Abstract
Background
Little is known on the burden of co-infections and superinfections in a specific setting such as the respiratory COVID-19 sub-intensive care unit. This study aims to (i) assess the prevalence of concurrent and superinfections in a respiratory sub-intensive care unit, (ii) evaluate the risk factors for superinfections development and (iii) assess the impact of superinfections on in-hospital mortality.
Methods
Single-center retrospective analysis of prospectively collected data including COVID-19 patients hospitalized in a newly established respiratory sub-intensive care unit managed by pneumologists which has been set up from September 2020 at a large (1200 beds) University Hospital in Rome. Inclusion criteria were: (i) COVID-19 respiratory failure and/or ARDS; (ii) hospitalization in respiratory sub-intensive care unit and (iii) age > 18 years. Survival was analyzed by Kaplan–Meier curves and the statistical significance of the differences between the two groups was assessed using the log-rank test. Multivariable logistic regression and Cox regression model were performed to tease out the independent predictors for superinfections’ development and for mortality, respectively.
Results
A total of 201 patients were included. The majority (106, 52%) presented severe COVID-19. Co-infections were 4 (1.9%), whereas 46 patients (22%) developed superinfections, mostly primary bloodstream infections and pneumonia. In 40.6% of cases, multi-drug resistant pathogens were detected, with carbapenem-resistant Acinetobacter baumannii (CR-Ab) isolated in 47%. Overall mortality rate was 30%. Prior (30-d) infection and exposure to antibiotic therapy were independent risk factors for superinfection development whereas the development of superinfections was an independent risk factors for in-hospital mortality. CR-Ab resulted independently associated with 14-d mortality.
Conclusion
In a COVID-19 respiratory sub-intensive care unit, superinfections were common and represented an independent predictor of mortality. CR-Ab infections occurred in almost half of patients and were associated with high mortality. Infection control rules and antimicrobial stewardship are crucial in this specific setting to limit the spread of multi-drug resistant organisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-023-02315-9.
Keywords: COVID-19, Superinfections, MDR pathogens, Sub-intensive care unit, Acinetobacter baumannii, CAPA
Introduction
As of 28th July 2022, more than 570,000,000 people had been infected with SARS-CoV-2 worldwide, with more than 6,000,000 deaths [1]. Around 10% need hospitalization; respiratory failure, hyperinflammatory response, thrombotic events and superinfections are possible complications [2].
Viral respiratory illnesses predispose patients to bacterial and fungal infections; while this link has been widely described in relation with influenza pneumonia, it is still unclear the exact roles co-pathogens play in patients with COVID-19 [3]. Indeed, data available from the flu pandemics showed how bacterial co-infections and superinfections have a worse outcome than either infection on its own [4], leading the scientific community to consider antibiotic administration in patients admitted for influenza pneumonia [5].
During the first wave of COVID-19 pandemic, although few papers reported a low incidence of bacterial co-infections [6], most patients received antibiotics at disease onset [7, 8]. Later on, questions began to be asked on the relation between SARS-CoV-2 and co-pathogens [9, 10]. A living review ensuring regular updates every three months shows that co-infections and superinfections are encountered respectively in 4.9% and 16% of patients hospitalized for COVID-19 [11].
While the rate of co-infections has an important role in deciding whether to use empiric antibiotic therapy, superinfection rate tends to be higher in patients with prolonged hospital stay, worst clinical presentation, and need for Intensive Care Unit (ICU) [11]. Wide spectrum antibiotic use and reduced adherence to infection prevention practices contributed to a rapid spread of multidrug-resistant bacteria (MDR) [12, 13], which increase during hospital stay and represent over 70% of the isolates after 30 days of hospitalization [14].
In most centers in Italy, COVID-19 patients were admitted to ICU or ordinary wards based on their clinical conditions. Italian hospitals faced an unpreceded massive inflow in a short period of time [15]. In centers with more of 500 beds, semi-intensive units were set up, with integrated management between emergency medicine specialists, infectious diseases specialists, pulmonologists, and other specialties. In our Academic Hospital in Rome, a center with more than 1200 beds, starting from September 2020, a semi-intensive unit was set up managed by pneumologists.
To our knowledge, little is known about the prevalence of concurrent and superinfections in this peculiar setting.
Based on these premises, we carried out a study specifically targeting patients hospitalized in a respiratory sub-intensive care unit with the aims to (i) assess the prevalence and etiology of co- and superinfections, (ii) evaluate the risk factors for superinfection development and (iii) assess the impact of superinfections on in-hospital mortality.
Materials and methods
Study design
We performed a single-center, retrospective analysis of prospectively collected data on patients with COVID-19 pneumonia hospitalized in a respiratory sub-intensive respiratory care unit at Azienda Ospedaliero-Universitaria Policlinico Umberto I, Sapienza University of Rome, from November 2020 for the following 6 months, until April 2021.
Inclusion criteria were: (i) diagnosis of COVID-19 respiratory failure and/or acute respiratory distress syndrome (ARDS), (ii) hospitalization in a sub-intensive respiratory care unit for > 48 h and (iii) age > 18 years. Exclusion criteria included: age < 18 years, hospitalization in a sub-intensive respiratory care unit for < 48 h and missing data.
Diagnosis of SARS-CoV-2 infection was made with molecular analyses. Diagnosis of respiratory failure was made on clinical presentation and arterial blood gases (ABGs), a lung CT scan was performed to detect ground glass opacity and/or others lesions compatible with COVID-19 pneumonia. Acute respiratory distress syndrome was diagnosed on clinical and ABGs data according to Berlin definitions [16]. The decision to hospitalize in sub-intensive respiratory care unit was made on a clinical judgment by the emergency department or internal medicine doctors or infectious disease specialists in case of patients admitted from other wards.
Setting
Starting from September 2020, a sub-intensive respiratory care unit with 42 beds managed by pneumologists was set up in our 1200-bed Academic Hospital. Patients were admitted in case of severe respiratory failure and/or ARDS due to COVID-19 pneumonia requiring oxygen therapy and/or Helmet continuous positive airway pressure (CPAP) treatment or non-invasive mechanical ventilation (NIMV) (severe disease and critical disease according to WHO definitions [17]). Oxygen therapy was administered with Venturi Mask or high flow nasal cannula (HFNC) or with Helmet CPAP treatment or NIMV. In case of patients with tracheostomy, invasive ventilation was performed. Ventilation was performed in pressure support modality or assisted-controlled modality.
Patients required the use of continuous vital signs monitoring, and, in most cases, central venous catheter (CVC) or arterial catheters’ placement, total parenteral nutrition and, in some cases, sedation. Sedation was performed in case of non-adaptation to ventilation. Drugs administered in case of sedation were midazolam or dexmedetomidine. The level of sedation was related to the possibility of maintaining non-invasive ventilation.
We managed also critically patients with septic shock, or heart failure or requiring renal replacement, in this case with integrated management with nephrology specialists. We collaborated with an infectious disease specialist to treat superinfections.
Criteria for ICU-transfer was: if clinical conditions worsened despite ventilation and clinical care, an anesthesiologist consultation was required, and patients were transferred to ICU in case orotracheal intubation was needed.
Main differences with ICU setting were: (i) we did not manage intubated patients; (ii) most patients were not sedated and (iii) we have a different number of nurses, with one nurse assisting almost height patients during the work shift (nurse/patient ratio 1:8); (iv) ICU was managed by anesthesiologists.
Procedures
Nasopharyngeal swab samples were collected, and SARS-CoV-2 RNA was detected by using real time RT-PCR assay (RealStar SARS-CoV2 RT-PCR, Altona Diagnostics).
