Abstract
Objective.
Gut dysbiosis, defined as pathogenic alterations in the distribution and abundance of different microbial species, is associated with neuropathic pain in a variety of clinical conditions, but this has not been explored in the context of neuropathy in people with HIV (PWH).
Methods.
We assessed gut microbial diversity and dysbiosis in PWH and people without HIV (PWoH), some of whom reported distal neuropathic pain (DNP). DNP was graded on a standardized, validated severity scale. The gut microbiome was characterized using 16S rRNA sequencing and diversity was assessed using phylogenetic tree construction. Songbird analysis (https://github.com/mortonjt/songbird) was used to produce a multinomial regression model predicting counts of specific microbial taxa through metadata covariate columns.
Results.
Participants were 226 PWH and 101 PWoH, mean (SD) age 52.0 (13.5), 21.1% female, 54.7% men who have sex with men (MSM), 44.7% non-white. Among PWH, median (interquartile range, IQR) nadir and current CD4 were 174 (21, 302) and 618 (448, 822) respectively; 90% were virally suppressed on antiretroviral therapy. PWH and PWoH did not differ with respect to microbiome diversity as indexed by Faith’s phylogenetic diversity (PD). More severe DNP was associated with lower alpha diversity as indexed by Faith’s PD in PWH (Spearman’s ρ = 0.224, p = 0.0007), but not in PWoH (Spearman’s ρ = 0.032, p = 0.748). These relationships were not confounded by demographics or disease factors. In addition, the log-ratio of features identified at the genus level as Blautia to Lachnospira was statistically significantly higher in PWH with DNP than in PWH without DNP (t-test, p = 1.01e-3). Furthermore, the log-ratio of Clostridium features to Lachnospira features also was higher in PWH with DNP than in those without (t-test, p = 6.24e-5)
Conclusions.
Our results, in combination with previous findings in other neuropathic pain conditions, suggest that gut dysbiosis, particularly reductions in diversity and relative increases in the ratios of Blautia and Clostridium to Lachnospira, may contribute to prevalent DNP in PWH. Two candidate pathways for these associations, involving microbial pro-inflammatory components and microbially-produced anti-inflammatory short chain fatty acids, are discussed. Future studies might test interventions to re-establish a healthy gut microbiota and determine if this prevents or improves DNP.
Keywords: HIV, microbiome, neuropathic pain, gut dysbiosis
Introduction
Gut dysbiosis: Links to pain phenotypes
Gut dysbiosis, defined as pathogenic alterations in the distribution and abundance of different microbial species, has been shown in individuals with various pain conditions.18, 31, 32, 41 As an example, in fibromyalgia -- a syndrome considered by many to be one of neuropathic pain as it shares with sensory polyneuropathies symptoms of burning pain, prickling and touch-evoked allodynia21, and in which small fiber pathology has been found46 -- alterations in the gut microbiota also have been reported27. Another study found that the abundance of the Bifidobacterium and Eubacterium genera, which include microbes that participate in the metabolism of neurotransmitters, was significantly reduced in fibromyalgia patients who experience pain with neuropathic qualities7, 44.
Neuropathic pain associated with peripheral nerve injury also is linked to the gut microbiota. For example, germ-free mice and those pre-treated with antibiotics demonstrated reduced oxaliplatin-induced mechanical hyperalgesia40. Providing evidence that neuroinflammation is critical to these relationships, the dorsal root ganglia of antibiotic-treated mice showed reduced infiltration of macrophages, and lower levels of IL-6 and TNF-α compared to mice fed with water40. In another report, reciprocal gut microbiota transfers between C57BL/6 (B6) and 129SvEv (129) mice as well as antibiotic depletion showed gut microbial profiles to be causally linked to paclitaxel-induced pain sensitivity and resistance33. Microglia proliferated in the spinal cords of paclitaxel treated mice harboring a pain-sensitive gut microbiota, but not in mice with a pain-resistant gut microbiota. A third study found that an abnormal composition of the gut microbiota contributed to neuropathic pain susceptibility induced by spared nerve injury in rats52. Fecal microbiota transplantation from rats resilient to spared nerve injury pain resulted in reduced pain in pseudo-germ-free mice.
The burden of DNP in PWH
Distal sensory polyneuropathy (DSP), defined as the presence of symmetrical reduction in distal pin or vibratory sensation or deep tendon reflexes, and distal neuropathic pain (DNP), comprising symmetrical uncomfortable burning and stabbing pain, are common in HIV and their clinical impact in virally suppressed people with HIV (PWH) is substantial. For example, distal DNP in HIV-related DSP was associated with opioid use, depression, and low quality of life13, 22, 24, 47. DSP also is a major contributor to balance difficulties and falls. In a study of 2,647 PWH, those with DSP had a more than 5-fold increase in the odds of balance problems compared to those without DSP37. DNP is often treatment-resistant8. We showed in a recent longitudinal study of 254 PWH that polyneuropathy prevalence increased from 25.7% to 43.7% over 12 years, and of 173 participants initially pain-free, 42 (24.3%) had incident DNP11. Participants with DNP at follow-up had significantly worse quality of life and greater dependence in activities of daily living than those who remained pain-free11. DNP contributes to the burden of polypharmacy in older PWH42. These findings highlight the increasing burden of DSP and DNP in the growing population of older long-term PWH survivors despite viral suppression on cART.
We sought to characterize potential alterations, including differences in gut microbial diversity and dysbiosis in PWH with DNP.
Methods
Participants.
