Abstract
The distribution of quorum-sensing genes among strains from seven genomovars of the Burkholderia cepacia complex was examined by PCR. cepR and cepI were amplified from B. cepacia genomovars I and III, B. stabilis, and B. vietnamiensis. cepR was also amplified from B. multivorans and B. cepacia genomovar VI. bviIR were amplified from B. vietnamiensis. All genomovars produced N-octanoyl-l-homoserine lactone and N-hexanoyl-l-homoserine lactone. B. vietnamiensis and B. cepacia genomovar VII produced additional N-acyl-l-homoserine lactones.
Burkholderia cepacia is an opportunistic pathogen that infects patients with cystic fibrosis (CF) (10, 12, 13). Some patients infected with B. cepacia develop cepacia syndrome, a necrotizing, often fatal pneumonia sometimes associated with bacteremia (14). Colonization with B. cepacia correlates with an increased risk of mortality at all levels of pulmonary function (4). The transmissibility of B. cepacia between CF patients (11, 15, 20, 26) and intrinsic resistance to a wide variety of antibiotics (25) are of increasing concern in the CF community.
B. cepacia was originally classified in the genus Pseudomonas but was transferred to the genus Burkholderia in 1992 on the basis of rRNA sequence analysis (35). Recently, B. cepacia has been classified into genotypically distinct species or genomovars referred to as the “B. cepacia complex” (3, 31; Coeyne et al., submitted for publication). Genomovars are phenotypically similar but genotypically distinct groups of strains that show a low level of DNA hybridization. The B. cepacia complex currently includes seven genomovars referred to as B. cepacia genomovar I, B. multivorans (formerly genomovar II), B. cepacia genomovar III, B. stabilis (formerly genomovar IV), and B. vietnamiensis (also known as genomovar V) (3, 31, 32) and two newly identified genomovars, genomovars VI (3) and VII (Coeyne et al., submitted).
Currently it is not known if these Burkholderia species possess different virulence factors or regulate virulence factors differently and subsequently vary in their pathogenicity. Strains of the B. cepacia complex produce a number of potential virulence factors, including siderophores, proteases, lipase, hemolysins, and pili (reviewed in references 10, 12, and 13). Production of extracellular virulence factors does not likely correlate with specific genomovars, since the majority of B. cepacia complex isolates produce these factors. Three markers have been associated with transmissible isolates, including cable pili (28), which have been shown to mediate adherence to respiratory mucins (21); an open reading frame of unknown function with homology to transcriptional regulators, termed the B. cepacia epidemic strain marker (19); and a hybrid of insertion sequences IS402 and IS1356 (30). These three markers have been predominantly found in isolates of B. cepacia genomovar III (2).
Quorum sensing is a signaling mechanism used by bacteria for the coordinate regulation of genes (5, 9, 22, 34). Quorum sensing involves the production of autoinducer signaling molecules, which are normally N-acyl homoserine lactones (AHLs) in gram-negative bacteria, and a transcriptional regulator. Quorum sensing regulates virulence factors, motility, biofilm formation, plasmid transfer, and antibiotic resistance (5, 34).
We have previously described the B. cepacia CepIR quorum-sensing system that was identified in B. cepacia genomovar III strain K56-2 (16). The autoinducer synthase gene, cepI, directs the synthesis of N-octanoyl-l-homoserine lactone (OHL) and N-hexanoyl-l-homoserine lactone (HHL) (16, 17). The transcriptional regulator, CepR, has been shown to negatively regulate biosynthesis of the siderophore ornibactin and positively regulate protease, OHL, and HHL production (16, 17). A second autoinducer synthetase gene, bviI, was identified in B. vietnamiensis DBO1 using random TnMod mutagenesis (6). Quorum-sensing genes have also recently been described in another strain of B. vietnamiensis (B. Conway and E. P. Greenberg, Abstr. 5th Annu. Int. Burkholderia cepacia Working Group Meet., 2000, p. 17, http://www.go.to/cepacia). The objectives of the present study were to determine if the cepIR and bviIR genes were present in other genomovars of the B. cepacia complex and to determine the autoinducer profiles of representative strains in the B. cepacia complex.
