Abstract
Background
With ~ 50 million individuals suffering from post-COVID condition (PCC), low health related quality of life (HRQoL) is a vast problem. Common symptoms of PCC, that persists 3 months from the onset of COVID-19 are fatigue, shortness of breath and cognitive dysfunction. No effective treatment options have been widely adopted in clinical practice. Hyperbaric oxygen (HBO2) is a candidate drug.
Methods
The objective of this interim analysis is to describe our cohort and evaluate the safety of HBO2 for post covid condition. In an ongoing randomised, placebo-controlled, double blind, clinical trial, 20 previously healthy subjects with PCC were assigned to HBO2 or placebo. Primary endpoints are physical domains in RAND-36; Physical functioning (PF) and Role Physical (RP) at 13 weeks. Secondary endpoints include objective physical tests. Safety endpoints are occurrence, frequency, and seriousness of Adverse Events (AEs). An independent data safety monitoring board (DSMB) reviewed unblinded data. The trial complies with Good Clinical Practice. Safety endpoints are evaluated descriptively. Comparisons against norm data was done using t-test.
Results
Twenty subjects were randomised, they had very low HRQoL compared to norm data. Mean (SD) PF 31.75 (19.55) (95% Confidence interval; 22.60–40.90) vs 83.5 (23.9) p < 0.001 in Rand-36 PF and mean 0.00 (0.00) in RP. Very low physical performance compared to norm data. 6MWT 442 (180) (95% CI 358–525) vs 662 (18) meters p < 0.001. 31 AEs occurred in 60% of subjects. In 20 AEs, there were at least a possible relationship with the study drug, most commonly cough and chest pain/discomfort.
Conclusions
An (unexpectedly) high frequency of AEs was observed but the DSMB assessed HBO2 to have a favourable safety profile. Our data may help other researchers in designing trials.
Trial Registration
ClinicalTrials.gov: NCT04842448. Registered 13 April 2021, https://clinicaltrials.gov/ct2/show/NCT04842448. EudraCT: 2021-000764-30. Registered 21 May 2021, https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-000764-30/SE
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-023-08002-8.
Keywords: Long COVID, Post COVID condition, HRQoL, RCT, Clinical trial, Hyperbaric oxygen, HBOT, Safety
Background
With more than 500 million confirmed cases of COVID-19 and 10% of infected individuals suffering persistent symptoms, patient-reported low health related quality of life has become a vast problem for individuals, health care systems and society for years to come [1].
Post COVID condition (PCC), also known as Long COVID is commonly defined as having a history of probable or confirmed SARS-CoV-2 infection, and persistent symptoms 3 months from the onset of COVID-19 [2]. Common symptoms are fatigue, shortness of breath and cognitive dysfunction [3]. Mechanisms are still an enigma but suggested mechanisms include auto-immune disease such as dysregulated T-cell activation, chronic oxidative stress, mitochondrial dysfunction, and endothelial dysfunction [4].
No effective evidence-based treatment options for the underlying condition have been widely adopted in clinical practice and many patients seek expensive “remedies” for self-management [5]. Hyperbaric oxygen (HBO2) is a possibly effective drug but has not been evaluated for safety and efficacy in compliance with International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use-Good Clinical Practice (ICH-GCP) for PCC in clinical trials. It has been suggested to be effective in similar conditions such as Fibromyalgia and Chronic Fatigue syndrome [6, 7]. HBO2 has become increasingly popular off-label, a couple of case reports/series are published and RCTs are on the way [8–10]. Since the first submission of this manuscript one RCT including 73 subjects have shown an improvement of neurocognitive function and symptoms in PCC with 40 sessions of HBO2 at 2 Bar for 90 min with five-minute air breaks every 20 min [11]. Our hypothesis for use of HBO2 in PCC is based on the hyperoxic-hypoxic paradox with activation of Hypoxia Inducible Factor 1 and 2 (HIF-1 and HIF-2) and downstream regulation of hypoxia and inflammatory pathways [12, 13]. The rationale for using fewer and less frequent sessions is based on the above hypotheses, previous clinical experience and from experimental research including own unpublished data. The safety profile of HBO2 is well known for accepted indications but has not been described in compliance with ICH-GCP and is not an accepted treatment for patients diagnosed with PCC [14]. The aim of the interim analysis was to evaluate safety of HBO2 for our cohort by evaluating reported adverse events (AE) and serious adverse events (SAE) in compliance with ICH-GCP.
