Abstract
The PRXamide neuropeptides have been described in both protostome and deuterostome species, including all major groups of the Panarthropoda. Best studied are the insect PRXamides consisting of three genes: pk/pban, capa, and eth, each encoding multiple short peptides that are cleaved post-translationally. Comparisons of genome and transcriptome sequences reveal that while retaining its fundamental ancestral organization, the products of the pk/pban gene have undergone significant change in the insect Order Diptera. Basal dipteran pk/pban genes are much like those of other holometabolous insects, while more crown species have lost two peptide coding sequences including the otherwise ubiquitous pheromone biosynthesis activating neuropeptide (PBAN). In the genomic model species Drosophila melanogaster, one of the remaining peptides (hugin) plays a potentially novel role in feeding and locomotor regulation tied to circadian rhythms. Comparison of peptide coding sequences of pk/pban across the Diptera pinpoints the acquisition or loss of the hugin and PBAN peptide sequences respectively, and provides clues to associated changes in life history, physiology, and/or behavior. Interestingly, the neural circuitry underlying pk/pban function is highly conserved across the insects regardless of the composition of the pk/pban gene. The rapid evolution and diversification of the Diptera provide many instances of adaptive novelties from genes to behavior that can be placed in the context of emerging selective pressures at key points in their phylogeny; further study of changing functional roles of pk/pban may then be facilitated by the high-resolution genetic tools available in Drosophila melanogaster.
Keywords: diptera, pheromone biosynthesis activating protein, hugin, diapause hormone, capa
Neuropeptides (NPs) are short signaling peptides that are produced and released by neurons of the central nervous system, acting as juxtacrine, paracrine, and/or endocrine signals. NP genes can be recognized across taxa and share several key features (Jékely 2013, Mirabeau and Joly 2013, Elphick et al. 2018). At the synapse, NPs are packaged in dense core vesicles distinct from the clear vesicles containing small molecule transmitters; NPs can also be released from nonsynaptic areas of the cell membrane. NP genes encode an mRNA that is translated into a longer prepropeptide and subsequently enzymatically cleaved into multiple functional NPs with post-translational modifications (e.g., C-terminal amidation; Mains and Eipper 1999a). Recognition sites for peptide cleavage and post-translational modifications are often conserved across taxa as well. Nonetheless, functional NPs generated from the prepropeptide are often taxon-specific, and within species may vary according to cell type and developmental stage. Thus, a single NP gene may play multiple roles both within and between species. Additionally, NPs act through G-protein coupled receptors, and cross-reactivity of multiple related products of a single NP gene may occur (Mains and Eipper 1999b).
Evolutionary History of Neuropeptides
Neuropeptide signaling is ubiquitous and highly conserved, with representatives of two metazoan NP gene families present even in single celled choanoflagellates, the likely sister group to the Metazoa. Cnidaria and Ctenophora have many NP genes in their genomes but most do not appear homologous to those of the Bilateria (Hayakawa et al. 2019); rather, most extant neuropeptide signaling systems have arisen, expanded, and diversified in the Bilateria (Li et al. 1999, Hewes and Taghert 2001, Burke et al. 2006, Conzelmann et al. 2013, Jékely 2013, Mirabeau and Joly 2013, Roch and Sherwood 2014, Elphick et al. 2018, Yañez-Guerra et al. 2022). Based on the widespread presence of NPs it is likely that as a group they serve fundamental core functions while at the same time remaining amenable to further adaptation and diversification. The availability of genome and transcriptome sequences for an increasing number of organisms allows comparisons of identified NP systems and reconstruction of changes in their makeup across taxa in relation to adaptations associated with species diversification. These analyses can thus provide a framework for understanding how even a highly conserved regulatory system has been differentially influenced by taxon-specific selection for life history, physiology, and behavior.
This paper begins as a review of the insect PRXamide neuropeptide system, followed by analysis of publicly available transcriptomes and genomes in one insect Order, the Diptera. Peptides in this family have been the subject of studies in diverse species such as cockroaches, locusts, moths, and flies; in particular, in the genomic model organism Drosophila melanogaster (Meigen, Diptera, Drosophilidae) PRXamide cells and circuits have been dissected in tremendous detail. Intriguingly, along with fundamentally conserved features of this system, Drosophila PRXamide structure and function illustrate some apparent novelties. Pairing this high-resolution template with comparative studies, especially in species carefully chosen for their place in the dipteran phylogeny and potentially key life history traits, will provide a sandbox for explorations of the nature of conservation, divergence, and evolutionary novelty in a framework encompassing adaptive modifications of genes, neurons, circuits, and behaviors.
Structure of PRXamide Genes
The PRXamide NPs are ubiquitous across the protostomes (Jékely 2013, Mirabeau and Joly 2013; reviewed in Jurenka 2015, Elphick et al. 2018). In the Insecta where they are best characterized, PRXamide genes are highly conserved. Pheromone biosynthesis activating neuropeptide (PBAN) and 1–3 pyrokinins (PK), all characterized by an FXPRLamide C-terminus, are encoded on the pk/pban gene (termed hugin in Diptera, pban in Lepidoptera; Veenstra 2014). The pyrokinins (PKs) are confusingly referred to by several different monikers, including the subesophageal ganglion neuropeptides (α- β- and γ- SGNP) and hugin-γ/hugin (Sato et al. 1993, Bader, Wegener et al. 2007, Jurenka and Nusawardani 2011, Fodor et al. 2017). The capa gene encodes 1–2 periviscerokinins (referred to as CAPA-PVKs) that can be identified by a C-terminal sequence of FPRV/Iamide (Hull et al. 2021). Both capa and pk/pban genes also typically encode a tryptopyrokinin (trypto-PK) with a C-terminal sequence of WFGPRLamide; these peptides are designated diapause hormones DH1 and DH2, respectively (Jurenka 2015). Finally, ecdysis triggering hormone (ETH, usually encoded in two copies on the eth gene) is characterized by a longer consensus sequence, FFLKASKNVPRIamide or FFLKASKSVPRIamide (Melcher et al. 2006, Jurenka 2015). A regulator of ecdysis, the eth gene arose at the base of the Panarthropoda (de Oliveira et al. 2019). It is also not a true neuropeptide as it is produced by nonneuronal inka cells in the epitracheal gland (Žitňan et al. 1996); thus, the biology and evolution of eth will not be considered further here.
The deuterostome ortholog of insect PRXamides is neuromedin U (Predel and Nachman 2006, Lindemans et al. 2009, Jurenka 2015). In addition to conservation of neuropeptide genes, the G-protein coupled receptors (GPCRs) for PRXamides have coevolved with their ligands such that cross-phyla reactivity of ligands and receptors is possible. For example, the neuromedin U peptide is capable of binding and activating the PBAN GPCR (Choi et al. 2003).
Evolutionary History of PRXamides in Arthropods
The evolutionary history of PRXamides in arthropods includes duplications across and within genes. A duplication of the phylogenetically oldest PRXamide gene (likely retained as the modern eth gene) gave rise to a gene with properties of both pk/pban and capa, termed pk/capa and containing both PK and PVK sequences (although in some species only PVKs have been identified; Neupert, Russell et al. 2009, Veenstra et al. 2012). A pk/capa gene has been identified in all of the major arthropod groups: the chelicerates, myriapods, and Pancrustacea (Derst et al. 2016). In species of tick (Acari) and Crustacea these neuropeptides are expressed in tissues including the nervous system (CNS) and feeding structures, and like the PRXamides of insects are capable of regulating muscle contraction (Torfs et al. 2001, Xiong et al. 2022). PKs also play neuromodulatory roles in the neural circuitry of the stomatogastric ganglion in the crustacean Cancer borealis (Stimpson, Decapoda, Cancridae) (Saideman et al. 2007, Dickinson et al. 2015, Yang et al. 2015).
A single gene encoding PKs, PVKs, and a newly acquired trypto-PK is observed in basal Hexapoda and in Xilbalbanus tulumensis (Yager, Nectiopoda, Speleonectidae), a species of the Remipedia and likely sister group to the Hexapoda (Derst et al. 2016, Diesner et al. 2021). Duplication of this gene into a PVK-encoding gene containing a trypto-PK (DH1) sequence (capa) and a separate PK-encoding gene (pk/pban; subsequently acquiring its own trypto-PK (DH2) sequence) occurred twice in basal Hexapoda, once in the Diplura (Campodea augens (Silvestri, Diplura, Campodeidae)) and in the apterygote insects belonging to the Zygentoma (Thermobia domestica (Packard, Zygentoma, Lepismatidae)). The latter is thought to represent the common ancestor possessing separate capa and pk/pban genes that are characteristic of extant insects. Interestingly, while species of the basal hexapod group Collembola possess a single gene, it produces two splice isoforms, one with PVKs and one with PKs. This separation of PVK and PK peptide production by one means or another appears to be ubiquitous in insects; as Derst et al suggest, early hexapods seem to have undergone strong selection for independent regulation of PRXamide gene products.
