Abstract
Streptococcus intermedius is associated with deep-seated purulent infections. In this study, we investigated expression and functional activities of antigen I/II in S. intermedius. The S. intermedius antigen I/II appeared to be cell surface associated, with a molecular mass of approximately 160 kDa. Northern blotting indicated that the S. intermedius NCTC 11324 antigen I/II gene was transcribed as a monocistronic message. Maximum expression was seen during the early exponential phase. Insertional inactivation of the antigen I/II gene resulted in reduced hydrophobicity during early exponential phase, whereas no effect was detected during mid- and late exponential phases. Binding to human fibronectin and laminin was reduced in the isogenic mutant, whereas binding to human collagen types I and IV and to rat collagen type I was not significant for either the wild type or the mutant. Compared to the wild type, the capacity of the isogenic mutant to induce interleukin 8 (IL-8) release by THP-1 monocytic cells was significantly reduced. The results indicate that the S. intermedius antigen I/II is involved in adhesion to human receptors and in IL-8 induction.
Streptococcus intermedius belongs to the anginosus group of streptococci. Members of this group are residents of the oral cavity and gastrointestinal and urogenital tracts but are also associated with suppurative infections at various clinical sites (17, 39, 49). S. intermedius shows tropism for infections of the brain and liver (49). Like other oral streptococci, S. intermedius may be implicated as a causative agent of infective endocarditis (11, 39, 49). For both abscesses and infective endocarditis, molecular interactions with host components represent presumptive virulence factors (2, 51).
Bacterial interactions with host components are most often associated with surface proteins. The oral streptococci express a family of structurally and antigenically related surface proteins termed antigen I/II. These proteins have received a variety of names according to the strains or species in which they were identified, such as antigen B (40), Sr (35), I/II (20), and PAc (37) from Streptococcus mutans; SpaA from Streptococcus sobrinus (25, 45); and SspA and SspB from two tandemly arranged genes in Streptococcus gordonii (7). Multifunctional activities are attributed to the antigen I/II family, i.e., binding to soluble extracellular matrix glycoproteins (41) and to host cell receptors (42, 47), coaggregation with other microorganisms (4, 15, 24), interactions with salivary glycoproteins (3, 9, 12, 19, 21, 27, 36), and activation of monocytic cells (1, 5, 6). Members of the antigen I/II family may, however, exhibit functional species specificity. Differences in antigen I/II binding properties, mechanisms, and affinities have, for instance, been described for S. gordonii and S. mutans (18). Enzyme-linked immunosorbent assays (32), DNA hybridization studies (30), and homologous PCR-amplified sequences (6, 30) indicate that the anginosus group of streptococci also expresses an antigen I/II-like protein. In S. intermedius, limited information is available on the structure, expression and function of this protein. The present aim was to study expression and functional activities of antigen I/II in S. intermedius.
MATERIALS AND METHODS
Bacterial strains and media.
Three S. intermedius strains were included in this study: the type strain NCTC 11324, CCUG 43515 (a brain abscess isolate), and CCUG 28191 (a dental plaque isolate). S. mutans OMZ 175 was used as a positive control in the adhesion to collagen and activation assays. Strains were stored at −70°C in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) supplemented with 15% (vol/vol) glycerol. Cells prepared for immunoblotting, adhesion, activation, and hydrophobicity assays were grown in brain heart infusion broth. Type strain NCTC 11324 was subjected to transformation. Todd-Hewitt broth (Difco Laboratories) containing 5% heat-inactivated horse serum was then used as the growth medium. Streptococci were incubated at 37°C under microaerophilic conditions. Escherichia coli containing the E. coli-Streptococcus shuttle vector pSF151 (44) was grown in Luria-Bertani broth (Difco Laboratories) supplemented with kanamycin (Km) (50 μg/ml; Sigma-Aldrich AS, Oslo, Norway). For the selection of S. intermedius transformants, Km was used at a final concentration of 500 μg/ml.
DNA and RNA isolation.
pSF151 was isolated from E. coli with the Plasmid Maxi kit (Qiagen GmbH, Hilden, Germany), following the manufacturer's recommendations for high-copy-number plasmids. This integration plasmid replicates in E. coli but not in streptococci and expresses Km resistance in both organisms.
S. intermedius chromosomal DNA was isolated by the modified cetyltrimethylammonium bromide method, as previously described (38).
Total RNA from S. intermedius NCTC 11324 was isolated with the RNeasy mini kit (Qiagen) according to the manufacturer's recommendations, except that the cells were incubated at 37°C for 30 min in 100 μl of lysis buffer containing 20 mg of lysozyme/ml and 100 U of mutanolysin/ml.
PCR amplification.
The forward primer PF (5′-GTCAGTGGCAACGATTTATCCAA) and the reverse primer PR (5′-AATAATTCGTTGAACCGGCAAGA) were designed to amplify a 254-bp sequence of the S. intermedius antigen I/II gene. The amplified fragment corresponded to a conserved sequence downstream of the proline-rich region in S. mutans antigen I/II (30). The final PCR volume of 100 μl contained 245 ng of DNA template, 3 U of Dynazyme (Finnzymes Oy, Espoo, Finland), 0.8 mM dNTP mixture, and 1× Dynazyme buffer with 1.5 mM MgCl2. The following parameters for PCR amplification were used: 94°C for 3 min (initial denaturing period), 94°C for 30 s (denaturing period), 55°C for 1 min (annealing period), 72°C for 1 min (extension period) for 25 cycles, and 72°C for 5 min (final extension period).
Insertional inactivation.