All patients received oxygen therapy and/or HFNC or CPAP or NIMV treatment based on respiratory failure severity.
Clinical criteria for diagnostic cultures and infection management
Screening for multi-drug resistant (MDR) bacteria [methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VRE), or carbapenem resistant (CR) gram-negative bacilli] colonization was systematically performed by means of rectal swab, at admission to the sub-intensive respiratory unit and every 7 days afterwards.
Respiratory samples included sputum and/or tracheobronchial aspirate (when feasible) and were collected on clinical bases, i.e., in the presence of augmented or modified respiratory secretions or when a pulmonary superinfection was suspected because of worsening of respiratory changes, with or without fever.
Additional microbiological procedures (i.e., blood or urine cultures) were performed during hospitalization in case of suspected superinfections, as requested by the attending physicians or the Infectious Disease (ID) consultant. Tracheobronchial aspirate (TBA) galactomannan (GM) and fungal culture were performed based on clinical suspicion of Aspergillus spp superinfection.
In case of suspected superinfections, all patients received empiric antibiotic therapy whereas definitive antibiotic therapy was based on the results of cultures. The clinical approach to bacterial and fungal superinfections was managed by a dedicated ID physician (author name, AO).
Microbiological methods
Isolated colonies from biological samples were identified by the Matrix-Assisted Laser Desorption Ionization–Time Of Flight Mass Spectrometry (MALDI-TOF MS) system (Bruker Daltonik GmbH, Bremen, Germany). Antimicrobial susceptibility tests were performed with Vitek 2 automated system (bioMérieux, Marcy l’Etoile, France) or with the MicroScan system (Beckman Coulter, Brea, CA, USA), according to the manufacturer’s instructions.
Fungal cultures were incubated for 7 days at 30 °C on Sabouraud selective media, whereas GM test in serum and TBA was performed according to manufacturer’s instructions (Platelia Aspergillus EIA, Bio-Rad).
Definitions
Respiratory failure was diagnosed in case of a value of PaO2 < 60 Torr at room air at ABGs, whereas PaO2/FiO2 ratio (P/F ratio) was used as an indicator of severity, according to Berlin definitions [16]. PaO2/FiO2 was recollected at hospital admission and daily. Only P/F at hospital admission was included into statistical analysis. COVID-19 pneumonia was diagnosed by clinical data, ABGs and lung CT scan performed for all patients at hospital admission [8]. Severe and critical disease were defined according to WHO definitions [17].
Prior (30-d) infections referred to infections diagnosed within 30 days before hospital admission; prior (30-d) antibiotic exposure included the receival of antibiotic therapy in the 30 days before diagnosis of secondary infections.
Co-infections were defined as infection onset less than 48 h from admission or present at hospital admission whereas superinfections were diagnosed by clinical signs of infections and a positive culture on blood, urine, and respiratory specimens, after 48 h from hospital admission [11, 18]. MDR and extensively drug-resistant pathogens (XDR) were defined based on susceptibility to different classes of antibiotics [19].
Pulmonary Aspergillosis following COVID-19 was defined according to the recently proposed definition [20].
Infections were defined according to the standard definitions of the ECDC [21]. The likely or ascertained source of infection was indicated by the attending physician or by the ID consultant in the medical record and defined in accordance with guidelines [21, 22]. Primary bloodstream infection (BSI) was defined as BSI occurring in patients without a recognized source of infection. Catheter-related related BSI (CR-BSI) was defined if the semiquantitative culture of the catheter tip was positive for the same microorganism isolated from the blood [23].
Mortality referred to in-hospital death for all causes, while 14-d survival estimates were performed in patients with superinfections.
The study was conducted according to the guidelines of the Declaration of Helsinki. The study was approved by the local Ethics Committee of Azienda Ospedaliera Universitaria Policlinico Umberto I, Rome (ID Prot. 109/2020). Given the retrospective nature of the study, specific informed consent was waived. However, at hospital admission, all the patients provided general consent for the use of their clinical data in future studies.
Statistical analysis
The data were given as medians with interquartile ranges (IQR) for continuous variables and as simple frequencies, proportions, and percentages for categorical variables. Mann–Whitney test was used for unpaired samples. Dichotomous variables were compared using Fisher's exact tests or chi-square test statistics, as appropriate. Survival was analyzed by Kaplan–Meier curves and the statistical significance of the differences between the 2 groups was assessed using the log-rank test. Multivariable logistic regression and Cox regression model were performed to tease out the independent predictors for superinfections’ development and for mortality, respectively. All statistical analyses were performed with STATA/IC (StataCorp, version 15).
Results
General population
A total of 201 patients were included in the study, with a median age of 67 years (IQR 56–80); 106 (52%) presented with severe COVID-19. General features of the study population are shown in Table 1.
Table 1.
Baseline characteristics of patients and type of superinfections
| Variable | Total population N = 201 |
Non infection group N = 155 |
Infection group N = 46 |
p value |
|---|---|---|---|---|
| Age, median (IQR), years | 67 (56–80) | 66 (55–77) | 77 (65–83) | 0.0005 |
| Sex (M), n (%) | 127 (63) | 100 (64) | 27 (58%) | 0.49 |
| Demographics | ||||
| Autoimmune disease, n (%) | 3 (1.4) | 2 (1.2) | 1 (2.1) | 0.54 |
| Chronic steroid/immunosuppressive therapy, n (%) | 17 (8.4) | 9 (5.8) | 8 (17.3) | 0.029 |
| Diabetes, n (%) | 45 (22) | 29 (18.7) | 16 (34.7) | 0.027 |
| Chronic ischemic heart disease, n (%) | 43 (21) | 24 (15.4) | 19 (41.3) | 0.0004 |
