Abstract
Colorectal cancer (CRC) is one of the most common malignant tumor types occurring in the digestive system. The incidence of CRC has exhibits yearly increases and the mortality rate among patients with CRC is high. The Wnt/β-catenin signaling pathway, which is associated with carcinogenesis, is abnormally activated in CRC. Most patients with CRC have adenomatous polyposis coli mutations, while half of the remaining patients have β-catenin gene mutations. Therefore, targeting the Wnt/β-catenin signaling pathway for the treatment of CRC is of clinical value. In recent years, with in-depth research on the Wnt/β-catenin signaling pathway, inhibitors have been developed that are able to suppress or hinder the development and progression of CRC. In the present review, the role of the Wnt/β-catenin signaling pathway in CRC is summarized, the research status on Wnt/β-catenin pathway inhibitors is outlined and potential targets for inhibition of this pathway are presented.
Keywords: colorectal cancer, Wnt/β-catenin signaling pathway, occurrence, recurrence, inhibitors, targeted therapy
1. Introduction
As the fourth most common malignant cancer type worldwide, colorectal cancer (CRC) is the second leading cause of cancer-related death. Its high morbidity and mortality rates indicate that this disease has a serious impact on human health, with the third (10%) and second (9.4%) highest morbidity and mortality rates, respectively, for all tumors globally in 2020 (1,2). In the past, CRC was mainly treated by surgery, chemotherapy and radiotherapy, but recurrence and distant metastasis after treatment remain challenges in the treatment of CRC (3,4). It has been demonstrated that the Wnt/β-catenin signaling pathway has an important role in the development, progression, metastasis and recurrence of CRC (5-7). In most patients with CRC, the Wnt/β-catenin signaling pathway has abnormal levels of activation (8) and blocking the Wnt/β-catenin signaling pathway may inhibit the development, progression, invasion and metastasis of CRC. The development of therapeutic drugs that target the Wnt/β-catenin signaling pathway is of current interest in the research community. Therefore, the Wnt/β-catenin signaling pathway is expected to become a target for the treatment of CRC.
2. Current status of CRC treatments
The development and procession of CRC are complex processes that are related to several individual factors, including lifestyle, environmental factors and genetics, but the exact mechanism remains to be fully elucidated, while a previous study has indicated that individuals with adenomas have a higher risk of developing CRC (9). Among the different risk factors, heredity is an independent risk factor for CRC and patients without a family history only occasionally develop CRC. Although several CRC-related genetic factors remain elusive, mutations in genes associated with CRC (such as single nucleotide polymorphisms) have been successfully identified (10). Thus, more in-depth studies are required to confirm the genetic mechanisms of CRC. Of note, due to the insidious early symptoms of CRC, at the time of diagnosis, half of the patients have already progressed to the advanced stage of the disease. The prognosis of patients is poor and the 5-year overall survival (OS) rate is <15% (11,12).
In recent years, with progress in the related fields of immunology and oncology, members of the T-cell regulator CD28 superfamily, including programmed cell death protein-1 (PD-1), programmed cell death ligand-1 (PD-L1) and their inhibitors, represent new targets in strategies for anti-CRC therapy (13). Compared with traditional therapy, such as chemotherapy and radiotherapy alone, PD-1/PD-LI inhibitors significantly shorten the time to remission and there is a lower rate of adverse events following treatment (3,14). However, patients with tumors that display microsatellite stability and a low proficiency of mismatch repair, which includes the vast majority of patients with advanced CRC, do not exhibit any signs of obvious remission, and most do not respond to immunotherapy (15,16). Therefore, research on the Wnt pathway is of practical significance and value, particularly in light of the current status of therapeutic treatments.
3. Overview of the Wnt signaling pathway
It has been demonstrated that the Wnt signaling pathway, the transforming growth factor-β (TGF-β) signaling pathway and the Hedgehog signaling pathway all have a role in colorectal tumors (17,18). Among them, the Wnt signal transduction pathway, which leads to tumor invasion, recurrence and metastasis, is abnormally activated in most patients with CRC and it has an important role in the development and progression of CRC (19).
The Wnt protein family is involved in numerous cellular functions and comprises 19 secreted glycoproteins, which may transmit extracellular signals intracellularly through cell surface receptors; therefore, these proteins participate in a wide variety of biological processes, such as embryonic development and organ formation, cell proliferation, cell differentiation and stem cell self-renewal (20,21). The Wnt signaling pathway may be generally divided into the following two categories: The β-catenin-dependent canonical pathway and the β-catenin-independent noncanonical pathway (22).
Wnt/β-catenin canonical signaling pathway
The Wnt/β-catenin signaling pathway is the classical pathway of Wnt signaling and its abnormal activation may lead to the development, progression, invasion, metastasis and recurrence of different types of tumor, including CRC, in humans (8). The key mechanism underlying the Wnt-induced promotion of tumor-cell proliferation and neoplasia is β-catenin protein stabilization and nuclear translocation, which is mediated by Wnt.
In the absence of Wnt activation, the destruction complex composed of glycogen synthase kinase 3-β (GSK-3β), casein kinase 1α (CK1α), adenomatous polyposis coli (APC) and Axin may enhance the ubiquitination of β-catenin through regulation of the β-transducin-repeat-containing protein, which mediates ubiquitin ligase E3 and eventually leads to the absorption and degradation of β-catenin in the cytoplasm (23). In the case of Wnt activation, Wnt binds to the transmembrane Frizzled (FZD) receptor and the low-density lipoprotein receptor-related protein (LRP)5 and LRP6 coreceptors to trigger the dissociation of GSK-3β from the destruction complex. At the same time, Dishevelled (DVL) and Axin accumulate in the cell membrane, inhibiting the formation of the destruction complex (24,25). Based on this, β-catenin cannot be degraded and tends to be stabilized, accumulate in the cytoplasm and translocate to the nucleus to bind to the T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) family of transcription factors and coactivators. This binding induces the transcription of genes important for Wnt final activity, including c-Myc, survivin, c-Jun, cyclin D1 and Wnt1-inducible-signaling pathway protein 1 (Wisp1), which leads to the development and progression of CRC (7,20). The specific mechanism is as follows: Proto-oncogenes, such as c-Jun, cyclin D1 and c-Myc, promote cell proliferation, differentiation and maturation and eventually lead to tumorigenesis. While survivin is a new member of the apoptosis suppressor protein family, it is specifically expressed only in tumor and embryonic tissues, and it promotes tumor cell differentiation, proliferation, infiltration and metastasis. Wisp1 inhibits the caspase cascade reaction and suppresses apoptosis, thereby inducing tumorigenesis.
Noncanonical signaling pathway
The main noncanonical Wnt signaling pathways are the Wnt/Ca2+ (7) and Wnt/planar cell polarity (PCP) signaling pathways (26). After Wnt binds to FZD receptors and tyrosine kinase-like coreceptors, DVL aggregates on the cell membrane and initiates the Wnt/Ca2+ and Wnt/PCP signaling pathways, respectively. In the PCP signaling pathway, DVL activates disheveled-associated activator of morphogenesis 1 (DAAM1), DAAM1 dissociates RhoA and RhoA activates Rho-associated kinase, which affects the formation of the cytoskeleton. On the other hand, DVL activates Ras-related C3 botulinum toxin substrate (Rac) and Rac may activate c-Jun N-terminal kinase, which ultimately affects gene transcription (27). However, the Wnt/Ca2+ signaling pathway mainly relies on the activation of phospholipase C, which promotes the generation of calcium ions and ultimately affects DNA transcription.
Of note, Wnt binding to FZD-activated DVL causes calcium to be released from the endoplasmic reticulum and activates calcium-binding proteins, including protein kinase C and calcium/calmodulin-dependent protein kinase II, which enhances the phosphorylation of TCF/LEF (27-29). This phosphorylation blocks the canonical Wnt pathway and inhibits the expression of its downstream target genes, which suppresses the development of colorectal tumors (27-29). There is also a clear interaction between the canonical and noncanonical Wnt pathways, wherein the noncanonical pathway may reduce the occurrence of colorectal tumors by blocking the canonical pathway (7,26-29).
4. Role of the Wnt/β-catenin signaling pathway in the development and progression of CRC
The maintenance of normal intestinal function and stem-cell homeostasis requires normal activation of Wnt signaling. It has been verified that inhibition of the Wnt/β-catenin signaling pathway may lead to a lack of intestinal stem cells (30), whereas the number of intestinal stem cells increases at the bottom of intestinal crypts (31), where the activity of the Wnt/β-catenin signaling pathway is highest (32,33). These findings indicate that Wnt/β-catenin signaling has a critical role in the maintenance and self-renewal of intestinal stem cells, particularly epithelial stem cells at the base of intestinal crypts (34,35).
It has been indicated that abnormal activation of the Wnt/β-catenin signaling pathway is a cause of CRC development and progression, and most patients with CRC have mutations in this pathway (19). Abnormal activation of the Wnt/β-catenin pathway may lead to accumulation of the β-catenin protein in the nucleus, causing massive cell proliferation (7,36,37). Initially, this leads to the formation of adenomas, but with the accumulation of several mutations, adenomas eventually develop into CRC (7,36). In patients with sporadic CRC, a β-catenin mutation may be found in 10% of patients, while the rate of mutation in the APC gene is up to 80% in patients (7,37). The absorption and degradation of β-catenin is suppressed in patients that have an APC mutation (7,37,38). This suppression causes further accumulation of β-catenin in the cytoplasm and an increase in the amount of it translocating into the nucleus, both of which contribute to the progression of CRC (7,36,37). In addition, the Wnt/β-catenin signaling pathway may induce tumor resistance and targeted inhibition of the Wnt/β-catenin pathway in digestive tract tumors may enhance the tumors' sensitivity to chemotherapeutic drugs (39,40). Wnt/β-catenin signaling may also induce epithelial-mesenchymal transition (EMT), which has a vital role in the physiological processes of intestinal and CRC stem cells (41,42). More importantly, abnormal activation of the Wnt/β-catenin signaling pathway and accumulation of β-catenin has been confirmed in >80% of patients with CRC (43). Therefore, inhibiting Wnt/β-catenin signaling and the accumulation of β-catenin in the nucleus may inhibit the development, progression, invasion, metastasis and recurrence of CRC. Overall, the Wnt/β-catenin signaling pathway may become an effective target for CRC treatment and studies investigating how to inhibit this pathway are of practical and clinical significance.
5. Advances in anti-colorectal tumor therapy targeting the canonical Wnt signaling pathway
Studies investigating the targeting of the Wnt signaling pathway and blocking its signal transduction have indicated that this pathway may stabilize the formation of the destruction complex, promote the degradation of β-catenin and interfere with β-catenin-dependent transcription factors, all of which hinder the development, progression, invasion, metastasis and recurrence of colorectal tumors (44). Research aimed at inhibiting the Wnt/β-catenin signaling pathway may provide treatments that benefit patients with CRC by improving their quality of life and prolonging their survival time. Inhibitors of the Wnt/β-catenin signaling pathway that have been discovered in previous years are summarized below and are outlined in Table I and Fig. 1.
Table I.
Inhibitors of the Wnt/β-catenin signaling pathway.
| A, Wnt ligands and receptor inhibitors
| |||||||
|---|---|---|---|---|---|---|---|
| Name of the inhibitor | Molecular target | Effect on target | Trial phase | Interventions | Trial identifier | Status | (Refs.) |
| OMP-18R5 | Frizzled | Inhibition | Phase I | Drugs: OMP-18R5 combined with paclitaxel | NCT01973309 | Completed | (83) |
| OMP-54F28 | Frizzled | Inhibition | i) Phase I; and ii) phase I | i) Drugs: OMP-54F28, Nab-Paclitaxel andGemcitabine; and ii) Drugs: OMP-54F28 Paclitaxel, and Carboplatin | i) NCT02050178; and ii) NCT02092363 | i) Completed; and ii) Completed | (86,87) |
| LGK974 | Porcupine | Inhibition | Phase I | Drug: LGK974 | NCT01351103 | Recruiting | (106) |
| IWP | Porcupine | Inhibition | Preclinical | - | - | - | (107) |
| Wnt-C59 | Porcupine | Inhibition | Preclinical | - | - | - | (108-114) |
| ETC-159 | Porcupine | Inhibition | Preclinical | - | - | - | (115,117) |
| NVP-TNKS656 | Axin | Activation | Preclinical | - | - | - | (119,120) |
| NVP-XAV939 | Axin | Activation | Preclinical | - | - | - | (121-127) |
| IWR | Axin | Activation | Preclinical | - | - | - | (128,129) |
| G244-LM | Axin | Activation | Preclinical | - | - | - | (131,132) |
| G007-LK | Axin | Activation | Preclinical | - | - | - | (133-136) |
| JW55, JW67, JW74 | Axin | Activation | Preclinical | - | - | - | (136) |
| Shizukaol D | Axin | Activation | Preclinical | - | - | - | (108,137) |
|
| |||||||
| B, β-catenin-TCF/LEF complex inhibitors
| |||||||
| Name of the inhibitor | Molecular target | Effect on target | Trial phase | Interventions | Trial identifier | Status | (Refs.) |
| PKF118-310 | β-catenin/TCF | Inhibition | Preclinical | - | - | - | (45) |
| PKF115-584 | β-catenin/TCF | Inhibition | Preclinical | - | - | - | (46-48) |
| CWP232228 | β-catenin/TCF | Inhibition | Preclinical | - | - | - | (49-51) |
| 2,4-DAQ | β-catenin/TCF | Inhibition | Preclinical | - | - | - | (56-58) |
| DHME | β-catenin/TCF | Inhibition | Preclinical | - | - | - | (59) |
| PNU-74654 | β-catenin/TCF4 | Inhibition | Preclinical | - | - | - | (60) |
| GB1874 | β-catenin/TCF4 | Inhibition | Preclinical | - | - | - | (61) |
| ICAT | β-catenin/TCF | Inhibition | Preclinical | - | - | - | (62) |
| NCB-0846 | β-catenin/TCF4 | Inhibition | Preclinical | - | - | - | (64-67) |
| LF3 | β-catenin/TCF4 | Inhibition | Preclinical | - | - | - | (68,69) |
| NSAIDs | β-catenin-TCF/LEF | Inhibition | Phase III | Drug: Aspirin | NCT02607072 | Recruiting | (71-74) |
|
| |||||||
| C, Natural compounds
| |||||||
| Name of the inhibitor | Molecular target | Effect on target | Trial phase | Interventions | Trial identifier | Status | (Refs.) |
| Curcumin | β-catenin | Inhibition | i) Phase I; ii) phase I; and iii) phase II | i) Drug: Curcumin; Drug: 5-flurorouracil; ii) Drug: Curcumin; Drug: Irinotecan; and iii) Drug: Capecitabine; Drug: Curcumin; Radiation: Radiation therapy | i) NCT02724202; ii) NCT01859858; and iii) NCT00745134 | i) Unknown; ii) Completed; and iii) Terminated | - |
| Genistein | GSK-3β | Activation | Phase I and II | Drug: Genistein | NCT01985763 | Completed | (144) |
| Vitamin D | β-catenin | Inhibition | i) Not applicable; and ii) phase I | i) Cholecalciferol; and ii) Drug: Vitamin D3 | i) NCT02603 757; and ii) NCT02172651 | i) Completed; and ii) Recruiting | - |
| Brucine | β-catenin | Inhibition | Preclinical | - | - | - | (151,152) |
| Berberine | β-catenin | Inhibition | Preclinical | - | - | - | (153,154) |
| Ginsenosides | β-catenin | Inhibition | Preclinical | - | - | - | (155-158) |
| Tanshinone IIA | HIF1-α | Inhibition | Preclinical | - | - | - | (159) |
| Z-ajoene | β-catenin | Inhibition | Preclinical | - | - | - | (160,161) |
| Artemisinin | β-catenin | Inhibition | Preclinical | - | - | - | (163-165) |
| Nerigoside | GSK-3β | Activation | Preclinical | - | - | - | (166) |
| Vicenin-2 | β-catenin-TCF/LEF | Inhibition | Preclinical | - | - | - | (167) |
| Ginkgolide C | β-catenin | Inhibition | Preclinical | - | - | - | (168) |
| Resveratrol | β-catenin | Inhibition | i) Phase I; and ii) phase I | i) Drug: SRT501; and ii) Dietary supplement: Grapes | i) NCT00920803; and ii) NCT00578396 | i) Completed; and ii) Withdrawn | (170) |
| Andrographolide analog 3A1 | β-catenin-TCF/LEF | Inhibition | Preclinical | - | - | - | (171) |
| DIF-3 | GSK-3β | Activation | Preclinical | - | - | - | (172-174) |
| Z86 | GSK-3β | Activation | Preclinical | - | - | - | (175) |
|
| |||||||
| D, Other Wnt/β-catenin signaling pathway inhibitors
| |||||||
| Name of the inhibitor | Molecular target | Effect on target | Trial phase | Interventions | Trial identifier | Status | (Refs.) |
| KYA1797K | Axin | Activation | Preclinical | - | - | - | (176-178) |
| BC2059 | β-catenin | Inhibition | Preclinical | - | - | - | (179,180) |
| ICG-001 | β-catenin/TCF | Inhibition | Preclinical | - | - | - | (181-183) |
| Pyrvinium | CK1α | Activation | Preclinical | - | - | - | (184,185,187) |
| YW2065 | Axin | Activation | Preclinical | - | - | - | (186) |
| SR3029 | CK1 | Inhibition | Preclinical | - | - | - | (188) |
| BBI608 | STAT3 | Inhibition | i) Phase III; ii) phase II; iii) phase I and II; and iv) phase II | i) Drug: BBI608; Other: Best supportivecare; ii) Drugs: BBI608, Panitumumab, Capecitabine, Cetuximab; iii) Drug: Napabucasin; Drug: Pembrolizumab; and iv) Drug: Nivolumab; Drug: BNC 105; Drug: BBI608 | i) NCT01830621; ii) NCT01776307; iii) NCT02851004; and iv) NCT03647839 | i) Completed; ii) Completed; iii) Terminated; and iv) Completed | (193) |
| SM08502 | CLK | Inhibition | Preclinical | - | - | - | (194) |
| ST2825 | MYD88 | Inhibition | Preclinical | -(201) | - | - | (195) |
| FH535 | β-catenin | Inhibition | Preclinical | - | - | - | (196,197) |
| Acetyltransferase P300 inhibitor | TRIB3 | Inhibition | Preclinical | - | - | - | (198,199) |
| Apatinib | GSK-3β | Activation | Preclinical | - | - | - | (200) |
| Obatoclax | Bcl-2 | Inhibition | Preclinical | - | - | - | (201) |
| NSC668036 | β-catenin | Inhibition | Preclinical | - | - | - | (210) |
| Apicularen, Bafilomycin | ATPase | Inhibition | Preclinical | - | - | - | (211,212) |
| DK419, P5091 | β-catenin | Inhibition | Preclinical | - | - | - | (213,214) |
| 36-077 | PIK3C3/VPS34 kinase | Inhibition | Preclinical | - | - | - | (215) |
| OVOL2 | β-catenin/TCF | Inhibition | Preclinical | - | - | - | (216) |
| Niclosamide | Frizzled | Inhibition | FDA-approved | - | - | - | (217) |
| Pimozide | β-catenin | Inhibition | FDA-approved | - | - | - | (218,219) |
| Ethacrynic acid | β-catenin/LEF-1 | Inhibition | FDA-approved | - | - | - | (220) |
-, not available. TCF/LEF, T-cell factor/lymphoid enhancer-binding factor; DHME, dehydroxyhispolon methyl ether; ICAT, inhibitor of β-catenin and TCF; NSAIDs, nonsteroidal anti-inflammatory drugs; CK1, casein kinase 1; STAT3, signal transducer and activators of transcription 3; MYD88, myeloid differentiation factor 88; TRIB3, tribbles homolog 3; PIK3C3/VPS34, phosphoinositide-3-kinase, class 3/vacuolar protein sorting 34; CLK, CDC-like kinase; FDA, Food and Drug Administration.
