Abstract
Extended spectrum beta lactamases producing Enterobacteriaceae are a major player in the antibiotic resistance challenge. In general, the situation regarding antibiotic resistance in Austria is very good compared to many other countries. Perhaps this is why there is a lack of data on the distribution of ESBL genes in the clinical setting. The aim of this study was to collect data on ESBL genes from a larger sample of human non-invasive clinical isolates from one region in Austria. In total, 468 isolates from different sample materials isolated at the Medical University of Graz from 2017 were examined. The most frequent organisms were Escherichia coli and Klebsiella pneumoniae. Among the enzymes produced, CTX-M-15 was clearly dominant, exotic ESBLs were only represented by three Proteus mirabilis isolates harboring genes for VEB-6 and one P. mirabilis for CTX-M-2, respectively. Compared to other countries, the results are in line with the expectations. The data help to better classify the many studies from the non-clinical field in Austria and to shift the focus slightly away from the exotic results and sample sites.
Keywords: Escherichia coli, Klebsiella spp., epidemiology, antimicrobial resistances, ESBL, infection, third generation cephalosporins
1. Introduction
Extended spectrum beta lactamases (ESBLs), especially found in Enterobacteriaceae, are one of the big players in the rise in antibiotic resistance in Gram-negative bacilli. ESBLs are roughly defined as enzymes that lead to resistance to penicillins (at least) to third and/or fourth generation cephalosporins and aztreonam, and can be inhibited by beta-lactamase inhibitors. However, there are also variants that are insensitive to beta-lactamase inhibitors [1,2,3,4]. These were (and still are) evolved as a reaction to the introduction of modern cephalosporins. The first ESBLs could be observed in the enzyme family TEM and SHV, caused by mutations of already known non-ESBL variants in the 1980s [3,5]. In the following decades, new enzyme families with ESBL phenotype members have occurred. Nowadays, the CTX-M family is dominant worldwide with CTX-M-15 being the most often detected ESBL enzyme. While TEM ESBL has mostly disappeared, SHV ESBLs (close to the CTX-M family) are still one of the most common ESBLs found [5]. However, there are now dozens of other beta lactamase families that consist entirely of ESBL members, most of which belong to Ambler class A (like GES, VEB, BEL). However, some enzymes of Ambler class D (OXA-2 and OXA-10 derivates) also belong to the ESBL group of enzymes [1,3,5].
The successful spread of ESBL enzymes is also due to the high gene exchange within the Enterobacteriaceae and related groups like the Pseudomonadales. Many genes coding for ESBLs are found on mobile genetic elements (such as plasmids) and can be expressed in different species and genera. Therefore, ESBL enzymes are present in nearly all clinically important members of the Enterobacteriaceae family, with Escherichia coli and members of the genus Klebsiella being the most common isolates [6,7,8].
Bacteria that produce ESBL are by no means only found in the clinical setting. There are a number of studies that show that ESBL Enterobacteriaceae are colonizing the healthy normal population almost all around the world. These can be found in farm animals, pets and wildlife, on food, and in various habitats in the environment [8,9,10,11,12,13].
The studies from non-clinical areas are so numerous that sometimes a better picture of the situation outside the clinical setting (apart from the pure resistance data) is available. This is certainly true for Austria, for example, for the enzyme family GES, there is only evidence from water samples thus far [14,15,16,17,18,19].
The aim of this study is to provide an up-to-date overview of the predominant ESBL producers, enzymes, and co- resistance from human non-invasive isolates collected during one year (2017) as well as to be able to provide an up-to-date basis for the current studies from the non-clinical area.
2. Results
A total of 392 (83.76%) of the 468 isolates (from 423 patients, please see material and methods for details on the exclusion procedure) included in the analysis were E. coli followed by 54 (11.54%) Klebsiella pneumoniae isolates, and eight (1.71%) Proteus mirabilis. Other species were isolated five times or less often (Table 1). Isolates originated from various body sites can be summarized in six categories: the majority of isolates were isolated from the genito-urinary system (GUS; 343/468, 73.3%), followed by feces (39 isolates, 8.3%), wounds (34 isolates, 7.3%), skin (29 isolates, 6.2%), the upper (URT; 15 isolates 3.2%) and lower (LRT; eight isolates, 1.7%) respiratory tract.
Table 1.
