Abstract
Background: The plasmid−mediated tigecycline resistance gene tet(X4) confers a high level of resistance to tigecycline. The experiment aims to investigate the prevalence and characterization of tet(X4) in Escherichia coli isolates from chicken and pig farms in Hunan province, China. Methods: A total of six tet(X4) positive strains were identified in 257 E. coli derived from chicken samples in Xiangtan city (n = 2), pig samples in Xiangxiang city (n = 1), Chenzhou city (n = 2), and Zhuzhou city (n = 1). The presence of tet(X4) was directly detected by PCR assay, and then the broth dilution method determined the antimicrobial susceptibility profile of the tet(X4)−positive isolates. Genomic locations were identified by whole−genome sequencing (WGS) and bioinformatics. Results: Almost all tet(X4)−positive strains showed high resistance to multidrug, including tigecycline. Resistome analysis revealed many antibiotic resistance genes, including those with resistance to tetracyclines, β−lactams, phenicols, quinolones, lincosamides chloramphenicol, aminoglycosides and sulfamids. These tet(X4)−bearing strains exhibited six distract STs, such as ST10, 202, ST218, ST362, ST2077, ST7068. The plasmid replicon types carrying tet(X4) were the hybrid plasmid IncFIA(HI1)/IncHIA/IncHIB(R27) (5/6) and IncX1 (1/6). Conclusions: The presence of similar genetic environments in E. coli from different cities suggests there may be horizontal transmission pathways promoting the broad spread of drug−resistant genes in Hunan Province, putting great pressure on multidrug resistance monitoring.
Keywords: multidrug resistance, plasmid, tet(X4), chicken, pig, Escherichia coli
1. Introduction
Tigecycline is regarded as a ‘last resort’ antibiotic to treat clinical infection caused by multidrug−resistant (MDR) and even extensively drug−resistant bacteria [1]. However, novel plasmid−mediated high levels of tigecycline resistance genes tet(X3)/tet(X4) were discovered in Enterobacteriaceae and Acinetobacter isolates from animals and humans in China in 2019, which has drawn worldwide attention and has posed a major threat to public health [2,3]. Since then, diverse tet(X) genes, ranging from tet(X5) [4], tet(X6) [5], and tet(X7) [6] to tet(X13) [7], tet(X14) [8], and tet(X15) [9], have been described. The prevalence of the tet(X4) gene, the most widespread tet(X) variant [1], has posed a great challenge to public health. Furthermore, tet(X4)−bearing plasmids (e.g., IncX1, IncHI2, and IncFIA) have been identified worldwide from human, poultry, food, and environmental samples [10,11,12,13,14]. Most tet(X4)−bearing plasmids are multidrug−resistant plasmids with various replicon types (e.g., IncX1, IncFIA, IncHIA, and IncHIB) carrying various ISs (insert sequences), especially ISCR2 [15]. In addition, ISCR2 is adjacent to tet(X4) in most plasmids and plays an important role in the transmission of tet(X4) [16]. Mobile elements with multidrug resistance genes increase the possibility of transmission in the transportation chain and further give rise to the risk of problems in public health.
Chickens and pigs are the main farmed animals in Hunan Province, with the emergence of multidrug resistance genes conferring resistance to most antibiotics in farms, bringing great pressure to daily management and limiting the use of antibiotics. In this study, we describe the characteristics and molecular epidemiology of tet(X4)−positive E. coli isolates in chicken and pig farms in Hunan Province, China, to provide experimental data and a basis for drug resistance investigation and surveillance.
