Abstract
We studied the expression of cytokines, chemokines, and chemokine receptors by the RNase protection assay in chlamydia-pulsed dendritic cells to better understand their potent anti-chlamydial immunizing properties. We found that chlamydia-pulsed dendritic cells express a complex profile of inflammatory and immunomodulatory molecules. These include CCR-7, interleukin-12, and interferon-induced protein 10, molecules that might influence the homing of pulsed dendritic cells to the site of chlamydial infection and the induction of a local protective CD4+ Th1 cellular immunity.
Chlamydia trachomatis is an obligatory intracellular bacterial parasite that infects the oculogenital mucosal epithelium, causing trachoma, the world's leading cause of preventable blindness, and sexually transmitted diseases. Pelvic inflammatory disease is a serious sequalae of C. trachomatis infection of the female genital tract that can result in tubal blockage, infertility, or ectopic pregnancy (2, 4, 8, 13). The development of an efficacious vaccine against C. trachomatis oculogenital infection is likely to be key to the control of both trachoma and chlamydial sexually transmitted diseases. Despite considerable effort, however, there has been little favorable progress toward this end. Conventional vaccination approaches have produced disappointing results in their abilities to prevent infection of the mouse female genital tract (12, 14, 19), despite a modicum of success in controlling chlamydial infection of the respiratory tract (20). Solid protective immunity to genital rechallenge has been achieved only by infection or adoptive immunization with dendritic cells (DC) pulsed ex vivo with inactivated whole chlamydial organisms (6, 18). Interestingly, mice immunized with chlamydia-pulsed DC exhibit equivalent levels of protective immunity to that in mice that have spontaneously resolved a primary genital infection (18). Both infection-mediated protective immunity and immunity elicited following adoptive transfer of antigen-pulsed DC correlate with a chlamydia-specific CD4+ Th1-biased immune response characterized by the secretion of high levels of gamma interferon from local and splenic CD4+ T cells (6, 18). Recent studies have also indicated an important cooperative role for both CD4+ T cells and B cells in recall immunity in the murine model; however the mechanism(s) that mediates this cooperative effector function has not been described (11). Clearly, the use of ex vivo antigen-pulsed DC as a practical chlamydial vaccine is unsuited for use in humans. Nevertheless, the ability of ex vivo antigen-pulsed DC to elicit solid antichlamydial protective immunity at the genital mucosae is gratifying because it demonstrates that a more complete understanding of chlamydia-DC interactions may provide important information applicable to the development of a conventional antichlamydial vaccine.
In this work we have investigated cytokine, chemokine, and chemokine receptor gene expression in chlamydia-pulsed DC by the RNase protection assay (RPA). Our findings show that populations of chlamydia-pulsed DC that provide solid protective immunity following adoptive transfer to naive mice up regulate genes that mediate DC homing to lymphatic tissues and the recruitment and activation of T cells.
Chlamydiae, mice, and DC.
The mouse pneumonitis (MoPn) strain of C. trachomatis was grown in HeLa 229 cells. Infectious elementary bodies (EBs) were purified by density gradient centrifugation, and infection-forming units (IFU) were determined as previously described (3). Female C57BL/10 mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and used between 8 and 12 weeks of age. Bone marrow-derived DC were generated from C57BL/10 female mice (6 to 12 weeks old) using a modified version of the Inaba technique (7). Briefly, femurs were removed from mice and bone marrow cells were flushed from the femurs and cultured in Iscove modified Dulbecco medium (IMDM) (Life Technologies) supplemented with 10% fetal bovine serum, 10 μg of gentamicin sulfate per ml, 10 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF), per ml, and 103 U of interleukin-4 (IL-4) (PharMingen) per ml at 2 × 106 cells/ml in 100-mm tissue culture dishes. On day 3 of culture, nonadherent cells were removed and fresh medium containing GM-CSF and IL-4 was added. On day 5 of culture, DC were separated from the remaining contaminating macrophages by transferring the nonadherent and loosely adherent DC cells to new culturing plates and were then incubated at 37°C for 2 h. This procedure was repeated, and nonadherent DC were further purified by density gradient centrifugation in metrizamide gradients (Sigma) prepared in cell culture medium. The purity of density gradient-purified DC was assessed by flow cytometry (Becton Dickinson) after staining with anti-I-Ab, anti-CD86, anti-CD40, anti-CD11b, anti-Gr1, anti-CD3, anti-CD19, and anti-Pan NK. Isolated DC showed positive staining for I-Ab, CD86, CD40, and CD11b and were negative for CD3, CD19, and Gr-1 (data not shown).