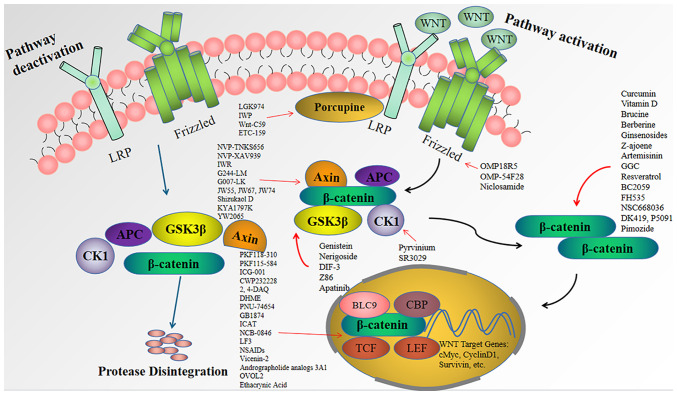
Figure 1.
Schematic illustration of the Wnt/β-catenin signaling pathway. Inactive: In cells where the Wnt signaling pathway is inactive, β-catenin is degraded by the proteasome and cannot enter the nucleus. Active: In the presence of Wnt ligands, disruption of complex dissociation leads to β-catenin accumulation in the nucleus and activation of transcription by binding to TCF/LEF. This binding causes transcription of Wnt downstream target genes, including c-Myc, survivin, cyclin D1, etc., which promotes cell proliferation, differentiation and maturation, leading to CRC development. Porcupine inhibitors such as LGK974 can suppress palmitoylation of Wnt proteins and block Wnt signaling. OMP-18R5 and others can block Wnt/β-catenin signaling by binding to Frizzled. NVP-TNKS656, Genistein and SR3029 can act on Axin, GSK3β and CK1, respectively, to stabilize the 'damage complex' and promote the degradation of β-catenin, thus blocking Wnt signaling. Compounds such as curcumin can inhibit Wnt signaling by directly acting on β-catenin and increasing β-catenin degradation, thus blocking Wnt signaling. PKF118-310 and others can effectively inhibit the formation of β-catenin-TCF complex, and suppress the activation of Wnt downstream target genes, and thus, inhibit the development of CRC. LRP, low density lipoprotein receptor-related protein; CK1, casein kinase 1; APC, adenomatous polyposis coli; DVL, dishevelled; CBP, cAMP response element-binding protein; TCF/LEF, T-cell factor/lymphoid enhancer-binding factor.
Inhibitors of the β-catenin-TCF complex
The TCF/LEF family is a group of transcription factors that bind DNA through high mobility group motifs, and they recruit the coactivator β-catenin protein to enhance the expression of the genetic elements they target. The typical Wnt signaling pathway is activated by a combination of β-catenin and TCF/LEF transcription factors, which then activates target genes that are downstream of the Wnt pathway, such as cyclin D1 and c-Myc and other proto-oncogenes, leading to cell proliferation and mediating the development and progression of tumors (20). Therefore, inhibiting the formation of the β-catenin-TCF/LEF complex may interrupt the expression of downstream target genes and block the formation, invasion and metastasis of colorectal tumors.
PKF115 -584, PKF118 -310 and CWP232228
Paluszczak et al (45) indicated that the compounds PKF115-584 (perylene; carbonic acid derivative) and PKF118-310 (toxoflavin) may effectively inhibit the formation of the β-catenin-TCF complex, further inhibiting the proliferation of CRC cells and thereby exerting antitumor effects. In addition, previous studies demonstrated that the compound PKF115-584 inhibited the proliferation of CRC cells in a dose-dependent manner, as did the compound CGP049090 (cercosporin), and the two were also able to interfere with the interaction between β-catenin and APC (46-48). CWP232228, as a highly selective inhibitor of the Wnt/β-catenin signaling pathway, antagonizes β-catenin and TCF binding in the nucleus. Kim et al (49) confirmed that CWP232228 treatment had a dose-dependent cytotoxic effect in HCT-116 human colon cancer cells and blocked the growth of mouse xenogeneic colon cancer cells. Furthermore, CWP232228 was reported to interrupt the growth of bulk breast cancer stem cells (CSCs) in patients with breast cancer (50) and suppress the self-renewal capability of liver CSCs and reduce tumorigenicity in vitro and in vivo (51).
The Ras gene has a critical role in the development and progression of tumors in humans. Clinically, the Kirsten rat sarcoma viral oncogene (KRAS) mutation is the most common mutation of the Ras gene and patients with a KRAS mutation have unfavorable prognosis and a higher level of tumor invasion and metastasis (52). Approximately half of the patients with colon cancer express the KRAS mutation, and in CRC cells expressing activated KRAS, the Wnt/β-catenin signaling pathway is activated (53). Furthermore, KRAS mutation disrupts the negative feedback mechanism of its target microRNA (miR)-139-5p and Wnt signaling, resulting in downregulation of the tumor suppressor miR-139-5p and promotion of tumor growth (54). Hence, the simultaneous downregulation of β-catenin and KRAS is key to blocking the development and progression of CRC. Mologni et al (55) indicated that the combination of PKF115-584 and the KRAS inhibitor S-trans was superior to any monotherapy in arresting cell growth, causing cell death and downregulating c-Myc and survivin.
2,4-diamino-quinazoline (2,4-DAQ) and dehydroxyhispolon methyl ether (DHME)
Studies including that by Chen et al (56) indicated that 2,4-DAQ derivatives are potent inhibitors of β-catenin-TCF/LEF-mediated transcription and proliferation in CRC cells and xenograft models (56,57). Further research indicated that 2,4-DAQ is able to reduce the expression of the β-catenin target genes c-Myc, Axin2 and LGR5, and inhibit the proliferation and viability of CSCs. More importantly, 2,4-DAQ may reduce the migratory and invasive activities of tumor cells, suppress the growth of xenograft tumors and reduce the final tumor weights with few side effects and low drug toxicity (58).
DHME, a chemically synthesized derivative of the fungal polyphenol hispolon has been indicated to block signaling of the Wnt canonical pathway by inhibiting the transcriptional activity of the β-catenin/TCF complex. It exhibits strong apoptosis-inducing activity and has a significant role in blocking the development and progression of CRC (59).
PNU-74654, GB1874 and inhibitor of b-catenin and TCF (ICAT)
PNU-74654 is a small molecule that disrupts Wnt pathway signaling by suppressing the interaction between β-catenin and TCF4. Of note, Wu et al (60) found that pleomorphic adenoma gene like-2 (PLAGL2) has a role in promoting CRC cells by upregulating and stabilizing the level of β-catenin and promoting the translocation of β-catenin into the nucleus, and as PNU-74654 blocks PLAGL2 activity in CRC cells, it further prevents the development, progression and metastasis of CRC. GB1874 blocks Wnt signaling by targeting the β-catenin-TCF4 protein-protein interaction, thereby inhibiting xenograft tumor growth in vivo and CRC stem cell proliferation in vitro (61).
ICAT was found to be a polypeptide that downregulates the expression of genes that are targeted by the Wnt pathway, such as cyclin D1 and Myc, by blocking the β-catenin-TCF interaction, which significantly reduces the growth of CRC cells and tumor formation (62). Furthermore, patients with CRC and high ICAT levels have a favorable prognosis. The study provides a scientific basis for treatment based on ICAT and opens a new avenue for their use in patients with CRC.
NCB-0846 and LF3
Traf2 and Nck-interacting protein kinase (TNIK) is an important component of the β-catenin/TCF-4 transcription complex, which is crucial for the development and progression of CRC (63). Downregulation of TNIK reduces the transcriptional activity of the β-catenin/TCF-4 complex, thereby inhibiting the growth of CRC and xenograft tumor cells (64). The TNIK inhibitor NCB-0846 reduced the expression of Wnt target genes and inhibited the development of colorectal tumors and the stemness of CRC cells. Furthermore, NCB-0846 also inhibited oncogenic proteins, such as FMS-like tyrosine kinase 3, platelet-derived growth factor-α and cyclin-dependent kinase 2/cyclin A2 (64). Of note, NCB-0846 inhibited lung cancer metastasis by downregulating the expression of TGF-β receptor type-I and blocking the TGF-β pathway and the SMAD signaling pathway (65,66). Of note, it has been indicated that in patients with CRC carrying a KRAS mutation, the expression of the antiapoptotic protein BCL-XL is increased, and the BCL-XL inhibitor ABT-263 synergized with NCB-0846 to induce cell death and inhibit tumor cell proliferation (67).
LF3, a novel inhibitor of the β-catenin and TCF4 interaction, blocks the self-renewal capacity of CSCs (68). LF3 significantly inhibits tumor cell hypermobility and progression of the cell cycle. Its inhibitory effect on tumor cells is dose-dependent and it has a certain therapeutic effect in Wnt/β-catenin-dependent cancers (69).
Nonsteroidal anti-inflammatory drugs (NSAIDs)
NSAIDs may reduce the transcriptional activity of the β-catenin-TCF/LEF complex and inhibit the classic Wnt/β-catenin signaling pathway (70). Previous studies have confirmed that after treatment with diclofenac, celecoxib and sulindac, the degradation of intracellular β-catenin was increased, β-catenin translocation to the nucleus was reduced, and the expression of Wnt target genes, such as Axin2, cyclin D1 and c-Myc, was also reduced (71,72), thus suppressing the proliferation of tumor cells and inducing their apoptosis. Egashira et al (73) found that 2,5-dimethyl celecoxib, an analog of celecoxib, reduced the expression of Wnt target genes and further exhibited a significant antitumor effect. More importantly, repeated use of 2,5-dimethyl celecoxib was able to significantly reduce the size and number of tumors without any drug toxicity, suggesting that 2,5-dimethyl celecoxib is a new potential anticancer treatment. Of note, the combination of an NSAID (sulindac) and rexinoid (bexarotene) resulted in a durable amelioration of familial adenomatous polyposis, thereby preventing the development of CRC (74). Furthermore, a clinical trial is currently investigating the role of aspirin as an adjunctive drug for the prevention of postoperative CRC recurrence and metastasis (NCT02607072).
Wu et al (75) indicated the presence of positive feedback between cyclooxygenase 2 (Cox-2)/prostaglandin E2 (PGE2) and the Wnt pathway, namely Cox-2 promotes the proliferation and self-renewal of CSCs through PGE2, thereby activating the Wnt pathway. However, NSAIDs may inhibit the activity of Cox-2, interfere with the positive feedback between Cox-2/PGE2 and the Wnt pathway and restrict the development and progression of tumors. However, the incidence of adverse events associated with traditional NSAID treatment is high and due to their extent, they may even affect the quality of life of patients. Therefore, to reduce the adverse effects of NSAIDs, the lowest effective dose should be used and the duration of treatment should be limited. Furthermore, the choice of drug type and dose should be individualized and the patient's underlying conditions should be fully considered. For instance, attention should be paid to the dual cardiovascular and gastrointestinal risks in elderly patients. Selective Cox-2 inhibitors should be used in patients with gastrointestinal risk factors, and enteric tablet slow release reduces the direct damage to the gastric mucosa as the drug passes through the gastrointestinal tract. NSAIDs should be used with caution if the patient is at risk of adverse cardiovascular events and the concomitant use of two or more NSAIDs should be avoided (76-78).
Wnt ligands and receptor inhibitors
OMP-18R5 and OMP-54F28
Wnt ligands bind to corresponding receptors, such as FZD receptors and LRP5 and LRP6 coreceptors, thereby initiating Wnt/β-catenin signaling and allowing β-catenin to accumulate in the cytoplasm, translocate into the nucleus and activate downstream target genes to induce tumor growth (79). OMP-18R5 (vantictumab) is a monoclonal antibody that binds to members of the FZD family and inhibits the cascade of tumorigenesis and progression by blocking Wnt/β-catenin signaling (80). It has been indicated that OMP-18R5 significantly inhibited the growth of CRC cells and xenograft tumors, and OMP-18R5 synergized with irinotecan (80). In other tumor models, such as those of pancreatic cancer and gastric cancer, OMP-18R5 inhibited the growth of tumor tissue (81,82). It is worth noting that in a phase Ib clinical trial of OMP-18R5 for breast cancer, although there was a good response rate, the common occurrence of fractures limited its development in the clinic (83).