2. Results and Discussion
2.1. Bacteria Isolates
A total of six isolates positive for the tet(X4) gene were collected from four cities located far apart, with the prevalence rate of tet(X4) positivity at 2.33% (6/257). These tet(X4)−positive strains were from chicken fecal samples from Xiangtan city (2/80) and pig fecal samples from Chenzhou city (2/75), Xiangxiang city (1/52), and Zhuzhou city (1/50), with prevalence rates of 2.5%, 2.67%, 1.93%, and 2%, respectively (Table S1). This prevalence of tet(X4)−positive strains are low compared to reports from chicken or pig farms in Jiangsu (18.24%) [17], Shandong (66.7%) [2], and Shanghai (12.24%) [18] in China, which may be due to the insufficient scope of the survey and the number of samples, as well as the need to remain vigilant and strengthen the surveillance in Hunan Province. According to previous reports, plasmid−mediated tet(X3) was detected in food samples in Acinetobacter from 2015–2018 in Hunan province, China. The emergence of the tet(X4) gene was first reported in these cities and may be attributed to daily farm management and potential transmission.
2.2. Antimicrobial Susceptibility Testing and Resistance Genes
According to MICs (minimum inhibitory concentrations) compared with the resistance point in CLSI, these tet(X4)−positive isolates showed resistance to multiple drugs; they were all resistant to ampicillin (≥256 mg/L), florfenicol (≥64 mg/L), gentamicin (≥64 mg/L), nalidixic (≥8 mg/L), trimethoprim–sulfamethoxazole (>16 mg/L), and tigecycline (≥4 mg/L), but susceptible to amikacin (0.5 mg/L), cefotaxime (≤0.125 mg/L), meropenem (≤0.5 mg/L), and colistin (≤0.25 mg/L) (Table 1). It is noteworthy that the MICs of these strains toward polymyxin, amikacin, and cefotaxime were not very different, but the MIC of strain 22a16 toward meropenem was four times the MIC of the other strains toward meropenem, which may indicate a trend of resistance to carbapenem antibiotics in the farm. The resistance phenotype could, in most cases, be explained by the carriage of the corresponding resistance genes. In addition, the genotype analysis of antimicrobial resistance genes (ARGs) revealed 21 ARGs for seven antimicrobial classes (beta−lactam, tetracycline, aminoglycoside, sulfonamides, phenicol, fluoroquinolone, and lincosamide) that were discovered in six strains (Figure 1). blaTEM−1 (6/6), tet(X4) (6/6), floR (5/6), lnu(G) (5/6), and qnrS1 (5/6) were the most common ARGs in the six isolates. Strain 22a10, belonging to ST10, showed the most resistance genes. Interestingly, although the blaTEM−1 resistance gene existed in six strains, and the blaLAP−2 resistance gene existed in strain 22a62 in particular, these tet(X4)−positive strains did not show resistance to cefotaxime. Six tet(X4)−carrying strains exhibited high resistance to multiple classes of antibiotics, especially tigecycline, which could bring about great difficulty in clinical treatment.
Table 1.
The MIC of 6 tet(X4)−positive E. coli strains.
| Strain | Antimicrobial Agents (mg/L) a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TGC | AMP | AMK | CPL | CTX | NAL | FFC | COL | MEM | GEN | STX | |
| 22a10 | 4 | >256 | 0.5 | 256 | 0.06 | >256 | 128 | 0.25 | 0.06 | >128 | >16 |
| 22a16 | 8 | >256 | 0.5 | 128 | 0.06 | 32 | 128 | 0.125 | 0.5 | >128 | >16 |
| 22a22 | 8 | >256 | 0.5 | 256 | 0.06 | >256 | 128 | 0.125 | 0.06 | 64 | >16 |
| 22a62 | 4 | >256 | 0.5 | 128 | 0.125 | >256 | 64 | 0.125 | 0.03 | >128 | >16 |
| 22a66 | 8 | 256 | 0.5 | 128 | 0.06 | 64 | 128 | 0.125 | 0.06 | >128 | >16 |
| 22a232 | 4 | >256 | 0.5 | 256 | 0.125 | 8 | 128 | 0.125 | 0.06 | >128 | >16 |
a Abbreviations and resistance breakpoints: TGC—tigecycline (R > 2 mg/L), AMP—ampicillin (R > 8 mg/L), AMK—amikacin (R > 16 mg/L), CPL—chloramphenicol (R > 8 mg/L), CTX—cefotaxime (R > 2 mg/L), NAL—nalidixic acid (R > 4 mg/L), FFC—florfenicol (R > 16 mg/L), COL—colistin (R > 2 mg/L), MEM—meropenem (R > 8 mg/L), GEN—gentamicin (R > 4 mg/L), SXT—trimethoprim−sulfamethoxazole (R > 4 mg/L). Bold formatting indicates resistance to the respective antimicrobial agents.
