Abstract
Prediction model has been the focus of studies since the last century in the diagnosis and prognosis of various diseases. With the advancement in computational technology, machine learning (ML) has become the widely used tool to develop a prediction model. This review is to investigate the current development of prediction model for the risk of cardiovascular disease (CVD) among type 2 diabetes (T2DM) patients using machine learning. A systematic search on Scopus and Web of Science (WoS) was conducted to look for relevant articles based on the research question. The risk of bias (ROB) for all articles were assessed based on the Prediction model Risk of Bias Assessment Tool (PROBAST) statement. Neural network with 76.6% precision, 88.06% sensitivity, and area under the curve (AUC) of 0.91 was found to be the most reliable algorithm in developing prediction model for cardiovascular disease among type 2 diabetes patients. The overall concern of applicability of all included studies is low. While two out of 10 studies were shown to have high ROB, another studies ROB are unknown due to the lack of information. The adherence to reporting standards was conducted based on the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) standard where the overall score is 53.75%. It is highly recommended that future model development should adhere to the PROBAST and TRIPOD assessment to reduce the risk of bias and ensure its applicability in clinical settings. Potential lipid peroxidation marker is also recommended in future cardiovascular disease prediction model to improve overall model applicability.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01741-7.
Keywords: Type 2 diabetes mellitus, Cardiovascular disease, Machine learning, Prediction model
Introduction
Machine learning is a branch of computer science that uses existing data to predict future responds when new data is provided [1]. By utilizing artificial intelligence, pattern recognizing, and computational statistics, the training of prediction model can improve its overall performance and make decisions based on new set settings or situations.
Early prediction of cardiovascular disease (CVD) among diabetes patients were created based on logistic regression, but machine learning has been used as a predictive model for its flexibility and variability [2]. Regression models are made based on a hypothesis and a fixed model structure but machine learning search for the optimal fit based on different algorithms [3]. Various machine learning algorithms are used in creating predictive model such as neural networks (NN), support vector machine (SVM), decision tree (DT), and k-nearest neighbours (k-NN) [4]. The building of predictive model using machine learning approach will require extra steps that include the model training and validation. Through repeated training and testing of models, different algorithms can only be compared among each other to find out the best performing model or algorithm.
The performance of machine learning model also affected by the predictors or risk factors used in the model [5]. Several risk factors that involve in the development of atherosclerosis which lead to CVD in individuals with T2DM were include hypertension, insulin resistance, hyperglycaemia, obesity, and dyslipidaemia [6]. In addition, recent studies have shown that T2DM patients have higher risk in developing CVD due to lipid peroxidation where free radicals or reactive oxygen species (ROS) attacked polyunsaturated fats (PUFAs) [7, 8]. Polyunsaturated acyl group of phospholipids lose its hydrogen to form a highly reactive radical, followed by the reaction with oxygen to form a peroxyl radical [9]. The peroxyl group is then obtain hydrogen from other phospholipids to form a lipid hyperperoxide [10]. The peroxide will react with other organic substrate such as another phospholipid [11]. As the result, production of electrophilic molecules such as malondialdehyde (MDA) increases causing oxidative stress [12]. The cytotoxicity of these molecules can cause complications such as aging and atherosclerosis by binding to DNA, proteins, or other nucleophilic molecules. These damages induce cell death and eventually progress into cardiovascular complications [11]. Since the increase of level of lipid peroxide molecules and oxidative stress known for causing CVD, the level of these biomolecules can be potentially used as predictors in the development of prediction model for cardiovascular disease among diabetes patients.
Previous studies have provided the basis to build a disease prediction model by machine learning [13]. Machine learning (ML) approach offers the opportunity to identify patients at greater risk of T2DM complications [14] while prediction models built using ML techniques improve cardiovascular disease prediction and reducing the number of screenings required when compared with the ACC/AHA Pooled Cohort Equations (PCE) calculator alone [15].
In the Action to Control Cardiovascular Risk in Diabetes Study (ACCORD) and the Veterans Affairs Diabetes Trial (VADT) trials, a ML analysis provided evidence supporting the diabetes treatment guideline recommendation of intensive glucose lowering in diabetes patients with low cardiovascular risk and additionally suggested benefits of intensive glycaemic control in some individuals at higher cardiovascular risk [16]. Moreover, an unsupervised ML clustering method could address T2DM patients with heterogeneous clinical indicators and identify groups with different types of coronary plaque and degrees of coronary stenosis, allowing patient stratification [17]. In addition, a ML approach demonstrated high performance in identifying metabolic-associated fatty liver disease (MAFLD) patients with prevalent cardiovascular disease based on the easy-to-obtain patient parameters [18]. Finally, incorporating genome-wide polygenic risk score (gPRS) and serum metabolite data enhances diabetes risk prediction [19]. The application of the cardiovascular diabetes prediction model can assist in clinical settings such as decision-making, clinical management in diabetes care, and patient communication to reduce the risk of cardiovascular complications among diabetes patients [20]. In this context, this systematic review is to identify the available machine learning-based prediction models for diabetic cardiovascular disease.