OMP-54F28 (ipafricept) is a fusion protein of FZD8 and immunoglobulins, and inhibits Wnt signaling by competing with FZD8 receptors for ligands (84). In addition, OMP-54F28 has been indicated to inhibit tumor cell proliferation to a greater extent when combined with chemotherapeutic drugs, such as gemcitabine, as compared with its use as a monotherapy (84). A phase I study in patients with advanced solid tumors suggested that OMP-54F28 prolonged survival in both patients with desmoid and germ cell carcinoma; furthermore, the patients remained stable and the drug was well tolerated (85). In a phase Ib clinical trial of OMP-54F28 combined with paclitaxel and gemcitabine for the treatment of metastatic pancreatic cancer, the rate of patients who had a clinical benefit was 81%, with 12 cases of complete response (46.2%) and 9 cases of partial response (34.6%). The median progression-free survival (PFS) was 5.9 months and the median OS was 9.7 months (86). In a clinical trial of OMP-54F28 combined with paclitaxel and carboplatin for ovarian cancer, the overall response rate was 75.7%, PFS was 10.3 months and OS was 33 months (87). The above studies confirmed the effectiveness of OMP-54F28-mediated inhibition of tumor growth, invasion and metastasis. In addition, Le et al (88) reported that OMP-18R5 and OMP-54F28 significantly inhibited the growth of xenograft tumors and blocked Wnt signaling at the tumor epithelial-stromal boundary. It was evident that both OMP-18R5 and OMP-54F28 inhibit tumor growth; however, the associated risk of fractures should be noted. However, the addition of bone protectants, such as alendronate, may reduce bone toxicity and bone loss after Wnt ligand and receptor inhibitor therapy (89).
Porcupine (PPN) inhibitors LGK974, IWP, Wnt-C59 and ETC-159
The secretion of Wnt proteins requires palmitoylation. Palmitoylation is the attachment of the 16-carbon saturated fatty acid palmitate as a lipid donor to one or more cysteine residues in a protein to correctly guide signal transduction between targets and enhance the efficiency and specificity of signal transmission (90). PPN protein, a member of the membrane-bound O acyltransferase family that is located in the endoplasmic reticulum, is a multiple transmembrane protein that is required for the palmitoylation of Wnt proteins (91,92). Therefore, targeted inhibition of the PPN protein may effectively block excessive activation of the Wnt signaling pathway.
LGK974 is a PPN inhibitor that mitigates Wnt palmitate transferase activity to block Wnt signaling. Bagheri et al (93) demonstrated that combinations of LGK974 and aspirin may lead to cell cycle arrest of CRC cell lines and induce apoptosis. In vivo studies have demonstrated that LGK974 inhibits tumor-cell invasion, metastasis and xenograft tumor growth (94-97). Of note, a recent study reported that the chemotherapeutic drug 5-fluorouracil (5-FU) increases both the translation and transcription of the sequence-specific transcription factor p53, which may promote Wnt3 transcription and activate the Wnt/β-catenin signaling pathway, ultimately leading to the recurrence of CRC (98). When combining LGK974 with 5-FU for the treatment of CRC, 5-FU-induced CSC activation was significantly inhibited and the rate of tumor recurrence was significantly decreased, indicating that the combination of Wnt inhibitors with 5-FU may improve the therapeutic effects of treatment compared with chemotherapy alone (98).
In other types of cancer, LGK974 complexed with cyclodextrin may synergistically decrease the expression of Wnt target genes in lung cancer organoids and transplants, reduce the enterotoxicity of the drug and improve the safety of drug therapy (99). In the treatment of neuroendocrine tumors, LGK974 reduced cell viability by causing cell cycle arrest in the G1 and G2/M phases (100). In glioblastoma and ovarian cancer, LGK974 exhibited synergistic effects when combined with chemotherapeutic agents (101,102). LGK974 also inhibited the growth of prostate cancer xenografts (103). Of note, a previous preclinical study confirmed that LGK974 had a strong inhibitory effect on ring finger protein (RNF)43-mutated cancer cells (104), while ~18% of patients with CRC have RNF43 mutations (105). A recent phase I clinical trial confirmed the safety and efficacy of LGK974 for the treatment of advanced solid tumors; of note, the study found that LGK974 enhances the activity of immune checkpoint inhibitors but may affect immune-cell recruitment in tumors (106).
IWP is a PPN inhibitor that may inhibit the palmitoylation of Wnt3A. In addition, IWP may suppress the phosphorylation of LRP5/6 and DVL2 and stabilize the Axin2 destruction complex to block Wnt signaling. Mo et al (107) verified the therapeutic effect of IWP in gastric cancer cells. The results indicated that IWP decreased the invasion and metastasis ability of MKN28 cells. Wnt-C59 is also a PPN protein that blocks the acylation of Wnt by inhibiting the coprecipitation of Wnt3A and the carrier protein Wntless, thereby blocking the activation of Wnt signaling (108,109). Studies including that by Boulter et al (110) have indicated that Wnt-C59 has an important role in inhibiting tumor cell proliferation and promoting tumor cell apoptosis (110,111). Furthermore, Zhang et al (112) confirmed that Wnt-C59 may block EMT and suppress the growth of xenograft tumors. Of note, Wnt-C59 also exerts anti-inflammatory effects by blocking the interaction of β-catenin with nuclear factor κB (NF-κB) (113,114). NF-κB has a vital role in the development, angiogenesis and metastasis of cancer and is closely related to the development of resistance to anticancer drugs. Hence, there is potential clinical value in performing further clinical trials on Wnt-C59.
ETC-159 is a novel oral PNN inhibitor that may block the secretion and activity of all Wnts. In addition, xenotransplantation in patients with CRC with R-spondin translocation was markedly inhibited (115). Zhong et al (116) confirmed that the combined use of ETC-159 and the pan-PI3K inhibitor GDC-0941 markedly suppressed the growth of RNF43-mutant xenografts in vivo by downregulating cell proliferation and glucose metabolism. More importantly, Kaur et al (117) found that the combination treatment of ETC-159 with the poly(ADP-ribose) polymerase (PARP) inhibitor olaparib resulted in the inhibition of tumor-selective DNA repair pathways.
Tankyrase (TNKS) small molecule inhibitors
TNKS, a PARP that promotes the degradation of destruction complexes through the ubiquitin-proteasome pathway, is key to increasing the breakdown of Axin, which leads to the activation of the Wnt signaling pathway. Of note, suppression of TNKS increased the stability of Axin, decreased the degradation of the destruction complex and decreased the cytoplasmic accumulation and translocation of β-catenin into the nucleus, thereby blocking the activation of the Wnt/β-catenin signaling pathway (118).
NVP-TNKS656 and NVP-XAV939
Both NVP-TNKS656 and NVP-XAV939 are TNKS inhibitors, of which NVP-TNKS656 selectively inhibits TNKS2. Both are able to stabilize Axin, lessen the accumulation of β-catenin in the cytoplasm and block the activation of Wnt signaling (119,120). Previous studies have reported that NVP-TNKS656 and NVP-XAV939 may inhibit the growth of colorectal tumors to a greater extent than other drugs (120). In a study in which NVP-XAV939 was combined with 5-FU/cisplatin (DDP)-treated SW480 and SW620 colon cancer cells, Axin levels increased, cytoplasmic and nuclear β-catenin levels decreased and SW480 cell apoptosis induced by 5-FU/DDP increased (121). Of note, Yu et al (122) reported that knockdown of caudal-related homeobox transcription factor 2 (CDX2) in colon cancer cells promoted cell proliferation and tumor formation in vivo, while XAV939 significantly inhibited colon cancer cell proliferation and tumor formation after CDX2 knockdown. In addition, XAV939 inhibited the expression of β-catenin, cyclin D1 and c-Myc induced by overexpression of ubiquitin-specific protease 6 N-terminal-like protein, thereby slowing colon tumor progression (123). More importantly, XAV939 also reversed the inhibition of Axin expression and the activation of β-catenin caused by prohibitin 1 (PHB1) deficiency (124). PHB1 is an important regulator of gene transcription and may block the Wnt/β-catenin signaling pathway and the formation of intestinal tumors. In addition, XAV939 may also reduce the degree of drug resistance in chemotherapy-resistant colon tumor cells (125,126) and overcome the resistance to 5-FU observed in CRC cells carrying a short truncating APC mutation (127), which is expected to be a therapeutic strategy for chemotherapy-resistant CRC.
IWRs
IWR is a TNKS inhibitor that is able to impair the self-renewal capacity of CSCs. A study investigating the tumor-suppressive effect of IWR-1 combined with doxorubicin indicated that in vitro, IWR-1 and doxorubicin have synergistic effects and reverse the drug resistance of tumor stem cells; in vivo, IWR-1 synergistically inhibits tumor-cell proliferation with doxorubicin and considerably slows tumor progression (128). A study by Cheng et al (129) confirmed that Numb is related to EMT and its knockdown promoted EMT through the Wnt signaling pathway, thereby accelerating the formation of colon tumors; furthermore, IWR-1 attenuated EMT caused by Numb knockdown to inhibit the development and progression of colon tumors (129). Of note, it was recently indicated that the TNKS inhibitor bis-quinazolinone 5/Cpd 5 is superior to IWR-1 based on its inhibitory activity and stability, providing a new basis for the development of TNKS inhibitors (130).
G007-LK and G244-LM
G007-LK and G244-LM are also TNKS inhibitors. Studies have confirmed that G007-LK and G244-LM are able to completely block Wnt/β-catenin signaling and inhibit the growth of colorectal tumors caused by APC mutations. Their key mechanism is to block the cell cycle and reduce colony formation (131,132). In addition, G007-LK is well tolerated and inhibits Wnt signaling and the proliferation of LGR5+ intestinal stem cells, which hinders the formation of intestinal tumors (133). Furthermore, in glioblastoma, G007-LK decreased the expression of Wnt/β-catenin signaling pathway-related proteins and genes in CSCs, suppressing cell proliferation and tumor formation (134). Of note, G007-LK may also block Wnt/β-catenin signaling in melanoma cells and increase their sensitivity to anti-PD-1 immunotherapy, and when used in combination with immunotherapy, it has a synergistic effect that is dependent on the absence of β-catenin in tumor cells (135). A study on G007-LK combined with PI3K (BKM120) and EGFR (erlotinib) inhibitors indicated that this combination synergistically suppressed tumor growth in patients with CRC, implying that combinations of TNKS inhibitors with PI3K and EGFR inhibitors may expand the treatment options for CRC (136), which warrants additional in-depth studies.
Other TNKS small molecule inhibitors
The TNKS inhibitors JW55, JW67 and JW74 (136) block Wnt pathway signaling and promote the degradation of β-catenin, further decreasing the expression of Wnt target genes, inducing apoptosis and inhibiting tumor cell growth in a dose- and time-dependent manner (108,137).
Natural compounds targeting the Wnt signaling pathway
Numerous natural compounds have been proven to block the Wnt/β-catenin signaling pathway by acting on different targets, further changing the pathology of CRC and effectively preventing and treating CRC.
Curcumin
Curcumin is a natural compound derived from the rhizome of turmeric. In recent years, it has been indicated that curcumin inhibits the proliferation of various types of cancer cells, and its key mechanism relies on blocking the Wnt signal transduction pathway. Previous studies have demonstrated that curcumin promotes the stalling of p53- and p21-independent cells in the G2/M phase and induces apoptosis in HCT-116 colon cancer cells (138-140). In addition, the curcumin-mediated reduction of cell proliferation and increase of apoptosis may also be achieved by inhibiting CDX2, an intestinal-specific nuclear transcription factor in the Wnt/β-catenin signaling pathway (141,142). It is worth noting that a phase I trial initially explored the efficacy and safety of curcumin in combination with 5-FU in 5-FU-resistant metastatic colon cancer (NCT02724202) and in combination with irinotecan for the treatment of metastatic CRC (NCT01859858). In addition, a clinical trial evaluating whether curcumin is beneficial for patients with rectal cancer prior to chemotherapy and radiotherapy (NCT00745134) is in progress, indicating that curcumin is a promising traditional medicine for the treatment of CRC.
Genistein
Genistein is a soybean-derived isoflavone and phytoestrogen that has a key role in blocking Wnt/β-catenin signaling by upregulating GSK-3β and E-cadherin expression (143). A phase I/II clinical trial (NCT01985763) indicated that genistein combined with chemotherapy is an effective treatment for metastatic CRC, with good safety and tolerability (144). Of note, genistein also reverses chemoresistance (145) and is expected to have a role in the treatment of chemoresistant CRC.
Vitamin D
Previous epidemiological studies have demonstrated that the amount of vitamin D in the active form is inversely associated with the risk of CRC (146), and vitamin D deficiency increases the risk and mortality of patients with CRC (147,148). A study by Fernández-Barral et al (149) indicated that 1,25-(OH)2D3 reduced the proliferation of colon CSCs and inhibited the development and progression of colon cancer. Of note, a study evaluating the anti-colorectal tumor activity of vitamin D-nanoemulsion (NVD) revealed that NVD induced apoptosis in HCT-116 cells in a dose- and time-dependent manner, and this apoptotic effect was mediated by the targeted inhibition of β-catenin (150). Recently, a pilot study examined the effect of vitamin D supplementation on the survival of patients with stage II-III CRC undergoing chemotherapy (NCT02603757). In addition, a phase I trial investigating the vitamin D receptor in relation to CRC is ongoing (NCT02172651).
Other natural compounds
Brucine is an active alkaloid present in the natural plant Strychnos nux-vomica tree and it has been demonstrated to reduce the level of β-catenin and the expression of c-Myc in colon cancer cells (151). Furthermore, it has been indicated that strychnine may also diminish tumor cell proliferation and promote apoptosis by downregulating the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway (152), making it a good antitumor agent. Berberine (BBR) is a quaternary ammonium alkaloid isolated from the traditional Chinese medicine Coptidis Rhizoma for the treatment of diarrhea and gastroenteritis. Berberine is able to bind to the nuclear receptor retinoid X receptor α to mediate the degradation of β-catenin, thereby inhibiting the proliferation of CRC cells and the growth of xenograft tumors (153). Liu et al (154) reported that BBR also decreased the expression of PD-L1 on the surface of tumor cells, disrupting the tumor immune escape pathway and further enhancing immunity to tumor-infiltrating T cells, thereby exerting a marked antitumor effect. These findings provide a new basis for the use of BBR in tumor immunotherapy.
Ginsenosides are steroid compounds that are derived from the plant ginseng. Previous studies have confirmed that ginsenosides may inhibit the proliferation of tumor cells and suppress their self-renewal ability by blocking Wnt/β-catenin signaling (155,156). A study by Yuan et al (157) found that 20(S)-ginsenoside Rh2 inhibited CRC lymph node metastasis in a dose-dependent manner in vivo. It is worth noting that in breast cancer models, ginsenosides may increase the sensitivity of tumor cells to chemotherapeutic drugs, thereby improving the efficacy of chemotherapy (158). Tanshinone IIA is one of the main active components of the traditional Chinese herbal medicine Salvia miltiorrhiza. It has been confirmed that it is able to downregulate the level of hypoxia-inducible factors-1α (HIF-1α) and block the translocation of β-catenin into the nucleus, which subsequently blocks the activation of TCF/LEF to decrease angiogenesis (159). This finding provides a new therapeutic strategy for the development and progression of CRC. Z-ajoene, a simple sulfur-containing compound extracted from garlic, has been reported to inhibit CRC cell growth. Z-ajoene also facilitates β-catenin degradation by regulating CK1α activity (160). Studies using Z-ajoene for the treatment of gastric cancer suggested that the proliferation of MGC-803 gastric cancer cells was considerably inhibited (161,162). Artemisinin is an active sesquiterpene extracted from Artemisia annua. It has been confirmed that artemisinin blocks Wnt/β-catenin signaling and diminishes the proliferation, invasion and migration of tumor cells (163-165).
