Abstract
Mycotoxins are secondary metabolites produced by specific fungi. More than 400 different mycotoxins are known in the world, and the concentration of these toxins in food and feed often exceeds the acceptable limit, thus causing serious harm to animals and human body. At the same time, modern industrial agriculture will also bring a lot of environmental pollution in the development process, including the increase of heavy metal content, and often the clinical symptoms of low/medium level chronic heavy metal poisoning are not obvious, thus delaying the best treatment opportunity. However, the traditional ways of detoxification cannot completely eliminate the adverse effects of these toxins on the body, and sometimes bring some side effects, so it is essential to find a new type of safe antidote. Trace element selenium is among the essential mineral nutrient elements of human and animal bodies, which can effectively remove excessive free radicals and reactive oxygen species in the body, and has the effects of antioxidant, resisting stress, and improving body immunity. Selenium is common in nature in inorganic selenium and organic selenium. In previous studies, it was found that the use of inorganic selenium (sodium selenite) can play a certain protective role against mycotoxins and heavy metal poisoning. However, while it plays the role of antioxidant, it will also have adverse effects on the body. Therefore, it was found in the latest study that selenium yeast could not only replace the protective effect of sodium selenite on mycotoxins and heavy metal poisoning, but also improve the immunity of the body. Selenium yeast is an organic selenium source with high activity and low toxicity, which is produced by selenium relying on the cell protein structure of growing yeast. It not only has high absorption rate, but also can be stored in the body after meeting the physiological needs of the body for selenium, so as to avoid selenium deficiency again in the short term. However, few of these studies can clearly reveal the protective mechanism of yeast selenium. In this paper, the detoxification mechanism of selenium yeast on mycotoxins and heavy metal poisoning was reviewed, which provided some theoretical support for further understanding of the biological function of selenium yeast and its replacement for inorganic selenium. The conclusions suggest that selenium yeast can effectively alleviate the oxidative damage by regulating different signaling pathways, improving the activity of antioxidant enzymes, reversing the content of inflammatory factors, regulating the protein expression of apoptosis-related genes, and reducing the accumulation of mycotoxins and heavy metals in the body.
Keywords: Selenium yeast, Mycotoxins, Heavy metals, Oxidative stress
Introduction
Mycotoxins are toxic secondary metabolites produced by certain fungi, which mainly grow in soil, hay, decaying vegetation, and grains, and can cause biochemical, physiological, and pathological changes in many species [1]. Mycotoxins were first discovered by certain fungal scientists in the early 1960s during an outbreak of disease X in British turkeys. At that time, nearly 100,000 turkeys were killed because peanuts in their food were heavily contaminated with Aspergillus flavus, a mycotoxin-producing mold [2]. Other mycotoxin-producing fungi include Aspergillus, penicillium, and Fusarium. Different molds produce different mycotoxins, mainly including ochratoxin A (OTA), deoxynivalenol (DON), T-2 toxin, zearalenone (ZEA, ZEN), and aflatoxin B1 (AFB1) [3]. Currently, more than 400 mycotoxins are recognized and their number is constantly growing. After all, we live in a world in crisis—from volatile financial markets to panic over feed and food safety. Instability and inconsistencies in agriculture and the feed industry are causing uncertainty around the world. It should be noted that more than 25% of the world’s cereals are contaminated with mycotoxins and such contaminated components pose a serious threat to the health and performance of animals. Experience shows that often dangerous diseases caused by harmful substances (e.g., aflatoxin, ochratoxins) produced as a result of improper storage (e.g., moisture) or non-compliance with technological regimes during harvesting cause veterinarians to misdiagnose the causes of diseases, e.g., coli, when in fact ochratoxin overloads the animal’s liver and causes secondary coli infection.
Heavy metal poisoning refers to poisoning caused by heavy metal elements or their compounds with a relative atomic mass greater than 65, mainly including arsenic, chromium, cadmium, and lead poisoning, which is mainly caused by exposure to or eating a large amount of mining, smelting, industrial, and other sewage wastes or abandoned industrial products [4]. With the development of new technologies in agricultural industry, heavy metal pollution needs more and more attention. The frequent exposure and accumulation of heavy metals in organisms will cause serious health problems and affect the normal operation of a series of organs such as the brain, liver, and reproductive organs [5]. Some heavy metals (such as mercury, lead, and cadmium) are able to accumulate in the food chain due to their long presence in the environment. At present, the safety of cultivated land and water polluted by heavy metals is seriously threatening the normal life of animals and people all over the world. The effects of acute heavy metal exposure at toxic levels are usually known and can be treated promptly. However, the impact of low/moderate levels of chronic heavy metal exposure on health is unlikely to be determined because they may be subclinical and pathogenic effects may only manifest clinically over time in the guise of diagnosable diseases or other symptoms attributable to aging, thus delaying treatment [6]. At the same time, chelation therapy is commonly used to treat heavy metal poisoning in clinic. However, the widely used heavy metal chelating agent, dimercaptosuccinate, is not only poor in water solubility, low in oral bioavailability, but also short in half-life, which seriously limits its clinical application [7].
Nutrition is one of the most important factors affecting animal health. The forage used to feed livestock can be contaminated with a variety of harmful substances, including secondary metabolites of toxin-causing fungi and heavy metals waste from factories. Therefore, detoxification of domestic animal mycotoxin and heavy metal poisoning is an important problem. A substantial body of research demonstrates that mycotoxins and heavy metals can harm the organism by producing too many reactive oxygen species and oxidative stress [8–11]. Therefore, the current research focuses on the use of antioxidants like vitamin E, vitamin C, proanthocyanidins, curcumin, and some trace elements like selenium and zinc to combat the oxidative damage caused to the body by these chemicals by weakening the destructive effect of reactive oxygen species and oxidative stress. It has been shown that simultaneous supplementation of various antioxidants can minimize the oxidative stress associated with ingestion of feed contaminated with mycotoxins. The best results are to be expected when using a mixture of antioxidants, not just the individual ingredients. In addition to the use of antioxidants, some scientists have also found that Saccharomyces cerevisiae, a microbial feed additive classified as probiotics, can absorb fungal toxins (aflatoxin B1, ochratoxin A, and zearalenone) in contaminated feed to mitigate the negative effects. Saccharomyces cerevisiae RC016 is a promising candidate for a feed additive formulation that improves animal growth and intestinal immune system. It has been shown that Saccharomyces cerevisiae RC016 can improve intestinal health in weaned piglets by alleviating small intestinal inflammation caused by deoxynivalenol poisoning [12]. Meanwhile, it was found that Lactobacillus rhamnosus bacteria can reduce the harmful effects of deoxynivalenol on the intestine of young pigs and has anti-inflammatory and antioxidant effects. They alleviate histopathological changes, limit the increase in the expression of pro-inflammatory cytokines, and reduce the permeability of the epithelium [13]. LGG supplementation can also reduce the damage of DON on the antioxidant system of piglet kidney [14]. At the same time, it was noticed that L. rhamnosus bacteria do not bind deoxynivalenol and only to a small extent cause the decomposition of this mycotoxin.
Trace element selenium is an important component of many important antioxidant enzymes in vivo, including glutathione peroxidase, thioredoxin reductase, and iodothyronine deiodinase. As a part of enzymes that catalyze oxidation and reduction (REDOX) reactions, selenium protects cells from the harmful effects of free radicals and plays an important antioxidant role in the normal life activities of animals [15]. Selenium can exert its detoxification effect on mycotoxin poisoning by reducing reactive oxygen species production and mitochondrial dysfunction, enhancing cell viability and function, and inhibiting the immune response (see Fig. 1). More and more experimental results show that selenium is also a nutrient with immunomodulatory properties, which can regulate the immune function changes caused by mycotoxins. It has been proved that selenium limits changes in cytokine and immunoglobulin gene expression induced by the action of deoxynivalenol [16]. Dietary supplementation of selenium prevents impaired humoral or mucosal immune function due to AFB1 [17]. In addition, Se can further counteract the immunotoxic effects of T-2 toxins on T lymphocytes [18].
Fig. 1.
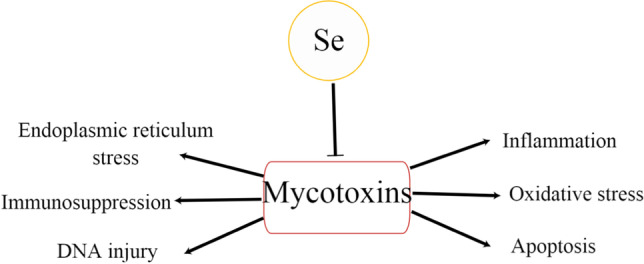
Effect of selenium on mycotoxin. The symbol “→” indicates activation and “⟞” indicates inhibition
Like other elements, selenium circulates in nature. Selenium has two different forms: inorganic selenium and organic selenium. Organic forms include selenate (Na2SeO4) and sodium selenite (Na2SeO3), while organic forms include selenomethionine (SeMet) and selenocysteine (SeC). After being ingested by the body, selenium is absorbed by the intestine and transported to the liver. In the liver, selenium is mainly metabolized to Sec. Then, Sec is combined into selenoprotein to form selenoprotein in the form of selenium-cysteine, which is the derivative of the simplest amino acid cysteine and can be used as a selenium source for other tissues in the body [19]. Selenoproteins are also involved in the development and regeneration of muscle tissue [20]. Selenium is also a component of two essential amino acids (selenomethionine and selenocysteine) that form key enzymes in many metabolic processes [21]. The discovery of the conversion of inorganic selenium organisms into organic derivatives (e.g., selenomethionine) by prion producing yeast cells suggests that prion producing yeast cells rich in organic forms of selenium create the possibility of obtaining selenium biocomplexes that could be used to produce dietary supplements for protein selenium in animals and humans [22]. In addition, selenium has many important functions in the human body. It can inhibit cancer cell division and protect neurons and cardiomyocytes [23]. It is also involved in the recovery of ascorbic acid from its oxidative metabolites, in DNA synthesis and apoptotic processes (i.e., programmed cell death), and plays an important anti-inflammatory role in autoimmune thyroiditis [24]. Dietary supplementation of selenium also contributes to the development of strong antioxidant defenses for maternal and developing embryos, and is positively associated with embryo survival and offspring development [25].
