Summary
Microorganisms and eukaryotic human cells coexist in synergistic relationships in nearly every niche of the human body. The female genital tract consisting of the vagina, uterus with its cervix and endometrium, uterine tubes and ovaries – harbors its own typical microbiota, which accounts for 9 % of the total bacterial population in females. To this organ system, we also assigned the microbiome of the placenta, which has not been studied much until now. Among the spectrum of microbial species, the female genital tract is mainly dominated by Lactobacillus species, which are considered to be one of the simplest yet most important microbial communities. However, this relationship between macro- and micro-organisms seems to have a number of physiological functions, e.g., the vaginal and cervical microbiota have unique impact on reproductive health. The aim of this review was to provide current view on female genital tract microbiota and its role in reproductive health. We describe in detail the association of vaginal or tubal epithelium with microbiota or the role of microbiota in normal placental function.
Keywords: Microbiota, Female genital tract, Reproductive functions, Lactobacillus spp
Introduction
Microorganisms and eukaryotic human cells coexist in synergistically in nearly every niche of the human body. The microbiota in the human body play a critical role in maintaining human wellbeing and are associated with the pathogenesis of various diseases [1]. These communities of microorganisms can be found in the skin, respiratory tract, alimentary tract, and other tissue sites, each with their own functional capabilities [2].
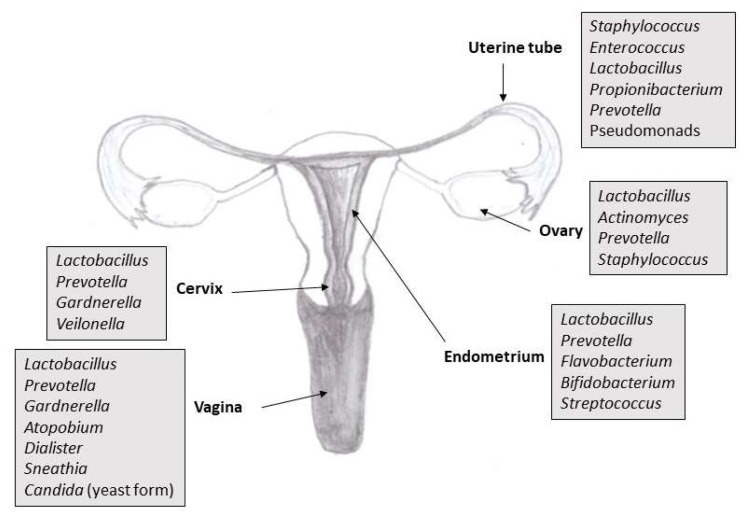
The goal of the International Human Microbiome Project, which began in 2007, was to analyze the genomic information of microorganisms in healthy adults. Samples from the nose, mouth, skin, intestines and vagina were taken from healthy adult volunteers. Microbes were identified based on the sequence of 16S ribosomal ribonucleic acid target regions, and gene content information was obtained by whole genome sequencing. Analyses have shown species variations as well as gene composition in individuals as well as within different sites on the human body. For example, the bacteria colonizing the intestinal tract are different from bacteria that colonize the oral cavity, skin, or other sites. The intestine is the locality with the most significant taxonomic and genetic diversity, and the vagina seems to be the least complex. Each microenvironment (e.g., intestine, skin surfaces, and vagina) has its own unique microbiome. Most people have so-called basic microbiome, which is 95 % identical for all individuals. The basic microbiome helps perform normal metabolic functions, stimulates innate immunity, prevents the colonization of unwanted pathogens [2,3]. In addition, an analysis of the microbiota within the female reproductive tract revealed a microbiota continuum along the whole tract, which is indicative of a non-sterile environment [4]. Female genital tract comprising the vagina, uterus (especial interest is focused on the cervix and endometrium), uterine tubes and ovaries. It harbors its own typical microbiota (Fig. 1), which accounts for 9 % of the total bacterial population in females [5,6].
Fig. 1.
An overview of the composition of a microbiota in the reproductive tract of healthy reproductive age woman
Among the spectra of microbial communities, the female genital tract microbiota, mainly dominated by Lactobacillus species, are considered to be one of the simplest yet most important microbial communities, and the cervicovaginal microbiota have vast impact on the reproductive health of females [7]. For example, lactobacilli help regulate the pH value of the vagina to inhibit the growth of other bacteria and to prevent undesirable microbial colonization and infection through their adhesion to the vaginal epithelial cells [8]. However, the composition, diversity, and dynamics of the microbiota within the uterine cavity of reproductive-aged women have not been fully unveiled, and as the uterine cavity seems to be an essential part of the female genital tract. More efforts are needed to further illustrate the interaction between microbial communities in the vagina and uterus. Current evidence have shown that female genital tract microbial communities are closely associated with gynecological disease. However, the impact of microbial communities in the uterine cavity on female fertility and the underlying mechanism still remain largely unclear [9,10].
Overview of methodical approaches in microbiota research
To investigate the composition of microbiota in the genital tract, samples are collected from individual locations (organs) throughout the genital tract of healthy reproductive-aged females. For vaginal microbiota analyses, most groups so far performed vaginal swabs and vaginal secretions sampled by swabs. Some groups take urine or vaginal aspirates with sterile catheter. Two types of samples are collected in order to analyze cervical microbiota, swabs of cervical mucus and endocervical swabs. Collection of these samples is noninvasive process, but in case of cervix samples the risk of contamination with vaginal species is higher [6]. The most common samples used in the detection of endometrial microbiota are endometrial swabs, endometrial biopsies and endometrial fluid sampled by catheter. These specimens are taken by invasively during laparoscopy or laparotomy from minimally invasive surgery, providing samples or through the cervical canal. The problem of transcervical samples might be their contamination by the vaginal microbiota. The specificity of sampling route was tested to determine the distribution of bacteria. The results of this research yielded high similarity of bacterial distribution in the transcervical samples to that in samples taken by opening the uterus during surgery [4]. To determine uterine tubes microbiota, samples are obtained by laparoscopic access, specifically by salpingectomy, by direct biopsies of the distal portion of the uterine tube during laparoscopic procedures. Currently, hysteroscopy is considered an ideal diagnostic procedure for assessing the vaginal walls, cervical canal, uterine cavity, endometrium, and tubal ostia [11].