Nerigoside (NG), derived from the Umbelliferae celery plant, facilitates the apoptosis of a variety of tumor cells, but its role in CRC has remained to be elucidated. Wen et al (166) indicated that the viability and colony formation of the CRC cell lines HT29 and SW620 were inhibited after NG treatment, and the invasive ability of the cells was also significantly reduced. NG was also observed to enhance the expression and stability of GSK3β to block the Wnt/β-catenin pathway signaling (166). More importantly, the drug toxicity associated with NG treatment is very low. Vicenin-2, an NG, blocks Wnt/β-catenin signaling by inhibiting the transcription of TCF/LEF (167). Ginkgolide C is an extract derived from Ginkgo biloba leaves that blocks the Wnt/β-catenin signaling cascade and exhibits strong anti-CRC effects (168). Resveratrol is an antitoxin produced by numerous plants when they are stimulated that may inhibit CRC cell proliferation and tumor formation by blocking the Wnt/β-catenin signaling cascade (169). A phase I trial suggested that micronized trans-resveratrol (SRT501) treatment significantly increased the level of the apoptosis marker caspase-3 in patients with CRC liver metastases, and SRT501 was well tolerated (170). In addition, a phase I trial is exploring whether resveratrol in grape skins is able to prevent colon cancer (NCT00578396).
Andrographolide analog 3A.1 is an analog the main active ingredient of the plant Andrographis paniculata. Previous studies suggested that analog 3A.1 significantly reduced the viability of human CRC HT29 cells and promoted their apoptosis by significantly suppressing the activity of the TCF/LEF promoter (171). DIF-3 is a small molecule compound identified in Dictyostelium discoideum. It may activate GSK3β and promote the degradation of β-catenin, thereby hindering cell proliferation and promoting apoptosis (172,173). It is worth noting that the use of DIF-3 in patients undergoing chemotherapy may significantly reduce the severity of adverse events such as nausea and vomiting (174). Z86 is a novel inhibitor of the Wnt signaling pathway. Its key mechanism is to block the phosphorylation of GSK-3β, thereby increasing the degradation of β-catenin, downregulating the expression of endogenous Wnt signaling target genes and ultimately suppressing CRC cell proliferation (175).
Other Wnt/β-catenin signaling pathway inhibitors
KYA1797K, BC2059 and ICG-001
KYA1797K is a β-catenin inhibitor that may bind Axin and suppress tumors by promoting the instability of β-catenin (176). It has been indicated that high expression of β-catenin and subunit of the oligosaccharyltransferase complex, homolog A/B (STT3A/B) in colon cancer tissue is related to poor prognosis (177). KYA1797K may inhibit the signal transduction of β-catenin/STT3, thereby inhibiting the proliferation of colon CSCs and inducing apoptosis. Of note, KYA1797K may also reduce the stability of PD-L1 on the surface of colon tumor cells, disrupt the immune evasion of colon tumors and contribute to the effectiveness of immunotherapies (177). In addition, KYA1797K suppresses CSC stemness and blocks the growth of tumor organoids and xenografts (178). BC2059 (tegatrabetan) is a novel β-catenin inhibitor that may reduce the levels of β-catenin in the cytoplasm and nucleus and reduce the transcriptional activity of the TCF4/LEF complex. Studies have indicated that BC2059 may prolong the OS of patients and the side effects are tolerable (179,180). The above studies demonstrated that BC2059 is a potent and safe inhibitor of β-catenin and is worthy of further study in colorectal tumor models.
ICG-001 is a small molecule β-catenin/TCF inhibitor and its target gene myeloid ecotropic viral integration site 1 (MEIS1) promotes the proliferation and metastasis of colorectal tumor cells. Studies have confirmed that ICG-001 significantly inhibits the stemness and metastasis of colorectal CSCs by inhibiting MEIS1, which reduces tumor formation and invasion (181). Of note, ICG-001 also reversed Wnt4-induced EMT and angiogenesis in vitro and in vivo, significantly inhibiting colorectal tumor formation (182,183). ICG-001 is expected to improve the poor prognosis of patients with CRC metastases.
Pyrvinium and SR3029
Pyrvinium is a small molecule that inhibits WNT signaling by activating CK1α (184). The inhibition of colorectal tumor growth by pyrvinium has been demonstrated, but the underlying mechanism remains to be fully elucidated (185). Zheng et al (185) found that pyrvinium blocks the growth of colorectal tumor cells by inhibiting the reactive oxygen species-mediated AKT-dependent signaling pathway. Of note, Yang et al (186) developed a new compound based on pyrvinium, YW2065 (pyrazole-4-carboxamide), which promotes the degradation of β-catenin by stabilizing Axin1. Furthermore, YW2065 exerted a strong anti-CRC effect both in vitro and in vivo, and YW2065 activated the tumor suppressor AMP-activated protein kinase, further enhancing the antitumor effects (186). A recent study confirmed that heat shock factor 1 (HSF1) promoted the proliferation of CRC cells by activating mTOR, which promotes the development and progression of CRC. After pyrvinium treatment, both the expression and activity of HSF1 were reduced, which slowed the growth of tumors (187).
SR3029 is a CK1 inhibitor. Amino-terminal enhancer of split (AES) has been indicated to suppress tumor metastasis in CRC. Wang et al (188) identified a CK1δ/ε-AES axis in CRC cells, in which CK1δ/ε promotes the degradation of AES and significantly increases the proliferation, invasion and migration of CRC cells by upregulating the expression of Wnt target genes. After treatment with SR3029, the growth of colorectal tumors and liver metastasis in mice was significantly diminished.
BBI608
Napabucasin (BBI608) is an orally administered inhibitor of signal transducer and activators of transcription (STAT)3 and cancer cell pluripotency. Abnormal expression and activation of STAT3 has been reported in various tumor diseases and STAT3 has been indicated to regulate β-catenin expression during CSC self-renewal. Various studies have confirmed that BBI608 may significantly inhibit tumor cell proliferation and colony formation (189-192). A phase III trial of napabucasin for refractory advanced CRC indicated no difference in OS between the treatment and placebo groups. However, STAT3 may be an important target for the treatment of CRC with elevated phosphorylated STAT3 levels (193). In addition, phase II clinical trials of BBI608 in combination with chemotherapy drugs for patients with advanced metastatic CRC are ongoing (NCT01776307, NCT02851004 and NCT03647839).
SM08502, ST2825 and FH535
SM08502 is a novel small molecule under investigation for the clinical treatment of solid tumors. SM08502 may block signaling of the Wnt pathway and downstream target gene expression by inhibiting the activity of CDC-like kinase (194). A phase I clinical trial of SM08502 for patients with advanced solid tumors is ongoing (NCT03355066). ST2825 is a myeloid differentiation factor 88 (MyD88) inhibitor. MyD88 is an adapter protein that is used for signal transduction in the IL-1β/toll-like receptor (TLR) pathway. A preclinical study indicated that MyD88 upregulates NF-κB and promotes Wnt/β-catenin signaling in intestinal tumor cells. However, after treatment with ST2825, the growth of intestinal tumor cells was inhibited and apoptosis was increased, which significantly hindered the development of intestinal tumors (195). FH535 is an inhibitor of the Wnt/β-catenin pathway and peroxisome proliferator-activated receptors with antitumor activity; thus, it has considerable anti-proliferative and proapoptotic effects (196). In addition, Tu et al (197) used FH535 to treat human CRC cells (DLD-1 and SW620 cells) and found that the cell cycle was arrested in the G2/M phase and the ability of cells to proliferate and migrate was significantly inhibited.
Acetyltransferase p300 inhibitor, apatinib and obatoclax
Hua et al (198) have demonstrated that tribbles homolog 3 (TRIB3) interacts with β-catenin and TCF4 to accelerate colon tumor formation and xenograft tumor growth. It was recently confirmed that acetyltransferase p300 inhibitors may promote the degradation of TRIB3, which not only reduces the proliferation of CRC tumor stem cells but also increases CD8+ T-cell infiltration and sensitizes patients with CRC to immune checkpoint inhibitor treatment (199). These studies imply that TRIB3 may become another key target for the treatment of CRC. Apatinib is a VEGFR2 inhibitor that exhibits anti-proliferative and proapoptotic effects in CRC. The key mechanism involves regulating the level of GSK-3β, which mediates the ubiquitination of β-catenin and further blocks Wnt signaling. In addition, apatinib may inhibit angiogenesis and reduce the development and progression of colorectal tumors (200).
Obatoclax is a B-cell CLL/Lymphoma 2 (BCL-2) antagonist that inhibits BCL-2, BCL-xL and MCL-1 to induce apoptosis. Obatoclax reduces the stability of β-catenin and downregulates the transcriptional activity of the TCF/LEF complex, significantly suppressing the growth of HCT-116 cells. More importantly, obatoclax significantly reduced the levels of survivin mRNA and survivin promoter activity, which induced apoptosis (201), and revealed the potential of obatoclax as a Wnt pathway antagonist for the treatment of CRC.
Inhibitors based on potential targets of the Wnt/β-catenin signaling pathway
Further possible CRC therapeutic targets have been discovered through various general studies. For example, Gan et al (202) found that knockdown of protein tyrosine phosphatase receptor type F may significantly down-regulate the expression of CSC-related genes downstream of the Wnt/β-catenin pathway to cause an antiproliferative effect in CRC cells. Song et al (203) found that sine oculis homeobox 1 (Six1), a homeodomain transcription factor, promotes Wnt/β-catenin pathway signaling. After Six1 knockdown, the growth of CRC cells was significantly decreased and cell metastasis and invasion were significantly weakened. p38α is a novel β-catenin-associated kinase that increases the proliferation, invasion and metastasis of colorectal tumor cells by promoting the transcription of β-catenin target genes. By contrast, targeted inhibition of p38α may inhibit the activity of CSCs and increase the sensitivity of tumor cells to chemotherapeutic drugs (204). Clinical trials for p38α inhibition are ongoing.
Mucin 13 (MUC13), a cell surface mucin, is highly expressed in patients with CRC and is associated with poor prognosis. Sheng et al (205) revealed a new role for MUC13 in the development and progression of colorectal tumors. MUC13 inhibits β-catenin degradation by interacting with GSK-3β and promoting canonical Wnt signaling. As tumor cells are less sensitive to immune checkpoint inhibitors (205), inhibition of MUC13 may benefit patients with CRC. It has been reported that overexpression of RNF6 in CRC cells promotes the phosphorylation of GSK-3β, which in turn upregulates the activation of the Wnt/β-catenin signaling pathway and increases the formation and metastasis of colorectal tumors. Therefore, inhibition of RNF6 is crucial in patients with CRC (206).
Lysine-specific histone demethylase 1 (LSD1) has been indicated be a potential target for cancer therapy. Peng et al (207) studied the anti-CRC activity of the LSD1 inhibitor ZY0511 when combined with 5-fluorouracil (5-HU). The results suggested that ZY0511 significantly inhibited the growth of CRC cells and induced apoptosis both in vivo and in vitro. Furthermore, ZY0511 synergized with 5-HU and significantly reduced the invasive and metastatic abilities of CRC cells. Further investigation indicated that the antitumor effect of ZY0511 was achieved by inhibiting the Wnt/β-catenin pathway signaling.
Zhu et al (208) reported that the ceramide synthase 5/TLR4/β-catenin/sterol O-acyltransferase 1 (SOAT1) axis was dysregulated in patients with CRC. SOAT1 is able to directly bind to β-catenin to increase cholesterol esterification and induce the development of CRC. Therefore, inhibition of this axis may be an effective therapeutic strategy for CRC. A recent study (209) determined increased levels of cis-homeodomain (HOX) (a circular RNA that stabilizes HOXC10) and HOXC10 in CRC cells, and the Wnt/β-catenin pathway may be activated through the cis-HOX-HOXC10 axis, which has a role in the development and metastasis of CRC. Of note, HOXC10 inhibitors are therapeutically effective only in APC wild-type CRC and not in tumors with nonsense mutations in APC (209). Therefore, the cis-HOX-HOXC10 axis may become a therapeutic target for APC wild-type CRC. In summary, the development of inhibitors for the above targets may be a new strategy to develop treatments for CRC.
Other less studied but potentially valuable inhibitors
NSC668036 is an organic inhibitor that has been demonstrated to inhibit β-catenin-driven gene transcription in vitro (210). Apicularen and bafilomycin act on ATPases and inhibit the activation of the Wnt pathway (211,212). DK419, a derivative of niclosamide, inhibits the growth of CRC cells and xenografts in vitro by blocking the Wnt/β-catenin signaling (213). P5091 attenuates Wnt pathway activity by promoting β-catenin degradation, which inhibits CRC cell proliferation and promotes apoptosis in vitro (214). 36-077, a Phosphoinositide-3-kinase, Class 3/Vacuolar Protein Sorting 34 (PIK3C3/VPS34) kinase inhibitor, reduces 5-FU resistance in CRC cells and induces autophagy in CRC cells by blocking Wnt/β-catenin pathway signaling (215). Ovo like zinc finger 2 (OVOL2) is a Wnt signaling inhibitor that decreases the expression of related target genes by promoting the recruitment of histone deacetylase 1 to the TCF4-β-catenin complex; however, the level of OVOL2 is reduced in patients with CRC. Therefore, upregulating the level of OVOL2 may be a strategy to suppress the development and progression of CRC (216). All the above-mentioned compounds may inhibit the signal transduction of the Wnt pathway, which further inhibits the development and progression of colorectal tumors.
Various drugs that are used to treat other diseases in the clinic have also been found to inhibit Wnt/β-catenin signaling. The safety of these drugs has been confirmed in clinical applications, and they may be effective and safe options for the treatment of Wnt/β-catenin signaling-dependent cancers. Niclosamide is an antiparasitic drug that may inhibit the binding of FZD receptors to Wnt ligands, thereby blocking Wnt/β-catenin signaling (217). Pimozide is a neuroleptic drug, but studies have indicated that it may also suppress the Wnt/β-catenin signaling pathway (218,219). Ethacrynic acid is a highly potent diuretic that was also found to inhibit the activity of the β-catenin/LEF-1 transcription complex in liver cancer cells, thereby reducing the expression of downstream target genes as part of its anticancer effect (220,221). Although the above-mentioned drugs have so far not been used for the treatment of CRC, their inhibitory effect on the Wnt/β-catenin pathway signaling and clinical drug safety make them favorable options for the treatment of CRC.
6. Summary and prospects
The Wnt protein family has a role in cell proliferation and differentiation, the self-renewal of stem cells and the transmission of extracellular signals intracellularly through cell-surface receptors, thereby driving the development and progression of tumors. The Wnt/β-catenin pathway is the classical Wnt signaling pathway and its dysregulation in malignant tumors has been widely reported, particularly in digestive system tumors. For instance, abnormal expression of β-catenin and other Wnt pathway proteins in the cytoplasm and nucleus of CRC cells is common. Therefore, inhibitors that target the Wnt/β-catenin signaling pathway have good prospects for tumor therapy, as they may act on different upstream and downstream Wnt-related factors to suppress or impede the development and progression of CRC.
For instance, a β-catenin-TCF/LEF complex inhibitor may downregulate the expression of proto-oncogenes, such as cyclin D1 and c-Myc, which are downstream of Wnt. This downregulation inhibits cell proliferation and promotes cell apoptosis, thereby inhibiting the formation, invasion and metastasis of colorectal tumors. Although the anti-CRC effects of numerous β-catenin-TCF/LEF complex inhibitors have been demonstrated in preclinical studies, their efficacy and safety in humans require to be confirmed in clinical trials.
There are numerous types of Wnt ligands and receptor inhibitors, and their antitumor mechanisms mainly involve blocking the binding of Wnt ligands to their corresponding receptors, such as FZD receptors and LRP5 and LRP6 coreceptors. This inhibits Wnt/β-catenin signaling, which further promotes the degradation of β-catenin in the cytoplasm, reduces β-catenin translocation into the nucleus and downregulates the expression of downstream target genes to inhibit the growth, invasion and metastasis of colorectal tumors. To date, much progress has been made in the research and development of Wnt ligands and receptor inhibitors, and there are ongoing clinical trials to assess their clinical benefits. Of note, Wnt ligands and receptor inhibitors may also synergize with chemotherapeutic drugs to increase the sensitivity of tumors to immunotherapy. These studies are expected to provide clinicians with effective and safe treatment strategies for patients with CRC, and an increased population of patients with CRC that benefit from treatment.