DC cultured for 5 days, panned, and isolated from density gradients were washed and plated at 2.5 × 106 DC/well in six-well tissue culture plates containing culture medium supplemented with 10 ng of GM-CSF per ml. DC were divided into the following groups and treated as follows: (i) no treatment (IMDM alone); (ii) 10 ng of lipopolysaccharide (LPS) (Escherichia coli strain O26:B6 [Sigma]) per ml, which served as a positive control for DC activation; (iii) latex beads (2.5% solids-latex, 0.585 μm in diameter [Polysciences, Inc.]), which served as a negative control for gene expression induced by endocytosis; and (iv) heat-inactivated (56°C for 30 min) MoPn EBs. LPS, latex beads, and EBs were all diluted in IMDM and the material added directly to the plates containing DC. The materials were mixed with DC by gently swirling the plates. The plates were then incubated at 37°C for 12 h, and DC were harvested and prepared for flow cytometry, RPA, or adoptive immunization.
Immunization with chlamydia-pulsed DC.
Female mice (five to eight animals per group) were immunized by the subcutaneous (s.c.), intraperitoneal (i.p.), or intravenous (i.v.) route. Treated or untreated DC were washed in cell culture medium without FBS and resuspended in Hanks balanced salt solution (HBSS) at 2.5 × 107 cells/ml. A 0.2-ml volume of these suspensions (5 × 106 cells) was used to inoculate mice by the different routes described above. Heat-inactivated EBs (2.5 × 108, an equivalent number of heat-inactivated HK EBs to that used to pulse DC) resuspended in 0.2 ml of cell culture medium without FBS or mice receiving no immunization were used as negative controls. A second immunization was administered 14 days after the primary immunization. One week following the second immunization, mice were injected s.c. with 2.5 mg of medroxyprogesterone acetate (Depo-Provera; Upjohn Co., Kalamazoo, Mich.) in 0.1 ml of saline to synchronize estrous cycles. Seven days later, the mice were challenged by injecting 5 μl of chlamydial MoPn (150 IFU, 10 50% infective doses) 10mM phosphate (pH 7.2) containing 0.25 M sucrose and 5 mM l-glutamic acid (SPG) directly into the vaginal vault by using a narrow-bore pipette. Mice that had resolved a primary chlamydial genital infection were rechallenged vaginally and served as a positive (infection immune) control. Protection was assessed by quantifying recoverable infectious organisms from cervicovaginal swabs at intervals following infectious challenge (days 3, 5, 7, 10, 14, 21, and 28). Briefly, mice were cultured by swabbing the vaginal vault (Calgiswab type 1; Hardwood Products Co., Guilford, Maine), samples were vortexed vigorously, and diluted in SPG. HeLa cells were inoculated with all dilutions in triplicate in 96-well tissue culture plates. The plates were centrifuged for 1 h at 700 × g and then rocked at 37°C for 30 min. Cells were washed with HBSS three times and fed 100 μl of minimal essential medium supplemented with 10% FBS and cycloheximide (10 ng/ml) per well. Cultures were incubated for 24 h and fixed in methanol. Chlamydial inclusions were detected by indirect-immunofluorescence staining using the chlamydia-LPS genus-specific monoclonal antibody EVI-H1 and quantified as IFU.
RPA.
DC total RNA was harvested by the TriZol RNA isolation method (Life Technologies) at 2, 12, 24, 48, and 72 h posttreatment. A multiprobe RPA system (PharMingen) was used to detect DC expression of macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-2, MIP-3α, MIP-3β, monocyte chemotactic protein 1, lymphotactin, eotaxin, RANTES (regulated on activation, normal T-cell expressed and secreted), interferon-induced protein 10 (IP-10), T-cell activation gene 3 (TCA-3), chemokine receptor 1 (CCR1), CCR1β, CCR3, CCR4, CCR5, CCR6, CCR7, IL-1α, IL-1β, IL-1 receptor antagonist, IL-6, IL-10, IL-12p35, IL-12p40, IL-18, gamma interferon, tumor necrosis factor alpha (TNF-α), migration inhibitory factor (MIF), and constitutive genes (internal controls) L32 and glyceraldehyd-3-phosphate dehydrogenase (GAPDH). Briefly, DNA templates encoding exonic sequences of the genes of interest were fused to a T7 promoter and used for T7 RNA polymerase-directed synthesis of highly specific 32P-labeled antisense RNA probes. Labeled RNA probes were hybridized in excess overnight at 56°C with 4 μg of target DC mRNA. The following day, free probe and other single-stranded RNA molecules were digested with RNases T1 and A. The remaining “RNase-protected” probes were purified, resolved on denaturing polyacrylamide gels based on size, and imaged by autoradiography. Protected probes at the appropriate sizes represent specific DC mRNA.