Traditionally, to investigate bacterial or fungal diversity, culture-dependent techniques were used. The bacterial composition of the vaginal microbiota was detected by microscopy after Gram staining of vaginal smear. Some standardized criteria based on Nugent scoring and Amsel’s criteria were used to analyze the presence of Gram-positive Lactobacillus, compared to Gram-negative and Gram-variable bacteria such as Gardnerella, Atopobium and Mobiluncus. The cultivation process allowed these microbes to be identified [12, 13]. Culture-based techniques have various disadvantages. Principally, culture conditions are unsuitable for all microbial species, which leads to many species being undetected. If microbial species have been isolated, they are unable to recognize and culture-based procedures are not adjustable to high-throughput investigation. The fact is that 1 % of bacteria survive and form colonies on agar plates. Special platforms based on DNA/RNA and protein analysis have been established to recognize the real diversity of the microbiota [14]. Most of our current knowledge on the genital microbiota is based on qualitative and semiquantitative descriptive research using cultivation-dependent methods.
The introduction of culture-independent molecular-based methods provided new information about the composition of genital flora and abnormal colonization, which has supplemented existing knowledge from culture-dependent techniques [13]. The identification of microbiota is based on genomic DNA analysis using these techniques. The DNA is isolated from specimen by a variety of next-generation sequencing tools. These procedures can also be used to isolate individual microbial species from mixed cultures. Following DNA isolation, two methods may be used to examine microbiota. The first is whole metagenome sequencing that focuses entirely on the sequencing of microbial DNA present in the sample. The second is marker gene sequencing that targets sequencing of a particular locus in all genomes. In this way the 16S rRNA gene is the most commonly used marker gene specific to archaea and bacteria, the 18S rRNA gene and 28S rRNA gene are specific for eukaryotic organisms and the internal transcribed spacer is specific for fungi. The complete procedure includes microbial DNA extraction, PCR amplification of the selected gene and next-generation sequencing. By such methods that do not rely on microbial cultivation, it is possible to determine the composition of the microbiota in the female genital tract at the species level much accurate compared with the culture-based techniques [6,13,15,16].
Healthy vaginal microbiota
The vaginal microbiota is a dynamic microbial ecosystem that regularly undergoes fluctuations throughout a female’s life and throughout the menstrual cycle [17]. A mutual relationship exists between woman reproductive physiology and vaginal microbiota. Over the last decades, female reproductive health has been at the forefront of research as strong evidence supports bidirectional relationships between the vaginal and cervical microenvironments and cervical cancer, viral acquisition and persistence, gynecologic and obstetric diseases and other benign conditions like cervical ectopy [18]. Physiological hormonal changes that start from birth and continue till post-menopause, may affect the vaginal microbiota. On the other hand, vaginal microbiota can also affect reproductive physiology [19]. The vaginal microbiota differs among individuals due to variations in sexual activity, vaginal douching / intravaginal washing, regional disparity and other factors [4,20]. A previous study has investigated the microbial compositions in three sites of the vagina, including the introitus, midpoint, and posterior fornix, and concluded that there was little variation in species across the three sampling sites, with Lactobacillus species being dominant in all sites [2].
From the past, the vaginal microbiota of healthy females in their reproductive age is defined as Lactobacillus dominated microflora, producing sufficient quantity of lactic acid with pH values < 4.5 [21]. The composition of the vaginal microbiota has critical implications for the susceptibility to sexually transmitted infections, miscarriage, and spontaneous preterm delivery [22–28]. The mechanism is in part attributed to the action of Lactobacillus spp., many of which provide broad-spectrum protection via antimicrobial substances, mainly lactic acid, narrow-ranging bacteriocins and wide-ranging hydrogen peroxide. These products are suggested to play various important roles in host defense [29–31].
The composition and structure of the vaginal microbiota have been described adequately using conventional techniques (microscopy, cultivation) and non-conventional techniques, especially sequencing. Although often dominated by lactobacilli, the vaginal microbiota is also frequently composed of a collection of facultative and obligate anaerobes. Based on the abundance and composition of vaginal bacterial species in reproductive age females detected by molecular based techniques, five major microbial community state types (CSTs) are established. These CSTs are differentiated by dominant species and pH values. CST-I, CST-II, CST-III and CST-V are characterized by abundance of Lactobacillus crispatus, L. gasseri, L. inners and L. jensenii, respectively. CST-IV harbors higher ratios of strictly anaerobic bacterial genera Prevotella, Atopobium, Dialister, Gardnerella and Sneathia and low levels of genus Lactobacillus. Depending on the Lactobacillus abundance, average pH values range from 4.0 ± 0.3 (CST-I) to 5.3 ± 0.6 (CST-IV) [21, 32]. Two sub-states exist in CST-IV. The CST-IV-A is characterized by species of genera Anaerococcus, Peptoniphilus, Corynebacterium, Prevotella, Finegoldia and Streptococcus. The CST-IV-B contains Atopobium, Gardnerella, Sneathia, Mobiluncus, Megasphera and other taxa of order Clostridiales [31, 32]. Among these five states, the CST-I, II, III and V exist in 89.7 % white women, 80.2 % Asian women, 61.9 % Afroamerican women and 59.6 % Hispanic women. The CST-IV state predominates in healthy Afroamerican and Hispanic women (40 %), but also represents the most common dysbiosis state (bacterial vaginosis) [32]. The vagina of child has CST-IV of vaginal microbiota and neutral pH value. Some reproductive aged women show switching between CSTs over a short span, other remain consistent. These conversions are elicited by menstruation [33, 34]. Post-menopause leads to shift of vaginal microbiota from lactobacilli to microbial diversity (CST-IV) and rise in pH [35]. Additionally, a higher number of lactobacilli are found in pregnant when compared to non-pregnant females [36]. The above research results indicate that the vagina harbors a huge microecosystem containing billions of microbes. Data from 110 reproductive aged women revealed that the vagina contains 1010 – 1011 bacteria [4].