| Hypertension, n (%) | 93 (46) | 67 (43.2) | 26 (56.5) | 0.13 |
| Heart failure, n (%) | 21 (10.4) | 8 (5.1) | 13 (28.2) | 0.0001 |
| Atrial fibrillation, n (%) | 24 (11.9) | 14 (9) | 10 (21.7) | 0.034 |
| Vasculopathy, n (%) | 19 (9.4) | 15 (9.6) | 4 (8.6) | 0.99 |
| Cerebrovascular disease, n (%) | 12 (5.9) | 7 (4.5) | 5 (10.8) | 0.15 |
| Dementia, n (%) | 15 (7.4) | 9 (5.8) | 6 (13) | 0.10 |
| COPD, n (%) | 30 (14.9) | 22 (14.1) | 8 (17.3) | 0.63 |
| Chronic hepatopathy, n (%) | 9 (4.4) | 5 (3.2) | 4 (8.6) | 0.21 |
| Solid malignancy, n (%) | 17 (8.4) | 9 (5.8) | 8 (17.3) | 0.029 |
| Hematological malignancy, n (%) | 7 (3.4) | 5 (3.2) | 2 (4.3) | 0.66 |
| Chronic kidney disease, n (%) | 13 (6.4) | 7 (4.5) | 4 (8.6) | 0.27 |
| Obesity (BMI > 30), n (%) | 31 (15.4) | 26 (16.7) | 5 (10.8) | 0.48 |
| Charlson Comorbidity Index, median (IQR) | 3 (2–5) | 3 (1–4) | 5 (3.25–6) | 0.0001 |
| Prior (30-d) infections*, n (%) | 13 (6.4) | 4 (2.5) | 9 (19.5) | 0.0003 |
| Prior (30-d) chemotherapy, n (%) | 2 (0.9) | 1 (0.6) | 1(2.1) | 0.4 |
| Prior (30-d) hospitalization, n(%) | 27 (13.4) | 17 (10.9) | 10 (21.7) | 0.24 |
| Central line catheter, n (%) | 12 (5.9) | 3 (1.9) | 9 (19.5) | 0.0001 |
| Total parental nutrition, n (%) | 12 (5.9) | 3 (1.9) | 9 (19.5) | 0.0001 |
| Severe COVID-19**, n (%) | 106 (52.7) | 76 (49) | 30 (66) | 0.06 |
| First PaO2/FiO2 ratio at ED admission, median (IQR) | 300 (248–333) | 305 (252–338) | 271 (213–320) | 0.06 |
| Transferred from ICU, n (%) | 2 (0.9) | 1 (0.6) | 1(2.1) | 0.4 |
| Symptoms at COVID onset, n (%) | ||||
| Fever | 143 (59) | 112 (72) | 31 (67.3) | 0.57 |
| Cough | 66 (32) | 59 (38) | 7 (15.2) | 0.039 |
| Dyspnea | 114 (56) | 86 (55.4) | 28 (60.8) | 0.6 |
| Laboratory parameters at admission | ||||
| Leucocytes (× 103/mm3), median (IQR) | 7050 (4905–10,590) | 7110 (5120–10,150) | 6900 (4570–11,410) | 0.9 |
| Neutrophils (× 103/mm3), median (IQR) | 5585 (3660–8990) | 5580 (3660–8640) | 5610 (3690–9640) | 0.79 |
| Lymphocyte (× 103/mm3), median (IQR) | 760 (532,5–1020) | 760 (540–1040) | 690 (490–990) | 0.49 |
| Platelet (× 103/mm3), median (IQR) | 195 (156.25–256.75) | 196 (1257–256) | 190 (151–270) | 0.98 |
| Creatinine, median (IQR) mg/dl | 1 (0.8–1.2) | 0.9 (0.8–1.1) | 1.1 (0.9–1.4) | 0.075 |
| Albuminemia, median (IQR) g/dl | 3.7 (3.5–4) | 3.7(3.5–4) | 3.7 (3.1–3,9) | 0.9 |
| D-Dimer, median (IQR) U/l | 760 (473.2–1369.75) | 665.5 (431–1102) | 1281 (756–2335) | 0.0001 |
| CRP, median (IQR) mg/dl | 5.4 (1.7–10) | 5.5 (2–10.3) | 5.7 (1.2–10.4) | 0.65 |
| MDR colonization, n (%) | 23 (11) | 5 (3.2) | 18 (39) | 0.0001 |
| Long of in-hospital stay, median (IQR), days | 18 (12–26) | 16 (11–22) | 30 (19–45) | 0.001 |
| Prior antibiotic therapy (30-d), n (%) | 162 (80.5) | 116 (74) | 46 (100) | 0.0001 |
| Transfer to ICU | 12 (5.9) | 5 (3.2) | 7 (15.2) | 0.068 |
| Corticosteroid therapy, n (%) | 195 (97) | 149 (96.1) | 46 (100) | 0.33 |
| Mortality, n (%) | 61 (30.3) | 35 (22) | 26 (56) | 0.0001 |
| Pronation, n (%) | 17 (8.5) | 15 (9.7) | 2 (4.3) | 0.254 |
| Sedation, n (%) | 16 (8) | 10 (6.4) | 6 (13) | 0.155 |
| Respiratory failure treatment, n (%) | 0.008 | |||
| HFNC | 25 (12.4) | 19 (12.2) | 6 (13) | |
| Venturi mask | 51 (25.4) | 41 (26.4) | 10 (21.7) | |
| Helmet CPAP | 104 (51.7) | 85 (54.8) | 19 (41.3) | |
| NIMV | 21 (10.4) | 10 (6.4) | 11 (23.9) | |
| Superinfections episodes°, n (%) | 64 (100) | NA | 64 (100) | NA |
| Type of superinfections episodes, n (%) | ||||
| Primary bloodstream infection | 23 (35.9) | NA | 23 (35.9) | NA |
| Hospital-acquired pneumonia | 19 (29.6) | NA | 19 (29.6) | NA |
| Urinary infections | 18 (28.5) | NA | 18 (28.5) | NA |
| CR-BSI | 2 (3.1) | NA | 2 (3.1) | NA |
| Skin and soft tissues infections | 1 (1.5) | NA | 1 (1.5) | NA |
| Clostridoides difficile colitis | 1 (1.5) | NA | 1 (1.5) | NA |
Bold is used to emphasize relevant p values
COPD: chronic obstructive pulmonary disease; ED: Emergency department; CRP: C-reactive protein; MDR: multi-drug resistant; ICU: intensive care unit; CR-BSI: catheter-related bloodstream infection. NA: not applicable. 30-d: 30 days before infection development
*Prior (30-d) infections referred to infections diagnosed within 30 days before hospital admission
**Severe COVID-19 was defined according to WHO definitions [17]
°Superinfections episodes: the total number is different because several patients experienced > 1 infective episode (46 patients for a total of 64 episodes). HFNC: High Flow Nasal Cannula; CPAP: Continuous Positive Airway Pressure; NIMV: Non-invasive mechanical ventilation
The majority of patients (156, 77%) was admitted to our sub-intensive care unit directly from the emergency room, 43 (21%) from ordinary wards because of respiratory worsening and only 2 (0.9%) from ICU. Twelve patients (5.9%) were transferred to ICU. Co-infections were 4 (1.9%), all due to Mycoplasma pneumoniae whereas 46 patients (22%) developed a superinfection. Overall mortality rate was 30%.
Study population was further divided into two groups according to the development of superinfection [Infection (n = 46, 22%) and No-infection (n = 155, 78%) groups, respectively].
Comparison between survivors and non-survivors
As shown in Table 2, patients who died presented higher prevalence of comorbidities, and, consequently, a higher CCI than survivors (p = 0.0001). Moreover, non-survivors presented a higher percentage of infection in last 30-d before sub-intensive care unit admission (14.9% vs 2.8%, p = 0.036), severe COVID-19 at presentation (72% vs 44%, p = 0.004) and colonization by MDR pathogens (24% vs 5.7%, p = 0.004). At hospital admission, non-survivors presented a lymphocytes count, platelets count and albuminemia significantly lower (p = 0.001, 0.004 and 0.0033, respectively) and C-reactive protein, D-dimer, and creatinine significantly higher than patients who survived (p = 0.02, 0.0022 and 0.02, respectively). The development of superinfections was more common in non-survivors (42% vs 14%, p = 0.0001). Likewise, sedation use was more frequent in non-survivors (22% vs 2.1%, p < 0.0001). Non-survivors were treated more frequently with NIMV and less commonly with venturi mask (29.5% vs 2.1% and 9.8% vs 32%, respectively).
Table 2.
