Abstract
Biostimulants play an important role in promoting crop growth and development and improving fruit yield, but their influence on fruit quality in horticulture plants is still unclear. In this study, four types of biostimulants, Ainuo (AN), Aigefu (AG), Weiguo (WG), and Guanwu Shuang (GS) were applied to the fruit surface of ‘Yinhongli’ plum at 60 and 75 days after anthesis to investigate their effect on carbohydrates and biosynthesis of anthocyanins, and also analyze the relationship between sugar and anthocyanin accumulation during fruit color change to ripening. Results showed that all biostimulant treatments significantly improved fruit appearance quality, and increased single fruit weight and TSS/TA. Cyanidin 3-O-glucoside and cyanidin 3-O-rutinoside, are the most important anthocyanins in the red skin of the ‘Yinhongli’ plum, and no anthocyanin was detected in the green skin. In addition, WG and GS treatments significantly increased the expression of structural genes involved in anthocyanin biosynthesis compared with the control, especially chalcone synthase (CHS) and flavonoid 3-O-glucosyltransferase (UFGT) at 95-105 d after anthesis, leading to anthocyanin accumulation 10 days earlier than the control. Correlation analysis showed that there was a significant correlation between total sugar and anthocyanin content during fruit coloring and ripening.
Keywords: biostimulants, anthocyanins, carbohydrates, Plum (Prunus salicina L.), Principal component analysis (PCA)
1 Introduction
Plum (Prunus domestica Lindl.) is highly favored by consumers due to its various varieties, colors, fresh taste, rich nutrition, and natural antioxidant functional components (Roussos et al., 2016; Liu et al., 2020). Fruit variety and yield are important factors affecting the fruit quality (Tomić et al., 2019; Hamdani et al., 2022). Chinese plums (Prunus salicina Lindl) and European plums (Prunus domestica Lindl) are the main production cultivars of plums. Compared with European plums, which are mostly used for processing, Chinese plums are mainly used for fresh food due to their crispier and sweeter flesh. Plum fruits have red, green, yellow, and purplish-black, which greatly contributes to the visual quality of the fruits. Anthocyanins are the main pigments responsible for plums, and usually give bright colors to fruit (Jaakola, 2013; Ge et al., 2022). Anthocyanins, one kind of flavonoid, are products from the phenylpropanoid biosynthesis pathway, which have very important nutritional and pharmacological values (Kalt et al., 2019; Ghattamaneni et al, 2019).
The anthocyanin biosynthesis pathway has been elucidated in a variety of plant species, such as apples (Li et al., 2022), peaches (Khan et al, 2021), and strawberries (Shen et al., 2020). Numerous studies have shown that anthocyanin accumulation is influenced by complex interactions of environmental and developmental factors, such as light, temperature, and the level of sugar, and plant hormones (Nardozza et al., 2019; Fang et al., 2021; Gao et al., 2021). Previous studies found that hot air and UV-C treatments promoted the synthesis of anthocyanins and PAs by enhancing the activities and expressions of enzymes involved in phenylpropanoid metabolism during peach fruit development (Zhou et al., 2020a). Fulvic acid applied to the foliage or soil of ‘Flordaprince’ peach trees significantly increased fruit yield, fruit size, soluble solids content, and anthocyanin concentration in the pericarp (EI-Razek et al, 2012). Carbohydrates, especially sugars, also induce anthocyanin accumulation by interacting with MBW complex genes in plant species (Payyavula et al., 2013). Sugars (glucose, fructose, sucrose) serve both as the main precursor for the biosynthesis of phenylpropanoids and as signal substances that regulate their synthesis (Das et al., 2012). However, Different kinds of sugar in the biosynthesis of anthocyanins in plums are still unknown.
‘Yinhongli’ plum, belonging to the Chinese plum, is a local variety of Yibin City, Sichuan province, with crisp and sweet flesh and easily separated stone. Unlike other plum varieties, the peel of ‘Yinghongli’ is only partially red and mostly green when ripe. Importantly, the size of the red area of the peel directly affects the perception of the fruit and the selling price. Consumers prefer fruit with the more red area because it has higher bioactive substances (Gramza-Michałowska et al., 2019; Chen et al., 2021). Therefore, some agronomic practices have been attempted to improve fruit quality and coloration, including the spraying of biostimulants (Soppelsa et al., 2019; Francesca et al., 2020; Distefano et al., 2022). Biostimulants are natural substances, according to the officially definition of the EU (EU, 2019), that can enhance the absorption and utilization of nutrients by stimulating the natural physiological process of plants, including humic acid, seaweed extract, protein hydrolysate, amino acids, chitosan and its derivatives, microbial and bacterial preparations, etc. (Battacharyya et al., 2015). In recent years, biostimulants have been reported to promote plant vigor (Dong et al., 2020), as well as strawberry fruit quality (Weber et al., 2018; Rahman et al., 2018), apricot (Tarantino et al., 2018), and sweet cherry (Gonçalves et al., 2020). However, the application of biostimulants in other horticultural crops has not been seldom studied. In this study, four types of biostimulants were applied to ‘Yinhong’ plum’ to investigate their effects on fruit quality and anthocyanins accumulation. The results will lay the foundation for further studies on the regulation mechanism of sugar on anthocyanin accumulation in ‘Yinhongli’ plum.
2 Materials and methods
2.1 Plant material and treatments
The field experimental were carried out in an orchard in Yibin city, Sichuan Province, China (28°28’N, 28°54’E). The altitude is about 800 meters, the annual average temperature is 14.9 ℃, the sunshine hours are 970 hours, and the frost-free period is more than 300 days. The soil had a pH of 4.4, organic matter of 3.7%, total nitrogen (N) of 1.42g kg−1, total phosphorus (P) of 12 g kg−1, available P of 1.6 mg kg−1, and available K of 48 mg kg−1. Ten-year-old ‘Yinhongli’ plums were randomly selected for treatments. Four biostimulants products, Ainuo (AN, humic acid ≥30g/L, North China Pharmaceutical Group Ainuo Co., Ltd.), Aigefu (AF, seaweed extract ≥20g/L, Cape South Africa Co., Ltd.), Weiguo (WG, amino acids ≥100g/L, Guangdong Weisheng greenhouse Technology Co., Ltd.), and Guanwu Shuang (GS, amino acids ≥110g/L, Syngenta China Investment Co., Ltd.), were sprayed on the leaves and fruits of ‘Yinhongli’ plum at 60 d and 75 d after anthesis. The recommended concentration of the products was used, and the trees that were not sprayed were used as controls (CK). Each treatment was designed with three replications and three trees per replicate.
At least 27 fruits were collected for each treatment at 60 d, 75 d, 80 d, 90 d, and 105 d (ripening) after anthesis (DAA), respectively. All samples were brought back to the laboratory in ice boxes as soon as possible. After single fruit weight, longitudinal, transverse diameter and color, peel and flesh were separatedand frozen with liquid nitrogen, then stored at −80 °C.
2.2 Determination of fruit quality parameters
Ten fruits were randomly selected for each treatment, and the color of each fruit was measured on both symmetrical sides at the equator (mid-latitude line) using a colorimeter CR-10 (Konica Minolta, Tokyo, Japan) as described previously (Mcguire, 1992). Fruit firmness was determined using a GY-4 digital fruit sclerometer (GY-4; Jinyang, Beijing, China), and total soluble solids were quantified using a digital refractometer (ARP-TD32, Airui pu, China). The soluble sugar content, and titratable acid content (in terms of malic acid) of the fruit were determined by the method of Dong et al. (2002). All index measurements were designed with three biological repetitions.