In most modern insect species, especially within the Holometabola, the makeup of the capa and pk/pban genes and their loci of expression and function are generally conserved. However, given the long evolutionary history and diversity of the insects, variation in the NPs encoded by these genes does exist. For example, large-scale rearrangements in peptide coding sequences coupled with gene duplications has been reported in species of two polyneopteran groups, the Dictyoptera and Orthoptera (Predel et al 2001, Redeker et al. 2017, Hao et al. 2019). The genome of the grasshopper Locusta migratoria (Orthoptera) contains three genes encoding multiple copies of trypto-PKs, two encoding several PKs including PBANs, and one capa gene encoding PVKs the trypto-PK DH1 (Redeker et al. 2017). Later, two more DH1 trypto-PK genes with multiple peptide sequences were isolated in additional species of Orthoptera and Blattodea (Hao et al. 2019). Grasshoppers also appear to have entirely lost coding sequences for the DH2 trypto-PK typically encoded on the pk/pban gene, leaving DH1 peptides solely responsible for inducing egg diapause. Loss of DH2 has also occurred independently within the Heteroptera and Neodiptera (Redeker et al. 2017, Ahn and Choi 2018, Hao et al. 2019). The number of non-PBAN PKs (α- β- and γ-SGNP is also variable: β- and γ-SGNP are found in many insect taxa, while α-SGNP only found in the sister groups Lepidoptera and Trichoptera; Apterygota, Heteroptera and some Homoptera. In contrast, the coding sequence for PBAN appears to be nearly ubiquitous across the Insecta, with the exception of the higher Diptera as demonstrated below.
PRXamide-Expressing Neurons
Early studies using extracts of the subesophageal ganglion of the ventral nervous system (more recently termed the gnathal ganglion or GNG) identified this region of the central nervous system as a key source of signals inducing production of diapause eggs and pheromone biosynthesis. These signaling peptides were eventually identified as DH2 and PBAN, both encoded on the pk/pban gene (Hasegawa 1964, Fukuda and Takeuchi 1967, Raina et al. 1989, Teal et al. 1989). Subsequent studies showed that the GNG contains pk/pban expressing cells of two types: interneurons whose axons are restricted to the CNS, and efferent neurons with axons projecting through peripheral nerves to neurohemal organs. The latter-produced peptides act as endocrine factors released into the hemolymph while the former act as neurotransmitters via synaptic or perisynaptic connections with other neurons (Teal et al. 1989). Efferent neurosecretory cell bodies are found as serially homologous pairs or bilateral clusters along the ventral midline of the GNG in each neuromere: the mandibular (Md), maxillary (Mx) and labial (Lb). Ablation studies in the silk moth Bombyx mori (Linnaeus, Lepidoptera, Bombycidae) showed that while these median neurosecretory cells all express the pk/pban gene, they do not process the prepropeptide into identical complements of mature peptides. Median neurosecretory cells of the mandibular and maxillary neuromeres (MMd and MMx) are required for initiation of pheromone biosynthesis, while MLb is necessary for inducing diapause (Ichikawa et al. 1996; using the nomenclature of Davis et al. 1996). This finding has been confirmed using antibodies specific to DH and PBAN peptides, demonstrating that while all express and produce the entire PBAN prepropeptide, Bombyx MMd and MMx release mature PBAN peptide but not DH while MLb is responsible for release of DH but not PBAN (Ma et al. 2000, Hagino et al. 2010). Similarly, Locusta migratoria (Linnaeus, Orthoptera, Acrididae) MLb cells produce many trypto-PK peptides while PBAN and other PKs are restricted to MMd and MMx (Redeker et al. 2017); differential peptide production has also been demonstrated in Periplaneta americana (Linnaeus, Dictyoptera, Blattidae) (Predel et al. 2007). Median neurosecretory neuron capa gene expression and peptide production can also be variable across species; in addition to expression of the pk/pban gene, the capa gene is also expressed in MLb in the moth Manduca sexta (Linnaeus, Lepidoptera, Sphingidae) (Loi and Tublitz 2004). Curiously, the MMd and MMx usually share PRXamide gene expression/peptide production characteristics while the MLb differs; this is likely related to the morphology of these neurons’ projection in which MMd and MMx are very similar while that of MLb targets some of the same tissues but via a unique trajectory as described below.
Additionally, PRXamides are nearly always produced and released by serially homologous neurosecretory neurons in the abdominal ganglia. These neurons are usually capa-expressing although rarely they express the pk/pban gene as well. PRXamide producing neurons can also be observed in thoracic ganglia and brain although this is more variable by species as well as developmental stage (Loi and Tublitz 2004, Predel and Wegener 2006).
Both GNG and abdominal median neurosecretory cells are ancient and can be observed with PRXamide immunostaining even in the basal apterygote Lepisma saccharina (Linnaeus, Zygentoma, Lepismatidae) and at least one nonhexapod member of the Pancrustacea (Xibalbanus tulumensis, Remipedia) (Diesner et al. 2021). Specifically, the GNG is an important locus of cells and circuits that regulate the animal’s physiology, life cycle, and autonomic functions somewhat analogous to the vertebrate brainstem (Hückesfeld et al. 2021). GNG median neurosecretory cells characteristically project to the retrocerebral complex in the head, specifically the corpora cardiaca (CC). In addition to releasing PRXamides onto the CC, these neurons also form dense secretory neurohemal networks on the surfaces of nerves and the GNG itself. The morphology of the abdominal neurosecretory neurons is also stereotyped, with axons leaving the ganglia via peripheral nerves to the perivisceral organs (PVOs) forming neurohemal surfaces on nerves along the way (Davis et al. 1996, Golubeva et al. 1997).
Anatomy of these efferent median neurosecretory neurons is particularly well-described in a number of moth species and can be used as a template for further comparisons (Fig. 1; Kingan et al. 1992, Davis et al. 1996, Golubeva et al. 1997). Anti-PBAN immunostaining and backfills of peripheral nerves reveal that median neurosecretory cells possess dorsally projecting neurites that travel contralaterally and produce bilateral arborizations in the dorsal GNG and anteriorly near the subesophageal foramen. MMd and MMx cell axons leave the GNG via the maxillary nerve, enter the nervus corpora cardiaca V (NCCV) and end in the CC. They produce dense neurohemal networks along the way, forming a structure referred to as the corpus ventralis by (Golubeva et al. 1997). The precise anatomy and nomenclature of the cranial nerves utilized by the median neurosecretory cells vary by species, but the presence of MMd and MMx efferent outputs to the CC appears relatively invariant across the insects (Tips et al. 1993, Bader, Colomb et al. 2007, Predel et al. 2007, Hellmich et al. 2014).
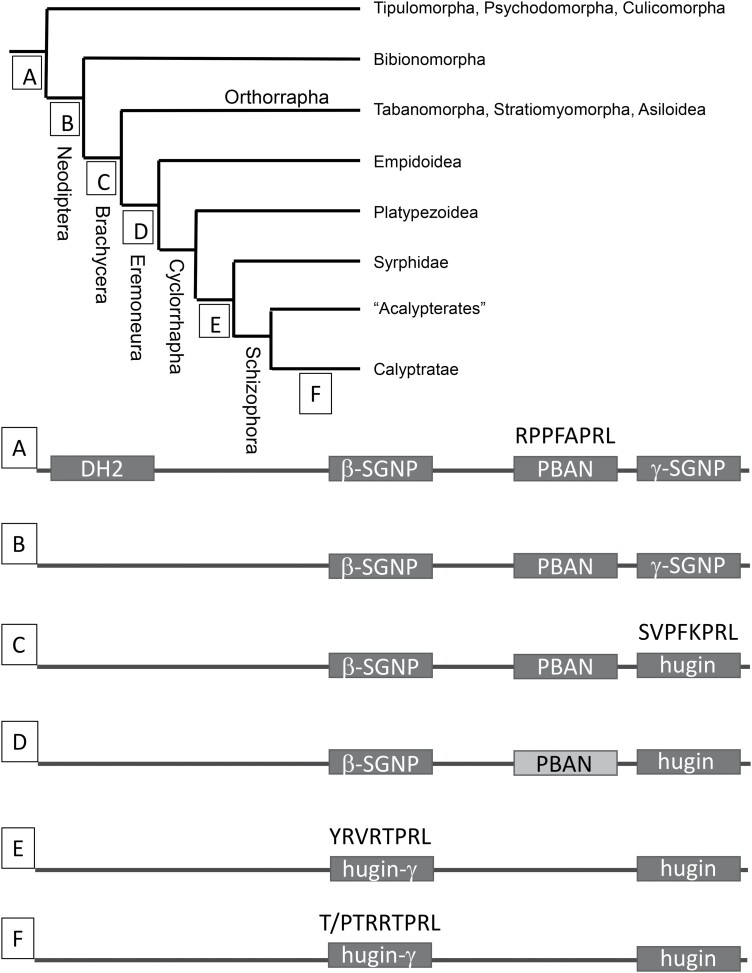
Fig. 1.
Changes in peptides coded by the pk/pban/hugin gene during the evolution of the Diptera. Letters A–F indicate correspondence of phylogenetic groups with mature pk/pban/hugin peptide sequences. DH2-diapause hormone 2; YRVRTPRL-hugin-γconsensus sequence in Syrphidae and acalypterate Schizophora; T/PTRRTPRL-hugin-γ consensus sequence in Calyptratae; SVPFKPRL-hugin consensus sequence in Brachycera; RPPFAPRL-PBAN consensus sequence in Tipulomorpha, Psychodomorpha, Culicomorpha, Bibionomorpha, and Orthorrapha. Lighter shaded box in the D gene sequence indicates PBAN-like peptide in Empidoidea and Platypezoidea that has lost the consensus sequence.