In addition, selenium has been experimentally and clinically proven to be effective against viral diseases. Selenium is an oxidizing agent, which easily reacts with the sulfhydryl group in the active center of the viral protein disulfide isomerase, thus inactivating it. This causes the hydrophobic spikes of the virus to lose their ability to interact with the dithionyl group of membrane proteins, and the virus is unable to enter the cytoplasm of healthy cells. The same mechanism underlies the action of oxidants with apparent antiviral activity [26]. Selenomionine at higher than physiological concentrations was found to resist replication of porcine Delta coronavirus in porcine renal epithelial cells and enhance mitochondrial antiviral signaling protein (MAVS) protein expression and interferon regulatory factor-3 (IRF-3) phosphorylation [27]. Therefore, selenium may be considered a promising antiviral drug and may be effective in the treatment of COVID-19. For example, when isolating samples from COVID-19 patients, Moghaddam et al. found that 39% of survivors had low serum selenium levels, while 65% of patients who died of COVID-19 showed severe selenium deficiency compared with survivors [28]. As mentioned above, Im et al. also found that 42% of hospitalized patients with COVID-19 showed selenium deficiency with moderate pneumonia [29]. Therefore, adequate selenium supplementation may reduce oxidative stress by restoring antioxidant enzymes, reducing cell death and clotting pathways, and protecting endothelial cells, thus having an overall protective effect on the lungs and other organs to mitigate the impact of COVID-19 on the body [30].
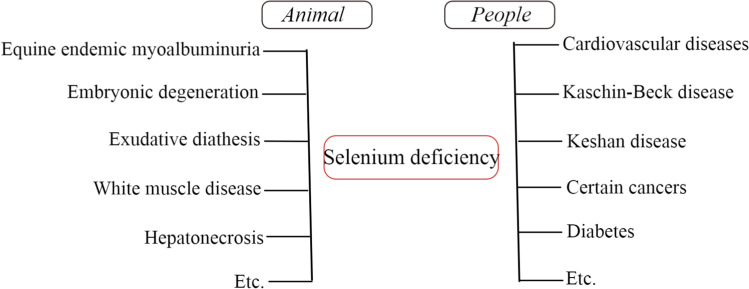
According to the survey, most parts of China are in the selenium deficiency environment, and selenium deficiency often causes a variety of diseases, as shown in Fig. 2 [31–33]. Moreover, selenium deficiency can also cause serious pathological changes in body organs and tissues. The study found that selenium deficiency can cause kidney damage and fibrosis in the body, and the Wnt/β-catenin pathway may play an important role [34]. At the same time, selenium deficiency also affects the morphology and structure of liver cells and mitochondria, and causes structural damage and fibrosis in liver tissues [35]. Selenium deficiency also causes diarrhea by disrupting intestinal flora, and through intestinal flora causes pathological changes such as intestinal inflammation, autophagy, endoplasmic reticulum stress, apoptosis, tight junctions, and abnormal smooth muscle contraction [36]. However, even exposure to higher concentrations of selenium also can be toxic to the body. Selenium is essential for the proper production of mammalian sperm cells and for sperm maturation. Sperm production is always affected when dietary selenium levels are too high or too low, which may affect semen quality decline and sterility [37]. It was found that high doses of selenium would increase the oxidative stress in yeast, thus increasing the process of lipid peroxidation. The experiment also found that higher levels of oxidized glutathione (GSSG) were obtained from yeast biomass aqueous solution supplemented with selenium (40–60 mg/L). The low concentrations of GR observed in yeast showed that the two examined yeast strains have a reduced ability to effectively convert emerging GSSG to its reduced form. GR was responsible for maintaining adequate levels of reduced glutathione in the cytoplasm of cells [38].
Fig. 2.
Diseases caused by selenium deficiency
Due to its ability to affect various diseases by enhancing antioxidant capacity and regulating immune cells as well as pathogens such as viruses, selenium supplements may become a high-dose supplement to treat various diseases, especially the body’s sensitivity to selenium supplementation and the relatively low price of selenium preparation, which may lead to new ideas for solving medical problems such as cancer treatment and HIV treatment. It is possible that new and effective methods will be developed to prevent these diseases, which may save more resources than treatment alone.
Selenium yeast is a high-quality organic selenium source with high absorption rate, low toxicity, and wide safety range generated by the substitution of sulfur element in the protein structure of growing yeast cells [39]. The studies have found that adding selenium yeast to the diet can prevent various pathological conditions caused by oxidative stress [40–43]. At present, although some studies have shown that inorganic selenium sources, such as sodium selenite (SS), which are mainly used as dietary selenium source additives, have significant detoxification effects on mycotoxins and heavy metal poisoning [44–47], they are still limited by acute toxicity, accelerated oxidation, and low bioavailability in the application process. So far, some developed countries have prohibited their use in animal diets [48]. Meanwhile,most tests have found that selenium in SY has higher bioavailability and can improve its antioxidant activity more available than selenium in SS after comparison [49–51]. This is because the biological potency and safety of selenium yeast products have been greatly improved in the production process, making them easier to be absorbed and utilized by the body.
At present, although selenium yeast has been widely used in the production of selenium-rich products, e.g., selenium-rich eggs, the mechanism of selenium yeast against oxidative stress induced by mycotoxins and heavy metal poisoning to protect the body from oxidative damage is still little known. Therefore, this review aims to analyze and summarize the detoxification mechanisms of selenium yeast to mycotoxins and heavy metal poisoning, and will help to inform future studies investigating the effects of selenium yeast.
Major Toxicity Mechanisms Induced by Mycotoxins and Heavy Metals
To date, various studies have shown that once animals ingest feed contaminated with mycotoxins, multiple adverse effects can be induced in vivo, e.g., hepatororal and renal toxicity, reproductive toxicity, cardiotoxicity, neurotoxicity, and immunotoxicity [52–54]. The accumulation of heavy metals in the body will also lead to the occurrence of the above diseases [55–57]. However, any study on the effects of mycotoxins on organisms usually involves articles on a single substance. In fact, feed may be contaminated by several mycotoxins at the same time, which increases their toxicity. For example, mycotoxins mixed with aflatoxin and deoxynivalenol could aggravate liver damage and immune dysfunction, leading to a decline in pig growth [58]. Mycotoxins mixed with deoxynivalenol and aflatoxin B1 could reduce the ileal apparent digestibility of nutrients in the feed of weaned pigs, and affects the growth performance and nutrient digestibility of weaned piglets [59]. The main mechanism of its toxicity is described below.
Oxidative Stress
Reactive oxygen species (ROS) are oxygen free radicals in organisms, including oxygen and highly active molecules containing oxygen, such as superoxide anion (O2·−), hydroxyl radical (HO·), nitric oxide (·NO), hydrogen peroxide (H2O2), and peroxynitrite (ONOO) [60], which are by-products of mitochondria and other organelles in cells [61]. The body will constantly produce reactive oxygen species during metabolism. Under normal circumstances, the production and clearance of active oxygen in the body are in dynamic balance, so the body will not express obvious oxidative damage. While the body accumulates too much reactive oxygen species and exceeds the threshold of cell antioxidant capacity, it can induce oxidative stress [62]. Oxidative stress is a negative effect produced by free radicals in the body. Under this condition, active substances will cause oxidative damage to biological macromolecules in living cells, such as proteins, carbohydrates, lipids, and DNA [63]. In addition, oxidative stress can accelerate cell aging [64], induce cell apoptosis [65], and eventually lead to the occurrence of many diseases, such as cancer, cardiovascular disease, and neurodegenerative diseases [66–68].
AFB1 is the most toxic of all aflatoxins, which can induce oxidative stress in the testes, causing pathological damage and dysfunction of the testes [69]. It has been reported that AFB1 was able to significantly increase the production of intracellular ROS and decrease the level of glutathione, thereby activating several signal pathways related to inflammatory response to induce oxidative stress in cells [70]. Similarly, the OTA exposure significantly downregulated the expression of the antioxidant enzyme genes, decreased the activity of the antioxidant enzymes, and increased the intracellular ROS levels to cause oxidative stress [71]. Meanwhile, the oxidative damage of DNA could also be directly induced by OTA [72]. Moreover, ZEA could also damage the cytoskeletal structure of the mouse TM4 Sertoli cells via the oxidative stress-autophagy-ER stress pathway [73]. Fu et al. found that ZEA can cooperate with LPS to increase the accumulation of ROS and malondialdehyde (MDA) in bovine mammary epithelial cells (MAC-T), and reduce their mitochondrial membrane potential, superoxide dismutase (SOD), and glutathione (GSH) levels, to aggravate cytotoxicity [74]. T-2 toxin exposure could cause oxidative stress–induced cytotoxicity by significantly reducing cell viability, increasing MDA levels, and reducing glutathione peroxidase (GSH-Px), SOD, and catalase (CAT) activity [75]. All these findings suggest that cellular oxidative stress is one of the modes of virulence induced by mycotoxins.
Oxidative stress is also one of the main toxic mechanisms of heavy metals. It has been found that cadmium could accumulate within the nephron and induce the dysfunction of the mitochondrial electron transport chain, leading to electron leakage and the production of reactive oxygen species, and ultimately leading to oxidative damage to DNA, proteins, and lipids [76]. Furthermore, lead exposure was also able to increase ROS levels, decrease antioxidant enzyme activity in vivo, and impair oocyte maturation and fertilization by inducing oxidative stress, leading to the decline of fertility in female mice [77]. Ma et al. also found that adding arsenic to the diet could inhibit the Nrf2-Keap1 pathway in the liver and kidney to induce oxidative stress [78]. The levels of lipid peroxidation and protein carbonyl group content in mice exposed to hexavalent chromium (Cr(VI)) were increased, and a large number of ROS were produced in the body. At the same time, the activities of SOD, glutathione S-transferase, GSH, total mercaptan (TT), CAT, and cholinesterase were decreased, which induced oxidative stress leading to liver cell damage, causing the increase of aspartate aminotransferase and alanine aminotransferase [79].