The relationship between intravaginal douching and vaginal health is interesting, whereas nearly half of American women reported washing the inside of the vagina during the past month (based on a sample of sexually active women living in Los Angeles) [37]. According to some studies, intravaginal washing is associated with decreased vaginal colonization with beneficial lactobacilli [38]. The use of commercial vaginal douching products containing vinegar, iodine or baking soda is likely not be beneficial for vaginal health. Hesham et al. [39] demonstrate in vitro that vaginal douching products increase vaginal epithelial cell death and secretion of pro-inflammatory cytokines, suggesting the potential for epithelial disruption. After exposure to vinegar-based douche, Lactobacillus crispatus and L. jensenii - two classic beneficial lactobacilli - induce greater production of the pro-inflammatory cytokine IL6. In vivo, analyses of vaginal fluid cytokine levels demonstrates higher levels of pro-inflammatory cytokines in women who use douching products [40]. Based on these findings, vaginal douching could potentially increase the risk of all genitourinary infections, including urinary tract infections, and support clinical recommendations to avoid douching [39].
In addition to bacteria, the vaginal microbiota of healthy reproductive aged women also contains fungi that form vaginal mycobiota. Using culture-dependent techniques researchers isolated vaginal fungi in approximately 20 % of the asymptomatic women. Predominant species Candida albicans (72–91 %) was followed by non-albicans Candida species (C. glabrata, C. tropicalis, C. parapsilosis) [41]. Studies based on sequencing techniques yielded Candida species in significantly higher frequency, which accounted for 64.5 % of the participants involved. The predominant part of this mycobiota was C. albicans (82 %), followed by C. dubliniensis, C. parapsilosis, C. krusei, Candida sp. V104616 [42]. Studies based on co-culturing of vaginal yeast and bacteria suggested that bacteria inhibit Candida yeast to hyphae switch, maintain low numbers of Candida in vagina and compete with yeast cells for adhesion sites on epithelial receptors owing to its higher affinity. Lactobacillus abundance and low Candida number along with their interactions play an important role in maintaining microbiota balance [43, 44].
Overlap of urogenital and vaginal microbiota
Recent studies applying culture-independent methods have allowed for the detection of a quantifiable and diverse urinary microbiota [45–49], and these findings have been validated with quantitative enhanced culture methods [50,51]. Several organisms commonly found in the vagina have been observed in urine samples [47,52]. Bacterial strains isolated from the urinary bladder and vagina have been found to be functionally and phylogenetically similar [53]. In one study, voided urine samples demonstrated more similarity to paired vaginal swabs than to paired supra-pubic needle aspirates or trans-urethral catheterized samples [54]. This suggests that the microbiota of some types of urine samples may more closely resemble vaginal microbiota than other urine sample types; however, there is also similarity at the genus level between paired vaginal and trans-urethral catheterized samples [55]. Given the overlap between the genitourinary and vaginal microbiota, it was hypothesized that voided urine may be used as a proxy for vaginal community assessment in research studies utilizing 16S rRNA gene amplicon sequencing. To evaluate the use of urine as a proxy for vaginal swabs, Brown et al. [56] compared the microbiota of paired mid-vaginal swabs with the microbiota of urine samples collected using “clean-catch” or “random-catch” methods from reproductive-aged females, and paired mid-vaginal swabs and random-catch urine samples from peri/post-menopausal women. The first void of the initial urine stream is collected for random-catch urine samples, while for urine collected via the clean-catch method, the labia are cleaned with an antibacterial wipe and mid-stream urine is collected. While the microbiota of both random-catch and clean-catch urine samples might be similar in composition to vaginal microbiota because of shared species, RC urine may contain a higher proportion of vulvovaginal bacteria due to contamination from the urine stream washing over the labia, resulting in a better proxy of the vaginal microbiota than clean-catch urine. To our knowledge, clean-catch and random-catch urine samples have not been assessed in conjunction with the vaginal microbiota and, although the concordance between the urinary and vaginal microbiota of peri/post-menopausal women has been studied [55], they have not been evaluated separately from reproductive-age women. Peri/post-menopausal women have different vaginal [57, 58] and urinary [59] microbiota compared to reproductive-age women, and may carry lower bacterial loads [60]. These differences may affect the extent to which the genitourinary and vaginal microbiota overlap.
Interaction between vaginal epithelium and vaginal microbiota
More than 50 different species of bacteria may live in a woman's vagina, with lactobacilli being the predominant microorganism found in healthy adult females [61]. Some studies, however, describe more than 200 bacterial species of normal and the abnormal vaginal microbiota influenced by genes, ethnic background and environmental and behavioral factors [62]. Lactobacilli are relevant as a barrier to infection and are important in the impairment of colonization by pathogens, owing to competitive adherence to adhesion sites in the vaginal epithelium and their capacity to produce antimicrobial compounds. Due this, women without vaginal Lactobacillus strains may be susceptible to non-indigenous and potentially harmful microorganisms [61]. The human vaginal microbiota is a critical determinant of vaginal health. These communities live in close association with the vaginal epithelium and rely on host tissues for resources [63].