Intravenous immunization with chlamydia-pulsed DC is the optimum route for eliciting protective immunity.
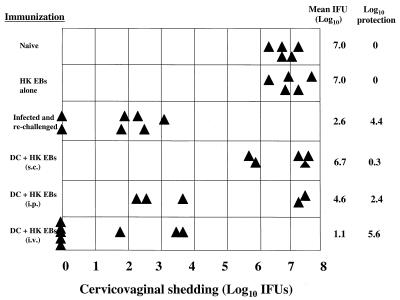
We initially studied whether the route of immunization with chlamydia-pulsed DC had an effect on the level of antichlamydial protective immunity generated at the genital mucosae. We found that i.v. immunization with chlamydia-pulsed DC was superior to either the s.c. or i.p. route (Fig. 1). Compared to naive control animals, mice immunized i.v. with chlamydia-pulsed DC exhibited a 5.6-log-unit reduction in infectious burden following chlamydial challenge. Five of the eight challenged mice in the i.v.-immunized group were culture negative 7 days postchallenge, and the three culture-positive mice exhibited a marked reduction in chlamydial burden compared to naive controls. Interestingly, this level of protection was equivalent to that found in mice that had resolved a primary genital infection and were then rechallenged (4.6-log-unit reduction). Mice immunized by the s.c. or i.p. route exhibited only marginal levels of protection (s.c. = 0.3 log unit) or were partially protected (i.p. = 2.4 log units), respectively. There was also marked variation in the level of protective immunity generated in mice immunized by the i.p. route; three of five mice were highly protected, whereas no protection was observed the other two. Mice immunized i.v. with heat-inactivated EBs alone and challenged intravaginally were not protected.
FIG. 1.
Intravenous delivery of DC pulsed with nonviable chlamydiae elicits strong protective immunity against infectious intravaginal challenge. Mice received either no immunization (naive), 150 IFU of C. trachomatis EBs (MoPn strain) delivered intravaginally (infected), 2.5 × 108 heat-inactivated MoPn EBs in 0.2 ml of HBSS delivered i.v. (HK EB), or 5 × 106 DC pulsed with 2.5 × 108 heat-inactivated MoPn EBs (DC + HK EB) delivered in 0.2 ml of HBSS s.c., i.p., and i.v. One week following the booster immunization, all mice were challenged intravaginally with 150 IFU of MoPn EBs in 5 μl of SPG. Protection was assessed by quantifying the chlamydial IFU recovered from cervicovaginal swabs (shown on the x axis). Day 4 postchallenge data are shown here. Triangles represent individual mice.
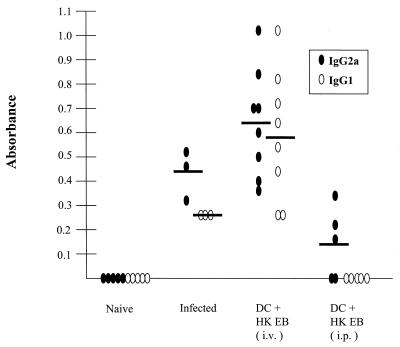
We next assayed the sera of immunized mice by enzyme-linked immunosorbent assay ELISA for chlamydia-specific immunoglobulin G1 (IgG1) and IgG2a antibodies as a way of indirectly ascertaining whether they generated a CD4+ Th1 immune response (Fig. 2). All mice in the infected and rechallenged group and the i.v.-immunized group produced a chlamydia-specific IgG2a response indicative of a type 1 CD4+ response. In contrast, only three of the five mice immunized by the i.p. route had detectable antichlamydial antibodies that were exclusively of the IgG2a (Th1) isotype. The mice with chlamydia-specific IgG2a serum antibodies were the same animals that exhibited significant levels of protective immunity following intravaginal challenge (Fig. 1). All mice immunized s.c. with chlamydia-pulsed EBs and which were not protected following chlamydial challenge were negative by ELISA for chlamydial antibody (data not shown). In conclusion, the results clearly demonstrate that delivery of chlamydia-pulsed DC by the i.v. route is superior for eliciting a protective immune response against chlamydial infection of the genital mucosae. The reasons for these findings are not known, but it is possible that i.v.-administered antigen-pulsed DC home efficiently to regional or mesenteric lymph nodes, where they interact with T cells that are capable of homing to the genital mucosae.