The distribution of cepIR and bviIR was determined in representative strains of the B. cepacia complex by PCR (Table 1; Fig. 1). The oligonucleotide primers and PCR conditions used are listed in Table 2. Genomic DNA was isolated from cultures grown in Luria-Bertani (LB) broth (Life Technologies, Burlington, Ontario, Canada) as described by Ausubel et al. (1). Taq polymerase and oligonucleotide primers were purchased from Life Technologies. PCRs were carried out in 50-μl volumes with the following amounts of reagents: 3.2 pmol of primer, 250 ng of DNA, 2.5 U of Platinum Taq Polymerase, a 0.2 mM concentration of each deoxynucleotide triphosphate (Amerisham Pharmacia Biotech, Inc., Baie d'urfé, Quebec, Canada), 3 mM MgCl2, 5 μl of 10× buffer, and 10 μl of Q solution (Qiagen, Mississauga, Ontario, Canada). PCR products were separated on 0.8% agarose gels in Tris-acetate buffer. The plasmid pSLA3.2 (16) containing the cepIR genes was used as a positive control template for cepI and cepR amplification. The plasmids p824-E-3, which contains bviI, and p823-E-9, which contains bviR, were used as positive controls for amplification of bviI and bviR, respectively. The plasmid pUCP28T (23) was used a negative control for all PCRs.
TABLE 1.
Presence of quorum-sensing genes in strains of the B. cepacia complex
| Species and strain | Source (location) or genotype | cepI | cepR | bviI | bviR | cepI reporter bioassay | Reference or source |
|---|---|---|---|---|---|---|---|
| B. cepacia genomovar I | |||||||
| ATCC 25416 | Onion (United States) | +a | + | −b | − | + | 18 |
| ATCC 17759 | Soil (Trinidad) | + | + | − | − | + | 18 |
| CEP509 | CF (Australia) | + | + | − | − | + | 18 |
| B. multivorans | |||||||
| C5393 | CF (Canada) | − | + | − | − | + | 18 |
| 249-2 | Laboratory (United States) | − | + | − | − | + | 18 |
| LMG13010 | CF (Belgium) | − | + | − | − | + | 18 |
| B. cepacia genomovar III | |||||||
| J2315 | CF (United Kingdom) | + | + | − | − | + | 18 |
| K56-2 | CF (Canada) | + | + | − | − | + | 18 |
| C5424 | CF (Canada) | + | + | − | − | + | 18 |
| K56-I2 | cepI::tmp | + | + | − | − | + | 16 |
| K56-R2 | cepR::Tn5-OT182 | + | + | − | − | + | 16 |
| B. stabilis | |||||||
| LMG14294 | CF (Belgium) | + | + | − | − | + | 18 |
| LMG14291 | CF (Belgium) | + | + | − | − | + | 18 |
| LMG07000 | Blood (Sweden) | + | + | − | − | + | 18 |
| B. vietnamiensis | |||||||
| PC259 | CF (United States) | + | + | + | + | + | 18 |
| LMG15232 | CF (Sweden) | + | + | + | + | + | 18 |
| LMG10929 | Rice (Vietnam) | + | + | + | + | + | 18 |
| G4c | Environment | + | + | + | + | + | B. Conway |
| DBO1c | Environment | + | + | + | + | + | 33 |
| B. cepacia genomovar VI | |||||||
| LMG18943 | CF | − | + | − | − | + | 3 |
| LO6 | CF | − | + | − | − | + | 3 |
| B. cepacia genomovar VII | |||||||
| ATCC 53266 | Soil (United States) | − | − | − | − | + | T. Coenye |
| CEP996 | CF (Australia) | − | − | − | − | + | T. Coenye |
| AMMD | Biocontrol strain | − | − | − | − | + | T. Coenye |
+, presence of the gene or activity in the autoinducer bioassay.
−, absence of the gene or the activity in the autoinducer bioassay.
Typed as B. vietnamiensis by the Cystic Fibrosis Foundation B. cepacia research laboratory and repository (J. LiPuma, personal communication).
FIG. 1.