Methods
Trial design, setting, participants, and interventions
Prospective randomised, parallel arms, placebo-controlled, double blind, clinical trial at Karolinska University Hospital, Sweden.
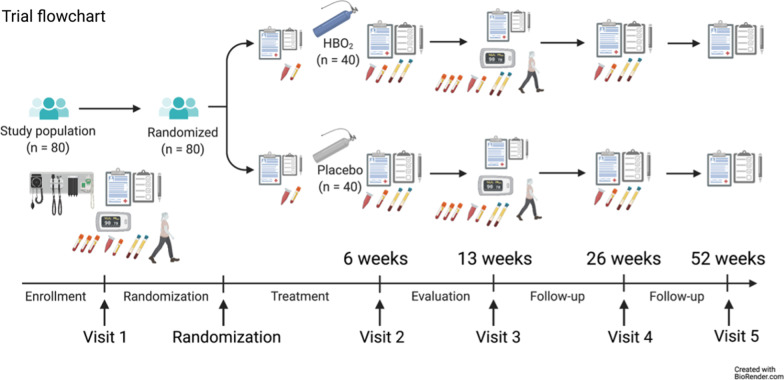
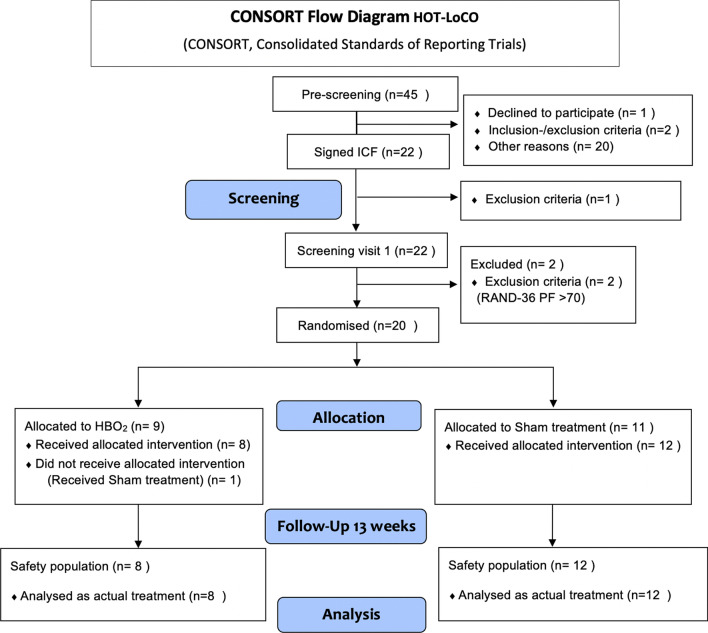
We plan to enroll 80 previously healthy subjects diagnosed with PCC (U09.9) randomised (1:1) to HBO2 or placebo (sham treatment), maximum ten treatments within 6 weeks from randomisation (Fig. 1). HBO2 was administered at 2.4 Bar for 90 min with two five minutes air breaks. Sham treatment with air was administered by increasing pressure to 1.35 Bar and then reduce to 1.2 Bar. All treatments were given in monoplace chambers (Sechrist, USA). The trial adheres to Consolidated Standards of Reporting Trials (CONSORT) guidelines [15]. The first subject was enrolled September 4 2021 and the interim safety analysis was conducted according to protocol when 20 subjects were followed up 13 weeks, April 28 2022. The CONSORT flow diagram shows the progress of the trial, allocation to treatment according to randomisation. One subject received the wrong allocation and has been removed from the group it guessed, this subject guessed HBO2 (Fig. 2). The protocol includes a detailed description and rationale for the primary and main secondary endpoints, including patient reported outcomes (PRO) in line with Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) SPIRIT-PRO Extension Guidelines [16]. The protocol is available with open access [10].
Fig. 1.
Trial flowchart of the HOT-LoCO trial
Fig. 2.