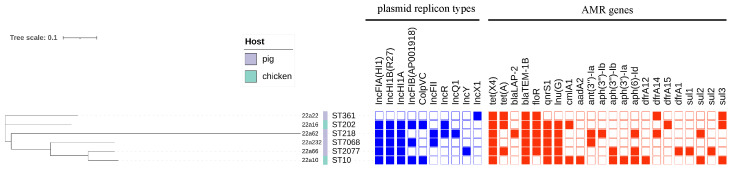
Figure 1.
The phylogenetic tree and genomic features of six tet(X4)−positive E. coli isolates. The heatmap in different colors depicts the presence or absence of the plasmid replicon types (blue) and antimicrobial−resistance (AMR) genes (red). The whole genome sequence data of six E. coli strains have been submitted to NCBI under the BioProject accession number PRJNA898522.
2.3. Multilocus Sequence Typing and Plasmid Replicons
These tet(X4)−positive strains were sequenced through whole−genome sequencing and used for phylogenetic tree analysis and other bioinformatics analyses. The phylogenetic tree analysis revealed that six tet(X4)−carrying isolates had genetic diversity (Table S2) and were distributed into six distinct STs (sequence types): ST10, ST202, ST361, ST218, ST2077, and ST7068 (Figure 1). Although the isolates originated from the same locations, the ST types were all different, which could suggest that transmission of the tet(X4) gene was extensive in various ST types. Importantly, one tet(X4)−positive E. coli, named 22a10, belonged to ST10, which was regarded as the main ST type for transferring tet(X4) [1].
Through bioinformatics analysis, type IV secretion systems (virB) were found in almost all isolates, related to the transferability of bacteria and the contribution of tet(X4) to other bacteria [19] (Figure 2). The PlasmidFinder analysis of six isolates identified 10 distinct plasmid replicons: IncFIA (HI1), IncHIA, IncHIB (R27), ColpVC, IncFIB (AP001918), IncFII, IncQ1, IncR, IncY, and IncX1 (Figure 1). Interestingly, IncFIA, IncHIA, and IncHIB were identified in most of these isolates, and these plasmid replicons normally constitute a hybrid plasmid [1]. Although the whole−genome sequences of strain 22a22 containing pure IncX1 replicon alone differed significantly compared with other strains of hybrid plasmid type, they showed relatively high similarity in the upstream and downstream sequences of the tet(X4) and blaTEM−1 resistance genes (Figure 2). These isolates were obtained from different sources and collections while sharing the same plasmid replicon, which illustrated that the hybrid plasmid IncFIA(HI1)/IncHIA/IncHIB (R27) was the dominant tet(X4)−carrying plasmid replicon playing an important role in the spread of the tet(X4) resistance gene [15]. Importantly, the IncFIA (HI1)/IncHIA/IncHIB (R27) hybrid plasmid p22a62 shared 99% identity at 88% coverage with the plasmids pSX8G−tetX4 (MW940625), pSY3626 (CP059284), pT16R−1 (CP046717), and pE−T306 (CP090284) in E. coli SX8G, E. coli SY3626, E. coli T16R−1, and E. coli E−T306 derived from pork, chicken meat, pet dog, and human in China, respectively. In addition, plasmid p22a62 also showed 99% identity at 73% coverage to plasmid pHNGS471−2 (CP089511) in Klebsiella pneumoniae strain GD21SC417 isolated from Jiangsu Province in China (Figure 2). The high similarity of the tet(X4)−bearing plasmid between animals and humans indicated that the plasmid had achieved a wide distribution among different origins, and the transmission of multidrug resistance, including tigecycline resistance, required more control and monitoring to reduce the potential risk of public food health problems [20].