2.3 Determination of anthocyanin content
Total anthocyanin content was detected according to the method described by Olivares et al. (2017). About 1 g Frozen skin samples were ground into fine powder in liquid nitrogen, extracted with methanol: HCl (99:1, v/v), and ultrasonic extraction was carried out at 40°C for 60 min. The extract was centrifuged at 8000×g for 15 min. The pH of the supernatant was adjusted to 1 or 4.5. The absorbance was measured at 520 nm and 700 nm, respectively.
The anthocyanin components of skin were extracted according to the method of Chen et al. (2007) with slight modification. Under dark conditions, 0.5 g of frozen red and green skin samples were ground on ice, dissolved in 1.5 mL of 1% HCl-methanol, mixed until homogenized, and extracted at 4°C for 12 h. The extracts were centrifuged at 10,000 × g at 4 ℃ for 10 min, and the supernatant was filtered with a microporous membrane (0.45μm) for high-performance liquid chromatography (HPLC, Agilent 1260 Series) analysis, and Comatex C18 column (250 mm x 4.6 mm, 5 μm, Plainfield, USA), column temperature 30 ℃. The elution system consisted of a mobile phase A (ultra-pure water) with a gradient elution procedure at a flow rate of 1.0 mL·min-1, injection volume of 10.0 μL, column temperature of 30 ℃, and detection wavelength of 520 nm. Anthocyanin components were identified by comparison the peak with of standard sample cyanidin 3-O-glucoside (Cy3G) and cyanidin 3-O-rutinoside(Cy3R, bought from Solarbio, Beijing, China). Quantification of anthocyanin was performed based on the peak area of the sample recorded at 520 nm.
2.4 Determination of the content of glucose, fructose, sucrose, and sorbitol
The extraction and determination of sucrose, glucose, fructose, and sorbitol were carried out according to the methods of Wu et al. (2007), with slight modifications. 1.0 g of the sample was added to 4 mL of ultrapure water, and heated at 80 °C for 15 min. After centrifugation and filtering, the supernatant was analyzed on an Agilent 1260 HPLC system (Agilent Technologies), equipped with a Thermo NH2 column (4.6 mm ×250 mm, 5 μm, Angela Technologies, Shanghai, China). The mobile phase consisted of acetonitrile/water (80:20, v/v)) at a flow rate of 1.0 mL/min, and the column temperature was 40°C.
2.5 Principal component analysis and comprehensive evaluation
The data were analyzed using SPSS 26.0 for principal component analysis (PCA) to build the model.
The PCA reduces the complexity of the data and finds the most important features. Assuming that there are n samples in a practical problem. Each sample has p indexes which are regarded as p random variables and recorded as X1, X2 … Xp. Through PCA, p indicators are standardized, reduced dimensions, performed linear regression and formed k principal components F1, F2 … Fk (k ≤p) according to equation. The scores of different treatment samples were calculated and ranked to evaluate the sensory quality.
2.6 Gene expression analysis in skin by quantitative RT-PCR (qRT-PCR)
Candidate gene sequences related to anthocyanin synthesis and sugar metabolism were searched fome Genbank. for in the ‘Sanyueli’ plum and Prunus domestica gene sequence libraries using the TBtools tool, and BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for comparison (details are shown in Table S1 ).
Total RNA was extracted using the RNA prep Pure polysaccharide polyphenol plant total RNA (TIANGEN biotech, Beijing, China) and the Prime ScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time) kit (TaKara, Dalian, China) for reverse transcription. All steps were performed according to the reagent instructions. Quantitative PCR was conducted using the TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) (TaKara, Dalian, China). The reaction procedure was 95 ℃ for 30 s, 95 ℃ for 5 s, and TM for 30 s for 40 cycles. Each treatment was designed with three biological repetitions. The melting curve was analyzed, and the relative expression levels of each gene were calculated by the 2-ΔΔCt method.
2.7 Statistical analysis
All values are mean ± SD of three biological repeats. Duncan’s method was used to test the significance using SPSS 26.0 (SPSS, Inc., Chicago, IL, USA). Figures were elaborated using SigmaPlot 14.0 (Systat, San Jose, CA, USA).
3 Results
3.1 Effect of biostimulants on fruit quality
Compared to the control (CK), the application of biostimulants differentially stimulated the fruit growth ( Table 1 ), with the increase of fruit weight in treatments of AN, WG and GS at ripening (105 DAA), accompanied with no significant change of firmness. AN, AG and GS treatments increased TSS, the WG and GS treatments decreased TA content, resulting in improvement of TSS/TA ratio in all treatments. The sugar/acid ratio is an important indicator determining fruit quality.
Table 1.
Effects of biostimulants on the fruit appearance quality.
| Treatment | Fruit weight (g/fruit) | Firmness (kg.cm−2) | TSS (%) | TA (%) | TSS/TA |
|---|---|---|---|---|---|
| CK | 45.88 ± 2.91b | 16.90 ± 1.21ab | 9.41 ± 0.07c | 0.87 ± 0.03a | 10.80 ± 0.37c |
| AN | 51.30 ± 1.76a | 19.14 ± 2.48a | 11.83 ± 0.06b | 0.79 ± 0.06ab | 15.11 ± 1.13b |
| AG | 45.29 ± 2.74b | 17.28 ± 2.36ab | 12.15 ± 0.28ab | 0.77 ± 0.05ab | 15.78 ± 1.01ab |
| WG | 51.43 ± 2.38a | 13.54 ± 1.30ab | 9.72 ± 0.12c | 0.69 ± 0.03c | 14.03 ± 0.42b |
| GS | 54.17 ± 1.80a | 14.42 ± 2.22b | 12.37 ± 0.24a | 0.67 ± 0.08d | 18.79 ± 2.68a |
Values are mean ± SD (n=10). Different letters indicate significantlences among treatments at p< 0.05 by one-way ANOVA test. Treatments: CK, control; AN, humic acid; AF, seaweed extract; WG, seaweed extract; GS, amino acid.
3.2 Effects of biostimulants on the coloration and anthocyanins content
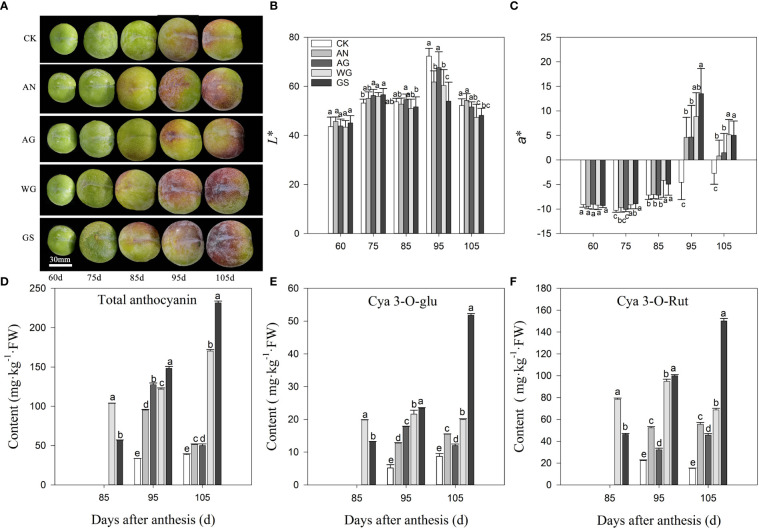
After biostimulant application, the skin color of ‘Yinhongli’ plum turned light red at 85 DAA, while the control treatment appeared green. The skin turned color which was 10 d earlier than that of CK ( Figure 1A ). Correspondingly, the a* values of all treatments were significantly higher than that of CK at 95 DAA and 105 DAA ( Figures 1B, C ). Application of biostimulants significantly increased the content of total anthocyanins and anthocyanin components in the skin ( Figures 1D–F ). Total anthocyanins content was almost undetectable in the skin of ‘Yinhongli’ plum at 85 DAA. During fruit ripening, the total anthocyanin content in CK, WG and GS treatments reached the peak at 95 DAA and then decreased. WG and GS treatments reached the peak at 105 DAA, which were much higher than that in other treatments. The main anthocyanin component in red skin detected was cyanidin 3-O-rutinoside, followed by cyanidin 3-O-glucoside, and no anthocyanin was tested in the green skin. At 105 DAA (fruit ripening stage), the GS treatment had the maximum content of anthocyanin, with 51.79 mg·kg-1 FW cyanidin 3-O-glucoside and 150.1 mg·kg-1 FW cyanidin 3-O-rutinoside, which was significantly higher than the other treatments.