CONSORT Flow diagram of the Safety analysis
Randomisation and blinding
Eligible subjects were randomised in a 1:1 allocation, stratified by disease severity in relation to RAND-36 and gender in blocks (blinded to all study personnel) to either HBO2 or Placebo. A computer-based generated randomisation tool (randomizer.at) is used and only delegated staff specifically involved in the treatment and an unblinded monitor have access to the code. The placebo protocol is well established, and even experienced divers cannot differ between “sham treatment” and HBO2 [17].
Endpoints
Primary endpoints are physical domains Physical functioning (PF) and Role Physical (RP) in RAND-36 at 13 weeks. Secondary endpoints are the objective physical tests 6-min walk test (6MWT) and 30 s chair stand (CST), EQ-5D and Reactive hyperemia Index (RHI) at 13 weeks. Safety endpoints are occurrence, frequency, and seriousness of Adverse Events (AE) [10].
Statistical analysis
Safety endpoints are evaluated descriptively. The number and percentage of patients reporting AEs, and the number of AEs reported are presented. Listings with the events tabulated by system organ class and preferred term are available as supplementary material (Additional file 1).
The number of patients experiencing an AE compared descriptively between groups. All patients with AEs listed individually with subject number in addition to type of event, start and stop time, duration, seriousness, severity, action taken, relationship to trial drug and outcome of AE was presented to the members of the DSMB.
Baseline characteristics of the first 20 subjects are presented as mean ± standard deviation (SD) or number (n) and fraction (%) (Table 1).
Table 1.
Demographic characteristics for the safety analysis cohort (N = 20)
| Female sex | 18 (90%) |
| Body mass index (BMI) | 23.3 (7.27) |
| Ex-smoker | 4 (20%) |
| Never smoker | 16 (80%) |
| Work/study before COVID-19 (%) | 95.25 (17.13) |
| Work/study at baseline (%) | 25.0 (36.27) |
| Education post 2nd, 3 years or more | 17 (85%) |
| Physical activity (min/week) | 145.5 (128.2) |
| Fully vaccinated | 13 (65%) |
| Months since COVID-19 onset | 17.15 (1.599) |
| Positive SARS-CoV-2 antibody | 8 (40%) |
Baseline data is compared with available norm data from reference populations in Sweden for RAND-36 and EQ-5D and published international reference values for 6MWT and 30 s CST [18]. For RAND-36 the mean of a Swedish reference group with mean age of 57 (20.1) (n = 3422) has been used without adjustment for age and sex [19]. For comparison of EQ-5D the mean of age and sex matched reference values have been used. For RHI no age and sex matched reference data was found and therefore compared with a healthy reference population with mean age 48 [14, 20]. Statistical data analyses and graphs were performed using GraphPad Prism 8.4.3.
All data for efficacy continuous endpoints at baseline are presented using mean, SD, and 95% confidence interval. Comparisons against norm data was done using unpaired t-test.
No adjustment for multiplicity was done since results are descriptive. A p value < 0.05 was considered statistically significant. All reported p values are two-sided. All bar graphs are presented as mean and CI. Significant difference I presented as: *p < 0.05; **p < 0.01; ***p < 0.001.
Safety and adverse events
Collection of Adverse events (AE) and Serious Adverse Events (SAE) data was started directly after inclusion and recorded until Visit 3. Only SAE was collected outside the treatment period (after Visit 2). Ongoing AE and SAE at the end of Visit 3 will be followed up during long-term follow-up until the subject’s last visit. The definition, handling, follow-up, and reporting of AEs are defined in the original protocol (pp. 34–38). The safety endpoints were evaluated by an independent Data Safety Monitoring Board (DSMB) in the context of the trial design and currently existing information about Long COVID and HBOT. The DSMB is composed of three experts in their respective disciplines of medicine, clinical trial methodology and conduct. The DSMB reviewed the data at the predetermined interim analysis of 20 subjects with safety data available. A charter delineating their guidelines for operating and rules for terminating individual subjects, a portion of or the full trial prematurely was drawn up and agreed upon before the trial started. The members of the DSMB, meeting plan and responsibilities are specified in the original protocol (pp. 6 and 44). The DSMB meeting consists of two parts: An open part where the principal investigator and monitor summarised current status and experience form the trial. During the second, closed part, only the DSMB members discussed safety data (Additional file 2, protocol from DSMB meeting). The DSMB-members had access to data one week before the meeting and had a dialogue with the senior statistician. All study personnel that participate in the assessment of symptoms and objective findings are blinded to the allocated treatment and have only accessed baseline data and AEs for the whole group.