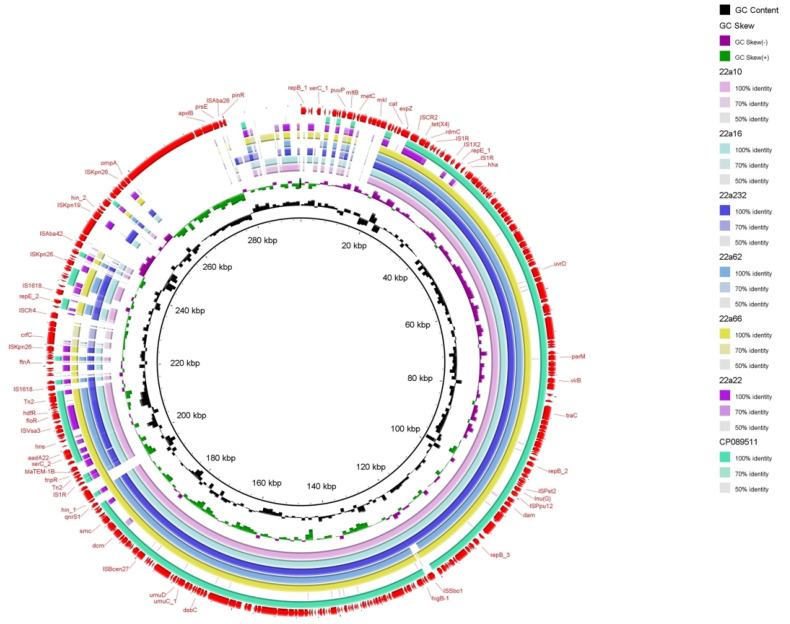
Figure 2.
Circular comparison of the tet(X4)−bearing plasmids with other closely related plasmids pRT18−1 (MT219824) from the NCBI database. The outmost ring represents the reference plasmid with its gene positions.
2.4. Genetic Contexts and Conjugation
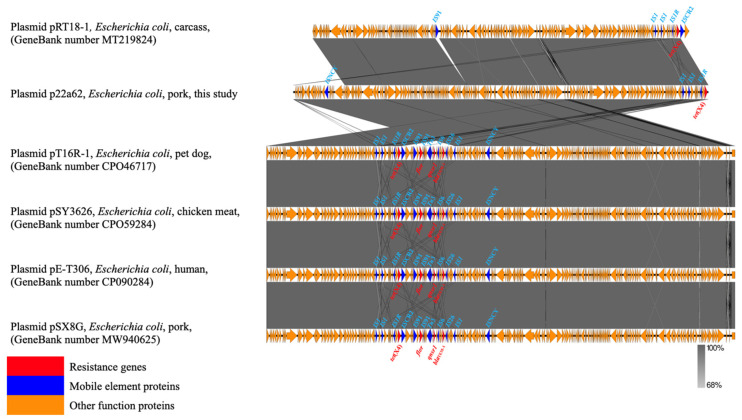
Six tet(X4)−harboring strains showed the same genetic environment compared with the structure ISCR2/tet(X4)/RdmC/hp/IS1R of the original tet(X4)−carrying plasmid pRT18−1 (MT219824) (Figure 2 and Figure 3). On the one hand, ISCR2 was regarded as the most normal mobile element on the upstream−flanking region of tet(X4) [21]. On the other hand, IS1 mobile elements have also been reported for the transmission of tet(X4) [22]. The transmission of tet(X4) by two accustomed IS elements calls for increased monitoring, and this identical group of genetic contexts that occurred in most farms may indicate that the structure ISCR2−tet(X4)−RdmC−hp−IS1R was conducive to the spread of tet(X4) [22,23]. Furthermore, there were other types of mobile elements, such as ISVsa3 (Figure 2), which could have mediated tet(X4) transfer in conjugation and conferred a mild fitness cost [2], and Tn2 transposon, which was most abundant in blaTEM−harboring strains [24].
Figure 3.