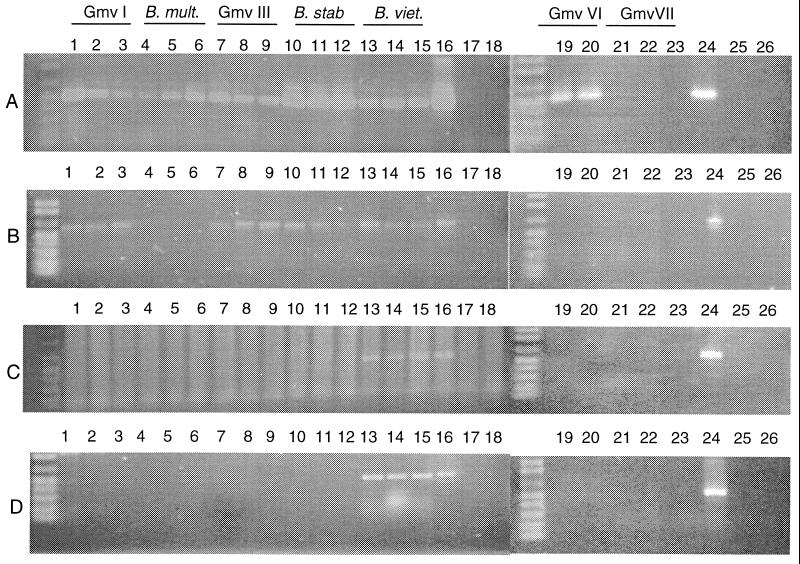
Detection of cepIR and bviIR genes in strains of the B. cepacia complex by PCR. (A) cepR; (B) cepI; (C) bviI; (D) bviR. Lanes 1, ATCC 25416; lanes 2, ATCC 17759; lanes 3, CEP 509; lanes 4, C5393; lanes 5, 249-2; lanes 6, LMG13010; lanes 7, K56-2; lanes 8, J2315; lanes 9, C5424; lanes 10, LMG14294; lanes 11, LMG07000; lanes 12, LMG14291; lanes 13, PC259; lanes 14, LMG16232; lanes 15, LMG10929; lanes 17, pUCP28T; lanes 18, H2O control; lanes 19, LMG18943; lanes 20, L06; lanes 21, ATCC 53266; lanes 22, AMMD; lanes 23, CEP996; lanes 25, pUCP28T; lanes 26, H2O control. Lanes 16 and 24 contain the positive controls pSLA3.2 (A and B) p824E-9 (C), and p824E-3 (D). Abbreviations: Gmv, genomovar; B. mult., B. multivorans; B. stab., B. stabilis; B. viet., B. vietnamiensis.
TABLE 2.
Primers and PCR conditions for amplification of quorum-sensing genes in B. cepacia
| Gene | Primer | Sequence | Annealing temp (°C)a | Amplicon size (bp) |
|---|---|---|---|---|
| cepI | CEPI1 | 5′-GCGGATCC-121-ACCAGACGCCCATCTACCTGCTTCG-3′134b | 59 | 598 |
| CEPI2 | 6995′-GTTACCAGTTACAGGCTCCTC-3′679 | |||
| cepI | CEPI1 | 5′-GCGGATCC-121-ACCAGACGCCCATCTACCTGCTTCG-3′134 | 59 | 278 |
| CEPI3 | 3905′-GTATCTGCTGAACTCGCTGTTC-3′379 | |||
| cepR | CEPR1 | 5′-CGGGATCC-1347-GAGAAAGAATGGAACTGCGC-3′1366 | 55 | 866 |
| CEPR2 | 22175′-TCAGCAGAAGCTCGAGCAGAT-3′2197 | |||
| cepR | CEPR1 | 5′-CGGGATCC-1347-GAGAAAGAATGGAACTGCGC-3′1366 | 55 | 494 |
| CEPR3 | 18455′-ATGAAGCGGCTCAGCGAAT-3′1824 | |||
| cepR | CEPR1 | 5′-CGGGATCC-1347-GAGAAAGAATGGAACTGCGC-3′1366 | 55 | 575 |
| CEPR4 | 19925′-TTGTTCACGTGGAAGTTGAC-3′1973 | |||
| bviI | BVII1 | 13415′-CGCAAAGTATCGGCATAAGG-3′1322 | 55 | 600 |
| BVII2 | 8465′-CTGTTCGTCGATCTCGATCCC-3′866 | |||
| bviR | BVIR1 | 32315′-GGAATTTGACGGTGCGGTCG-3′3212 | 55 | 471 |
| BVIR2 | 27605′-ATGCTGCAGTCCAACTATCC-3′2779 |
PCR conditions were 94°C for 3 min (1 cycle) and 94°C for 1 min, annealing at the indicated temperature for 1 min, and 72°C for 1 min (30 cycles).