MLb efferent neurons have large, distinct soma with axons that ascend through the contralateral circumesophageal connective into the tritocerebrum and exit via the NCC-3 cranial nerve to the CC. The axons produce dense neurohemal terminals on the CC and then proceed to the corpora allata (CA) and dorsal aorta. Innervation of the CC by MLb appears highly conserved, although the additional innervation of the CA and aorta is not always reported. Interestingly, additional outputs of this neuron have been reported in some species. Most notably, Bräunig (1991) reports that in addition to targeting the CC, the MLb of the orthopteran Locusta migratoria produces ascending outputs that innervate the pharyngeal dilator muscles in the head while descending processes exit the abdominal ganglia and form neurohemal surfaces on nerves associated with the heart. Descending MLb processes to the VNC that exit via segmental nerves have also been observed in the cockroach (Predel et al. 2007), and innervation of the pharynx by MMd and MMx but not MLb occurs in Drosophila melanogaster (Bader, colomb et al. 2007). Given the broad but patchy distribution of these neuron morphologies, it is possible that pharyngeal and descending efferent outputs of pk/pban expressing neurons are more common but underreported due to the small number of insects investigated to date, or that they are remnants of an ancestral innervation pattern, lost in many insects but retained in others, perhaps capable of reappearing in some taxa via a process such as generative homology where the developmental program is retained and can be redeployed and (Butler and Saidel 2000).
The second group of pk/pban expressing cells in the GNG are interneurons (INs) that provide processes to the brain, VNC, or both. In moths, INs that express the pk/pban gene are restricted to the maxillary neuromere (Blackburn et al. 1992, Kingan et al. 1992, Davis et al. 1996, Golubeva et al. 1997, Sun et al. 2005), however, INs are observed in the mandibular and/or labial neuromeres in other species (Hellmich et al. 2014). Together these neurons have a characteristic innervation pattern: descending processes travel along the lateral margin of the entire VNC, producing arbors along the way and ending with a broad arbor in the terminal abdominal ganglion. Ascending neurons arborize in the tritocerebrum and around the esophageal foramen, and travel dorsally through the pars intercerebralis (PI) and branch in an arc across the superior medial protocerebrum (Tips et al. 1993, Choi et al. 2001, Sun et al. 2005, Bader, Wegener et al. 2007, Predel et al. 2007, Choi et al. 2009, Ahn and Choi 2018, Hull et al. 2021).
Order Diptera: PRXamides Under Rapid Diversification
The genomic model organism Drosophila melanogaster has an unusual modification to the pk/pban gene: loss of the PBAN coding sequence, which is present in all other insects investigated to date including basal Diptera. and acquisition of the hugin peptide that functions in feeding and circadian locomotor activity (Schoofs et al. 2014, Choi et al. 2015, Schwarz et al. 2021). The Diptera are a diverse and speciose insect Order that has undergone three rapid bursts of diversification: the first in the most basal clades (i.e., the former ‘Nematocera’), the second in the early Brachycera, and the third in the Schizophora, a group contained within the modern Cyclorrhapha. This last radiation occurred relatively recently in the Tertiary (around 65–40 mya). The Cyclorrhapha or ‘higher flies’ include Drosophila melanogaster and many other familiar species such as house flies and flesh flies. These insects are characterized by autapomorphies associated with molecular, cellular, and physiological processes. Students of developmental biology are well aware of the duplication and divergence of the Hox3 gene into zerknüllt (zen), which is expressed in a novel embryonic tissue, the amnioserosa, and bicoid (bcd), one of the first axis patterning genes in the embryo (Schmidt-Ott 2000, Simpson 2002). Cyclorrhaphan maggot larvae are also unique in the loss of an external head and the formation of a puparium from the last instar exoskeleton rather than forming a pupal cuticle at metamorphosis. Additionally, the cyclorraphan subgroup Schizophora have a novel head structure, the ptilinum, that inflates with hemolymph to push through the puparium at adult emergence (Wiegmann and Yeates 2017, Bayless et al. 2021). As these examples illustrate, the Cyclorrhapha underwent a large-scale reorganization of developmental mechanisms during their radiation, and it would not be surprising if other seemingly fundamental processes were radically modified in comparison to other insects and more basal Diptera.
One way to investigate the relationship between adaptions and divergence of conserved cellular, molecular and physiological processes is to consult the wealth of publicly available genomes and transcriptomes available online. Using this approach, the pk/pban gene coding sequence was identified in 155 dipteran species, and alignment of putative mature neuropeptides was performed to assess conservation and divergence of consensus sequences. This data was then mapped onto a published phylogeny of Diptera and key locations of change in the pk/pban coding sequence identified.
Materials and Methods
The phylogeny of Diptera published by (Wiegmann et al. 2011) was used to devise a strategy for genome, transcriptome, and protein sequence searches so that the location of pk/pban alterations could be pinpointed to distinct clades (e.g., Neodiptera, Orthorrhapha etc.). The website InsectBase 2.0 (http://v2.insect-genome.com/; Yang et al. 2022) was used as a starting point for searches by gene name (pban or hugin) for protein sequences from major taxonomic groups of Diptera. These protein sequences were used to search the National Center for Biotechnology Information (NCBI) Nucleotide and Sequence Read Archive (SRA) databases at the National Center for Biotechnology Information website (Sayers et al. 2022) using the tblastn search tool (Altschul et al.1990). Retrieved sequences were aligned using the Clustal Omega web tool (Sievers et al. 2011). The WebLogo tool was used for visualization of peptide consensus sequences (Schneider and Stephens 1990, Crooks et al. 2004). Mature peptide sequences were identified using criteria employed in previous studies of the dipteran pk/pban gene (Bader, Wegener et al. 2007, Choi et al. 2015): the C-terminal sequence (PRXGK/R) specifically characteristic of the pyrokinins, and N-terminal cleavage sites consisting of mono- or dibasic residues (K, R).
Results
Early studies posited that the primary functional peptide sequence in the Drosophila pk/pban gene (called the hugin gene in this species) is derived from the ancestral PBAN sequence. Comparisons of pk/pban coding sequences gleaned from publicly available genomes and transcriptomes across the Diptera instead support a scenario in which the PBAN peptide was lost entirely and the neighboring γ-SGNP sequence diverged into the hugin consensus sequence (SVPFKPRL) at the base of the Neodiptera (C, Fig. 2; Supplementary Data 1 [online only]). As observed for most insects the PBAN sequence is present in basal Diptera and resides between β-SGNP and γ-SGNP. It has a conserved consensus C-terminal sequence (RPPFAPRL; C, Fig. 2). Beginning with the Eremoneura, the PBAN consensus sequence begins to diverge but retains the PRXamide motif (D, Fig. 2; Supp. Data 1 [online only]). Complete loss of the PBAN consensus sequence including the PRXamide motif is found to have occurred in the ancestor of the Syrphidae and Schizophora (E, Fig. 2). At the same time, the β-SGNP sequence acquired the conserved γ-hugin consensus sequence for this group (YRVRTPRL) later modified to a T/PTRRTPRL consensus sequence characteristic of the calypterates (Kean et al. 2002, Bader, Wegener et al. 2007). The hugin sequence is still preceded at its N-terminus by the dibasic cleavage site (KK) that in more basal Diptera follows the amidation target glycine (G) of the PBAN sequence. The hugin peptide also contains 8 amino acids including the PRX motif which is more typical of γ-SGNP than of PBAN, the latter of which is characteristically around 30 amino acids long (Jurenka 2015).
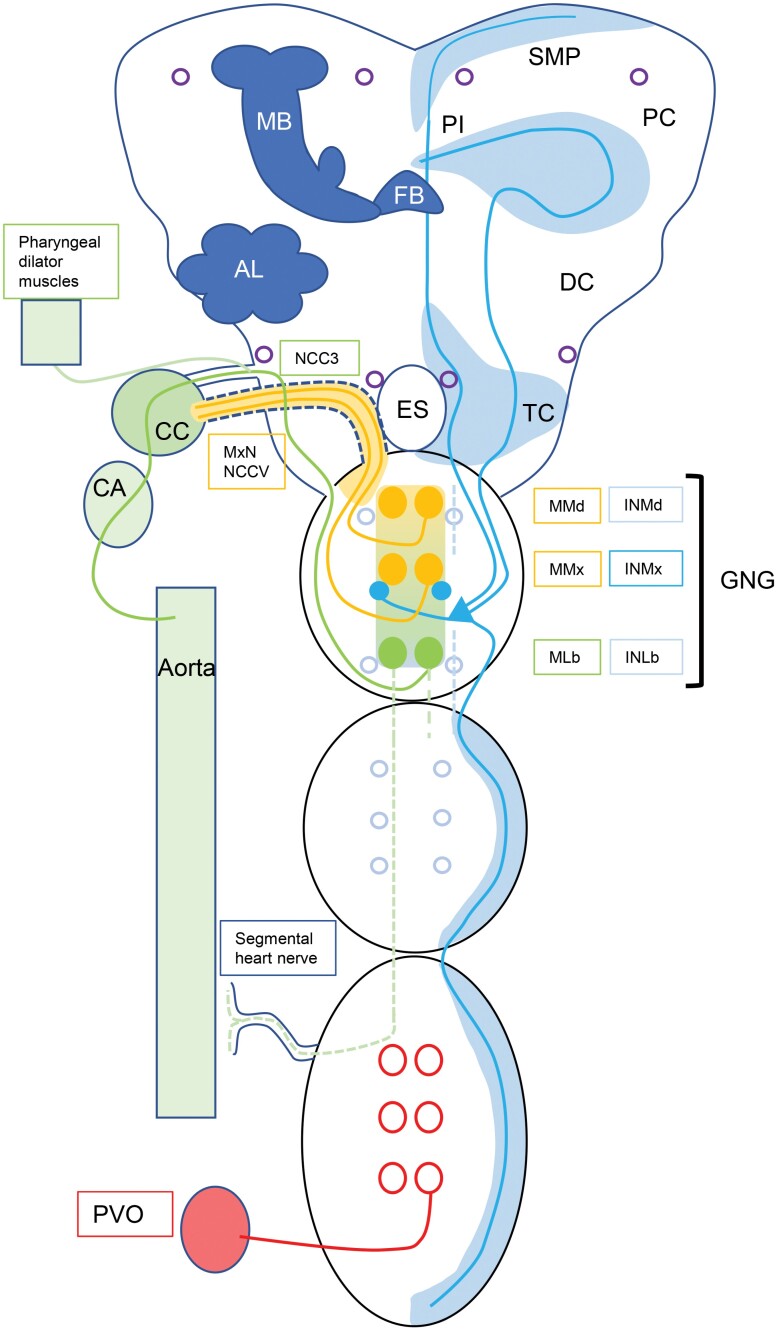
Fig. 2.