Apoptosis
Apoptosis is also known as programmed cell death. OTA was able to increase cellular oxidative stress and apoptosis rates by activating the phosphatidylinositol 3-kinase/threonine kinase (PI3K/AKT) signaling pathway to hinder cell proliferation and development [80]. It was also found that OTA could also induce endoplasmic reticulum stress and reactive oxygen species generation by activating NADPH oxidase and calpain [81]. Meanwhile, ZEA could significantly decrease the transcription and expression of the anti-apoptotic protein Bcl-2 and the increase of the pro-apoptotic protein Bax, to induce apoptosis through the mitochondrial apoptosis pathway [82]. Furthermore, Long et al. also indicated that ZEA could also cause mouse epithelial cell apoptosis through ER stress [83]. In addition, the study also found that the cytotoxicity induced by DON could increase the expression of genes and proteins related to apoptosis and inflammation in cells [84]. In vitro, DON could induce significant morphological changes in subject cells undergoing the Caspase-3-related pathway, leading to apoptosis [85]. AFB1 could promote cell apoptosis and cell cycle arrest in G2-M phase through oxidative stress, and activate the phosphorylation of nuclear factor-kappa B (NF-ĸB) in microglia in mouse spinal cord to induce cell apoptosis [86]. Fumonisin (FB1) could also increase the ROS levels of cells by affecting the Keap1-Nrf2 pathway-related factors to cause inflammation and cell apoptosis, and finally destroy the testicular tissue structure and affect the formation of sperm [87]. In summary, mytoxins can cause apoptosis both in vitro and in vivo.
The experiment showed that cadmium could activate the mitochondrial-mediated intrinsic apoptosis pathway and JNK (c-Jun N-terminal kinase), extracellular signal-regulated kinase (ERK), and p38 MAPK (mitogen-activated protein kinase) pathways to reduce cell viability and increase apoptosis rate [88]. Meanwhile, cadmium could also induce hepatocyte apoptosis and tissue damage through inflammatory reaction [89]. Even at very low lead concentrations, Pb was equally toxic to monocytes and macrophages, which shows a decline in cell viability and an increase in the number of apoptotic cells [90]. Zhou also found that lead could increase the expression of PPARγ and stimulate the cleavage of caspase-3 and PARP to induce cytotoxicity [91]. In addition, the mRNA and protein levels of apoptosis- and autophagy-related genes were significantly increased in cells exposed to Arsenic trioxide (As2O3) [92]. Inorganic arsenic (As3+) could exert its cytotoxicity in neuronal cells through downstream regulated autophagy-dependent apoptosis pathway via Akt inactivation/AMPK activation, ultimately leading to cell death [93]. To sum up, apoptosis is one of the mechanisms of heavy metals induced toxicity as well.
Immunologic Injury
Mycotoxins also have immunotoxicity, which seriously affects the immune function of the body. It was found that ZEA could induce T lymphocyte apoptosis through excessive activation of MAPK pathway [94]. Islam also found that ZEA could reduce the level of IgM and tumor necrosis factor (TNF)-ɑ, and increase the level of IgE and interleukin (IL)-6 in vivo. In addition, ZEA could also induce splenocyte apoptosis by regulating the ratio of Bax to Bcl-2 [95]. Recent studies have also shown that ZEA could also attenuate macrophage-mediated innate immunity by reducing LPS-activated macrophages’ production of pro-inflammatory mediators, cytokines, and NLRP3 inflammasome [96]. At the same time, AFB1 could induce the increase of inflammatory cytokine interferon (IFN)-γ, IL-1β, as well as the decrease of anti-oxidant enzyme activity to cause damage to intestinal immune function and barrier function of broilers [97]. Mycotoxins can also cause atrophy and failure of immune organs and other histopathological changes [98]. Khan et al. significantly detected leucopenia and the decrease of IgY and IgA concentrations in serum, as well as the weightlessness of thymus, spleen, and bursa in chicks after OTA exposure [99]. To sum up, mycotoxin exposure can induce immunosuppression.
For heavy metals, heavy metal poisoning can also cause a strong immunosuppressive reaction in the body. Qu found in the study that cadmium exposure could trigger oxidative stress and obvious pathological damage in spleen tissue [100]. At the same time, cadmium exposure significantly inhibited the phagocytic activity of chicken peritoneal macrophages, and caused the increase of apoptosis, accumulation of reactive oxygen species, morphological changes, and other pathological damage, and promoted the transcription of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-ɑ) in macrophages under the stimulation of LPS [101]. Similarly, lead treatment also significantly inhibited the activities of SOD, GPX, and CAT and increased the accumulation of nitric oxide (NO) and MDA to induce oxidative stress and activate MAPK/NF-ĸB signal pathway to cause splenic necrosis [102]. Arsenic can disrupt lymphocyte morphology, reduce cell viability, cause abnormal proportions of T lymphocyte subsets, and induce regulatory T cell (Tregs) dysfunction to cause immune damage [103]. Liu et al. found that chickens exposed to arsenic showed a reduced IFN-γ/IL-4 ratio and an increased IL-17 level, which indicated an imbalanced immune response in vivo [104]. In conclusion, heavy metals can induce immune changes in the body.
Histological Injury
Mycotoxin poisoning also has significant effects on histomorphology. DON exposure can significantly reduce intestinal villus height and increase recess depth [105]. Popescu found that local necrosis, sinus dilatation, and inflammatory parenchyma infiltration occurred in the liver of piglets fed the basic diet contaminated with the mixture of OTA and AFB1 [106]. In the livers of piglets exposed to fumonisin, scattered necrosis, swelling, and scattered focal hyperplasia of hepatocytes were observed. Meanwhile, mild focal interstitial lymphoid tissue cell infiltration were shown in the lung and mild focal fibrosis were founded in visceral pleura [107]. To sum up, mycotoxin poisoning can induce tissue necrosis, inflammatory cell infiltration, and focal proliferation and so on.
The study found that with the increase of dietary cadmium supplement, the liver tissue of laying hens was significantly damaged. That is, liver tissue had periportal fibrosis, bile duct hyperplasia, and periportal inflammatory cell infiltration [108]. In the testis of lead-exposed chickens, obvious gaps were found between convoluted seminiferous tubules, and some convoluted seminiferous tubules, basement membrane, and blood testis barrier were damaged. The reproductive epithelium became thinner, the number of spermatogenic cells decreased significantly, the nucleus of spermatogenic cells shrank, and a large number of swollen mitochondria and autophages could be seen [46]. Sections of the arsenic-induced cardiotoxicity group contained a variety of lesions, including extensive necrosis, inflammatory cell infiltration, cytoplasmic vacuolation, minor fibrous swelling, soft interstitial edema, and muscle fiber loss and so on [109]. In conclusion, heavy metals can lead to significant pathological changes in tissue morphology.
Detoxification Mechanism of Selenium Yeast to Mycotoxin Poisoning
In modern farming, mycotoxins are likely to be inadvertently ingested by the body, so safe, efficient, and novel detoxification methods are crucial. To date, much research has been conducted in this regard, and because detoxification processes are often accompanied by the loss of feed palatability and nutritional value, the addition of protective nutrients or additives is a good way to reduce mycotoxin toxicity. It has found that selenium has a certain protective effect on the oxidative damage caused by mycotoxin poisoning. However, because the use of inorganic selenium often brings potential harm, further research on the detoxification mechanism of selenium yeast to mycotoxin poisoning can be used to solve this part of the problem (see Table 1).
Table 1.
Mechanism of selenium yeast on mycotoxins poisoning
| Mycotoxins | Site of injury | Protective mechanism of selenium yeast | Ref. |
|---|---|---|---|
| OTA | Broiler cecum | Regulates TLR4/MYD88 signal pathway, inhibits NF-ĸB expression, and increases the expression of tight junction related genes; significantly reduces MDA and IL-1β, IL-6 and IFN-γ level, increases GSH, SOD activity, and IL-10 level, and antagonizes the intestinal barrier damage | [110] |
| Broiler liver, kidney | Reduces the content of ALT and MDA induced by OTA, and reverses the reduction of T-AOC, GSH-Px, and T-SOD, improves the activity of antioxidant enzymes; activates PI3K/AKTNrf2/Keap1 signaling pathway to improve the antioxidant defense system, significantly upregulates the gene expression of Nrf2 and its target genes | [111, 112] | |
| AFB1 | Mouse immune system | Improves the growth performance, antioxidant capacity, IL-2 and IFN-γ content, increases IL-2 and IFN-γ and GSH-Px1 mRNA levels | [113] |
| Duck immune system | Increases lymphocyte proliferation and acidity α—the positive rate of naphthyl acetate esterase (ANAE+); improves the levels of IL-2 and IL-6, and reverses the adverse effects of AFB1 on relative immune organ weight | [114] | |
| ZEA | Mouse liver, kidney | Reduces ALT, AST, urea, and uric acid; increases the levels of MDA, GSH-Px, and SOD in serum; and reverses the damage caused by ZEA | [115] |
| Mouse testis | Improves the decrease of epididymal index and testicular index; decreases the content of MDA in testis, increases the activities of SOD and GPx, reverses the increase of mRNA expression levels of Bax and caspase 3 and mRNA expression levels of BCL2, vimentin and cadherin 2 | [116] | |
| DON | Broiler immune system | Increases the number of CD3 (+), CD4 (+), CD8 (+) T cells and IgM (+) B cells in the blood | [117] |
Anti-inflammatory and Anti-oxidation
ROS, MDA, GSH-Px, and SOD are the key indicators of the overall antioxidant capacity of the body. As a result of selenium is an important component of GSH-Px in the body, and antioxidant enzymes are widely found in the immune system, selenium also has a certain role on inflammatory factors. Zhou et al. found that T-2 toxin resulted in significantly higher levels of IL-6, IL-1β, and TNF-α in serum and cartilage [118]. Meanwhile, the ROS levels of T-2 toxin were decreased after treatment with organic selenium [119]. It has been reported that selenium-rich yeast could reverse the levels of cytokines IFN-γ, IL-10, IL-6, and IL-1β by inhibiting TLR4/MYD88 signaling and prolonging NF-ĸB signaling, to antagonize the oxidative stress and inflammation induced by OTA exposure, and reduce intestinal pathological damage [110]. Similarly, selenium-rich yeast also can detoxify AFB1-induced toxicity. That is, SY could significantly improve the antioxidant capacity, IL-2, and IFN-γ content, and increase the IL-2, IFN-γ, and GSH-Px1 mRNA levels [113]. Selenium could also reduce DON-induced oxidative damage by increasing glutathione peroxidase activity [120]. In addition, the increase of MDA level and the decrease of GSH-Px and SOD content in serum caused by ZEA exposure also returned to the normal level after treatment with selenium-enriched yeast [115] In view of this, selenium yeast can inhibit oxidative stress and inflammatory reaction by regulating the activation of signal pathways, the levels of antioxidant enzyme, and the content of inflammatory factors caused by mycotoxin poisoning.