Methods
Systematic searches and the development of this review was guided by Preferred Reporting Items for Systematic Review and Meta-analyses Protocols (PRISMA-P) [21]. A protocol has been registered at The International Prospective Register of Systematic Reviews (PROSPERO) under the reference ID CRD42022337764. Criteria of the studies are outlined based on PICOTS framework [22].
Participants (P)
Studies that involve patients that are diagnosed with T2DM. These patients include individuals with or without cardiovascular complications. Studies that involve other types of diabetes were not included in this review. There were no eligibility restrictions on age, population, gender, ethnicity, geographic location of participants. Studies that include other diabetes complications are included.
Interventions (I)
Only predictive modelling studies that clearly describe the use of machine learning (ML) in prognosis and diagnosis models are included. Therefore, studies without the clear demonstration of ML-based prediction model will be excluded. Studies that include supervised, unsupervised or combination of both are accepted as the interventions to build a predictive model. Since different studies’ aim may or may not require external validation, thus, the three study types that are, prediction models development studies without external validation, prediction model development studies with external validation, and external model validation studies with or without model updating were included [23].
Outcomes (O)
The effects and properties of prediction models were observed and measured in this review based on the reported metrics, including c-statistics or classification measurements such as, the accuracy, sensitivity, and specificity. Secondary outcomes that were observed were study design, population, predictors, and model types.
Time (T)
The search was limited to publications from 1st January 2017 to 14th April 2022 to ensure data were up to date within five years from this study.
Settings (S)
Only studies published in English were included.
Search strategies
A uniform systematic search was performed in two databases including SCOPUS and Web of Science. Relevant articles from the references were searched manually. The search terms are based on the PICOTS list in Table 1.
Table 1.
Selection criteria of predictive modelling studies in PICOTS format
| Participants (P) | Intervention (I) | Comparison (C) | Outcomes (O) | Timeframe (T) | Settings (S) | Other limitations | |
|---|---|---|---|---|---|---|---|
| Inclusion criteria | Patients with T2DM | ML-based predictive modelling including supervised and unsupervised machine learning or combination of both | N/A | Study designs, population, predictors, and models used, quality validation of models | From 1st January 2017 to date | N/A | Language = English |
| Exclusion criteria | Patients with other types of diabetes or pre-diabetes | Prediction models without specific use of ML |
Data extraction and risk of bias assessment
The extraction had been performed according to the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) statement [24, 25]. TRIPOD statement had also been used for reporting adherence. ROB for each studies included was carried out based on Prediction Model Risk of Bias Assessment Tool (PROBAST) [26] (Additional files 1, 2).
Results
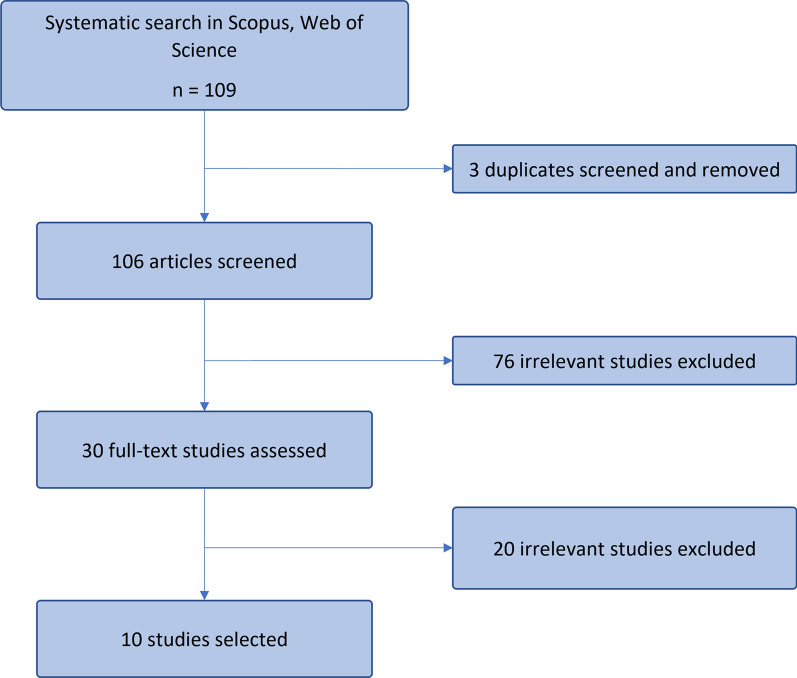
Of 109 articles reviewed, only 10 were selected in this review article (Fig. 1). The general characteristics of each included study are described in Table 2. All studies included in this review were cohort studies all over the world except for only one is from cross-sectional study that conducted in China (Table 2). Majority of the studies were based on the European/Caucasian population (Germany, Greece, Sweden, Denmark, Australia, and United States), but only two studies were based on the Chinese population.
