Abstract
BACKGROUND
The effect of CYP2C19 genotype on treatment outcomes with ticagrelor or prasugrel as compared to clopidogrel is unclear.
METHODS
Databases through February 19th, 2020 were searched for studies reporting the effect of CYP2C19 genotype on ischemic outcomes during ticagrelor or prasugrel versus clopidogrel treatment. Study eligibility required outcomes reported for CYP2C19 genotype status and clopidogrel and alternate P2Y12 inhibitors in coronary artery disease (CAD) patients with at least 50% undergoing percutaneous coronary intervention (PCI). The primary analysis consisted of randomized controlled trials (RCTs). A secondary analysis was conducted by adding non-RCTs to the primary analysis. The primary outcome was a composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, and severe recurrent ischemia. Meta-analysis compared the two drug regimens and tested interaction with CYP2C19 genotype.
RESULTS
Of 1,335 studies identified, 7 RCTs were included (15,949 patients, mean age 62 years, 77% had PCI, 98% had acute coronary syndromes). Statistical heterogeneity was minimal and risk of bias was low. Ticagrelor and prasugrel as compared to clopidogrel resulted in a significant reduction in ischemic events (relative risk (RR) 0.70; 95%CI 0.59–0.83) in CYP2C19 loss-of-function carriers but not in non-carriers (RR 1.0; 95%CI 0.80–1.25). The test of interaction based on CYP2C19 genotype status was statistically significant (p=0.013) suggesting that CYP2C19 genotype modified the effect. An additional 4 observational studies were found and adding them to the analysis provided the same conclusions (p value of the test of interaction=<0.001).
CONCLUSIONS
The effect of ticagrelor or prasugrel compared to clopidogrel in reducing ischemic events in patients with CAD who predominantly undergo PCI is primarily based on the presence of CYP2C19 loss-of-function carrier status. These results support genetic testing prior to prescribing P2Y12 inhibitor therapy.
Keywords: meta-analysis, CYP2C19 genotype, ischemic outcomes, ticagrelor, prasugrel, clopidogrel, systematic review
CONDENSED ABSTRACT
To determine the effect of CYP2C19 genotype on ischemic outcomes with ticagrelor or prasugrel as compared to clopidogrel a meta-analysis was conducted in patients with coronary artery disease who predominantly underwent PCI. There were 7 randomized clinical trials identified with 15,949 patients, 77% had percutaneous coronary intervention and 98% had acute coronary syndromes. Ticagrelor and prasugrel as compared to clopidogrel resulted in a significant 30% reduction in ischemic events in CYP2C19 loss-of-function carriers but not in non-carriers. The overall beneficial effect of ticagrelor and prasugrel when compared to clopidogrel was modified by CYP2C19 genotype supporting the role of pharmacogenetic testing.
Tweet:
CYP2C19 genetic testing can identify loss of function patients who would benefit from ticagrelor or prasugrel while non-carriers could be prescribed clopidogrel after PCI
INTRODUCTION
Clopidogrel is a prodrug and is primarily metabolized by the cytochrome P450 enzyme CYP2C19 resulting in an active metabolite that blocks the platelet P2Y12 receptor inhibiting platelet aggregation (1). Patients with CYP2C19 loss-of-function (LOF) genotype are unable to metabolize clopidogrel effectively and hence are at an increased risk of cardiovascular (CV) ischemic events (2). The Food and Drug Administration therefore advises medical practitioners to prescribe alternative antiplatelet therapy that are not predominantly metabolized by CYP2C19 such as ticagrelor or prasugrel for patients who are CYP2C19 poor metabolizers (3). Ticagrelor and prasugrel have both been demonstrated in randomized clinical trials (RCTs) to be superior to clopidogrel in reducing ischemic outcomes in patients with acute coronary syndromes (ACS) (4,5). However, it is not clear whether the presence of CYP2C19 LOF genotype influenced outcomes in these RCTs. It remains uncertain whether the benefit of alternate P2Y12 inhibitors occurs primarily in the patients who are CYP2C19 LOF carriers and not in non-carriers. Treatment with alternate P2Y12 inhibitors as compared to clopidogrel is more expensive, results in increased bleeding complications and adverse effects such as dyspnea in the case of ticagrelor, therefore it may be advantageous to individualize antiplatelet therapy based on CYP2C19 genotype (4). A recent CYP2C19 genotype-guided P2Y12 inhibitor therapy strategy study demonstrated non-inferiority in reducing a composite of ischemic and bleeding events as compared to treating all patients with ticagrelor after percutaneous coronary intervention (PCI) for myocardial infarction (6). In this study, patients in the genotype-guided group who were CYP2C19 LOF carriers received ticagrelor and non-carriers received clopidogrel, therefore the results were suggestive that treating non-carriers with clopidogrel was as efficacious as treating them with ticagrelor. However, CYP2C19 genotyping information was not available for patients receiving ticagrelor in the standard of care arm that if available would have allowed evaluation of the test of interaction based on CYP2C19 genotype status in our study described below. In the recently completed TAILOR PCI trial, the point estimate suggests a 34% reduction in ischemic events in CYP2C19 LOF carriers receiving ticagrelor compared to clopidogrel, with a 95% confidence interval (CI) of 0.43–1.02 (7). The upper boundary of which may be due to lack of power given that the sample size calculation of this trial was based on a 50% treatment effect.
Considering that these RCTs may not have been conclusive largely due to lack of power, we conducted a systematic review and meta-analysis to examine the association of CYP2C19 genotype and clinical outcomes in patients with coronary artery disease (CAD) who predominantly underwent PCI with CYP2C19 genotyping information available and compared outcomes of those treated with ticagrelor or prasugrel versus clopidogrel.
METHODS
The study was considered exempt by the Mayo Clinic Institutional Review Board. The inclusion and exclusion criteria of this systematic review (Table 1) and statistical analysis plan were defined a priori. The reporting of this systematic review follows the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (8).
Table 1.
Inclusion and exclusion criteria applied for review of all studies extracted using initial search strategy.
| Inclusion criteria |
|---|
|
|
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
|
ACS- Acute coronary syndromes,
CAD- Coronary artery disease,
PCI- Percutaneous coronary intervention,
MACE- Major adverse cardiovascular events
Literature Search
A systematic literature search was conducted in several databases including Ovid MEDLINE® and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid Cochrane Central Register of Controlled Trials, and Scopus from inception to February 19th, 2020. Controlled vocabulary supplemented with keywords ‘CYP2C19’ and ‘clopidogrel’, ‘ticagrelor’, or ‘prasugrel’ was used to search for studies in human adults. The detailed search strategy is outlined in the Supplemental Appendix.
Study Selection and Data Extraction
The citations identified by the initial search were evaluated in two rounds by two investigators (GM, SS) independently for inclusion. The first round of evaluation consisted of title and abstract review and the second round consisted of full-text review. Any discrepancies were adjudicated by a third investigator (RL). The inclusion and exclusion criteria used are described in Table 1.
Data were extracted in duplicate (GM, SS) to a standardized data collection file with prespecified fields, including: study identifiers, genotyping information, baseline characteristics, study design parameters and clinical outcomes. The target primary efficacy endpoint was defined as the composite cardiovascular death, myocardial infarction, stroke, stent thrombosis and severe recurrent ischemia. Similarly, the safety endpoint was defined as rate of major or minor bleeding based on TIMI (Thrombolysis in myocardial infarction) criteria. If a study did not report these endpoints, the available endpoint most similar in definition was abstracted. Disagreements in data abstraction were resolved by consensus or by a third evaluator (RL) if consensus was not reached.
Risk of Bias and Certainty in the Evidence
The risk of bias was assessed using the Cochrane tool for assessing risk of bias in RCTs (9). The certainty in evidence about an outcome was evaluated using the GRADE approach (10). In this approach, RCTs provide high certainty evidence that could be rated down for risk of bias, imprecision (wide confidence intervals), inconsistency (i.e., heterogeneity), indirectness (surrogate outcomes or extrapolation from other populations) or publication bias (11).