Anti-apoptosis
Similarly, selenium deficiency could also aggravate oxidative stress injury induced by AFB1 via reducing the level of antioxidant enzymes, upregulating apoptosis genes (Caspase-3 and Caspase-9) and downregulating anti-apoptosis genes and several selenium proteins [121]. After adding selenium yeast, the cell survival rate could be increased by 10 times, and the cells could be effectively protected from AFB1-induced killing [122]. In addition, selenium yeast could also significantly improve the expression of apoptosis-related genes in OTA poisoning, showing anti-apoptotic effects in vivo and in vitro. For chicken, cell apoptosis and oxidative damage induced by OTA exposure could also return to normal levels after the use of selenium yeast [111]. The main mechanism is that SY could not only reverse the decrease of antioxidant enzyme activity induced by OTA, but also protect the decrease of Bcl-2 protein level and the increase of Caspase-3 and Bax protein expression by activating Nrf2/Keap1 and PI3K/AKT signaling pathways, so as to restore normal kidney and liver conditions [112]. For ZEA exposure, Long et al. found that selenium yeast could inhibit the apoptosis and testicular damage in germ cells by enhancing the antioxidant capacity and changing the expression levels of apoptosis-related genes in mice as well [116]. At the same time, it was found to prevent apoptosis and oxidative stress caused by ZEA through inhibiting ER stress [123]. In conclusion, selenium yeast can inhibit the apoptosis caused by mycotoxins via significantly reducing oxidative stress and regulating apoptosis-related proteins.
Enhance the Immune Function
In the study of the antagonistic effect of selenium on mycotoxins, it was also found that selenium could enhance the immune function. For example, dietary selenium could protect from AFB1-induced humoral or mucosal immune function damage, as well as restoring the content of IgM, IgG, IgA, and sIgA [17]. Furthermore, Huang et al. found that the usage of organic selenium increased the production of T cell proliferation and interleukin-2 in vivo to mitigate the immunotoxicity of AFB1 [124]. Another experiment showed that adding selenium yeast to the diet could counterbalance the adverse effects of duck AFB1 poisoning on growth performance and immunity by improving the levels of IL-2 and IL-6 and the relative weight of immune organs [114]. As far as DON is concerned, the diet supplemented with selenium yeast could increase the induced reduction of B lymphocytes and T lymphocytes in blood [117]. Of course, the enhanced immune function of selenium yeast is still related to its antioxidant activity [125]. These studies could suggest that selenium yeast may be a promising supplement to protect humans and animals from the decreased immunity induced by mycotoxins.
Among the numerous challenges facing the breeding industry, nutritional supplementation can be used as an effective and safe way to address the problem of exposure to mycotoxins in livestock and poultry. As a potential antioxidant, a trace element selenium is an essential component of several essential enzymes in the body. Owing to high safety, high bioavailability, and low toxicity of selenium yeast, it can be used as a good choice for mycotoxin detoxification. This may help in the future to improve both human and animal health, while reducing the inevitable economic losses.
Detoxification Mechanism of Heavy Metal Poisoning
Heavy metal poisoning is a common public health problem. Chelation therapy has been the main treatment for heavy metal poisoning; i.e., chelating agents combine with metal ions to form chelators to enhance their clearance from the body. However, metal chelating agents have some disadvantages, which can redistribute heavy metals from other tissues to the brain, increasing its neurotoxicity and other adverse effects [126]. Therefore, it is very momentous to propose a safe and effective new chelating agent. Studies have found that selenium has antioxidant properties and can prevent animals and humans from the harm of heavy metals [127–130]. Due to the adverse effects of inorganic selenium on the body, further research on the detoxification mechanism of selenium yeast against heavy metals poisoning provides the future direction to solve this problem (see Table 2).
Table 2.
Mechanism of selenium yeast on heavy metals poisoning
| Heavy metals | Injury site | Protective mechanism of selenium yeast | Ref. |
|---|---|---|---|
| Cd | Layers kidney | Significantly increases the activities of SOD, GSH-Px, and CAT in serum and liver, decreases the level of MDA to inhibit oxidative stress; reverses the expression of MLKL, RIp1, RIP3, ERK, JNK, and P38 mRNA induced by Cd, and increases the expression level of caspase8 mRNA | [131] |
| Broiler liver | Inhibits the expression of RIP1, RIP3, and MLKL, prevents the accumulation of cadmium in the kidney to reduce necrosis; activates the expression of miR-26a-5p, downregulates the expression of PTEN, upregulates PI3K/AKT signal pathway, to inhibit oxidative stress; specifically reduces the expression level of HSP60, HSP70 and HSP90 | [132] | |
| Layers liver | Significantly reduces cadmium accumulation, NO production, inducible nitric oxide synthase (iNOS) activity, inflammatory factors, HSPs (HSP 27, 40, 60, 70, and 90) mRNA and protein expression levels induced by cadmium to inhibit inflammation | [133] | |
| Layers liver | Downregulates the levels of NLRP3, Caspas-1 and IL-1 β and IL-18, restores antioxidant level and selenoprotein expression level, and reduces hepatocyte apoptosis | [134] | |
| Porcine jejunal epithelial cells | Significantly improves cell viability and protects cells from cadmium-induced DNA breakage and apoptosis | [135] | |
| Pb | Broiler skeletal muscles | Reverses the increase of nitric oxide concentration and iNOS activity induced by pb, and reduces the expression level of IL-1β, IL-4, IL-10, IFN-γ | [136] |
| Cr | Broiler spleen | Increases the index of spleen organs; increases the level of SOD and GSH, reduces the content of MDA, and alleviate the histopathological damage; significantly increases the content of T-globulin IgA, IgM, and IgG, and reduces the expression of proinflammatory cytokines | [137] |
| Broiler kidney | Alleviates the morphological and structural damage of renal tubules and glomeruli, and reduces the organ index, creatinine level and blood urea nitrogen level of the kidney; increases the levels of SOD and GSH, reduces the level of MDA to alleviate oxidative stress; recovers the levels of p53, c-Myc, Bax, Cyt-c, caspase-9, and caspase-3 | [138] |
Anti-inflammatory and Anti-oxidation
The anti-inflammatory and antioxidant effects of selenium yeast can also combat the toxic damage of heavy metals. Selenium yeast can protect Cd-induced oxidative damage by inhibiting the decrease of antioxidant enzyme activity caused by cadmium exposure, and downregulating MAPK pathway and improving caspase8 mRNA expression level [131]. Chen et al. found that selenium yeast could also inhibit the expression of receptor interacting protein-1 (RIP1), receptor interacting protein-3 (RIP3), and mixed lineage kinase domain–like protein to significantly block the accumulation of cadmium in the kidney, and inhibit oxidative stress and reduce cadmium-induced necrosis by regulating PTEN (phosphatase and tensin homolog deleted on chromosome 10)/PI3K/AKT signal pathway induced by miR26a-5p [132]. Moreover, selenium yeast also has an antagonistic effect on cadmium-induced inflammatory damage. That is, selenium yeast could reverse the increase of inflammatory factors and heat shock proteins (HSPs) caused by cadmium exposure to reduce the accumulation of cadmium in the liver [133]. For chicken skeletal muscle inflammation caused by excessive lead poisoning, selenium yeast could inhibit Ras/ERK pathway as well as the content of IL-1β, IL-4, IL-10, IFN-γ, and other inflammatory factors to alleviate it [136]. Finally, SY may also have a protective effect against the Cr6+-induced immune suppression and inflammation response by regulating the NF-ĸB signaling pathway [137]. Therefore, according to the above studies, SY can be used as a potential therapeutic agent for heavy metal–induced inflammatory damage.
Anti-apoptosis
Selenium also has some antagonistic effects on apoptosis induced by heavy metal poisoning. Cadmium exposure induced the increase of Bax, caspase-9, p53, cytochrome c (Cyt-c) mRNA levels, Bax/Bcl-2 ratio, caspase-3 mRNA, and protein levels [139]. However, the application of selenium yeast was found to reduce the cadmium-induced apoptosis in chicken liver cells by increasing the antioxidant levels and the expression of selenoprotein [134]. At the cellular level, selenium yeast can also repair cadmium-induced DNA damage [135]. In addition, selenium yeast can also inhibit the dysfunction and apoptosis caused by hexavalent chromium. First, it can reduce the level of malondialdehyde and alleviate chromium-induced oxidative stress by increasing the level of superoxide dismutase and glutathione. Second, it can restore the expression of p53, Bax, Cyt-c, caspase-9, caspase-3, Bcl-2, and other genes induced by chromium to normal [138]. Overall, selenium yeast can alleviate heavy metal–induced cytotoxicity by inhibiting oxidative stress and reversing the protein expression levels of apoptosis-related genes. However, the more detailed mechanisms still need to be summarized through more studies.
Compared with traditional metal chelating agents, natural antioxidants are easy to obtain, are reasonable in price, and have few or no side effects [126]. Thus, they can be used as a new method to solve heavy metal poisoning. Selenium yeast is a good choice for new chelators due to its potent antioxidant activity, high safety, high bioavailability, and low toxicity. This provides new ideas for the future field of heavy metal toxicology research.
Summary
This review mainly presents the protective effect of selenium yeast on effectively reducing the toxicity of animal mycotoxins and heavy metals, which shows that selenium yeast has a certain detoxification effect. Selenium yeast can effectively alleviate oxidative damage by regulating different signal pathways, improving the activity of antioxidant enzymes, reversing the content of inflammatory factors, regulating the protein expression of apoptosis-related genes, and reducing the accumulation of mycotoxins and heavy metals in the body. The main detoxification mechanism is as follows, as shown in Fig. 3.
Fig. 3.