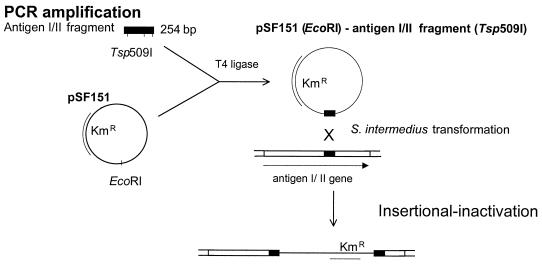
The strategy for inactivation of the antigen I/II gene in S. intermedius NCTC 11324 is shown in Fig. 1. The PCR-amplified fragment of the antigen I/II gene (targeting insert) was extracted from an 0.7% agarose gel by the high-speed centrifugation method (52), purified with glass milk following the recommended protocol (Geneclean; BIO 101 Inc., Carlsbad, Calif.), and digested with Tsp509I. Two fragments of 100 or 80 bp with Tsp509I ends were generated. pSF151 was digested with EcoRI and ligated to the Tsp509I-restricted PCR product. The ligation mixture (10 μg of pSF151 and 0.75 μg of the PCR product) was used to transform S. intermedius NCTC 11324. We applied the transformation method described by Håvarstein et al. (14), except that the cells were grown for 1 h before the ligation reaction mixture was added. The synthetic competence-stimulating peptide 11325 (200 ng/ml) was added to 1 ml of 10-fold-diluted S. intermedius culture. The incubation continued for 3 h before the cells were plated on Todd-Hewitt–horse serum agar containing Km. Transformants on the selective plates were obtained after 48 h of microaerophilic incubation at 37°C.
FIG. 1.
Strategy used for insertional inactivation of the S. intermedius antigen I/II gene. The antigen I/II gene sequence amplified by PCR was cut with Tsp509I, and the resulting fragments were ligated to pSF151 digested with EcoRI. The ligation mixture was used to transform S. intermedius NCTC 11324. Kmr mutants were selected.
Immunoblotting.
S. intermedius and the Km-selected mutants were harvested in early, mid-, and late exponential phases. The cells were washed twice with phosphate-buffered saline (pH 7.4) (PBS), resuspended in extraction buffer (0.05 M Tris, pH 6.8; 2% sodium dodecyl sulfate; 10% glycerol; 0.1 M dithiothreitol; and 0.004% pyronin), and boiled for 3 min. The culture supernatants were lyophilized and resuspended in loading buffer. Polypeptides were separated by sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis with the discontinuous buffer system described by Laemmli (23) and were electroblotted onto nitrocellulose membranes. The blots were incubated with rabbit anti-I/II immunoglobulin G (IgG) raised against purified Sr (anti-SR I/II) from S. mutans OMZ 175. Antibody binding was revealed with alkaline phosphatase-conjugated goat anti-rabbit IgG and enzyme substrate (5-bromo-4-chloro-3-indolylphosphate and Nitro Blue Tetrazolium; both from Sigma).
Southern blotting.
Chromosomal DNA isolated from S. intermedius NCTC 11324 and the isogenic mutant IB08981 (described below) was digested with XbaI or PstI. The DNA fragments were then electrophoresed on an 0.7% agarose gel and were transferred to a positively charged nylon membrane (Boehringer GmbH, Mannheim, Germany). The randomly primed method was used for labeling of the pSF151 probe. The antigen I/II probe was labeled by PCR with the PF and PR primers, following the cycle conditions described above. The probes were labeled with digoxigenin-11-dUTP (Boehringer), as recommended by the manufacturer. Prehybridization and hybridization were performed with DIG Easy Hyb (Boehringer) at 45°C. Chemiluminescent substrate was used for the detection of hybridization signals on X-ray films.
Northern blotting.
Total RNA (2 μg) from S. intermedius NCTC 11324 extracted at early, mid-, and late exponential phases was electrophoresed on a 0.7% formaldehyde gel and transferred to a positively charged nylon membrane. Prehybridization and hybridization with the antigen I/II probe were performed at 60°C, following the procedure described above for Southern blotting.
As a control for the amount of RNA applied in the wells, the membrane was stripped and hybridized with a 16S rRNA probe (46). This probe was labeled by PCR as described above, using the forward primer 16S F (5′-AGATGGACCTGCGTTGTATTAGC) and the reverse primer 16S R (5′-AACGCTCGGGACCTACGTATTAC). The relative antigen I/II transcription levels were calculated by densitometry.
Immunofluorescence microscopy.
S. intermedius NCTC 11324 and the isogenic mutant were grown to exponential phase, harvested, and washed in PBS. The cells were streaked and dried on microscope slides and treated with normal goat serum (1.5%; Sigma) to block nonspecific binding. The slides were incubated with anti-SR I/II for 1 h, washed, and incubated with goat anti-rabbit IgG-biotin 1:200 (7.5 μl/ml) for 30 min. Fluorescence was obtained by adding cyanine 2 fluorescent-labeled streptavidin (Fluorolink Cy2-Streptavidin; Amersham Pharmacia Biotech, Uppsala, Sweden) 1:1,000 in 10% bovine serum albumin (BSA; Sigma) for 30 min. Microscopic observations and image acquisition were performed with a confocal scanning laser microscope (CSLM). CSLM software was used to generate extended-focus images.
Hydrophobicity assay.
Surface hydrophobicity of S. intermedius NCTC 11324 and the isogenic mutant IB08981 was measured by the hexadecane affinity method, as described by Westergren and Olsson (48). Cells at early, mid-, and late exponential phases were washed twice with PBS and resuspended to an optical density at 450 nm (OD450) of 1.0. Volumes of 1.2-ml bacterial suspensions were mixed with 75, 150, or 200 μl of hexadecane. The OD450 in the aqueous phase was then measured, and the relative reduction in hydrophobicity was calculated.
Adhesion assay.
S. intermedius NCTC 11324 and the isogenic mutant IB08981 were radioactively labeled by overnight growth in the presence of [methyl-3H]thymidine (6 μCi/ml; 85 Ci/mmol; Amersham Corp.), giving between 1 × 10−3 to 4 × 10 −3 disintegrations per min per cell. The cells were collected by centrifugation, washed twice with PBS, and resuspended to an OD600 of 2.4. Cell suspensions were sonicated for 10 s at a power output of 20%. Microscopic examination confirmed the disruption of the streptococcal chains.