Statistical Analysis
For each study, the most adjusted effect size was extracted. If unavailable, we extracted an unadjusted effect (i.e., a 2×2 table). Effect sizes were pooled across studies using the random-effects model (12) due to heterogeneity of study populations and settings. The pooled effect was expressed as a relative risk (RR) with 95% confidence intervals. Analysis was conducted separately for CYP2C19 LOF carriers and non-carriers to explore the interaction between genotype and treatment effect, as well as a combined analysis that pooled both genotypes together to compare the two different drug regimens. The primary analysis included only RCTs. A secondary analysis was conducted by adding non-RCTs to the analysis. Heterogeneity was assessed using the I-squared statistic which represents the percentage of heterogeneity non-attributable to chance. An interaction test was conducted as described by Altman and Bland (13) with 2 tailed p value < 0.05 considered statistically significant. Analysis was done using STATA version 16.
RESULTS
The initial literature search identified 1,335 potential citations. Of them, 1,303 (97.6%) were excluded during the initial screening, most commonly because they were not original studies (n=599), failed to report CV outcomes (n=274), or did not report genotyping information (n=186). The remaining 32 publications underwent full text review and data extraction. Of them, 21 were further excluded, most commonly because the study design did not allow for assessment of outcomes based on CYP2C19 LOF carrier and non-carrier status. Figure 1 shows the details of the search. Finally, data from the recently presented TAILOR-PCI trial was added to the systematic review, resulting in 12 studies from 7 RCTs and 4 non-RCTs (7,14–24). Two publications by Mega et al. were sub-analyses from a single RCT as each one compared CYP2C19 LOF carriers to non-carriers within a given P2Y12 receptor inhibitor regimen (Figure 1) (14,23). The characteristics of studies included are summarized in Table 2.
Figure 1.
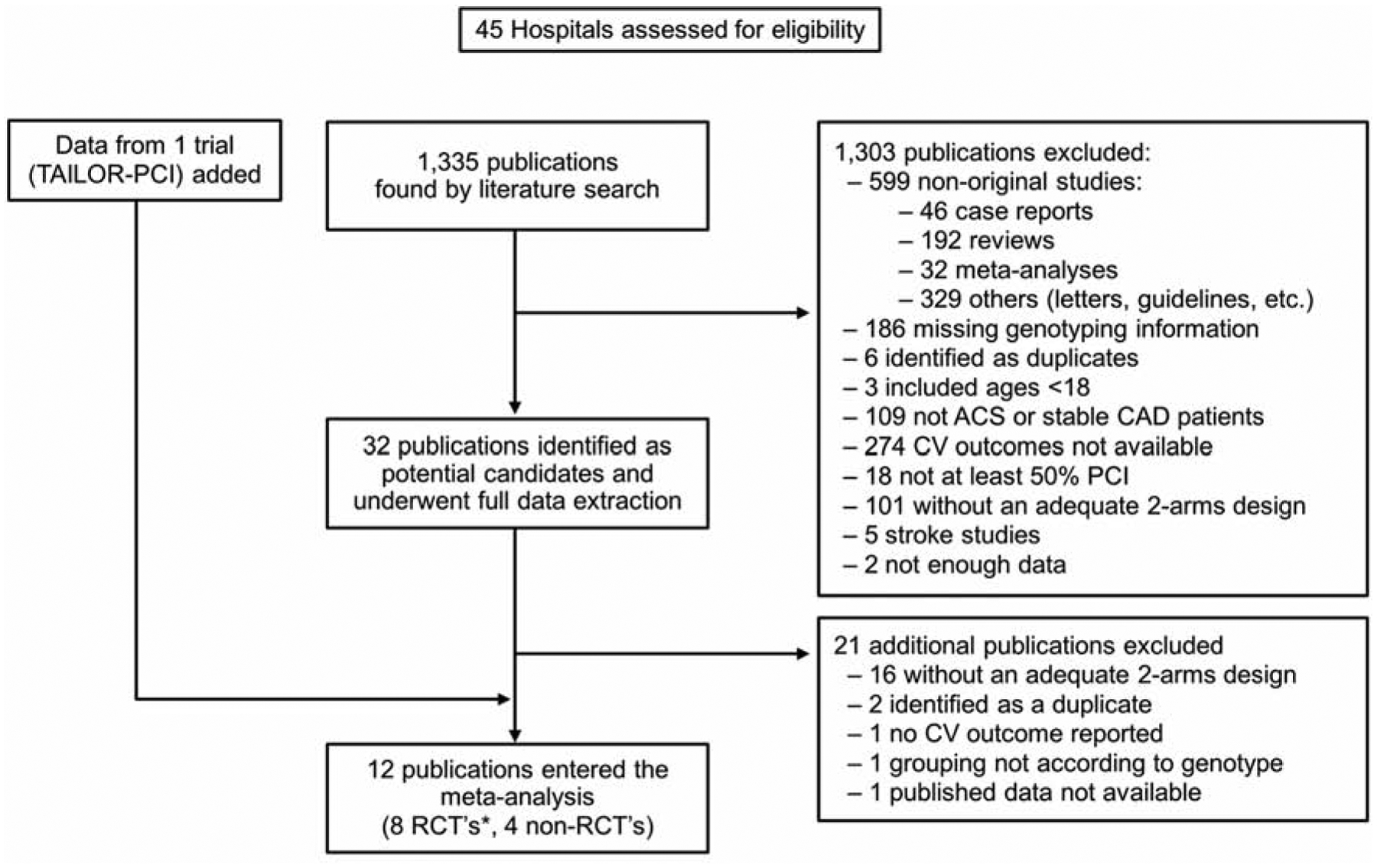
Flowchart Summary of Study Selection for Meta-Analysis According to Inclusion/Exclusion Criteria. RCT, Randomized Controlled Trial; ACS, Acute Coronary Syndrome; CAD, Coronary Artery Disease; CV, Cardiovascular; PCI, Percutaneous Coronary Intervention.
Table 2.
Characteristics of Studies Included in the Meta-analysis
| Source | Type | Subgroups reported | Ischemic outcome† | Bleeding outcome | Alleles genotyped | Maximum FU (months) | Clopidogrel loading/maintenance doses | Alternate therapy | Alternate loading/maintenance doses | Mean age | Male % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mega 2009,(14) Mega 2009(23) |
RCT | LOF and non-LOF | CVD, MI, CVA | Major or minor | CYP2C19, CYP2C9, CYP2B6, CYP1A2, CYP3A5, CYP1A12 | 15 | 300/75 | Prasugrel | 60/10 | 60 | 72 |
| Wallent in 2010(15) | RCT | LOF and non-LOF | CVD, MI, CVA | Major | CYP2C19 (*2, *3, *4, *5, *6, *7, *8) | 12 | 300–600/75 | Ticagrelor | 180/90 | 63 | 70 |
| Deiman 2016(16) | Non-RCT | LOF only | CVD, MI, CVA, ST, re-PCI | -- | CYP2C19 (*2, *3) | 18 | 300/75 | Prasugrel | --/10 | 67 | 74 |
| Dong 2016(17) | RCT | LOF only | DTH, MI, CVA, revasc | -- | CYP2C19 (*2, *3) | 1 | 600/75 | Ticagrelor | 180/90 | 67 | 80 |
| Ogawa 2016(18) | RCT | LOF and non-LOF | CVD, MI, CVA | Major or minor | CYP2C19 (*2, *3) | 6 | 300/75 | Prasugrel | 20/3.75 | 64 | 81 |
| Zhang 2016(19) | RCT | LOF only | DTH, MI, CVA | Major or minor | CYP2C19 (*2, *3) | 6 | 600/75 | Ticagrelor | 180/90 | 70 | 50 |
| Chen 2017(20) | Non-RCT | LOF only | CVD, MI, CVA | Any bleeding event | CYP2C19 (*2) | 12 | 300/75 | Ticagrelor | --/90 | 60 | -- |
| Cavallari 2018(21) | Non-RCT | LOF and non-LOF | DTH, MI, CVA | -- | CYP2C19 (*2, *3) | 12 | 300/75 | Prasugrel, ticagrelor, high-dose clopidogrel | --/-- | 63 | 67 |
| Lee 2018(22) | Non-RCT | LOF and non-LOF | DTH, MI, CVA, TIA, ST, UA-hosp | Clinically significant | CYP2C19 (*2, *3) | 12 | -- | Prasugrel or ticagrelor | --/-- | -- | |
| Xiong 2015(24) | RCT | LOF only | MACE | Major or minor | CYP2C19 (*2) | 1 | 600/150 | Ticagrelor | 180/90 | 67 | 71 |
| Pereira 2020(7) | RCT | LOF only | CVD, MI, CVA, ST, SRI | Major or minor | CYP2C19 (*2, *3) | 12 | 300–600/75 | Ticagrelor | 180/90 | 62 | 76 |
CVD=Cardiovascular-related death, MI=myocardial infarction, CVA=cerebrovascular accident, ST=stent thrombosis, re-PCI=repeat PCI, revasc=revascularization (PCI or CABG), TIA=transient ischemic attack, UA-hosp=hospitalization for unstable angina, MACE=major adverse cardiovascular event, SRI=severe recurrent ischemia.