Comparison of the genetic context of tet(X4) with those of closely related sequences. The extent and direction of genes are shown by arrows labeled with gene names. Red arrows—resistance genes; blue arrows—mobile elements proteins; yellow arrows—other functional proteins.
Plasmids carrying tet(X4) from six strains were successfully transferred to E. coli C600, with transfer frequencies ranging from 10−6 to 10−4. This result indicated that the tet(X4) genes were located on a plasmid, and the hybrid−type replicons showed greater transfer compared with strain 22a22, which harbored IncX1−type replicons. Additionally, the horizontal dissemination of tet(X4) by conjugative plasmids or other mobilizable genetic elements existed in most chicken and pig farms in Hunan province, China, which may have accelerated the transmission of tet(X4).
In summary, this is the first time that tet(X4) has been detected in farms in these cities in 2021, in addition to the prevalence of tet(X4)−positive E. coli at a low percentage compared with other provinces in China. Furthermore, these tet(X4)−harboring strains carrying similar genetic structures with extremely similar resistance phenotypes, plasmid types, and genetic environments were seen over a wide area. In addition, these tet(X4)−positive E. coli also had very high similarity to tet(X4)−positive strains detected in pets, farm animals, and humans in other provinces, piqued our research interest. According to what we know, the breeders in each farm are generally fixed and have no contact with each other, and the medications used in the farms are different. We hypothesize that (1) this plasmid type and gene structure are more prevalent in and conducive to the process of drug resistance transmission, and (2) the environment acts as a major reservoir of drug resistance genes, with some of these potential transmission pathways facilitating the transmission of drug resistance genes. However, this experiment suffers from insufficient sample data and requires further investigation and research.
3. Materials and Methods
3.1. Sample Collection and tet(X) Detection
A total of 257 samples were obtained in 2021 from chicken fecal samples in Xiangtan City (n = 80) and pig fecal samples in Chenzhou City (n = 75), Xiangxiang City (n = 52), and Chaling City (n = 50). All samples were kept in an icebox and transported to the laboratory. Then, the samples were cultured on MacConkey agar (Land Bridge, Beijing, China) and incubated at 37 °C overnight. A single pink clone was randomly selected [25]. The tet(X4) gene was identified in all isolates by PCR assay and Sanger sequencing using the primes described previously in Table S3 [26]. Species identification was confirmed with the 16srRNA gene [27].
3.2. Antimicrobial Susceptibility Testing
The tet(X4)−positive isolates were subjected to antimicrobial susceptibility testing for 11 antimicrobial agents (tigecycline, ampicillin, amikacin, chloramphenicol, cefotaxime, nalidixic acid, florfenicol, colistin, meropenem, gentamicin, and trimethoprim–sulfamethoxazole) using the broth dilution method and interpreted according to the American Clinical and Laboratory Standards [28]. E. coli strain ATCC 25922 served as a quality control strain.
3.3. Whole−Genome Sequencing and Analysis
DNA was extracted from the tet(X4)−positive isolates using the TIANamp Bacteria DNA Kit DP302 (Tiangen Biotech, Beijing, China). The whole−genome sequence of strains was determined using Illumina HiSeq 2500 (Illumina, United States). The draft genome sequences of six tet(X4)−positive E. coli were assembled by Spades 3.14 [29]. The assembled genomes sequences were annotated using PATRIC3.6.9 (https://patricbrc.org/, accessed on 8 August 2022). The sequence types and plasmid replicon types were analyzed using the CGE server (https://cge.cbs.dtu.dk/services/), and the phylogenetic trees were generated using Parsnp (Harvest v1.1.2, https://github.com/marbl/parsnp) and visualized using iTOL (https://itol.embl.de). Ultimately, to visualize the comparative genetic features, Easyfig v2.2.3 was used to generate linear comparison figures (http://mjsull.github.io/Easyfig).