Certain natural compounds and their derivatives extracted from traditional Chinese medicines have been indicated to change the pathology of CRC and effectively prevent and treat CRC by acting on different targets in the Wnt/β-catenin signaling pathway. In addition, studies have indicated that natural compounds have low pharmacological toxicity and fewer adverse events in patients. Of note, when certain natural compounds are combined with chemotherapeutic drugs, they may synergistically exert antitumor effects, while also limiting the adverse effects of chemotherapy. In short, these natural compounds deserve more thorough research to explore their anti-CRC mechanisms and to determine how combinations may benefit patients with CRC.
There are numerous other substances that block Wnt/β-catenin pathway signaling, and there have also been newly identified targets within the Wnt/β-catenin signaling pathway. Furthermore, even drugs that have been approved for the treatment of other diseases have demonstrated significant anti-CRC effects.
Although further advances in research have led to the identification of numerous CRC targets in the classical Wnt signaling pathway, which has provided additional options to treat colorectal tumors, there are potential drawbacks to inhibiting the Wnt pathway. Previous studies have indicated that the Wnt/β-catenin signaling pathway is involved in numerous biological processes, such as osteoblast differentiation, neurodevelopment and insulin secretion; therefore, inhibition of the Wnt/β-catenin signaling pathway may cause dysfunction in the body, leading to adverse events such as fragility-related bone fractures, neurodegenerative changes and metabolic dysregulation (7,222). More importantly, research on the classical Wnt signaling pathway still presents various challenges. For instance, the Wnt/β-catenin signaling pathway affects CRC cells through a complex mechanism that involves multiple receptors and protein families. In addition, mutations in downstream targets of the Wnt/β-catenin signaling pathway create difficulties in the research progress, as mutations in each factor may lead to abnormal activation of this pathway. Of note, most of the compounds targeting the Wnt/β-catenin pathway are currently only at the preclinical stage and no new drugs have been developed for use in patients. However, research on molecules that target the Wnt pathway is promising and worthwhile.
The present review comprehensively summarized the progress of research on the Wnt signaling pathway and related active compounds with the aim of providing new targets and ideas for the development of effective anti-CRC drugs with low toxicity. With the progress of research, an increased number of anti-CRC drugs that target the Wnt canonical signaling pathway will be developed in the future, providing further therapeutic options and individualized therapy regimens for patients with CRC. This will allow clinicians to increase the population of patients with CRC who benefit from treatment.
Acknowledgments
Not applicable.
Funding Statement
The corresponding author (KD) was supported by the National Natural Science Foundation of China (grant no. 81600511), Science Foundation of Sichuan Health and Family Planning Commission (grant no. 20PJYY0314), Sichuan Science and Technology Program (grant nos. 22ZD YF1618 and 2021YFH0005), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant nos. 2019-051 and 2021-150) and Operation Funding of Sichuan University-University of Oxford Huaxi Joint Centre for Gastrointestinal Cancer (grant no. 161200021).
Availability of data and materials
Not applicable.
Authors' contributions
YC and KD contributed to the conception and design of the review. YC wrote the first draft of the manuscript. KD and MC wrote sections of the manuscript. All authors contributed to manuscript revision. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Liu C, Zhu S, Liang X, Zhang Q, Luo X, Yuan L, Song L. PD-1/PD-L1 immune checkpoint blockade-based combinational treatment: Immunotherapeutic amplification strategies against colorectal cancer. Int Immunopharmacol. 2021;96:107607. doi: 10.1016/j.intimp.2021.107607. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Cheng X, Xu X, Chen D, Zhao F, Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473–481. doi: 10.1016/j.biopha.2018.11.082. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy N, Kurzrock R. Targeting the Wnt/betacatenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi: 10.1016/j.ctrv.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 10.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee YH, Kung PT, Wang YH, Kuo WY, Kao SL, Tsai WC. Effect of length of time from diagnosis to treatment on colorectal cancer survival: A population-based study. PLoS One. 2019;14:e0210465. doi: 10.1371/journal.pone.0210465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee A, Pathak S, Subramanium VD, G D, Murugesan R, Verma RS. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov Today. 2017;22:1224–1232. doi: 10.1016/j.drudis.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 14.Payandeh Z, Khalili S, Somi MH, Mard-Soltani M, Baghbanzadeh A, Hajiasgharzadeh K, Samadi N, Baradaran B. PD-1/PD-L1-dependent immune response in colorectal cancer. J Cell Physiol. 2020;235:5461–5475. doi: 10.1002/jcp.29494. [DOI] [PubMed] [Google Scholar]
- 15.Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015;5:16–18. doi: 10.1158/2159-8290.CD-14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva VR, Santos LS, Dias RB, Quadros CA, Bezerra DP. Emerging agents that target signaling pathways to eradicate colorectal cancer stem cells. Cancer Commun (Lond) 2021;41:1275–1313. doi: 10.1002/cac2.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voorneveld PW, Kodach LL, Jacobs RJ, van Noesel CJ, Peppelenbosch MP, Korkmaz KS, Molendijk I, Dekker E, Morreau H, van Pelt GW, et al. The BMP pathway either enhances or inhibits the Wnt pathway depending on the SMAD4 and p53 status in CRC. Br J Cancer. 2015;112:122–130. doi: 10.1038/bjc.2014.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nusse R, Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–149. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taciak B, Pruszynska I, Kiraga L, Bialasek M, Krol M. Wnt signaling pathway in development and cancer. J Physiol Pharmacol. 2018 Jul 4;69 doi: 10.26402/jpp.2018.2.07. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Zhang Q, Li F, Wang C, Yang C, Yu H. β-TrCP-mediated ubiquitination and degradation of Dlg5 regulates hepatocellular carcinoma cell proliferation. Cancer Cell Int. 2019;19:298. doi: 10.1186/s12935-019-1029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBruine ZJ, Xu HE, Melcher K. Assembly and architecture of the Wnt/β-catenin signalosome at the membrane. Br J Pharmacol. 2017;174:4564–4574. doi: 10.1111/bph.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi J, Lee HJ, Saquet A, Cheng XN, Shao M, Zheng JJ, Shi DL. Autoinhibition of Dishevelled protein regulated by its extreme C terminus plays a distinct role in Wnt/β-catenin and Wnt/planar cell polarity (PCP) signaling pathways. J Biol Chem. 2017;292:5898–5908. doi: 10.1074/jbc.M116.772509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schatoff EM, Leach BI, Dow LE. Wnt signaling and colorectal cancer. Curr Colorectal Cancer Rep. 2017;13:101–110. doi: 10.1007/s11888-017-0354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gómez-Orte E, Sáenz-Narciso B, Moreno S, Cabello J. Multiple functions of the noncanonical Wnt pathway. Trends Genet. 2013;29:545–553. doi: 10.1016/j.tig.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 28.De A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim Biophys Sin (Shanghai) 2011;43:745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 29.Chae WJ, Bothwell ALM. Canonical and Non-Canonical Wnt signaling in immune cells. Trends Immunol. 2018;39:830–847. doi: 10.1016/j.it.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, Clevers H. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol. 2012;32:1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 32.Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, de Punder K, Angers S, Peters PJ, Maurice MM, Clevers H. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- 33.Chen M, Lin W, Li N, Wang Q, Zhu S, Zeng A, Song L. Therapeutic approaches to colorectal cancer via strategies based on modulation of gut microbiota. Front Microbiol. 2022;13:945533. doi: 10.3389/fmicb.2022.945533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Tóth B, Kondo A, Massasa EE, Itzkovitz S, Kaestner KH. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature. 2018;557:242–246. doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn M. Wnt signaling in stem cells and cancer stem cells: A tale of two coactivators. Prog Mol Biol Transl Sci. 2018;153:209–244. doi: 10.1016/bs.pmbts.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Aceto GM, Catalano T, Curia MC. Molecular aspects of colorectal adenomas: The interplay among microenvironment, oxidative stress, and predisposition. Biomed Res Int. 2020;2020:1726309. doi: 10.1155/2020/1726309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bright-Thomas RM, Hargest R. APC, beta-Catenin and hTCF-4; an unholy trinity in the genesis of colorectal cancer. Eur J Surg Oncol. 2003;29:107–117. doi: 10.1053/ejso.2002.1331. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Shay JW. Multiple roles of APC and its therapeutic implications in colorectal cancer. J Natl Cancer Inst. 2017;109:djw332. doi: 10.1093/jnci/djw332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu N, Shen C, Luo Y, Xia L, Xue F, Xia Q, Zhang J. Upregulated miR-130a increases drug resistance by regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell. Biochem Biophys Res Commun. 2012;425:468–472. doi: 10.1016/j.bbrc.2012.07.127. [DOI] [PubMed] [Google Scholar]
- 40.Shen DY, Zhang W, Zeng X, Liu CQ. Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 2013;104:1303–1308. doi: 10.1111/cas.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26:570–579. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 42.Roy S, Majumdar AP. Signaling in colon cancer stem cells. J Mol Signal. 2012;7:11. doi: 10.1186/1750-2187-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sebio A, Kahn M, Lenz HJ. The potential of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther Targets. 2014;18:611–615. doi: 10.1517/14728222.2014.906580. [DOI] [PubMed] [Google Scholar]
- 44.Tai D, Wells K, Arcaroli J, Vanderbilt C, Aisner DL, Messersmith WA, Lieu CH. Targeting the WNT signaling pathway in cancer therapeutics. Oncologist. 2015;20:1189–1198. doi: 10.1634/theoncologist.2015-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paluszczak J, Kleszcz R, Studzińska-Sroka E, Krajka-Kuźniak V. Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells. Mol Cell Biochem. 2018;441:109–124. doi: 10.1007/s11010-017-3178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gekas C, D'Altri T, Aligué R, González J, Espinosa L, Bigas A. β-Catenin is required for T-cell leukemia initiation and MYC transcription downstream of Notch1. Leukemia. 2016;30:2002–2010. doi: 10.1038/leu.2016.106. [DOI] [PubMed] [Google Scholar]
- 47.Li L, Peng W, Zhou Q, Wan JP, Wang XT, Qi HB. LRP6 regulates Rab7-mediated autophagy through the Wnt/β-catenin pathway to modulate trophoblast cell migration and invasion. J Cell Biochem. 2020;121:1599–1609. doi: 10.1002/jcb.29394. [DOI] [PubMed] [Google Scholar]
- 48.Matsuzaki S, Darcha C. Involvement of the Wnt/β-catenin signaling pathway in the cellular and molecular mechanisms of fibrosis in endometriosis. PLoS One. 2013;8:e76808. doi: 10.1371/journal.pone.0076808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JY, Park G, Krishnan M, Ha E, Chun KS. Selective Wnt/β-catenin Small-molecule Inhibitor CWP232228 impairs tumor growth of colon cancer. Anticancer Res. 2019;39:3661–3667. doi: 10.21873/anticanres.13514. [DOI] [PubMed] [Google Scholar]
- 50.Jang GB, Hong IS, Kim RJ, Lee SY, Park SJ, Lee ES, Park JH, Yun CH, Chung JU, Lee KJ, et al. Wnt/β-Catenin small-molecule inhibitor CWP232228 preferentially inhibits the growth of breast cancer stem-like cells. Cancer Res. 2015;75:1691–1702. doi: 10.1158/0008-5472.CAN-14-2041. [DOI] [PubMed] [Google Scholar]
- 51.Kim JY, Lee HY, Park KK, Choi YK, Nam JS, Hong IS. CWP232228 targets liver cancer stem cells through Wnt/β-catenin signaling: A novel therapeutic approach for liver cancer treatment. Oncotarget. 2016;7:20395–20409. doi: 10.18632/oncotarget.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kazi A, Xiang S, Yang H, Delitto D, Trevino J, Jiang RHY, Ayaz M, Lawrence HR, Kennedy P, Sebti SM. GSK3 suppression upregulates β-catenin and c-Myc to abrogate KRas-dependent tumors. Nat Commun. 2018;9:5154. doi: 10.1038/s41467-018-07644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong CC, Xu J, Bian X, Wu JL, Kang W, Qian Y, Li W, Chen H, Gou H, Liu D, et al. In colorectal cancer cells with mutant KRAS, SLC25A22-Mediated Glutaminolysis Reduces DNA demethylation to increase WNT signaling, stemness, and drug resistance. Gastroenterology. 2020;159:2163–2180.e6. doi: 10.1053/j.gastro.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Du F, Cao T, Xie H, Li T, Sun L, Liu H, Guo H, Wang X, Liu Q, Kim T, et al. KRAS Mutation-Responsive miR-139-5p inhibits colorectal cancer progression and is repressed by Wnt signaling. Theranostics. 2020;10:7335–7350. doi: 10.7150/thno.45971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mologni L, Brussolo S, Ceccon M, Gambacorti-Passerini C. Synergistic effects of combined Wnt/KRAS inhibition in colorectal cancer cells. PLoS One. 2012;7:e51449. doi: 10.1371/journal.pone.0051449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Z, Venkatesan AM, Dehnhardt CM, Dos Santos O, Delos Santos E, Ayral-Kaloustian S, Chen L, Geng Y, Arndt KT, Lucas J, et al. 2,4-Diamino-quinazolines as inhibitors of beta-catenin/Tcf-4 pathway: Potential treatment for colorectal cancer. Bioorg Med Chem Lett. 2009;19:4980–4983. doi: 10.1016/j.bmcl.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 57.Dehnhardt CM, Venkatesan AM, Chen Z, Ayral-Kaloustian S, Dos Santos O, Delos Santos E, Curran K, Follettie MT, Diesl V, Lucas J, et al. Design and synthesis of novel diaminoquinazolines with in vivo efficacy for beta-catenin/T-cell transcriptional factor 4 pathway inhibition. J Med Chem. 2010;53:897–910. doi: 10.1021/jm901370m. [DOI] [PubMed] [Google Scholar]