Fig. 1.
PRISMA flow diagram for the inclusion of cardiovascular diabetes prediction models from 1st January 2017 till 14th April 2022
Table 2.
General characteristics of the included studies in the systematic review of cardiovascular diabetes prediction models
| References | Study designs | Population | Predictors | Model types | Outcome |
|---|---|---|---|---|---|
| [31] | Cohort | Greece | Body mass index (BMI), Hba1c, fasting blood glucose (FBG), lipid profile (LP), age, smoking habit, hypertension (HPT), pulse pressure, lipid-lowering therapy, parental history of diabetes | XGBoost | Development of prediction model for fatal or non-fatal incidence in T2DM individuals |
| [32] | Cohort | Denmark | Disease codes, prescription of insulin and analogues, and prescription of blood glucose lowering drugs | Logistic ridge regression, random forest, decision tree gradient boosting | Prediction of individuals at elevated risk of developing T2DM comorbidities |
| [29] | Cohort | Greece | Age, diabetes duration, Hba1c, blood pressure (BP), FBG, LP, smoking habit, sex, HPT, lipid-lowering therapy | HWNN, SOM, BLR, FFN, CART, RF, NB | Development of prediction model for fatal or non-fatal incidence in T2DM individuals |
| [30] | Cohort | United States | BMI, BP, age, sex, hypertensions, heart and diabetic complications, other nosology, insulin, sugar-lowering drugs, other drugs | XGBoost, DT, RF, LR, Dummy, kNN, multinominal, complement and Bernoulli’s NB | Prediction of individuals at elevated risk of developing T2DM comorbidities |
| [13] | Cohort | United States, Greece | Age, diabetes duration, BMI, BP, Hba1c, FBG, LP, HPT, ACE inhibitor, sex, diabetic parents, retinopathy, calcium antagonists, diuretics, B-blockers, smoking habit, proteinuria, hypolipid diet, aspirin, diet, sulphonyl urea, diguanide, insulin | ANN, binary logistic model, logistic model tree, Bayes net, DT, naïve Bayes | Assess the ability and performance of six machine learning models in prediction T2DM and CVD complications |
| [33] | Cohort | China | Sex, age, race, total cholesterol, high density lipoprotein (HDL), systolic BP, anti-HPT treatment, diabetes, and smoking habit | Knowledge learning symbiosis (KLS) | Development of prediction model for CVD risk in T2DM individuals |
| [34] | Cohort | Sweden | Sex, systolic BP, BMI, smoking habit, diagnosis of atrial fibrillation, myocardial and stroke history, HbA1c, HDL, total cholesterol, duration of type 2 diabetes, microalbuminuria, macroalbuminuria | Cox gradient boosting machine learning (GBM) | Assess eighty cardiovascular and inflammatory proteins for biomarker discovery and the prediction of major cardiovascular events in T2DM |
| [28] | Cross-sectional | China | Sex, age, marital status, educational level, monthly income, diabetes duration, insulin treatment, HbA1c, FBG, LP, BP, BMI, anxiety, depression, smoking habit, and drinking habit | Deep neural network | Development of a CVD risk prediction model based on the bio-psycho-social contributors in T2DM patients |
| [35] | Cohort | Australia | Age, sex, admission episode, discharge dates and disease codes | LR, SVM, DT, RF, NB, kNN | Development of prediction model for CVD risk in T2DM individuals |
| [36] | Cohort | United States | BMI, age, and fasting plasma glucose | SVM, kNN | Development of prediction model for CVD risk in T2DM individuals |
The most common predictor used in the predictive model was HbA1c, which six out of ten studies included in their model, followed by body mass index (BMI) where 50% used in their model and medical history or disease, which only included in three articles (Table 2). Other predictors were sex, age, heart rate, blood pressure, lipid profile, fasting blood glucose, waist circumference, parental history of diabetes, patients’ smoking or drinking habits and the treatment of the patients received such as insulin treatment and lipid-lowering treatment. Among the 18 predictors involved in this study, the top five predictors are BMI, anxiety, depression, total cholesterol, and systolic blood pressure.
There were several models or algorithms that have been reported to predict cardiovascular diabetes so far such as support vector machine (SVM), decision tree (DT), random forest (RF), Naïve Bayes (NB), linear regression (LR), Self-Organizing Maps (SOM), and knowledge learning symbiosis (KLS) [27]. Gradient boosting models have been reported in four studies that included extreme gradient boosting, cox gradient boosting, and decision tree gradient boosting. Other reported models were neural networks and k-nearest neighbour from another three studies (Table 2).
Model performance
From the review, not all studies reported their model performance using the same metrics of evaluation. Based on Table 3, neural network model [28] has the best performance which achieve 87.5% accuracy, 88.06% sensitivity, 87.23% specificity and AUC of 0.91. The precision of the model was not reported but based on the confusion matrix provided, it is 76.6%. In addition, previous studies [13, 28, 29] have shown that the overall performance of neural network is better than gradient boosting.