Characteristics of survivors and non-survivors
| Variable | Non-survivors N = 61 |
Survivors N = 140 |
p value |
|---|---|---|---|
| Age, median (IQR), years | 82 (73–87) | 61 (53–72) | 0.0001 |
| Sex (M), n (%) | 36 (59.4) | 91 (65) | 0.43 |
| Demographics | |||
| Chronic steroid/immunosuppressive therapy, n (%) | 4 (6.5) | 13 (9.2) | 0.59 |
| Diabetes, n (%) | 19 (31.1) | 26 (18.5) | 0.0008 |
| Chronic ischemic heart disease, n (%) | 27 (44) | 16 (11.4) | 0.0001 |
| Hypertension, n (%) | 37 (60.6) | 56 (40) | 0.008 |
| Heart failure, n (%) | 16 (26.2) | 5 (3.5) | 0.0001 |
| Atrial fibrillation, n (%) | 17 (27.8) | 7 (5) | 0.0001 |
| Vasculopathies, n (%) | 15 (24.5) | 4 (2.8) | 0.0001 |
| COPD, n (%) | 12 (19.6) | 18 (12.8) | 0.28 |
| Solid malignancy, n (%) | 12 (19.6) | 5 (3.5) | 0.0001 |
| Hematological malignancy, n (%) | 1 (1.6) | 6 (4.2) | 0.99 |
| Chronic kidney disease, n (%) | 7 (11.4) | 6 (4.2) | 0.01 |
| Obesity (BMI > 30), n (%) | 7 (11.4) | 24 (17.1) | 0.39 |
| Charlson CI, median (IQR) | 5 (4–6) | 2 (1–4) | 0.0001 |
| Prior (30-d) infections°, n (%) | 9 (14.7) | 4 (2.8) | 0.0036 |
| Prior hospitalization, n (%) | 14 (22.8) | 13 (9.2) | 0.0129 |
| Central line catheter, n (%) | 9 (14.7) | 3 (2.1) | 0.0009 |
| Total parental nutrition, n (%) | 10 (16.3) | 2 (1.4) | 0.0001 |
| Severe COVID, n (%) | 44 (72.1) | 62 (44.2) | 0.004 |
| PaO2/FiO2 ratio, median (IQR) | 248 (210–310) | 310 (271–343) | 0.0001 |
| Transfer from ICU, n (%) | 0 (0) | 2 (1.4) | 0.345 |
| Transfer to ICU, n (%) | 10 (16.4) | 2 (1.4) | < 0.0001 |
| Prior (30-d) antibiotic therapy, n (%) | 54 (88.5) | 108 (77.1) | 0.08 |
| MDR colonization, n (%) | 15 (24.5) | 8 (5.7) | 0.0004 |
| Superinfection, n (%) | 26 (42.6) | 20 (14.2) | 0.0001 |
| Corticosteroid therapy for SARS-CoV2, n (%) | 61 (100) | 134 (95) | 0.18 |
| Pronation, n (%) | 5 (8.2) | 12 (8.6) | 0.930 |
| Sedation, n (%) | 13 (22.0) | 3 (2.1) | < 0.0001 |
| Respiratory failure treatment, n (%) | |||
| HFNC | 7 (11.5) | 18 (12.9) | < 0.0001 |
| Venturi mask | 6 (9.8) | 45 (32.1) | |
| CPAP | 30 (49.2) | 74 (52.9) | |
| NIMV | 18 (29.5) | 3 (2.1) | |
| Laboratory parameters at admission | |||
| Leucocytes (× 103/mm3) median (IQR) | 6865 (4485–10,732) | 7050 (5072–9547) | 0.85 |
| Neutrophile (× 103/mm3) median (IQR) | 5535 (3682–9890) | 5585 (3607–8275) | 0.53 |
| Lymphocyte (× 103/mm3) median (IQR) | 555 (427–875) | 840 (642–1077) | 0.0001 |
| Platelet (× 103/mm3) median (IQR) | 171 (138–232) | 198,5 (163–261) | 0.04 |
| Creatinine, median (IQR) mg/dl | 1.2 (0.86–1.5) | 0.9 (0.8–1.09) | 0.02 |
| Albumin, median (IQR) g/dl | 3.6 (3.3–3.8) | 3.8 (3.5–4) | 0.0033 |
| D-dimer, median (IQR) U/l | 1179 (735–1894) | 624 (433–1109) | 0.0022 |
| CRP, median (IQR) mg/dl | 8,375(3.3–13) | 4.2 (1.4–9) | 0.02 |
Bold is used to emphasize relevant p values
COPD: chronic obstructive pulmonary disease; Charlson CI: Charlson Comorbidity Index; P/F ratio: PaO2/FiO2 ratio; CRP: C-reactive protein; MDR: multi-drug resistant; ICU: intensive care unit. 30-d: 30 days before infection development. HFNC: High Flow Nasal Cannula; CPAP: Continuous Positive Airway Pressure; NIMV: Non-invasive mechanical ventilation. °: Prior (30-d) infections referred to infections diagnosed within 30 days before hospital admission
At multivariable analysis, independent risk factors for in-hospital mortality were the development of superinfections [OR 3.04 (1.16–7.96), p = 0.024], age > 65 years [OR 5.25 (1.36–19.84), p = 0.014], CCI > 5 [OR 4.81 (1.81–12.71), p = 0.002], lymphocytes count < 750/mmc [OR 3.08 (1.21–7.84), p = 0.018], use of sedation [OR 6.68 (1.36–32.71), p = 0.019] and NIMV [OR 11.81 (2.04–68.16), p = 0.002] (Table 3).
Table 3.
Multivariate analysis of factors associated with in-hospital mortality
| OR (95% CI) | p value | |
|---|---|---|
| Superinfections | 3.04 (1.16–7.96) | 0.024 |
| Age > 65 years | 5.25 (1.36–19.84) | 0.014 |
| Severe COVID-19 | 2.09 (0.83–5.26) | 0.116 |
| CCI > 5 | 4.81 (1.81–12.71) | 0.002 |
| Lymphocytes < 750/mmc | 3.08 (1.21–7.84) | 0.018 |
| Sedation | 6.68 (1.36–32.71) | 0.019 |
| Respiratory failure treatment | ||
| Venturi mask (ref) | 1 | – |
| HFNC | 2.38 (0.49–11.60) | 0.281 |
| CPAP | 1.92 (0.54–6.78) | 0.308 |
| NIMV | 11.81 (2.04–68.16) | 0.006 |
Bold is used to emphasize relevant p values
CCI: Charlson Comorbidity Index, HFNC: High Flow Nasal Cannula, CPAP: Continuous Positive Airway Pressure, NIMV: Non-invasive mechanical ventilation
Comparison between infection and no-infection groups
Patients with superinfections presented a higher rate of severe COVID-19 on admission, albeit not significant (66% vs 49%, p = 0.6), were older [77 (65–83) vs 66 (55–77) years, p = 0.0005] and had a higher rate of diabetes, chronic ischemic heart disease, chronic heart failure, atrial fibrillation and malignancy, with a higher Charlson Comorbidity Index (CCI) (p = 0.0001) (Table1). The number of patients coming from or transferred to the ICU did not differ between the two groups.
Chronic corticosteroids therapy (17% vs 5%, p = 0.029) and antibiotic exposure in the previous 30-d before superinfections development (100% vs 74%, p = 0.0001) were more common in the Infection group. Given the severity of COVID-19 pneumonia, corticosteroid treatment was similar in both groups (100% vs 96.1%, p = 0.33). Colonization with MDR pathogens was higher in the group of patients with super-infections (39% vs 3.2%, p = 0.0001). No differences were observed for pronation and sedation use in the two groups, whereas NIMV was more frequent in patients developing superinfections (23.9% vs 6.4%).
Mortality rate was higher in patients with superinfections (56% vs 22%, p = 0.0001). Furthermore, they presented a longer in-hospital stay [30 (19–45) vs 16 (11–22) days, p = 0.001].
At multivariable analysis, prior (30-d) infection [OR 3.95 (1.00–15.7), p = 0.05] and exposure to antibiotic therapy in last 30-d [OR 4.82 (1.28–18.1), p = 0.020] were independent risk factors for new onset superinfections development (Table 4).