FIG. 2.
Serum antibody titers following infection or immunization with chlamydia-pulsed DC. Sera were collected from naive mice, infected mice (35 days postinfection), and 14 days following two immunizations (administered 14 days apart) with chlamydia-pulsed DC (DC + HK EB) by i.v. and i.p. injection. Chlamydia-specific IgG1 and IgG2a titers were determined by ELISA. Naive mice did not elicit a chlamydia-specific IgG1 or IgG2a response. Infected mice elicited a predominantly IgG2a response. All mice immunized with chlamydia-pulsed DC by i.v. injection elicited a strong chlamydia-specific IgG2a and IgG1 response with higher titers than did mice that had been infected and resolved infection for 35 days. Three of the five mice immunized by i.p. injection elicited a chlamydia-specific IgG2a response. Ovals represent individual mice (solid ovals, IgG2a; open ovals, IgG1). Solid horizontal bars indicate the mean absorbance. Absorbance is shown on the y axis; 1:256 dilution of all sera is indicated on the x axis.
Chlamydia-pulsed DC differentially express genes that promote DC migration, activation, and recruitment of T cells.
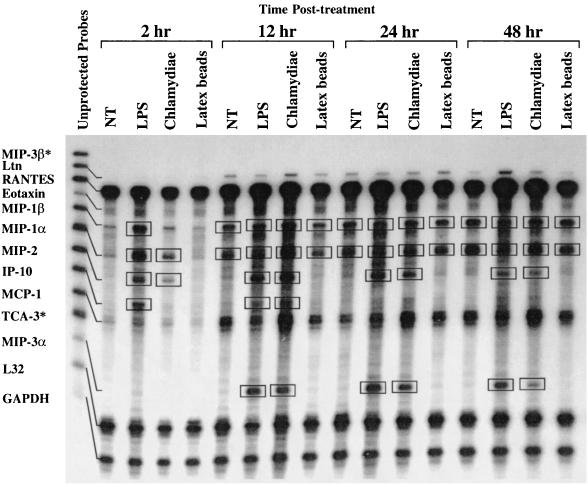
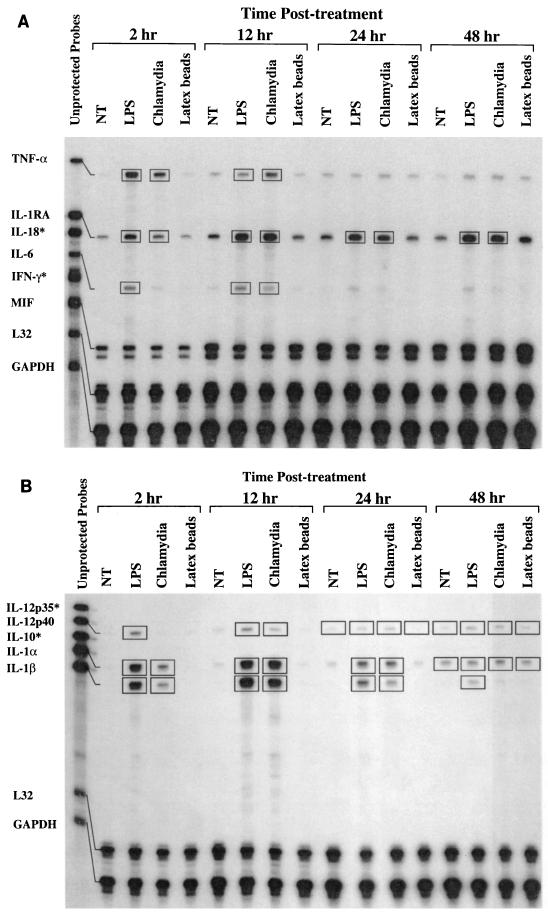
The ability of immature DC to traffic from peripheral tissues such as the epithelium to draining regional lymph nodes is critical for the generation of an immune response (1, 5, 15–17). DC migration to lymphatic tissue is regulated by chemokines, chemokine receptors, and cytokines (1, 21). To ascertain if chlamydia-pulsed DC expressed immunomodulatory molecules that function in the homing and induction of T-cell immunity, we used RPA to examine the kinetics of DC chemokine, chemokine receptor, and cytokine gene expression following endocytosis of chlamydiae. Untreated DC, DC treated with E. coli LPS, and DC that had endocytosed 500-nm-diameter latex beads were included in the assays as negative and positive controls and as a control for induction of gene expression by the phagocytic process, respectively. Genes that were up regulated are identified in the figures by the boxes. The results show that MIP-1α, MIP-2, TNF-α, IL-1α, IL-1β, and IL-1RA were expressed by 2 h after treatment with chlamydiae as well as LPS and remained continuously expressed for as long as 48 h (Fig. 3 and 4A and B). In contrast, MIP-3α and IL-12p40 were expressed at 12 h and showed similar continuous expression (Fig. 3 and 4B). IP-10 and IL-6 were transiently expressed, being detected at 12 h but not at 48 h (Fig. 3 and 4A). CCR1, CCR1β, CCR2, CCR3, CCR4, CCR5, and