SARS-CoV-2 vaccination. Thromb Res 2021;203:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunter PR. Thrombosis after covid-19 vaccination. BMJ 2021;373:n958. [DOI] [PubMed] [Google Scholar]
- 21. Torjesen I. Covid-19: risk of cerebral blood clots from disease is 10 times that from vaccination, study finds. BMJ 2021;373:n1005. [DOI] [PubMed] [Google Scholar]
- 22. Schulz JB, Berlit P, Diener HC, Gerloff C, Greinacher A, Klein C et al. COVID-19 vaccine-associated cerebral venous thrombosis in Germany: a descriptive study. medRxiv. 2021:2021.04.30.21256383. [DOI] [PMC free article] [PubMed]
- 23. Pawlowski C, Rincón-Hekking J, Awasthi S, Pandey V, Lenehan P, Venkatakrishnan AJ et al. Cerebral venous sinus thrombosis is not significantly linked to COVID-19 vaccines or non-COVID vaccines in a large multi-state health system. J Stroke Cerebrovasc Dis 2021;30:105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.