Table 4.
Multivariate analysis evaluating independent risk factors associated with the onset of superinfections
| OR (95% CI) | p value | |
|---|---|---|
| Age > 65 years | 1.6 (0.60–4.56) | 0.322 |
| Charlson CI > 5 | 2.28 (0.89–5.83) | 0.084 |
| Prior infections (30-d)* | 3.95 (1.00–15.7) | 0.050 |
| Sedation | 1.58 (0.43–5.7) | 0.487 |
| Severe COVID-19 | 1.46 (0.66–3.25) | 0.346 |
| Prior antibiotic therapy (30-d)** | 4.82 (1.28–18.1) | 0.020 |
| Respiratory failure treatment | ||
| Venturi mask (ref) | 1 | – |
| HFNC | 1.27 (0.34–4.66) | 0.717 |
| Helmet CPAP | 0.69 (0.25–1.92) | 0.488 |
| NIMV | 2.12 (0.55–8.12) | 0.271 |
Bold is used to emphasize relevant p values
Charlson CI: Charlson Comorbidity Index; HFNC: High Flow Nasal Cannula; CPAP: Continuous Positive Airway Pressure; NIMV: Non-invasive mechanical ventilation
*Prior (30-d) infections referred to infections diagnosed within 30 days before hospital admission
**Prior (30-d) antibiotic exposure included the receival of antibiotic therapy in the 30 days before diagnosis of secondary infections
Subjects with superinfections
Overall, 46 patients (22%) developed superinfections, with the different causative pathogens shown in Supplementary Table 1. Several patients experienced > 1 infective episode and therefore we recorded 64 infections. Primary BSI was the most frequent superinfection with 23 episodes (35.9%), followed by pneumonia (29.6%), urinary infections (28.5%), CR-BSI (3.1%), skin/soft tissue infections and Clostridioides difficile infection (1.5% each).
First infective episode was diagnosed after a median of 14.5 days (IQR 7–22) from sub-intensive unit admission. In 23 cases (40.6%), MDR pathogens were detected, with CR Acinetobacter baumannii (CR-Ab) isolated in 11 (47%) of them. Others MDR involved were MRSA, VRE and CR Klebsiella pneumoniae (CR-Kp) (6 cases, 21.5%; 4 cases, 17.3% and 2 cases, 13% respectively). All the strains of A. baumannii were resistant to meropenem; all but one exhibited colistin susceptibility. Cefiderocol susceptibility was available only for 6 strains; all the tested strains were susceptible at disk diffusion method (Supplementary Table2).
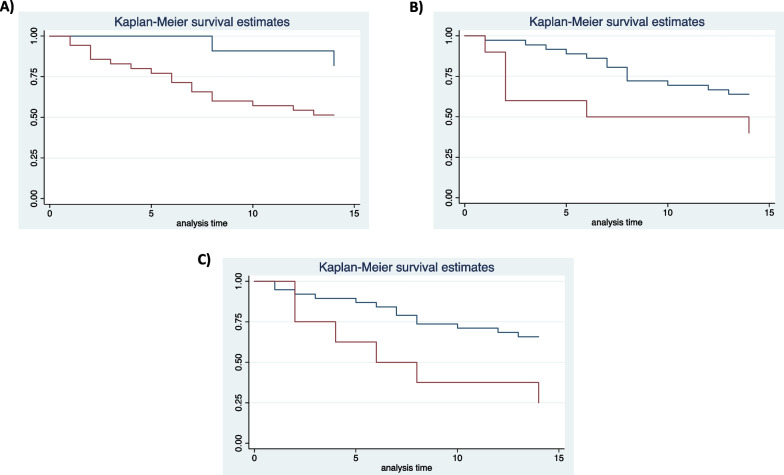
Kaplan–Meier survival curves at 14 days from the superinfection showed different rates of mortality in patients with age > 65 years, septic shock at infection onset and XDR A. baumannii as etiologic agents of first infective episode (Fig. 1a–c).
Fig. 1.
14-d survival estimates in patients with superinfections according to age (a age > 65-y, red line; age < 65-y blue line), severity of infection (b presence of septic shock, red line; absence of septic shock, blue line) and causative agent (c XDR-Ab, red line; pathogens other than XDR-Ab, blue line)
At multivariable analysis, only XDR Acinetobacter baumannii resulted independently associated with 14-d mortality [OR 5 (1.3–19.1), p = 0.018] (Table 5).
Table 5.
Multivariate analysis of risk factors associated with mortality at 14-d from infection onset
| HR (95% CI) | p value | |
|---|---|---|
| CCI > 5 | 1.1 (0.3–3.6) | 0.87 |
| Severe COVID | 1.5 (0.5–4.6) | 0.44 |
| Female sex | 0.2 (0.05–0.7) | 0.021 |
| Septic shock | 2 (0.5–6.9) | 0.255 |
| First infective episode due to XDR A. baumannii | 5 (1.3–19.1) | 0.018 |
| Age > 65 years | 3.6 (0.5–22) | 0.162 |
Bold is used to emphasize relevant p values
CCI: Charlson Comorbidity Index; XDR: extended drug resistant
Discussion
This study presents the prevalence and the clinical impact of co-infections and superinfections in COVID-19 patients hospitalized in a respiratory sub-intensive care unit. We showed that (i) co-infections were uncommon while the development of superinfections occurred in almost one quarter of patients and were independently associated with mortality; (ii) MDR organisms were isolated in approximately 40% of patients, with CR-Ab accounting for almost half of cases and (iii) CR-Ab as the causative agent of the first infective episode was associated with early (14-d) mortality.
To the best of our knowledge, the prevalence and characteristics of superinfections in COVID-19 patients hospitalized in a respiratory sub-intensive care unit are not well understood and represent a significant knowledge gap in literature data.
Indeed, from the spread of SARS-CoV-2 pandemic, several studies reported the rates of bacterial, viral and/or fungal infections in COVID-19 patients, especially in ICU. A study conducted during the first pandemic wave reported a high rate of incidence and prevalence of co-infections during COVID-19 [24]. This argument seemed to be consistent with previously demonstrated association between other viral infections, such as influenza, and bacterial co-infections, especially with Staphylococcus aureus [25], thus justifying the use of antimicrobials. Under these circumstances, in the clinical practice also COVID-19 patients had been routinely treated with empirical antibiotic therapy, especially in case of critical illness during 2020.
Nevertheless, according to the following literature [11–26] co-infections during COVID-19 were considered uncommon. Our report confirmed a low rate of bacterial co-infection (1.9%), with Mycoplasma pneumoniae as the etiologic agent in all the cases, although concerns were raised about diagnosing M. pneumoniae infection in COVID-19 patients only by means of serology diagnostic testing. These data are in accordance with our previous data collection referred to the first pandemic wave in which Mycoplasma pneumoniae was responsible for the 1.1% of co-infections [27] and sustain, in line with the literature and according to antimicrobial stewardship principles, [28] the possibility of an antibiotic sparing approach in the early phase of COVID-19 pneumonia.
Overall, 46 patients (22%) developed superinfections. Our data seems in line with literature [29, 30] in terms of development of superinfections. Indeed, recent reports showed an overall prevalence of bacterial superinfections of 16% in COVID-19 patients [31], ranging from 4.8 to 42.8%, with the highest prevalence in critically ill patients.