Groundplan of pk/pban/hugin expressing neurons and their peripheral targets. Light blue open circles and blue/green dotted lines – neurons and processes in the GNG and VNC that are observed in some but not all species. Purple open circles – neurons observed in the brain of some but not all species. Brain structures: MB-mushroom bodies, FB-fan-shaped body, AL-antennal lobe, SMP-superior median protocerebrum, DC-deutocerebrum TC-tritocerebrum, ES-esophageal foramen. GNG-gnathal ganglion: MMd, MMx, MLb-median mandibular, maxillary, and labial efferent neurons. INMd, INMx, and INLb-mandibular, maxillary, and labial interneurons. Nerves: NCC3-nervus corpora cardiaca 3, NCCV-nervus corpora cardiaca 5, MxN-maxillary nerve. Peripheral targets: CC-corpora cardiaca, CA-corpora allata, PVO-perivisceral organ.
Previous authors have introduced several lines of evidence suggesting that γ-hugin (residing in the N-terminal direction from PBAN and termed β-SGNP in most insects) does not produce a functional mature peptide in Drosophila (Predel and Nachman 2006, Choi et al. 2015); however, the PRXamide motif and C-terminal cleavage and amidation sites as well as the C-terminal consensus sequences are conserved, suggesting that this sequence may still play an adaptive role in these insects (Fig. 2).
The hugin peptide sequence of Orthorrapha and Eremoneura is also highly conserved (SVPFKPRL) although single amino acid substitutions characterize some taxa (e.g., SVQFKPRL in the higher Calyptratae). In Drosophila melanogaster the hugin peptide plays a potentially novel role in regulation of circadian locomotor rhythms and feeding behaviors (Sun et al. 2014, Schoofs et al. 2014, King et al. 2017, Schwarz et al. 2021). Given the conserved sequence motif of the hugin peptide it is likely that these functions are supported by the hugin peptide throughout the Brachycera. Interestingly, the vertebrate ortholog of pk/pban and hugin, neuromedin U, has also been shown to regulate feeding in rodents (Martinez and O’Driscoll 2015), perhaps suggesting that his NP system is amenable to incorporation into regulatory pathways for control of feeding behaviors.
Potential for Novel Functions of Dipteran PRXamides
Multiple, sometimes overlapping functions of the peptide products of the pk/pban and capa genes have been identified in a diverse array of insect species. Early studies of the physiology of visceral muscle contraction in species of Dictyoptera and Orthoptera insects identified several classed of neuropeptides. Those referred to as myotropins, locustapyrokinins, or leucopyrokinins were later shown to be encoded by the pk/pban gene and were subsequently isolated in other species (Tips et al. 1993, Predel et al. 2001, Yaginuma and Niimi 2016). PBAN was identified first in Lepidoptera as the signal initiating pheromone biosynthesis by the pheromone glands, a finding that has since been replicated in species outside of the Lepidoptera (Kitamura et al. 1989, Raina et al. 1989, Tillman et al. 1999, Choi and Vander Meer 2012). The peptide MRCH (melanization and reddish coloration hormone) was identified in the moth Spodoptera litura (Fabricius, Lepidoptera, Noctuidae) as a regulator of environmentally-controlled larval cuticle pigmentation and later shown to correspond to the PBAN neuropeptide of Bombyx mori (Matsumoto et al. 1990). Similarly, in brachyceran Diptera, pyrokinins decrease the time needed for puparium formation and cuticle tanning (Zdarek et al. 1997). These studies hint at a general role in cuticle physiology during development, although it should be noted that in these and other physiological and immunocytochemical studies the exact identity of the PK, and even the difference between PKs and PVKs, was difficult to determine due to the sequence similarities among the peptides.
The trypto-PKs DH1 and DH2 encoded by the capa and pk/pban genes act on the ovaries of adult female Lepidoptera and are transferred to developing oocytes, where they regulate production of eggs that will begin diapause after fertilization (Yamashita 1996, Hao et al. 2019). In species in which diapause occurs in the pupa rather than the egg, DH2 appears to initiate the end of diapause rather than the beginning (Xu and Denlinger 2003). Periviscerokinin was initially isolated as a facilitator of visceral muscle contraction in Periplaneta americana, as a cardioaccelatory peptide in Manduca sexta (CAP2b), and as diuretic or anti-diuretic hormones in several insect species.
Insect genomes contain four GPCRs whose products have different binding affinities to each peptide encoded by the pk/pban and capa genes. More than one peptide typically can bind each receptor (reviewed by Jurenka 2015); although the functions of the additional 2–3 pyrokinins encoded by the pk/pban gene are poorly understood, their ability to bind these GPCRs suggests they may participate in some aspect of the functions already described for the better-known peptides (Ma et al. 1996, Kean et al. 2002, Choi et al. 2003). In Drosophila melanogaster, binding assays reveal that both hugin and PBAN peptides bind with high affinity to PK GPCRs (Choi et al. 2003), suggesting that the loss of the PBAN peptide and gain of hugin functionality need not require dramatic changes in structure and function of the cognate receptor. Rather, a newly acquired peptide such as hugin may tie ancestral functions of pyrokinins to a novel function, as discussed below.
Hugin Peptides and Neurons in Diptera
A closer look at hugin expressing neurons afforded by the genetic tools available in Drosophila melanogaster confirms the conserved nature of the pk/pban neurosecretory circuits and uncovers high-resolution details of cell structure and function. Early studies using a polyclonal antibody against the C-terminus of PBAN detected hugin protein in larval ventral midline cells in each neuromere of the GNG and in abdominal ganglia (Choi et al. 2001). The abdominal neurons project to the perivisceral organs in the periphery, while those of the GNG have processes in the protocerebrum and tritocerebrum of the brain, the ventral nerve cord, and corpora cardiaca of the retrocerebral complex (termed the ring gland (RG) in Diptera). A similar pattern was observed in adults, with the addition of a pair of immunopositive neurons in the protocerebrum and a small group of neurons in the tritocerebrum. A closer look at individual hugin-expressing neurons in the mandibular and maxillary neuromeres of the GNG reveals that each neuron innervates one of four targets: efferent neurons project to the RG or pharyngeal dilator muscles (PH), and INs target the superior medial protocerebrum (PC) or have descending axons (VNC). All have fine processes in the dorsal GNG and TC around the esophageal foramen (Bader, colomb et al. 2007, Bader, Wegener et al. 2007; Mizuno et al. 2021).
An interesting and potentially novel feature of Drosophila GNG neurosecretory neurons is the loss of hugin gene expression in MLb and redeployment of these neurons in a new circuit in which they instead express capa. Single cell neuroanatomical reconstructions identify efferent neurons innervating the RG and PH that appear to reside only in the mandibular and maxillary neuromeres. (Melcher and Pankratz 2005, Hückesfeld et al. 2016). A pair of large ventromedial neurons reside in the labial neuromere that express capa but not hugin (Predel and Wegener 2006). As described above, MLb frequently has a different peptidergic content from the MMx and MMd cells, including expression of both capa and pban in the moth Manduca sexta (Loi and Tublitz 2004, Neupert, Huetteroth et al. 2009). Despite co-expression of both PRXamide genes the lepidopteran MLb has the ‘typical’ morphology of projections through NCCIII to the CC, CA and aorta (Ichikawa et al. 1995, Davis et al. 1996, Golubeva et al. 1997). The same is true for basal dipteran mosquitos, where MLb co-expresses capa and pk/pban and efferents from all three GNG neuromeres project to the ring gland (Hellmich et al. 2014). Drosophila capa-expressing MLb have processes in the ring gland that are reported to provide dendrites to adipokinetic hormone (AKH) cells in the CC. Additionally, they have a potentially novel efferent output onto the foregut proventriculus (Kean et al. 2002, Melcher and Pankratz 2005, Koyama et al. 2021). CAPA-PVKs are well established diuretic or anti-diuretic factors that typically act on the Malphigian tubules or the gut but a direct projection from the GNG to the gut has not been reported in other insects (Sajadi et al. 2020, Zandawala et al. 2021).
A detailed connectome of hugin-producing neurons is being constructed in Drosophila as well. Interneurons are characterized by mixed pre- and postsynaptic processes, and all neurons receive input in the GNG. One source of GNG inputs is Gr66a gustatory afferents from the pharynx, external head and abdomen that are specifically tuned to detect bitter, aversive substances (Melcher and Pankratz 2005, Bader, Wegener et al. 2007, Schlegel et al. 2016). Additionally, hugin neurons receive input from descending PI neurons in the dendritic field of the ventrolateral esophageal foramen (Schlegel et al., 2016, King et al. 2017). The PC hugin neurons form a complex circuitry in the medial and superior protocerebrum, with reciprocal connections with the median neurosecretory cells of the PI and inputs from neurons that also target the PI. Finally, VNC neurons are both post- and presynaptic within the VNC suggesting a neuromodulatory role that is sensitive to local feedback (Miroschnikow et al. 2018).