Figure 1.
Effect of biostimulants on skin color (A), L* value (B), a* value (C), total anthocyanin content (D), and contents of anthocyanin components (E, F). Values are mean ± SD. (n =3). Different letters indicate significantly different values at p< 0.05.
3.3 Effect of biostimulants on fruit carbohydrates
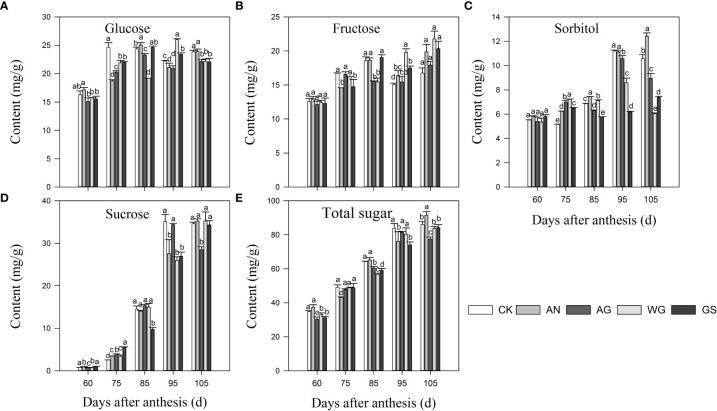
Four soluble sugars, glucose, fructose, sucrose, and sorbitol, were separated from the pulp of the ‘Yinhongli’ plum ( Figure 2 ). The highest sucrose content was found in ‘Yinhongli’ plum fruits at maturity, followed by glucose and fructose. Glucose and fructose accumulated rapidly from young fruits to green fruits at 60 d and 75 DAA. At 85-95 DAA, there was a significant decrease in glucose and fructose in CK, AG, WG, and GS treatments, while sorbitol content accumulated substantially at this time. At 105 DAA, the sorbitol content decreased significantly. The content of sorbitol was consistently lower during the fruit ripening. At 105 DAA, the fructose content was higher in AW and AX treatments compared to other treatments, which were 20.32 mg/g and 21.78 mg/g, respectively. Sucrose contents in all treatments fruit increased throughout the ripening process of the ‘Yinhongli’ plum and were higher than other soluble sugars at 105 DAA, indicating that the ‘Yinhongli’ plum belongs to the sucrose accumulation type.
Figure 2.
Effects of biostimulants on the content of glucose (A), fructose (B), sorbitol (C), sucrose (D), and total sugar (E). Values are mean ± SD (n =3). Different letters indicate significantly different values at p< 0.05.
3.4 Evaluation of fruit quality based on PCA
The eigenvalues and contribution rates of the principal components (PC) are the basis of selecting the PCs; the larger the eigenvalues, the more information the PC contains. In this experiment, PCA was performed with 11 measured variables (Fruit weight, Firmness, TSS/TA, L*, a*, cyanidin 3-O-rutinoside, cyanidin 3-O-glucoside, total anthocyanins, glucose, fructose, sorbitol, Sucrose, and total sugar). The variance contribution rate of PC of ‘Yinhongli’ plum fruit quality variation showed in Tables 2 - 4 . The first three PCs gave eigenvalues greater than 1.0 and explain 85.73% of the total variation. As shown in Table 3 , the contribution rate of the first PC accounted for 58.61% of the total variance. Fruit weight (X1), TSS/TA (X3), a* (X5), cyanidin 3-O-glucoside (X6), cyanidin 3-O-rutinoside (X7), total anthocyanins (X8), Fructose (X10) and Sucrose (X12) primarily reflected PC1 with contributing rates of 0.763, 0.823, 0.936, 0.880, 0.932, 0.947, 0.735 and 0.076, respectively. These variables are important indexes to evaluate the fruit quality of ‘Yinhongli’ plum. The second PC explained 27.91% of the total variation, with Firmness (X2), Sucrose (X12) and total sugar (X13) contributed 0.857, 0.928, and 0.948, respectively. The results indicated that the sugar of the ‘Yinhongli’ plum is the main information source of PC2. The contribution rate of a* (X5) is 0.070, which is the color index of sugar content, representing 8.65% of the total variation. The scores of the three PCs showed that the score of GS treatment was the highest (0.60), followed by AN (0.35) and WG (0.29), indicating the higher comprehensive quality of the GS treatment.
Table 2.
Eigen values and contribution rates of principal components.
| Component | Initial eigenvalues |
Contribution rate of variance (%) |
Cumulative variance contribution rate (%) |
|---|---|---|---|
| 1 | 7.620 | 58.613 | 58.613 |
| 2 | 3.628 | 27.909 | 86.522 |
| 3 | 1.124 | 8.645 | 95.167 |
| 4 | .628 | 4.833 | 100.000 |
| 5 | 5.240E-16 | 4.031E-15 | 100.000 |
| 6 | 3.423E-16 | 2.633E-15 | 100.000 |
| 7 | 1.597E-16 | 1.229E-15 | 100.000 |
| 8 | -2.422E-17 | -1.863E-16 | 100.000 |
| 9 | -9.772E-17 | -7.517E-16 | 100.000 |
| 10 | -2.172E-16 | -1.670E-15 | 100.000 |
| 11 | -3.930E-16 | -3.023E-15 | 100.000 |
| 12 | -4.510E-16 | -3.470E-15 | 100.000 |
| 13 | -6.237E-16 | -4.798E-15 | 100.000 |
Table 4.
Principal component scores after standardization.
| Sample | F1 | F2 | F3 | F | Rank |
|---|---|---|---|---|---|
| CK | -1.02 | -0.11 | -0.23 | -0.68 | 5 |
| AN | -0.27 | 1.71 | 0.16 | 0.35 | 2 |
| AG | 0.14 | -2.23 | 0.15 | -0.55 | 4 |
| WG | 0.58 | 0.08 | -1.05 | 0.29 | 3 |
| GS | 0.56 | 0.55 | 0.97 | 0.60 | 1 |
Table 3.
Component matrix.
| Parameters | Contribution rate | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Fruit weight(X1) | 0.763 | 0.638 | 0.100 |
| Firmness(X2) | -0.410 | 0.857 | -0.210 |
| TSS/TA(X3) | 0.823 | -0.091 | 0.519 |
| L*(X4) | -0.979 | 0.001 | 0.202 |
| a*(X5) | 0.936 | -0.012 | 0.070 |
| cyanidin 3-O-glucoside(X6) | 0.880 | 0.161 | 0.314 |
| cyanidin 3-O-rutinoside(X7) | 0.932 | 0.151 | 0.298 |
| Total anthocyanins(X8) | 0.947 | 0.168 | -0.137 |
| Glucose(X9) | -0.797 | 0.561 | 0.208 |
| Fructose(X10) | 0.735 | 0.486 | -0.300 |
| Sorbitol(X11) | -0.754 | 0.299 | 0.567 |
| Sucrose(X12) | 0.076 | 0.928 | -0.243 |
| Total sugar(X13) | -0.258 | 0.948 | 0.187 |
PC, principal component; X1….X13, 13 random variables. L*represents the relative brightness, a*represents the balance between green and red.