Table 3.
Performance of the proposed models reported using various metrics of evaluation including accuracy, sensitivity, specificity, precision, C-value, and area under the curve
| References | Best performing model | Accuracy (%) | Sensitivity (%) | Specificity (%) | Precision (%) | Area under the curve |
|---|---|---|---|---|---|---|
| [31] | XGBoost | NA | 71.00 ± 23.85 | NA | NA | 71.13 ± 11.69 |
| [32, 34] | Gradient Boosting Machine | NA | 79.1 | 55.8 | NA | 0.69–0.825 |
| [29] | Hybrid Wavelet Neural Network (HWNN) | 83.04 ± 8.22 | 29.50 ± 23.15 | 87.30 ± 9.73 | NA | 67.64 ± 15.09 |
| [30] | XGBoost | 84.5 | 85 | NA | 84.5 | NA |
| [13] | Ensembles of ANN | 80.20 | NA | NA | NA | 0.849 |
| [33] | Knowledge Learning Symbiosis (KLS) | NA | NA | NA | NA | NA |
| [28] | Neural network | 87.50 | 88.06 | 87.23 | 76.6 | 0.91 |
| [35] | Logistic regression, support vector machine | 83.33 | 83.33 | 83.33 | 83.33 | 0.81 |
| [36] | Support vector machine | 96.93 | 92.87 | NA | 94.44 | NA |
Gradient boosting algorithm was indicated as the second-best performing algorithms after neural network based on the performance metrics provided. A cohort study conducted in US [30] has shown gradient boosting with the highest performance with 84.5% accuracy, 85% sensitivity, and precision of 84.5% compared to other models. This is supported with previous study conducted in Greece [31] that extreme gradient boosting (XGBoost) has the potential to handle the imbalanced medical dataset. In that study, the best reported model was based on XGBoost with sensitivity of 71.00% (CI 74.15, 94.85) and AUC of 0.71 (CI 0.59, 0.83). The third performing model are the only comparable models with complete performance data are LR and SVM models [35]. The two models in this study have the same performance with 83.33% accuracy, 83.33% sensitivity, 83.33 specificity, 83.33% precision, and AUC of 0.81. SVM model developed in Swedish cohort [36] also reported to perform better compared to k-nearest neighbour with 96.93% accuracy, 92.87% sensitivity, and 94.44% precision.
Risk of bias assessment
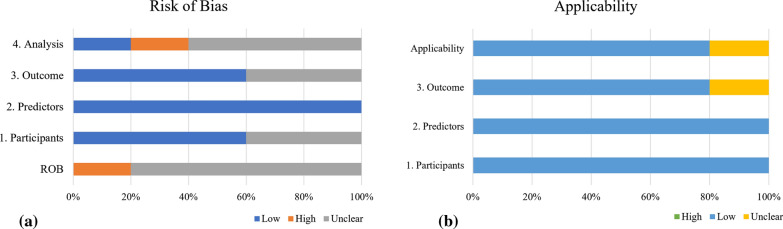
From Fig. 2, two out of 10 included studies have high risk-of bias, another eight have unclear risk-of-bias. The elevated risk is from the participant domain, which the inclusion and exclusion criteria are often not reported. All included studies have low risk in the predictor’s domain. As in the outcome domain, all studies have minimal risk, while another four studies have an unclear risk due to the lack of information about the time interval between predictor assessment and outcome determination. Whereas another six studies have unclear risk in their analysis due to the lack of multivariable analysis and four studies did not discuss about sampling controls. Although there is no study with low overall risk of bias, the concern of applicability for all models developed are low because the included participants and settings, definition, timing, or assessment of predictors, and the outcome definition, timing, or determination in all studies match the review question.
Fig. 2.
Prediction Model Risk of Bias Assessment Tool (PROBAST) for the studies included in this review. a The risk of bias of the 10 included studies. b The applicability of the 10 included studies
Adherence to reporting standards
The overall percentage of adherence to reporting standards based on the TRIPOD assessment is 53.75% with 13 out of 31 items with less than 50% adherence (Fig. 3). However, there are four out of the 13 items have 0% of adherence, which are sample size calculation, participant characteristics, full prediction model, and model usage guide. Other items with less than 50% reporting adherence are outcome blinding, predictors blinding, missing data, risk groups, flow of participants, unadjusted association, and model performance. The rationale, objectives, study design, setting, and model building and validation have a 100% reporting adherence. The lack of reporting adherence about full prediction model and model usage rendered the model unapplicable in real life clinical settings (Table 4).
Fig. 3.
Adherence of included studies to Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) assessment
Table 4.