Underlined region represents BamHI site.
Two primer combinations were used to amplify cepR. An 866-bp amplicon containing the complete open reading frame of cepR was amplified using primer set one, CEPR1 and CEPR2, and a 494-bp product containing the N-terminal 163 of 239 amino acids of CepR was amplified with primer set two, CEPR1 and CEPR3. With the exception of strains CEP509 (genomovar I) and C5393 (genomovar III), primer set one amplified cepR in the genomovar I, B. multivorans, genomovar III, B. stabilis, and B. vietnamiensis strains examined (data not shown). cepR was not amplified from strains of either genomovar VI or VII using these primers (data not shown). The primers CEPR1 and CEPR3 amplified an approximately 500-bp product in all strains with the exception of the three genomovar VII strains (Fig. 1A; Table 1). CEPR1 in combination with CEPR4, which amplifies a 575-bp product, also resulted in a negative PCR with the three genomovar VII strains (data not shown).
A 598-bp fragment containing the N-terminal 173 of 202 amino acids of CepI was amplified with the primers CEPI1 and CEPI2 in strains of genomovars I and III, B. stabilis, and B. vietnamiensis but not in B. multivorans or genomovars VI and VII (Fig. 1B). CEPI1 and CEPI3, which amplify a 278-bp product containing the first 93 amino acids of CepI, amplified this product from the same strains (data not shown).
Amplicons of cepI and cepR from one strain from each genomovar were cloned into the Topo vector pCR 2.1 (Invitrogen, Carlsbad, Calif.), and the nucleotide sequences were determined with the ABI PRISM DyeDeoxy Termination Cycle Sequencing System using AmpliTaq DNA polymerase (Perkin-Elmer Corp.) and the M13 universal primers and primers internal to cepIR. Reactions were performed with the ABI1371A DNA sequencer at the University Core DNA Services (University of Calgary). Sequence alignments were performed using DNAMAN Sequence Analysis Software (Lynnon Biosoft, Vandreuil, Quebec, Canada). The 866-bp cepR PCR product was sequenced, with the exception of genomovar VI LO6. In this instance the 494-bp amplicon containing only the first 163 amino acids of the predicted cepR open reading frame was cloned and sequenced. The predicted amino acid sequences were compared to those of genomovar III, strain K56-2 CepI and CepR (Table 3). The percent identity for CepR ranged from 99% in B. vietnamiensis PC259 to 92% in genomovar VI strain LO6. The percent identity for CepI ranged from 96% in B. vietnamiensis strain PC259 to 90% in B. stabilis strain LMG14291. These results indicate that cepI and cepR are highly conserved among the strains examined in most of the genomovars in the B. cepacia complex.
TABLE 3.
Percent identity between predicted amino acid sequences of genomovar III CepIR and CepIR homologues in other genomovars of the B. cepacia complex
| Genomovar or species | Strain | % Identitya
|
|
|---|---|---|---|
| CepR | CepI | ||
| Genomovar I | ATCC 17759 | 94 (226/239)a | 95 (166/173) |
| B. multivorans | LMG13010 | 97 (232/239) | NDb |
| B. stabilis | LMG14291 | 95 (228/239) | 90 (156/173) |
| B. vietnamiensis | PC259 | 99 (237/239) | 96 (167/173) |
| Genomovar VI | LO6 | 92 (151/163) | ND |
Values in parentheses represent the number of amino acids identical out of the total number of amino acids compared to CepI or CepR from K56-2.