Detachable microtiter plates (MaxiSorp; Nunc A/S, Roskilde, Denmark) were coated with human fibronectin (2 μg/well; Sigma), human laminin (10 μg/well; Sigma), human collagen type I (2.5 μg/well; Sigma), human collagen type IV (2.5 μg/well; Sigma), or rat collagen type I (5 μg/well; Collaborative Biomedical Products, Bedford, Mass.), all diluted into 100 μl. Fibronectin was diluted in 0.05 M Tris-buffered saline (pH 7.5), laminin was diluted in PBS, and collagen was diluted in 0.01 M acetic acid. Wells coated with Tris-buffered saline, PBS, or acetic acid were used as respective controls. The plates were incubated at room temperature for 2 h. After incubation the plates were washed four times with PBS. Unspecific protein-binding sites were blocked with 200 μl of 1% BSA in PBS by incubation at room temperature for 30 min. The content of the plates was then aspirated, and 50 μl of 0.2% PBS–BSA and 50 μl of the bacterial suspension were added to each well. The plates were incubated with gentle shaking at 37°C for 2 h. Unbound cells were removed by washing the plates four times with PBS containing 0.05% Tween 20 (Sigma). The wells were then detached and transferred to separate vials containing 5 ml of scintillation liquid. Radioactivity was measured as the number of disintegrations per minute in a liquid scintillation analyzer (Packard 1900 TR, Packard Instrument Company, Meriden, Conn.).
Activation and IL-8 assay.
THP-1 monocytic cells were cultured in RPMI 1640 medium supplemented with 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml) in the presence of 10% heat-inactivated fetal calf serum. Prior to activation experiments, cells were washed twice with serum-free and antibiotic-free RPMI 1640. THP-1 cells (2 × 105 per well) were incubated at 37°C in serum-free and antibiotic-free RPMI 1640 with or without various amounts of live S. intermedius NCTC 11324 or the isogenic mutant IB08981. After 4 h of incubation the supernatants were harvested by centrifugation and were used to estimate cytokine release by a heterologous two-site sandwich enzyme-linked immunosorbent assay. This was achieved using a capture anti-human interleukin-8 (IL-8) monoclonal antibody (BioSource International, Camarillo, Calif.) at a concentration of 0.1 μg per well and a biotinylated anti-IL-8 (BioSource International) at a concentration of 0.05 μg per well. Tetramethylbenzidine was used as the substrate of streptavidin-horseradish peroxidase, and the absorbance was read at 450 nm. Cell viability was examined by dye uptake. S. mutans OMZ 175 was used as a control, as both whole cells and antigen I/II from this strain stimulate IL-8 production (47). THP-1 cells were activated with S. mutans OMZ 175 cells, antigen I/II from S. mutans OMZ 175, or with 4-h-incubation bacterial supernatants harvested from wells in the absence of THP-1 cells. The release rate of antigen I/II in the activation medium was estimated by immunoblotting with reference to serial dilutions of purified S. mutans OMZ 175 antigen I/II. Antigen I/II was detected in blots of activation supernatants with biotinylated rabbit anti-SR I/II prepared with NHS-LC biotin and used according to the manufacturer's instructions (Pierce, Oud Beijerland, The Netherlands).
Statistical analysis.
Student's t test was used for statistical analysis, and P values of <0.05 were considered significant.
RESULTS
Antigen I/II expression.
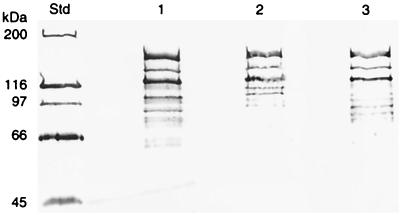
Multiple-protein bands of different strains of S. intermedius cell extracts reacted with anti-SR I/II from S. mutans OMZ 175. The highest-molecular-mass band corresponded to approximately 160 kDa (Fig. 2). Analysis of the S. intermedius NCTC 11324 supernatant revealed faint bands of size similar to that of some of the bands from the cell extract (Fig. 3).
FIG. 2.
Immunoblot detection of antigen I/II in S. intermedius with antibodies raised to S. mutans OMZ 175 antigen I/II (anti-I/II SR). Lanes: 1, S. intermedius NCTC 11324; 2, S. intermedius CCUG 28191; 3, S. intermedius CCUG 43515. Std, Standard Protein Molecular Weight Marker (Bio-Rad). Molecular masses are given in kilodaltons.
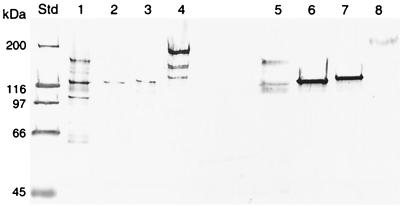
FIG. 3.
Immunoblot analysis of antigen I/II expression in the cell extract (lanes 1 to 4) and supernatant (lanes 5 to 8) of S. intermedius NCTC 11324 (1, 5), the S. intermedius isogenic mutants IB08981 (2, 6) and IB08982 (3, 7), and S. mutans OMZ 175 (4, 8). Std, Standard Protein Molecular Weight Marker (Bio-Rad). Molecular masses are given in kilodaltons.
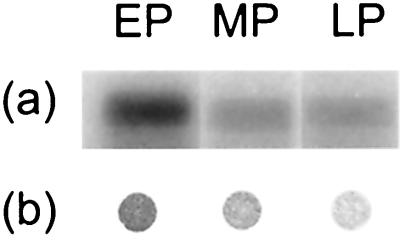
Northern blotting of RNA isolated from S. intermedius NCTC 11324 demonstrated that the antigen I/II probe hybridized with a single band of approximately 4.2 kb (Fig. 4). This transcript corresponds to a coding region of approximately 1,400 amino acids or an estimated molecular mass of about 160 kDa, suggesting that antigen I/II in S. intermedius is transcribed as a monocistronic message. Relative to the early exponential phase, the antigen I/II transcription level was reduced by 40% in the mid- and late exponential phases (Fig. 5a). The 16S rRNA band intensities were similar in the three stages of growth, confirming the uniformity of RNA amount loaded per well. Dot blotting of S. intermedius NCTC 11324 cell extract showed that antigen I/II expression coordinated well with expression of the mRNA (Fig. 5b).