The studies reported on a total of 15,949 patients that were enrolled in RCTs (mean age was 62 years, 71% were males, 77% underwent PCI, 98% had ACS, 25% had diabetes and 34% were smokers) and 18,808 patients in both RCTs and non-RCTs (mean age was 62 years, 71% were males, 80% underwent PCI, 94% had ACS, 26% had diabetes, 34% were smokers).
Risk of Bias
The details of risk of bias assessment are summarized in Table 3 for RCTs and Table 4 for non-RCTs and definitions of risk of bias indicators are outlined in Supplemental Table 1. Most RCTs had adequate randomization approaches and concealment of allocation, while degree of blinding varied. Most non-RCTs had appropriate selection and ascertainment approaches, while adjustment for confounding and blinded assessments were typically lacking. Overall, the global risk of bias for both ischemic and bleeding outcomes in the RCTs was low, and in the non-RCTs was high.
Table 3.
Assessments of the Risk of Bias in Randomized Clinical Trials Included in the Meta-analysis
| Source | Randomization sequence | Allocation concealment | Blinding of patients | Blinding of caregivers | Blinding of outcome assessors | Blinding of analysts | Percent lost to follow-up |
|---|---|---|---|---|---|---|---|
| Mega 2009(14) | adequate | adequate | adequate | adequate | adequate | inadequate | 1.2 |
| Mega 2009(23) | adequate | adequate | adequate | adequate | adequate | inadequate | 0.75 |
| Wallentin 2010(15) | adequate | adequate | adequate | adequate | inadequate | inadequate | 0 |
| Dong 2016(17) | adequate | inadequate | inadequate | inadequate | inadequate | inadequate | 0 |
| Ogawa 2016(18) | adequate | adequate | adequate | inadequate | adequate | inadequate | 0 |
| Zhang 2016(19) | adequate | inadequate | inadequate | inadequate | inadequate | inadequate | 0 |
| Xiong 2015(24) | adequate | inadequate | inadequate | inadequate | inadequate | inadequate | 0 |
| Pereira 2020(7) | adequate | adequate | inadequate | inadequate | adequate | Inadequate | 2.9 |
Table 4.
Assessments of the Risk of Bias in Non-RCT Studies included in the Meta-analysis
| Source | Exposed cohort selection | Control cohort selection | Ascertainment of exposure | Ascertainment of outcome | Groups comparable in characteristics | Analysis adjusted for important confounders | Assessment of outcome blinded | Follow-up sufficient for outcome to occur | Percent lost to followup |
|---|---|---|---|---|---|---|---|---|---|
| Deiman (2016) | inadequate | inadequate | adequate | adequate | adequate | inadequate | inadequate | adequate | unknown |
| Chen (2017) | adequate | adequate | adequate | adequate | adequate | inadequate | inadequate | adequate | unknown |
| Cavallari (2018) | adequate | adequate | adequate | adequate | adequate | adequate | inadequate | adequate | unknown |
| Lee (2018) | adequate | adequate | adequate | adequate | inadequate | inadequate | inadequate | adequate | unknown |
Meta-Analysis
Ischemic outcomes:
Meta-analysis of 7 RCTs enrolling 6,409 CYP2C19 LOF carriers demonstrated a statistically significant reduction in the risk of ischemic events (RR 0.70; 95% CI, 0.59–0.83) (Figure 2a) with the use of ticagrelor or prasugrel (7.0%; 223 events in 3,172 patients) as compared to clopidogrel (10.3%; 335 events in 3,237 patients). There was no similar significant reduction observed in meta-analysis of 4 studies enrolling 9,540 non-carriers (RR 1.00; 95% CI 0.80–1.25; alternate therapy 8.8%, 419/4,781; clopidogrel 9.2%, 439/4,759) (Figure 2a). The test of interaction based on CYP2C19 genotype status was statistically significant (p=0.013) suggesting that CYP2C19 genotype modifies the effect. In a secondary analysis, all studies (RCTs and non-RCTs) were analyzed (Figure 2b). The results were consistent with the main analysis demonstrating a significant reduction in the risk of ischemic events in CYP2C19 LOF carriers treated with ticagrelor and prasugrel (11 studies) compared to clopidogrel and but no difference in non-carriers (6 studies) (p value of the test of interaction=<0.001).
Figure 2a.
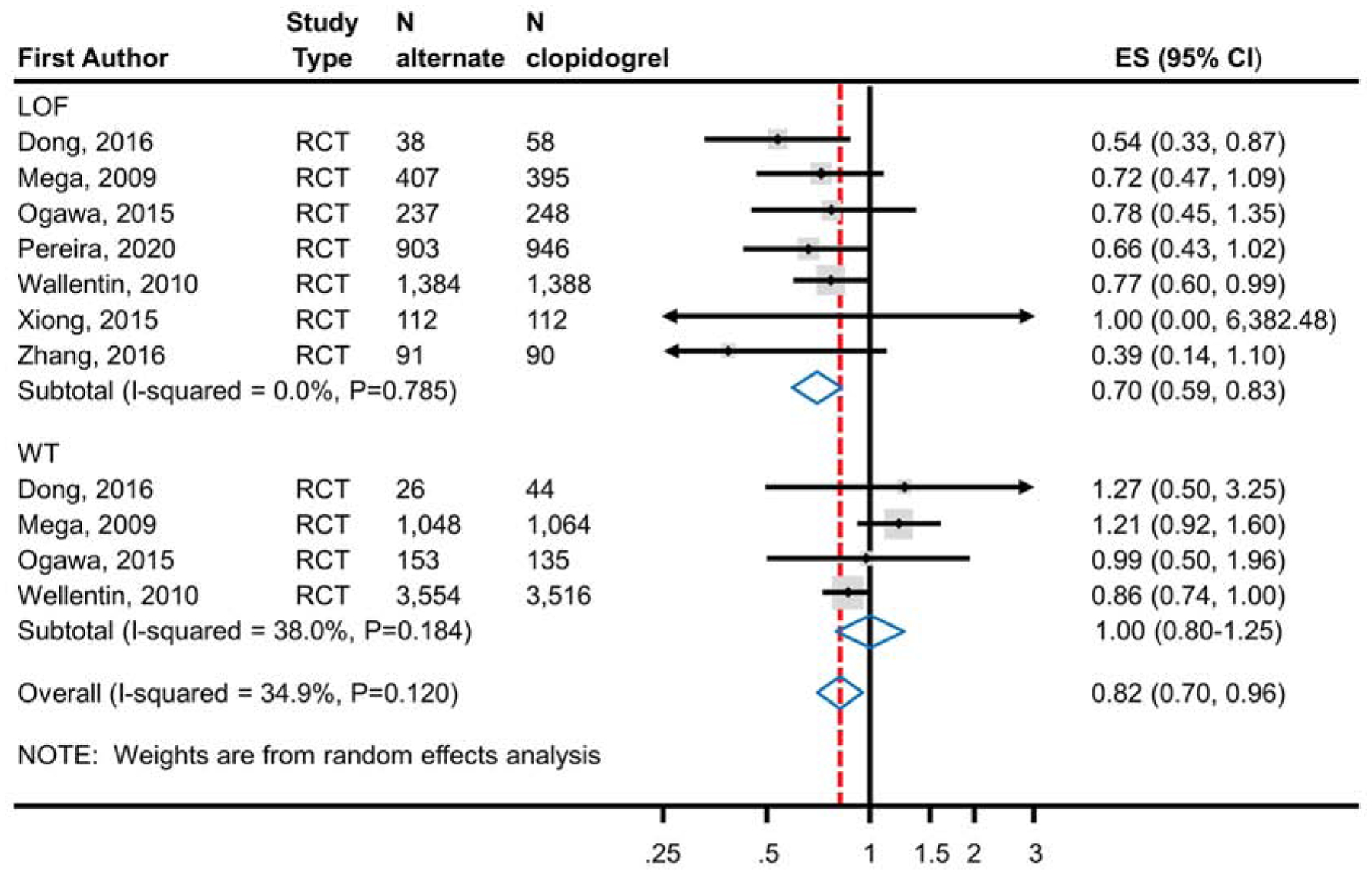
Analysis of Ischemic Events in RCTs Comparing Alternate P2Y12 Inhibitors to Clopidogrel Treatment According to CYP2C19 Genotype Status. Meta-analysis of ischemic event risk in CAD patients predominantly after PCI treated with an alternate P2Y12 inhibitor or clopidogrel. The top panel analyzes patients identified as CYP2C19 LOF carriers; the bottom panel analyzes those identified as non-carriers. Risk ratios less than 1 indicate better outcomes for alternative therapy; risk ratios greater than 1 indicate better outcomes for clopidogrel. The test for interaction between genotype status and treatment effect was significant (p=0.013) indicating a statistically significant difference in effect based on genotype. ES, Effect Size; LOF, Loss-of-Function; RCT, Randomized Controlled Trial; CAD, Coronary Artery Disease; PCI, Percutaneous Coronary Intervention.