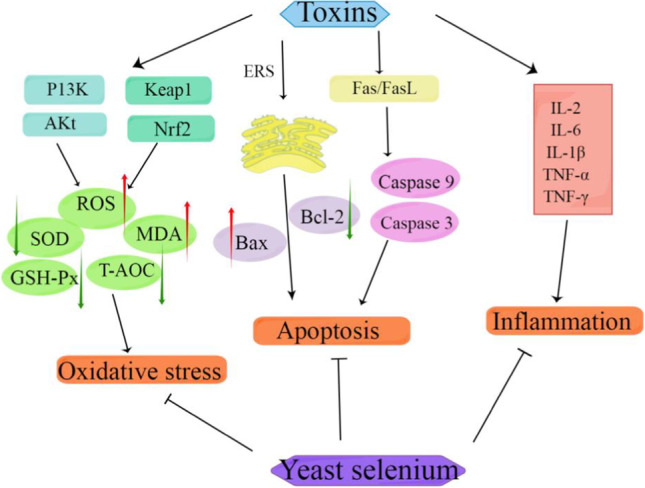
Detoxification mechanism of selenium yeast on mycotoxins and heavy metals poisoning [140]. The symbol “→” indicates activation and “⟞” indicates inhibition. The red arrow indicates that the concentration or level of the indicator increases or rises, while the green arrow indicates that the concentration or level of the indicator decreases or falls
Inevitably, now we live in a world of feed and food safety crises. Instability and inconsistency in the agricultural and feed industries are creating uncertainty around the world. Whether mycotoxins or heavy metals, long-term exposure to feed and food can cause serious health problems and even death in animals. Under normal circumstances, the symptoms of acute poisoning are more obvious, but often those with low concentration of chronic toxin poisoning not only the symptoms of slow, but also bring greater harm to the body. With the continuous development of industrial and agricultural technology, many studies have found that the traditional detoxification methods of mycotoxin and heavy metal poisoning have many shortcomings. They not only can not completely eliminate the toxicity of these toxins, but also have serious adverse effects on the body. Therefore, it is important to develop a new antidote to avoid these problems.
Through the analysis of the toxic mechanism of mycotoxins and heavy metal poisoning through a number of research data, it is found that they mainly play toxic effects by causing oxidative stress in the body. Therefore, adding antioxidants to feed and utilizing their powerful antioxidant properties can play a protective role for animal body. At present, a variety of antioxidants such as vitamin E and vitamin C, proanthocyanidins, curcumin, and some trace elements such as selenium and zinc are the focus of research.
Since selenium can affect various diseases by improving antioxidant capacity and regulating immune cells as well as pathogens such as viruses, selenium supplements may become a highly effective supplement for treating various diseases, especially the body’s sensitivity to selenium supplementation and the relatively low price of selenium preparations, which may bring new ideas for the clinical use of selenium. According to previous studies, inorganic sodium selenite can detoxify toxic substances in the body. However, because of its high toxicity and low utilization rate, the use of inorganic sodium selenite has been banned in some developed countries. It has been found that selenium yeast, a highly active organic selenium source with low toxicity, not only has a high absorption rate, but also can store selenium after meeting the physiological needs of the body. Therefore, the use of selenium yeast as a new antidote to fungal toxins and heavy metal poisoning will be more safe and efficient detoxification, will have better protection against oxidative damage, and further improve the body’s immune function. At present, although there are many studies on the effect of selenium yeast on reducing the toxicity of mycotoxins and heavy metals, its more detailed mechanism has not been fully obtained, so more experiments are still needed to analyze.
Authors’ Contributions
Writing—original draft preparation, H.S.; writing—review and editing, J.C. D.X., supervision, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant Nos.32273074, 31972746, 31772809, and 31872538) and through a Key Grant Project of Liaoning Provincial Department of Education (Grant No. LJKZ0632).
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huiying Sun, Jia Chen, and Dongwei Xiong contributed equally to this work and should be considered co-first authors
Contributor Information
Huiying Sun, Email: 2022240765@stu.syau.edu.cn.
Jia Chen, Email: 2020200157@stu.syau.edu.cn.
Dongwei Xiong, Email: 2022200183@stu.syau.edu.cn.
Miao Long, Email: longmiao@syau.edu.cn.
References
- 1.Pleadin J, Frece J, Markov K. Mycotoxins in food and feed. Adv Food Nutr Res. 2019;89:297–345. doi: 10.1016/bs.afnr.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Dhakal A, Hashmi MF, Sbar E. StatPearls [Internet]. Treasure Island. StatPearls Publishing; 2022. Aflatoxin Toxicity. [PubMed] [Google Scholar]
- 3.Karlovsky P, Suman M, Berthiller F, et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016;32:179–205. doi: 10.1007/s12550-016-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JJ, Kim YS, Kumar V. Heavy metal toxicity: an update of chelating therapeutic strategies. J Trace Elem Med Biol. 2019;54:226–231. doi: 10.1016/j.jtemb.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Kannappan S, Ramisetty B. Engineered whole-cell-based biosensors: sensing environmental heavy metal pollutants in water-a review. Appl Biochem Biotechnol. 2022;194:1814–1840. doi: 10.1007/s12010-021-03734-2. [DOI] [PubMed] [Google Scholar]
- 6.Perrelli M, Wu R, Liu DJ, et al. Heavy metals as risk factors for human diseases - a Bayesian network approach. Eur Rev Med Pharmacol Sci. 2022;26:9275–9310. doi: 10.26355/eurrev_202212_30681. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Yao Q, Zhu W, et al. Biomimetic antidote nanoparticles: a novel strategy for chronic heavy metal poisoning. Aaps Pharmscitech. 2022;24(1):12. doi: 10.1208/s12249-022-02466-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Chang J, Zhou M, et al. Cypermethrin induces cell injury in primary cortical neurons of C57BL/6 mice by inhibiting Nrf2/ARE signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:1469–1475. doi: 10.12122/j.issn.1673-4254.2019.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omidifar N, Nili-Ahmadabadi A, Nakhostin-Ansari A, et al. The modulatory potential of herbal antioxidants against oxidative stress and heavy metal pollution: plants against environmental oxidative stress. Environ Sci Pollut Res Int. 2021;28:61908–61918. doi: 10.1007/s11356-021-16530-6. [DOI] [PubMed] [Google Scholar]
- 10.Paithankar JG, Saini S, Dwivedi S, et al. Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere. 2021;262:128350. doi: 10.1016/j.chemosphere.2020.128350. [DOI] [PubMed] [Google Scholar]
- 11.Santos EV, Fontes DO, Benfato M, et al. Mycotoxin deactivator improves performance, antioxidant status, and reduces oxidative stress in nursery pigs fed diets containing mycotoxins. J Anim Sci. 2021;99(10):skab277. doi: 10.1093/jas/skab277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia GR, Dogi CA, Poloni VL, et al. Beneficial effects of Saccharomyces cerevisiae RC016 in weaned piglets: in vivo and ex vivo analysis. Benef Microbes. 2019;10:33–42. doi: 10.3920/BM2018.0023. [DOI] [PubMed] [Google Scholar]
- 13.Bai Y, Ma K, Li J, et al. Lactobacillus rhamnosus GG ameliorates DON-induced intestinal damage depending on the enrichment of beneficial bacteria in weaned piglets. J Anim Sci Biotechnol. 2022;13:90. doi: 10.1186/s40104-022-00737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma K, Bai Y, Li J, et al. Lactobacillus rhamnosus GG ameliorates deoxynivalenol-induced kidney oxidative damage and mitochondrial injury in weaned piglets. Food Funct. 2022;13:3905–3916. doi: 10.1039/d2fo00185c. [DOI] [PubMed] [Google Scholar]
- 15.Kielczykowska M, Kocot J, Pazdzior M, et al. Selenium - a fascinating antioxidant of protective properties. Adv Clin Exp Med. 2018;27:245–255. doi: 10.17219/acem/67222. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Zuo Z, Deng J, et al. Protective role of selenium in immune-relevant cytokine and immunoglobulin production by piglet splenic lymphocytes exposed to deoxynivalenol. Biol Trace Elem Res. 2018;184:83–91. doi: 10.1007/s12011-017-1160-6. [DOI] [PubMed] [Google Scholar]
- 17.He Y, Fang J, Peng X, et al. Effects of sodium selenite on aflatoxin B1-induced decrease of ileal IgA+ cell numbers and immunoglobulin contents in broilers. Biol Trace Elem Res. 2014;160:49–55. doi: 10.1007/s12011-014-0035-3. [DOI] [PubMed] [Google Scholar]
- 18.Salimian J, Arefpour MA, Riazipour M, et al. Immunomodulatory effects of selenium and vitamin E on alterations in T lymphocyte subsets induced by T-2 toxin. Immunopharmacol Immunotoxicol. 2014;36:275–281. doi: 10.3109/08923973.2014.931420. [DOI] [PubMed] [Google Scholar]
- 19.Ha HY, Alfulaij N, Berry MJ, et al. From selenium absorption to selenoprotein degradation. Biol Trace Elem Res. 2019;192:26–37. doi: 10.1007/s12011-019-01771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesolowski LT, Semanchik PL, White-Springer SH. Beyond antioxidants: selenium and skeletal muscle mitochondria. Front Vet Sci. 2022;9:1011159. doi: 10.3389/fvets.2022.1011159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kieliszek M, Bierla K, Jimenez-Lamana J, et al. Metabolic response of the yeast candida utilis during enrichment in selenium. Int J Mol Sci. 2020;21(15):5287. doi: 10.3390/ijms21155287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kieliszek M, Blazejak S, Kurek E. Binding and CONVERSION OF SELENIum in Candida utilis ATCC 9950 yeasts in bioreactor culture. Molecules. 2017;22(3):352. doi: 10.3390/molecules22030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieliszek M, Bano I, Zare H. A Comprehensive review on selenium and its effects on human health and distribution in Middle Eastern countries. Biol Trace Elem Res. 2022;200:971–987. doi: 10.1007/s12011-021-02716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieliszek M, Bano I. Selenium as an important factor in various disease states - a review. Excli J. 2022;21:948–966. doi: 10.17179/excli2022-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pappas AC, Zoidis E, Chadio SE. Maternal selenium and developmental programming. Antioxidants (Basel) 2019;8(5):145. doi: 10.3390/antiox8050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mal'Tseva VN, Goltyaev MV, Turovsky EA, et al. Immunomodulatory and anti-inflammatory properties of selenium-containing agents: their role in the regulation of defense mechanisms against COVID-19. Int J Mol Sci. 2022;23(4):2360. doi: 10.3390/ijms23042360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren Z, Jia G, He H, et al. Antiviral effect of selenomethionine on porcine deltacoronavirus in pig kidney epithelial cells. Front Microbiol. 2022;13:846747. doi: 10.3389/fmicb.2022.846747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moghaddam A, Heller RA, Sun Q, et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Im JH, Je YS, Baek J, et al. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majeed M, Nagabhushanam K, Prakasan P, et al. Can selenium reduce the susceptibility and severity of SARS-CoV-2?-A comprehensive review. Int J Mol Sci. 2022;23(9):4809. doi: 10.3390/ijms23094809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Cheng W, Zhang L. Maternal selenium deficiency suppresses proliferation, induces autophagy dysfunction and apoptosis in the placenta of mice. Metallomics. 2021;13(11):mfab058. doi: 10.1093/mtomcs/mfab058. [DOI] [PubMed] [Google Scholar]