- 58.Chang TS, Lu CK, Hsieh YY, Wei KL, Chen WM, Tung SY, Wu CS, Chan MWY, Chiang MK. 2,4-Diamino-Quinazoline, a Wnt signaling inhibitor, suppresses gastric cancer progression and metastasis. Int J Mol Sci. 2020;21:5901. doi: 10.3390/ijms21165901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan HC, Hsieh YC, Li LH, Chang CC, Janoušková K, Ramani MV, Subbaraju GV, Cheng KT, Chang CC. Dehydroxyhispolon methyl ether, a hispolon derivative, inhibits WNT/β-Catenin signaling to elicit human colorectal carcinoma cell apoptosis. Int J Mol Sci. 2020;21:8839. doi: 10.3390/ijms21228839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu L, Zhou Z, Han S, Chen J, Liu Z, Zhang X, Yuan W, Ji J, Shu X. PLAGL2 promotes epithelial-mesenchymal transition and mediates colorectal cancer metastasis via β-catenin-dependent regulation of ZEB1. Br J Cancer. 2020;122:578–589. doi: 10.1038/s41416-019-0679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Low JL, Du W, Gocha T, Oguz G, Zhang X, Chen MW, Masirevic S, Yim DGR, Tan IBH, Ramasamy A, et al. Molecular docking-aided identification of small molecule inhibitors targeting β-catenin-TCF4 interaction. iScience. 2021;24:102544. doi: 10.1016/j.isci.2021.102544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu J, Wang Z, Chen J, Yu Z, Zhang J, Li W, Lin M, Yang X, Liu H. Overexpression of ICAT inhibits the progression of colorectal cancer by binding with β-Catenin in the cytoplasm. Technol Cancer Res Treat. 2021;20:15330338211041253. doi: 10.1177/15330338211041253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masuda M, Uno Y, Ohbayashi N, Ohata H, Mimata A, Kukimoto-Niino M, Moriyama H, Kashimoto S. TNIK inhibition abrogates colorectal cancer stemness. Nat Commun. 2016;7:12586. doi: 10.1038/ncomms12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamada T, Masuda M. Emergence of TNIK inhibitors in cancer therapeutics. Cancer Sci. 2017;108:818–823. doi: 10.1111/cas.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugano T, Masuda M, Takeshita F, Motoi N, Hirozane T, Goto N, Kashimoto S, Uno Y, Moriyama H, Sawa M, et al. Pharmacological blockage of transforming growth factor-β signalling by a Traf2- and Nck-interacting kinase inhibitor, NCB-0846. Br J Cancer. 2021;124:228–236. doi: 10.1038/s41416-020-01162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sekita T, Yamada T, Kobayashi E, Yoshida A, Hirozane T, Kawai A, Uno Y, Moriyama H, Sawa M, Nagakawa Y, et al. Feasibility of targeting Traf2-and-Nck-Interacting kinase in synovial sarcoma. Cancers (Basel) 2020;12:1258. doi: 10.3390/cancers12051258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung HR, Oh Y, Na D, Min S, Kang J, Jang D, Shin S, Kim J, Lee SE, Jeong EM, et al. CRISPR screens identify a novel combination treatment targeting BCL-XL and WNT signaling for KRAS/BRAF-mutated colorectal cancers. Oncogene. 2021;40:3287–3302. doi: 10.1038/s41388-021-01777-7. [DOI] [PubMed] [Google Scholar]
- 68.Zhao L, Sun L, Lu Y, Li F, Xu H. A small-molecule LF3 abrogates β-catenin/TCF4-mediated suppression of NaV 1.5 expression in HL-1 cardiomyocytes. J Mol Cell Cardiol. 2019;135:90–96. doi: 10.1016/j.yjmcc.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang L, Zhu Q, Neuenschwander M, Specker E, Wulf-Goldenberg A, Weis WI, von Kries JP, Birchmeier W. A small-molecule antagonist of the β-Catenin/TCF4 interaction blocks the self-renewal of cancer stem cells and suppresses tumorigenesis. Cancer Res. 2016;76:891–901. doi: 10.1158/0008-5472.CAN-15-1519. [DOI] [PubMed] [Google Scholar]
- 70.Gurpinar E, Grizzle WE, Piazza GA. NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res. 2014;20:1104–1113. doi: 10.1158/1078-0432.CCR-13-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sareddy GR, Kesanakurti D, Kirti PB, Babu PP. Nonsteroidal anti-inflammatory drugs diclofenac and celecoxib attenuates Wnt/β-catenin/Tcf signaling pathway in human glioblastoma cells. Neurochem Res. 2013;38:2313–2322. doi: 10.1007/s11064-013-1142-9. [DOI] [PubMed] [Google Scholar]
- 72.Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, Li Y, Chen X, Keeton AB, Abadi AH, et al. Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/β-catenin signaling. Mol Cancer Ther. 2013;12:1848–1859. doi: 10.1158/1535-7163.MCT-13-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Egashira I, Takahashi-Yanaga F, Nishida R, Arioka M, Igawa K, Tomooka K, Nakatsu Y, Tsuzuki T, Nakabeppu Y, Kitazono T, Sasaguri T. Celecoxib and 2,5-dimethylcelecoxib inhibit intestinal cancer growth by suppressing the Wnt/β-catenin signaling pathway. Cancer Sci. 2017;108:108–115. doi: 10.1111/cas.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bowen CM, Walter L, Borras E, Wu W, Ozcan Z, Chang K, Bommi PV, Taggart MW, Thirumurthi S, Lynch PM, et al. Combination of sulindac and bexarotene for prevention of intestinal carcinogenesis in familial adenomatous polyposis. Cancer Prev Res (Phila) 2021;14:851–862. doi: 10.1158/1940-6207.CAPR-20-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu M, Guan J, Li C, Gunter S, Nusrat L, Ng S, Dhand K, Morshead C, Kim A, Das S. Aberrantly activated Cox-2 and Wnt signaling interact to maintain cancer stem cells in glioblastoma. Oncotarget. 2017;8:82217–82230. doi: 10.18632/oncotarget.19283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol. 2020;180:114147. doi: 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grosser T, Ricciotti E, FitzGerald GA. The cardiovascular pharmacology of nonsteroidal anti-inflammatory drugs. Trends Pharmacol Sci. 2017;38:733–748. doi: 10.1016/j.tips.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walker C, Biasucci LM. Cardiovascular safety of non-steroidal anti-inflammatory drugs revisited. Postgrad Med. 2018;130:55–71. doi: 10.1080/00325481.2018.1412799. [DOI] [PubMed] [Google Scholar]
- 79.Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature. 2017;545:238–242. doi: 10.1038/nature22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Morris JP, IV, Yan W, Schofield HK, Gurney A, Simeone DM, Millar SE, Hoey T, Hebrok M, Pasca di Magliano M. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Res. 2013;73:4909–4922. doi: 10.1158/0008-5472.CAN-12-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flanagan DJ, Barker N, Costanzo NSD, Mason EA, Gurney A, Meniel VS, Koushyar S, Austin CR, Ernst M, Pearson HB, et al. Frizzled-7 is required for Wnt signaling in gastric tumors with and without apc mutations. Cancer Res. 2019;79:970–981. doi: 10.1158/0008-5472.CAN-18-2095. [DOI] [PubMed] [Google Scholar]
- 83.Diamond JR, Becerra C, Richards D, Mita A, Osborne C, O'Shaughnessy J, Zhang C, Henner R, Kapoun AM, Xu L, et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res Treat. 2020;184:53–62. doi: 10.1007/s10549-020-05817-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J, Stagg R, Kapoun AM, Xu L, Uttamsingh S, et al. A First-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin Cancer Res. 2017;23:7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 86.Dotan E, Cardin DB, Lenz HJ, Messersmith W, O'Neil B, Cohen SJ, Denlinger CS, Shahda S, Astsaturov I, Kapoun AM, et al. Phase Ib study of wnt inhibitor ipafricept with gemcitabine and nab-paclitaxel in patients with previously untreated stage IV pancreatic cancer. Clin Cancer Res. 2020;26:5348–5357. doi: 10.1158/1078-0432.CCR-20-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore KN, Gunderson CC, Sabbatini P, McMeekin DS, Mantia-Smaldone G, Burger RA, Morgan MA, Kapoun AM, Brachmann RK, Stagg R, et al. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 2019;154:294–301. doi: 10.1016/j.ygyno.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Le PN, Keysar SB, Miller B, Eagles JR, Chimed TS, Reisinger J, Gomez KE, Nieto C, Jackson BC, Somerset HL, et al. Wnt signaling dynamics in head and neck squamous cell cancer tumor-stroma interactions. Mol Carcinog. 2019;58:398–410. doi: 10.1002/mc.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Madan B, McDonald MJ, Foxa GE, Diegel CR, Williams BO, Virshup DM. Bone loss from Wnt inhibition mitigated by concurrent alendronate therapy. Bone Res. 2018;6:17. doi: 10.1038/s41413-018-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 91.Torres VI, Godoy JA, Inestrosa NC. Modulating Wnt signaling at the root: Porcupine and Wnt acylation. Pharmacol Ther. 2019;198:34–45. doi: 10.1016/j.pharmthera.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 92.Nile AH, Hannoush RN. Fatty acylation of Wnt proteins. Nat Chem Biol. 2016;12:60–69. doi: 10.1038/nchembio.2005. [DOI] [PubMed] [Google Scholar]
- 93.Bagheri M, Tabatabae Far MA, Mirzaei H, Ghasemi F. Evaluation of antitumor effects of aspirin and LGK974 drugs on cellular signaling pathways, cell cycle and apoptosis in colorectal cancer cell lines compared to oxaliplatin drug. Fundam Clin Pharmacol. 2020;34:51–64. doi: 10.1111/fcp.12492. [DOI] [PubMed] [Google Scholar]
- 94.Hayashi M, Baker A, Goldstein SD, Albert CM, Jackson KW, McCarty G, Kahlert UD, Loeb DM. Inhibition of porcupine prolongs metastasis free survival in a mouse xenograft model of Ewing sarcoma. Oncotarget. 2017;8:78265–78276. doi: 10.18632/oncotarget.19432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rudy SF, Brenner JC, Harris JL, Liu J, Che J, Scott MV, Owen JH, Komarck CM, Graham MP, Bellile EL, et al. In vivo Wnt pathway inhibition of human squamous cell carcinoma growth and metastasis in the chick chorioallantoic model. J Otolaryngol Head Neck Surg. 2016;45:26. doi: 10.1186/s40463-016-0140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li J, Wu G, Xu Y, Li J, Ruan N, Chen Y, Zhang Q, Xia Q. Porcupine Inhibitor LGK974 Downregulates the Wnt signaling pathway and inhibits clear cell renal cell carcinoma. Biomed Res Int. 2020;2020:2527643. doi: 10.1155/2020/2527643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho YH, Ro EJ, Yoon JS, Mizutani T, Kang DW, Park JC, Il Kim T, Clevers H, Choi KY. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation. Nat Commun. 2020;11:5321. doi: 10.1038/s41467-020-19173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guimaraes PPG, Tan M, Tammela T, Wu K, Chung A, Oberli M, Wang K, Spektor R, Riley RS, Viana CTR, et al. Potent in vivo lung cancer Wnt signaling inhibition via cyclodextrin-LGK974 inclusion complexes. J Control Release. 2018;290:75–87. doi: 10.1016/j.jconrel.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin XF, Spoettl G, Maurer J, Nölting S, Auernhammer CJ. Inhibition of Wnt/β-Catenin signaling in neuroendocrine tumors in vitro: Antitumoral effects. Cancers (Basel) 2020;12:345. doi: 10.3390/cancers12020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suwala AK, Koch K, Rios DH, Aretz P, Uhlmann C, Ogorek I, Felsberg J, Reifenberger G, Köhrer K, Deenen R, et al. Inhibition of Wnt/beta-catenin signaling downregulates expression of aldehyde dehydrogenase isoform 3A1 (ALDH3A1) to reduce resistance against temozolomide in glioblastoma in vitro. Oncotarget. 2018;9:22703–22716. doi: 10.18632/oncotarget.25210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boone JD, Arend RC, Johnston BE, Cooper SJ, Gilchrist SA, Oelschlager DK, Grizzle WE, McGwin G, Jr, Gangrade A, Straughn JM, Jr, Buchsbaum DJ. Targeting the Wnt/β-catenin pathway in primary ovarian cancer with the porcupine inhibitor WNT974. Lab Invest. 2016;96:249–259. doi: 10.1038/labinvest.2015.150. [DOI] [PubMed] [Google Scholar]
- 103.Bland T, Wang J, Yin L, Pu T, Li J, Gao J, Lin TP, Gao AC, Wu BJ. WLS-Wnt signaling promotes neuroendocrine prostate cancer. iScience. 2021;24:101970. doi: 10.1016/j.isci.2020.101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N, Lu B, Hsieh MH, Bagdasarian L, Meyer R, et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci USA. 2013;110:12649–12654. doi: 10.1073/pnas.1307218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, Li J, Wang R, Zhang L, Fu G, Wang X, Wang Y, Fang C, Zhang D, Du D, et al. Frequent RNF43 mutation contributes to moderate activation of Wnt signaling in colorectal signet-ring cell carcinoma. Protein Cell. 2020;11:292–298. doi: 10.1007/s13238-020-00691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodon J, Argilés G, Connolly RM, Vaishampayan U, de Jonge M, Garralda E, Giannakis M, Smith DC, Dobson JR, McLaughlin ME, et al. Phase 1 study of single-agent WNT974, a first-in-class Porcupine inhibitor, in patients with advanced solid tumours. Br J Cancer. 2021;125:28–37. doi: 10.1038/s41416-021-01389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mo ML, Li MR, Chen Z, Liu XW, Sheng Q, Zhou HM. Inhibition of the Wnt palmitoyltransferase porcupine suppresses cell growth and downregulates the Wnt/β-catenin pathway in gastric cancer. Oncol Lett. 2013;5:1719–1723. doi: 10.3892/ol.2013.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blyszczuk P, Müller-Edenborn B, Valenta T, Osto E, Stellato M, Behnke S, Glatz K, Basler K, Lüscher TF, Distler O, et al. Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur Heart J. 2017;38:1413–1425. doi: 10.1093/eurheartj/ehw116. [DOI] [PubMed] [Google Scholar]
- 109.Najdi R, Proffitt K, Sprowl S, Kaur S, Yu J, Covey TM, Virshup DM, Waterman ML. A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities. Differentiation. 2012;84:203–213. doi: 10.1016/j.diff.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, Ridgway RA, Samuel K, Van Rooijen N, Barry ST, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125:1269–1285. doi: 10.1172/JCI76452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katoh M. Multi-layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β-catenin signaling activation (Review) Int J Mol Med. 2018;42:713–725. doi: 10.3892/ijmm.2018.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang J, Cai H, Sun L, Zhan P, Chen M, Zhang F, Ran Y, Wan J. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J Exp Clin Cancer Res. 2018;37:225. doi: 10.1186/s13046-018-0864-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jang J, Song J, Sim I, Kwon YV, Yoon Y. Wnt-Signaling Inhibitor Wnt-C59 suppresses the cytokine upregulation in multiple organs of lipopolysaccharide-induced endotoxemic mice via reducing the interaction between β-Catenin and NF-κB. Int J Mol Sci. 2021;22:6249. doi: 10.3390/ijms22126249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jang J, Song J, Sim I, Yoon Y. Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells. Korean J Physiol Pharmacol. 2021;25:307–319. doi: 10.4196/kjpp.2021.25.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J, Jeyaraj DA, Pendharkar V, Ghosh K, Virshup IH, et al. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35:2197–2207. doi: 10.1038/onc.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhong Z, Sepramaniam S, Chew XH, Wood K, Lee MA, Madan B, Virshup DM. PORCN inhibition synergizes with PI3K/mTOR inhibition in Wnt-addicted cancers. Oncogene. 2019;38:6662–6677. doi: 10.1038/s41388-019-0908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaur A, Lim JYS, Sepramaniam S, Patnaik S, Harmston N, Lee MA, Petretto E, Virshup DM, Madan B. WNT inhibition creates a BRCA-like state in Wnt-addicted cancer. EMBO Mol Med. 2021;13:e13349. doi: 10.15252/emmm.202013349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shirai F, Mizutani A, Yashiroda Y, Tsumura T, Kano Y, Muramatsu Y, Chikada T, Yuki H, Niwa H, Sato S, et al. Design and discovery of an orally efficacious spiroindolinone-based tankyrase inhibitor for the treatment of colon cancer. J Med Chem. 2020;63:4183–4204. doi: 10.1021/acs.jmedchem.0c00045. [DOI] [PubMed] [Google Scholar]