Figure 2b.
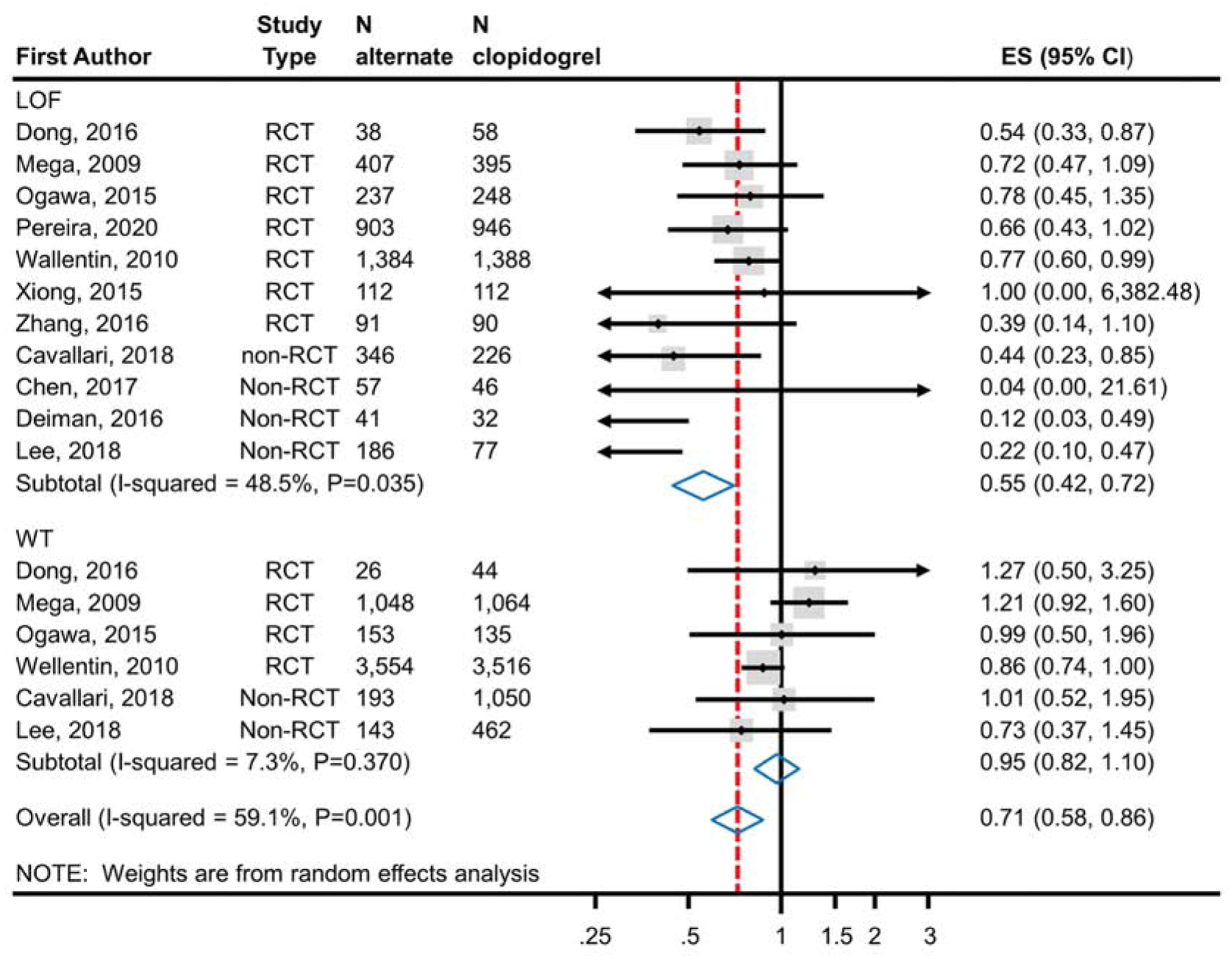
Analysis of Ischemic Events in RCTs and Non-RCTs Comparing Alternate P2Y12 Inhibitors to Clopidogrel Treatment According to CYP2C19 Genotype Status. Meta-analysis of ischemic event risk in CAD patients predominantly after PCI treated with an alternate P2Y12 inhibitor or clopidogrel. The top panel analyzes patients identified as CYP2C19 LOF carriers; the bottom panel analyzes those identified as non-carriers. Risk ratios less than 1 indicate better outcomes for alternative therapy; risk ratios greater than 1 indicate better outcomes for clopidogrel. The test for interaction between CYP2C19 genotype status and treatment effect was significant (p<0.001) indicating a statistically significant difference in effect based on genotype. ES, Effect Size; LOF, Loss-of-Function; RCT, Randomized Controlled Trial; CAD, Coronary Artery Disease; PCI, Percutaneous Coronary Intervention.
Bleeding outcome:
Meta-analysis of 6 RCTs enrolling 6,309 CYP2C19 LOF carriers showed no significant difference in the risk of major and minor bleeding (RR 0.91; 95% CI, 0.64–1.30) with the use of ticagrelor or prasugrel (6.7%; 210 events in 3,132 patients) as compared to clopidogrel (6.8%; 215 events in 3,177 patients) (Figure 3a). The difference was also not significant in the meta-analysis of 3 RCTs enrolling 9,466 CYP2C19 LOF non-carriers (RR 0.99; 95% CI 0.86–1.14; alternate therapy 7.9%, 376/4,754; clopidogrel 8.0%, 375/4,712) (Figure 3a). The results were also consistent with the secondary analysis that included non-RCTs (Figure 3b); that is, the reduction in the risk of the bleeding outcome was not statistically significant in meta-analysis of 9 studies with CYP2C19 LOF carriers and in 4 studies with non-carriers. Test of interaction based on CYP2C19 genetic status was non-significant in both analyses (p=0.67 in RCTs, p=0.92 in all studies).