We recruited PWH and PWoH from community sources at a single site in San Diego, CA for studies of neurological complications of HIV, including DSP and DNP. Inclusion criteria were HIV positive or negative confirmed by serology. Exclusion criteria were active neurological illnesses other than those related to HIV, and active psychiatric (e.g., psychosis) or substance use disorder that might interfere with completing study evaluations. All participants signed an IRB-approved written consent and the study was approved by the UCSD Human Subjects Protection Committee (IRB).
Clinical examination.
Physical findings of distal sensory polyneuropathy (DSP) were assessed using a validated neurological examination administered by trained nurses12. Similarly, DNP and its severity were evaluated using a validated self-report tool12. Evaluations included clinical examination for neuropathy signs (bilateral distal vibration, sharp and touch loss) and self-reported neuropathy symptoms (pain, numbness/sensory loss, paresthesias). Moderate or worse neuropathy was defined as two or more clinical signs of neuropathy from the list above. DNP was defined as burning, aching, or shooting symptoms and classified into five categories of clinician-rated pain severity: none, slight (occasional, fleeting), mild (frequent), moderate (frequent, disabling), and severe (constant, daily, disabling, requiring analgesic medication or other pain medication). Participants were characterized as men who have sex with men (MSM) based on self report. MSM status was of particular interest since it has been shown previously to be strongly associated with differences in the gut microbiome2, 37.
Characterization of the gut microbiome.
Stool was collected according to a standardized protocol. Participants unable to provide specimen at the on-site visit were provided with a kit to collect and freeze stool off site and return it within 24-hours. Stool samples are aliquoted into 5 equal parts, one gram was homogenized and processed in a nucleic acid preservative, and stored at −80C for 16S DNA sequencing. Gut microbial diversity was characterized using 16S rRNA sequencing. Gut microbial diversity was indexed by Faith’s phylogenetic diversity (PD)14. Microbiome beta diversity calculations were performed using robust Aitchison principal components analysis on the unrarefied table and PERMANOVA through the Adonis method in QIIME2.1, 26, 30.
16S rRNA Gene Sequencing.
DNA extraction and 16S rRNA amplicon sequencing were done using Earth Microbiome Project (EMP) standard protocols (http://www.earthmicrobiome.org/protocols-and-standards/16s)6. DNA was extracted with the Qiagen MagAttract PowerSoil DNA kit as previously described25. Amplicon PCR was performed on the V4 region of the 16S rRNA gene using the primer pair 515f to 806r with Golay error-correcting barcodes on the reverse primer. Amplicons were barcoded and pooled in equal concentrations for sequencing. The amplicon pool was purified with the QIAGEN DNeasy UltraClean Microbial Kit (GmbH, Hilden, Germany) and sequenced on the Illumina MiSeq sequencing platform (Illumina, San Diego, CA, USA). Sequence data were demultiplexed and minimally quality filtered using the Qiita defaults. Sequence data were demultiplexed and minimally quality filtered using the QIIME 1.9.1 script split_libraries_fastq.py, with a Phred quality threshold of 3 and default parameters (per Qiita recommendations)4 to generate per-study FASTA sequence files. Data generated in this study has been deposited on Qiita under study ID 11135. Sequencing data associated with this study have been deposited to EBI/ENA with accession number ERP122366. Phylogenetic tree construction was performed using the align_to_tree_mafft_fasttree command in the QIIME2 phylogeny plugin. Taxonomic assignment of microbial features was done through QIIME2 (https://doi.org/10.1186/s40168-018-0470-z) using a Naïve Bayes classifier trained on the GreenGenes 13_8 99% OTU database (https://doi.org/10.1038/ismej.2011.139).
HIV disease and treatment characteristics.
Clinical and laboratory data were ascertained via comprehensive neuromedical evaluations consisting of a structured clinician-administered interview, physical and neurological examinations and standard laboratory assays as previously described16. Levels of HIV RNA in plasma were measured via reverse transcriptase-polymerase chain reaction (Amplicor, Roche Diagnostics, Indianapolis, IN) and were considered undetectable below the lower limit of quantitation of 50 copies/ml. As is the convention in the literature and as has been previously validated10, nadir CD4+ T-cells were by self-report and current CD4 by flow cytometry in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory.
Statistical Analyses.
Group differences in background characteristics (i.e., demographics, neuropsychiatric and neuromedical characteristics) and microbiome alpha diversity were examined using analysis of variance (ANOVA), Wilcoxon/Kruskal-Wallis tests, and Chi-square statistics as appropriate. Variables were log10-transformed as needed to ensure normality of the distributions. We conducted one primary comparison, comparing microbiome diversity to DNP severity, stratified by HIV serostatus. The comparison was done using Spearman’s ρ with DNP severity as the independent variable. All other analyses were secondary. Covariates examined included demographics and MSM status; the latter has been shown previously to be strongly associated1 with differences in the gut microbiome2, 37. Multivariable models including these covariates were performed using standard least squares analysis. Analyses were conducted using JMP Pro® version 15.0.0 (SAS Institute Inc., Cary, NC, 2018). Multinomial regression was performed through Songbird on the full feature table excluding microbial features present in fewer than 10% of samples28. Model construction included DNP, HIV status, & MSM status. The interaction of DNP and HIV status was included in addition to their individual effects. We found that adding additional parameters such as sex did not result in better fit models quantified by calculating the pseudo Q2 value compared to a null model. Differential coefficients from this regression were used to determine log-ratios of taxa abundances that differentiate between groups through t-tests.
Results
Demographics and HIV disease and neuropathy characteristics.