Functions of Drosophila Hugin
Initial studies of Drosophila hugin function focused on regulation of feeding as manipulation of GNG hugin neuron activity can disrupt or initiate feeding behaviors (Melcher and Pankratz 2005, Melcher et al. 2007, Sun et al. 2014, Hückesfeld et al. 2016). This function appears to be especially linked to aversive gustatory cues, supported by the inputs to GNG hugin neurons from aversive Gr66a-expressing gustatory neurons as described previously. Further investigations began to untangle roles of specific GNG hugin neurons: locomotor speed is specifically regulated by the VNC projecting hugin neurons while the remaining neurons are required for suppression of feeding and onset of locomotion (Schoofs et al. 2014). A potential adaptive scenario for hugin in this role is revealed by the necessity of hugin expressing neurons for larval evasion when confronted with bacterial pathogen-infected food (Surendran et al. 2017).
Recently, a novel population of hugin producing interneurons (HuginTS in the third thoracic segment) was found to respond to rising glucose levels by inhibiting diuretic hormone 44 (DH44) producing neurons and promoting cessation of feeding behavior (Oh et al. 2021). Median neurosecretory cells in the PI produce (DH44) and send descending processes to the ventrolateral esophageal foramen neuropil where they overlap with the dendrites of GNG hugin neurons expressing the DH44 receptor (McKellar 2016, King et al. 2017, Schwarz et al. 2021). Circadian release of DH44 is an important regulator of neurons that control sleep: activity rhythms suggesting that hugin neurons also contribute to daily behavioral rhythms (Cavanaugh et al. 2014). GNG hugin neurons with axons in the VNC are presynaptic to motor neurons providing a means for contributing to circadian regulation of locomotor patterns (King et al. 2017). The modulation of motor output by pyrokinins is well-documented in systems such as the stomatogastric ganglion of the crab Cancer borealis, potentially suggesting conservation of this particular role (Saideman et al. 2007). Drosophila hugin efferents projecting to the CA of the ring gland also express the receptor for DH44 and thus are likely to participate in circadian functions of this neuroendocrine gland (Mizuno et al. 2021). Finally, GNG hugin neurons decrease activity and suppress circadian activity under sleep deprivation conditions, possibly via direct connections between ascending hugin interneurons and sleep homeostasis regulating neurons in the protocerebrum (Schwarz et al. 2021).
Evolutionary Significance of Divergence of PK/PBAN Peptides
The pk/pban gene of Diptera has retained its ancestral structure but has also undergone significant evolutionary divergence during the evolution of the Diptera. How have the higher flies compensated for the lack of PBAN, and how did the novel functions of hugin arise? The PBAN peptide is highly conserved across the entire Insecta and plays an equally conserved role in the regulation of pheromone biosynthesis (reviewed by Jurenka 2015). In some moth species, PBAN release and subsequent pheromone biosynthesis occur on a circadian basis (reviewed by Groot 2014, Iglesias et al. 1999, Tawata and Ichikawa 2001, Závodská et al. 2009). This suggests that the pk/pban expressing neural circuitry in the GNG is ancestrally linked to circadian networks in the bran, likely facilitating the incorporation of the newly acquired hugin peptide into this regulatory network as observed in Drosophila. The adaptive significance of the novel function of hugin in the higher Diptera remains to be determined, and a search for similar changes in the pk/pban gene in other insects should provide insight.
Loss of the PBAN coding sequence in the ancestor of Syrphidae and Schizophora is especially curious, as pheromones play an important role in fly behaviors including attraction of the opposite sex. What aspects of pheromone physiology biology differ in the higher flies when compared with other insects? An intriguing study in Drosophila demonstrates that circadian pheromone biosynthesis in the absence of the PBAN peptide is instead directly regulated by release of pigment-dispersing factor (pdf) from circadian rhythm generating clock neurons in the brain (Krupp et al. 2013). Loss of the PBAN sequence in the ancestor of Syrphidae + Schizophora could be explained by the loss of its adaptive function as a regulator of pheromone biosynthesis following recruitment of the pdf peptide for this role.
Another factor that may have influenced the regression and loss of the PBAN peptide is the tissue responsible for pheromone production. In the higher flies, pheromone biosynthesis occurs in large epidermal secretory cells called oenocytes while basal Diptera possess PBAN-responsive abdominal pheromone glands like those observed in most other insects (Blomquist et al. 1987, Spiegel et al. 2016). Perhaps oenocytes are more amenable to direct regulation by the circadian clock, contributing to the obsolescence of the PBAN peptide in higher Diptera.
Finally, pheromone biosynthesis by some schizophoran species appears to be uniquely regulated by ecdysteroid hormones, as shown by studies of Drosophila melanogaster and Musca domestica (Linnaeus, Diptera, Muscidae). In contrast, juvenile hormone is more typically employed as a regulator of pheromone biosynthesis, (Blomquist et al. 1987, Tillman et al. 1999). This rather dramatic change in endocrine regulatory pathways may also have contributed to the loss of the role of PBAN.
The Order Diptera is ideal for continued studies of neuropeptide evolution. First, the genetic model species Drosophila melanogaster provides a means for high resolution dissection of genetic, cellular, and physiological processes. Second, the diversity of species can be employed in comparative studies to identify the adaptive significance of divergence of the PBAN gene in terms of life history, ecology, and behavior. This approach has already been used to trace the many genetic and developmental novelties of Drosophila to their evolutionary origins (e.g., the origin of the Drosophila anterior determining gene bicoid as reviewed by McGregor 2005). Further novelties in neuropeptide systems are likely present and waiting to be studied. Utilizing the wealth of genomes and transcriptomes now available, changes in gene sequence may be mapped not only onto phylogeny, but onto nervous system function, ecology, and behavior. Once such a framework is in place, emerging technologies such as CRISPR may be used to gain a more detailed mechanistic understanding of cellular and molecular systems to species in addition to Drosophila.
Author Contribution
SMF Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review and editing.
References Cited
- Ahn, S. -J., and Choi M. -Y.. . 2018. Identification and characterization of capa and pyrokinin genes in the brown marmorated stink bug, Halyomorpha halys (Hemiptera): gene structure, immunocytochemistry, and differential expression. Arch. Insect. Biochem. Physiol. 99: e21500. doi: 10.1002/arch.21500 [DOI] [PubMed] [Google Scholar]
- Altschul, S. F., Gish W., Miller W., Myers E. W., and Lipman D. J.. . 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Bader, R., Colomb J., Pankratz B., Schröck A., Stocker R. F., and Pankratz M. J.. . 2007. Genetic dissection of neural circuit anatomy underlying feeding behavior in Drosophila: distinct classes of hugin-expressing neurons. J. Comp. Neurol. 502: 848–856. doi: 10.1002/cne.21342 [DOI] [PubMed] [Google Scholar]
- Bader, R., Wegener C., and Pankratz M.. . 2007. Comparative neuroanatomy and genomics of hugin and pheromone biosynthesis activating neuropeptide (PBAN). Fly (Austin). 1: 228–231. [DOI] [PubMed] [Google Scholar]
- Bayless, K. M., Trautwein M. D., Meusemann K., Shin S., Petersen M., Donath A., Podsiadlowski L., Mayer C., Niehuis O., Peters R. S., . et al. 2021. Beyond Drosophila: resolving the rapid radiation of schizophoran flies with phylotranscriptomics. BMC Biol. 19: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn, M. B., Kingan T. G., Raina A. K., and Ma M. C.. . 1992. Colocalization and differential expression of PBAN- and FMRFamide-like immunoreactivity in the subesophageal ganglion of Helicoverpa zea (Lepidoptera: Noctuidae) during development. Arch. Insect. Biochem. Physiol. 21: 225–238. doi: 10.1002/arch.940210306 [DOI] [Google Scholar]
- Blomquist, G. J., Dillwith J. W., and Adams T. S.. . 1987. Biosynthesis and endocrine regulation of sex pheromone production in Diptera, pp. 217–250. InPrestwich G. D. and Blomquist G. J. (eds.), Pheromone biochemistry. Academic Press,Orlando. [Google Scholar]
- Bräunig, P. 1991. A suboesophageal ganglion cell innervates heart and retrocerebral glandular complex in the locust. J. Exp. Biol. 156: 567–582. doi: 10.1242/jeb.156.1.567 [DOI] [Google Scholar]