- 119.Kulak O, Chen H, Holohan B, Wu X, He H, Borek D, Otwinowski Z, Yamaguchi K, Garofalo LA, Ma Z, et al. Disruption of Wnt/β-Catenin signaling and telomeric shortening are inextricable consequences of tankyrase inhibition in human cells. Mol Cell Biol. 2015;35:2425–2435. doi: 10.1128/MCB.00392-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arqués O, Chicote I, Puig I, Tenbaum SP, Argilés G, Dienstmann R, Fernández N, Caratù G, Matito J, Silberschmidt D, et al. Tankyrase Inhibition Blocks Wnt/β-Catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Clin Cancer Res. 2016;22:644–656. doi: 10.1158/1078-0432.CCR-14-3081. [DOI] [PubMed] [Google Scholar]
- 121.Wu X, Luo F, Li J, Zhong X, Liu K. Tankyrase1 inhibitior XAV939 increases chemosensitivity in colon cancer cell lines via inhibition of the Wnt signaling pathway. Int J Oncol. 2016;48:1333–1340. doi: 10.3892/ijo.2016.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu J, Liu D, Sun X, Yang K, Yao J, Cheng C, Wang C, Zheng J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019;10:26. doi: 10.1038/s41419-018-1263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun K, He SB, Yao YZ, Qu JG, Xie R, Ma YQ, Zong MH, Chen JX. Tre2 (USP6NL) promotes colorectal cancer cell proliferation via Wnt/β-catenin pathway. Cancer Cell Int. 2019;19:102. doi: 10.1186/s12935-019-0823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alula KM, Delgado-Deida Y, Jackson DN, Venuprasad K, Theiss AL. Nuclear partitioning of Prohibitin 1 inhibits Wnt/β-catenin-dependent intestinal tumorigenesis. Oncogene. 2021;40:369–383. doi: 10.1038/s41388-020-01538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang T, Ning K, Lu TX, Hua D. Elevated expression of TrpC5 and GLUT1 is associated with chemoresistance in colorectal cancer. Oncol Rep. 2017;37:1059–1065. doi: 10.3892/or.2016.5322. [DOI] [PubMed] [Google Scholar]
- 126.Xu J, Lv G, Xu B, Jiang B. Overexpression of UBE2M through Wnt/β-Catenin signaling is associated with poor prognosis and chemotherapy resistance in colorectal cancer. Transl Cancer Res. 2020;9:5614–5625. doi: 10.21037/tcr-20-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Siraj AK, Kumar Parvathareddy S, Pratheeshkumar P, Padmaja Divya S, Ahmed SO, Melosantos R, Begum R, Concepcion RMJA, Al-Sanea N, Ashari LH, et al. APC truncating mutations in Middle Eastern Population: Tankyrase inhibitor is an effective strategy to sensitize APC mutant CRC To 5-FU chemotherapy. Biomed Pharmacother. 2020;121:109572. doi: 10.1016/j.biopha.2019.109572. [DOI] [PubMed] [Google Scholar]
- 128.Martins-Neves SR, Paiva-Oliveira DI, Fontes-Ribeiro C, Bovée JVMG, Cleton-Jansen AM, Gomes CMF. IWR-1, a tankyrase inhibitor, attenuates Wnt/β-catenin signaling in cancer stem-like cells and inhibits in vivo the growth of a subcutaneous human osteosarcoma xenograft. Cancer Lett. 2018;414:1–15. doi: 10.1016/j.canlet.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 129.Cheng C, Huang Z, Zhou R, An H, Cao G, Ye J, Huang C, Wu D. Numb negatively regulates the epithelial-to-mesenchymal transition in colorectal cancer through the Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2020;318:G841–G853. doi: 10.1152/ajpgi.00178.2019. [DOI] [PubMed] [Google Scholar]
- 130.Okunlola FO, Akawa OB, Subair TI, Omolabi KF, Soliman MES. Unravelling the mechanistic role of quinazolinone pharmacophore in the inhibitory activity of bis-quinazolinone derivative on tankyrase-1 in the treatment of colorectal cancer (CRC) and non-small cell lung cancer (NSCLC): A computational approach. Cell Biochem Biophys. 2022;80:1–10. doi: 10.1007/s12013-021-01027-3. [DOI] [PubMed] [Google Scholar]
- 131.Lau T, Chan E, Callow M, Waaler J, Boggs J, Blake RA, Magnuson S, Sambrone A, Schutten M, Firestein R, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73:3132–3144. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- 132.Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review) Int J Oncol. 2017;51:1357–1369. doi: 10.3892/ijo.2017.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Norum JH, Skarpen E, Brech A, Kuiper R, Waaler J, Krauss S, Sørlie T. The tankyrase inhibitor G007-LK inhibits small intestine LGR5+ stem cell proliferation without altering tissue morphology. Biol Res. 2018;51:3. doi: 10.1186/s40659-017-0151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kierulf-Vieira KS, Sandberg CJ, Waaler J, Lund K, Skaga E, Saberniak BM, Panagopoulos I, Brandal P, Krauss S, Langmoen IA, Vik-Mo EO. A small-molecule tankyrase inhibitor reduces glioma stem cell proliferation and sphere formation. Cancers (Basel) 2020;12:1630. doi: 10.3390/cancers12061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Waaler J, Mygland L, Tveita A, Strand MF, Solberg NT, Olsen PA, Aizenshtadt A, Fauskanger M, Lund K, Brinch SA, et al. Tankyrase inhibition sensitizes melanoma to PD-1 immune checkpoint blockade in syngeneic mouse models. Commun Biol. 2020;3:196. doi: 10.1038/s42003-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Solberg NT, Waaler J, Lund K, Mygland L, Olsen PA, Krauss S. TANKYRASE inhibition enhances the antiproliferative effect of PI3K and EGFR inhibition, mutually affecting β-CATENIN and AKT signaling in colorectal cancer. Mol Cancer Res. 2018;16:543–553. doi: 10.1158/1541-7786.MCR-17-0362. [DOI] [PubMed] [Google Scholar]
- 137.Tang L, Zhu H, Yang X, Xie F, Peng J, Jiang D, Xie J, Qi M, Yu L. Shizukaol D, a dimeric sesquiterpene isolated from chloranthus serratus, represses the growth of human liver cancer cells by modulating wnt signalling pathway. PLoS One. 2016;11:e0152012. doi: 10.1371/journal.pone.0152012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pricci M, Girardi B, Giorgio F, Losurdo G, Ierardi E, Di Leo A. Curcumin and colorectal cancer: From basic to clinical evidences. Int J Mol Sci. 2020;21:2364. doi: 10.3390/ijms21072364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weng W, Goel A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin Cancer Biol. 2022;80:73–86. doi: 10.1016/j.semcancer.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Villegas C, Perez R, Sterner O, González-Chavarría I, Paz C. Curcuma as an adjuvant in colorectal cancer treatment. Life Sci. 2021;286:120043. doi: 10.1016/j.lfs.2021.120043. [DOI] [PubMed] [Google Scholar]
- 141.Jiang X, Li S, Qiu X, Cong J, Zhou J, Miu W. Curcumin inhibits cell viability and increases apoptosis of SW620 human colon adenocarcinoma cells via the caudal type homeobox-2 (CDX2)/Wnt/β-catenin pathway. Med Sci Monit. 2019;25:7451–7458. doi: 10.12659/MSM.918364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bian Y, Wang G, Zhou J, Yin G, Liu T, Liang L, Liang L, Yang X, Zhang W, Ni K, et al. Astragalus membranaceus (Huangqi) and Rhizoma curcumae (Ezhu) decoction suppresses colorectal cancer via downregulation of Wnt5/β-Catenin signal. Chin Med. 2022;17:11. doi: 10.1186/s13020-021-00564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu X, Yu N, Zhang Y, Ye Y, Sun W, Ye L, Wu H, Yang Z, Wu L, Wang F. Radix Tetrastigma hemsleyani flavone exhibits antitumor activity in colorectal cancer via Wnt/β-catenin signaling pathway. Onco Targets Ther. 2018;11:6437–6446. doi: 10.2147/OTT.S172048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pintova S, Dharmupari S, Moshier E, Zubizarreta N, Ang C, Holcombe RF. Genistein combined with FOLFOX or FOLFOX-Bevacizumab for the treatment of metastatic colorectal cancer: Phase I/II pilot study. Cancer Chemother Pharmacol. 2019;84:591–598. doi: 10.1007/s00280-019-03886-3. [DOI] [PubMed] [Google Scholar]
- 145.Křížová L, Dadáková K, Kašparovská J, Kašparovský T. Isoflavones. Molecules. 2019;24:1076. doi: 10.3390/molecules24061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dou R, Ng K, Giovannucci EL, Manson JE, Qian ZR, Ogino S. Vitamin D and colorectal cancer: Molecular, epidemiological and clinical evidence. Br J Nutr. 2016;115:1643–1660. doi: 10.1017/S0007114516000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wesselink E, Kok DE, Bours MJL, de Wilt JHW, van Baar H, van Zutphen M, Geijsen AMJR, Keulen ETP, Hansson BME, van den Ouweland J, et al. Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am J Clin Nutr. 2020;111:1007–1017. doi: 10.1093/ajcn/nqaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim H, Lipsyc-Sharf M, Zong X, Wang X, Hur J, Song M, Wang M, Smith-Warner SA, Fuchs C, Ogino S, et al. Total Vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology 2021. 2021;161:1208–1217.e9. doi: 10.1053/j.gastro.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fernández-Barral A, Costales-Carrera A, Buira SP, Jung P, Ferrer-Mayorga G, Larriba MJ, Bustamante-Madrid P, Domínguez O, Real FX, Guerra-Pastriá L, et al. Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J. 2020;287:53–72. doi: 10.1111/febs.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Razak S, Afsar T, Almajwal A, Alam I, Jahan S. Growth inhibition and apoptosis in colorectal cancer cells induced by Vitamin D-Nanoemulsion (NVD): Involvement of Wnt/β-catenin and other signal transduction pathways. Cell Biosci. 2019;9:15. doi: 10.1186/s13578-019-0277-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151.Ren H, Zhao J, Fan D, Wang Z, Zhao T, Li Y, Zhao Y, Adelson D, Hao H. Alkaloids from nux vomica suppresses colon cancer cell growth through Wnt/β-catenin signaling pathway. Phytother Res. 2019;33:1570–1578. doi: 10.1002/ptr.6347. [DOI] [PubMed] [Google Scholar]
- 152.Seshadri VD. Brucine promotes apoptosis in cervical cancer cells (ME-180) via suppression of inflammation and cell proliferation by regulating PI3K/AKT/mTOR signaling pathway. Environ Toxicol. 2021;36:1841–1847. doi: 10.1002/tox.23304. [DOI] [PubMed] [Google Scholar]
- 153.Ruan H, Zhan YY, Hou J, Xu B, Chen B, Tian Y, Wu D, Zhao Y, Zhang Y, Chen X, et al. Berberine binds RXRα to suppress β-catenin signaling in colon cancer cells. Oncogene. 2017;36:6906–6918. doi: 10.1038/onc.2017.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Y, Liu X, Zhang N, Yin M, Dong J, Zeng Q, Mao G, Song D, Liu L, Deng H. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm Sin B. 2020;10:2299–2312. doi: 10.1016/j.apsb.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ham SW, Kim JK, Jeon HY, Kim EJ, Jin X, Eun K, Park CG, Lee SY, Seo S, Kim JY, et al. Korean Red ginseng extract inhibits glioblastoma propagation by blocking the Wnt signaling pathway. J Ethnopharmacol. 2019;236:393–400. doi: 10.1016/j.jep.2019.03.031. [DOI] [PubMed] [Google Scholar]
- 156.Kim D, Park M, Haleem I, Lee Y, Koo J, Na YC, Song G, Lee J. Natural product ginsenoside 20(S)-25-Methoxyl-Dammarane-3β, 12β, 20-Triol in cancer treatment: A review of the pharmacological mechanisms and pharmacokinetics. Front Pharmacol. 2020;11:521. doi: 10.3389/fphar.2020.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yuan Y, Wang J, Xu M, Zhang Y, Wang Z, Liang L, Sun P. 20(S)-ginsenoside Rh2 as agent for the treatment of LMN-CRC via regulating epithelial-mesenchymal transition. Biosci Rep. 2020;40:BSR20191507. doi: 10.1042/BSR20191507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hashemi F, Zarrabi A, Zabolian A, Saleki H, Farahani MV, Sharifzadeh SO, Ghahremaniyeh Z, Bejandi AK, Hushmandi K, Ashrafizadeh M, Khan H. Novel strategy in breast cancer therapy: Revealing the bright side of ginsenosides. Curr Mol Pharmacol. 2021;14:1093–1111. doi: 10.2174/1874467214666210120153348. [DOI] [PubMed] [Google Scholar]
- 159.Sui H, Zhao J, Zhou L, Wen H, Deng W, Li C, Ji Q, Liu X, Feng Y, Chai N, et al. Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 2017;403:86–97. doi: 10.1016/j.canlet.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 160.Li H, Jeong JH, Kwon SW, Lee SK, Lee HJ, Ryu JH. Z-Aj oene Inhibits Growth of Colon Cancer by Promotion of CK1α Dependent β-Catenin Phosphorylation. Molecules. 2020;25:703. doi: 10.3390/molecules25030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhu ML, Zheng Z, Lou EZ, Zhao KT, He SY, Chen JY. Anti-gastric cancer effects of Z Ajoene and its molecular mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2021;37:514–519. doi: 10.12047/j.cjap.6122.2021.069. In Chinese. [DOI] [PubMed] [Google Scholar]
- 162.Li N, Zeng A, Wang Q, Chen M, Zhu S, Song L. Regulatory function of DNA methylation mediated lncRNAs in gastric cancer. Cancer Cell Int. 2022;22:227. doi: 10.1186/s12935-022-02648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tong Y, Liu Y, Zheng H, Zheng L, Liu W, Wu J, Ou R, Zhang G, Li F, Hu M, et al. Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget. 2016;7:31413–31428. doi: 10.18632/oncotarget.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang CZ, Wan C, Luo Y, Zhang CF, Zhang QH, Chen L, Liu Z, Wang DH, Lager M, Li CH, et al. Effects of dihydroartemisinin, a metabolite of artemisinin, on colon cancer chemoprevention and adaptive immune regulation. Mol Biol Rep. 2022;49:2695–2709. doi: 10.1007/s11033-021-07079-1. [DOI] [PubMed] [Google Scholar]
- 165.Gong RH, Yang DJ, Kwan HY, Lyu AP, Chen GQ, Bian ZX. Cell death mechanisms induced by synergistic effects of halofuginone and artemisinin in colorectal cancer cells. Int J Med Sci. 2022;19:175–185. doi: 10.7150/ijms.66737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wen SY, Chen YY, Deng CM, Zhang CQ, Jiang MM. Nerigoside suppresses colorectal cancer cell growth and metastatic potential through inhibition of ERK/GSK3β/β-catenin signaling pathway. Phytomedicine. 2019;57:352–363. doi: 10.1016/j.phymed.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 167.Yang D, Zhang X, Zhang W, Rengarajan T. Vicenin-2 inhibits Wnt/β-catenin signaling and induces apoptosis in HT-29 human colon cancer cell line. Drug Des Devel Ther. 2018;12:1303–1310. doi: 10.2147/DDDT.S149307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang MH, Ha IJ, Lee SG, Lee J, Um JY, Ahn KS. Ginkgolide C promotes apoptosis and abrogates metastasis of colorectal carcinoma cells by targeting Wnt/β-catenin signaling pathway. IUBMB Life. 2021;73:1222–1234. doi: 10.1002/iub.2532. [DOI] [PubMed] [Google Scholar]
- 169.Pashirzad M, Johnston TP, Sahebkar A. Therapeutic effects of polyphenols on the treatment of colorectal cancer by regulating Wnt β-Catenin signaling pathway. J Oncol. 2021;2021:3619510. doi: 10.1155/2021/3619510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases-safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 2011;4:1419–1425. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reabroi S, Chairoungdua A, Saeeng R, Kasemsuk T, Saengsawang W, Zhu W, Piyachaturawat P. A silyl andrographolide analogue suppresses Wnt/β-catenin signaling pathway in colon cancer. Biomed Pharmacother. 2018;101:414–421. doi: 10.1016/j.biopha.2018.02.119. [DOI] [PubMed] [Google Scholar]