Participants were 226 PWH and 101 PWoH (Table 1). In the two groups combined, the mean (SD) age was 52.0 (13.5); 21.1% were females, 54.7% were men who have sex with men (MSM) and 44.7% were non-white. Among PWH, median (interquartile range, IQR) nadir and current CD4 were and 174 (21, 302) and 618 (448, 822) respectively; 90% were virally suppressed on antiretroviral therapy. Distal sensory polyneuropathy (DSP) was more frequent in PWH than PWoH (26.2 vs 9.9%). DNP was more common (34.1% vs 11.9%; p = 1.14e-5) and severe in PWH than PWoH. DNP was much more frequent in those who had DSP (34.8%) than in those without (19.6%; p = 0.0008).
Table 1.
Participant demographics and neuropathy characteristics by HIV serostatus
| PWoH | PWH | p | |
|---|---|---|---|
| N | 101 | 226 | 0.374 |
| Age – years (mean SD) | 51 ± 16.7 | 52.4 ± 11.8 | 0.238 |
| Sex female – N (%) | 41 (23.3%) | 28 (12.4%) | <0.001 |
| MSM – N (%) | 23 (22.8%) | 154 (69.4%) | <0.001 |
| Ethnicity Black – N (%) | 20 (19.8%) | 44 (19.5%) | 0.858 |
| Hispanic | 20 (19.8%) | 50 (22.1%) | |
| Non-Hispanic White | 57 (56.4%) | 124 (54.9%) | |
| Other | 4 (4.0%) | 8 (3.5%) | |
| Distal sensory polyneuropathy – N (%) | 10 (9.9%) | 66 (26.2%) | <0.001 |
| Distal neuropathic pain – N (%) | 12 (11.9%) | 77 (34.0%) | <0.001 |
PWH and PWoH did not differ with respect to microbiome diversity as indexed by Faith’s PD (12.4 ± 3.96 vs 12.2 ± 3.80; p = 0.572). In PWH, more severe DNP was associated with lower diversity as indexed by Faith’s PD (Spearman’s ρ = 0.224, p = 0.0007), with a monotonic decrease in diversity for each increase in pain severity. This was not the case for PWoH (ρ = 0.032, p = 0.748) (Figure 1). Among PWH, both neuropathic paresthesias and neuropathic sensory loss also were associated with lower Faith’s PD (ρ = −0.146, p = 0.0283 and ρ = −0.180, p = 0.0069, respectively). These relationships were not confounded by demographics, sexual orientation or HIV disease factors. In particular, sex, which differed between PWH and PWoH, was non-significant in multivariable models.
Figure 1.
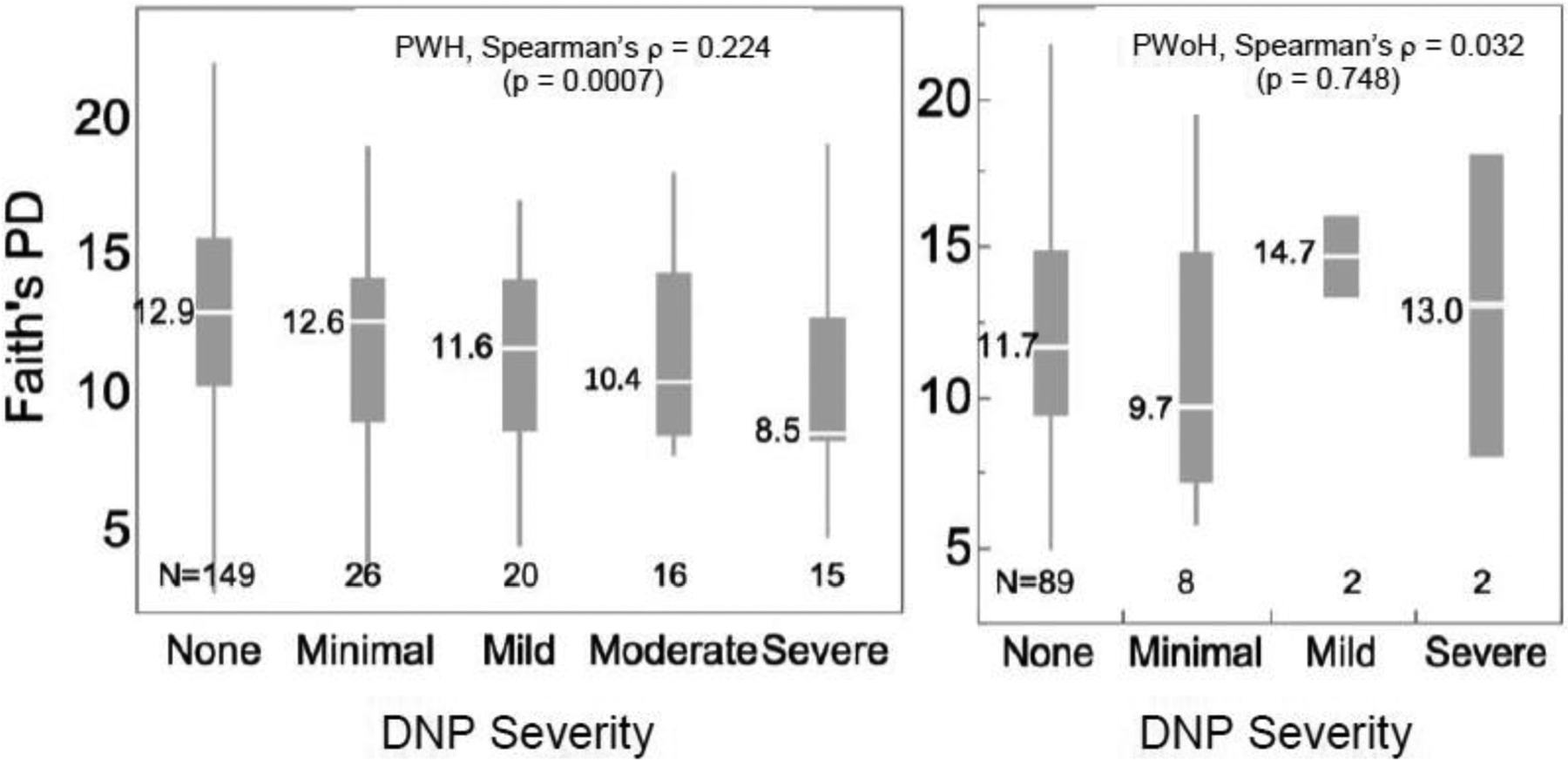
Among PWH, there was a dose-response relationship such that worse pain was associated with a stepwise reduction in alpha diversity as indexed by Faith’s PD (Spearman’s ρ = 0.224, p = 0.0007). Among PWoH, there was no significant relationship (Spearman’s ρ = 0.032, p = 0.748). Box plots show for each group the median (central white line), 25th and 75th percentiles (box) and 5th and 95th percentiles (whiskers). Values to the left of each box plot are the medians.