FIG. 4.
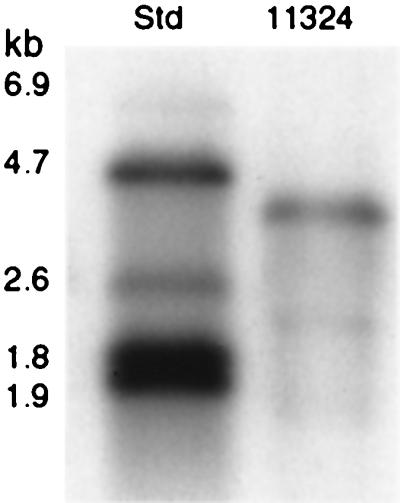
Northern blot analysis of total RNA from S. intermedius NCTC 11324 (lane 11324) hybridized with the antigen I/II digoxigenin-labeled probe. Std, Standard RNA Molecular Weight Marker II (Roche Diagnostics). kb, kilobases.
FIG. 5.
S. intermedius NCTC 11324 growth-related antigen I/II transcription and expression. (a) Northern blot analysis of total RNA hybridized with the antigen I/II probe. (b) Immunoblot detection of antigen I/II in the cell extract with anti-I/II SR. EP, early exponential phase; MP, mid-exponential phase; LP, late exponential phase.
Immunofluorescence microscopy.
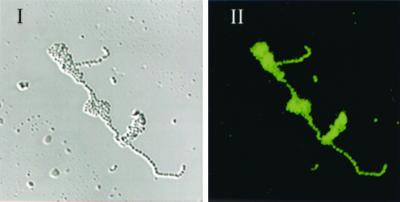
Immunofluorescence microscopy revealed a surface-localized protein in the wild type that reacted with S. mutans OMZ 175 anti-SR I/II (Fig. 6), whereas no surface protein was discernible in the mutant (not shown).
FIG. 6.
CSLM image of the S. intermedius NCTC 11324. (I) Cells present in a selected field of view. (II) Fluorescence detection with antibodies raised to S. mutans OMZ 175 antigen I/II.
Construction of antigen I/II isogenic mutants.
Eight colonies with resistance to Km were obtained. The transformation frequency was 0.7 × 10−4%. Two variants of isogenic mutants expressing truncated proteins of approximately 125 or 130 kDa were obtained (Fig. 3). The two variants were expected, since two Tsp509I-restricted fragments of 100 or 80 bp were added to the ligation mixture used to inactivate the antigen I/II gene (Fig. 1). The truncated protein in the two variants was found predominantly in the supernatant, as opposed to the wild-type antigen I/II, which was found primarily in the whole-cell extract (Fig. 3). A mutant expressing the lowest molecular mass of the truncated protein, IB08981, was randomly selected for functional characterization.
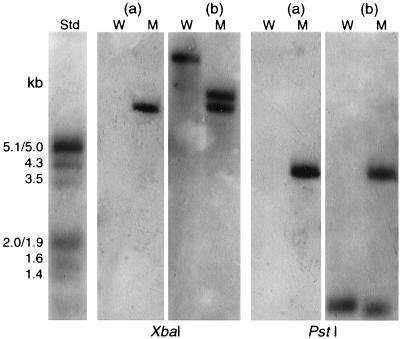
Disruption of the antigen I/II gene in IB08981 was verified by Southern hybridization of XbaI- and PstI-digested chromosomal DNAs with probes for the antigen I/II gene and for pSF151 (Fig. 7). Sequences recognized by XbaI and PstI are present in the pSF151 multiple-restriction site (44). The pSF151 probe hybridized with a 7.4-kb XbaI fragment and a 3.8-kb PstI fragment of IB08981 but not with the wild type (Fig. 7a). In the wild type, the antigen I/II probe hybridized with single XbaI or PstI fragments of approximately 14.0 and 0.8 kb, respectively. In the isogenic mutant, this probe hybridized with XbaI fragments of 7.4 and 9.8 kb and with PstI fragments of 0.7 and 3.8 kb (Fig. 7b).
FIG. 7.
Southern blot analysis of XbaI- and PstI-digested chromosomal DNA from the wild-type S. intermedius NCTC 11324 (W) and the isogenic mutant IB08981 (M), probed with pSF151 (a) or antigen I/II (b) digoxigenin-labeled probes. Std, Standard DNA Molecular Weight Marker III (Roche Diagnostics). kb, kilobases.
Surface hydrophobicity.
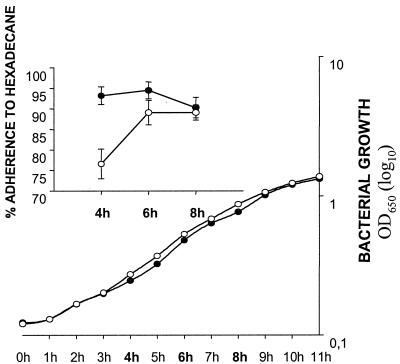
Cell hydrophobicity as determined by adsorption to hexadecane was calculated as the percentage of loss in optical density relative to that of the initial bacterial suspension. Maximum bacterial adsorption occurred with 150 μl of hexadecane. The mutant was 18% less hydrophobic than the wild type during early exponential phase (Fig. 8). No significant difference was observed during mid- and late exponential phases.
FIG. 8.
Hydrophobicity of S. intermedius NCTC 11324 (●) and the isogenic mutant IB08981 (○) during growth (top graph). Hydrophobicity was calculated as percent adsorption to hexadecane. Growth was monitored by measuring OD650 (bottom graph). The x axis for both graphs indicates time in hours (h). Error bars indicate standard deviations of triplicate samples.