PROBAST results
| Study | ROB | Applicability | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants | Predictors | Outcome | Analysis | Participants | Predictors | Outcome | ROB | Applicability | |
| Athanasiou et al. 2020 | − | + | + | + | + | + | + | − | + |
| Dworzynski et al. 2020 | + | + | + | − | + | + | + | − | + |
| Zarkogianni et al. 2018 | − | + | + | ? | + | + | + | − | + |
| Derevitskii and Kovalchuk 2020 | + | + | + | ? | + | + | + | ? | + |
| Dalakleidi et al. 2017 | ? | + | + | ? | + | + | + | ? | + |
| Mei and Xia 2019 | + | + | + | ? | + | + | + | ? | + |
| Nowak et al. 2018 | + | + | ? | + | + | + | + | ? | + |
| Chu et al. 2021 | + | + | ? | − | + | + | + | − | + |
| Hossain et al. 2021 | + | + | ? | ? | + | + | + | ? | + |
| Miao et al. 2020 | ? | + | ? | ? | + | + | + | ? | + |
“+” indicates low ROB/low concern regarding applicability; “−” indicates high ROB/high concern regarding applicability; and “?” indicates unclear ROB/unclear concern regarding applicability
ROB risk of bias
Discussion
This review identified ten machine learning models that were developed for predicting cardiovascular disease among diabetic patients conducted mostly among European population. Even though the prevalence of cardiovascular diabetic was high in Asian countries, only two included studies were conducted among the Chinese population but none from the Malay or Indian population. In 2019, 44.2% of Malaysian patients presented with acute coronary syndrome had diabetes which is the second common cardiovascular risk factor (CVRF) after hypertension (61.9%) [37]. Thus, this highlighted the importance of conducting predictive model studies of diabetic cardiovascular disease for Malaysian since its population also pose a higher risk at younger age than the European population [38]. Furthermore, a sharp increase of T2DM treatment cost from USD 232 billion in 2007 to USD 966 billion in 2021 with the high prevalence of the disease worldwide is causing concerns on its burden in the lower-income nations [39]. It is known that most T2D patients do not require insulin for the rest of their life, but the complications developed from T2D eventually increase the economic burden on the patients and the healthcare system worldwide [1, 40]. Thus, it is essential to develop cardiovascular diabetes prediction model to effectively reduce the morbidity and further complication as well as the economic burden especially in Asian countries.
The artificial neural network model (ANN) reported by Dalakleidia and Zarkogianni [13] showed that ANN performed better than other algorithms such as NB, decision tree, and logistic model when working with the imbalanced nature of medical datasets. Imbalanced dataset is when the distribution of classes is unequal that leads to the situation where one class out represent the other. This suggest that receiver operating curves, precision-recall curves, and cost curves are necessary when imbalanced datasets are involved [41]. This nature of medical datasets lead to prediction bias towards larger disjuncts and misclassification of the smaller disjuncts [42]. To avoid class imbalance, oversampling of the minority class such as the use of Synthetic Minority Oversampling Technique (SMOTE) can help improving the overall accuracy of a model [14]. In addition, three out of ten studies in this review have supported that neural network can be used to construct predictive models for diabetic cardiovascular disease. However, the three studies that involve neural network did not include the use of gradient boosting algorithm, thus, these models were not compared based on their accuracy, sensitivity, specificity, and precision.
Before machine learning was introduced, prediction models were developed using classical statistics such as logistic regression. The Framingham Heart Studies (FHS) is one of the most famous examples of a prediction model for cardiovascular disease that applies logistics regression [43] and the focus on diabetes mellitus as a risk factor of CVD emerged after years of follow up studies [44, 45]. Other than logistic regression, a classic statistical model such as the cox regression model was also applied in the development of CVD prediction model for diabetic patients. For example, a study that incorporated the patient population and electronic medical record (EMR) data in US [46] developed a cox regression model with a c-statistic of 0.782 and the model reported in Ley et al. [47] achieved a c-statistic of 0.73 (0.72–0.74). While classical statistic has been applied in various medical disciplines from CVD to cancer studies [48–50], machine learning model is advantageous when working with pattern recognition other than just projection based on existing data [51].
Predictors in a predictive model are important as it affects the performance when dealing with new datasets. However, not all studies mention about the impact of the predictors involved in their models. Out of ten articles reviewed, only four studies summarized the most important factors for their model and BMI were reported as the top five key factors. BMI has been used as an obesity indicator, which is directly linked to cardiovascular disease and diabetes [52]. With the increasing availability of fast food and processed food, the general eating habit and diet of most people are known to become less healthy due to increasing carbohydrates and fat intake. This phenomenon worsens in recent years as part of the urbanization [53]. Body mass index reflects the diet of an individual which is also known to be a strong factor in causing cardiovascular complications among diabetic patients [54]. Although its contribution to the prediction models was not reported in all included studies, six out of ten articles used BMI as one of their predictors. Although family history is known to be a major risk factor in the development of the CVD, but none of the study included this predictor. This might be due that family history of CVD has been excluded in these studies [55, 56]. In the past few decades, the number of studies on lipid peroxidation is increasing due to its association with cardiovascular disease through lipid alteration [8, 57]. The earliest mention of lipid peroxidation with extensive discussion is in 1958 by Lundberg [58]. Since then, more studies about the autoxidation of lipid were published. Even though the relationship between increased lipid peroxidation level in diabetic patients and risk of CVD is well known [59], no studies included in this systematic review included any lipid peroxidation marker as a predictor in model development.