CCR6 were not expressed at any time points following the uptake of chlamydial organisms (Fig. 5). Expression of CCR7 by DC was observed under all culture conditions; however, only chlamydia-pulsed DC expressed CCR7 at 48 h (Fig. 5). The ability of EBs to dramatically up regulate the expression of such a large number of proinflammatory and immunomodulatory molecules is impressive. At present it is unknown what components of the organism might mediate this response. An obvious candidate is chlamydial LPS; however, we do not believe this to be the case because chlamydial LPS differs structurally from the positive-control enteric LPS in that it possess unusual long-chain fatty acids that are known to result in its low to undetectable endotoxic activity (10). Nevertheless, we cannot exclude this possibility, and wed did not test chlamydial LPS directly because of the difficulty in purifying sufficient amounts of the molecule from native chlamydial organisms.
FIG. 3.
DC pulsed with chlamydiae differentially express chemokines important for recruitment and activation of T cells and immature DC. RNA was harvested from DC for RPA analysis at 2, 12, 24, and 48 h following no treatment (NT) or treatment with LPS, chlamydiae (heat-inactivated MoPn EBs), or latex beads. Unprotected mRNA probes for selected chemokines are shown in the left-hand lane and named along the y axis. Treatment groups and time points are listed at the top of the gel. L32 and GAPDH are constitutive genes that serve as internal controls. Boxed bands represent differentially expressed genes. ∗, no mRNA detected.
FIG. 4.
DC pulsed with chlamydiae differentially express Th1 cytokines. RNA was harvested from DC for RPA analysis at 2, 12, 24, and 48 h following no treatment (NT) or treatment with LPS, chlamydiae (heat-inactivated MoPn EBs), or latex beads. Unprotected mRNA probes for selected cytokines are shown in the left-hand lane in A and B. Treatment groups and time points are listed at the top of the gels. L32 and GAPDH are internal controls. Boxed bands represent differentially expressed genes. ∗, no mRNA detected.
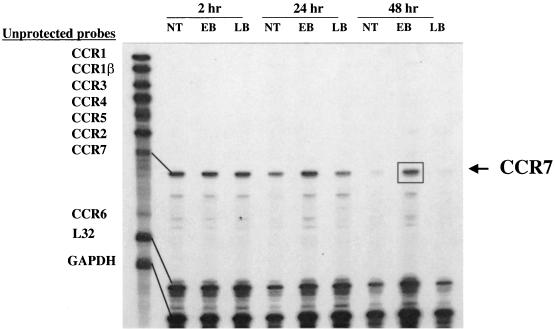
FIG. 5.
Expression of CCR7 mRNA by chlamydia-pulsed DC. RNA was harvested from DC for RPA analysis at 2, 24, and 48 h following no treatment (NT) or treatment with heat-inactivated chlamydial EBs or latex beads (LB). Unprotected probes for chemokine receptors 1 to 7 are shown in the left-hand lane. The boxed band indicates increased longevity of expression of the CCR7 mRNA by DC pulsed with nonviable chlamydiae for 48 h.