Binding to matrix proteins.
Adhesion to rat collagen type I and to human collagen types I and IV was not significant for either the wild type or the isogenic mutant IB08981 (Table 1). As S. mutans OMZ 175 has been shown to bind to human collagen (28), this strain was included as a control. Compared with the wild type, adherence of the mutant to human fibronectin was reduced by 75% and to human laminin by 35% (Table 1).
TABLE 1.
Adherence to extracellular matrix proteinsb
| Streptococcal strain | Mean no. of cells bound (106) (SD)
|
||||
|---|---|---|---|---|---|
| Human fibronectin (n = 4) | Human laminin (n = 2) | Human collagen type I (n = 2) | Human collagen type IV (n = 2) | Rat collagen type I (n = 2) | |
| S. intermedius | |||||
| NCTC 11324 | 1.56 (0.47) | 5.63 (0.04) | NS | NS | NS |
| Isogenic mutant IB08981 | 0.36 (0.18)a | 3.60 (0.41)a | NS | NS | NS |
| S. mutans | |||||
| OMZ 175 | NT | NT | 21.72 (4.33) | 15.7 (1.24) | 25.5 (3.00) |
Significantly different from S. intermedius NCTC 11324 (P < 0.05).
NT, not tested. NS, counts not significantly different from background values.
Cytokine release by THP-1 cells after stimulation with the wild type and the isogenic mutant.
THP-1 cells were incubated at time zero in RPMI 1640 alone or with streptococcal cells at various concentrations. The stimulus was maintained for 4 h, which was compatible with a minimal cell viability rate of 60%. During the activation period, the number of bacteria increased twofold. The constitutive IL-8 release level was negligible. Up-regulation of IL-8 release was effective but variable among the streptococcal strains used in this study (Table 2). Compared with the wild type, the isogenic mutant IB08981 exhibited a significantly reduced capacity to stimulate the release of IL-8 (up to 32%). The amount of the truncated form of antigen I/II released by the mutant in the activation medium was estimated. We found that the approximate amount of antigen released during a 4-h activation period by the mutant was the equivalent of 0.5 μg/ml. Neither antigen I/II at this concentration nor 4-h-incubation bacterial supernatants were able to stimulate IL-8 release by THP-1 cells (not shown).
TABLE 2.
IL-8 release from THP-1 cells stimulated by S. intermedius or S. mutansa
| Streptococcal strain | CFU/wellb | IL-8 concn (SD) (n = 3)c |
|---|---|---|
| S. intermedius NCTC 11324 | 2 × 107 | 1.57 (0.05) |
| 4 × 106 | 1.03 (0.01) | |
| S. intermedius IB08981 | 2 × 107 | 1.07 (0.03)d |
| 4 × 106 | 0.85 (0.00)d | |
| S. mutans OMZ 175 | 2 × 107 | 1.50 (0.04) |
| Negative control | 0 | 0.04 |
Supernatants were tested after 4 h of incubation, which was consistent with a 60% THP-1 cell viability in the presence of a streptococcal concentration of 2 × 107 CFU/well.
Bacterial CFU/well at time zero.
The results are expressed as IL-8 mean concentration values in nanograms per milliliter.
Significantly different from S. intermedius NCTC 11324 (P < 0.05). Comparison between similar CFU values.
DISCUSSION
By immunoblotting, the molecular mass of antigen I/II in S. intermedius NCTC 11324 was estimated to be approximately 160 kDa. Two other strains, one from dental plaque and one from a brain abscess, expressed an antigenically related protein with similar molecular mass. In other oral streptococci for which the entire protein sequence is known, such as S. mutans, S. gordonii, and Streptococcus sobrinus, the reported molecular mass ranges from 170 to 215 kDa (7, 20, 35, 37, 45). These proteins show the conserved LPXTG C-terminal motif, found in gram-positive C-terminal-anchored proteins. S. mutans GS 5 varies, however, in that the molecule has a molecular mass of 155 kDa and is found mainly in the culture supernatant. This is attributed to a frameshift mutation in the antigen I/II-encoding gene, resulting in loss of the C-terminal cell wall anchoring domain (34). In S. intermedius NCTC 11324, a similar mutation to explain the lower molecular mass is unlikely. The isogenic mutant IB08981 expressed a truncated antigen I/II gene product, approximately 35 kDa smaller than that of the wild type. This difference corresponds to the calculated value between the C-terminal and the target sequence in S. mutans strains expressing the entire molecule. Thus, the difference in size is most likely due to differences upstream of the insertion site. The immunoblotting results corroborate this reasoning, since the amount of the protein was comparatively larger in the cell extract of the wild type than in the mutants, whereas in the mutants the protein was found almost exclusively in the supernatant. This may indicate that the protein is anchored in S. intermedius NCTC 11324, while disruption of antigen I/II caused loss of the C-terminal anchoring part and thus concomitant release of the truncated polypeptide. Immunofluorescence microscopy of the wild type supported the notion of a surface-located protein. However, a definitive conclusion as to the presence of a C-terminal anchoring region of antigen I/II in S. intermedius requires further investigation.
Antigen I/II in the cell extracts and supernatants of the S. intermedius wild type appeared as multiple bands in the immunoblot. Similar patterns have also been observed for S. gordonii (7), S. mutans (21), and S. sobrinus (22) antigen I/II proteins. Such multiple-band patterns are suggested to represent proteolytic degradation products.
Northern hybridization with the antigen I/II probe revealed a major band with a size compatible with that of the 160-kDa protein, indicating that the gene is transcribed as a monocistronic message. This has also been reported for the homologues sspA and sspB in S. gordonii (8, 10).