Current trial status
The first subject was included in September 2021. 40 subjects have been randomised and 28 have completed 13 weeks follow-up (Visit 3) by December 25, 2022. The second interim analysis will be performed when 40 subjects have completed Visit 3, according to current plan, Q1 2023.
Results
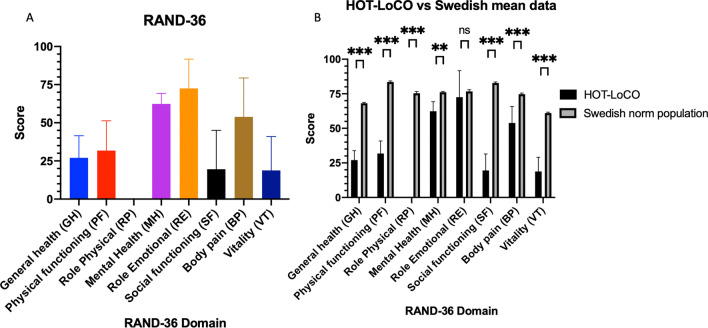
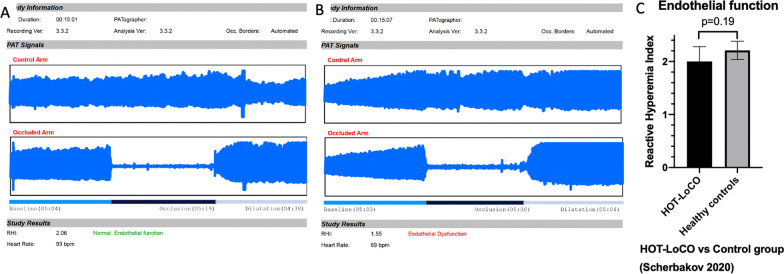
Twenty subjects had safety data available at 13 weeks. Demographic characteristics are presented in Table 1. Self-reported HRQoL in RAND-36 was very low in physical domains at baseline compared to Swedish norm data; PF 31.75(19.55) vs 83.5(23.9) (95% Confidence interval 22.60–40.90) p < 0.001, RP 0(0) vs 75.4(37.6) p < 0.001 and statistically significantly lower in all domains except Role emotional (RE) (Fig. 3). Self-reported HRQoL in EQ-5D was very low; index 0.36 (0.22) (95% CI 0.25–0.46) vs 0.87(95% CI 0.82–0.92) p < 0.001 and visual analogue scale (VAS) 39.1 (16.75)) (95% CI 31.26–46.94) vs 85.1 (1.05)) (95% CI 81–89) p < 0.001 compared to age and sex matched norm data (Fig. 4). Performance in physical tests were very low at baseline compared to international norm data; 6MWT 442 (180) (95% CI 357.7–525.8) vs 662 (18) meters and CST 13(5.1) (95% CI 10.51–15.29) vs 25 (1.23) (95% CI 22.95–27.60) stands in 30 s (Fig. 5). Baseline data of RHI in the 20 subjects in the interim analysis; 35% of the subjects have RHI < 1.67 i.e., endothelial dysfunction and 30% RHI 1.67–2.10 i.e., borderline ED at baseline. While numerically lower, this did not reach statistical significance compared to a ten-year older control group (Fig. 6).
Fig. 3.
Baseline RAND-36 was very low in Physical domains PF and RP (A) and all domains except RE (B) compared to a Swedish reference population. Results presented as mean and SD
Fig. 4.