3.5 Effect of biostimulants on glucose metabolism genes
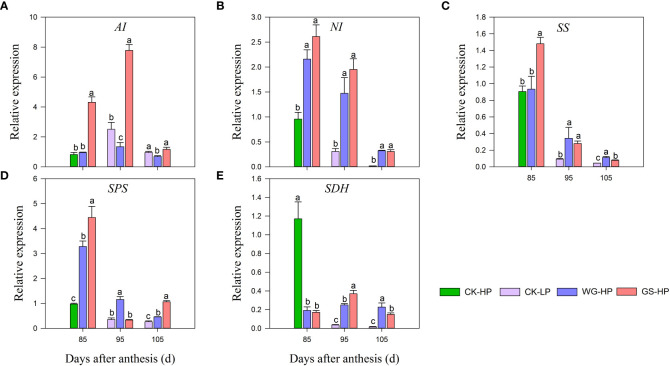
qRT-PCR was performed to determine the expression levels of genes involved in sugar metabolic, such as acid invertase (AI), neutral invertase (NI), sucrose synthase (SS), sucrose phosphate synthase (SPS), and Succinate dehydrogenase (SDH) ( Figure 3 ). During the fruit ripening, a continuous decrease in the transcript level was observed in NI, SS, and SPS in CK, with low expression occurring at the fruit ripening stage. While after treatments all structural genes were significantly up-regulated compared with CK. The high expression of SS and SPS transcript levels of structural genes at the early stage of coloration promoted the accumulation of sucrose content, which was consistent with the rapid increase of sucrose content at 85-95 DAA. The opposite was true for glucose and fructose contents ( Figure 2 ), indicating that this is the stage of rapid sucrose accumulation and the decomposition rate of sucrose reduces. The expression levels of SS and SPS decreased late in fruit development, sucrose was partially broken down into glucose and fructose, and glucose and fructose content started to increase. Upon control treatment, the expression of sugar metabolism structural genes AI and SDH peaked at 85 DAA (only green skin expression) and continued to down-regulate expression after coloration. WG and GS-treated red skin first increased and then decreased, but overall SDH transcript levels were lower.
Figure 3.
Transcript levels of sugar-related genes in different treatments. CK-LP means green skin of ‘Yinhongli’plum, the rest are red skin. Values are mean ± SD (n =3). Different letters indicate significantly different values at p< 0.05. AI, acid invertase (A); NI, neutral invertase (B); SS, sucrose synthase (C); SPS, sucrose phosphate synthase (D); SDH, Succinate dehydrogenase (E).
3.6 Effect of biostimulants on genes expression involved in anthocyanin biosynthesis
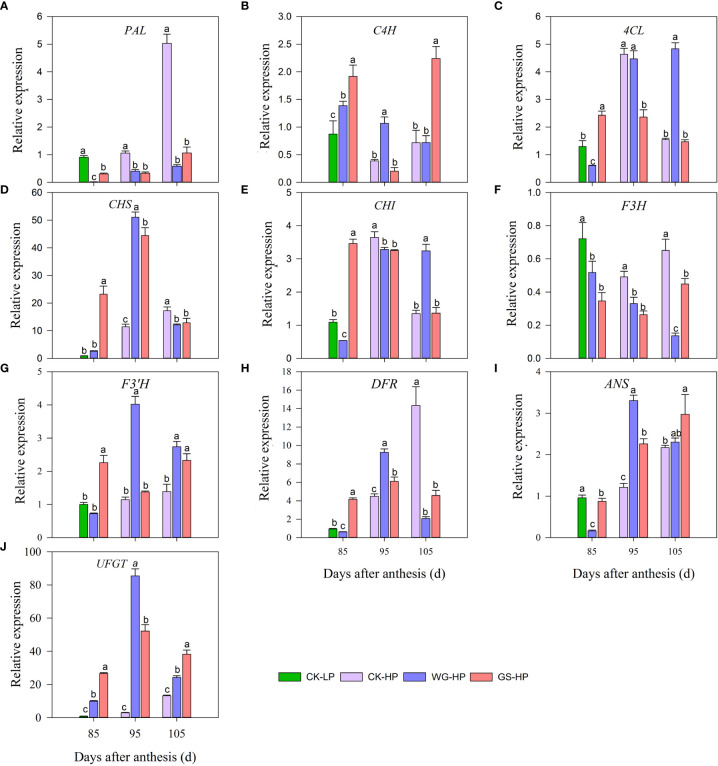
The expression levels of structural genes in anthocyanin metabolic pathways were further investigated in red skin of treatment WG, GS, and CK by qRT-PCR ( Figure 4 ). At 85 DAA, the structural genes phenylalanine ammonialyase (PAL), namate-4-hydroxylase (C4H), 4-coumaroyl: CoA-ligase (4CL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavanone 3′-hydroxylase(F3’H), dihydroflavonol 4-reductase (DFR) and UDP-glucose: flavonoid 3-O-glucosyltransferase (UFGT) in anthocyanin metabolic pathway of ‘Yinhongli’ plum treated by WG and GS were all expressed in red skin while CK was only expressed in the green skin. GS treatment had the highest expression level in most anthocyanin synthesis genes, such as C4H, 4CL, CHS, CHI, F3’H, DFR, and UFGT. While CK had the highest expression level of F3H and the lowest expression level of UFGT. At 95 DAA, fruit skin in treatment WG had the highest expression level of most genes, such as C4H, CHS, F3’H, DFR, and ANS. While at 105 DAA, the expression of anthocyanin structural genes F3’H, anthocyanidin synthase(ANS), and UFGT were all up-regulated after treatment with biostimulant compared to untreated CK. Therefore, this led to an increase in anthocyanin accumulation in ‘Yinhongli’ plum. UFGT is a precursor enzyme of anthocyanin synthesis, which catalyzes the glycosylation of anthocyanin to form a stable anthocyanin-3-monoglycoside. At 85-105 DAA, the expression of structural gene UFGT was up-regulated after biostimulant application compared to control.
Figure 4.
Transcript levels of anthocyanin-related genes in different treatments. CK-LP means green skin of ‘Yinhongli’plum, the rest are red skin. Values are mean ± SD (n =3). Different letters indicate significantly different values at p< 0.05. Phenylalanine ammonialyase, PAL (A), namate-4-hydroxylase, C4H (B), 4-coumaroyl: CoA-ligase, 4CL (C), chalcone synthase, CHS (D), chalcone isomerase, CHI (E), flavanone 3-hydroxylase, F3H (F), flavanone 3′-hydroxylase, F3’H (G), dihydroflavonol 4-reductase, DFR (H) anthocyanidin synthase, ANS (I); UDP-glucose: flavonoid 3-O-glucosyltransferase, UFGT (J).
3.7 Correlation analysis between anthocyanins and sugars
Pearson correlation coefficients between anthocyanins and sugars calculated and listed in Table 5 . In CK, the content of glucose and fructose showed positive correlation with the content of total anthocyanin. While for WG and GS treatments, sucrose and total sugar contents were positively correlated with total anthocyanin content. Sucrose and total anthocyanin content accumulated continuously during fruit ripening ( Figure 1D and Figure 2D ), which was consistent with the trend of skin color change ( Figure 1A ). In addition, the expression level of PAL, CHS, DFR, ANS and UFGT showed significant correlation with anthocyanin content ( Table 2 ), 4CL and CHI showed negative correlation with anthocyanin content, while only expression levels of PAL and ANS in GS treatment were highly significantly positively correlated with anthocyanin content.
Table 5.