- Burke, R. D., Angerer L. M., Elphick M. R., Humphrey G. W., Yaguchi S., Kiyama T., Liang S., Mu X., Agca C., Klein W. H., . et al. 2006. A genomic view of the sea urchin nervous system. Dev. Biol. 300: 434–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, A. B., and Saidel W. M.. . 2000. Defining sameness: historical, biological, and generative homology. BioEssays 22: 846–853. doi: [DOI] [PubMed] [Google Scholar]
- Cavanaugh, D. J., Geratowski J. D., Wooltorton J. R. A., Spaethling J. M., Hector C. E., Zheng X., Johnson E. C., Eberwine J. H., and Sehgal A.. . 2014. Identification of a circadian output circuit for rest: activity rhythms in Drosophila. Cell. 157: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, M. -Y., Fuerst E. -J., Rafaeli A., and Jurenka R.. . 2003. Identification of a G protein-coupled receptor for pheromone biosynthesis activating neuropeptide from pheromone glands of the moth Helicoverpa zea. Proc. Natl. Acad. Sci.. 100: 9721–9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, M. -Y., Rafaeli A., and Jurenka R. A.. . 2001. Pyrokinin/PBAN-like peptides in the central nervous system of Drosophila melanogaster. Cell Tissue Res. 306: 459–465. doi: 10.1007/s00441-001-0467-x [DOI] [PubMed] [Google Scholar]
- Choi, M. -Y., Raina A., and Vander Meer R. K.. . 2009. PBAN/pyrokinin peptides in the central nervous system of the fire ant, Solenopsis invicta. Cell Tissue Res. 335: 431–439. [DOI] [PubMed] [Google Scholar]
- Choi, M. -Y., Sanscrainte N. D., Estep A. S., Vander Meer R. K., and Becnel J. J.. . 2015. Identification and expression of a new member of the pyrokinin/pban gene family in the sand fly Phlebotomus papatasi. J. Insect Physiol. 79: 55–62. doi: 10.1016/j.jinsphys.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Choi, M. -Y., and Vander Meer R. K.. . 2012. Ant trail pheromone biosynthesis is triggered by a neuropeptide hormone. PLoS One. 7: e50400. doi: 10.1371/journal.pone.0050400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann, M., Williams E. A., Krug K., Franz-Wachtel M., Macek B., and Jékely G.. . 2013. The neuropeptide complement of the marine annelid Platynereis dumerilii. BMC Genomics. 14: 906. doi: 10.1186/1471-2164-14-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks, G. E., Hon G., Chandonia J. M., and Brenner S. E.. . 2004. WebLogo: a sequence logo generator. Genome Res. 14: 1188–1190. doi: 10.1101/gr.849004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, N. T., Homberg U., Teal P. E. A., Altstein M., Agricola H. -J., and Hildebrand J. G.. . 1996. Neuroanatomy and immunocytochemistry of the median neuroendocrine cells of the subesophageal ganglion of the tobacco hawkmoth, Manduca sexta: immunoreactivities to PBAN and other neuropeptides. Microsc. Res. Tech. 35: 201–229. [DOI] [PubMed] [Google Scholar]
- Derst, C., Dircksen H., Meusemann K., Zhou X., Liu S., and Predel R.. . 2016. Evolution of neuropeptides in non-pterygote hexapods. BMC Evol. Biol. 16: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira, A. L., Calcino A., and Wanninger A.. . 2019. Ancient origins of arthropod moulting pathway components. eLife. 8: e46113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, P. S., Kurland S. C., Qu X., Parker B. O., Sreekrishnan A., Kwiatkowski M. A., Williams A. H., Ysasi A. B., and Christie A. E.. . 2015. Distinct or shared actions of peptide family isoforms: II. Multiple pyrokinins exert similar effects in the lobster stomatogastric nervous system. J. Exp. Biol. 218: 2905–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesner, M., Bläser M., Eckardt S., Iliffe T. M., Boelen Theile E., and Predel R.. . 2021. Expression pattern of CAPA/pyrokinin neuropeptide genes in Remipedia and silverfish: rapid differentiation after gene duplication in early Hexapoda, followed by strong conservation of newly established features in insects. Peptides. 144: 170610. doi: 10.1016/j.peptides.2021.170610 [DOI] [PubMed] [Google Scholar]
- Elphick, M. R., Mirabeau O., and Larhammar D.. . 2018. Evolution of neuropeptide signalling systems. J. Exp. Biol. 221: jeb151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor, J., Köblös G., Kákai A., Kárpáti Z., Molnár B. P., Dankó T., Bozsik G., Bognár C., Szőcs G., and Fónagy A.. . 2017. Molecular cloning, mRNA expression and biological activity of the pheromone biosynthesis activating neuropeptide (PBAN) from the European corn borer, Ostrinia nubilalis: the DH-PBAN gene of Ostrinia nubilalis. Insect. Mol. Biol. 26: 616–632. [DOI] [PubMed] [Google Scholar]
- Fukuda, S., and Takeuchi S.. . 1967. Diapause factor-producing cells in the suboesophageal ganglion of the silkworm, Bombyx mori L. Proc. Jpn. Acad. 43: 51–56. [DOI] [PubMed] [Google Scholar]
- Golubeva, E., Kingan T. G., Blackburn M. B., Masler E. P., and Raina A. K.. . 1997. The distribution of PBAN (pheromone biosynthesis activating neuropeptide)-like immunoreactivity in the nervous system of the gypsy moth, Lymantria dispar. Arch. Insect. Biochem. Physiol. 34: 391–408. [Google Scholar]
- Groot, A. T. 2014. Circadian rhythms of sexual activities in moths: a review. Front. Ecol. Evol. 2(43): 1–21. doi: 10.3389/fevo.2014.00043 [DOI] [Google Scholar]
- Hagino, A., Kitagawa N., Imai K., Yamashita O., and Shiomi K.. . 2010. Immunoreactive intensity of FXPRL amide neuropeptides in response to environmental conditions in the silkworm, Bombyx mori. Cell Tissue Res. 342: 459–469. doi: 10.1007/s00441-010-1083-4 [DOI] [PubMed] [Google Scholar]
- Hao, K., Tu X., Ullah H., McNeill M. R., and Zhang Z.. . 2019. Novel Lom-dh genes play potential role in promoting egg diapause of Locusta migratoria L. Front. Physiol. 10: 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa, K. 1964. Studies on the mode of action of the diapause hormone in the silkworm, Bombyx mori L. II content of diapause hormone in the suboesophageal ganglion. J. Exp. Biol. 41: 855–863. doi: 10.1242/jeb.41.4.855 [DOI] [PubMed] [Google Scholar]
- Hayakawa, E., Watanabe H., Menschaert G., Holstein T. W., Baggerman G., and Schoofs L.. . 2019. A combined strategy of neuropeptide prediction and tandem mass spectrometry identifies evolutionarily conserved ancient neuropeptides in the sea anemone Nematostella vectensis. PLoS One. 14: e0215185. doi: 10.1371/journal.pone.0215185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmich, E., Nusawardani T., Bartholomay L., and Jurenka R.. . 2014. Pyrokinin/PBAN-like peptides in the central nervous system of mosquitoes. Cell Tissue Res. 356: 39–47. doi: 10.1007/s00441-013-1782-8 [DOI] [PubMed] [Google Scholar]
- Hewes, R. S., and Taghert P. H.. . 2001. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 11: 1126–1142. doi: 10.1101/gr.169901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückesfeld, S., Peters M., and Pankratz M. J.. . 2016. Central relay of bitter taste to the protocerebrum by peptidergic interneurons in the Drosophila brain. Nat. Commun. 7: 12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückesfeld, S., Schlegel P., Miroschnikow A., Schoofs A., Zinke I., Haubrich A. N., Schneider-Mizell C. M., Truman J. W., Fetter R. D., Cardona A., . et al. 2021. Unveiling the sensory and interneuronal pathways of the neuroendocrine connectome in Drosophila. eLife. 10: e65745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull, J. J., Brent C. S., Choi M. -Y., Mikó Z., Fodor J., and Fónagy A.. . 2021. Molecular and functional characterization of pyrokinin-like peptides in the Western tarnished plant bug Lygus hesperus (Hemiptera: Miridae). Insects. 12: 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa, T., Hasegawa K., Shimizu I., Katsuno K., Kataoka H., and Suzuki A.. . 1995. Structure of neurosecretory cells with immunoreactive diapause hormone and pheromone biosynthesis activating neuropeptide in the silkworm, Bombyx mori. Zool. Sci. 12: 703–712. [Google Scholar]
- Ichikawa, T., Shiota T., Shimizu I., and Kataoka H.. . 1996. Functional differentiation of neurosecretory cells with immunoreactive diapause hormone and pheromone biosynthesis activating neuropeptide of the moth, Bombyx mori. Zool. Sci. 13: 21–25. [Google Scholar]
- Iglesias, F., Jacquin-Joly E., Marco M. -P., Camps F., and Fabrias G.. . 1999. Temporal distribution of PBAN-like immunoreactivity in the hemolymph of Mamestra brassicae females in relation to sex pheromone production and calling behavior. Arch. Insect. Biochem. Physiol. 40: 80–87. doi: [DOI] [Google Scholar]
- Jékely, G. 2013. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl. Acad. Sci. 110: 8702–8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurenka, R. 2015. The PRXamide neuropeptide signalling system, pp. 123–170. InAdvances in insect physiology, vol. 49. Elsevier. [Google Scholar]