The human vaginal surface lined by stratified squamous epithelium has a large surface area (mean 87.46 cm2, measured by vinyl polysiloxane casting) and is the first mucosal surface contacted by sexually transmitted pathogens [64]. The stratified vaginal epithelium undergoes differentiation and contains several distinct layers: the deepest and mitotically active basal layer, the parabasal layer, the intermediate layer where the glycogen amount increase, and the superficial layer, where cells continuously die. During the regulated cell death of the vaginal epithelial cells, glycogen is released on the surface of the epithelium. This glycogen is metabolized and their product – lactic acid - forms acidic microenvironment of vagina [65]. However, the interaction between lactobacilli and other microorganisms and the vaginal epithelium and the entire vaginal microenvironment is much more complex than it might seem at first glance. The superficial layers of the vaginal epithelium provide a unique microenvironment that maintains vaginal health by fostering endogenous lactobacilli and retaining critical mediators of acquired and innate immunity, as also leukocytes may also penetrate and traverse the superficial layer following placement in the vaginal lumen [66]. It seems that the vaginal epithelial cells actively regulate membrane adhesiveness to co-ordinate bacterial adhesion. From biochemical point of view, bacterial adhesion forces were dramatically decreased by depleting the epithelial cell membrane of cholesterol or sub-membrane cortical actin [67].
Key nutrients for lactobacilli include sugars produced when glycogen is degraded. But most genital isolates of lactobacilli are not able to use glycogen as an energy source in vitro. An important role during glycogen degradation plays α-amylase enzyme, which has been demonstrated in vaginal fluid. Until now, it is unclear whether α-amylases are produced solely by the vaginal epithelium, bacteria in the vagina, or both [68, 69].
The understanding of the interaction between normal vaginal microflora and vaginal epithelium is crucial for new methods of bacterial vaginosis treatment. Vaginosis is defined as a condition experienced by most women at least once in their lifetime. This condition arises due to the imbalance in the microbiome of the vaginal ecosystem. Most of the pathogens of this disease are organisms which are commonly found in a normal healthy vagina. Recent studies have provided insights into the relationship between the vaginal microbiome environment and bacterial vaginosis symptoms. In the Lactobacillus-dominated vaginal microbiome, various antimicrobial substances are produced, including lactic acid, bacteriocins, and hydrogen peroxide, which play essential roles in protecting against potential pathogens [70]. The standard of care treatment for bacterial vaginosis is antibiotics. Recently, vaginal microbiome transplant and vaginosis treatment with probiotics, efforts to restore the normalcy in the vaginal environment, are becoming new and popular treatments [70, 71].
Cervical microbiota
The microbiota correlation among vaginal, cervical and endometrial samples within an individual is very strong. These findings have been shown by Wee et al. [72] who has analyzed cervical mucus samples of healthy women and determined dominance of genus Lactobacillus (L. crispatus, L. inners), accounted for 97.56 %. In this study quantitative PCR method was used. In another study cervical mucus samples were analyzed by sequencing and shotgun sequencing and dominance of Lactobacillus was confirmed. Several studies exhibited higher diversity of bacterial genera in endocervical samples. In one study prevalence of Lactobacillus and Prevotella was reported [73]. Another study published Lactobacillus was dominant genus, followed by Gardnerella, Veilonella, Prevotella, Sneathia, Fusobacterium [74]. Winters et al. [75] reported dominance of Acinetobacter (49 %), followed by Pseudomonas, Cloacibacterium and Lactobacillus. Chen et al. [4] analyzed endocervical samples of healthy women and women with different conditions by 16S rRNA sequencing. Lactobacillus was the most recovered genus (almost 75 %), followed by Gardnerella, Streptococcus, Atopobium, Prevotella and Pseudomonas. Some studies underline the link among high diversity of species within cervical microbiota and different gynecological issues.
Persistent high-risk human papillomavirus infection (HPV) is undoubtedly the main carcinogen leading to cervical intraepithelial neoplasia and cervical cancer. However, studies have found that not all patients with cervical HPV infection experience development into cancer. According to recent studies of Wang et al. [76] and Lin et al. [77], the relationship between cervical microbial diversity or dysbiosis may be related to the severity of high-risk HPV infection and cervical intraepithelial neoplasia as a precancerous condition.
Endometrial microbiota
Until recently, the endometrium was considered a sterile environment. Several studies have now reported that the endometrium harbors a functional microbiome in physiological conditions. Detection of bacteria through culture-independent techniques of endometrial samples have confirmed a unique microbiota harboring 100 to 1000 times less bacterial amount than that of the vagina. This low biomass microbiota is characterized by high diversity. Like the normal vaginal microbiota, the endometrium of healthy asymptomatic women is often colonized by lactobacilli that represent dominant group of bacteria [78–80].
The configuration of healthy bacterial microbiota is not clearly defined as some studies suggest Lactobacillus to be dominant and representative genus of healthy endometrium and some other studies point to other bacterial genera [6]. Franasiak et al. [81] examined endometrial samples and detected Lactobacillus and Flavobacterium the most abundant genera. Analysis of endometrial fluid samples of fertile women revealed the highest incidence of Lactobacillus (71.1 %), followed by Gardnerella, Bifidobacterium, Streptococcus and Prevotella [60]. Garcia-Velasco et al. [82] classified 2 types of endometrial microbiota, Lactobacillus-dominated with >90 % of Lactobacillus spp. and non-Lactobacillus-dominated with <90 % of Lactobacillus spp. plus >10 % of other bacteria. However, non-Lactobacillus-dominated microbiota have been identified in the genital tract of healthy and asymptomatic women, suggesting that in the absence of pathological signs, this microbiota could be considered normal [80].
The similarity between bacterial genera in vaginal and endometrial samples of healthy women supports the hypothesis that uterine cavity is colonized mainly by vaginal bacteria coming from the vagina by ascending route. Specific physiochemical or biological conditions in the uterus of some women may lead to colonization by the bacterial community that differs significantly from the vaginal community. In such cases, collection and analysis of endometrial samples is important for diagnostics of the microbial state of the uterus [80].