Chong et al. reported an overall time to diagnose pulmonary superinfections of 10 days (2–21 days) from initial hospitalization and 9 days (4–18 days) after ICU admission [32]. Likewise, in our study, the first infective episode was diagnosed after a median of 14.5 days from sub-intensive unit admission.
COVID-19 severity could in part explain the association between critical conditions and superinfections. Ripa M et al. concluded that factors associated with superinfections were low baseline lymphocyte count, baseline P/F ratio, and ICU admission [33], while Falcone and colleagues showed that invasive mechanical ventilation, carbapenem-resistant Enterobacterales (CRE) intestinal colonization, immunomodulatory agents (tocilizumab/baricitinib), high C-reactive protein on admission and previous treatment with piperacillin/tazobactam were risks factors for bacterial and fungal superinfections [14]. We confirmed the association between superinfections development and a previous exposure to antibiotic therapy and prior infection. Conversely, although NIMV was more frequent in patients developing superinfections, at multivariable analysis the mode of respiratory failure treatment did not predict superinfections in our population.
We reported an overall mortality rate of 30%, with a higher rate in patients with superinfections, especially when sustained by CR-Ab. The development of superinfections represented an independent risk factor for in-hospital mortality, together with age > 65 years, CCI > 5, severe COVID-19 and a count of lymphocytes < 750/mmc on admission. In a previous study conducted in 24 ICUs, trends of clinical, ventilatory and laboratory parameters throughout the entire ICU stay showed different slopes between survivors and non-survivors [34]. Furthermore, in our study sedation and NIMV were associated with mortality. This data could be explained considering that sedation and NIMV were used when other treatments (i.e., Helmet CPAP) failed or respiratory failure worsened despite Helmet CPAP treatment, suggesting a more severe respiratory condition. As a matter of fact, in most cases patients treated with NIMV and sedation presented severe ARDS.
As reported for patients hospitalized in ICU [31–35], in the present report MDR pathogens including MRSA, VRE, CR-A. baumannii and CR-K. pneumoniae were detected in 40.6% of cases, with CR-Ab, a common colonizer in the ICU setting, isolated in almost half of cases. This prevalence could be explained by considering that patients hospitalized in our sub-intensive unit presented several risk factors usually associated with A. baumannii infections, such as prolonged hospitalization, need of intensive care, presence of devices, older age, prior colonization, and prior use of antibiotics [36].
MDR in Gram-negative bacilli represent a major threat to human health and significantly contribute to global deaths attributable to and associated with bacterial antimicrobial resistance [37]. With this regard, superinfections caused by MDR Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii have been increasingly reported during the hospital stays of patients with severe COVID-19 [38].
Indeed, a recent study by Falcone and colleagues during the first pandemic wave in Italy (March–April 2020) showed that MDR organisms caused 65.1% of superinfections, slightly higher than our report. Interestingly, the rate of MDR infections increased during the hospital stay, from 50% in the first 7 days up to 70.4% if hospitalized for > 30 days. Although the majority of MDR organisms were represented by CRE, the increase during hospitalization mostly regarded non fermenters such as P. aeruginosa, A. baumannii and S. maltophilia [14].
These findings are in line with other reports showing that more than 60% of patients with COVID-19 who had a bacterial infection carried a highly resistant organism [18]. Furthermore, emerging data from the United States Centers for Disease Control and Prevention suggests that pandemic has resulted in rising rates of antimicrobial resistance, including CR-Ab [39].
More recently, national Italian data evaluating superinfections caused by CRE in hospitalized patients with COVID-19 from March to December 2020 found that the majority of infections occurred in the ICU, although almost one third of episodes occurred in medical wards; however, no data on sub-intensive respiratory units was present. Thirty-day mortality was 33.3% [40].
In a previous study, we evaluated the impact of COVID-19 on MDR Gram-negatives BSIs in a single ICU: while before pandemic patients were more likely to present CR-Kp BSIs, after COVID-19 CR-Ab BSIs were more commonly observed [41]. Furthermore, CR-Ab colonization was associated with MDR A. baumannii infection in COVID-19 patients [13].
Many hospitals have reported MDR outbreaks in ICU during the COVID-19 pandemic, mostly caused by Gram-negative bacteria or Candida auris [42]. Carbapenem-resistant Acinetobacter baumannii seems to be the main pathogen involved, with several outbreaks reported worldwide [42, 43].
Another Italian study compared colonization and infection rates before and after COVID-19; authors found that in the COVID-19 period, the incidence rate ratios of colonization and infection with CR-Ab increased 7.5- and 5.5-fold, respectively. Genome sequencing showed that all CR-Ab strains belonged to the CC92/IC2 clonal lineage [44]
More recently, a systematic review and meta-analysis analyzed the impact of the COVID-19 pandemic on MDR organisms across different healthcare settings by providing data on antimicrobial resistance before or during the COVID-19 pandemic. Authors found that the pandemic was not associated with a change in the incidence or proportion of MRSA or VRE; conversely, although not significant, an increase of resistant Gram-negatives (i.e., ESBL, CRE, MDR or carbapenem-resistant Pseudomonas aeruginosa or Acinetobacter baumannii) was observed, particularly in settings where enhanced infection control policies and/or antimicrobial stewardship programs were not reported. Furthermore, there was a small increase in the proportion of infections due to resistant Acinetobacter spp [18].
The apparent increase in incidence of Gram-negative, but not Gram-positive, antimicrobial resistance suggests that antibiotic prescription may play an important role in selecting MDR organisms, especially in the light of the high use of beta-lactam/beta-lactamase inhibitors and third generation cephalosporins in patients with COVID-19 [18].
In most of the reported cases of MDR A. baumannii infections in COVID-19 patients, high rates of mortality were observed [13, 45]. Our data confirm this trend; in fact, 75% of patients affected by a CR-Ab superinfections died, and CR-Ab was independently associated with 14-d mortality.
In our study, MRSA was detected in 6/64 (9.4%) infective episodes, mostly represented by BSIs. Our results are in line with those recently observed by Falcone et al., who found that MRSA accounted for 5.5% of superinfections episodes [14]. Conversely, these data are lower than the reported percentage of S. aureus isolates (including those resistant to methicillin) in patients with severe influenza, which accounted for up to 22–28% of total cases of pulmonary co- and superinfections and were associated with a high mortality rate [4, 46].
The low percentage of pulmonary Aspergillosis (1.9%) is in line with the data recently observed in our hospital context, where we found COVID-19 associated pulmonary aspergillosis (CAPA) in only a minority of ICU patients [47]. The incidence of CAPA is widely variable, ranging from less than 1% to more than 30% in critically ill patients [48, 49]; nevertheless, most data refer to the ICU setting. Accordingly, there is the need to elucidate the prevalence of this condition even in different settings, such as ours.
Our study presents some limitations: (i) this is a single-center retrospective observational study with a limited number of patients, that does not include all hospitalized patients; (ii) patients usually received empiric antibiotic therapy at admission, so that the prevalence of co-infections could be possibly underestimated, even if administering antibiotics was a common practice worldwide in that phase of pandemic and we reported similar prevalence of literature; (iii) lack of control group, such as COVID-19 patients hospitalized in ordinary wards or ICUs or patients without COVID-19; (iv) we were unable to investigate with specific microbiological analyses whether the isolation of CR-Ab was a cluster. Moreover, we developed this study in an emergency setting in the middle phase of the second wave, so we should acknowledge that the hospital surge during the pandemic may have affected patients’ outcome.