ND, Not determined because not detectable by PCR.
bviI was identified in B. vietnamiensis strain DBO1 as DBO6R using a random plasposon mutagenesis strategy, as previously described (6). TnMod-KmO was introduced from Escherichia coli DH5α into DBO1 by triparental mating with E. coli HB101 (pRK2013) (7). The DNA fragment containing the plasposon's site of insertion was cloned by performing a total genomic DNA digestion with PstI (Life Technologies) Bethesda, Md. (a restriction enzyme that does not cut within the plasposon), purifying the digested products with Gene-Clean (Bio 101, Santa Clara, Calif). ligating with T4 DNA ligase (Life Technologies), electroporating into E. coli DH5α, and selecting on LB medium containing 50 μg of kanamycin per ml. The DNA sequence flanking the plasposon's site of insertion was determined using the primers JD45 (5′-ACGCTCAGTGGAACG-3′) and JD48 (5′-TTCCCGTTGAATATGGC-3′) and an ABI 377 DNA sequencer. The TnMod-KmO plasposon was inserted 301 bp from the start of the bviI open reading frame. The original cloned DNA fragment containing the rescued plasposon did not contain the complete sequence of the cognate response regulator bviR; therefore, the genomic DNA from B. cepacia complex strain DBO6R was digested with BamHI in order to isolate a larger DNA fragment flanking TnMod-KmO. This fragment was similarly cloned as described above. The DNA sequence of bviR was obtained using a combination of primer walking and from EcoRI subclones constructed in the nested deletion vector p824 (J. J. Dennis, and G. L. Zylstra, submitted for publication).
bviI encodes a 219-amino-acid protein with 36% identity to CepI over the first 204 amino acids (Fig. 2). The bviI open reading frame encodes a product that is is 17 amino acids longer than CepI. The bviR open reading frame encodes a protein of 237 amino acids that is 36% identical to CepR (Fig. 2). The primers BVII1 and BVII2 amplified a 600-bp product internal to bviI only in strains of B. vietnamiensis (Fig. 1C). The primers BVIR1 and BVIR2 amplified a 471-bp product internal to bviR in all representative strains from B. vietnamiensis but not in strains from the other genomovars (Fig. 1D).
FIG. 2.
Computer-generated alignment of the deduced amino acid sequence of CepI from B. cepacia K56-2 (accession no. AF019654) (16) with B. vietnamiensis BviI (accession no. AF296284) (A) and CepR from B. cepacia K56-2 (accession no. AF019654) (16) with B. vietnamiensis BviR (accession no. AF296284) (B). Sequence alignments were performed using DNAMAN Sequence Analysis Software (Lynnon Biosoft). Shaded areas indicate identical amino acids.
B. cepacia K56-I2, a cepI mutant, was used as a reporter strain to detect OHL production by each representative strain (16). When OHL is produced it binds CepR and restores protease production to K56-I2. Strains were streaked perpendicularly to the reporter strain grown on D-BHI (Becton Dickinson, Sparks, Md.)-milk agar (27), and protease production by the reporter was measured after incubation for 48 h. All of the strains tested were able to cross-feed the cepI reporter, suggesting that either OHL or other AHL molecules that can activate CepR are produced regardless of whether or not cepI or bviI was detectable by PCR using the indicated primers.
To determine the AHLs produced by strains of the various genomovars, an Agrobacter tumefaciens reporter previously shown to detect AHLs with 3-oxo-, 3-hydroxy-, and 3-unsubstituted side chains of all lengths, with the exception of N-butanoyl-l-HSL, was employed to examine AHL production in one strain of each genomovar. A. tumefaciens A136 does not contain a Ti plasmid coding for an autoinducer synthetase (36). This strain with plasmids pCF18, which harbors traR, and pCF372, with a traI-lacZ reporter, allows the detection of exogenous autoinducer production (8, 36). In the presence of AHLs, β-galactosidase activity observed from the traI-lacZ reporter is detected by a blue zone at the location of migration on thin-layer chromotography (TLC).