Uterine tubes and ovarian microbiota
Both uterine tubes and ovaries display highly variable microbial communities among women. In contrast with the acidity of vaginal pH value, these organs harbor a variety of bacteria growing in mildly alkaline conditions. Lactobacillus sp. is present in lower ratio than in vagina or cervix [4].
Pelzer et al. [83] have demonstrated using both culture-dependent and culture-independent techniques, that in the absence of infection, the human uterine tube is not a sterile site. Uterine tubes in asymptomatic women contain diverse microbial communities, which are affected by hormones and antibiotics, and display biogeographical tropism. Microbiota is represented by members of the phylum Firmicutes, most notably Staphylococcus sp., Enterococcus sp., and Lactobacillus sp. Other highly abundant and prevalent taxa include pseudomonads (Pseudomonas sp. and Burkholderia sp.) and known genital tract anaerobes Propionibacterium sp. and Prevotella sp. The results of microbial profiling are consistent with cultivation for most cohorts, with Staphylococcus sp. dominating both the culture-dependent and culture-independent results. Community profiles differ significantly between the left and the right uterine tubes. Lactobacillus sp., Enterococcus sp. and Prevotella sp. are more abundant within the left tube versus the right tube, whilst Staphylococcus sp. is more abundant within the right tube. The microbial community within the ampulla demonstrates a significantly greater abundance of Enterococcus sp. and P. acnes when compared to the isthmus [83]. For the sake of the complexity of knowledge, we will add that in contrast with the vaginal epithelium, the simple columnar epithelium of the uterine tubes contains numerous specific cells of the immune system. As our previous research shows, these immunologically active cells are mostly regulatory T- lymphocytes [84]. The occurrence of these intraepithelial T- lymphocytes together with secretion of tubal fluid containing cytokines and chemokines by tubal epithelial cells [85] cause pathogen inhibition inside uterine tube. Probably because of both mentioned factors, the presence of lymphatic follicles in the wall of uterine tubes, as the sign of chronic inflammation, is rare and is present during histological examination only in 2.1 % of surgically removed uterine tubes [86].
Miles et al. [73] detected Bacteroides, Corynebacterium, Lactobacillus, Coproccocus and Hymenobacter in uterine tubes, and Lactobacillus, Corynebacterium, Escherichia, Blaudia in ovarian samples surgically removed during total hysterectomy and bilateral salpingo-oopherectomy. Further analyses of ovarian follicular fluid by Pelzer et al. [87] revealed L. iners, Actinomyces spp., Corynebacterium auromuosum, Fusobacterium sp., Prevotella, or Staphylococcus sp. being colonisation more prevalent in the left than in the right ovary. Based on this finding we can suppose that the follicular fluid is not sterile. Microorganisms colonizing follicular fluid and the ensuing cytokine response could be a further as yet unrecognized cause and/or predictor of adverse assisted reproduction techniques outcomes and infertility.
Zhou et al. [88] analyzed the diversity and composition of the microbiota from 25 ovarian cancer tissues and 25 normal distal uterine tube tissues by 16S rRNA sequencing. Results of sequencing showed that the diversity and richness indexes were significantly decreased in ovarian cancer tissues compared to tissues from normal distal uterine tubes. The ratio of Proteobacteria and Firmicutes was notably increased in ovarian cancer, which revealed that microbial composition change might be associated with the process of ovarian cancer development. The authors assume that the microbial composition change may be involved in the initiation and progression of ovarian cancer via influencing and regulating the local immune microenvironment of uterine tubes except for regular pathways [88].
Placental microbiota
The placenta exhibits its own unique microbiome, with a low abundance but a metabolically rich microbiome. The combination of 16S rDNA and whole-genome shotgun metagenomic techniques indicate that the placental microbiota largely consists of nonpathogenic commensal microbiota from the phyla of Firmicutes, Tenericutes, Proteobacteria, Bacteroidetes, and Fusobacteria. By such composition, the placental microbiota mostly resembles the microbiota of the oral cavity and the deep endometrium of non-pregnant women rather that of the adjacent vaginal microbiota [2, 89]. Since fetal viability, growth and development are completely dependent on optimal placental function, the recent finding of a placental microbiome in healthy pregnancy may implicate a role for the bacteria in normal fetal growth and development [90].
Conclusion
Results of culture-dependent and culture-independent studies have revealed composition, diversity and functions of healthy female genital tract microbiota and its relation to the physiology and pathophysiology of reproductive health. In a balanced microecosystem, homeostasis and mutualism characterize the relationship between the microbiota and the human host. This balance plays important role in reproductive health, whereas its disruption by variations of internal and/or external factors leads to dysbiosis and higher risk for diseases. The understanding of female genital tract microbiota provides new options of clinical diagnostics for infertility, new approaches for assisted reproduction techniques and new treatments for gynecological disorders, such as probiotics and microbiome transplantation.