Participants self-identifying as MSM had higher Faith’s PD than non-MSM (13.0 ± 3.94 vs 11.3 ± 3.65; p = 1.07e-4). Rates of DSP (56.1% versus 67.9%, p = 0.121) and DNP (65.9% versus 66.0%, p = 0.985) were not different in MSM and non-MSM PWH, however, MSM PWH had less severe DNP than non-MSM (ordered comparison, p = 0.0243). In a stepwise, mixed, multivariable regression (p to enter 0.1, p to exit 0.1; minimum AICc) examining DNP severity, MSM, and their interaction as predictors of PD in PWH, MSM was significantly associated with higher PD (p = 0.0058), and DNP severity remained significant as well (p = 0.0019), but the interaction was not (p = 0.572). MSM were more likely to report numbness than non-MSM, but there was no difference for DNP or paresthesias. The relationship between DNP severity and Faith’s PD in MSM PWH was not significant. Faith’s PD also was lower in those with exam findings of DSP than in those without DSP among PWH (11.9 ± 3.99 vs 13.2 ± 3.81; p = 0.0189) but not in PWoH (12.1 ± 3.88 vs 12.2 ± 3.80; p = 0.138).
Microbiome diversity did not differ by on/off ART (Off, N = 11, mean [SD] diversity 13.3 [3.82] versus on N = 216, 12.4 [3.96], p = 0.4511), regimen type (NNRTI/INSTI 13.0 [0.581], NNRTI/NRTI 13.1 [3.75], NRTI 14.2 [3.32], NRTI/II, 12.7 [4.23], PI/INSTI 15.2 [1.61], PI/NRTI 12.1 [4.10], p = 0.295; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; INSTI, integrase strand inhibitor; PI, protease inhibitor) or current (r = 0.111, p = 0.0953) or nadir CD4 (r = 0.100, p = 0.0970). Female PWH (N = 28) had lower microbiome diversity than males (N = 198) (mean [SD] 10.9 [3.90] versus 12.7[3.93], p = 0.0259). In a multivariable model, DNP (p = 0.00675) and sex (p = 0.0480) were both significantly associated with microbiome diversity (full model p = 0.0051). Older females (a surrogate for menopausal status) had higher diversity than younger (r = 0.317, p = 0.0080). Among female PWH, age did not confound the relationship between DNP and microbiome diversity (age p = 0.00158; DNP p = 0.0109; whole model p = 0.0012).
Figure 2 shows a robust Aitchison PCA (RPCA) beta diversity ordination that demonstrates a microbial separation of samples by the various levels in this study. PERMANOVA of all samples show that beta diversity differences were highly statistically significant for HIV status, MSM status, and the interaction of HIV status and DNP but not for DNP by itself (Table 2). Thus, accounting for the effects of other terms in the multivariable model, HIV emerged as significantly associated with diversity, indicating that confounding between HIV and DNP (DNP more frequent in PWH than PWoH; odds ratio, 95% confidence interval 3.83 [1.98, 7.43]) and between HIV and MSM (more PWH than PWoH were MSM; OR 10.5 [6.03, 18.2]) was responsible for the lack of difference in the univariable analysis. A second RPCA beta diversity analysis on only MSM subjects showed a statistically significant difference between those with DNP and those without (p = 0.028).
Figure 2.