- 32.Wang N, Tan HY, Li S, et al. Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxid Med Cell Longev. 2017;2017:7478523. doi: 10.1155/2017/7478523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada BK, Alfulaij N, Seale LA. The impact of selenium deficiency on cardiovascular function. Int J Mol Sci. 2021;22(19):10713. doi: 10.3390/ijms221910713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin T, Tao J, Chen Y, et al. Selenium deficiency leads to changes in renal fibrosis marker proteins and Wnt/beta-catenin signaling pathway components. Biol Trace Elem Res. 2022;200:1127–1139. doi: 10.1007/s12011-021-02730-1. [DOI] [PubMed] [Google Scholar]
- 35.Qiao L, Lin X, Zhao Y et al (2022) Short-term dietary selenium deficiency induced liver fibrosis by inhibiting the Akt/mTOR signaling pathway in rats. Biol Trace Elem Res. 10.1007/s12011-022-03453-7 [DOI] [PubMed]
- 36.Wang F, Sun N, Zeng H, et al. Selenium deficiency leads to inflammation, autophagy, endoplasmic reticulum stress, apoptosis and contraction abnormalities via affecting intestinal flora in intestinal smooth muscle of mice. Front Immunol. 2022;13:947655. doi: 10.3389/fimmu.2022.947655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chauychu-Noo N, Thananurak P, Boonkum W, et al. Effect of organic selenium dietary supplementation on quality and fertility of cryopreserved chicken sperm. Cryobiology. 2021;98:57–62. doi: 10.1016/j.cryobiol.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Kieliszek M, Blazejak S, Bzducha-Wrobel A, et al. Effect of selenium on growth and antioxidative system of yeast cells. Mol Biol Rep. 2019;46:1797–1808. doi: 10.1007/s11033-019-04630-z. [DOI] [PubMed] [Google Scholar]
- 39.Kieliszek M, Blazejak S, Gientka I, et al. Accumulation and metabolism of selenium by yeast cells. Appl Microbiol Biotechnol. 2015;99:5373–5382. doi: 10.1007/s00253-015-6650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Wu C, Chen D, et al. Selenium-enriched yeast alleviates oxidative stress-induced intestinal mucosa disruption in weaned pigs. Oxid Med Cell Longev. 2020;2020:5490743. doi: 10.1155/2020/5490743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Chen D, Yu B, et al. Influences of selenium-enriched yeast on growth performance, immune function, and antioxidant capacity in weaned pigs exposure to oxidative stress. Biomed Res Int. 2021;2021:5533210. doi: 10.1155/2021/5533210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo CH, Hsia S, Shih MY, et al. Effects of selenium yeast on oxidative stress, growth inhibition, and apoptosis in human breast cancer cells. Int J Med Sci. 2015;12:748–758. doi: 10.7150/ijms.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo J, Li X, Li X, et al. Selenium-rich yeast protects against aluminum-induced peroxidation of lipide and inflammation in mice liver. Biometals. 2018;31:1051–1059. doi: 10.1007/s10534-018-0150-2. [DOI] [PubMed] [Google Scholar]
- 44.Xiong Y, Li B, Li J, et al. Sodium selenite attenuates zearalenone-induced apoptosis through inhibition of endoplasmic reticulum stress in goat trophoblast cells. Biometals. 2022;35:699–710. doi: 10.1007/s10534-022-00394-5. [DOI] [PubMed] [Google Scholar]
- 45.Wang F, Shu G, Peng X, et al. Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Int J Environ Res Public Health. 2013;10:2834–2844. doi: 10.3390/ijerph10072834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H, Wang Y, An Y, et al. Selenium alleviates oxidative stress and autophagy in lead-treated chicken testes. Theriogenology. 2019;131:146–152. doi: 10.1016/j.theriogenology.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Ansar S, Abudawood M, Hamed SS, et al. Sodium selenite protects against silver nanoparticle-induced testicular toxicity and inflammation. Biol Trace Elem Res. 2017;175:161–168. doi: 10.1007/s12011-016-0759-3. [DOI] [PubMed] [Google Scholar]
- 48.Tsai CF, Wu JY, Hsu YW. Protective effects of rosmarinic acid against selenite-induced cataract and oxidative damage in rats. Int J Med Sci. 2019;16:729–740. doi: 10.7150/ijms.32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jing CL, Dong XF, Wang ZM, et al. Comparative study of DL-selenomethionine vs sodium selenite and seleno-yeast on antioxidant activity and selenium status in laying hens. Poult Sci. 2015;94:965–975. doi: 10.3382/ps/pev045. [DOI] [PubMed] [Google Scholar]
- 50.Liu G, Zhao Y, Cao S, et al. Relative bioavailability of selenium yeast for broilers fed a conventional corn-soybean meal diet. J Anim Physiol Anim Nutr (Berl) 2020;104:1052–1066. doi: 10.1111/jpn.13262. [DOI] [PubMed] [Google Scholar]
- 51.Konkol D, Korzeniowska M, Rozanski H, et al. The use of selenium yeast and phytobiotic in improving the quality of broiler chicken meat. Foods. 2021;10(11):2558. doi: 10.3390/foods10112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan R, Ghazali FM, Mahyudin NA, et al. Aflatoxin biosynthesis, genetic regulation, toxicity, and control strategies: a review. J Fungi (Basel) 2021;7(8):606. doi: 10.3390/jof7080606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alshannaq A, Yu JH. Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Public Health. 2017;14(6):632. doi: 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ropejko K, Twaruzek M. Zearalenone and its metabolites-general overview, occurrence, and toxicity. Toxins (Basel) 2021;13(1):35. doi: 10.3390/toxins13010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan W, Yang N, Li X. Advances in understanding how heavy metal pollution triggers gastric cancer. Biomed Res Int. 2016;2016:7825432. doi: 10.1155/2016/7825432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rzymski P, Tomczyk K, Rzymski P, et al. Impact of heavy metals on the female reproductive system. Ann Agric Environ Med. 2015;22:259–264. doi: 10.5604/12321966.1152077. [DOI] [PubMed] [Google Scholar]
- 57.Rehman K, Fatima F, Waheed I, et al. Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem. 2018;119:157–184. doi: 10.1002/jcb.26234. [DOI] [PubMed] [Google Scholar]
- 58.Weaver AC, See MT, Hansen JA, et al. The use of feed additives to reduce the effects of aflatoxin and deoxynivalenol on pig growth, organ health and immune status during chronic exposure. Toxins (Basel) 2013;5:1261–1281. doi: 10.3390/toxins5071261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holanda DM, Yiannikouris A, Kim SW. Investigation of the efficacy of a postbiotic yeast cell wall-based blend on newly-weaned pigs under a dietary challenge of multiple mycotoxins with emphasis on deoxynivalenol. Toxins (Basel) 2020;12(8):504. doi: 10.3390/toxins12080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Almeida A, de Oliveira J, Da SPL, et al. ROS: basic concepts, sources, cellular signaling, and its implications in aging pathways. Oxid Med Cell Longev. 2022;2022:1225578. doi: 10.1155/2022/1225578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui X, Zhang Y, Lu Y, et al. ROS and endoplasmic reticulum stress in pulmonary disease. Front Pharmacol. 2022;13:879204. doi: 10.3389/fphar.2022.879204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poljsak B, Suput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sadasivam N, Kim YJ, Radhakrishnan K, et al. Oxidative stress, genomic integrity, and liver diseases. Molecules. 2022;27(10):3159. doi: 10.3390/molecules27103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faraonio R. Oxidative stress and cell senescence process. Antioxidants (Basel) 2022;11(9):1718. doi: 10.3390/antiox11091718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu C, Zhang C, Cui X, et al. Trichosanthin inhibits cervical cancer by regulating oxidative stress-induced apoptosis. Bioengineered. 2021;12:2779–2790. doi: 10.1080/21655979.2021.1930335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shaito A, Aramouni K, Assaf R, et al. Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front Biosci (Landmark Ed) 2022;27:105. doi: 10.31083/j.fbl2703105. [DOI] [PubMed] [Google Scholar]
- 67.Teleanu DM, Niculescu AG, Lungu II, et al. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci. 2022;23(11):5938. doi: 10.3390/ijms23115938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azmanova M, Pitto-Barry A. Oxidative stress in cancer therapy: friend or enemy? Chembiochem. 2022;23:e202100641. doi: 10.1002/cbic.202100641. [DOI] [PubMed] [Google Scholar]