Acknowledgements
This work was supported by The Slovak Research and Development Agency Grant No. APVV-18-0499, by the Ministry of Health of the Slovak Republic under the grant number 2019/34-UPJŠ-6 and by Ministry of Education of Slovak Republic under the grant number KEGA 002UK-4/2022.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Young VB. The Role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 2017;356:j831. doi: 10.1136/bmj.j831. [DOI] [PubMed] [Google Scholar]
- 2.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liptáková A, Predný J, Buc M, Slobodníková L, Jalili N, Krčméry V, Koreň J, et al. Medical Microbiology. 1st Edition. Bratislava, Herba: 2019. p. 952. (in Slovak) [Google Scholar]
- 4.Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017;8:875. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno I, Simon C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod Med Biol. 2018;18(1):40–50. doi: 10.1002/rmb2.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punzón-Jiménez P, Labarta E. The impact of the female genital tract microbiome in women health and reproduction: a review. J Assist Reprod Genet. 2021;38(10):2519–2541. doi: 10.1007/s10815-021-02247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witkin SS, Linhares IM. Why do lactobacilli dominate the human vaginal microbiota? BJOG. 2017;124(4):606–611. doi: 10.1111/1471-0528.14390. [DOI] [PubMed] [Google Scholar]
- 8.Boris S, Barbés C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000;2(5):543–546. doi: 10.1016/S1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 9.White BA, Creedon DJ, Nelson KE, Wilson BA. The vaginal microbiome in health and disease. Trends Endocrinol Metab. 2011;22(10):389–393. doi: 10.1016/j.tem.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenbaum S, Greenbaum G, Moran-Gilad J, Weintraub AY. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am J Obstet Gynecol. 2019;220(4):324–335. doi: 10.1016/j.ajog.2018.11.1089. [DOI] [PubMed] [Google Scholar]
- 11.Vitale SG, Carugno J, D'Alterio MN, Mikuš M, Patrizio P, Angioni S. A New Methodology to Assess Fallopian Tubes Microbiota and Its Impact on Female Fertility. Diagnostics. 2022;12(6):1375. doi: 10.3390/diagnostics12061375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amegashie CP, Gilbert NM, Peipert JF, Allsworth JE, Lewis WG, Lewis AL. Relationship between nugent score and vaginal epithelial exfoliation. PLOS ONE. 2017;12(5):e0177797. doi: 10.1371/journal.pone.0177797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma M, Chopra C, Mehta M, Sharma V, Mallubhotla S, Sistla S, Sistla JC, et al. An Insight into Vaginal Microbiome Techniques. Life. 2021;11(11):1229. doi: 10.3390/life11111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirota I, Zarek SM, Segars JH. Potential influence of the microbiome on infertility and assisted reproductive technology. Semin Reprod Med. 2014;32(1):35–42. doi: 10.1055/s-0033-1361821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui L, Morris A, Ghedin E. The human mycobiome in health and disease. Genome Med. 2013;5:63. doi: 10.1186/gm467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JS, Spakowicz DJ, Hong BY, Petersen LM, Demkowicz P, Chen L, Leopold SR, et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat Commun. 2019;10(1):5029. doi: 10.1038/s41467-019-13036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pekmezovic M, Mogavero S, Naglik JR, Hube B. Host-Pathogen Interactions during Female Genital Tract Infections. Trends Microbiol. 2019;27(12):982–996. doi: 10.1016/j.tim.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Łaniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol. 2020;17(4):232–250. doi: 10.1038/s41585-020-0286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farage MA, Miller KW, Sobel JD. Dynamics of the vaginal ecosystem-hormonal influences. Infectious Diseases: Research and Treatment. 2010;3:IDRT-S3903. doi: 10.4137/IDRT.S3903. [DOI] [Google Scholar]
- 20.Chen X, Lu Y, Chen T, Li R. The female vaginal microbiome in health and bacterial vaginosis. Front Cell Infect Microbiol. 2021;11:631972. doi: 10.3389/fcimb.2021.631972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. 2015;6:81. doi: 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown SE, Robinson CK, Shardell MD, Holm JB, Ravel J, Ghanem KG, Brotman RM. Assessing the concordance between urogenital and vaginal microbiota: can urine specimens be used as a proxy for vaginal samples? Front Cell Infection Microbiol. 2021;11:671413. doi: 10.3389/fcimb.2021.671413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin DH. The microbiota of the vagina and its influence on women's health and disease. Am J Med Sci. 2012;343(1):2–9. doi: 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, Cone RA, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol. 2015;6:164. doi: 10.3389/fphys.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, Padavattan N, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity. 2017;46(1):29–37. doi: 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noel-Romas L, Grobler A, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356(6341):938–945. doi: 10.1126/science.aai9383. [DOI] [PubMed] [Google Scholar]
- 27.Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, Ravel J. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun. 2019;10(1):1305. doi: 10.1038/s41467-019-09285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Memar M, Bobdiwala S, Fourie H, Mannino R, Lee YS, Smith A, Marchesi JR, et al. The association between vaginal bacterial composition and miscarriage: a nested case-control study. BJOG. 2020;127(2):264–274. doi: 10.1111/1471-0528.15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 200;16(9):1809–1813. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 30.Aldunate M, Tyssen D, Johnson A, Zakir T, Sonza S, Moench T, Cone R, et al. Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother. 2013;68(9):2015–2025. doi: 10.1093/jac/dkt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards VL, Smith SB, McComb EJ, Tamarelle J, Ma B, Humphrys MS, Cajer P, et al. The cervicovaginal microbiota-host interaction modulates chlamydia trachomatis infection. mBio. 2019;10(4):e01548–19. doi: 10.1128/mBio.01548-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravel J, Gajer P, Abdo Z, Frney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, Koenig SSK, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4(132):132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, Ross FJ, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver M, Viscidi R, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21(5):450–458. doi: 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacha-Herrera D, Vasco G, Cruz-Betancourt C, Galarza JM, Barragán V, Machado A. Vaginal microbiota evaluation and lactobacilli quantification by qPCR in pregnant and non-pregnant women: a pilot study. Front Cell Infect Microbiol. 