- Jurenka, R., and Nusawardani T.. . 2011. The pyrokinin/pheromone biosynthesis-activating neuropeptide (PBAN) family of peptides and their receptors in Insecta: evolutionary trace indicates potential receptor ligand-binding domains: pyrokinin/PBAN ligands and receptors. Insect. Mol. Biol. 20: 323–334. [DOI] [PubMed] [Google Scholar]
- Kean, L., Cazenave W., Costes L., Broderick K. E., Graham S., Pollock V. P., Davies S. A., Veenstra J. A., and Dow J. A. T.. . 2002. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282: R1297–R1307. [DOI] [PubMed] [Google Scholar]
- King, A. N., Barber A. F., Smith A. E., Dreyer A. P., Sitaraman D., Nitabach M. N., Cavanaugh D. J., and Sehgal A.. . 2017. A peptidergic circuit links the circadian clock to locomotor activity. Curr. Biol. 27: 1915–1927.e5. doi: 10.1016/j.cub.2017.05.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingan, T. G., Blackburn M. B., and Raina A. K.. . 1992. The distribution of pheromone-biosynthesis-activating neuropeptide (PBAN) immunoreactivity in the central nervous system of the corn earworm moth, Helicoverpa zea. Cell Tissue Res. 270: 229–240. doi: 10.1007/bf00328008 [DOI] [Google Scholar]
- Kitamura, A., Nagasawa H., Kataoka H., Inoue T., Matsumoto S., Ando T., and Suzuki A.. . 1989. Amino acid sequence of pheromone-biosynthesis-activating neuropeptide (PBAN) of the silkworm. Biochem. Biophys. Res. Commun. 163: 520–526. [DOI] [PubMed] [Google Scholar]
- Koyama, T., Terhzaz S., Naseem M. T., Nagy S., Rewitz K., Dow J. A. T., Davies S. A., and Halberg K. V.. . 2021. A nutrient-responsive hormonal circuit mediates an inter-tissue program regulating metabolic homeostasis in adult Drosophila. Nat. Commun. 12: 5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp, J. J., Billeter J. -C., Wong A., Choi C., Nitabach M. N., and Levine J. D.. . 2013. Pigment-dispersing factor modulates pheromone production in clock cells that influence mating in Drosophila. Neuron. 79: 54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Kim K., and Nelson L. S.. . 1999. FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. Brain Res. 848: 26–34. [DOI] [PubMed] [Google Scholar]
- Lindemans, M., Janssen T., Husson S. J., Meelkop E., Temmerman L., Clynen E., Mertens I., and Schoofs L.. . 2009. A neuromedin-pyrokinin-like neuropeptide signaling system in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 379: 760–764. doi: 10.1016/j.bbrc.2008.12.121 [DOI] [PubMed] [Google Scholar]
- Loi, P. K., and Tublitz N. J.. . 2004. Sequence and expression of the CAPA/ CAP2b gene in the tobacco hawkmoth, Manduca sexta. J. Exp. Biol. 207: 3681–3691. doi: 10.1242/jeb.01186 [DOI] [PubMed] [Google Scholar]
- Ma, P. W. K., Garden R. W., Niermann J. T., O’ Connor M., Sweedler J. V., and Roelofs W. L.. . 2000. Characterizing the Hez-PBAN gene products in neuronal clusters with immunocytochemistry and MALDI MS. J. Insect Physiol. 46: 221–230. [DOI] [PubMed] [Google Scholar]
- Ma, P. W. K., Roelofs W. L., and Jurenka R. A.. . 1996. Characterization of PBAN and PBAN-encoding gene neuropeptides in the central nervous system of the corn earworm moth, Helicoverpa zea. J. Insect Physiol. 42: 257–266. [Google Scholar]
- Mains, R., and Eipper B.. . 1999a. The neuropeptides. InSiegel G. J., Agranoff B. W., Albers R. W., et al. (eds.), Basic neurochemistry: molecular, cellular and medical aspects, 6th ed. Lippencott-Raven, Philadelphia. [Google Scholar]
- Mains, R., and Eipper B.. . 1999b. Neuropeptide Receptors. InSiegel G. J., Agranoff B. W., Albers R. W., et al. (eds.), Basic neurochemistry: molecular, cellular and medical aspects, 6th ed. Lippencott-Raven, Philadelphia. [Google Scholar]
- Martinez, V. G., and O’Driscoll L.. . 2015. Neuromedin U: a multifunctional neuropeptide with pleiotropic roles. Clin. Chem. 61: 471–482. doi: 10.1373/clinchem.2014.231753 [DOI] [PubMed] [Google Scholar]
- Matsumoto, S., Kitamura A., Nagasawa H., Kataoka H., Orikasa C., Mitsui T., and Suzuki A.. . 1990. Functional diversity of a neurohormone produced by the suboesophageal ganglion: molecular identity of melanization and reddish colouration hormone and pheromone biosynthesis activating neuropeptide. J. Insect Physiol. 36: 427–432. doi: 10.1016/0022-1910(90)90060-s [DOI] [Google Scholar]
- McGregor, A. P. 2005. How to get ahead: the origin, evolution and function of bicoid. BioEssays. 27: 904–913. doi: 10.1002/bies.20285 [DOI] [PubMed] [Google Scholar]
- McKellar, C. E. 2016. Motor control of fly feeding. J. Neurogenet. 30: 101–111. doi: 10.1080/01677063.2016.1177047 [DOI] [PubMed] [Google Scholar]
- Melcher, C., Bader R., and Pankratz M. J.. . 2007. Amino acids, taste circuits, and feeding behavior in Drosophila: towards understanding the psychology of feeding in flies and man. J. Endocrinol. 192: 467–472. doi: 10.1677/joe-06-0066 [DOI] [PubMed] [Google Scholar]
- Melcher, C., Bader R., Walther S., Simakov O., and Pankratz M. J.. . 2006. Neuromedin U and its putative Drosophila homolog hugin. PLoS Biol. 4: e68. doi: 10.1371/journal.pbio.0040068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher, C., and Pankratz M. J.. . 2005. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 3: e305. doi: 10.1371/journal.pbio.0030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabeau, O., and Joly J. -S.. . 2013. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl. Acad. Sci 110: E2028–E2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroschnikow, A., Schlegel P., Schoofs A., Hueckesfeld S., Li F., Schneider-Mizell C. M., Fetter R. D., Truman J. W., Cardona A., and Pankratz M. J.. . 2018. Convergence of monosynaptic and polysynaptic sensory paths onto common motor outputs in a Drosophila feeding connectome. eLife. 7: e40247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno, Y., Imura E., Kurogi Y., Shimada‐Niwa Y., Kondo S., Tanimoto H., Hückesfeld S., Pankratz M. J., and Niwa R.. . 2021. A population of neurons that produce hugin and express the diuretic hormone 44 receptor gene projects to the corpora allata in Drosophila melanogaster. Dev. Growth Differ. 63: 249–261. doi: 10.1111/dgd.12733 [DOI] [PubMed] [Google Scholar]
- Neupert, S., Huetteroth W., Schachtner J., and Predel R.. . 2009. Conservation of the function counts: homologous neurons express sequence-related neuropeptides that originate from different genes. J. Neurochem. 111: 757–765. doi: 10.1111/j.1471-4159.2009.06361.x [DOI] [PubMed] [Google Scholar]
- Neupert, S., Russell W. K., Predel R., Russell D. H., Strey O. F., Teel P. D., and Nachman R. J.. . 2009. The neuropeptidomics of Ixodes scapularis synganglion. J. Proteomics. 72: 1040–1045. doi: 10.1016/j.jprot.2009.06.007 [DOI] [PubMed] [Google Scholar]
- Oh, Y., Lai J. S.-Y., Min S., Huang H.-W., Liberles S. D., Ryoo H. D., and Suh G. S. B.. . 2021. Periphery signals generated by Piezo-mediated stomach stretch and Neuromedin-mediated glucose load regulate the Drosophila brain nutrient sensor. Neuron. 109: 1979–1995.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predel, R., Eckert M., Pollák E., Molnár L., Scheibner O., and Neupert S.. . 2007. Peptidomics of identified neurons demonstrates a highly differentiated expression pattern of FXPRLamides in the neuroendocrine system of an insect. J. Comp. Neurol. 500: 498–512. [DOI] [PubMed] [Google Scholar]
- Predel, R., and Nachman R. J.. . 2006. The FXPRLamide (Pyrokinin/PBAN) peptide family, pp. 207–212. InKastin A. J., (ed.), Handbook of biologically active peptides. Elsevier,Amsterdam. [Google Scholar]
- Predel, R., Nachman R. J., and Gäde G.. . 2001. Myostimulatory neuropeptides in cockroaches: structures, distribution, pharmacological activities, and mimetic analogs. J. Insect Physiol. 47: 311–324. doi: 10.1016/s0022-1910(00)00129-3 [DOI] [PubMed] [Google Scholar]
- Predel, R., and Wegener C.. . 2006. Biology of the CAPA peptides in insects. Cell. Mol. Life Sci. 63: 2477–2490. doi: 10.1007/s00018-006-6187-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina, A. K., Jaffe H., Kempe T. G., Keim P., Blacher R. W., Fales H. M., Riley C. T., Klun J. A., Ridgway R. L., and Hayes D. K.. . 1989. Identification of a neuropeptide hormone that regulates sex pheromone production in female moths. Science. 244: 796–798. doi: 10.1126/science.244.4906.796 [DOI] [PubMed] [Google Scholar]
- Redeker, J., Bläser M., Neupert S., and Predel R.. . 2017. Identification and distribution of products from novel tryptopyrokinin genes in the locust, Locusta migratoria. Biochem. Biophys. Res. Commun. 486: 70–75. doi: 10.1016/j.bbrc.2017.02.135 [DOI] [PubMed] [Google Scholar]