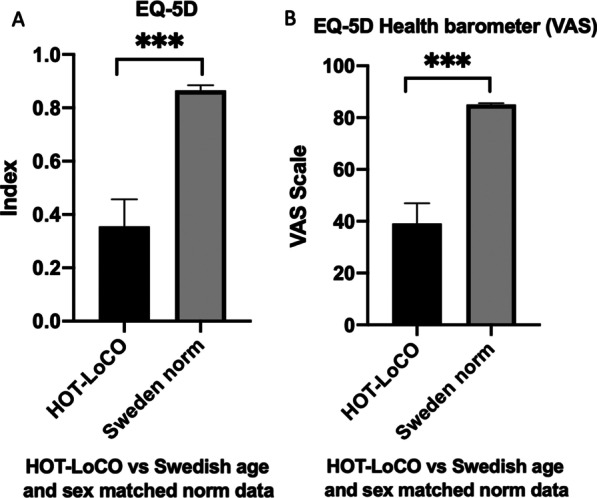
Baseline EQ-5D was very low in index (A) and VAS (B) compared to Swedish age and sex matched norm data
Fig. 5.
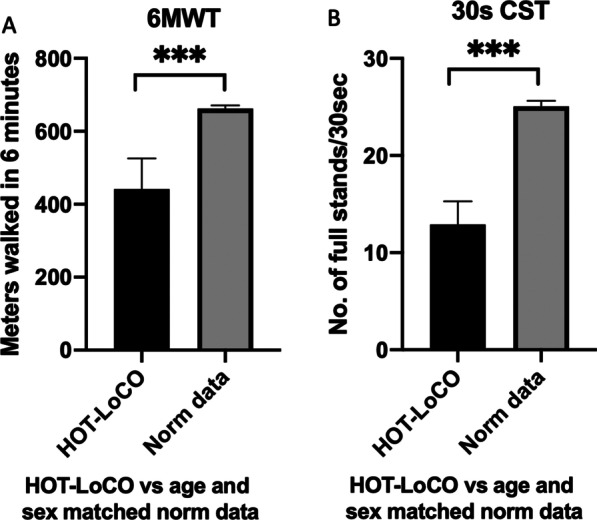
Baseline 6MWT (A) and 30 s CST (B) was very low compared to international age and sex matched norm data
Fig. 6.
Typical recordings of RHI measurements of ED (A) vs normal endothelial function (B) and Baseline RHI compared to an older control group (C). A and B shows typical recordings of the EndoPAT 2000 measurement, the “control arm” is measured on the right index finger and “occluded arm” is measured on the left index finger. C Shows mean and SD of RHI in our cohort compared to a previously published control group (n = 20) with mean age 48 ± 14 (Scherbakov 2020)
Thirty-one AEs were recorded, at least one in 60% of subjects. No SAE was reported. Most AE were grade 1, 6 were grade 2. In 20 AEs, there was at least a possible relationship with the study drug. The most common AE was cough and chest pain/discomfort. All AE were transient. (Additional file 1, AE listings).
Discussion
Our results show that the self-reported HRQoL is extremely low in our cohort compared to previously published data on Long COVID [21]. The reference data for RAND-36 and EQ-5D are based on Swedish populations matched for age and sex but not adjusted for level of education. Our cohort consists of highly educated subjects, therefore HRQoL is expected to be even higher but may also explain the very low result of Role Physical (expectations). Most of our subjects were infected during the first wave 2020 and were unvaccinated at the time. There may be a selection bias depending on this fact and there has also been a selection of patients referred to our PCC clinic; only the most serious cases were accepted. According to our protocol with “sham treatment” we are not able to adjust the dose with pressure, only time. For some subjects the time was reduced due to cough or chest discomfort during treatment and some subjects was not able to complete all ten treatments. A protocol that allows a lower pressure or individually adjusted may be beneficial for compliance. The previously published RCT reported no significant difference in side effects between the groups (35.1% and 38.9%, p = 0.739 in the HBO2 and control groups respectively) and no discontinuation of the treatment due to side effects [11]. Given the frailty of this group it’s possible that AEs occurred in the placebo treatment group due to the effort of participation or by breathing non humidified air. Alternative explanations for the higher rate of adverse events are difference in disease severity or difference in treatment protocols. Very few in our cohort would be able to accept treatments on two consecutive days, due to severe fatigue.
Conclusions
HBO2 appears to have a favorable safety profile for PCC considering the absence of SAE but an unexpected high frequency of AE was observed. Most of them were mild and all of them were transient. We speculate that frequency of AE could be reduced by individual dosing. This safety analysis enables further investigation of the efficacy of HBO2 within the HOT-LoCO trial and may help other researchers in designing trials.