2020;10:303. doi: 10.3389/fcimb.2020.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JM, Poirot E, Hess KL, Brown S, Vertucci M, Hezareh M. Motivations for intravaginal product use among a cohort of Women in Los Angeles. PLoS One. 2016;11(3):e0151378. doi: 10.1371/journal.pone.0151378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lokken EM, Manguro GO, Abdallah A, Ngacha C, Shafi J, Kiarie J, Jaoko W, et al. Association between vaginal washing and detection of Lactobacillus by culture and quantitative PCR in HIV-seronegative Kenyan women: a cross-sectional analysis. Sex Transm Infect. 2019;95(6):455–461. doi: 10.1136/sextrans-2018-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hesham H, Mitchell AJ, Bergerat A, Hung K, Mitchell CM. Impact of vaginal douching products on vaginal Lactobacillus, Escherichia coli and epithelial immune responses. Sci Rep. 2021;11(1):23069. doi: 10.1038/s41598-021-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alcaide ML, Rodriguez VJ, Brown MR, Pallikkuth S, Arheat K, Martinez O, Roach M, et al. High levels of inflammatory cytokines in the reproductive tract of women with BV and engaging in intravaginal douching: A cross-sectional study of participants in the women interagency HIV study. AIDS Res Hum Retrovir. 2017;33(4):309–317. doi: 10.1089/aid.2016.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barousse MM, Van Der Pol BJ, Fortenberry D, Orr D, Fidel PL., Jr Vaginal yeast colonisation, prevalence of vaginitis, and associated local immunity in adolescents. Sex Transm Infect. 2004;80(1):48–53. doi: 10.1136/sti.2002.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drell T, Lillsaar T, Tummeleht L, Simm J, Aaspollu A, Saarma I, Salumets A, et al. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS One. 2013;8(1):e54379. doi: 10.1371/journal.pone.0054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72(9):4996–5003. doi: 10.1128/IAI.72.9.4996-5003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalia N, Singh J, Kaur M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann Clin Microbiol Antimicrob. 2020;19(1):5. doi: 10.1186/s12941-020-0347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson DE, Van Der Pol B, Dong Q, Revanna KV, Fan B, Easwaran S, Sodergren E, et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PLoS One. 2010;5(11):e14116. doi: 10.1371/journal.pone.0014116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong Q, Nelson DE, Toh E, Diao L, Gao X, Fortenberry JD, Van Der Pol B. The microbial communities in male first catch urine are highly similar to those in paired urethral swab specimens. PLoS One. 2011;6(5):e19709. doi: 10.1371/journal.pone.0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, Kliethermes S, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283–14. doi: 10.1128/mBio.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller ER, Wolfe AJ, Brubaker L. Female urinary microbiota. Curr Opin Urol. 2017;27(3):282–286. doi: 10.1097/MOU.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adebayo AS, Ackermann G, Bowyer RCE, Wells PM, Humphreys G, Knight R, Spector TD, et al. The urinary tract microbiome in older women exhibits host genetic and environmental influences. Cell Host Microbe. 2020;28(2):298–305.e3. doi: 10.1016/j.chom.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 50.Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coorevits L, Heytens S, Boelens J, Claeys G. The resident microflora of voided midstream urine of healthy controls: standard versus expanded urine culture protocols. Eur J Clin Microbiol Infect Dis. 2017;36(4):635–639. doi: 10.1007/s10096-016-2839-x. [DOI] [PubMed] [Google Scholar]
- 52.Thomas-White KJ, Kliethermes S, Rickey L, Wolfe AJ, Brubaker L. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am J Obstet Gynecol. 2017;216(1):55.e1–55.e16. doi: 10.1016/j.ajog.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas-White K, Forster SC, Kumar N, Van Kuiken M, Putonti C, Stares MD, Hilt EE, et al. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat Commun. 2018;9(1):1557. doi: 10.1038/s41467-018-03968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, FitzGerald MP, Mueller ER, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50(4):1376–83. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Komesu YM, Dinwiddie DL, Richter HE, Carper B, Gantz MG. Defining the relationship between vaginal and urinary microbiomes. Am J Obstet Gynecol. 2020;222(2):154.e1–154.e10. doi: 10.1016/j.ajog.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown SE, Robinson CK, Shardell MD, Holm JB, Ravel J, Ghanem KG, Brotman RM. Assessing the concordance between urogenital and vaginal microbiota: can urine specimens be used as a proxy for vaginal samples? Front Cell Infect Microbiol. 2021;11:671413. doi: 10.3389/fcimb.2021.671413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gliniewicz K, Schneider GM, Ridenhour BJ, Williams CJ, Song Y, Farage MA, Miller K, et al. Comparison of the vaginal microbiomes of premenopausal and postmenopausal women. Front Microbiol. 2019;10:193. doi: 10.3389/fmicb.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shardell M, Gravitt PE, Burke AE, Ravel J, Brotman RM. Association of vaginal microbiota with signs and symptoms of the genitourinary syndrome of menopause across reproductive stages. J Gerontol A Biol Sci Med Sci. 2021;76(9):1542–1550. doi: 10.1093/gerona/glab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ammitzb⊘ll N, Bau BPJ, Bundgaard-Nielsen C, Villadsen AB, Jensen AM, Leutscher PDC, Glavind K, et al. Pre- and postmenopausal women have different core urinary microbiota. Sci Rep. 2021;11(1):2212. doi: 10.1038/s41598-021-81790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holm JB, Humphrys MS, Robinson CK, Settles ML, Ott S, Fu L, Yang H, et al. Ultrahigh-throughput multiplexing and sequencing of >500-base-pair amplicon regions on the Illumina HiSeq 2500 platform. mSystems. 2019;4(1):e00029–19. doi: 10.1128/mSystems.00029-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amin M, Moradi Choghakabodi P, Alhassan Hamidi M, Najafian M, Farajzadeh Sheikh A. In vitro antimicrobial activities of metabolites from vaginal Lactobacillus strains against Clostridium perfringens isolated from a woman's vagina. J Chin Med Assoc. 2017;80(1):29–33. doi: 10.1016/j.jcma.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 62.Mendling W. Vaginal microbiota. Adv Exp Med Biol. 2016;902:83–93. doi: 10.1007/978-3-319-31248-4_6. [DOI] [PubMed] [Google Scholar]