Correlation between the anthocyanins content and sugar content and gene expression level.
| Total anthocyanin content | |||
|---|---|---|---|
| CK | WG | GS | |
| Glucose | 0.945** | 0.220 | -0.925** |
| Fructose | 0.929** | 0.611 | 0.369 |
| Sucrose | -0.245 | 0.946** | 0.974** |
| Sorbitol | -0.603 | -0.609 | 0.947** |
| Total sugar | 0.749 | 0.790* | 0.989** |
| PAL | 0.979** | 0.892** | 0.816** |
| C4H | 0.474 | -0.914** | 0.118 |
| 4CL | -0.952** | 0.767* | -0.812** |
| CHI | -0.931** | 0.710* | -0.887** |
| CHS | 0.855* | -0.047 | -0.290 |
| F3H | 0.663 | -0.918** | 0.483 |
| F3’H | 0.552 | 0.401 | 0.038 |
| DFR | 0.997** | -0.076 | 0.196 |
| ANS | 0.956** | 0.475 | 0.936** |
| UFGT | 0.948** | -0.014 | 0.396 |
** and * represent significant correlation at P<0.01 and P< 0.05, respectively.
4 Discussion
There was increasing evidence that biostimulant application increases single fruit weight, color, and sugar content (Annalisa et al., 2018; Mannino et al., 2020). In general, firmness, colour, sugar-acid ratio are considered important parameters for evaluating the quality of plums and determining their market value (Xu et al., 2020). Similar results were observed in our study. Principal component analysis (PCA) has been widely used for fruit quality evaluation of apples (Zhang et al., 2020), plums (Milošević and Milosevic, 2012), and other fruits (Finnegan and O’Beirne, 2015). The results of the PCA of 11 physiological indexes showed that the comprehensive quality of fruit of the biostimulation treatment was higher than that of the control ( Table 4 ). In detail, Fruit weight (X1), TSS/TA (X3), a* (X5), cyanidin 3-O-glucoside (X6), cyanidin 3-O-rutinoside (X7), total anthocyanins (X8), Fructose (X10) and Sucrose (X12) reflected PC1, and the contribution rate accounted for 38.40% of the total variance. It can be used as the basis to evaluate the fruit flavor of ‘Yinhongli’ plum. a*(X5) dominated the third PC, representing 8.65% of the total variation. Similarly, the results of this study showed that Weiguo and Guanwu Shuang treatments significantly reduced L* and increased a* value compared to the control, which meant that fruitsdarker, redder and mature (Champa et al., 2015). In addition, the both treatments prompted the fruit to turn color earlier.
Carbohydrates are closely related to fruit quality and provide the carbon and energy basis for fruit taste, texture, flavor (Barrett et al., 2010). The type and content of soluble sugars affect fruit flavor (Guan et al., 2015), which is an important non-visual property for consumers (Awad and Jager, 2003; Giovannoni, 2004). Sucrose and sorbitol are important means of carbohydrate transport, moving from leaves to sink (fruits, roots, and stems) in the form of bast loading and converting to glucose and fructose. The intensity of sweetness depends on the specific sugar spectrum, and sucrose is the main contributor to sweetness (Cirilli et al., 2016). In this study, we found that the glucose and fructose contents of ‘Yinhongli’ plum fruit were high at 60-85 DAA ( Figure 3 ). With the fruit ripening, sucrose accumulated, and the sucrose content was the highest at 105 DAA. Sorbitol is the main translocated soluble sugar in pear fruits (Mesa et al., 2016), including apple (Zhang et al., 2016), while sucrose is the main sugar transport in peach fruit (Aslam et al, 2019). Our resultst ( Figure 3D ) showed that ‘Yinhongli’ plum is sucrose accumulating, a result that is consistent with previous studies (Dugalic et al., 2014). Ren et al. (2021) found that a decrease in AI and NI activity during late fruit development resulted in lower sucrose catabolism and rapid sucrose accumulation. The present study is consistent with those of the above study results that AI and NI invertases transcript levels were higher at the initial stage of color conversion, which provided plentiful energy for fruit development. Therefore, changes in AI and NI transcript levels may play a major role in regulating soluble sugar metabolism.
The anthocyanin concentration and composition are important factors, which determined the color of plum fruits. The major anthocyanins in Japanese plum (P. salicina) fruit are cyanidin 3-galactoside (cy3-gal) and cyanidin 3-acetyl-glucoside (cy3-ace-glu) (Sahamishirazi et al, 2017), however, European plum (Prunus domestica L.) are mainly cyanidin 3-rutinoside (cy3-rut), peonidin 3-rutinoside (pe3-rut), 3-glucoside (cy3-glu), and peonidin 3-glucoside (pe3-glu) (Usenik et al., 2009). Our study demonstrated the plum cultivar, ‘Yinhongli’ plum, only contained two major anthocyanins: cyanidin 3-O-glucoside and cyanidin 3-O-rutinoside. These results are in agreement with previous results for ‘Taoxingli’ (Prunus salicina L.) varieties (Xu et al., 2020). And the variation trend of Cy3G was consistent with that of total anthocyanin, and no anthocyanin was detected in the green skin ( Figures 1D–F ). Anthocyanin belongs to flavonoids, the biosynthesis of its metabolites is regulated by some structural genes, and the accumulation of anthocyanins in fruit ultimately depends on the regulation of genes (Feng et al., 2010). Previous studies have reported that CHS, F3H, DFR, ANS, and UFGT are key genes for anthocyanin biosynthesis in fruits (Tsuda et al., 2004; Kim et al., 2005; Bashandy et al., 2015; Wu, et al., 2018). Similarly, this study found that the expression levels of anthocyanin structural genes increased with fruit maturation in the control treatment after fruit started to turn color, and CHS, DFR, ANS, and UFGT were highly significantly and positively correlated with total anthocyanin content ( Table 2 ). Kobayashi et al. (2001) found that UFGT was only highly expressed in the skin of red varieties, whereas inhibition of UFGT gene transcriptional activity resulted in green skin (Wang et al., 2013). In this study, biostimulants increased the transcript levels of CHS and UFGT in the mid and latter stages of the skin of ‘Yinhongli’ plum fruits. Weiguo and Guangwu Shuang treatments promoted the rapid coloration of skin in the middle stage of color change, while the highest expression was observed in the Weiguo treatment at 105 DAA. This is likely due to anthocyanin synthesis-related genes, which were located upstream of anthocyanin synthesis, and biostimulants induce abundantly expression of the related genes, and the skin produces anthocyanin synthesis-related enzyme proteins. At this time, even if the upstream genes are no longer expressed in large amounts, the activity of the existing enzymes can maintain anthocyanin synthesis. However, at the same time, the large amount of anthocyanin synthesized may also feedback to inhibit the expression of the upstream genes, resulting in the peak of total anthocyanin content not coinciding with the period of peak gene expression. Studies have reported that seaweed and protein hydrolysate biostimulant products can enhance the intensity and area of skin coloration of ‘Jonathan’ apples at fruit ripening (Soppelsa et al., 2018). In the present study, biostimulant application significantly up-regulated the expression of structural genes in the anthocyanin synthesis pathway and also promoted the accumulation of total anthocyanin, which may be the cause of the poor skin coloration of CK-treated fruits. However, this did not change the two-sided color characteristics of the ‘Yinhongli’ plum fruit, which may be determined by genetic factors. Further studies are needed to validate this point.