Figure 3a.
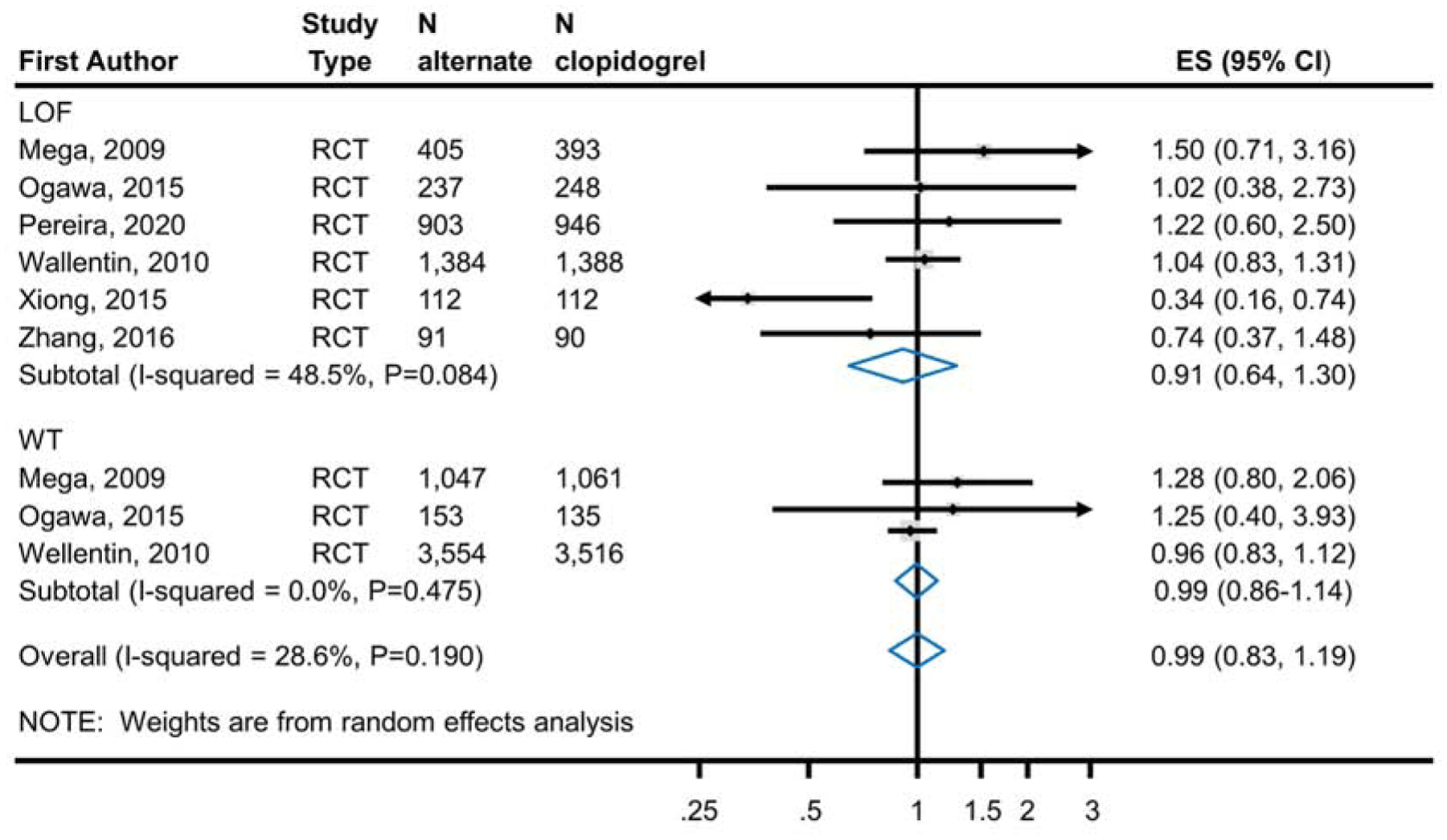
Analysis of Bleeding Events in RCTs Comparing Alternate P2Y12 Inhibitors to Clopidogrel Treatment According to CYP2C19 Genotype. Meta-analysis of bleeding event risk in CAD patients predominantly after PCI treated with an alternate P2Y12 inhibitor or clopidogrel. The top panel analyzes patients identified as CYP2C19 LOF carriers; the bottom panel analyzes those identified as non-carriers. Risk ratios less than 1 indicate better outcomes for alternative therapy; risk ratios greater than 1 indicate better outcomes for clopidogrel. The test for interaction between metabolizer type and treatment effect was non-significant (p=0.67) indicating no statistically significant evidence for a differential effect of alternative therapies based on genotype. ES, Effect Size; LOF, Loss-of-Function; RCT, Randomized Controlled Trial; CAD, Coronary Artery Disease; PCI, Percutaneous Coronary Intervention.
Figure 3b.
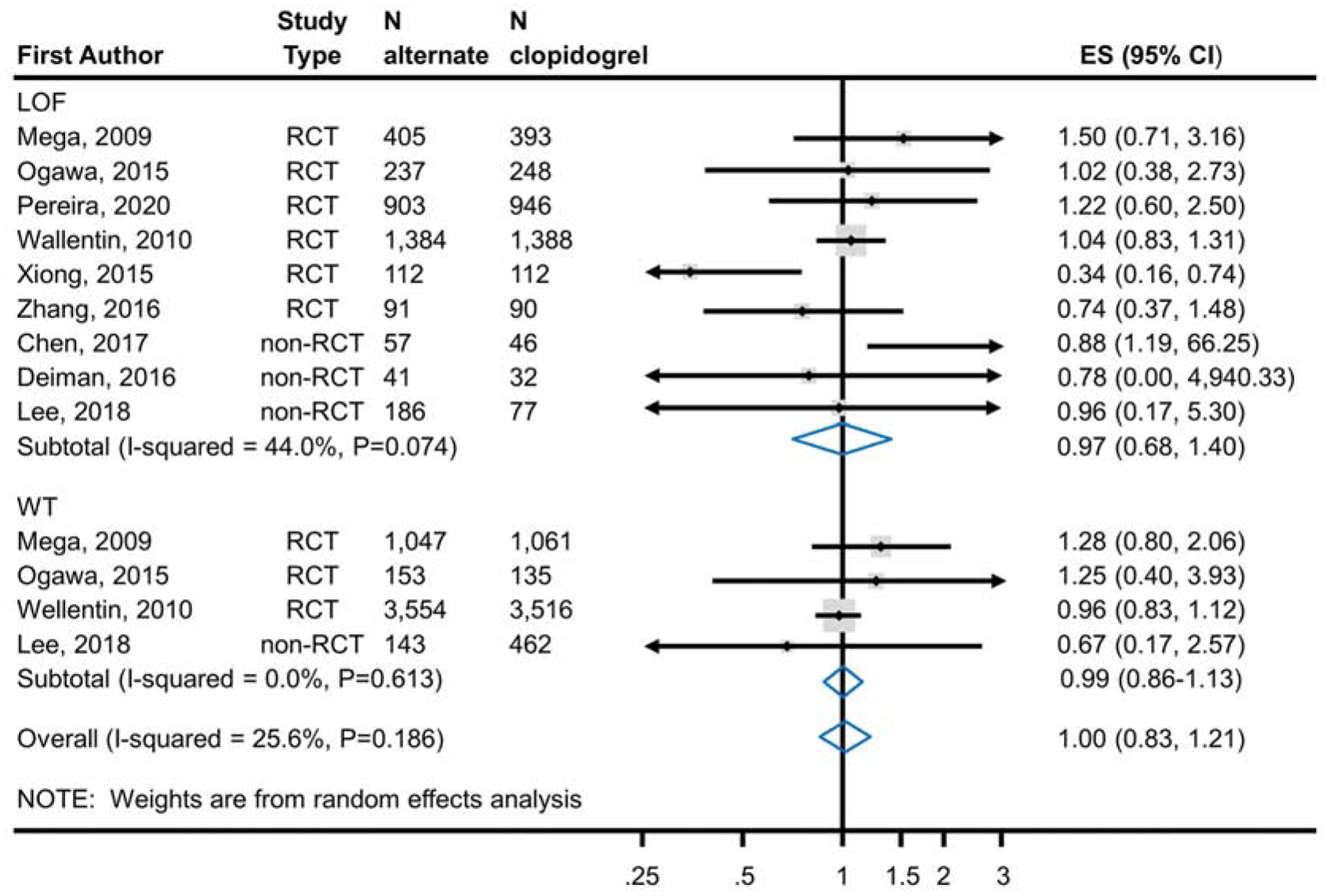
Analysis of Bleeding Events in RCTs and Non-RCTs Comparing Alternate P2Y12 Inhibitors to Clopidogrel Treatment According to CYP2C19 Genotype. Meta-analysis of bleeding event risk in CAD patients predominantly after PCI treated with an alternate P2Y12 inhibitor or clopidogrel. The top panel analyzes subjects identified as CYP2C19 LOF carriers; the bottom panel analyzes those identified as non-carriers. Risk ratios less than 1 indicate better outcomes for alternative therapy; risk ratios greater than 1 indicate better outcomes for clopidogrel. The test for interaction between metabolizer type and treatment effect was non-significant (p=0.92) indicating no statistically significant evidence for a differential effect of alternative therapies based on genotype. ES, Effect Size; LOF, Loss-of-Function; RCT, Randomized Controlled Trial; CAD, Coronary Artery Disease; PCI, Percutaneous Coronary Intervention.