Nevertheless, our study offers important explanations on the prevalence and clinical impact of superinfections in COVID-19 patients hospitalized in a highly intensive care setting other than ICU. Accordingly, we believe that additional studies specifically focused on respiratory sub-intensive care units are warranted, in order to elucidate similitudes and differences, if any, between this special setting and the ICU.
Conclusion
In conclusion, we showed that in a COVID-19 respiratory sub-intensive care unit, superinfections were common and represented an independent predictor of mortality. Conversely, co-infections had a low prevalence, supporting an antibiotic sparing strategy in the first phase of SARS-CoV-2 infection. Indeed, exposure to antibiotic therapy was independently associated with superinfections’ development. MDR organisms were found in almost half of patients with superinfections, and CR-Ab as the causative agent was associated with high mortality. For all these reasons, infection control rules and antibiotic stewardship programs represent critical points also in sub-intensive care units to limit the spread of MDR organisms.
Supplementary Information
Additional file 1. Supplementary Table 1. Pathogens isolated for type and site of infection. Supplementary Table 2. Antimicrobial susceptibility testing of the 11 CR-Ab isolates causing superinfections.
Acknowledgements
Authors thank all the nurse staff for their assistance to COVID-19 patients.
Abbreviations
- ICU
Intensive Care Unit
- MDR
Multidrug-resistant bacteria
- ARDS
Acute respiratory distress syndrome
- ABGs
Arterial blood gases
- CPAP
Continuous positive airway pressure
- CVC
Central venous access
- NIMV
Non-invasive mechanical ventilation
- XDR
Extensively drug-resistant pathogens
- HFNC
High flow nasal cannula
- MRSA
Methicillin-resistant Staphylococcus aureus
- VRE
Vancomycin-resistant Enterococcus faecium
- CR
Carbapenem resistant
- TBA
Tracheobronchial aspirate
- GM
Galactomannan
- IQR
Interquartile ranges
- CCI
Charlson Comorbidity Index
- CAPA
COVID-19 associated pulmonary aspergillosis
Author contributions
Conception and design of study: AI, AO, PP. Data acquisition: AI, GS, AT, DP. Analysis and data interpretation: AO. Drafting and revision of the manuscript: AI, AO, MV, CMM and PP. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Data are available upon request from corresponding author.
Declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki. The study was approved by the local Ethics Committee of Azienda Ospedaliera Universitaria Policlinico Umberto I, Rome (ID Prot. 109/2020). The need for written informed consent was waived by the local Ethics Committee of Azienda Ospedaliera Universitaria Policlinico Umberto I due to retrospective nature of the study. However, at hospital admission, all the patients provided general consent for the use of their clinical data in future studies.
Consent for publication
Not applicable.
Competing interests
Authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alessandra Iacovelli and Alessandra Oliva have contributed equally as first author.
References
- 1.WHO—World Health Organization. https://covid19.who.int/.
- 2.Bartoletti M, Azap O, Barac A, Bussini L, Ergonul O, Krause R, Paño-Pardo JR, Power NR, Sibani M, Szabo BG, Tsiodras S, Verweij PE, Zollner-Schwetz I, Rodríguez-Baño J. ESCMID COVID-19 living guidelines: drug treatment and clinical management. Clin Microbiol Infect. 2022;28(2):222–238. doi: 10.1016/j.cmi.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold FW, Fuqua JL. Viral respiratory infections: a cause of community-acquired pneumonia or a predisposing factor? Curr Opin Pulm Med. 2020;26(3):208–214. doi: 10.1097/MCP.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 4.Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;23(8):1041. doi: 10.3389/fmicb.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J. COVID-19 scientific advisory group rapid response report 2020; May 6, 2020. Alberta Health Services.
- 8.WHO. Clinical management of COVID-19. World Health Organization. Interim guidance 27 May, 2020.
- 9.Bengoechea JA, Bamford CG. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020;12(7):e12560. doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–40. 10.1016/0196-6553(88)90053-3. Erratum in: Am J Infect Control 1988;16(4):177. [DOI] [PubMed]
- 11.Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, Soucy JR, Daneman N. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel A, Emerick M, Cabunoc MK, Williams MH, Preas MA, Schrank G, Rabinowitz R, Luethy P, Johnson JK, Leekha S. Rapid spread and control of multidrug-resistant gram-negative bacteria in COVID-19 patient care units. Emerg Infect Dis. 2021;27(4):1234–1237. doi: 10.3201/eid2704.204036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo A, Gavaruzzi F, Ceccarelli G, Borrazzo C, Oliva A, Alessandri F, Magnanimi E, Pugliese F, Venditti M. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalized in intensive care unit. Infection. 2022;50(1):83–92. doi: 10.1007/s15010-021-01643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falcone M, Tiseo G, Giordano C, Leonildi A, Menichini M, Vecchione A, Pistello M, Guarracino F, Ghiadoni L, Forfori F, Barnini S, Menichetti F, Pisa COVID-19 Study Group Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021;76(4):1078–1084. doi: 10.1093/jac/dkaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deana C, Rovida S, Orso D, Bove T, Bassi F, De Monte A, Vetrugno L. Learning from the Italian experience during COVID-19 pandemic waves: be prepared and mind some crucial aspects. Acta Biomed. 2021;92(2):e2021097. doi: 10.23750/abm.v92i2.11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.WHO—World Health Organization. https://www.who.int/publications/i/item/clinical-care-of-severe-acute-respiratory-infections-tool-kit.
- 18.Langford BJ, So M, Simeonova M, Leung V, Lo J, Kan T, Raybardhan S et al. Antimicrobial resistance in patients with COVID-19: a systematic review and meta-analysis. https://ssrn.com/abstract=4099404 or 10.2139/ssrn.4099404. [DOI] [PMC free article] [PubMed]
- 19.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 20.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, Klimko N, Lass-Flörl C, Oladele RO, Vinh DC, Zhu LP, Böll B, Brüggemann R, Gangneux JP, Perfect JR, Patterson TF, Persigehl T, Meis JF, Ostrosky-Zeichner L, White PL, Verweij PE, Cornely OA. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Centre for Disease Prevention and Control. EU case definitions. https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions. Retrieved 15 Dec 2022.
- 22.Centers for Disease Control and Prevention website. National Healthcare Safety Network (NHSN). Bloodstream Infection (BSI) Events. Available online: https://www.cdc.gov/nhsn/psc/bsi/index.html. Retrieved 15 Dec 2022.