In this study, we discovered that anthocyanin accumulation continues in the ‘Yinhongli’ plum started before the fruit ripened, and sugar accumulation lasted until the fruit ripened. soluble sugars are considered important signals for the regulate anthocyanin synthesis in addition to playing an important role in carbohydrate metabolism and fruit quality (Farcuh et al., 2018; Turkyilmaz et al., 2019). In our work, there was a significant correlation between total sugar and total anthocyanin contents ( Table 4 ). Changes in carbohydrate and anthocyanin content during fruit ripening were associated with biostimulants on carbohydrate and anthocyanin-related gene expression. Studies have revealed that sucrose as an exogenous substance can regulate the expression of anthocyanin synthesis genes in the skin, and the expression of the structural anthocyanin genes CHS, CHI, F3H, F3’H, DFR, and UFGT were significantly up-regulated in Arabidopsis treated with exogenous sucrose, glucose and fructose (Boss et al., 1996; Sheng et al., 2005). Sugar induces the expression of genes related to anthocyanin synthesis and promotes anthocyanin synthesis (Meng et al., 2018). Sucrose accumulates continuously during fruit ripening of ‘Yinhongli’, which provides sufficient energy for sugar and anthocyanin metabolism ( Figures 2D ). In this study, We found that the transcript levels of most structural genes (CHS, F3’H, ANS and UFGT) in the anthocyanin synthesis pathway of the ‘Yinhongli’ plum were up-regulated after biostimulant treatment compared to the untreated control, which was consistent with the expression levels of genes related to sugar metabolism. This implies the importance of these genes in anthocyanin and sugar biosynthesis. Wu et al. (2018) found that 1-methylcyclopropene upregulated the transcript levels of PAL, CHS, F3H and ANS, and the corresponding anthocyanin content increased. Furthermore, this study found that biostimulant treatment significantly increased the accumulation of carbohydrates and anthocyanins in ‘Yinhongli’ plum fruits compared with CK treatment, which contributed to the early fruit turning color prematurely. These results pointed to the possibility that the accumulated sugars in the ‘Yinhongli’ plum play an important role in the induction of anthocyanin biosynthesis and the expression of regulatory genes, which are the leading causes of pigment formation in the skin of ‘Yinhongli’ plum.
5 Conclusion
Overall, the treatment of biostimulants promoted the accumulation of carbohydrates and improved the fruit coloration, and increased the nutritional value and marketability of ‘Yinhongli’ plum fruits. This did not change the two-sided color characteristics of the skin. Weiguo (Amino acid) and Guangwu Shuang (Amino acid) treatments significantly induced the up-regulation of gene expression of structural genes related to sugar metabolism and increased sugar content in fruit, while promoting the accumulation of anthocyanin in the skin. Biostimulant treatment induced high expression of the structural genes CHS and UFGT in the middle and late stages, which promoted the fruit color change of ‘Yinhongli’ plum in advance. These findings provide a direction for further studies on the interactions between anthocyanin and sugar metabolism synthesis pathways in the ‘Yinhongli’ plum.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://blast.ncbi.nlm.nih.gov/Blast.
Author contributions
Among the authors in the list, LY and YZP performed experiments, and data analysis and wrote the manuscript. DL and HX conducted the experiment design and revised the manuscript. QX, YH, WZ and CBP performed data analysis. JW and XLL conducted project Management and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding Statement
This study was financially supported by the Sichuan Science and Technology Project (2019ZYD061 and 2021NZZJ0013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1074965/full#supplementary-material
References
- Annalisa T., Francesco L., Grazia D., Giuseppe L. (2018). Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of ‘Orange rubis®’ apricot (Prunus armeniaca l.) cultivar in two consecutive years. Sci. Hortic. 39, 26–34. doi: 10.1016/J.SCIENTA.2018.04.055 [DOI] [Google Scholar]
- Aslam M. M., Deng L., Wang X., Wang Y., Pan L., Wang Z., et al. (2019). Expression patterns of genes involved in sugar metabolism and accumulation during peach fruit development and ripening. Sci. Hortic. 257, 108633. doi: 10.1016/j.scienta.2019.108633 [DOI] [Google Scholar]
- Awad M. A., Jager A. D. (2003). Influences of air and controlled atmosphere storage on the concentration of potentially healthful phenolics in apples and other fruits. Postharvest Biol. Technol. 27 (1), 53–58. doi: 10.1016/S0925-5214(02)00189-8 [DOI] [Google Scholar]
- Barrett D. M., Beaulieu J. C., Shewfelt R. (2010). Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci 50, 369–389. doi: 10.1080/10408391003626322 [DOI] [PubMed] [Google Scholar]
- Bashandy H., Pieti Inen M., Carvalho E., Lim K., Elomaa P., Martens S., et al. (2015). Anthocyanin biosynthesis in gerbera cultivar ‘Estelle’ and its acyanic sport ‘Ivory’. Planta. 242 (3), 601–611. doi: 10.1007/s00425-015-2349-6 [DOI] [PubMed] [Google Scholar]
- Battacharyya D., Babgohari M. Z., Rathor P., Prithiviraj B. (2015). Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 196, 39–48. doi: 10.1016/j.scienta.2015.09.012 [DOI] [Google Scholar]
- Boss P. K., Davies C., Robinson S. P. (1996). Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Mol. Biol. 32 (3), 565–569. doi: 10.1007/BF00019111 [DOI] [PubMed] [Google Scholar]
- Champa W. A. H., Gill M. I. S., Mahajan B. V. C., Arora N. K. (2015). Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera l.) cv. flame seedless. J. Food Sci. Technol. 52 (6), 3607–3616. doi: 10.1007/s13197-014-1422-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. J., Lei Y., Liu W. J., Zhang J., Wang N., Chen X. S. (2021). Research progress of fruit color development in apple (Malus domestica borkh.). Plant Physiol. Biochem. 162, 267–279. doi: 10.1016/j.plaphy.2021.02.033 [DOI] [PubMed] [Google Scholar]
- Chen F., Sun Y., Zhao G., Liao X., Hu X., Wu J., et al. (2007). Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography-mass spectrometry. Ultrasonics Sonochem. 14 (6), 767–778. doi: 10.1016/j.ultsonch.2006.12.011 [DOI] [PubMed] [Google Scholar]
- Cirilli M., Bassi D., Ciacciulli A. (2016). Sugars in peach fruit: A breeding perspective. Hortic. Res. 3, 15067. doi: 10.1038/hortres.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P. K., Dong H. S., Choi S. B., Park Y. I. (2012). Sugar-hormone cross-talk in anthocyanin biosynthesis. Mol. Cells 34 (6), 501–507. doi: 10.1007/s10059-012-0151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano M., Steingass C. B., Leonardi C., Giuffrida F., Schweiggert R., Mauro R. P. (2022). Effects of a plant-derived biostimulant application on quality and functional traits of greenhouse cherry tomato cultivars. Food Res. Int. 157, 111218. doi: 10.1016/j.foodres.2022.111218 [DOI] [PubMed] [Google Scholar]
- Dong L., Lurie S., Zhou H. W. (2002). Effect of 1-methylcyclopropene on ripening of ‘Canino’ apricots and ‘Royal zee’ plums. Postharvest Biol. Technol. 24 (2), 135–145. doi: 10.1016/S0925-5214(01)00130-2 [DOI] [Google Scholar]