Heterogeneity, Publication Bias and Certainty in the Evidence
Statistical heterogeneity of treatment effect was overall minimal (I-squared value under 50% in all analyses when stratified by CYP2C19 genotype). Publication bias could not be statistically assessed due to the small number of studies per each stratified analysis. The certainty in the estimates of the ischemic outcome was high. The certainty in the estimates of the bleeding outcome was low likely due to lack of power based on the lower number of bleeding events in each genotype category as reflected by the wide confidence intervals.
DISCUSSION
Main Findings
In the current meta-analysis, CYP2C19 LOF carriers with CAD who predominantly had ACS and underwent PCI had improved ischemic outcomes when treated with ticagrelor or prasugrel as compared to those receiving clopidogrel. This beneficial effect was not observed in CYP2C19 LOF non-carriers (Central Illustration). Our findings suggest that the reduction in ischemic events observed with alternate P2Y12 inhibitors when compared to clopidogrel in clinical trials is likely due to substantially reduced events in CYP2C19 LOF carriers and not in non-carriers. This meta-analysis supports genetic testing for selection of P2Y12 inhibitors in this patient population, validates the model of personalized medicine and is proof of concept for a precision medicine approach to adopting optimal and safe therapies in cardiovascular disease (25). The increasing acceptance of genetic testing is reflected in the 2020 ESC guidelines for acute coronary syndromes that recommend as Class IIb, consideration of de-escalation of P2Y12 receptor inhibitor treatment (e.g. with a switch from prasugrel or ticagrelor to clopidogrel) for ACS patients deemed unsuitable for potent platelet inhibition using CYP2C19 genotyping (26). This perspective is also reflected in a state-of-the-art expert consensus statement that advocates either de-escalation or escalation of dual anti-platelet therapy based clinical and procedural characteristics and results of platelet function and CYP2C19 genetic testing (27).
Central Illustration.
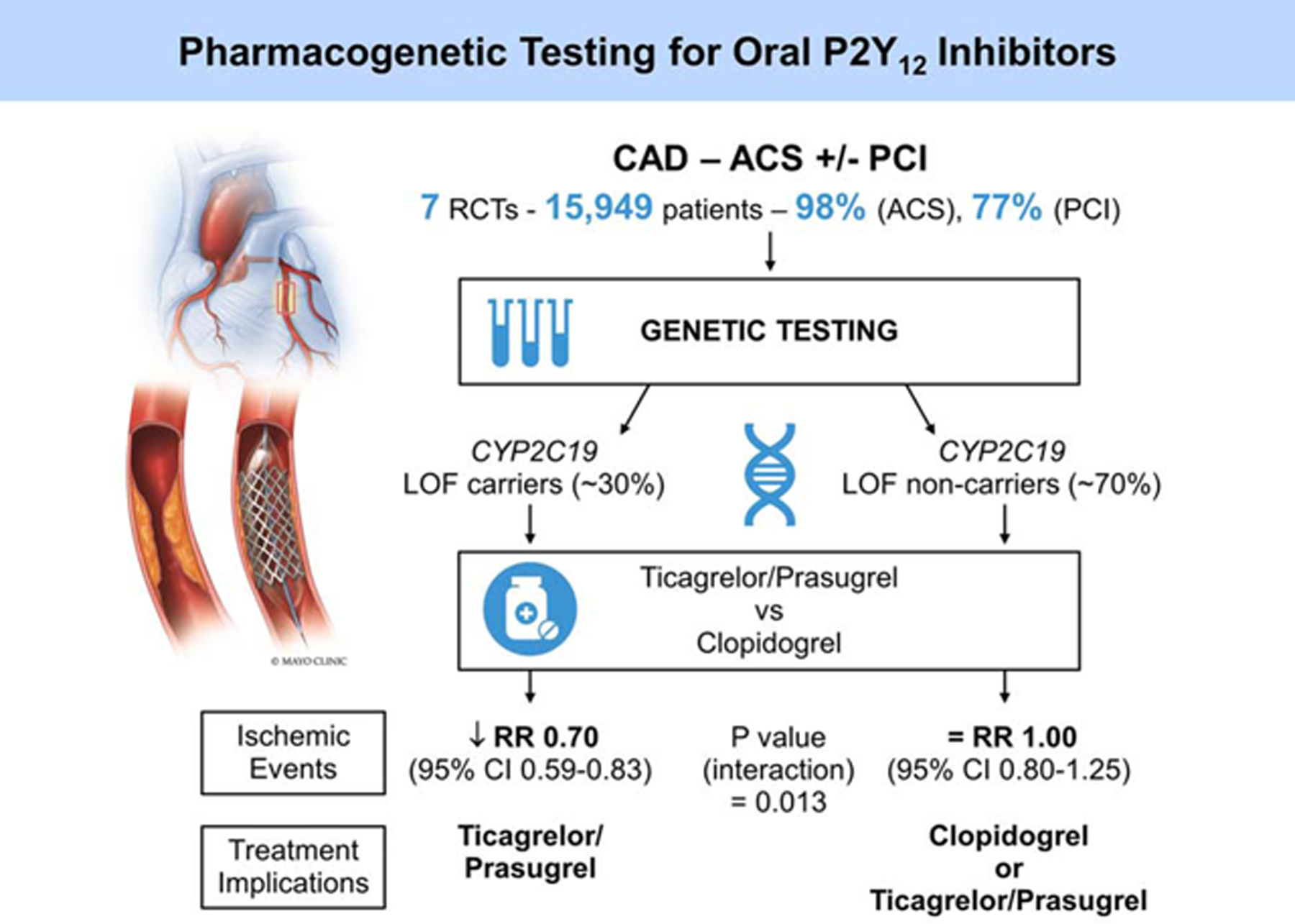
A Proposed Algorithm Utilizing CYP2C19 Pharmacogenetic Testing to Individualize Oral P2Y12 Inhibitor Therapy in Patients with Coronary Artery Disease (CAD) based on the Meta-Analysis Results. ACS, Acute Coronary Syndromes; PCI, Percutaneous Coronary Intervention; RCT, Randomized Controlled Trial; LOF, Loss-of-Function; RR, Relative Risk.