The effect of the growth phase on the expression of the S. intermedius antigen I/II gene was investigated by Northern blotting and immunoblotting. Maximal expression was observed in the early phase of exponential growth. The two tandemly arranged genes for antigen I/II in S. gordonii, sspA and sspB, have also been shown to be regulated in response to growth. Interestingly, the transcriptional activity differed for the two genes. Transcription of the sspA gene increased throughout growth, whereas sspB activity decreased in the stationary phase (10).
The present study revealed potential virulence factors associated with the S. intermedius antigen I/II by construction of antigen I/II isogenic mutants. To our knowledge, this is the first report where genetic recombination strategies are used to assist in the functional characterization of S. intermedius genes. The limited range of strains within the anginosus group that seem to be amenable to natural transformation probably explains why (16). The recent identification of competence-stimulating peptides in this group has, however, brought a new perspective to the application of gene transfer methods in S. intermedius (14). The use of synthetic competence-stimulating peptides for induction of Streptococcus pneumoniae competence indicates, for instance, that the conditions required for competence development can be more relaxed and can be extended to strains traditionally refractory to transformation (33).
The transformation efficiency of S. intermedius NCTC 11324 in the presence of the synthetic competence-stimulating peptide 11325 was approximately 2% (not shown) when using the streptococcal shuttle vector pVA838. As expected, insertion duplication with pSF151-targeting insert was lower (0.7 × 10−4%), as this event generally is less efficient and dependent on a variety of factors, such as ligation efficiency of the plasmid and insert, as well as the length of the insert. In S. pneumoniae, fragments as small as 96 bp have been found to target homologous insertion with high specificity (26). In our study we show insertion inactivation of the antigen I/II gene with homologous inserts of 80 and 100 bp. This is particularly useful in cases where only a short sequence of the gene of interest is known. At the time of our experiment, the known nucleotide sequence of the S. intermedius antigen I/II gene was restricted to a short segment of 350 bp (30).
Inactivation of the antigen I/II-encoding gene in S. mutans resulted in mutants with hydrophobicity reduced by more than 80% (13, 37). In our study, disruption of the S. intermedius antigen I/II gene affected hydrophobicity during the early phase of exponential growth but not during the mid- and late exponential phases. During the early exponential phase, a reduction by 18% was observed. Like in S. mutans (31), molecules other than antigen I/II may also affect surface hydrophobicity in S. intermedius. The variation with growth phase may result from differences in expression of hydrophobic surface-related molecules by S. intermedius during growth.
Antigen I/II was associated with the ability of S. intermedius to adhere to immobilized fibronectin. The adherence of streptococci to fibronectin is purported as a pivotal step in the pathogenesis of endocarditis and may be an important pathogenic determinant in the formation of abscesses (2, 50). Although various surface proteins may be involved in such binding, antigen I/II seems to play a major role, since the mutant expressing a truncated non-cell-anchored protein showed an approximately 75% reduction in adhesion to fibronectin.
Several strains of oral streptococci, particularly those isolated from the heart valves of patients with endocarditis, express receptors that interact specifically with laminin (43). Such interaction may be critical for the bacterial colonization of damaged tissue. We found that antigen I/II was associated with the binding of S. intermedius NCTC 11324 to laminin. The isogenic mutant exhibited a 35% reduction in adherence to laminin.
Adhesion of S. intermedius to immobilized human collagen types I and IV, as well as to rat collagen type I, was not significant for either the wild type or the mutant. Isogenic S. gordonii mutants which lack antigen I/II demonstrate, however, diminished adherence to collagen type I (29). This indicates that antigen I/II function may vary between the two species.
The role of S. mutans antigen I/II in adhesion to soluble extracellular matrix components, such as fibronectin, laminin, and collagen type I, has previously been reported (41). Differences in binding domains and conformation for soluble forms as employed in the latter study and for immobilized forms as used in our study restrict, however, further comparative analyses.
Activation experiments as performed for the present study suggested that S. intermedius antigen I/II may play a nonnegligible role in the activation of monocytes. The mutant was less efficient in inducing the release of IL-8 from THP-1 monocytic cells than was the wild type, notwithstanding the fact that a feeble amount of the truncated form of antigen I/II might be released in the activation medium.
The inactivation of the antigen I/II-encoding gene in S. intermedius NCTC 11324 provided evidence for a potential role of this protein in S. intermedius pathogenesis. It seems to be involved, at least in part, in binding to fibronectin and laminin and in inducing IL-8 release from monocytes. Other oral streptococci are also commonly associated with systemic infections. S. oralis, S. gordonii, Streptococcus sanguis, and S. mutans have, for instance, been implicated in the etiology of infective endocarditis. Unlike other oral streptococci, however, the anginosus group is frequently associated with severe purulent infections. Identification of antigen I/II common, as well as species-specific, functions and responses to variable environmental conditions, might have future clinical implications.
ACKNOWLEDGMENTS
We thank P. Gaustad for the gift of the competence-stimulating peptide 11325 and helpful advice on the S. intermedius transformation assay. We are grateful to M. Axelsson, S. Stig, M. Weidemann, and C. Affolter for excellent technical assistance. We also thank O. Schreus for technical help with the immunofluorescence microscopy.