- 69.Lin LX, Cao QQ, Zhang CD, et al. Aflatoxin B1 causes oxidative stress and apoptosis in sheep testes associated with disrupting rumen microbiota. Ecotoxicol Environ Saf. 2022;232:113225. doi: 10.1016/j.ecoenv.2022.113225. [DOI] [PubMed] [Google Scholar]
- 70.Ma J, Liu Y, Guo Y, et al. Transcriptional profiling of aflatoxin B1-induced oxidative stress and inflammatory response in macrophages. Toxins (Basel) 2021;13(6):401. doi: 10.3390/toxins13060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia-Perez E, Ryu D, Lee C, et al. Ochratoxin A induces oxidative stress in HepG2 cells by impairing the gene expression of antioxidant enzymes. Toxins (Basel) 2021;13(4):271. doi: 10.3390/toxins13040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang TY, Kong L, Hao JX, et al. Effects of ochratoxin A exposure on DNA damage in porcine granulosa cells in vitro. Toxicol Lett. 2020;330:167–175. doi: 10.1016/j.toxlet.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 73.Zheng W, Wang B, Si M, et al. Zearalenone altered the cytoskeletal structure via ER stress- autophagy- oxidative stress pathway in mouse TM4 Sertoli cells. Sci Rep. 2018;8:3320. doi: 10.1038/s41598-018-21567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu Y, Jin Y, Tian Y, et al. Zearalenone promotes LPS-induced oxidative stress, endoplasmic reticulum stress, and accelerates bovine mammary epithelial cell apoptosis. Int J Mol Sci. 2022;23(18):10925. doi: 10.3390/ijms231810925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang JY, Du JJ, Zhang YF, et al. Vitamin E and selenium partially prevent cytotoxicity, oxidative stress and DNA damage induced by T-2 toxin in bovine Leydig cells. Theriogenology. 2022;189:255–261. doi: 10.1016/j.theriogenology.2022.06.028. [DOI] [PubMed] [Google Scholar]
- 76.Yan LJ, Allen DC. Cadmium-induced kidney injury: oxidative damage as a unifying mechanism. Biomolecules. 2021;11(11):1575. doi: 10.3390/biom11111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang X, Xing X, Zhang Y, et al. Lead exposure activates the Nrf2/Keap1 pathway, aggravates oxidative stress, and induces reproductive damage in female mice. Ecotoxicol Environ Saf. 2021;207:111231. doi: 10.1016/j.ecoenv.2020.111231. [DOI] [PubMed] [Google Scholar]
- 78.Ma Y, Shi Y, Wu Q, et al. Dietary arsenic supplementation induces oxidative stress by suppressing nuclear factor erythroid 2-related factor 2 in the livers and kidneys of laying hens. Poult Sci. 2021;100:982–992. doi: 10.1016/j.psj.2020.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma Y, Li S, Tang S, et al. Clusterin protects against Cr(VI)-induced oxidative stress-associated hepatotoxicity by mediating the Akt-Keap1-Nrf2 signaling pathway. Environ Sci Pollut Res Int. 2022;29:52289–52301. doi: 10.1007/s11356-022-19118-w. [DOI] [PubMed] [Google Scholar]
- 80.Zhang TY, Sun XF, Li L, et al. Ochratoxin A exposure impairs porcine granulosa cell growth via the PI3K/AKT signaling pathway. J Agric Food Chem. 2019;67:2679–2690. doi: 10.1021/acs.jafc.8b06361. [DOI] [PubMed] [Google Scholar]
- 81.Sheu ML, Shen CC, Chen YS, et al. Ochratoxin A induces ER stress and apoptosis in mesangial cells via a NADPH oxidase-derived reactive oxygen species-mediated calpain activation pathway. Oncotarget. 2017;8:19376–19388. doi: 10.18632/oncotarget.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai G, Si M, Li X, et al. Zearalenone induces apoptosis of rat Sertoli cells through Fas-Fas ligand and mitochondrial pathway. Environ Toxicol. 2019;34:424–433. doi: 10.1002/tox.22696. [DOI] [PubMed] [Google Scholar]
- 83.Long M, Chen X, Wang N, et al. Proanthocyanidins protect epithelial cells from zearalenone-induced apoptosis via inhibition of endoplasmic reticulum stress-induced apoptosis pathways in mouse small intestines. Molecules. 2018;23(7):1508. doi: 10.3390/molecules23071508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang R, Li R, Dai P, et al. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ Pollut. 2019;251:689–698. doi: 10.1016/j.envpol.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 85.Chen Q, Cui Y, Zhao J, et al. Cellular apoptosis induced by deoxynivalenol. Indian J Microbiol. 2022;62:61–69. doi: 10.1007/s12088-021-00965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Y, Wang S, Luo H, et al. Aflatoxin B1 induces microglia cells apoptosis mediated by oxidative stress through NF-kappaB signaling pathway in mice spinal cords. Environ Toxicol Pharmacol. 2022;90:103794. doi: 10.1016/j.etap.2021.103794. [DOI] [PubMed] [Google Scholar]
- 87.Ouyang H, Zhu H, Li J, et al. Fumonisin B(1) promotes germ cells apoptosis associated with oxidative stress-related Nrf2 signaling in mice testes. Chem Biol Interact. 2022;363:110009. doi: 10.1016/j.cbi.2022.110009. [DOI] [PubMed] [Google Scholar]
- 88.Cao X, Fu M, Bi R, et al. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere. 2021;263:128346. doi: 10.1016/j.chemosphere.2020.128346. [DOI] [PubMed] [Google Scholar]
- 89.Salama SA, Arab HH, Hassan MH, et al. Cadmium-induced hepatocellular injury: modulatory effects of gamma-glutamyl cysteine on the biomarkers of inflammation, DNA damage, and apoptotic cell death. J Trace Elem Med Biol. 2019;52:74–82. doi: 10.1016/j.jtemb.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Metryka E, Kupnicka P, Kapczuk P, et al. Lead (Pb) Accumulation in human THP-1 monocytes/macrophages in vitro and the influence on cell apoptosis. Biol Trace Elem Res. 2021;199:955–967. doi: 10.1007/s12011-020-02215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou L, Wang S, Cao L, et al. Lead acetate induces apoptosis in Leydig cells by activating PPARgamma/caspase-3/PARP pathway. Int J Environ Health Res. 2021;31:34–44. doi: 10.1080/09603123.2019.1625034. [DOI] [PubMed] [Google Scholar]
- 92.Shao YZ, Zhao HJ, Wang Y, et al. The apoptosis in arsenic-induced oxidative stress is associated with autophagy in the testis tissues of chicken. Poult Sci. 2018;97:3248–3257. doi: 10.3382/ps/pey156. [DOI] [PubMed] [Google Scholar]
- 93.Fu SC, Lin JW, Liu JM, et al. Arsenic induces autophagy-dependent apoptosis via Akt inactivation and AMPK activation signaling pathways leading to neuronal cell death. Neurotoxicology. 2021;85:133–144. doi: 10.1016/j.neuro.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 94.Cai G, Sun K, Xia S, et al. Decrease in immune function and the role of mitogen-activated protein kinase (MAPK) overactivation in apoptosis during T lymphocytes activation induced by zearalenone, deoxynivalenol, and their combinations. Chemosphere. 2020;255:126999. doi: 10.1016/j.chemosphere.2020.126999. [DOI] [PubMed] [Google Scholar]
- 95.Islam MR, Kim JW, Roh YS, et al. Evaluation of immunomodulatory effects of zearalenone in mice. J Immunotoxicol. 2017;14:125–136. doi: 10.1080/1547691X.2017.1340371. [DOI] [PubMed] [Google Scholar]
- 96.Lee PY, Liu CC, Wang SC, et al. Mycotoxin zearalenone attenuates innate immune responses and suppresses NLRP3 inflammasome activation in LPS-activated macrophages. Toxins (Basel) 2021;13(9):593. doi: 10.3390/toxins13090593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sarker MT, Wan X, Yang H, et al. Dietary lycopene supplementation could alleviate aflatoxin B(1) induced intestinal damage through improving immune function and anti-oxidant capacity in broilers. Animals (Basel) 2021;11(11):3165. doi: 10.3390/ani11113165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bulgaru CV, Marin DE, Pistol GC, et al. Zearalenone and the immune response. Toxins (Basel) 2021;13(4):248. doi: 10.3390/toxins13040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan SA, Venancio EJ, Ono MA, et al. Effects of subcutaneous ochratoxin-A exposure on immune system of broiler chicks. Toxins (Basel) 2019;11(5):264. doi: 10.3390/toxins11050264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qu KC, Wang ZY, Tang KK, et al. Trehalose suppresses cadmium-activated Nrf2 signaling pathway to protect against spleen injury. Ecotoxicol Environ Saf. 2019;181:224–230. doi: 10.1016/j.ecoenv.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 101.Zhang D, Yang XY, Qin YZ, et al. Antagonistic effect of N-acetyl-L-cysteine against cadmium-induced cytotoxicity and abnormal immune response on chicken peritoneal macrophages. Ecotoxicol Environ Saf. 2020;206:111185. doi: 10.1016/j.ecoenv.2020.111185. [DOI] [PubMed] [Google Scholar]
- 102.Jiayong Z, Shengchen W, Xiaofang H, et al. The antagonistic effect of selenium on lead-induced necroptosis via MAPK/NF-kappaB pathway and HSPs activation in the chicken spleen. Ecotoxicol Environ Saf. 2020;204:111049. doi: 10.1016/j.ecoenv.2020.111049. [DOI] [PubMed] [Google Scholar]
- 103.Chen J, Jiang J, Liu Y, et al. Arsenite induces dysfunction of regulatory T cells through acetylation control of the Foxp3 promoter. Hum Exp Toxicol. 2021;40:35–46. doi: 10.1177/0960327120934533. [DOI] [PubMed] [Google Scholar]
- 104.Liu J, Wang Y, Zhao H, et al. Arsenic (III) or/and copper (II) exposure induce immunotoxicity through trigger oxidative stress, inflammation and immune imbalance in the bursa of chicken. Ecotoxicol Environ Saf. 2020;190:110127. doi: 10.1016/j.ecoenv.2019.110127. [DOI] [PubMed] [Google Scholar]