AHLs were extracted from 20-ml cultures grown in tryptic soy broth (Becton Dickinson) from one representative strain of each genomovar. Supernatants were extracted twice with equal volumes of acidified ethyl acetate (0.1 ml of glacial acetic acid per liter). Ethyl acetate was removed by rotary evaporation, and the residue was resuspended in 2 ml ethyl acetate, dried over N2 gas, and resuspended in 100 μl of acidified ethyl acetate. TLC bioassays were performed as described elsewhere with modifications (24). Samples were spotted onto C18 reversed-phase TLC plates (20 by 20 cm; Whatman) and developed using methanol-water (60:40, vol/vol). The plates were overlaid with a A. tumefaciens A136 culture prepared as follows. A 3-ml overnight culture was diluted 1/100 into 30 ml of LB and grown to log phase. Cells were pelleted by centrifugation, resuspended in 20 ml of AT (29)–0.5% glucose medium, and incubated for 30 min. This culture was added to 150 ml of AT supplemented with 0.7% agar and 5-bromo-4chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (60 μg/ml). TLC plates were incubated for 24 h at 30°C. Synthetic N-hexanoyl-HSL, N-octanoyl-HSL and N-decanoyl-HSL (Fluka) were used as reference standards.
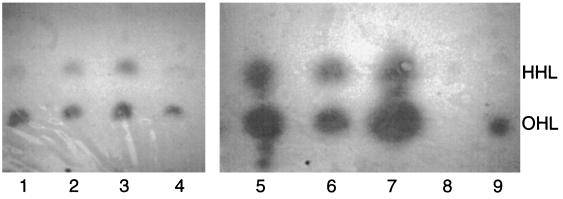
As previously reported (16, 17), B. cepacia K56-2 produces OHL and HHL. AHLs with Rf values corresponding to those of synthetic OHL and HHL were detected in extracts in all of the other strains examined (Fig. 3). In addition to OHL and HHL, B. vietnamensis also produced two other AHLs that may be N-decanoyl-HSL and N-dodecanoyl-HSL. Production of these four AHLs by B. vietnamiensis G4 has previously been reported (Conway and Greenberg, Abstr. 5th Annu. Int. Burkholderia cepacia Working Group Meet.). Since bviI was only amplified by PCR in B. vietnamensis it is likely that this gene is responsible for the production of one or both of these AHLs. The genomovar VII strain also produced another AHL that migrates between OHL and HHL on the TLC plate (Fig. 3).
FIG. 3.
TLC of acyl-HSLs. Ethyl acetate extracts were chromatographed on C18 reversed-phase thin-layer plates developed with methanol-water (60:40, vol/vol). The spots were visualized using the A. tumefaciens reporter strain. Samples from each genomovar were chromatographed as follows: lane 1, B. cepacia genomovar I (strain CEP509); lane 2, B. multivorans (strain C5393); lane 3, B. cepacia genomovar III (strain K56-2); lane 4, B. stabilis (strain LMG14294); lane 5, B. vietnamiensis (strain G4); lane 6, genomovar VI (strain LMG18943); lane 7, genomovar VII (strain CEP996); lane 8, synthetic N-hexanoyl-HSL; lane 9, synthetic N-octanoyl-HSL.
These studies suggest that the cepIR genes are widely distributed in all genomovars of the B. cepacia complex and that B. vietnamiensis has at least two sets of quorum-sensing genes. In the strains examined, the cepI genes were shown to be highly conserved (>90% identical at the amino acid level) in B. cepacia genomovars I and III, B. stabilis, and B. vietnamiensis. cepR was also highly conserved in strains of genomovar VI and B. multivorans, suggesting that these strains likely contain the cepIR genes but that the cepI genes are too divergent to be amplified by the selected primers. Since genomovar VII strains also produce OHL and HHL, it is likely that they have cepIR homologues but that these genes may not be as closely related to the cepIR homologues in the other genomovars. It is also possible, however, that genomovar VII contains a different AHL synthase gene that also directs the synthesis of OHL and HHL in addition to the unidentified molecule with activity in the A. tumefaciens reporter assay.