REFERENCES
- 1.Al-Okla S, Chatenay-Rivauday C, Klein J P, Wachsmann D. Involvement of α5β1 integrins in interleukin 8 production induced by oral viridans streptococcal protein I/IIf in cultured endothelial cells. Cell Microbiol. 1999;1:157–168. doi: 10.1046/j.1462-5822.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 2.Baddour L M. Virulence factors among gram-positive bacteria in experimental endocarditis. Infect Immun. 1994;62:2143–2148. doi: 10.1128/iai.62.6.2143-2148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady L J, Piacentini D A, Crowley P J, Oyston P C, Bleiweis A S. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect Immun. 1992;60:1008–1017. doi: 10.1128/iai.60.3.1008-1017.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks W, Demuth D R, Gil S, Lamont R J. Identification of a Streptococcus gordonii SspB domain that mediates adhesion to Porphyromonas gingivalis. Infect Immun. 1997;65:3753–3758. doi: 10.1128/iai.65.9.3753-3758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatenay-Rivauday C, Yamodo I, Sciotti M A, Ogier J A, Klein J P. The A and the extended V N-terminal regions of streptococcal protein I/IIf mediate the production of tumour necrosis factor alpha in the monocyte cell line THP-1. Mol Microbiol. 1998;29:39–48. doi: 10.1046/j.1365-2958.1998.00881.x. [DOI] [PubMed] [Google Scholar]
- 6.Chatenay-Rivauday C, Yamodo I, Sciotti M A, Troffer-Charlier N, Klein J P, Ogier J A. TNF-alpha release by monocytic THP-1 cells through cross-linking of the extended V-region of the oral streptococcal protein I/II. J Leukoc Biol. 2000;67:81–89. doi: 10.1002/jlb.67.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Demuth D R, Duan Y, Brooks W, Holmes A R, McNab R, Jenkinson H F. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol Microbiol. 1996;20:403–413. doi: 10.1111/j.1365-2958.1996.tb02627.x. [DOI] [PubMed] [Google Scholar]
- 8.Demuth D R, Duan Y, Jenkinson H F, McNab R, Gil S, Lamont R J. Interruption of the Streptococcus gordonii M5 sspA/sspB intergenic region by an insertion sequence related to IS1167 of Streptococcus pneumoniae. Microbiology. 1997;143:2047–2055. doi: 10.1099/00221287-143-6-2047. [DOI] [PubMed] [Google Scholar]
- 9.Demuth D R, Lammey M S, Huck M, Lally E T, Malamud D. Comparison of Streptococcus mutans and Streptococcus sanguis receptors for human salivary agglutinin. Microb Pathog. 1990;9:199–211. doi: 10.1016/0882-4010(90)90022-i. [DOI] [PubMed] [Google Scholar]
- 10.El-Sabaeny A, Demuth D R, Park Y, Lamont R J. Environmental conditions modulate the expression of the sspA and sspB genes in Streptococcus gordonii. Microb Pathog. 2000;29:101–113. doi: 10.1006/mpat.2000.0369. [DOI] [PubMed] [Google Scholar]
- 11.Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10:257–285. doi: 10.1093/clinids/10.2.257. [DOI] [PubMed] [Google Scholar]
- 12.Hajishengallis G, Koga T, Russell M W. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res. 1994;73:1493–1502. doi: 10.1177/00220345940730090301. [DOI] [PubMed] [Google Scholar]
- 13.Harrington D J, Russell R R. Multiple changes in cell wall antigens of isogenic mutants of Streptococcus mutans. J Bacteriol. 1993;175:5925–5933. doi: 10.1128/jb.175.18.5925-5933.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Håvarstein L S, Hakenbeck R, Gaustad P. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J Bacteriol. 1997;179:6589–6594. doi: 10.1128/jb.179.21.6589-6594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes A R, McNab R, Jenkinson H F. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect Immun. 1996;64:4680–4685. doi: 10.1128/iai.64.11.4680-4685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob A E, Horton W A, Drucker D B. Genetic transformation in some cariogenic Streptococcus milleri. Microbios. 1989;60:167–175. [PubMed] [Google Scholar]
- 17.Jacobs J A. The ‘Streptococcus milleri’ group: Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius. Rev Med Microbiol. 1997;8:73–80. [Google Scholar]
- 18.Jenkinson H F, Demuth D R. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol Microbiol. 1997;23:183–190. doi: 10.1046/j.1365-2958.1997.2021577.x. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson H F, Terry S D, McNab R, Tannock G W. Inactivation of the gene encoding surface protein SspA in Streptococcus gordonii DL1 affects cell interactions with human salivary agglutinin and oral actinomyces. Infect Immun. 1993;61:3199–3208. doi: 10.1128/iai.61.8.3199-3208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly C, Evans P, Bergmeier L, Lee S F, Progulske-Fox A, Harris A C, Aitken A, Bleiweis A S, Lehner T. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 1989;258:127–132. doi: 10.1016/0014-5793(89)81632-1. [DOI] [PubMed] [Google Scholar]
- 21.Koga T, Okahashi N, Takahashi I, Kanamoto T, Asakawa H, Iwaki M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990;58:289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuykindoll R J, Holt R G. Characterization of a P1-deficient strain of Streptococcus mutans that expresses the SpaA protein of Streptococcus sobrinus. Infect Immun. 1996;64:3652–3658. doi: 10.1128/iai.64.9.3652-3658.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lamont R J, Gil S, Demuth D R, Malamud D, Rosan B. Molecules of Streptococcus gordonii that bind to Porphyromonas gingivalis. Microbiology. 1994;140:867–872. doi: 10.1099/00221287-140-4-867. [DOI] [PubMed] [Google Scholar]