- 105.de Souza M, Baptista A, Valdiviezo M, et al. Lactobacillus spp. reduces morphological changes and oxidative stress induced by deoxynivalenol on the intestine and liver of broilers. Toxicon. 2020;185:203–212. doi: 10.1016/j.toxicon.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 106.Popescu RG, Bulgaru C, Untea A, et al. The effectiveness of dietary byproduct antioxidants on induced CYP genes expression and histological alteration in piglets liver and kidney fed with aflatoxin B1 and ochratoxin A. Toxins (Basel) 2021;13(2):148. doi: 10.3390/toxins13020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ali O, Mezes M, Balogh K, et al. Fumonisin B series mycotoxins' dose dependent effects on the porcine hepatic and pulmonary phospholipidome. Toxins (Basel) 2022;14(11):803. doi: 10.3390/toxins14110803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu MK, Li HY, Bai LH, et al. Histological changes, lipid metabolism, and oxidative and endoplasmic reticulum stress in the liver of laying hens exposed to cadmium concentrations. Poult Sci. 2020;99:3215–3228. doi: 10.1016/j.psj.2019.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao Z, Li J, Zheng B, et al. Ameliorative effects and mechanism of crocetin in arsenic trioxide-induced cardiotoxicity in rats. Mol Med Rep. 2020;22:5271–5281. doi: 10.3892/mmr.2020.11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang S, Li L, Yu L, et al. Selenium-enriched yeast reduces caecal pathological injuries and intervenes changes of the diversity of caecal microbiota caused by Ochratoxin-A in broilers. Food Chem Toxicol. 2020;137:111139. doi: 10.1016/j.fct.2020.111139. [DOI] [PubMed] [Google Scholar]
- 111.Li K, Cao Z, Guo Y, et al. Selenium yeast alleviates ochratoxin A-induced apoptosis and oxidative stress via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in the kidneys of chickens. Oxid Med Cell Longev. 2020;2020:4048706. doi: 10.1155/2020/4048706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li P, Li K, Zou C, et al. Selenium yeast alleviates ochratoxin A-induced hepatotoxicity via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in chickens. Toxins (Basel) 2020;12(3):143. doi: 10.3390/toxins12030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu L, Chen F, Qin S, et al. Effects of selenium-enriched yeast improved aflatoxin B1-induced changes in growth performance, antioxidation capacity, IL-2 and IFN-gamma contents, and gene expression in mice. Biol Trace Elem Res. 2019;191:183–188. doi: 10.1007/s12011-018-1607-4. [DOI] [PubMed] [Google Scholar]
- 114.He J, Zhang KY, Chen DW, et al. Effects of vitamin E and selenium yeast on growth performance and immune function in ducks fed maize naturally contaminated with aflatoxin B1. Livest Sci. 2013;152:200–207. doi: 10.1016/j.livsci.2012.12.018. [DOI] [Google Scholar]
- 115.Long M, Yang S, Zhang W, et al. The influence of selenium yeast on hematological, biochemical and reproductive hormone level changes in Kunming mice following acute exposure to zearalenone. Biol Trace Elem Res. 2016;174:362–368. doi: 10.1007/s12011-016-0725-0. [DOI] [PubMed] [Google Scholar]
- 116.Long M, Yang S, Wang Y, et al. The protective effect of selenium on chronic zearalenone-induced reproductive system damage in male mice. Molecules. 2016;21(12):1687. doi: 10.3390/molecules21121687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levkut M, Revajova V, Levkutova M, et al. Leukocytic responses of broilers following dietary contamination with deoxynivalenol and/or treatment by dietary selenium supplementation. Br Poult Sci. 2009;50:181–187. doi: 10.1080/00071660802710090. [DOI] [PubMed] [Google Scholar]
- 118.Zhou X, Wang Z, Chen J, et al. Increased levels of IL-6, IL-1beta, and TNF-alpha in Kashin-Beck disease and rats induced by T-2 toxin and selenium deficiency. Rheumatol Int. 2014;34:995–1004. doi: 10.1007/s00296-013-2862-5. [DOI] [PubMed] [Google Scholar]
- 119.Liu Y, Dong R, Yang Y, et al. Protective effect of organic selenium on oxidative damage and inflammatory reaction of rabbit kidney induced by T-2 toxin. Biol Trace Elem Res. 2021;199:1833–1842. doi: 10.1007/s12011-020-02279-5. [DOI] [PubMed] [Google Scholar]
- 120.Wang X, Zuo Z, Zhao C, et al. Protective role of selenium in the activities of antioxidant enzymes in piglet splenic lymphocytes exposed to deoxynivalenol. Environ Toxicol Pharmacol. 2016;47:53–61. doi: 10.1016/j.etap.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 121.Zhao L, Feng Y, Deng J, et al. Selenium deficiency aggravates aflatoxin B1-induced immunotoxicity in chick spleen by regulating 6 selenoprotein genes and redox/inflammation/apoptotic signaling. J Nutr. 2019;149:894–901. doi: 10.1093/jn/nxz019. [DOI] [PubMed] [Google Scholar]
- 122.Pfliegler WP, Pusztahelyi T, Pocsi I. Mycotoxins - prevention and decontamination by yeasts. J Basic Microbiol. 2015;55:805–818. doi: 10.1002/jobm.201400833. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Hu B, Wang M, et al. Selenium protects against zearalenone-induced oxidative stress and apoptosis in the mouse kidney by inhibiting endoplasmic reticulum stress. Oxid Med Cell Longev. 2020;2020:6059058. doi: 10.1155/2020/6059058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hao S, Hu J, Song S, et al. Selenium alleviates aflatoxin B(1)-induced immune toxicity through improving glutathione peroxidase 1 and selenoprotein S expression in primary porcine splenocytes. J Agric Food Chem. 2016;64:1385–1393. doi: 10.1021/acs.jafc.5b05621. [DOI] [PubMed] [Google Scholar]
- 125.Abbas AO, Alaqil AA, Mehaisen G, et al. Effect of organic selenium-enriched yeast on relieving the deterioration of layer performance, immune function, and physiological indicators induced by heat stress. Front Vet Sci. 2022;9:880790. doi: 10.3389/fvets.2022.880790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Amadi CN, Offor SJ, Frazzoli C, et al. Natural antidotes and management of metal toxicity. Environ Sci Pollut Res Int. 2019;26:18032–18052. doi: 10.1007/s11356-019-05104-2. [DOI] [PubMed] [Google Scholar]
- 127.Wai KM, Umezaki M, Umemura M, et al. Protective role of selenium in the shortening of telomere length in newborns induced by in utero heavy metal exposure. Environ Res. 2020;183:109202. doi: 10.1016/j.envres.2020.109202. [DOI] [PubMed] [Google Scholar]
- 128.Eddie-Amadi BF, Ezejiofor AN, Orish CN, et al. Zinc and selenium mitigated heavy metals mixture (Pb, Al, Hg and Mn) mediated hepatic-nephropathy via modulation of oxido-inflammatory status and NF-kB signaling in female albino rats. Toxicology. 2022;481:153350. doi: 10.1016/j.tox.2022.153350. [DOI] [PubMed] [Google Scholar]
- 129.Xue H, Cao H, Xing C, et al. Selenium triggers Nrf2-AMPK crosstalk to alleviate cadmium-induced autophagy in rabbit cerebrum. Toxicology. 2021;459:152855. doi: 10.1016/j.tox.2021.152855. [DOI] [PubMed] [Google Scholar]
- 130.Cong Y, Chi Q, Teng X, et al. The protection of selenium against cadmium-induced mitochondrial damage via the cytochrome P450 in the livers of chicken. Biol Trace Elem Res. 2019;190:484–492. doi: 10.1007/s12011-018-1557-x. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y, Chen H, Chang W, et al. Protective effects of selenium yeast against cadmium-induced necroptosis via inhibition of oxidative stress and MAPK pathway in chicken liver. Ecotoxicol Environ Saf. 2020;206:111329. doi: 10.1016/j.ecoenv.2020.111329. [DOI] [PubMed] [Google Scholar]
- 132.Chen H, Li P, Shen Z, et al. Protective effects of selenium yeast against cadmium-induced necroptosis through miR-26a-5p/PTEN/PI3K/AKT signaling pathway in chicken kidney. Ecotoxicol Environ Saf. 2021;220:112387. doi: 10.1016/j.ecoenv.2021.112387. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y, Liu J, Chen R, et al. The antagonistic effects of selenium yeast (SeY) on cadmium-induced inflammatory factors and the heat shock protein expression levels in chicken livers. Biol Trace Elem Res. 2020;198:260–268. doi: 10.1007/s12011-020-02039-5. [DOI] [PubMed] [Google Scholar]
- 134.Pengcheng X, Xu S, Wei C, et al. Yeast selenium exerts an antioxidant effect by regulating the level of selenoprotein to antagonize Cd-induced pyroptosis of chicken liver. Biol Trace Elem Res. 2022;200:4079–4088. doi: 10.1007/s12011-021-02984-9. [DOI] [PubMed] [Google Scholar]
- 135.Lynch SJ, Horgan KA, White B, et al. Selenium source impacts protection of porcine jejunal epithelial cells from cadmium-induced DNA damage, with maximum protection exhibited with yeast-derived selenium compounds. Biol Trace Elem Res. 2017;176:311–320. doi: 10.1007/s12011-016-0828-7. [DOI] [PubMed] [Google Scholar]
- 136.Liu Z, Zhang F, Lu P, et al. Selenium-yeast alleviated inflammatory damage caused by lead via inhibiting Ras/ERK pathway and inflammatory factors in chicken skeletal muscles. Biol Trace Elem Res. 2019;190:493–500. doi: 10.1007/s12011-018-1558-9. [DOI] [PubMed] [Google Scholar]
- 137.Zhao Y, Hao D, Zhang H, et al. Selenium-enriched yeast relieves hexavalent chromium toxicity by inhibiting NF-kappaB signaling pathway in broiler spleens. Animals (Basel) 2022;12(2):146. doi: 10.3390/ani12020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao Y, Zhang H, Hao D, et al. Selenium regulates the mitogen-activated protein kinase pathway to protect broilers from hexavalent chromium-induced kidney dysfunction and apoptosis. Ecotoxicol Environ Saf. 2022;239:113629. doi: 10.1016/j.ecoenv.2022.113629. [DOI] [PubMed] [Google Scholar]
- 139.Liu S, Xu FP, Yang ZJ, et al. Cadmium-induced injury and the ameliorative effects of selenium on chicken splenic lymphocytes: mechanisms of oxidative stress and apoptosis. Biol Trace Elem Res. 2014;160:340–351. doi: 10.1007/s12011-014-0070-0. [DOI] [PubMed] [Google Scholar]
- 140.Fang M, Hu W, Liu B. Protective and detoxifying effects conferred by selenium against mycotoxins and livestock viruses: a review. Front Vet Sci. 2022;9:956814. doi: 10.3389/fvets.2022.956814. [DOI] [PMC free article] [PubMed] [Google Scholar]