Although this review is quite comprehensive that followed the guideline of PRISM-P, the selection framework by PICOTS and addressed all the risk assessment bias using PROBAST and TRIPOD, but the search of this study was only performed on Scopus and WoS only. Meta-analysis also could not be done due to the limited number of articles and recent studies. To address the imbalanced nature of clinical data, which is very common in life sciences, precision-recall curve (PRC) is the recommended metric to display the true performance of a prediction model [60]. The sample size varies significantly among the prediction models discussed in this review, ranging from 560 in the study by Nowak and Carlsson [34] to more than 200,000 subjects in the study by Dworzynski, Aasbrenn [32]. The best model has the sample size of 834 subjects where the study with second best models involved 124,000 subjects.
For clinical practice, prediction models are required to be user friendly, and the presentation of the results also play a vital role in the communication between clinician and patients. Furthermore, to ensure the reliability and overall precision of a prediction model, external validation must be conducted using new datasets as the test data [61]. In the future, existing models can also be improved using newly collected data.
Conclusion
In this review, we discovered ten studies of cardiovascular disease prediction models among T2DM patients which used various machine learning approaches. The best model among the studies is the neural network model proposed by Chu and Chen [28] with AUC of 0.91. However, the precision of the model is only 76.6% and external validation is recommended to verify its performance when dealing with different datasets. External validation is a crucial step to ensure the applicability of a model in clinical settings [14]. This review shows that neural network has the best performance followed by gradient boosting machine to predict cardiovascular disease among diabetes patients. Future studies are recommended to include the comparison between neural network and gradient boosting machine using same datasets.
Supplementary Information
Additional file 1. PROBAST assessment form.
Additional file 2. TRIPOD assessment form.
Acknowledgements
The authors thank all volunteered participants, Endocrine and Cardio Clinic Staffs, UMBI and TMC staffs for their collaboration and contribution for the data collection. The authors also thank Universiti Kebangsaan Malaysia for funding, including infrastructure and utilities.
Abbreviations
- BP
Blood pressure
- BMI
Body mass index
- CVD
Cardiovascular disease
- CVRF
Cardiovascular risk factor
- FHS
Framingham Heart Studies
- LR
Linear regression
- SVM
Support vector machine
- RF
Random forest
- KLS
Knowledge learning symbiosis
- AUC
Area under the curve
- ML
Machine learning
- PRC
Precision-recall curve
- T2DM
Type 2 diabetes
Author contributions
Conceptualization: NA; data curation: OTK, HH; fund acquisition: NA; methodology: NA and OTK; writing—original draft: OTK; writing—review and editing: NA, OTK, NM, NAAM, SFC and RJ. All authors agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Universiti Kebangsaan Malaysia Research Grant GUP-2020-077.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest to report regarding the present study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 2.Dinh A, Miertschin S, Young A, Mohanty SD. A data-driven approach to predicting diabetes and cardiovascular disease with machine learning. BMC Med Inform Decis Mak. 2019;19(1):211. doi: 10.1186/s12911-019-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein BA, Navar AM, Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur Heart J. 2017;38(23):1805–1814. doi: 10.1093/eurheartj/ehw302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churpek MM, Yuen TC, Winslow C, Meltzer DO, Kattan MW, Edelson DP. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. 2016;44(2):368–374. doi: 10.1097/CCM.0000000000001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Saluja K, Goyal A, Vajpayee A, Tiwari V. Comparing the performance of machine learning algorithms using estimated accuracy. Meas Sens. 2022;24:100432. doi: 10.1016/j.measen.2022.100432. [DOI] [Google Scholar]
- 6.Joseph JJ, Deedwania P, Acharya T, Aguilar D, Bhatt DL, Chyun DA, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation. 2022;145(9):e722–e759. doi: 10.1161/CIR.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 7.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, et al. Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci. 2015;16(10):25234–25263. doi: 10.3390/ijms161025234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su L-J, Zhang J-H, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med Cell Longev. 2019;2019:5080843. doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratt DA, Tallman KA, Porter NA. Free radical oxidation of polyunsaturated lipids: new mechanistic insights and the development of peroxyl radical clocks. Acc Chem Res. 2011;44(6):458–467. doi: 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zielinski ZAM, Pratt DA. Lipid peroxidation: kinetics, mechanisms, and products. J Org Chem. 2017;82(6):2817–2825. doi: 10.1021/acs.joc.7b00152. [DOI] [PubMed] [Google Scholar]