- Roch, G. J., and Sherwood N. M.. . 2014. Glycoprotein hormones and their receptors emerged at the origin of metazoans. Genome Biol. Evol. 6: 1466–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saideman, S. R., Ma M., Kutz-Naber K. K., Cook A., Torfs P., Schoofs L., Li L., and Nusbaum M. P.. . 2007. Modulation of rhythmic motor activity by pyrokinin peptides. J. Neurophysiol. 97: 579–595. doi: 10.1152/jn.00772.2006 [DOI] [PubMed] [Google Scholar]
- Sajadi, F., Uyuklu A., Paputsis C., Lajevardi A., Wahedi A., Ber L. T., Matei A., and Paluzzi J. V.. . 2020. CAPA neuropeptides and their receptor form an anti-diuretic hormone signaling system in the human disease vector, Aedes aegypti. Sci. Rep. 10: 1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y., Oguchi M., Menjo N., Imai K., Saito H., Ikeda M., Isobe M., and Yamashita O.. . 1993. Precursor polyprotein for multiple neuropeptides secreted from the suboesophageal ganglion of the silkworm Bombyx mori: characterization of the cDNA encoding the diapause hormone precursor and identification of additional peptides. Proc. Natl. Acad. Sci. U. S. A. 5: 3251–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers, E. W., Bolton E. E., Brister J. R., Canese K., Chan J., Comeau D. C., Connor R., Funk K., Kelly C., Kim S.. et al. 2022. Database resources of the National Center for Biotechnology Information. Nucleic Acids Rep. 50 (D1): D20–D26. doi: 10.1093/nar/gkab1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel, P., Texada M. J., Miroschnikow A., Schoofs A., Hückesfeld S., Peters M., Schneider-Mizell C. M., Lacin H., Li F., Fetter R. D., . et al. 2016. Synaptic transmission parallels neuromodulation in a central food-intake circuit. eLife. 5: e16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ott, U. 2000. The amnioserosa is an apomorphic character of cyclorrhaphan flies. Dev. Genes Evol. 210: 373–376. doi: 10.1007/s004270000068 [DOI] [PubMed] [Google Scholar]
- Schneider, T. D., and Stephens R. M.. . 1990. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 18: 6097–6100. doi: 10.1093/nar/18.20.6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs, A., Hückesfeld S., Schlegel P., Miroschnikow A., Peters M., Zeymer M., Spieß R., Chiang A. -S., and Pankratz M. J.. . 2014. Selection of motor programs for suppressing food intake and inducing locomotion in the Drosophila brain. PLoS Biol. 12: e1001893. doi: 10.1371/journal.pbio.1001893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, J. E., King A. N., Hsu C. T., Barber A. F., and Sehgal A.. . 2021. Hugin+ neurons provide a link between sleep homeostat and circadian clock neurons. Proc. Natl. Acad. Sci. 118: e2111183118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers, F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J. D., Higgins D. G.. . 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. [DOI] [PMC free article] [PubMed]
- Simpson, P. 2002. Evolution of development in closely related species of flies and worms. Nat. Rev. Genet. 3: 907–907. doi: 10.1038/nrg947 [DOI] [PubMed] [Google Scholar]
- Spiegel, C. N., Dias D. B. S., Araki A. S., Hamilton J. G. C., Brazil R. P., and Jones T. M.. . 2016. The Lutzomyia longipalpis complex: a brief natural history of aggregation-sex pheromone communication. Parasites Vectors. 9: 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. -S., Zhang Q. -R., Zhang T. -Y., Zhu Z. -L., Zhang H. -M., Teng M. -K., Niu L. -W., and Xu W. -H.. . 2005. Developmental expression of FXPRLamide neuropeptides in peptidergic neurosecretory cells of diapause- and nondiapause-destined individuals of the cotton bollworm, Helicoverpa armigera. Gen. Comp. Endocrinol. 141: 48–57. doi: 10.1016/j.ygcen.2004.11.015 [DOI] [PubMed] [Google Scholar]
- Sun, F., Wang Y., Zhou Y., Van Swinderen B., Gong Z., and Liu L.. . 2014. Identification of neurons responsible for feeding behavior in the Drosophila brain. Sci. China Life Sci. 57: 391–402. doi: 10.1007/s11427-014-4641-2 [DOI] [PubMed] [Google Scholar]
- Surendran, S., Hückesfeld S., Wäschle B., and Pankratz M. J.. . 2017. Pathogen induced food evasion behavior in Drosophila larvae. J. Exp. Biol. 220: 1774–1780. [DOI] [PubMed] [Google Scholar]
- Tawata, M., and Ichikawa T.. . 2001. Circadian firing activities of neurosecretory cells releasing pheromonotropic neuropeptides in the silkmoth, Bombyx mori. Zool. Sci. 18: 645–649. doi: 10.2108/zsj.18.645 [DOI] [Google Scholar]
- Teal, P. E. A., Tumlinson J. H., and Oberlander H.. . 1989. Neural regulation of sex pheromone biosynthesis in Heliothis moths. Proc. Natl. Acad. Sci. 86: 2488–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman, J. A., Seybold S. J., Jurenka R. A., and Blomquist G. J.. . 1999. Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 29: 481–514. doi: 10.1016/s0965-1748(99)00016-8 [DOI] [PubMed] [Google Scholar]
- Tips, A., Schoofs L., Paemen L., Ma M., Blackburn M., Raina A., and De Loop A.. . 1993. Co-localization of locustamyotropin- and pheromone biosynthesis activating neuropeptide-like immunoreactivity in the central nervous system of five insect species. Comp. Biochem. Physiol. A Physiol. 106: 195–207. [Google Scholar]
- Torfs, P., Nieto J., Certiaens A., Boon D., Baggerman G., Poulos C., Waelkens E., Derua R., Caleron J., De Loof A., and Schoofs L.. . 2001. Pyrokinin neuropeptides in a crustacean. Isolation and identification in the white shrimp Penaeus vannamei. Eur. J. Biochem. 268: 149–154. [DOI] [PubMed] [Google Scholar]
- Veenstra, J. A. 2014. The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front. Physiol. 5(454): 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra, J. A., Rombauts S., and Grbić M.. . 2012. In silico cloning of genes encoding neuropeptides, neurohormones and their putative G-protein coupled receptors in a spider mite. Insect Biochem. Mol. Biol. 42: 277–295. [DOI] [PubMed] [Google Scholar]
- Wiegmann, B. M., Trautwein M. D., Winkler I. S., Barr N. B., Kim J. -W., Lambkin C., Bertone M. A., Cassel B. K., Bayless K. M., Heimberg, A. M.. et al. 2011. Episodic radiations in the fly tree of life. Proc. Natl. Acad. Sci. 108: 5690–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann, B. M., and Yeates D.. . 2017. Phylogeny of diptera, pp. 253–265. InKirk-Spriggs A. H. and Sinclair B. J. (eds.), Manual of afrotropical diptera, vol. 1. South African National Biodiversity Institute, Praetoria. [Google Scholar]
- Xiong, C., Wulff J. P., Nachman R. J., and Pietrantonio P. V.. . 2022. Myotropic activities of tick pyrokinin neuropeptides and analog in feeding tissues of hard ticks (Ixodidae). Front. Physiol. 12: 826399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W. -H., and Denlinger D. L.. . 2003. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect. Mol. Biol. 12: 509–516. doi: 10.1046/j.1365-2583.2003.00437.x [DOI] [PubMed] [Google Scholar]
- Yaginuma, T., and Niimi T.. . 2016. Pyrokinin, pp. 401–402. InTakei Y., Ando H., and Tsutsui K. (eds.), Handbook of hormones. Elsevier,Amsterdam. [Google Scholar]
- Yamashita, O. 1996. Diapause hormone of the silkworm, Bombyx mori: structure, gene expression and function. J. Insect Physiol. 42: 669–679. doi: 10.1016/0022-1910(96)00003-0 [DOI] [Google Scholar]
- Yañez-Guerra, L. A., Thiel D., and Jékely G.. . 2022. Premetazoan origin of neuropeptide signaling. Mol. Biol. Evol. 39: msac051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M., Dong J., Tang S., Chen X., Chen H., Duanmu H., He K., and Li F.. . 2022. InsectBase 2.0: a comprehensive gene resource for insects. Nucleic Acids Res. 50: D1040–D1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Nachman R. J., and Pietrantonio P. V.. . 2015. Molecular and pharmacological characterization of the Chelicerata pyrokinin receptor from the southern cattle tick, Rhipicephalus (Boophilus) microplus. Insect Biochem. Mol. Biol. 60: 13–23. doi: 10.1016/j.ibmb.2015.02.010 [DOI] [PubMed] [Google Scholar]
- Zandawala, M., Nguyen T., Balanyà Segura M., Johard H. A. D., Amcoff M., Wegener C., Paluzzi J. -P., and Nässel D. R.. . 2021. A neuroendocrine pathway modulating osmotic stress in Drosophila. PLoS Genet. 17: e1009425. doi: 10.1371/journal.pgen.1009425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Závodská, R., von Wowern G., Löfstedt C., Rosén W. Q., and Sauman I.. . 2009. The release of a pheromonotropic neuropeptide, PBAN, in the turnip moth Agrotis segetum, exhibits a circadian rhythm. J. Insect Physiol. 55: 435–440. [DOI] [PubMed] [Google Scholar]
- Zdarek, J., Nachman R. J., and Hayes T. K.. . 1997. Insect neuropeptides of the pyrokinin/pban family accelerate pupariation in the fleshfly (Sarcophaga bullata) larvae. Ann. N. Y. Acad. Sci. 814: 67–72. doi: 10.1111/j.1749-6632.1997.tb46145.x [DOI] [PubMed] [Google Scholar]
- Žitňan, D., Kingan T. G., Hermesman J. L., and Adams M. E.. . 1996. Identification of ecdysis-triggering hormone from an epitracheal endocrine system. Science. 271: 88–91. doi: 10.1126/science.271.5245.88 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.