Supplementary Information
Additional file 1. AE listing, AE Interim 1 Safety report.pdf.
Additional file 2. Protocol from DSMB meeting, DSMB protocol 2022-05-09.pdf.
Acknowledgements
Our sincere thanks to all subjects participating in the trial. Thanks to the unblinded staff at the hyperbaric unit, Carola Lernbäck, Birgitta Johansson, Johan Ohlberger, Annelie Kruthammar, Lovisa Liwenborg and Georgios Sidiras for managing the subjects. Thanks to the research nurses at KFE, Anna Schening, Anna Granström, Ola Friman and Pia Zetterqvist. Thanks to physiotherapists Ulrika Holdar and Anna Svensson-Raskh. Thanks to the head of ME Intensivvård Björn Persson for supporting the trial.
Abbreviations
- PCC
Post COVID condition
- HRQoL
Health related quality of life
- HBO2
Hyperbaric oxygen
- RAND-36
RAND-36 Questions Questionnaire for HRQoL
- PF
Physical functioning domain of RAND-36
- RP
Role physical domain of RAND-36
- AE
Adverse event
- SAE
Serious adverse event
- 6MWT
Six Minute Walk Test
- 30 s CST
30 Seconds Chair Stand Test
- EQ-5D
EuroQol Group 5 Questions Questionnaire for HRQoL
- RHI
Reactive Hyperemia Index (measurement of endothelial function)
- ICH-GCP
International Council for Harmonisation-Good Clinical Practice
- DSMB
Data Safety Monitoring Board
- VAS
Visual Analogue Scale
Author contributions
AK is the principal investigator and takes responsibility for the integrity of the data. AH, EB, SEG and SAE are sub-investigators, enrolling and evaluating subjects and collecting data. PL, MNB, JB, MS, MR and KRW are trial chairs, supervising subjects’ safety and conduct of the trial. JK is the trial statistician. AK and JK take responsibility for the accuracy of the data analysis. All authors contributed to the current submission and critically reviewed the manuscript. AK is corresponding author for this work and attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
Open access funding provided by Karolinska Institute. Swedish Heart–Lung foundation, Stockholm Council and Oura Health Oy. The trial is investigator initiated, sponsor is Karolinska University Hospital. None of the external funding bodies were involved in the trial design, collection, analysis, or interpretation of data or in writing the manuscript.
Availability of data and materials
An anonymised list of adverse events and the DSMB protocol is available as additional files. The full protocol is available with open access [10]. Anonymised lists of baseline data (blinded to intervention) on subject level will be available upon reasonable request. A full description of the intended use of the data must be sent to the corresponding author for review and approval. Participant consent for data sharing is conditioned and new ethics approval may be required.
Declarations
Ethics approval and consent to participate
The trial is conducted in accordance with The Declaration of Helsinki, ICH-GCP, local and national regulations. The trial was approved by The Swedish ethical review board (EPM no 2021-02634, amendment 2021-04572), approval 2021-05-25 and 2021-09-22 and The Swedish medical products agency (LV no 5.1-2020-36673), approval 2021-07-06. The trial was registered online (NCT04842448) and EudraCT number: 2021-000764-30 before start of the trial.
Consent for publication
All subjects have signed an informed consent form compliant with ICH-GCP, including information of dissemination and data sharing.