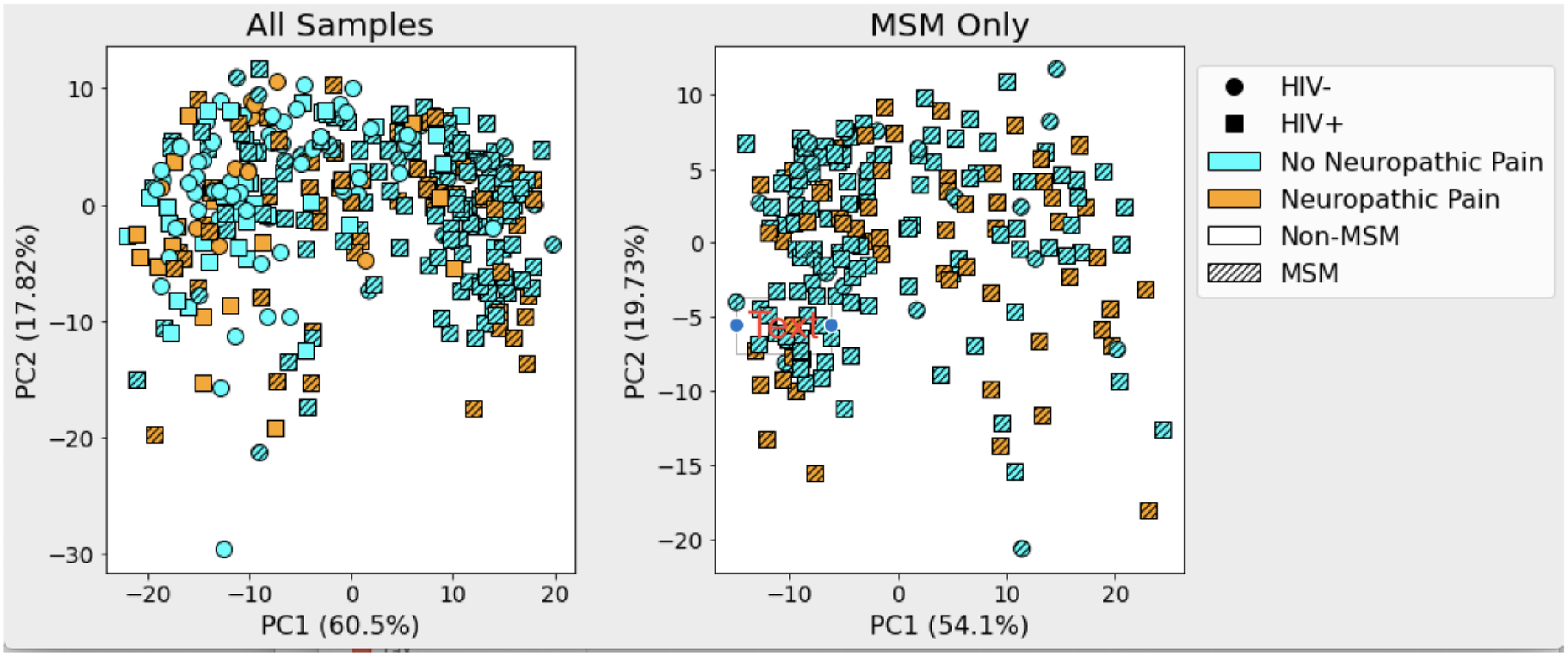
Robust Aitchison PCA plot showing beta diversity ordination of all samples (left) and of MSM only (right). In a multivariable model significant beta diversity effects were seen for HIV status (PWH > PWoH: p < 0.001), MSM status (p < 0.001), and the interaction of DNP × HIV (p = 0.023). Within MSM, participants with DNP were significantly different from those without DNP.
Table 2.
beta diversity differences were highly statistically significant for HIV status, MSM status, and the interaction of HIV status and DNP but not for DNP by itself
| Sum of Squares | Model F | p | |
|---|---|---|---|
| Neuropathic Pain | 4.27 | 1.49 | 0.210 |
| HIV Status | 20.8 | 7.25 | 0.001 |
| MSM Status | 70.8 | 24.7 | 0.001 |
| Pain × HIV Interaction | 8.28 | 2.89 | 0.023 |
Songbird analysis of the full cohort was used to produce a multinomial regression model predicting counts of specific microbial taxa through metadata covariate columns. Including both DNP and HIV status in this model resulted in regression coefficients indicating individual microbial taxa associations with DNP and HIV status relative to all other taxa. Taxonomic assignments of microbes were summarized according to genus-level identification in GreenGenes for downstream differential abundance analysis.
DNP:HIV+ coefficients from the regression were averaged for each genus and sorted to determine which genera were most associated with DNP and HIV status relative to all other microbes while taking into account prevalence and abundance. We used these genus rankings to choose numerator and denominator taxa rather than testing all possible combinations of microbial features to reduce the risk of reporting false positives. We noted Lachnospira as a genus that had a low average coefficient and high average prevalence, indicating that these microbes are relatively less associated with DNP:HIV+ status than other microbes – as such we use it as the denominator in log-ratio comparisons. Log-ratio of sets of taxa are compared rather than relative abundance to avoid issues of compositionality inherent to sequencing data (https://doi.org/10.1038/s41467-019-10656-5). Genera that were highly associated for use in the numerator were chosen the same way as the denominator. The log-ratio of features identified at the genus level as Blautia to Lachnospira was statistically significantly higher in PWH with DNP than in PWH without DNP (t-test, p = 1.01e-3) while retaining 89% of samples (Figure 3). Furthermore, the log-ratio of Clostridium features (2) to Lachnospira features also was higher in PWH with DNP than in those without (t-test, p = 6.24e-5). There were not enough samples from PWoH with DNP to make any meaningful statistical conclusions with this cohort.
Figure 3.