- 12.Ito F, Sono Y, Ito T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants. 2019;8(3):72. doi: 10.3390/antiox8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalakleidi K, Zarkogianni K, Thanopoulou A, Nikita K. Comparative assessment of statistical and machine learning techniques towards estimating the risk of developing type 2 diabetes and cardiovascular complications. Expert Syst. 2017;34(6):e12214. doi: 10.1111/exsy.12214. [DOI] [Google Scholar]
- 14.Nicolucci A, Romeo L, Bernardini M, Vespasiani M, Rossi MC, Petrelli M, et al. Prediction of complications of type 2 diabetes: a machine learning approach. Diabetes Res Clin Pract. 2022;190:110013. doi: 10.1016/j.diabres.2022.110013. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Campan A, Ren A, Eid WE. Automating and improving cardiovascular disease prediction using machine learning and EMR data features from a regional healthcare system. Int J Med Inform. 2022;163:104786. doi: 10.1016/j.ijmedinf.2022.104786. [DOI] [PubMed] [Google Scholar]
- 16.Edward JA, Josey K, Bahn G, Caplan L, Reusch JEB, Reaven P, et al. Heterogeneous treatment effects of intensive glycemic control on major adverse cardiovascular events in the ACCORD and VADT trials: a machine-learning analysis. Cardiovasc Diabetol. 2022;21(1):58. doi: 10.1186/s12933-022-01496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Yang Z-G, Wang J, Shi R, Han P-L, Qian W-L, et al. Unsupervised machine learning based on clinical factors for the detection of coronary artery atherosclerosis in type 2 diabetes mellitus. Cardiovasc Diabetol. 2022;21(1):259. doi: 10.1186/s12933-022-01700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drożdż K, Nabrdalik K, Kwiendacz H, Hendel M, Olejarz A, Tomasik A, et al. Risk factors for cardiovascular disease in patients with metabolic-associated fatty liver disease: a machine learning approach. Cardiovasc Diabetol. 2022;21(1):240. doi: 10.1186/s12933-022-01672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn S-J, Kim S, Choi YS, Lee J, Kang J. Prediction of type 2 diabetes using genome-wide polygenic risk score and metabolic profiles: a machine learning analysis of population-based 10-year prospective cohort study. eBioMedicine. 2022;86:104383. doi: 10.1016/j.ebiom.2022.104383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damen JAAG, Hooft L, Schuit E, Debray TPA, Collins GS, Tzoulaki I, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi: 10.1136/bmj.i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riva JJ, Malik KMP, Burnie SJ, Endicott AR, Busse JW. What is your research question? An introduction to the PICOT format for clinicians. J Can Chiropr Assoc. 2012;56(3):167–171. [PMC free article] [PubMed] [Google Scholar]
- 23.Moons KGM, de Groot JAH, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744. doi: 10.1371/journal.pmed.1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 25.Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 26.Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019;170(1):51–58. doi: 10.7326/M18-1376. [DOI] [PubMed] [Google Scholar]
- 27.Parfitt VJ, Desomeaux K, Bolton CH, Hartog M. Effects of high monounsaturated and polyunsaturated fat diets on plasma lipoproteins and lipid peroxidation in type 2 diabetes mellitus. Diabet Med. 1994;11(1):85–91. doi: 10.1111/j.1464-5491.1994.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 28.Chu H, Chen L, Yang X, Qiu X, Qiao Z, Song X, et al. Roles of anxiety and depression in predicting cardiovascular disease among patients with type 2 diabetes mellitus: a machine learning approach. Front Psychol. 2021;12:1189. doi: 10.3389/fpsyg.2021.645418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarkogianni K, Athanasiou M, Thanopoulou AC. Comparison of machine learning approaches toward assessing the risk of developing cardiovascular disease as a long-term diabetes complication. IEEE J Biomed Health Inform. 2018;22(5):1637–1647. doi: 10.1109/JBHI.2017.2765639. [DOI] [PubMed] [Google Scholar]
- 30.Derevitskii IV, Kovalchuk SV. Machine learning-based predictive modeling of complications of chronic diabetes. Procedia Comput Sci. 2020;178:274–283. doi: 10.1016/j.procs.2020.11.029. [DOI] [Google Scholar]
- 31.Athanasiou M, Sfrintzeri K, Zarkogianni K, Thanopoulou AC, Nikita KS. An explainable XGBoost-based approach towards assessing the risk of cardiovascular disease in patients with Type 2 diabetes mellitus. ArXiv. 2020. arXiv:2009.06629.