- 172.Takahashi K, Kikuchi H, Nguyen VH, Oshima Y, Ishigaki H, Nakajima-Shimada J, Kubohara Y. Biological activities of novel derivatives of differentiation-inducing factor 3 from dictyostelium discoideum. Biol Pharm Bull. 2017;40:1941–1947. doi: 10.1248/bpb.b17-00484. [DOI] [PubMed] [Google Scholar]
- 173.Totsuka K, Makioka Y, Iizumi K, Takahashi K, Oshima Y, Kikuchi H, Kubohara Y. Halogen-Substituted derivatives of dictyostelium differentiation-inducing factor-1 suppress serum-induced cell migration of human breast cancer MDA-MB-231 cells in vitro. Biomolecules. 2019;9:256. doi: 10.3390/biom9070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Efe Ertürk N, Taşcı S. The effects of peppermint oil on nausea, vomiting and retching in cancer patients undergoing chemotherapy: An open label quasi-randomized controlled pilot study. Complement Ther Med. 2021;56:102587. doi: 10.1016/j.ctim.2020.102587. [DOI] [PubMed] [Google Scholar]
- 175.Li X, Bai B, Liu L, Ma P, Kong L, Yan J, Zhang J, Ye Z, Zhou H, Mao B, et al. Novel β-carbolines against colorectal cancer cell growth via inhibition of Wnt/β-catenin signaling. Cell Death Discov. 2015;1:15033. doi: 10.1038/cddiscovery.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cha PH, Hwang JH, Kwak DK, Koh E, Kim KS, Choi KY. APC loss induces Warburg effect via increased PKM2 transcription in colorectal cancer. Br J Cancer. 2021;124:634–644. doi: 10.1038/s41416-020-01118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ruan Z, Liang M, Lai M, Shang L, Deng X, Su X. KYA1797K down-regulates PD-L1 in colon cancer stem cells to block immune evasion by suppressing the β-catenin/STT3 signaling pathway. Int Immunopharmacol. 2020;78:106003. doi: 10.1016/j.intimp.2019.106003. [DOI] [PubMed] [Google Scholar]
- 178.Cho YH, Ro EJ, Yoon JS, Kwak DK, Cho J, Kang DW, Lee HY, Choi KY. Small molecule-induced simultaneous destabilization of β-catenin and RAS is an effective molecular strategy to suppress stemness of colorectal cancer cells. Cell Commun Signal. 2020;18:38. doi: 10.1186/s12964-020-0519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Savvidou I, Khong T, Cuddihy A, McLean C, Horrigan S, Spencer A. β-Catenin Inhibitor BC2059 Is efficacious as monotherapy or in combination with proteasome inhibitor bortezomib in multiple myeloma. Mol Cancer Ther. 2017;16:1765–1778. doi: 10.1158/1535-7163.MCT-16-0624. [DOI] [PubMed] [Google Scholar]
- 180.Savvidou I, Khong T, Whish S, Carmichael I, Sepehrizadeh T, Mithraprabhu S, Horrigan SK, de Veer M, Spencer A. Combination of histone deacetylase inhibitor panobinostat (LBH589) with β-Catenin Inhibitor Tegavivint (BC2059) exerts significant anti-myeloma activity both in vitro and in vivo. Cancers (Basel) 2022;14:840. doi: 10.3390/cancers14030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Choi JH, Jang TY, Jeon SE, Kim JH, Lee CJ, Yun HJ, Jung JY, Park SY, Nam JS. The small-molecule Wnt Inhibitor ICG-001 efficiently inhibits colorectal cancer stemness and metastasis by suppressing MEIS1 Expression. Int J Mol Sci. 2021;22:13413. doi: 10.3390/ijms222413413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang D, Li Q, Shang R, Yao L, Wu L, Zhang M, Zhang L, Xu M, Lu Z, Zhou J, et al. WNT4 secreted by tumor tissues promotes tumor progression in colorectal cancer by activation of the Wnt/β-catenin signalling pathway. J Exp Clin Cancer Res. 2020;39:251. doi: 10.1186/s13046-020-01774-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Song Q, Han Z, Wu X, Wang Y, Zhou L, Yang L, Liu N, Sui H, Cai J, Ji Q, Li Q. β-Arrestin1 promotes colorectal cancer metastasis through GSK-3β/β-Catenin signaling-mediated epithelial-to-mesenchymal transition. Front Cell Dev Biol. 2021;9:650067. doi: 10.3389/fcell.2021.650067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li B, Orton D, Neitzel LR, Astudillo L, Shen C, Long J, Chen X, Kirkbride KC, Doundoulakis T, Guerra ML, et al. Differential abundance of CK1α provides selectivity for pharmacological CK1α activators to target WNT-dependent tumors. Sci Signal. 2017;10:eaak9916. doi: 10.1126/scisignal.aak9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zheng W, Hu J, Lv Y, Bai B, Shan L, Chen K, Dai S, Zhu H. Pyrvinium pamoate inhibits cell proliferation through ROS-mediated AKT-dependent signaling pathway in colorectal cancer. Med Oncol. 2021;38:21. doi: 10.1007/s12032-021-01472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang W, Li Y, Ai Y, Obianom ON, Guo D, Yang H, Sakamuru S, Xia M, Shu Y, Xue F. Pyrazole-4-Carboxamide (YW2065): A therapeutic candidate for colorectal cancer via dual activities of Wnt/β-Catenin signaling inhibition and AMP-Activated protein kinase (AMPK) activation. J Med Chem. 2019;62:11151–11164. doi: 10.1021/acs.jmedchem.9b01252. [DOI] [PubMed] [Google Scholar]
- 187.Song P, Feng L, Li J, Dai D, Zhu L, Wang C, Li J, Li L, Zhou Q, Shi R, et al. β-catenin represses miR455-3p to stimulate m6A modification of HSF1 mRNA and promote its translation in colorectal cancer. Mol Cancer. 2019;19:129. doi: 10.1186/s12943-020-01244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang Z, Zhou L, Wang Y, Peng Q, Li H, Zhang X, Su Z, Song J, Sun Q, Sayed S, et al. The CK1δ/ε-AES axis regulates tumorigenesis and metastasis in colorectal cancer. Theranostics. 2021;11:4421–4435. doi: 10.7150/thno.53901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB, Li CJ. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci USA. 2015;112:1839–1844. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.MacDonagh L, Gray SG, Breen E, Cuffe S, Finn SP, O'Byrne KJ, Barr MP. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 2018;428:117–126. doi: 10.1016/j.canlet.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 191.Han D, Yu T, Dong N, Wang B, Sun F, Jiang D. Napabucasin, a novel STAT3 inhibitor suppresses proliferation, invasion and stemness of glioblastoma cells. J Exp Clin Cancer Res. 2019;38:289. doi: 10.1186/s13046-019-1289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Beyreis M, Gaisberger M, Jakab M, Neureiter D, Helm K, Ritter M, Kiesslich T, Mayr C. The cancer stem cell inhibitor napabucasin (BBI608) shows general cytotoxicity in biliary tract cancer cells and reduces cancer stem cell characteristics. Cancers (Basel) 2019;11:276. doi: 10.3390/cancers11030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jonker DJ, Nott L, Yoshino T, Gill S, Shapiro J, Ohtsu A, Zalcberg J, Vickers MM, Wei AC, Gao Y, et al. Napabucasin versus placebo in refractory advanced colorectal cancer: A randomised phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:263–270. doi: 10.1016/S2468-1253(18)30009-8. [DOI] [PubMed] [Google Scholar]
- 194.Tam BY, Chiu K, Chung H, Bossard C, Nguyen JD, Creger E, Eastman BW, Mak CC, Ibanez M, Ghias A, et al. The CLK inhibitor SM08502 induces anti-tumor activity and reduces Wnt pathway gene expression in gastrointestinal cancer models. Cancer Lett. 2020;473:186–197. doi: 10.1016/j.canlet.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 195.Kajino-Sakamoto R, Fujishita T, Taketo MM, Aoki M. Synthetic lethality between MyD88 loss and mutations in Wnt/β-catenin pathway in intestinal tumor epithelial cells. Oncogene. 2021;40:408–420. doi: 10.1038/s41388-020-01541-3. [DOI] [PubMed] [Google Scholar]
- 196.Chen Y, Rao X, Huang K, Jiang X, Wang H, Teng L. FH535 inhibits proliferation and motility of colon cancer cells by targeting wnt/β-catenin signaling pathway. J Cancer. 2017;8:3142–3153. doi: 10.7150/jca.19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tu X, Hong D, Jiang Y, Lou Z, Wang K, Jiang Y, Jin L. FH535 inhibits proliferation and migration of colorectal cancer cells by regulating CyclinA2 and Claudin1 gene expression. Gene. 2019;690:48–56. doi: 10.1016/j.gene.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 198.Hua F, Shang S, Yang YW, Zhang HZ, Xu TL, Yu JJ, Zhou DD, Cui B, Li K, Lv XX, et al. TRIB3 Interacts With β-Catenin and TCF4 to increase stem cell features of colorectal cancer stem cells and tumorigenesis. Gastroenterology. 2019;156:708–721.e15. doi: 10.1053/j.gastro.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 199.Shang S, Yang YW, Chen F, Yu L, Shen SH, Li K, Cui B, Lv XX, Zhang C, Yang C, et al. TRIB3 reduces CD8(+) T cell infiltration and induces immune evasion by repressing the STAT1-CXCL10 axis in colorectal cancer. Sci Transl Med. 2022;14:eabf0992. doi: 10.1126/scitranslmed.abf0992. [DOI] [PubMed] [Google Scholar]
- 200.Cai X, Wei B, Li L, Chen X, Yang J, Li X, Jiang X, Lv M, Li M, Lin Y, et al. Therapeutic potential of apatinib against colorectal cancer by inhibiting VEGFR2-Mediated Angiogenesis and β-Catenin Signaling. Onco Targets Ther. 2020;13:11031–11044. doi: 10.2147/OTT.S266549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Or CR, Huang CW, Chang CC, Lai YC, Chen YJ, Chang CC. Obatoclax, a Pan-BCL-2 inhibitor, downregulates survivin to induce apoptosis in human colorectal carcinoma cells via suppressing WNT/β-catenin Signaling. Int J Mol Sci. 2020;21:1773. doi: 10.3390/ijms21051773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gan T, Stevens AT, Xiong X, Wen YA, Farmer TN, Li AT, Stevens PD, Golshani S, Weiss HL, Evers BM, Gao T. Inhibition of protein tyrosine phosphatase receptor type F suppresses Wnt signaling in colorectal cancer. Oncogene. 2020;39:6789–6801. doi: 10.1038/s41388-020-01472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Song W, Ma J, Lei B, Yuan X, Cheng B, Yang H, Wang M, Feng Z, Wang L. Sine oculis homeobox 1 promotes proliferation and migration of human colorectal cancer cells through activation of Wnt/β-catenin signaling. Cancer Sci. 2019;110:608–616. doi: 10.1111/cas.13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lepore Signorile M, Grossi V, Di Franco S, Forte G, Disciglio V, Fasano C, Disciglio V, Fasano C, Sanese P, De Marco K, et al. Pharmacological targeting of the novel β-catenin chromatin-associated kinase p38α in colorectal cancer stem cell tumorspheres and organoids. Cell Death Dis. 2021;12:316. doi: 10.1038/s41419-021-03572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sheng YH, Wong KY, Seim I, Wang R, He Y, Wu A, Patrick M, Lourie R, Schreiber V, Giri R, et al. MUC13 promotes the development of colitis-associated colorectal tumors via β-catenin activity. Oncogene. 2019;38:7294–7310. doi: 10.1038/s41388-019-0951-y. [DOI] [PubMed] [Google Scholar]
- 206.Li Q, Wang G, Tao J, Chen W. RNF6 promotes colorectal cancer invasion and migration via the Wnt/β-catenin pathway by inhibiting GSK3β activity. Pathol Res Pract. 2021;225:153545. doi: 10.1016/j.prp.2021.153545. [DOI] [PubMed] [Google Scholar]
- 207.Peng W, Zhang H, Tan S, Li Y, Zhou Y, Wang L, Liu C, Li Q, Cen X, Yang S, Zhao Y. Synergistic antitumor effect of 5-fluorouracil with the novel LSD1 inhibitor ZY0511 in colorectal cancer. Ther Adv Med Oncol. 2020;12:1758835920937428. doi: 10.1177/1758835920937428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhu Y, Gu L, Lin X, Zhang J, Tang Y, Zhou X, Lu B, Lin X, Liu C, Prochownik EV, Li Y. Ceramide-mediated gut dysbiosis enhances cholesterol esterification and promotes colorectal tumorigenesis in mice. JCI Insight. 2022;7:e150607. doi: 10.1172/jci.insight.150607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen Z, Wu J, Liu B, Zhang G, Wang Z, Zhang L, Wang K, Fan Z, Zhu P. Identification of cis-HOX-HOXC10 axis as a therapeutic target for colorectal tumor-initiating cells without APC mutations. Cell Rep. 2021;36:109431. doi: 10.1016/j.celrep.2021.109431. [DOI] [PubMed] [Google Scholar]
- 210.Wang C, Dai J, Sun Z, Shi C, Cao H, Chen X, Gu S, Li Z, Qian W, Han X. Targeted inhibition of disheveled PDZ domain via NSC668036 depresses fibrotic process. Exp Cell Res. 2015;331:115–122. doi: 10.1016/j.yexcr.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 211.Pradhan TR, Mohapatra DK. A synthetic study toward the core structure of (-)-apicularen A. Org Biomol Chem. 2018;16:8810–8818. doi: 10.1039/C8OB02301H. [DOI] [PubMed] [Google Scholar]
- 212.Mauvezin C, Neufeld TP. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy. 2015;11:1437–1438. doi: 10.1080/15548627.2015.1066957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang J, Mook RA, Jr, Ren XR, Zhang Q, Jing G, Lu M, Spasojevic I, Lyerly HK, Hsu D, Chen W. Identification of DK419, a potent inhibitor of Wnt/β-catenin signaling and colorectal cancer growth. Bioorg Med Chem. 2018;26:5435–5442. doi: 10.1016/j.bmc.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.An T, Gong Y, Li X, Kong L, Ma P, Gong L, Zhu H, Yu C, Liu J, Zhou H, et al. USP7 inhibitor P5091 inhibits Wnt signaling and colorectal tumor growth. Biochem Pharmacol. 2017;131:29–39. doi: 10.1016/j.bcp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 215.Kumar B, Ahmad R, Sharma S, Gowrikumar S, Primeaux M, Rana S, Natarajan A, Oupicky D, Hopkins CR, Dhawan P, Singh AB. PIK3C3 inhibition promotes sensitivity to colon cancer therapy by inhibiting cancer stem cells. Cancers (Basel) 2021;13:2168. doi: 10.3390/cancers13092168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ye GD, Sun GB, Jiao P, Chen C, Liu QF, Huang XL, Zhang R, Cai WY, Li SN, Wu JF, et al. OVOL2, an inhibitor of WNT signaling, reduces invasive activities of human and mouse cancer cells and is down-regulated in human colorectal tumors. Gastroenterology. 2016;150:659–671.e16. doi: 10.1053/j.gastro.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 217.Monin MB, Krause P, Stelling R, Bocuk D, Niebert S, Klemm F, Pukrop T, Koenig S. The anthelmintic niclosamide inhibits colorectal cancer cell lines via modulation of the canonical and noncanonical Wnt signaling pathway. J Surg Res. 2016;203:193–205. doi: 10.1016/j.jss.2016.03.051. [DOI] [PubMed] [Google Scholar]
- 218.Ren Y, Tao J, Jiang Z, Guo D, Tang J. Pimozide suppresses colorectal cancer via inhibition of Wnt/β-catenin signaling pathway. Life Sci. 2018;209:267–273. doi: 10.1016/j.lfs.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 219.Fako V, Yu Z, Henrich CJ, Ransom T, Budhu AS, Wang XW. Inhibition of wnt/β-catenin signaling in hepatocellular carcinoma by an antipsychotic drug pimozide. Int J Biol Sci. 2016;12:768–775. doi: 10.7150/ijbs.14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Al-Dali AM, Weiher H, Schmidt-Wolf IGH. Utilizing ethacrynic acid and ciclopirox olamine in liver cancer. Oncol Lett. 2018;16:6854–6860. doi: 10.3892/ol.2018.9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu Q, Zeng A, Liu Z, Wu C, Song L. Liver organoids: From fabrication to application in liver diseases. Front Physiol. 2022;13:956244. doi: 10.3389/fphys.2022.956244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Caspi M, Wittenstein A, Kazelnik M, Shor-Nareznoy Y, Rosin-Arbesfeld R. Therapeutic targeting of the oncogenic Wnt signaling pathway for treating colorectal cancer and other colonic disorders. Adv Drug Deliv Rev. 2021;169:118–136. doi: 10.1016/j.addr.2020.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.