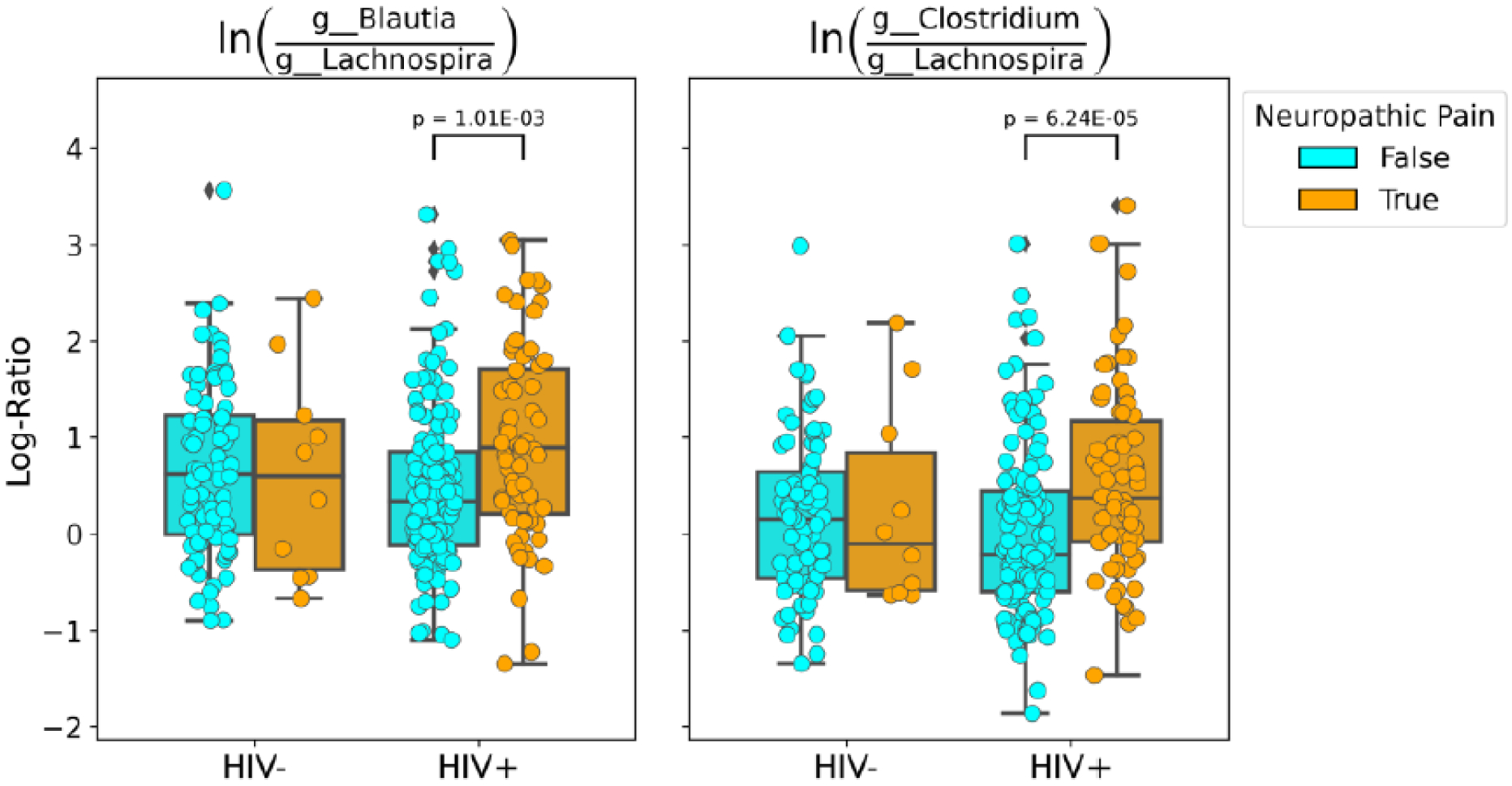
Log-ratio plots of g__Blautia features vs. g__Lachnospira features (left) and g__Clostridium features vs. g__Lachnospira features (right). Among PWH these log-ratios differed between those with DNP and those without.
Discussion
Our findings suggest that gut dysbiosis may contribute to prevalent DNP in PWH or may otherwise modulate the clinical phenotype of distal sensory polyneuropathy in HIV. These results are concordant with findings in other neuropathic pain conditions33, 40, 52. There was no evidence of confounding by measured covariates. In particular, MSM PWH, who had a more diverse gut microbiome, as shown in previous studies2, 37, did not show a significant association between generalized pain severity and gut microbial diversity. In other words, the DNP-gut microbiome diversity association appears to be specific for HIV-related DNP as compared to other pain conditions. The lack of a similar association in PWoH may reflect their qualitatively different microbiomes (independent of diversity) as shown in previous studies9, 15, or may be due to the low frequency of DNP in this group.
The specific mechanism by which the gut microbiome influences pain is not known. However, we offer several points in this regard. First, our findings are consistent with observations in other neuropathic pain conditions. Second, our findings have plausible pathophysiological underpinnings based on inflammatory microbial components, neuroprotective microbially-produced short chain fatty acids, reciprocal communications between the gut and the brain (the gut-brain axis), and commonalities in the brain regions influenced by the gut microbiome and those involved in pain processing5, 19, 29. We observed higher relative abundances of Blautia and Clostridium species in the DNP group. This is in agreement with a study of a neuropathic pain model in rats, chronic constriction injury (CCI), where rats with neuropathic pain had significantly increased Blautia compared to controls. Similarly, some Clostridium species (eg, C. scindens), were found in higher abundance in fibromyalgia patients with neuropathic pain27. Finally, Lachnospira produce short chain fatty acids (SCFAs)48 which are neuroprotective36 and anti-inflammatory49. Thus, relative reductions in Lachnospira abundance might reduce SCFAs, exacerbating inflammation and neural injury.
An additional mechanism by which the gut microbiome and DNP may be linked is through the autonomic nervous system (ANS). One key component of the gut-brain axis is the vagus nerve, which comprises large numbers of small, lightly myelinated and unmyelinated fibers, many regulating ANS function. Modulation of the CNS by the gut microbiome is mediated through the vagus by neuroimmune and neuroendocrine mechanisms5 43, 45, 50. Sensory polyneuropathy in PWH includes a prominent component of small fiber injury3, leading to ANS dysfunction23. Evidence from animal models supports that gut microbiota changes correlate with dysfunction of the ANS38. In addition, small fiber injury frequently manifests as neuropathic pain17. Thus, the composition and diversity of the gut microbiota may influence small fiber injury that causes both pain and ANS dysfunction.