- 23.Cleri DJ, Corrado ML, Seligman SJ. Quantitative culture of intravenous catheters and other intravascular inserts. J Infect Dis. 1980;141:781–786. doi: 10.1093/infdis/141.6.781. [DOI] [PubMed] [Google Scholar]
- 24.Kreitmann L, Monard C, Dauwalder O, Simon M, Argaud L. Early bacterial co-infection in ARDS related to COVID-19. Intensive Care Med. 2020;46(9):1787–1789. doi: 10.1007/s00134-020-06165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice TW, Rubinson L, Uyeki TM, Vaughn FL, John BB, Miller RR, Higgs E, Randolph AG, Smoot BE, Thompson BT, NHLBI ARDS Network Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40(5):1487–1498. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, Fernandez-Pittol M, Pitart C, Inciarte A, Bodro M, Morata L, Ambrosioni J, Grafia I, Meira F, Macaya I, Cardozo C, Casals C, Tellez A, Castro P, Marco F, García F, Mensa J, Martínez JA, Soriano A, COVID-19 Researchers Group Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliva A, Siccardi G, Migliarini A, Cancelli F, Carnevalini M, D'Andria M, Attilia I, Danese VC, Cecchetti V, Romiti R, Ceccarelli G, Mastroianni CM, Palange P, Venditti M. Co-infection of SARS-CoV-2 with Chlamydia or Mycoplasma pneumoniae: a case series and review of the literature. Infection. 2020;48(6):871–877. doi: 10.1007/s15010-020-01483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 30.Pickens CO, Gao CA, Cuttica MJ, Smith SB, Pesce LL, Grant RA, Kang M, Morales-Nebreda L, Bavishi AA, Arnold JM, Pawlowski A, Qi C, Budinger GRS, Singer BD, Wunderink RG, NU COVID Investigators Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am J Respir Crit Care Med. 2021;204(8):921–932. doi: 10.1164/rccm.202106-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurra N, Woodard PI, Gandrakota N, Gandhi H, Polisetty SR, Ang SP, Patel KP, Chitimalla V, Ali Baig MM, Samudrala G. Opportunistic infections in COVID-19: a systematic review and meta-analysis. Cureus. 2022;14(3):e23687. doi: 10.7759/cureus.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong WH, Saha BK, Ramani A, Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021;49(4):591–605. doi: 10.1007/s15010-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ripa M, Galli L, Poli A, Oltolini C, Spagnuolo V, Mastrangelo A, Muccini C, Monti G, De Luca G, Landoni G, Dagna L, Clementi M, Rovere Querini P, Ciceri F, Tresoldi M, Lazzarin A, Zangrillo A, Scarpellini P, Castagna A, COVID-BioB study group Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2021;27(3):451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanella A, Florio G, Antonelli M, Bellani G, Berselli A, Bove T, Cabrini L, Carlesso E, Castelli GP, Cecconi M, Citerio G, Coloretti I, Corti D, Dalla Corte F, De Robertis E, Foti G, Fumagalli R, Girardis M, Giudici R, Guiotto L, Langer T, Mirabella L, Pasero D, Protti A, Ranieri MV, Rona R, Scudeller L, Severgnini P, Spadaro S, Stocchetti N, Viganò M, Pesenti A, Grasselli G, COVID-19 Italian ICU Network Time course of risk factors associated with mortality of 1260 critically ill patients with COVID-19 admitted to 24 Italian intensive care units. Intensive Care Med. 2021;47(9):995–1008. doi: 10.1007/s00134-021-06495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahceci I, Yildiz IE, Duran OF, Soztanaci US, KirdiHarbawi Z, Senol FF, Demiral G. Secondary bacterial infection rates among patients with COVID-19. Cureus. 2022;14(2):e22363. doi: 10.7759/cureus.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou H, Yao Y, Zhu B, Ren D, Yang Q, Fu Y, Yu Y, Zhou J. Risk factors for acquisition and mortality of multidrug-resistant Acinetobacter baumannii bacteremia: a retrospective study from a Chinese hospital. Medicine. 2019;98:e14937. doi: 10.1097/MD.0000000000014937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel A, Emerick M, Cabunoc MK, et al. Rapid spread and control of multidrug-resistant Gram-negative bacteria in COVID-19 patient care units. Emerg Infect Dis. 2021;27:1234–1237. doi: 10.3201/eid2704.204036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Centers for Disease Control and Prevention. COVID-19: U.S. impact on antimicrobial resistance, special report 2022 [Internet]. National Center for Emerging and Zoonotic Infectious Diseases; 2022 Jun [cited 2022 Aug 9]. https://stacks.cdc.gov/view/cdc/117915.
- 40.Falcone M, Suardi LR, Tiseo G, Galfo V, Occhineri S, Verdenelli S, Ceccarelli G, Poli M, Merli M, Bavaro D, Carretta A, Nunnari G, Venanzi Rullo E, Trecarichi EM, Papalini C, Franco A, Del Vecchio RF, Bianco V, Punzi R, Francisci D, Rubino R, Torti C, Puoti M, Carbonara S, Cascio A, Saracino A, Santantonio T, Venditti M, Menichetti F. Superinfections caused by carbapenem-resistant Enterobacterales in hospitalized patients with COVID-19: a multicentre observational study from Italy (CREVID Study) JAC Antimicrob Resist. 2022;4(3):dlac064. doi: 10.1093/jacamr/dlac064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cogliati Dezza F, Arcari G, Alessi F, Valeri S, Curtolo A, Sacco F, Ceccarelli G, Raponi G, Alessandri F, Mastroianni CM, Venditti M, Oliva A. Clinical impact of COVID-19 on multi-drug-resistant gram-negative bacilli bloodstream infections in an intensive care unit setting: two pandemics compared. Antibiotics (Basel) 2022;11(7):926. doi: 10.3390/antibiotics11070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thoma R, Seneghini M, Seiffert SN, Vuichard Gysin D, Scanferla G, Haller S, Flury D, Boggian K, Kleger G-R, Filipovic M, et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida Auris during the coronavirus disease 2019 pandemic: Report of a Carbapenem-Resistant Acinetobacter Baumannii Outbreak and a Systematic Review of the Literature. Antimicrob Resist Infect Control. 2022;11:12. doi: 10.1186/s13756-022-01052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottesman T, Fedorowsky R, Yerushalmi R, Lellouche J, Nutman A. An outbreak of carbapenem-resistant Acinetobacter baumannii in a COVID-19 dedicated hospital. Infect Prev Pract. 2021;3(1):100113. doi: 10.1016/j.infpip.2021.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pascale R, Bussini L, Gaibani P, Bovo F, Fornaro G, Lombardo D, Ambretti S, Pensalfine G, Appolloni L, Bartoletti M, Tedeschi S, Tumietto F, Lewis R, Viale P, Giannella M. Carbapenem-resistant bacteria in an intensive care unit during the coronavirus disease 2019 (COVID-19) pandemic: a multicenter before-and-after cross-sectional study. Infect Control Hosp Epidemiol. 2022;43(4):461–466. doi: 10.1017/ice.2021.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangel K, Chagas TPG, De-Simone SG. Acinetobacter baumannii infections in times of COVID-19 pandemic. Pathogens. 2021;10(8):1006. doi: 10.3390/pathogens10081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacquot A, Luyt CE, Kimmoun A, Levy B, Baux E, Fluvalentine Study group Epidemiology of post-influenza bacterial pneumonia due to Panton-Valentine leucocidin positive Staphylococcus aureus in intensive care units: a retrospective nationwide study. Intensive Care Med. 2019;45(9):1312–1314. doi: 10.1007/s00134-019-05665-3. [DOI] [PubMed] [Google Scholar]
- 47.Oliva A, Ceccarelli G, Borrazzo C, Ridolfi M, Ettorre G, Alessandri F, Ruberto F, Pugliese F, Raponi GM, Russo A, Falletta A, Mastroianni CM, Venditti M. Comparison of clinical features and outcomes in COVID-19 and influenza pneumonia patients requiring intensive care unit admission. Infection. 2021;49(5):965–975. doi: 10.1007/s15010-021-01624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitaka H, Kuno T, Takagi H, Patrawalla P. Incidence and mortality of COVID-19-associated pulmonary aspergillosis: a systematic review and meta-analysis. Mycoses. 2021;64(9):993–1001. doi: 10.1111/myc.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong WH, Neu KP. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): a systematic review. J Hosp Infect. 2021;113:115–129. doi: 10.1016/j.jhin.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Table 1. Pathogens isolated for type and site of infection. Supplementary Table 2. Antimicrobial susceptibility testing of the 11 CR-Ab isolates causing superinfections.
Data Availability Statement
Data are available upon request from corresponding author.