The use of ticagrelor as compared to clopidogrel without a genotyping strategy in the PLATO trial decreased ischemic events (HR 0.84, 95% CI 0.77 to 0.92) in 18,624 patients with acute coronary syndromes with an overall ischemic event rate that was 9.8% in the ticagrelor and 11.7% in the clopidogrel groups (5). The PLATO genetic substudy, that included 10,285 patients from the original trial, suggested a reduction in ischemic events in CYP2C19 LOF carriers receiving ticagrelor compared with clopidogrel (HR 0.77, 95% CI 0·60 to 0·99) but not in noncarriers(15) (HR 0.86, 95% CI 0·74 to 1·01). Due to a non-significant interaction test (P=0.46) the authors concluded that ticagrelor was more efficacious than clopidogrel irrespective of CYP2C19 genotype status. The authors acknowledged that this genetic “sub-study was not prospectively powered.” No similar study examining the effect of CYP2C19 with the use of prasugrel had been performed to date.
Despite a FDA black box warning in the drug labeling information for clopidogrel, AHA/ACC clinical expert consensus guidelines do not support the routine practice of CYP2C19 genotyping prior to prescribing clopidogrel (28). It was unknown whether identifying CYP2C19 LOF carriers and prescribing alternative P2Y12 inhibitors such as ticagrelor or prasugrel based on CYP2C19 genotype reduced ischemic outcomes. The randomized trials and observational studies that followed the FDA warning were underpowered to address the question of whether or not to genotype and, therefore, lacked statistical significance to definitively demonstrate a role for genotyping. In the recently reported TAILOR PCI trial, treatment with ticagrelor as compared to clopidogrel in CYP2C19 LOF carriers did not result in a significant reduction of ischemic events at 12 months based on the prespecified analysis plan and the 50% treatment effect that the study had been powered to detect (7). Despite the occurrence of 89 ischemic events observed in this trial, which exceeded the 76 events anticipated to provide adequate power, the observed relative risk reduction was 34% instead of the estimated 50%, hence a borderline P value of 0.056 was observed. This meta-analysis of 7 RCTs enrolling 6,409 patients overcomes this limitation and demonstrates an overall risk reduction of 30% with alternate P2Y12 inhibitors as compared to clopidogrel, consistent with the treatment effect observed in TAILOR-PCI.
Practical Implications
These results suggest that genetic testing for identifying CYP2C19 LOF carriers and non-carriers could be beneficial prior to prescribing antiplatelet therapy resulting in the selection of an alternate P2Y12 inhibitor for the former and clopidogrel for the latter patients (Central Illustration). If alternate P2Y12 inhibitor therapy had reduced ischemic outcomes in non-carriers, then a ticagrelor or prasugrel-for-all approach irrespective of CYP2C19 genotype might have been preferred to clopidogrel. To the contrary, our meta-analysis results demonstrate that there was no difference in the rates of ischemic events when non-carriers were treated either with clopidogrel or alternate P2Y12 inhibitors. These results support the findings of the POPular Genetics trial in which all patients in one randomized group received ticagrelor and were compared to patients receiving genotype-guided P2Y12 inhibitors i.e. non-carriers receiving clopidogrel and LOF carriers receiving ticagrelor. This study demonstrated that such a targeted genotyping strategy was non-inferior to a ticagrelor-for-all approach with ischemic event rates of 4.6% and 4.7%, respectively, at 12 months (6).These studies imply that a large proportion of patients could safely receive clopidogrel given that CYP2C19 LOF non-carriers comprise approximately 50–70% of the population (1). CYP2C19 genotype can be incorporated with clinical variables in the form of a composite scoring system that could be helpful in identifying high risk patients and selecting appropriate oral P2Y12 inhibitor therapy as demonstrated in the recently published ABCD-GENE score (29).
In the PLATO trial, there was no significant difference observed in the rates of major bleeding between ticagrelor and clopidogrel treated patients although there was a higher risk for non-CABG major bleeding in the ticagrelor group (HR 1.19, 95% CI 1.02 to 1.38) (5). In the PLATO genetic sub-study, CYP2C19 genotype was shown to have no effect on major bleeding with P2Y12 inhibitor therapy (15), a finding that was similar to that observed in the TAILOR PCI clinical trial (7) and our meta-analysis. It is important to note that the PLATO genetic substudy did not report TIMI minor bleeding episodes by CYP2C19 genotype which may have attenuated the bleeding outcomes reported in this meta-analysis. Patients treated with prasugrel have a higher risk of major bleeding as compared to clopidogrel as demonstrated in the TRITON–TIMI 38 study (4) (HR 1.32, 95% CI, 1.03–1.68). This increased risk for major bleeding was not observed in the CYP2C19 genetic sub-studies of TRITON–TIMI 38 or in other studies comparing prasugrel to clopidogrel included in our meta-analysis. Although the risk for major bleeding may not be affected by CYP2C19 genotype, multiple prior studies have demonstrated a lower incidence of minor bleeding with clopidogrel than prasugrel or ticagrelor. For example, although Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding was reported to be lower in the genotype-guided group (9.9%) as compared to ticagrelor for all (12.8%) in POPular Genetics this effect was primarily driven by a reduction in BARC type 2 bleeding (HR 0.69, 95% CI, 0.53 to 0.89) rather than BARC type 3 or 5 bleeding (HR 1.23, 95% CI 0.71–2.13) (6). A similar increased risk for bleeding was observed in the TAILOR PCI trial when bleeding was assessed by the BARC classification in the CYP2C19 LOF per-protocol genotype-guided group that primarily received ticagrelor as compared to those who received clopidogrel (7). One of the potential advantages of genotype-guided P2Y12 inhibitor therapy in which large number of patients receive clopidogrel (given an approximate 50%−70% prevalence of CYP2C19 non-carriers) and the remainder receive more potent alternate P2Y12 inhibitors, is a lower risk of bleeding for this group of patients as compared to all patients receiving either ticagrelor or prasugrel. This beneficial effect was observed in POPular Genetics. Similar to prior studies, our meta-analysis did not demonstrate an effect of CYP2C19 genotype on bleeding outcomes likely due to reporting of bleeding complications by TIMI major or minor bleeding classification which does not result in reporting of actionable minor bleeding episodes that do not meet the drop in hemoglobin or hematocrit criteria of the TIMI classification but would be captured by BARC2 bleeding criteria for example. Furthermore, the bleeding results of the meta-analysis need to be interpreted with caution since the certainty in the estimates of the bleeding outcome was low.
The CYP2C19 *17 allele is considered a gain-of-function allele and has been shown, in some studies, to lead to enhanced response to clopidogrel (via platelet function testing) and perhaps a higher rate of bleeding events (30,31). However other studies have not demonstrated increased platelet inhibition or altered clinical outcomes in clopidogrel treated patients with the CYP2C19 *17 allele (32–35). Therefore its role in attenuating response to clopidogrel is controversial and guidelines do not recommending altering P2Y12 inhibitor therapy based on CYP2C19 *17 genotype (36).
Study Strengths and Limitations
The strengths of this meta-analysis relate to the comprehensive literature search, selecting and appraising studies by independent pairs of reviewers, and evaluating the whole body of evidence of randomized and nonrandomized studies with stratified analyses based on study design.
There are several limitations. First, there was incomplete reporting across studies. Not all studies reported results in CYP2C19 LOF non-carriers and the ischemic endpoints varied but most consisted of CV death, myocardial infarction and stroke. Most studies categorized subjects based on CYP2C19 *2/*3 alleles, but some had broader criteria. Nevertheless, despite such variation, we did not observe substantial statistical heterogeneity (the I-squared measure did not exceed 50% for any analysis stratified by genetic status). Second, we were unable to evaluate publication bias, which would have affected only the nonrandomized studies, considering that they are not required to be registered in trial registries such as clinicaltrials.gov. Third, combining the use of ticagrelor and prasugrel in the alternate P2Y12 inhibitor therapy group included in this meta-analysis may have attenuated the results of the ischemic outcomes given findings of a recent study that demonstrated a lower incidence of death, myocardial infarction or stroke in patients who received prasugrel as compared to ticagrelor (37). However, the differences in ischemic outcomes would have been similar in both CYP2C19 LOF patients and CYP2C19 non-carriers without altering the overall results observed. Furthermore, CYP2C19 LOF patients who received either ticagrelor or prasugrel have similar degree of platelet inhibition (38). Finally, bleeding outcomes according to BARC definition was not reported in the RCTs included in this meta-analysis other than TAILOR PCI which may limit the interpretation of bleeding risk with the use of the various P2Y12 inhibitors based on CYP2C19 genotype.