3.4. Conjugation Experiment
Transferability of the tet(X4) gene in the tet(X4)−positive strain was determined by conjugation experiment using E. coli C600 (streptomycin−resistant strain) as the recipient strain [25]. The donor and recipient strains were diluted to the 0.5 McFarland standard in Luria–Bertani (LB) broth; they were then mixed at a ratio of 1:3 and applied to a 0.22 μm filter, followed by coculture at 37 °C for 16 h. The transconjugants were screened on Mueller–Hinton agar plates containing 2 mg/L tigecycline and 1000 mg/L streptomycin. Subsequently, the transconjugants were confirmed by PCR with the primers in Table S3. The frequencies of conjugation transfer were calculated as a function of the number of transconjugants per recipient.
4. Conclusions
In conclusion, we isolated six tet(X4)−bearing strains from chicken and pork samples from various cities in Hunan Province, China. All tet(X4)−carrying strains exhibited high resistance to tigecycline and conferred resistance to multiple classes of antibiotics, which would bring about great difficulty in clinical treatment. Thus, we recommend using susceptible antibiotics to treat some bacterial infections in the investigated farms. Furthermore, the diversity of MLST types showed that tet(X4) genes have widespread sources in E. coli. Moreover, this study regarded the ISCR2−tet(X4)−RdmC−hp−IS1R structure as the dominant transmission potential pathway. Further attempts to reduce the risk of multidrug resistance transmission should focus on the mechanism mediated by mobile elements. In conclusion, the incidental transmission of multidrug resistance genes requires the rational use of antibiotics and improvements in strict daily management on the farm.
Acknowledgments
Thank you for the support and assistance provided by the Veterinary Medicine Engineering Center, College of Animal Medicine, Hunan Agricultural University.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12010147/s1, Table S1: The information of six tet(X4)-positive E. coli isolates; Table S2: The snps in six tet(X4)-positive strains.; Table S3: The prime that was used.
Author Contributions
Conceptualization, J.Y. and G.X.; methodology, N.X.; software, Z.J.; validation, C.J., Y.L. and W.C.; formal analysis, H.L.; investigation, J.Y.; resources, Y.L.; data curation, G.X.; writing—original draft preparation, J.Y.; writing—review and editing, J.L.; visualization, J.L.; supervision, Z.S.; project administration, Z.S.; funding acquisition, Z.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The whole genome sequence data of six E. coli strains have been submitted to NCBI under the BioProject accession number PRJNA898522.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Hunan Provincial Natural Science Foundation of China (2021JJ40234).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhang S., Wen J., Wang Y., Wang M., Jia R., Chen S., Liu M., Zhu D., Zhao X., Wu Y., et al. Dissemination and prevalence of plasmid−mediated high−level tigecycline resistance gene tet (X4) Front. Microbiol. 2022;13:969769. doi: 10.3389/fmicb.2022.969769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He T., Wang R., Liu D., Walsh T.R., Zhang R., Lv Y., Ke Y., Ji Q., Wei R., Liu Z., et al. Emergence of plasmid−mediated high−level tigecycline resistance genes in animals and humans. Nat. Microbiol. 2019;4:1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 3.Sun J., Chen C., Cui C.Y., Zhang Y., Liu X., Cui Z.H., Ma X.Y., Feng Y., Fang L.X., Lian X.L., et al. Plasmid−encoded tet(X) genes that confer high−level tigecycline resistance in Escherichia coli. Nat. Microbiol. 2019;4:1457–1464. doi: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L., Liu D., Lv Y., Cui L., Li Y., Li T., Song H., Hao Y., Shen J., Wang Y., et al. Novel Plasmid−Mediated tet(X5) Gene Conferring Resistance to Tigecycline, Eravacycline, and Omadacycline in a Clinical Acinetobacter baumannii Isolate. Antimicrob. Agents Chemother. 