Competing interests
AK and PL disclose funding from Swedish Heart–Lung foundation, Stockholm Council and Oura Health Oy for the current trial. MS discloses funding from Swedish Research Council and Dysautonomia International during the trial and previously from HLF. MS also disclose consulting fee from Swedish agency for health technology assessment of social services, speaker honoraria from Orion Pharma, Werfen and has filed a patent for pharmacological treatment in post-COVID POTS. JK disclose consulting fee for statistical work in this trial. AH, EB, SEG, SAE, JK, JB, MNB, MR declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2021;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- 4.Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown K, Yahyouche A, Haroon S, Camaradou J, Turner G. Long COVID and self-management. Lancet. 2022;399(10322):355. doi: 10.1016/S0140-6736(21)02798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efrati S, Golan H, Bechor Y, Faran Y, Daphna-Tekoah S, Sekler G, et al. Hyperbaric oxygen therapy can diminish fibromyalgia syndrome–prospective clinical trial. PLoS ONE. 2015;10(5):e0127012. doi: 10.1371/journal.pone.0127012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akarsu S, Tekin L, Ay H, Carli AB, Tok F, Simsek K, et al. The efficacy of hyperbaric oxygen therapy in the management of chronic fatigue syndrome. Undersea Hyperb Med. 2013;40(2):197–200. [PubMed] [Google Scholar]
- 8.Bhaiyat AM, Sasson E, Wang Z, Khairy S, Ginzarly M, Qureshi U, et al. Hyperbaric oxygen treatment for long coronavirus disease-19: a case report. J Med Case Rep. 2022;16(1):80. doi: 10.1186/s13256-022-03287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins T, Gonevski M, Clark C, Baitule S, Sharma K, Magar A, et al. Hyperbaric oxygen therapy for the treatment of long COVID: early evaluation of a highly promising intervention. Clin Med (Lond) 2021;21(6):e629–e632. doi: 10.7861/clinmed.2021-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kjellberg A, Abdel-Halim L, Hassler A, El Gharbi S, Al-Ezerjawi S, Bostrom E, et al. Hyperbaric oxygen for treatment of long COVID-19 syndrome (HOT-LoCO): protocol for a randomised, placebo-controlled, double-blind, phase II clinical trial. BMJ Open. 2022;12(11):e061870. doi: 10.1136/bmjopen-2022-061870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zilberman-Itskovich S, Catalogna M, Sasson E, Elman-Shina K, Hadanny A, Lang E, et al. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: randomized controlled trial. Sci Rep. 2022;12(1):11252. doi: 10.1038/s41598-022-15565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjellberg A, De Maio A, Lindholm P. Can hyperbaric oxygen safely serve as an anti-inflammatory treatment for COVID-19? Med Hypotheses. 2020;144:110224. doi: 10.1016/j.mehy.2020.110224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadanny A, Efrati S. The hyperoxic–hypoxic paradox. Biomolecules. 2020;10(6):958. doi: 10.3390/biom10060958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA. Hyperbaric Oxygen Therapy: Get the Facts: U.S. Food and Drug administration; 2021. https://www.fda.gov/consumers/consumer-updates/hyperbaric-oxygen-therapy-get-facts.
- 15.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT, et al. Guidelines for inclusion of patient-reported outcomes in Clinical Trial Protocols: the SPIRIT-PRO Extension. JAMA. 2018;319(5):483–494. doi: 10.1001/jama.2017.21903. [DOI] [PubMed] [Google Scholar]
- 17.Lansdorp CA, van Hulst RA. Double-blind trials in hyperbaric medicine: a narrative review on past experiences and considerations in designing sham hyperbaric treatment. Clin Trials. 2018;15(5):462–476. doi: 10.1177/1740774518776952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tveter AT, Dagfinrud H, Moseng T, Holm I. Health-related physical fitness measures: reference values and reference equations for use in clinical practice. Arch Phys Med Rehabil. 2014;95(7):1366–1373. doi: 10.1016/j.apmr.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Ohlsson-Nevo E, Hiyoshi A, Noren P, Moller M, Karlsson J. The Swedish RAND-36: psychometric characteristics and reference data from the Mid-Swed Health Survey. J Patient Rep Outcomes. 2021;5(1):66. doi: 10.1186/s41687-021-00331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherbakov N, Szklarski M, Hartwig J, Sotzny F, Lorenz S, Meyer A, et al. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020;7(3):1064–1071. doi: 10.1002/ehf2.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-a systematic review and meta-analysis. J Med Virol. 2022;94(1):253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. AE listing, AE Interim 1 Safety report.pdf.
Additional file 2. Protocol from DSMB meeting, DSMB protocol 2022-05-09.pdf.
Data Availability Statement
An anonymised list of adverse events and the DSMB protocol is available as additional files. The full protocol is available with open access [10]. Anonymised lists of baseline data (blinded to intervention) on subject level will be available upon reasonable request. A full description of the intended use of the data must be sent to the corresponding author for review and approval. Participant consent for data sharing is conditioned and new ethics approval may be required.