- Dong C. X., Wang G., Du M. H., Niu C. X., Zhang X. Y., Bao Z., et al. (2020). Biostimulants promote plant vigor of tomato and strawberry after transplanting. Sci. Hortic. 267, 109355. doi: 10.1016/j.scienta.2020.109355 [DOI] [Google Scholar]
- Dugalic K., Sudar R., Viljevac M., Josipovic M., Cupic T. (2014). Sorbitol and sugar composition in plum fruits influenced by climatic conditions. J. Agric. Sci.Technol. 16, 1145–11155. Available at: https://www.semanticscholar.org/paper/Sorbitol-and-Sugar-Composition-in-Plum-Fruits-by-Dugali%C4%87-Sudar/4ff136d41f479cfa261200527b0ddbdba1997de3. [Google Scholar]
- EI-Razek E. A., Abd-Allah A., Saleh M. (2012). Yield and fruit quality of Florida prince peach trees as affected by foliar and soil applications of humic acid. J. Adv. Res. 8 (12). Available at: https://www.semanticscholar.org/paper/Yield-and-Fruit-Quality-of-Florida-Prince-Peach-as-El-Razek-Abd-Allah/5da6bec04c65396614bbcae4e549dd963c0f0721. [Google Scholar]
- EU (2019). Regulation of the European parliament and of the council laying down rules on the making available on the market of EU fertilising products and amending regulations (EC) no 1069/2009 and (EC) no 1107/2009 and repealing regulation (EC) no 2003/2003. [Google Scholar]
- Fang Z. Z., Wang K. L., Jiang C. C., Zhou D. R., Lin Y. J., Pan S. L., et al. (2021). Postharvest temperature and light treatments induce anthocyanin accumulation in peel of ‘Akihime’ plum (Prunus salicina lindl.) via transcription factor PsMYB10.1. Postharvest Biol. Technol. 179, 111592. doi: 10.1016/j.postharvbio.2021.111592 [DOI] [Google Scholar]
- Farcuh M., Rivero R. M., Sadka A., Blumwald E. (2018). Ethylene regulation of sugar metabolism in climacteric and non-climacteric plums. Postharvest Biol. Technol. 139, 20–30. doi: 10.1016/j.postharvbio.2018.01.012 [DOI] [Google Scholar]
- Feng S., Wang Y., Yang S., Xu Y., Chen X. (2010). Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta. 232 (1), 245–255. doi: 10.1007/s00425-010-1170-5 [DOI] [PubMed] [Google Scholar]
- Finnegan E., O’Beirne D. (2015). Characterising deterioration patterns in fresh-cut fruit using principal component analysis. II: effects of ripeness stage, seasonality, processing and packaging. Postharvest Biol. Technol. 100, 91–98. doi: 10.1016/j.postharvbio.2014.09.009 [DOI] [Google Scholar]
- Francesca S., Arena C., Mele B. H., Carlo S., Rigano M. M. (2020). The use of a plant-based biostimulant improves plant performances and fruit quality in tomato plants grown at elevated temperatures. Agronomy. 10 (3), 363. doi: 10.3390/agronomy10030363 [DOI] [Google Scholar]
- Gao H. Y., Jiang H., Cui J. Y., You C. X., Li Y. Y. (2021). Review: The effects of hormones and environmental factors on anthocyanin biosynthesis in apple. Plant Sci. 312, 111024. doi: 10.1016/j.plantsci.2021.111024 [DOI] [PubMed] [Google Scholar]
- Ge M. Q., Sadeghnezhad E., Hakeem A., Zhong R., Wang P. P., Fang J. G., et al. (2022). Integrated transcriptomic and metabolic analyses unveil anthocyanins biosynthesis metabolism in three different color cultivars of grape (Vitis vinifera l.). Sci. Hortic. 305, 111418. doi: 10.1016/j.scienta.2022.111418 [DOI] [Google Scholar]
- Ghattamaneni N., Panchal S. K., Brown L. (2019). Cyanidin 3-glucoside from queen garnet plums and purple carrots attenuates dss-induced inflammatory bowel disease in rats. J. Funct. Foods. 56, 194–203. doi: 10.1016/j.jff.2019.01.028 [DOI] [Google Scholar]
- Giovannoni J. J. (2004). Genetic regulation of fruit development and ripening. Plant Cell. 16, 170–180. doi: 10.1105/tpc.019158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves B., Morais M. C., Sequeira A., Ribeiro C., Guedes F., Silva A. P., et al. (2020). Quality preservation of sweet cherry cv. 'staccato' by using glycine-betaine or Ascophyllum nodosum . Food Chem. 322 126713. doi: 10.1016/j.foodchem.2020.126713 [DOI] [PubMed] [Google Scholar]
- Gramza-Michałowska A., Bueschke M., Kulczy B., Gliszczyńska-Świgło A., Kmiecik D., Bilska A., et al. (2019). Phenolic compounds and multivariate analysis of antiradical properties of red fruits. J. Food Meas Charact. 13, 1–9. doi: 10.1007/s11694-019-00091-x [DOI] [Google Scholar]
- Guan Y. Z., Peace C., Rudell D., Verma S., Evans K. (2015). QTLs detected for individual sugars and soluble solids content in apple. Mol. Breed. 35, 1–13. doi: 10.1007/s11032-015-0334-1 [DOI] [Google Scholar]
- Hamdani A., Hssaini L., Bouda S., Adiba A., Razouk R. (2022). Japanese Plums behavior under water stress: impact on yield and biochemical traits. Heliyon 8, e09278. doi: 10.1016/j.heliyon.2022.e09278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola L. (2013). New insights into the regulation of anthocyanin biosynthesis in fruit. Trends Plant Sci. 18, 477–483. doi: 10.1016/j.tplants.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Kalt W., Cassidy A., Howard L. R., Krikorian R., Stull A. J., Tremblay F., et al. (2019). Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 11, 224–236. doi: 10.1093/advances/nmz065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I. A., Cao K., Guo J., Li Y., Wang Q., Wang L., et al. (2021). Identification of key gene networks controlling anthocyanin biosynthesis in peach flower. Plant Sci. 316, 111151. doi: 10.1016/j.plantsci.2021.111151 [DOI] [PubMed] [Google Scholar]
- Kim S., Yoo K. S., Pike L. M. (2005). Development of a PCR-based marker utilizing a deletion mutation in the dihydroflavonol 4-reductase (DFR) gene responsible for the lack of anthocyanin production in yellow onions (Allium cepa ). Theor. Apple Genet. 110 (3), 588. doi: 10.1007/s00122-004-1882-7 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Ishimaru M., Ding C. K., Yakushiji H., Goto N. (2001). Comparison of UDP-glucose:flavonoid 3-o-glucosyltransferase (UFGT) gene sequences between white grapes (Vitis vinifera) and their sports with red skin. Plant Sci. 160 (3), 543–550. doi: 10.1016/S0168-9452(00)00425-8 [DOI] [PubMed] [Google Scholar]
- Li H., Duan S., Sun W., Wang S., Zhang J., Song T., et al. (2022). Identification, through transcriptome analysis, of transcription factors that regulate anthocyanin biosynthesis in different parts of red-fleshed apple ‘may’ fruit. Hortic. Plant J. 8 (1), 11–21. doi: 10.1016/j.hpj.2021.07.001 [DOI] [Google Scholar]
- Liu W., Nan G., Nisar M. F., Wan C. (2020). Chemical constituents and health benefits of four chinese plum species. J. Food Quality. 4, 1–17. doi: 10.1155/2020/8842506 [DOI] [Google Scholar]
- Mannino G., Campobenedetto C., Vigliante I., Contartese V., Gentile C., Bertea C. M. (2020). The application of a plant biostimulant based on seaweed and yeast extract improved tomato fruit development and quality. Biomolecules. 10 (12). doi: 10.3390/biom10121662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcguire R. G. (1992). Reporting of objective color measurements. Hortscience. 27 (12), 1254–1255. doi: 10.21273/HORTSCI.27.12.1254 [DOI] [Google Scholar]