The bviIR genes were less related to K56-2 cepIR than any of the other cepIR homologues identified. Interestingly, only B. vietnamiensis contained sequences amplified by the primers designed to bviIR. Since B. vietnamiensis produces at least two AHL molecules in addition to OHL and HHL, it is likely that bviIR are involved in the production of these signals. Further studies are needed to determine the role of the cepIR and bviIR genes in virulence in the various species of the B. cepacia complex.
Nucleotide sequence accession numbers.
The nucleotide sequences of the cepIR and bviIR genes have been deposited in the GenBank and assigned the following accession numbers: AF296284, AF333002, AF333003, AF333004, AF333005, AF333006, AF333007, AF333008, and AF337814.
Acknowledgments
This study was supported by a grant from the Canadian Cystic Fibrosis Foundation. S.L. is the recipient of a studentship award from the Alberta Heritage Foundation for Medical Research.
We thank E. Mahenthiralingam for many of the Burkholderia strains used in this study.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 2.Clode F E, Kaufmann M E, Malnick H, Pitt T L. Distribution of genes encoding putative transmissibility factors among epidemic and nonepidemic strains of Burkholderia cepacia from cystic fibrosis patients in the United Kingdom. J Clin Microbiol. 2000;38:1763–1766. doi: 10.1128/jcm.38.5.1763-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coenye T, LiPuma J J, Henry D, Hoste B, Vandemeulebrouke K, Gillis M, Speert D P, Vandamme P. Burkholderia cepacia genomovar VI, a new member of the Burkholderia cepacia complex isolated from cystic fibrosis patients. Int J Syst Evol Microbiol. 2001;51:271–279. doi: 10.1099/00207713-51-2-271. [DOI] [PubMed] [Google Scholar]
- 4.Corey M, Farewell V. Determinants of mortality from cystic fibrosis in Canada, 1970–1989. Am J Epidemiol. 1996;143:1007–1017. doi: 10.1093/oxfordjournals.aje.a008664. [DOI] [PubMed] [Google Scholar]
- 5.de Kievit T R, Iglewski B H. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis J J, Zylstra G J. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl Environ Microbiol. 1998;64:2710–2715. doi: 10.1128/aem.64.7.2710-2715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figuski D H, Helenski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuqua C, Winans S C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 10.Gilligan P H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govan J R, Brown P H, Maddison J, Doherty C J, Nelson J W, Dodd M, Greening A P, Webb A K. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993;342:15–19. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- 12.Govan J R, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govan J R, Hughes J E, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 14.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 15.Johnson W M, Tyler S D, Rozee K R. Linkage analysis of geographic and clinical clusters in Pseudomonas cepacia infections by multilocus enzyme electrophoresis and ribotyping. J Clin Microbiol. 1994;32:924–930. doi: 10.1128/jcm.32.4.924-930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewenza S, Conway B, Greenberg E P, Sokol P A. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J Bacteriol. 1999;181:748–756. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewenza S, Sokol P A. Regulation of ornibactin synthesis and N-acyl-l-homoserine lactone production by CepR in Burkholderia cepacia. J Bacteriol. 2001;183:2212–2218. doi: 10.1128/JB.183.7.2212-2218.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahenthiralingam E, Coenye T, Chung J W, Speert D P, Govan J R W, Taylor P, Vandamme P. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J Clin Microbiol. 2000;38:910–913. doi: 10.1128/jcm.38.2.910-913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahenthiralingam E, Simpson D A, Speert D P. Identification and characterization of a novel DNA marker associated with epidemic Burkholderia cepacia strains recovered from patients with cystic fibrosis. J Clin Microbiol. 1997;35:808–816. doi: 10.1128/jcm.35.4.808-816.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pegues D A, Carson L A, Tablan O C, FitzSimmons S C, Roman S B, Miller J M, Jarvis W R. Acquisition of Pseudomonas cepacia at summer camps for patients with cystic fibrosis. Summer Camp Study Group. J Pediatr. 1994;124:694–702. doi: 10.1016/s0022-3476(05)81357-5. [DOI] [PubMed] [Google Scholar]