Pathways linking the gut microbiome to pain processing neural pathways via the gut-brain axis could mediate pain perception in neuropathy. There is overlap between brain regions and neurotransmitters affected by the gut microbiota and those that process pain. For example, chronic treatment with Lactobacillus rhamnosus JB-1 induced region-dependent alterations in GABA mRNA in the cingulate39. Indeed, the anterior cingulate cortex appears to be particularly important in processing the emotional and cognitive aspects of pain20, 51
Most research on gut microbiome changes related to antiretroviral treatment has focused on the impact of Immune recovery, rather than on the direct effects of different antiretroviral drugs. One small study of 16 patients showed different patterns of microbial changes in patients starting an efavirenz-containing regimen versus a protease inhibitor-based regimen34. Our analyses did not show effects of specific antiretroviral drugs or classes on microbial diversity.
A potential limitation in interpreting these results is that DNP may have been incorrectly attributed to DSP. We showed here that those with DNP were much more likely to have DSP than those without DNP. Additionally, we have previously reported that when examined with more sensitive measures of neuropathy including electrophysiology, the great majority of those with DNP in fact do have DSP35. Furthermore, we carefully elicited reports of pain that are typical for DSP-related DNP, rather than other neuropathic pain conditions. The number of PWoH with DNP was very small, limiting statistical power in this group. An additional limitation is the correlational nature of the study design, which precluded causal associations. However, other studies have shown that manipulation of the gut microbiome reduces susceptibility to neuropathic pain after nerve injury and chemotherapy, suggesting that the gut microbiome-pain connection is common to a variety of pain condition and is a causal factor. Finally, we acknowledge that the observed correlations might reflect the influence of unobserved confounding variables.
Future studies should evaluate the potential for interventions to re-establish a healthy gut microbiota, such as fecal transplantation or pre- or pro-biotics, to improve or prevent neuropathic pain.
Highlights.
Microbiome diversity was similar in people with HIV (PWH) and without HIV (PWoH).
Worse neuropathic pain accompanied lower microbiome diversity in PWH but not PWoH.
Blautia and Clostridium species were relatively more abundant in PWH with DNP.
Gut dysbiosis may contribute to prevalent neuropathic pain in PWH.
Re-establishing a healthy microbiota might reduce neuropathic pain.
Perspective.
The association of neuropathic pain in people with HIV with reduced gut microbial diversity and dysbiosis raises the possibility that re-establishing a healthy gut microbiota might ameliorate neuropathic pain in HIV by reducing pro-inflammatory and increasing anti-inflammatory microbial products.
Disclosures:
This publication was made possible by NIH grant P30MH062512.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
References
- 1.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecology. 26:32–46, 2001. [Google Scholar]
- 2.Boer CG, Radjabzadeh D, Medina-Gomez C, Garmaeva S, Schiphof D, Arp P, Koet T, Kurilshikov A, Fu J, Ikram MA, Bierma-Zeinstra S, Uitterlinden AG, Kraaij R, Zhernakova A, van Meurs JBJ. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. 10:4881, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boger MS, Hulgan T, Haas DW, Mitchell V, Smith AG, Singleton JR, Peltier AC. Measures of small-fiber neuropathy in HIV infection. Auton Neurosci. 169:56–61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 10:57–59, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 108:16050–16055, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6:1621–1624, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clos-Garcia M, Andres-Marin N, Fernandez-Eulate G, Abecia L, Lavin JL, van Liempd S, Cabrera D, Royo F, Valero A, Errazquin N, Vega MCG, Govillard L, Tackett MR, Tejada G, Gonzalez E, Anguita J, Bujanda L, Orcasitas AMC, Aransay AM, Maiz O, Lopez de Munain A, Falcon-Perez JM. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine. 46:499–511, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, McCutchan JA, Heaton RK, Ellis RJ. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 73:342–348, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis CL, Ma ZM, Mann SK, Li CS, Wu J, Knight TH, Yotter T, Hayes TL, Maniar AH, Troia-Cancio PV, Overman HA, Torok NJ, Albanese A, Rutledge JC, Miller CJ, Pollard RB, Asmuth DM. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr. 57:363–370, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. Aids. 25:1747–1751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis RJ, Diaz M, Sacktor N, Marra C, Collier AC, Clifford DB, Calcutt N, Fields JA, Heaton RK, Letendre SL, Group CNSATERS. Predictors of worsening neuropathy and neuropathic pain after 12 years in people with HIV. Ann Clin Transl Neurol. 7:1166–1173, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 67:552–558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I, Group CS. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 67:552–558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faith DP. Conservation evaluation and phylogenetic diversity. Biological Conservation. 61:1–10, 1992 [Google Scholar]
- 15.Gori A, Tincati C, Rizzardini G, Torti C, Quirino T, Haarman M, Ben Amor K, van Schaik J, Vriesema A, Knol J, Marchetti G, Welling G, Clerici M. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol. 46:757–758, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 17:3–16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hovaguimian A, Gibbons CH. Diagnosis and treatment of pain in small-fiber neuropathy. Curr Pain Headache Rep. 15:193–200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnsen PH, Hilpusch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, Goll R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 3:17–24, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Keltner JR, Connolly CG, Vaida F, Jenkinson M, Fennema-Notestine C, Archibald S, Akkari C, Schlein A, Lee J, Wang D, Kim S, Li H, Rennels A, Miller DJ, Kesidis G, Franklin DR, Sanders C, Corkran S, Grant I, Brown GG, Atkinson JH, Ellis RJ. HIV Distal Neuropathic Pain Is Associated with Smaller Ventral Posterior Cingulate Cortex. Pain Med. 18:428–440, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CE, Kim YK, Chung G, Jeong JM, Lee DS, Kim J, Kim SJ. Large-scale plastic changes of the brain network in an animal model of neuropathic pain. Neuroimage. 98:203–215, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Koroschetz J, Rehm SE, Gockel U, Brosz M, Freynhagen R, Tolle TR, Baron R. Fibromyalgia and neuropathic pain--differences and similarities. A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC Neurol. 11:55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krashin DL, Merrill JO, Trescot AM. Opioids in the management of HIV-related pain. Pain Physician. 15:ES157–168, 2012 [PubMed] [Google Scholar]