2019;64:e01326-19. doi: 10.1128/AAC.01326-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y.Y., Liu Y., Chen Y., Huang F.M., Chen R.C., Xiao Y.H., Zhou K. Sporadic Dissemination of tet(X3) and tet(X6) Mediated by Highly Diverse Plasmidomes among Livestock−Associated Acinetobacter. Microbiol. Spectr. 2021;9:e0114121. doi: 10.1128/Spectrum.01141-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soliman A.M., Ramadan H., Zarad H., Sugawara Y., Yu L., Sugai M., Shimamoto T., Hiott L.M., Frye J.G., Jackson C.R., et al. Coproduction of Tet(X7) Conferring High−Level Tigecycline Resistance, Fosfomycin FosA4, and Colistin Mcr−1.1 in Escherichia coli Strains from Chickens in Egypt. Antimicrob. Agents Chemother. 2021;65:e02084-20. doi: 10.1128/AAC.02084-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasparrini A.J., Markley J.L., Kumar H., Wang B., Fang L., Irum S., Symister C.T., Wallace M., Burnham C.D., Andleeb S., et al. Tetracycline−inactivating enzymes from environmental, human commensal, and pathogenic bacteria cause broad−spectrum tetracycline resistance. Commun. Biol. 2020;3:241. doi: 10.1038/s42003-020-0966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y., Chen Y., Liu Y., Guo Y., Zhou Y., Xiao T., Zhang S., Xu H., Chen Y., Shan T., et al. Identification of novel tetracycline resistance gene tet(X14) and its co−occurrence with tet(X2) in a tigecycline−resistant and colistin−resistant Empedobacter stercoris. Emerg. Microbes. Infect. 2020;9:1843–1852. doi: 10.1080/22221751.2020.1803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R., Peng K., Xiao X., Wang Y., Wang Z. Characterization of novel ISAba1−bounded tet(X15)−bearing composite transposon Tn6866 in Acinetobacter variabilis. J. Antimicrob. Chemother. 2021;76:2481–2483. doi: 10.1093/jac/dkab182. [DOI] [PubMed] [Google Scholar]
- 10.Ma J., Zhou W., Wu J., Liu X., Lin J., Ji X., Lin H., Wang J., Jiang H., Zhou Q., et al. Large−Scale Studies on Antimicrobial Resistance and Molecular Characterization of Escherichia coli from Food Animals in Developed Areas of Eastern China. Microbiol. Spectr. 2022;10:e0201522. doi: 10.1128/spectrum.02015-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W., Yang Z., Hu J., Wang B., Rong H., Li Z., Sun Y., Wang Y., Zhang X., Wang M., et al. Evaluation of culturable 'last−resort' antibiotic resistant pathogens in hospital wastewater and implications on the risks of nosocomial antimicrobial resistance prevalence. J. Hazard. Mater. 2022;438:129477. doi: 10.1016/j.jhazmat.2022.129477. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Y., Deng L., Zhou X., Zhang C., Hu Z., Chen Y., Zheng W. Prevalence and risk factors of tet(X4)−positive Enterobacteriaceae in human gut microbiota. J. Glob. Antimicrob. Resist. 2022;31:15–21. doi: 10.1016/j.jgar.2022.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Dong N., Zeng Y., Cai C., Sun C., Lu J., Liu C., Zhou H., Sun Q., Shu L., Wang H., et al. Prevalence, transmission, and molecular epidemiology of tet(X)−positive bacteria among humans, animals, and environmental niches in China: An epidemiological, and genomic−based study. Sci. Total Environ. 2022;818:151767. doi: 10.1016/j.scitotenv.2021.151767. [DOI] [PubMed] [Google Scholar]