In conclusion, the findings of the current meta-analysis confirm the beneficial trends observed in individual studies and support the use of genotyping to guide P2Y12 inhibitor therapy in patients with CAD especially with ACS and after PCI. The findings also support the concept of personalized medicine and justify the need for such studies in cardiovascular disease.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
Whether the benefit of alternate P2Y12 inhibitors such as ticagrelor or prasugrel as compared to clopidogrel occurs primarily in patients who are CYP2C19 loss-of-function carriers and not in non-carriers is unknown.
WHAT IS NEW?
This meta-analysis demonstrates that ticagrelor or prasugrel compared to clopidogrel significantly reduced ischemic events in CYP2C19 loss-of-function carriers but not in non-carrier patients with coronary artery disease after percutaneous coronary intervention (PCI).
WHAT IS NEXT?
Clopidogrel therefore can be safely used in the majority of patients and genetic testing prior to prescribing P2Y12 inhibitor therapy would be useful to guide selection of these agents for use after PCI.
ACKNOWLEDGEMENTS
Funding for this research was provided by NIH grants U01HL128606 and 3U01HL128606–03S1.
ABBREVIATIONS AND ACRONYMS
- ACS
acute coronary syndromes
- CAD
coronary artery disease
- CI
confidence interval
- CV
cardiovascular
- LOF
loss of function
- PCI
percutaneous coronary intervention
- RCTs
randomized controlled trials
- RR
relative risk
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
none
REFERENCES
- 1.Pereira NL, Weinshilboum RM. Cardiovascular pharmacogenomics and individualized drug therapy. Nat Rev Cardiol 2009;6:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira NL, Rihal CS, So DYF et al. Clopidogrel pharmacogenetics. Circ Cardiovasc Interv 2019;12:e007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes DR, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: Approaches to the FDA ‘Boxed Warning’. Circulation 2010;122:537–557. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott SD, Braunwald E, McCabe CH et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. New Engl J Med 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 5.Wallentin L, Becker RC, Budaj A et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 6.Claassens DMF, Vos GJA, Bergmeijer TO et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. New Engl J Med 2019;381:1621–1631. [DOI] [PubMed] [Google Scholar]
- 7.Pereira NL, Farkouh ME, So D et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: The TAILOR-PCI randomized clinical trial. JAMA 2020;324:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. http://www.prisma-statement.org/.
- 9.Higgins JPT, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkins D, Best D, Briss PA et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murad MH. Clinical practice guidelines: A primer on development and dissemination. Mayo Clin Proc 2017;92:423–433. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 13.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mega JL, Close SL, Wiviott SD et al. Cytochrome P-450 polymorphisms and response to clopidogrel. New Engl J Med 2009;360:354–362. [DOI] [PubMed] [Google Scholar]
- 15.Wallentin L, James S, Storey RF et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet (London, England) 2010;376:1320–1328. [DOI] [PubMed] [Google Scholar]
- 16.Deiman BALM Tonino PAL, Kouhestani K et al. Reduced number of cardiovascular events and increased cost-effectiveness by genotype-guided antiplatelet therapy in patients undergoing percutaneous coronary interventions in the Netherlands. Neth Heart J 2016;24:589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong P, Yang X, Bian S. Genetic polymorphism of CYP2C19 and inhibitory effects of ticagrelor and clopidogrel towards post-percutaneous coronary intervention (PCI) platelet aggregation in patients with acute coronary syndromes. Med Sci Monit 2016;22:4929–4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa H, Isshiki T, Kimura T et al. Effects of CYP2C19 allelic variants on inhibition of platelet aggregation and major adverse cardiovascular events in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. J Cardiol 2016;68:29–36. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhao Y, Pang M et al. High-dose clopidogrel versus ticagrelor for treatment of acute coronary syndromes after percutaneous coronary intervention in CYP2C19 intermediate or poor metabolizers: a prospective, randomized, open-label, single-centre trial. Acta Cardiologica 2016;71:309–316. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Zhang Y, Wang L et al. Effects of dual-dose clopidogrel, clopidogrel combined with tongxinluo capsule, and ticagrelor on patients with coronary heart disease and CYP2C19*2 gene mutation after percutaneous coronary interventions (PCI). Med Sci Monit 2017;23:3824–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallari LH, Lee CR, Beitelshees AL et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv 2018;11:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CR, Sriramoju VB, Cervantes A et al. Clinical outcomes and sustainability of using CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. Circ Genom Precis Med 2018;11:e002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mega JL, Close SL, Wiviott SD et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 2009;119:2553–60. [DOI] [PubMed] [Google Scholar]
- 24.Xiong R, Liu W, Chen L, Kang T, Ning S, Li J. A randomized controlled trial to assess the efficacy and safety of doubling dose clopidogrel versus ticagrelor for the treatment of acute coronary syndrome in patients with CYP2C19*2 homozygotes. Int J Clin Exp Med 2015;8:13310–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira NL, Sargent DJ, Farkouh ME, Rihal CS. Genotype-based clinical trials in cardiovascular disease. Nat Rev Cardiol 2015;12:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collet JP, Thiele H, Barbato E et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020. [DOI] [PubMed] [Google Scholar]
- 27.Sibbing D, Aradi D, Alexopoulos D et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y(12) receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1521–1537. [DOI] [PubMed] [Google Scholar]
- 28.Holmes DR, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA Clopidogrel Clinical Alert: Approaches to the FDA “Boxed Warning”: A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association. Circulation 2010;122:537–557. [DOI] [PubMed] [Google Scholar]
- 29.Angiolillo DJ, Capodanno D, Danchin N et al. Derivation, validation, and prognostic utility of a prediction rule for nonresponse to clopidogrel: The ABCD-GENE score. JACC Cardiovasc Interv 2020;13:606–617. [DOI] [PubMed] [Google Scholar]
- 30.Frére C, Cuisset T, Gaborit B, Alessi MC, Hulot JS. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. J Thromb Haemost 2009;7:1409–11. [DOI] [PubMed] [Google Scholar]
- 31.Sibbing D, Koch W, Gebhard D et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation 2010;121:512–8. [DOI] [PubMed] [Google Scholar]
- 32.Geisler T, Schaeffeler E, Dippon J et al. CYP2C19 and nongenetic factors predict poor responsiveness to clopidogrel loading dose after coronary stent implantation. Pharmacogenomics 2008;9:1251–9. [DOI] [PubMed] [Google Scholar]
- 33.Simon T, Verstuyft C, Mary-Krause M et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009;360:363–75. [DOI] [PubMed] [Google Scholar]
- 34.Sorich MJ, Polasek TM, Wiese MD. Systematic review and meta-analysis of the association between cytochrome P450 2C19 genotype and bleeding. Thromb Haemost 2012;108:199–200. [DOI] [PubMed] [Google Scholar]
- 35.Lewis JP, Stephens SH, Horenstein RB et al. The CYP2C19*17 variant is not independently associated with clopidogrel response. J Thromb Haemost 2013;11:1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott SA, Sangkuhl K, Stein CM et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clinical pharmacology and therapeutics 2013;94:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schüpke S, Neumann F-J, Menichelli M et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. New Engl J Med 2019;381:1524–1534. [DOI] [PubMed] [Google Scholar]
- 38.Franchi F, Rollini F, Rivas J et al. Prasugrel versus ticagrelor in patients with CYP2C19 loss-of-function genotypes. JACC: Basic to Translational Science 2020;5:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