- 23.Levine TD. Small Fiber Neuropathy: Disease Classification Beyond Pain and Burning. J Cent Nerv Syst Dis. 10:1179573518771703, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Liu X, Tang SJ. Interactions of Opioids and HIV Infection in the Pathogenesis of Chronic Pain. Front Microbiol. 7:103, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marotz C, Amir A, Humphrey G, Gaffney J, Gogul G, Knight R. DNA extraction for streamlined metagenomics of diverse environmental samples. Biotechniques. 62:290–293, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Martino C, Morton JT, Marotz CA, Thompson LR, Tripathi A, Knight R, Zengler K. A Novel Sparse Compositional Technique Reveals Microbial Perturbations. mSystems. 4, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minerbi A, Gonzalez E, Brereton NJB, Anjarkouchian A, Dewar K, Fitzcharles MA, Chevalier S, Shir Y. Altered microbiome composition in individuals with fibromyalgia. Pain. 160:2589–2602, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Morton JT, Marotz C, Washburne A, Silverman J, Zaramela LS, Edlund A, Zengler K, Knight R. Establishing microbial composition measurement standards with reference frames. Nat Commun. 10:2719, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology (Berl). 214:71–88, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H: vegan: Community Ecology Package.d R package version 2.5–3. Available at: https://CRAN.R-project.org/package=vegan
- 31.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am J Gastroenterol. 109:1831–1832, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Rajilic-Stojanovic M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 141:1792–1801, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Ramakrishna C, Corleto J, Ruegger PM, Logan GD, Peacock BB, Mendonca S, Yamaki S, Adamson T, Ermel R, McKemy D, Borneman J, Cantin EM. Dominant Role of the Gut Microbiota in Chemotherapy Induced Neuropathic Pain. Sci Rep. 9:20324, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray S, Narayanan A, Giske CG, Neogi U, Sonnerborg A, Nowak P. Altered Gut Microbiome under Antiretroviral Therapy: Impact of Efavirenz and Zidovudine. ACS Infect Dis. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson-Papp J, Morgello S, Vaida F, Fitzsimons C, Simpson DM, Elliott KJ, Al-Lozi M, Gelman BB, Clifford D, Marra CM, McCutchan JA, Atkinson JH, Dworkin RH, Grant I, Ellis R. Association of self-reported painful symptoms with clinical and neurophysiologic signs in HIV-associated sensory neuropathy. Pain. 151:732–736, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadler R, Cramer JV, Heindl S, Kostidis S, Betz D, Zuurbier KR, Northoff BH, Heijink M, Goldberg MP, Plautz EJ, Roth S, Malik R, Dichgans M, Holdt LM, Benakis C, Giera M, Stowe AM, Liesz A. Short-Chain Fatty Acids Improve Poststroke Recovery via Immunological Mechanisms. J Neurosci. 40:1162–1173, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakabumi DZ, Moore RC, Tang B, Delaney PA, Keltner JR, Ellis RJ. Chronic Distal Sensory Polyneuropathy is a Major Contributor to Balance Disturbances in Persons Living with HIV. J Acquir Immune Defic Syndr. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ Res. 120:312–323, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, Guarner F. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 4:17–27, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen S, Lim G, You Z, Ding W, Huang P, Ran C, Doheny J, Caravan P, Tate S, Hu K, Kim H, McCabe M, Huang B, Xie Z, Kwon D, Chen L, Mao J. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci. 20:1213–1216, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 10:1802–1805, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siefried KJ, Mao L, Cysique LA, Rule J, Giles ML, Smith DE, McMahon J, Read TR, Ooi C, Tee BK, Bloch M, de Wit J, Carr A, investigators Ps. Concomitant medication polypharmacy, interactions and imperfect adherence are common in Australian adults on suppressive antiretroviral therapy. AIDS. 32:35–48, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci. 36:7428–7440, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sumpton JE, Moulin DE. Fibromyalgia. Handb Clin Neurol. 119:513–527, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 61:364–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uceyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 136:1857–1867, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Uebelacker LA, Weisberg RB, Herman DS, Bailey GL, Pinkston-Camp MM, Stein MD. Chronic Pain in HIV-Infected Patients: Relationship to Depression, Substance Use, and Mental Health and Pain Treatment. Pain Med. 16:1870–1881, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanegas SM, Meydani M, Barnett JB, Goldin B, Kane A, Rasmussen H, Brown C, Vangay P, Knights D, Jonnalagadda S, Koecher K, Karl JP, Thomas M, Dolnikowski G, Li L, Saltzman E, Wu D, Meydani SN. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am J Clin Nutr. 105:635–650, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 3:858–876, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Telesford KM, Ochoa-Reparaz J, Haque-Begum S, Christy M, Kasper EJ, Wang L, Wu Y, Robson SC, Kasper DL, Kasper LH. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat Commun. 5:4432, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weizman L, Dayan L, Brill S, Nahman-Averbuch H, Hendler T, Jacob G, Sharon H. Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology. 91:e1285–e1294, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang C, Fang X, Zhan G, Huang N, Li S, Bi J, Jiang R, Yang L, Miao L, Zhu B, Luo A, Hashimoto K. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl Psychiatry. 9:57, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]