In summary, we show that DC pulsed with nonviable chlamydiae that generate a potent protective immune response following i.v. adoptive transfer induce a wide spectrum of immunomodulatory genes that includes genes encoding MIP-1α, MIP-2, MIP-3α, TNF-α, IL-1α, IL-1β, IL-1RA, IP-10, IL-6, IL-12p40, and CCR7. We believe that the most notable of these genes, in terms of the protective immunizing capabilities of antigen-pulsed DC, are likely to be CCR7, IL-12, and IP-10. The stable expression of CCR7 by chlamydia-pulsed DC may extend the duration of DC maturation following adoptive transfer, thereby allowing additional time for migration to local lymph nodes. Expression of IP-10 and IL-12 by chlamydia-pulsed DC, albeit transient, implies an ability to specifically traffic effector T cells to the genital mucosae and potentially promote their differentiation toward a CD4+ Th1 phenotype, a model that has recently been shown to function in the generation of protective immunity against the obligate intracellular eukaryotic pathogen Toxoplasma gondii (9). Clearly, however, these conclusions need to be supported by further experimentation that would include monitoring DC migration to the iliac and mesenteric lymph nodes and in vivo antibody-mediated depletion of IL-10 following adoptive immunization. Nevertheless, this work clearly demonstrates that DC pulsed with inactivated chlamydiae and administered i.v. elicit equivalent levels of protective immunity to that achieved by infection itself. These findings imply that eliciting protective immunity at the genital mucosae by more conventional vaccine strategies is feasible but will require both the identification of chlamydial protective antigens and the development of adjuvants capable of modulating local T-cell responses that favor the induction of CD4+ type 1 immunity.
REFERENCES
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Brunham R C, Peeling R, Maclean I, Kosseim M L, Paraskevas M. Chlamydia trachomatis-associated ectopic pregnancy: serologic and histologic correlates. J Infect Dis. 1992;165:1076–1081. doi: 10.1093/infdis/165.6.1076. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow J M, Yonekura M L, Richwald G A, Greenland S, Sweet R L, Schachter J. The association between Chlamydia trachomatis and ectopic pregnancy. A matched-pair, case-control study. JAMA. 1990;263:3164–3167. [PubMed] [Google Scholar]
- 5.Dieu M C, Vanbervliet B, Vicari A, Bridon J M, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igietseme J U, Ananaba G A, Bolier J, Bowers S, Moore T, Belay T, Eko F O, Lyn D, Black C M. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J Immunol. 2000;164:4212–4219. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 7.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones R B, Ardery B R, Hui S L, Cleary R E. Correlation between serum antichlamydial antibodies and tubal factor as a cause of infertility. Fertil Steril. 1982;38:553–558. doi: 10.1016/s0015-0282(16)46634-3. [DOI] [PubMed] [Google Scholar]
- 9.Khan I A, MacLean J A, Lee F S, Casciotti L, DeHaan E, Schwartzman J D, Luster A D. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 10.Kosma P. Chlamydial lipopolysaccharide. Biochim Biophys Acta. 1999;1455:387–402. doi: 10.1016/s0925-4439(99)00061-7. [DOI] [PubMed] [Google Scholar]
- 11.Morrison S G, Su H, Caldwell H D, Morrison R P. Immunity to murine chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal S, Barnhart K M, Wei Q, Abai A M, Peterson E M, de la Maza L M. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine. 1999;17:459–465. doi: 10.1016/s0264-410x(98)00219-9. [DOI] [PubMed] [Google Scholar]
- 13.Schachter J. Chlamydial infections. N Engl J Med. 1978;298:428–434. doi: 10.1056/NEJM197802232980805. [DOI] [PubMed] [Google Scholar]
- 14.Schnorr K L. Chlamydial vaccines. J Am Vet Med Assoc. 1989;195:1548–1561. [PubMed] [Google Scholar]
- 15.Shaffer A L, Yu X, He Y, Boldrick J, Chan E P, Staudt L M. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 16.Sozzani S, Allavena P, Vecchi A, Mantovani A. The role of chemokines in the regulation of dendritic cell trafficking. J Leukoc Biol. 1999;66:1–9. doi: 10.1002/jlb.66.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Steinman R M, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 18.Su H, Messer R, Whitmire W, Fischer E, Portis J C, Caldwell H D. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward M E. Chlamydial vaccines—future trends. J Infect. 1992;25(Suppl. 1):11–26. doi: 10.1016/0163-4453(92)91882-c. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D, Yang X, Berry J, Shen C, McClarty G, Brunham R C. DNA vaccination with the major outer-membrane protein gene induces acquired immunity to Chlamydia trachomatis (mouse pneumonitis) infection. J Infect Dis. 1997;176:1035–1040. doi: 10.1086/516545. [DOI] [PubMed] [Google Scholar]
- 21.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]