- 25.LaPolla R J, Haron J A, Kelly C G, Taylor W R, Bohart C, Hendricks M, Pyati J P, Graff R T, Ma J K, Lehner T. Sequence and structural analysis of surface protein antigen I/II (SpaA) of Streptococcus sobrinus. Infect Immun. 1991;59:2677–2685. doi: 10.1128/iai.59.8.2677-2685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M S, Seok C, Morrison D A. Insertion-duplication mutagenesis in Streptococcus pneumoniae: targeting fragment length is a critical parameter in use as a random insertion tool. Appl Environ Microbiol. 1998;64:4796–4802. doi: 10.1128/aem.64.12.4796-4802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S F, Progulske-Fox A, Erdos G W, Piacentini D A, Ayakawa G Y, Crowley P J, Bleiweis A S. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II) Infect Immun. 1989;57:3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu T, Gibbons R J. Binding of streptococci of the ‘mutans’ group to type 1 collagen associated with apatitic surfaces. Oral Microbiol Immunol. 1990;5:131–136. doi: 10.1111/j.1399-302x.1990.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 29.Love R M, McMillan M D, Jenkinson H F. Invasion of dentinal tubules by oral streptococci is associated with collagen recognition mediated by the antigen I/II family of polypeptides. Infect Immun. 1997;65:5157–5164. doi: 10.1128/iai.65.12.5157-5164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma J K, Kelly C G, Munro G, Whiley R A, Lehner T. Conservation of the gene encoding streptococcal antigen I/II in oral streptococci. Infect Immun. 1991;59:2686–2694. doi: 10.1128/iai.59.8.2686-2694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBride B C, Song M, Krasse B, Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984;44:68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moisset A, Schatz N, Lepoivre Y, Amadio S, Wachsmann D, Scholler M, Klein J P. Conservation of salivary glycoprotein-interacting and human immunoglobulin G-cross-reactive domains of antigen I/II in oral streptococci. Infect Immun. 1994;62:184–193. doi: 10.1128/iai.62.1.184-193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison D A. Streptococcal competence for genetic transformation: regulation by peptide pheromones. Microb Drug Resist. 1997;3:27–37. doi: 10.1089/mdr.1997.3.27. [DOI] [PubMed] [Google Scholar]
- 34.Murakami Y, Nakano Y, Yamashita Y, Koga T. Identification of a frameshift mutation resulting in premature termination and loss of cell wall anchoring of the PAc antigen of Streptococcus mutans GS-5. Infect Immun. 1997;65:794–797. doi: 10.1128/iai.65.2.794-797.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogier J A, Scholler M, Leproivre Y, Pini A, Sommer P, Klein J P. Complete nucleotide sequence of the sr gene from Streptococcus mutans OMZ 175. FEMS Microbiol Lett. 1990;56:223–227. doi: 10.1016/0378-1097(90)90155-j. [DOI] [PubMed] [Google Scholar]
- 36.Oho T, Yu H, Yamashita Y, Koga T. Binding of salivary glycoprotein-secretory immunoglobulin A complex to the surface protein antigen of Streptococcus mutans. Infect Immun. 1998;66:115–121. doi: 10.1128/iai.66.1.115-121.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okahashi N, Sasakawa C, Yoshikawa M, Hamada S, Koga T. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol Microbiol. 1989;3:673–678. doi: 10.1111/j.1365-2958.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 38.Petersen F C, Scheie A A. Genetic transformation in Streptococcus mutans requires a peptide secretion-like apparatus. Oral Microbiol Immunol. 2000;15:329–334. doi: 10.1034/j.1399-302x.2000.150511.x. [DOI] [PubMed] [Google Scholar]
- 39.Piscitelli S C, Shwed J, Schreckenberger P, Danziger L H. Streptococcus milleri group: renewed interest in an elusive pathogen. Eur J Clin Microbiol Infect Dis. 1992;11:491–498. doi: 10.1007/BF01960802. [DOI] [PubMed] [Google Scholar]
- 40.Russell R R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979;114:109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- 41.Sciotti M A, Yamodo I, Klein J P, Ogier J A. The N-terminal half part of the oral streptococcal antigen I/IIf contains two distinct binding domains. FEMS Microbiol Lett. 1997;153:439–445. doi: 10.1111/j.1574-6968.1997.tb12608.x. [DOI] [PubMed] [Google Scholar]
- 42.Soell M, Holveck F, Scholler M, Wachsmann R D, Klein J P. Binding of Streptococcus mutans SR protein to human monocytes: production of tumor necrosis factor, interleukin 1, and interleukin 6. Infect Immun. 1994;62:1805–1812. doi: 10.1128/iai.62.5.1805-1812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Switalski L M, Murchison H, Timpl R, Curtiss R D, Hook M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987;169:1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao L, LeBlanc D J, Ferretti J J. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene. 1992;120:105–110. doi: 10.1016/0378-1119(92)90016-i. [DOI] [PubMed] [Google Scholar]
- 45.Tokuda M, Okahashi N, Takahashi I, Nakai M, Nagaoka S, Kawagoe M, Koga T. Complete nucleotide sequence of the gene for a surface protein antigen of Streptococcus sobrinus. Infect Immun. 1991;59:3309–3312. doi: 10.1128/iai.59.9.3309-3312.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unnikrishnan M, Cohen J, Sriskandan S. Growth-phase-dependent expression of virulence factors in an M1T1 clinical isolate of Streptococcus pyogenes. Infect Immun. 1999;67:5495–5499. doi: 10.1128/iai.67.10.5495-5499.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vernier A, Diab M, Soell M, Haan-Archipoff G, Beretz A, Wachsmann D, Klein J P. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect Immun. 1996;64:3016–3022. doi: 10.1128/iai.64.8.3016-3022.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westergren G, Olsson J. Hydrophobicity and adherence of oral streptococci after repeated subculture in vitro. Infect Immun. 1983;40:432–435. doi: 10.1128/iai.40.1.432-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiley R A, Beighton D, Winstanley T G, Fraser H Y, Hardie J M. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): association with different body sites and clinical infections. J Clin Microbiol. 1992;30:243–244. doi: 10.1128/jcm.30.1.243-244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willcox M D. Potential pathogenic properties of members of the Streptococcus milleri group in relation to the production of endocarditis and abscesses. J Med Microbiol. 1995;43:405–410. doi: 10.1099/00222615-43-6-405. [DOI] [PubMed] [Google Scholar]
- 51.Willcox M D, Loo C Y, Harty D W, Knox K W. Fibronectin binding by Streptococcus milleri group strains and partial characterisation of the fibronectin receptor of Streptococcus anginosus F4. Microb Pathog. 1995;19:129–137. doi: 10.1006/mpat.1995.0052. [DOI] [PubMed] [Google Scholar]
- 52.Wu W, Welsh M J, Kaufman P B, Zhang H H. Methods in gene biotechnology. Boca Raton, Fla: CRC Press, Inc.; 1997. [Google Scholar]