- 32.Dworzynski P, Aasbrenn M, Rostgaard K, Melbye M, Gerds TA, Hjalgrim H, et al. Nationwide prediction of type 2 diabetes comorbidities. Sci Rep. 2020;10(1):1776. doi: 10.1038/s41598-020-58601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mei J, Xia E. Knowledge learning symbiosis for developing risk prediction models from regional EHR repositories. Stud Health Technol Inform. 2019;264:258–262. doi: 10.3233/SHTI190223. [DOI] [PubMed] [Google Scholar]
- 34.Nowak C, Carlsson AC, Ostgren CJ, Nystrom FH, Alam M, Feldreich T, et al. Multiplex proteomics for prediction of major cardiovascular events in type 2 diabetes. Diabetologia. 2018;61(8):1748–1757. doi: 10.1007/s00125-018-4641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hossain ME, Uddin S, Khan A. Network analytics and machine learning for predictive risk modelling of cardiovascular disease in patients with type 2 diabetes. Expert Syst Appl. 2021;164:113918. doi: 10.1016/j.eswa.2020.113918. [DOI] [Google Scholar]
- 36.Miao L, Guo X, Abbas HT, Qaraqe KA, Abbasi QH, editors. Using machine learning to predict the future development of disease. In: 2020 international conference on UK-China emerging technologies (UCET), 2020 20–21 Aug; 2020.
- 37.Ahmad WAW. Annual report of the NCVD-ACS registry, 2018–2019. National Cardiovascular Disease Database; 2022.
- 38.International Diabetes Federation. IDF diabetes atlas 2021: IDF; 2021.
- 39.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes—global burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020; 2020.
- 41.He H, Garcia EA. Learning from imbalanced data. IEEE Trans Knowl Data Eng. 2009;21(9):1263–1284. doi: 10.1109/TKDE.2008.239. [DOI] [Google Scholar]
- 42.Holte RC, Acker L, Porter BW, editors. Concept learning and the problem of small disjuncts. In: IJCAI; 1989.
- 43.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23(2):105–111. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 45.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 46.Williams BA, Geba D, Cordova JM, Shetty SS. A risk prediction model for heart failure hospitalization in type 2 diabetes mellitus. Clin Cardiol. 2020;43(3):275–283. doi: 10.1002/clc.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pylypchuk R, Wells S, Kerr A, Poppe K, Harwood M, Mehta S, et al. Cardiovascular risk prediction in type 2 diabetes before and after widespread screening: a derivation and validation study. Lancet. 2021;397(10291):2264–2274. doi: 10.1016/S0140-6736(21)00572-9. [DOI] [PubMed] [Google Scholar]
- 48.Chhatwal J, Alagoz O, Lindstrom MJ, Kahn CE, Jr, Shaffer KA, Burnside ES. A logistic regression model based on the national mammography database format to aid breast cancer diagnosis. AJR Am J Roentgenol. 2009;192(4):1117–1127. doi: 10.2214/AJR.07.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahlrot RH, Bangsø JA, Petersen JK, Rosager AM, Sørensen MD, Reifenberger G, et al. Prognostic role of Ki-67 in glioblastomas excluding contribution from non-neoplastic cells. Sci Rep. 2021;11(1):17918. doi: 10.1038/s41598-021-95958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuster-Garcia E, Lorente Estellés D, Álvarez-Torres MDM, Juan-Albarracín J, Chelebian E, Rovira A, et al. MGMT methylation may benefit overall survival in patients with moderately vascularized glioblastomas. Eur Radiol. 2021;31(3):1738–1747. doi: 10.1007/s00330-020-07297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bzdok D, Altman N, Krzywinski M. Statistics versus machine learning. Nat Methods. 2018;15(4):233–234. doi: 10.1038/nmeth.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circul Res. 2016;118(11):1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 53.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383(9933):1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gray N, Picone G, Sloan F, Yashkin A. Relation between BMI and diabetes mellitus and its complications among US older adults. South Med J. 2015;108(1):29–36. doi: 10.14423/SMJ.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolber MR, Scrimshaw C. Family history of cardiovascular disease. Can Fam Physician. 2014;60(11):1016. [PMC free article] [PubMed] [Google Scholar]
- 56.Valerio L, Peters RJ, Zwinderman AH, Pinto-Sietsma SJ. Association of family history with cardiovascular disease in hypertensive individuals in a multiethnic population. J Am Heart Assoc. 2016;5(12):e004260. doi: 10.1161/JAHA.116.004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxidative Med Cell Longev. 2019;2019:8267234. doi: 10.1155/2019/8267234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lundberg WO. Lipids of biologic importance: peroxidation products and inclusion compounds of lipids. Am J Clin Nutr. 1958;6(6):601–603. doi: 10.1093/ajcn/6.6.601. [DOI] [PubMed] [Google Scholar]
- 59.Bigagli E, Lodovici M. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxidative Med Cell Longev. 2019;2019:5953685. doi: 10.1155/2019/5953685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS ONE. 2015;10(3):e0118432. doi: 10.1371/journal.pone.0118432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ho SY, Phua K, Wong L, Bin Goh WW. Extensions of the external validation for checking learned model interpretability and generalizability. Patterns. 2020;1(8):100129. doi: 10.1016/j.patter.2020.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. PROBAST assessment form.
Additional file 2. TRIPOD assessment form.
Data Availability Statement
Not applicable.