- 63.France M, Alizadeh M, Brown S, Ma B, Ravel J. Towards a deeper understanding of the vaginal microbiota. Nat Microbiol. 2022;7:367–378. doi: 10.1038/s41564-022-01083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pendergrass PB, Belovicz MW, Reeves CA. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecol Obstet Invest. 2003;55(2):110–113. doi: 10.1159/000070184. [DOI] [PubMed] [Google Scholar]
- 65.Balko J, Tonar Z, Varga I. Memorix Histology. 1st Edition. Prague, Czech Republic: Triton; 2018. p. 556. [Google Scholar]
- 66.Anderson DJ, Marathe J, Pudney J. The structure of the human vaginal stratum corneum and its role in immune defense. Am J Reprod Immunol. 2014;71(6):618–623. doi: 10.1111/aji.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Younes JA, Klappe K, Kok JW, Busscher HJ, Reid G, van der Mei HC. Vaginal epithelial cells regulate membrane adhesiveness to co-ordinate bacterial adhesion. Cell Microbiol. 2016;18(4):605–614. doi: 10.1111/cmi.12537. [DOI] [PubMed] [Google Scholar]
- 68.Spear GT, McKenna M, Landay AL, Makinde H, Hamaker B, French AL, Lee BH. Effect of pH on cleavage of glycogen by vaginal enzymes. PLoS One. 2015;10(7):e0132646. doi: 10.1371/journal.pone.0132646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nunn KL, Clair GC, Adkins JN, Engbrecht K, Fillmore T, Forney LJ. Amylases in the human vagina. mSphere. 2020;5(6):e00943–20. doi: 10.1128/mSphere.00943-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu S, Hugerth LW, Schuppe-Koistinen I, Du J. The right bug in the right place: opportunities for bacterial vaginosis treatment. NPJ Biofilms Microbiomes. 2022;8(1):34. doi: 10.1038/s41522-022-00295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohankumar B, Shandil RK, Narayanan S, Krishnan UM. Vaginosis: Advances in new therapeutic development and microbiome restoration. Microb Pathog. 2022;168:105606. doi: 10.1016/j.micpath.2022.105606. [DOI] [PubMed] [Google Scholar]
- 72.Wee BA, Thomas M, Sweeney EL, Frentiu FD, Samios M, Ravel J, Gajer G, et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust N Z J Obstet Gynaecol. 2018;58(3):341–348. doi: 10.1111/ajo.12754. [DOI] [PubMed] [Google Scholar]
- 73.Miles SM, Hardy BL, Merrell DS. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil Steril. 2017;107(3):813–820.e1. doi: 10.1016/j.fertnstert.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 74.Pelzer ES, Willner D, Buttini M, Huygens F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie van Leeuwenhoek. 2018;111:933–943. doi: 10.1007/s10482-017-0992-6. [DOI] [PubMed] [Google Scholar]
- 75.Winters AD, Romero R, Gervasi MT, Gomez-Lopez N, Tran MR, Garcia-Flores V, Pacora P, et al. Does the endometrial cavity have a molecular microbial signature? Sci Rep. 2019;9(1):9905. doi: 10.1038/s41598-019-46173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Jiang Y, Liang Y, Wei L, Zhang W, Li L. Observation of the cervical microbiome in the progression of cervical intraepithelial neoplasia. BMC Cancer. 2022;22(1):362. doi: 10.1186/s12885-017-3359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin S, Zhang B, Lin Y, Lin Y, Zuo X. Dysbiosis of Cervical and Vaginal Microbiota Associated With Cervical Intraepithelial Neoplasia. Front Cell Infect Microbiol. 2022;12:767693. doi: 10.3389/fcimb.2022.767693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, Fredricks DN, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol. 2015;212(5):611.e1–9. doi: 10.1016/j.ajog.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moreno I, Codoñer FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazán J, Alonso R, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215(6):684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 80.Moreno I, Franasiak JM. Endometrial microbiota-new player in town. Fertil Steril. 2017;108(1):32–39. doi: 10.1016/j.fertnstert.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 81.Franasiak JM, Werner MD, Juneau CR, Tao X, Landis J, Zhan Y, Treff NR, et al. Endometrial microbiome at the time of embryo transfer: next-generation sequencing of the 16S ribosomal subunit. J Assist Reprod Genet. 2016;33(1):129–136. doi: 10.1007/s10815-015-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.García-Velasco JA, Budding D, Campe H, Malfertheiner SF, Hamamah S, Santjohanser C, Schuppe-Koistinen I, et al. The reproductive microbiome - clinical practice recommendations for fertility specialists. Reprod Biomed Online. 2020;41(3):443–453. doi: 10.1016/j.rbmo.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 83.Pelzer ES, Willner D, Buttini M, Hafner LM, Theodoropoulos C, Huygens F. The Fallopian tube microbiome: implications for reproductive health. Oncotarget. 2018;9(30):21541–21551. doi: 10.18632/oncotarget.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varga I, Miko M, Kachlík D, Žišková M, Danihel L’, Jr, Babál P. How many cell types form the epithelial lining of the human uterine tubes? Revision of the histological nomenclature of the human tubal epithelium. Ann Anat. 2019;224:73–80. doi: 10.1016/j.aanat.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 85.Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, Frechette GM, et al. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 2011;4(3):335–342. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hunt JL, Lynn AA. Histologic features of surgically removed fallopian tubes. Arch Pathol Lab Med. 2002;126(8):951–955. doi: 10.5858/2002-126-0951-HFOSRT. [DOI] [PubMed] [Google Scholar]
- 87.Pelzer ES, Allan JA, Cunningham K, Mengersen K, Allan JM, Launchbury T, Beagley K, et al. Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Hum Reprod. 2011;26:1799–1812. doi: 10.1093/humrep/der108. [DOI] [PubMed] [Google Scholar]
- 88.Zhou B, Sun C, Huang J, Xia M, Guo E, Li N, Lu H, et al. The biodiversity composition of microbiome in ovarian carcinoma patients. Sci Rep. 2019;9:1691. doi: 10.1038/s41598-018-38031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schoenmakers S, Steegers-Theunissen R, Faas M. The matter of the reproductive microbiome. Obstet Med. 2019;12(3):107–115. doi: 10.1177/1753495X18775899. [DOI] [PMC free article] [PubMed] [Google Scholar]