- Meng L. S., Xu M. K., Wan W., Yu F., Li C., Wang J. Y., et al. (2018). Sucrose signaling regulates anthocyanin biosynthesis through a MAPK cascade in Arabidopsis thaliana . Genetics. 210 (2), 607–619. doi: 10.1534/genetics.118.301470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa K., Serra. S., Masia A., Gagliardi F., Bucci D., Musacchi S. (2016). Seasonal trends of starch and soluble carbohydrates in fruits and leaves of ‘Abbé fétel’ pear trees and their relationship to fruit quality parameters. Sci. Hortic. 211, 60–69. doi: 10.1016/j.scienta.2016.08.008 [DOI] [Google Scholar]
- Milošević T., Milosevic N. (2012). Phenotypic diversity of autochthonous European (Prunus domestica l.) and damson (Prunus insititia l.) plum accessions based on multivariate analysis. Hortic. Sci. 39, 8–20. doi: 10.17221/99/2011-HORTSCI [DOI] [Google Scholar]
- Nardozza S., Boldingh H. L., Kashuba M. P., Feil R., Richardson A. C. (2019). Carbon starvation reduces carbohydrate and anthocyanin accumulation in red-fleshed fruit via trehalose 6, hosphate and. MYB27. Plant Cell Environ. 43 (4), 819–835. doi: 10.1111/pce.13699 [DOI] [PubMed] [Google Scholar]
- Olivares D., Contreras C., Mu Oz V., Rivera S., González-Agüero M., Retamales J., et al. (2017). Relationship among color development, anthocyanin and pigment-related gene expression in ‘Crimson seedless’ grapes treated with abscisic acid and sucrose. Plant Physiol. Biochem. 115, 286–297. doi: 10.1016/j.plaphy.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Payyavula R., Singh R., Navarre D. (2013). Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. J. Exp. Bot. 64, 5115–5131. doi: 10.1093/jxb/ert303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Sabir A. A., Mukta J. A., Khan M., Mohi-Ud-Din M., Miah M. G., et al. (2018). Plant probiotic bacteria bacillus and paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 8 (1). doi: 10.1038/s41598-018-20235-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Ran X., Zeng R., Chen J., Wang Y., Mao C., et al. (2021). Effects of sodium selenite spray on apple production, quality, and sucrose metabolism-related enzyme activity. Food Chem. 339, 127883. doi: 10.1016/j.foodchem.2020.127883 [DOI] [PubMed] [Google Scholar]
- Roussos P. A., Efstathios N., Intidhar B., Denaxa N. K., Tsafouros A. (2016). “Chapter 26–plum (Prunus domestica l. and p. salicina lindl.),” in Nutritional composition of fruit cultivars. Eds. Simmonds M. S. J., Preedy V. R. (San Diego: Academic Press; ). [Google Scholar]
- Sahamishirazi S., Moehring J., Claupein W., Graeff-Hoenninger S. (2017). Quality assessment of 178 cultivars of plum regarding phenolic, anthocyanin and sugar content. Food Chem. 214, 694–701. doi: 10.1016/j.foodchem.2016.07.070 [DOI] [PubMed] [Google Scholar]
- Sheng T., Keurentjes J., Bentsink L., Smeekens K. S. (2005). Sucrose-specific induction of anthocyanin biosynthesis in arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 139 (4), 1840–1852. doi: 10.1104/pp.105.066688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J. C., Shao W. L., Du Z. K., Lu H. F., Li J. M. (2020). Integrated metabolomic and transcriptomic analyses reveal differences in the biosynthetic pathway of anthocyanins in Fragaria nilgerrensis and Fragaria pentaphylla . Sci. Hortic. 271, 109476. doi: 10.1016/j.scienta.2020.109476 [DOI] [Google Scholar]
- Soppelsa S., Kelderer M., Casera C., Bassi M., Andreotti C. (2018). Use of biostimulants for organic apple production: Effects on tree growth, yield, and fruit quality at harvest and during storage. Front. Plant Sci. 9, 1342. doi: 10.3389/fpls.2018.01342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppelsa S., Kelderer M., Casera C., Bassi M., Andreotti C. (2019). Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy. 9 (9), 483. doi: 10.3390/agronomy9090483 [DOI] [Google Scholar]
- Tarantino A., Lops F., Disciglio G., Lopriore G. (2018). Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of 'Orange rubis' apricot (Prunus armeniaca l.) cultivar in two consecutive years. Sci. Hortic. 239, 26–34. doi: 10.1016/j.scienta.2018.04.055 [DOI] [Google Scholar]
- Tomić J., Stampar F., Glišić I., Jakopič J. (2019). Phytochemical assessment of plum (Prunus domestica l.) cultivars selected in Serbia. Food Chem. 299, 125113. doi: 10.1016/j.foodchem.2019.125113 [DOI] [PubMed] [Google Scholar]
- Tsuda T., Yamaguchi M., Honda C., Moriguchi T. (2004). Expression of anthocyanin biosynthesis genes in the skin of peach and nectarine fruit. J. Am. Soc. Hortic. Sci. 129 (6), 857–862. doi: 10.21273/JASHS.129.6.0857 [DOI] [Google Scholar]
- Turkyilmaz M., Hamzaoglu F., Ozkan M. (2019). Effects of sucrose and copigment sources on the major anthocyanins isolated from sour cherries. Food Chem. 281, 242–250. doi: 10.1016/j.foodchem.2018.12.089 [DOI] [PubMed] [Google Scholar]
- Usenik V., Štampar F., Veberič R. (2009). Anthocyanins and fruit colour in plums (Prunus domestica l.) during ripening. Food Chem. 114, 529–534. doi: 10.1016/j.foodchem.2008.09.083 [DOI] [Google Scholar]
- Wang Z., Dong M., Wang A., Li T., Jiang S., Li C. T. (2013). The methylation of the PcMYB10 promoter is associated with green-skinned sport in max red Bartlett pear. Plant Physiol. 162 (2), 885–896. doi: 10.1104/pp.113.214700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber N., Schmitzer V., Jakopic J., Stampar F. (2018). First fruit in season: seaweed extract and silicon advance organic strawberry ( fragaria × ananassa duch.) fruit formation and yield. Sci. Hortic. 242, 103–109. doi: 10.1016/j.scienta.2018.07.038 [DOI] [Google Scholar]
- Wu X. Q., An X. J., Yu M. L., Ma R. J., Yu Z. F. (2018). 1-methylcyclopropene treatment on phenolics and the antioxidant system in postharvest peach combined with the liquid chromatography/mass spectrometry technique. J. Agric. Food Chem. 66 25), 6364–6372. doi: 10.1021/acs.jafc.8b01757 [DOI] [PubMed] [Google Scholar]
- Wu J., Gao H., Lei Z., Liao X., Fang C., Wang Z., et al. (2007). Chemical compositional characterization of some apple cultivars. Food Chem. 103 (1), 88–93. doi: 10.1016/j.foodchem.2006.07.030 [DOI] [Google Scholar]
- Xu Y. H., Li S., Huan C., Jiang T., Zheng X. L., Brecht J. K. (2020). Effects of 1methylcyclopropene treatment on quality and anthocyanin biosynthesis in plum (prunus salicina cv. taoxingli) fruit during storage at a non-chilling temperature. Postharvest Biol. Technol. 169, 111291. doi: 10.1016/j.postharvbio.2020.111291 [DOI] [Google Scholar]
- Zhang X., Long H., Zhang J., Whiting M. D., Karkee M., Zhang Q. (2020). Determination of key canopy parameters for mass mechanical apple harvesting using supervised machine learning and principal component analysis (PCA). Biosyst. Eng. 193, 247–263. doi: 10.1016/j.biosystemseng.2020.03.006 [DOI] [Google Scholar]
- Zhang Y., Yan Y. J., Fu C. X., Li M., Wang Y. (2016). Zinc sulfate spray increases activity of carbohydrate metabolic enzymes and regulates endogenous hormone levels in apple fruit. Sci. Hortic. 211, 363–368. doi: 10.1016/j.scienta.2016.09.024 [DOI] [Google Scholar]
- Zhou D., Liu Q., Peng J., Tu S., Tu K. (2020. a). Metabolic analysis of phenolic profiles reveals the enhancements of anthocyanins and procyanidins in postharvest peach as affected by hot air and ultraviolet c. Postharvest. Biol. Technol. 167, 11122. doi: 10.1016/j.postharvbio.2020.111227 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://blast.ncbi.nlm.nih.gov/Blast.