- 21.Sajjan U, Wu Y, Kent G, Forstner J. Preferential adherence of cable-piliated Burkholderia cepacia to respiratory epithelia of CF knockout mice and human cystic fibrosis lung explants. J Med Microbiol. 2000;49:875–885. doi: 10.1099/0022-1317-49-10-875. [DOI] [PubMed] [Google Scholar]
- 22.Salmond G P, Bycroft B W, Stewart G S, Williams P. The bacterial ‘enigma’: cracking the code of cell-cell communication. Mol Microbiol. 1995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 23.Schweizer H P, Klassen T, Hoang T. Improved methods for gene analysis and expression in Pseudomonas spp. In: Nakazawa T, Furukawa K, Hass D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C.: ASM Press; 1996. pp. 229–237. [Google Scholar]
- 24.Shaw P D, Ping G, Daly S L, Cha C, Cronan J E, Jr, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson I N, Finlay J, Winstanley D J, Dewhurst N, Nelson J W, Butler S L, Govan J R. Multi-resistance isolates possessing characteristics of both Burkholderia (Pseudomonas) cepacia and Burkholderia gladioli from patients with cystic fibrosis. J Antimicrob Chemother. 1994;34:353–361. doi: 10.1093/jac/34.3.353. [DOI] [PubMed] [Google Scholar]
- 26.Smith D L, Gumery L B, Smith E G, Stableforth D E, Kaufmann M E, Pitt T L. Epidemic of Pseudomonas cepacia in an adult cystic fibrosis unit: evidence of person-to-person transmission. J Clin Microbiol. 1993;31:3017–3022. doi: 10.1128/jcm.31.11.3017-3022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokol P A, Ohman D E, Iglewski B H. A more sensitive plate assay for detection of protease production by Pseudomonas aeruginosa. J Clin Microbiol. 1979;9:538–540. doi: 10.1128/jcm.9.4.538-540.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Jiang R Z, Steinbach S, Holmes A, Campanelli C, Forstner J, Sajjan U, Tan Y, Riley M, Goldstein R. The emergence of a highly transmissible lineage of cbl+ Pseudomonas (Burkholderia) cepacia causing CF centre epidemics in North America and Britain. Nat Med. 1995;1:661–666. doi: 10.1038/nm0795-661. [DOI] [PubMed] [Google Scholar]
- 29.Tempe J, Petit A, Holsters M, van Montagu M, Schell J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: possible relation to transformation in crown gall. Proc Natl Acad Sci USA. 1977;74:2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyler S D, Rozee K R, Johnson W M. Identification of IS1356, a new insertion sequence, and its association with IS402 in epidemic strains of Burkholderia cepacia infecting cystic fibrosis patients. J Clin Microbiol. 1996;34:1610–1616. doi: 10.1128/jcm.34.7.1610-1616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R, Revets H, Lauwers S, Gillis M, Kersters K, Govan J R. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- 32.Vandamme P, Mahenthiralingam E, Holmes B, Coenye T, Hoste B, De Vos P, Henry D, Speert D P. Identification and population structure of Burkholderia stabilis sp. nov (formerly Burkholderia cepacia genomovar IV) J Clin Microbiol. 2000;38:1042–1047. doi: 10.1128/jcm.38.3.1042-1047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh T A, Ballou D P. Halogenated protocatechuates as substrates for protocatechuate dioxygenase from Pseudomonas cepacia. J Biol Chem. 1983;258:14413–14421. [PubMed] [Google Scholar]
- 34.Williams P, Camara M, Hardman A, Swift S, Milton D, Hope V J, Winzer K, Middleton B, Pritchard D I, Bycroft B W. Quorum sensing and the population-dependent control of virulence. Philos Trans R Soc Lond B Biol Sci. 2000;355:667–680. doi: 10.1098/rstb.2000.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yabuuchi E, Kosako Y, Oyaizu H, Yano I, Hotta H, Hashimoto Y, Ezaki T, Arakawa M. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol Immunol. 1992;36:1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Beaber J W, More M I, Fuqua C, Eberhard A, Winans S C. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J Bacteriol. 1998;180:5398–5405. doi: 10.1128/jb.180.20.5398-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]