- 14.Dao T.D., Kasuga I., Hirabayashi A., Nguyen D.T., Tran H.T., Vu H., Pham L.T.N., Vu T.M.H., Hasebe F., Nguyen H.T., et al. Emergence of mobile tigecycline resistance gene tet(X4)−harbouring Shewanella xiamenensis in a water environment. J. Glob. Antimicrob. Resist. 2022;28:140–142. doi: 10.1016/j.jgar.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Li R., Li Y., Peng K., Yin Y., Liu Y., He T., Bai L., Wang Z. Comprehensive Genomic Investigation of Tigecycline Resistance Gene tet(X4)−Bearing Strains Expanding among Different Settings. Microbiol. Spectr. 2021;9:e01633-21. doi: 10.1128/spectrum.01633-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D., Wang T., Shao D., Song H., Zhai W., Sun C., Zhang Y., Zhang M., Fu Y., Zhang R., et al. Structural diversity of the ISCR2−mediated rolling−cycle transferable unit carrying tet(X4) Sci. Total Environ. 2022;826:154010. doi: 10.1016/j.scitotenv.2022.154010. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Wang Q., Peng K., Liu Y., Xiao X., Mohsin M., Li R., Wang Z. Distribution and genomic characterization of tigecycline−resistant tet(X4)−positive Escherichia coli of swine farm origin. Microb. Genom. 2021;7:000667. doi: 10.1099/mgen.0.000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Lu M.J., Wang Z.Y., Jiang Y., Wu H., Pan Z.M., Jiao X. Tigecycline−resistant Escherichia coli ST761 carrying tet(X4) in a pig farm, China. Front. Microbiol. 2022;13:967313. doi: 10.3389/fmicb.2022.967313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhai W., Wang T., Yang D., Zhang Q., Liang X., Liu Z., Sun C., Wu C., Liu D., Wang Y. Clonal relationship of tet(X4)−positive Escherichia coli ST761 isolates between animals and humans. J. Antimicrob. Chemother. 2022;77:2153–2157. doi: 10.1093/jac/dkac175. [DOI] [PubMed] [Google Scholar]
- 20.Zhai W., Tian Y., Shao D., Zhang M., Li J., Song H., Sun C., Wang Y., Liu D., Zhang Y. Fecal Carriage of Escherichia coli Harboring the tet(X4)−IncX1 Plasmid from a Tertiary Class−A Hospital in Beijing, China. Antibiotics. 2022;11:1068. doi: 10.3390/antibiotics11081068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du P., Liu D., Song H., Zhang P., Li R., Fu Y., Liu X., Jia J., Li X., Fanning S., et al. Novel IS26−mediated hybrid plasmid harbouring tet(X4) in Escherichia coli. J. Glob. Antimicrob. Resist. 2020;21:162–168. doi: 10.1016/j.jgar.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z., Zhan Z., Shi C. International Spread of Tet(X4)−Producing Escherichia coli Isolates. Foods. 2022;11:2010. doi: 10.3390/foods11142010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Partridge S.R., Kwong S.M., Firth N., Jensen S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018;31:e00088-17. doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey J.K., Pinyon J.L., Anantham S., Hall R.M. Distribution of the blaTEM gene and blaTEM−containing transposons in commensal Escherichia coli. J. Antimicrob. Chemother. 2011;66:745–751. doi: 10.1093/jac/dkq529. [DOI] [PubMed] [Google Scholar]
- 25.Hu J., Yang J., Chen W., Liu Z., Zhao Q., Yang H., Sun Z., Chen X., Li J. Prevalence and Characteristics of mcr−1−Producing Escherichia coli in Three Kinds of Poultry in Changsha, China. Front. Microbiol. 2022;13:840520. doi: 10.3389/fmicb.2022.840520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun C., Cui M., Zhang S., Liu D., Fu B., Li Z., Bai R., Wang Y., Wang H., Song L., et al. Genomic epidemiology of animal−derived tigecycline−resistant Escherichia coli across China reveals recent endemic plasmid−encoded tet(X4) gene. Commun. Biol. 2020;3:412. doi: 10.1038/s42003-020-01148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava S., Singh V., Kumar V., Verma P.C., Srivastava R., Basu V., Gupta V., Rawat A.K. Identification of regulatory elements in 16S rRNA gene of Acinetobacter species isolated from water sample. Bioinformation. 2008;3:173–176. doi: 10.6026/97320630003173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI; Wayne, PA, USA: 2020. CLSI Supplement M100. [Google Scholar]
- 29.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: A new genome assembly algorithm and its applications to single−cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The whole genome sequence data of six E. coli strains have been submitted to NCBI under the BioProject accession number PRJNA898522.