Overview of the body site origins of the isolates.
| Isolates/Body Site (%) | ||||||
|---|---|---|---|---|---|---|
| Species (n) | GUS | Feces | Wound | Skin | URT | LRT |
| E. coli (392) | 309 (78.8%) | 21 (5.4%) | 27 (6.9%) | 22 (5.6%) | 9 (2.3%) | 4 (1.02%) |
| K. pneumoniae (54) | 28 (51.9%) | 9 (16.4%) | 2 (3.7%) | 7 (13%) | 5 (9.26%) | 3 (5.56%) |
| P. mirabilis (8) | 3 (37.5%) | 1 (12.5%) | 3 (37.5%) | - | 1 (12.5%) | - |
| E. cloacae (5) | 1 (20%) | 2 (40%) | 1 (20%) | - | - | 1(20%) |
| K. oxytoca (5) | - | 5 (100%) | - | - | - | - |
| C. braakii (1) | 1 (100%) | - | - | - | - | - |
| C. freundii (1) | - | - | 1 (100%) | - | - | - |
| C. koseri (1) | 1 (100%) | - | - | - | - | - |
| Salmonella spp. * (1) | - | 1 (100%) | - | - | - | - |
| sum (468) | 343 (73.3%) | 39 (8.3%) | 34 (7.3%) | 29 (6.2%) | 15 (3.2%) | 8 (1.7%) |
GUS: genito-urinary system; URT: upper respiratory tract; LRT: lower respiratory tract; * no species identification with MALDI was possible.
All isolates were resistant to ampicillin (AM including the natural resistance of most isolated species) as well as to cefuroxime (CXM) and cefotaxime (CTX). Five isolates of E. cloacae, four E. coli, one K. pneumoniae, and one P. mirabilis (in total 1.3% of all isolates) showed resistance to meropenem (MEM), which is therefore the most effective drug available in in vitro testing (Table 2).
Table 2.
Percent of isolates included in the statistical analysis showing resistance to the tested antibiotics.
| AM | AMC | TZP | CXM | CTX | CAZ | FEP | MEM | GM | SXT | CIP | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | 100 | 63.5 | 27.6 | 100 | 100 | 91.6 | 91.3 | 1.02 | 21.7 | 59.7 | 81.1 |
| K. pneumoniae | 100 | 88.9 | 74.1 | 100 | 100 | 94 | 96.3 | 1.85 | 61 | 77.8 | 85.19 |
| P. mirabilis | 100 | 37.5 | 12.5 | 100 | 100 | 87.5 | 100 | 12.5 | 62.5 | 75 | 87.5 |
| E. cloacae | 100 | 100 | 20 | 100 | 100 | 100 | 100 | 100 | 80 | 80 | 80 |
| K. oxytoca | 100 | 80 | 0 | 100 | 100 | 20 | 100 | 0 | 100 | 80 | 0 |
| C. braakii | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 100 | 0 | 100 |
| C. freundii | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 0 | 0 |
| C. koseri | 100 | 0 | 0 | 100 | 100 | 100 | 100 | 0 | 0 | 100 | 0 |
| Salmonella spp. | 100 | 100 | 0 | 100 | 100 | 100 | 0 | 0 | 100 | 0 | 100 |
| sum | 100 | 66.7 | 32.5 | 100 | 100 | 91.2 | 91.9 | 1.28 | 28.9 | 62.2 | 80.6 |
AM: ampicillin; AMC: amoxicillin/clavulanic acid; TZP: piperacillin/tazobactam; CXM: cefuroxime; CTX: cefotaxime; CAZ: ceftazidime; FEP: cefepime; MEM: meropenem; GM: gentamicin; SXT: trimethoprim/sulfamethoxazole; CIP: ciprofloxacin.
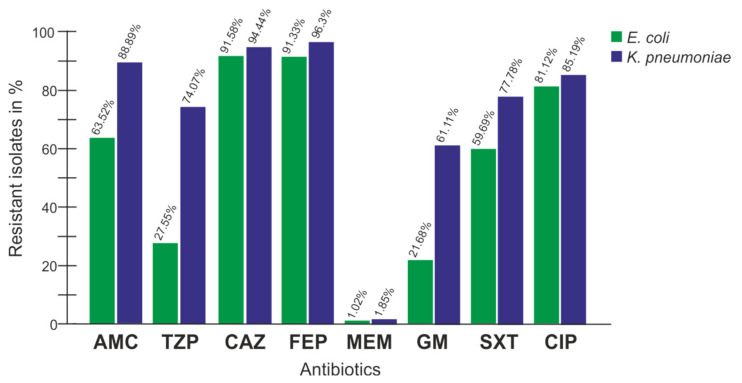
Highlighting the most important species E. coli and K. pneumoniae in comparison (Figure 1), the resistance patterns showed a high similarity with the exception of the ß-lactamase inhibitors and combinations (AMC, TZP) as well as GM. Generally, E. coli showed a lower percentage of resistance.
Figure 1.
Resistance profile comparison of E. coli (n = 392) and K. pneumoniae (n = 54). AMC: amoxicillin/clavulanic acid; TZP: piperacillin/tazobactam; CAZ: ceftazidime; FEP: cefepime; MEM: meropenem; GM: gentamicin; SXT: trimethoprim/sulfamethoxazole; CIP: ciprofloxacin.
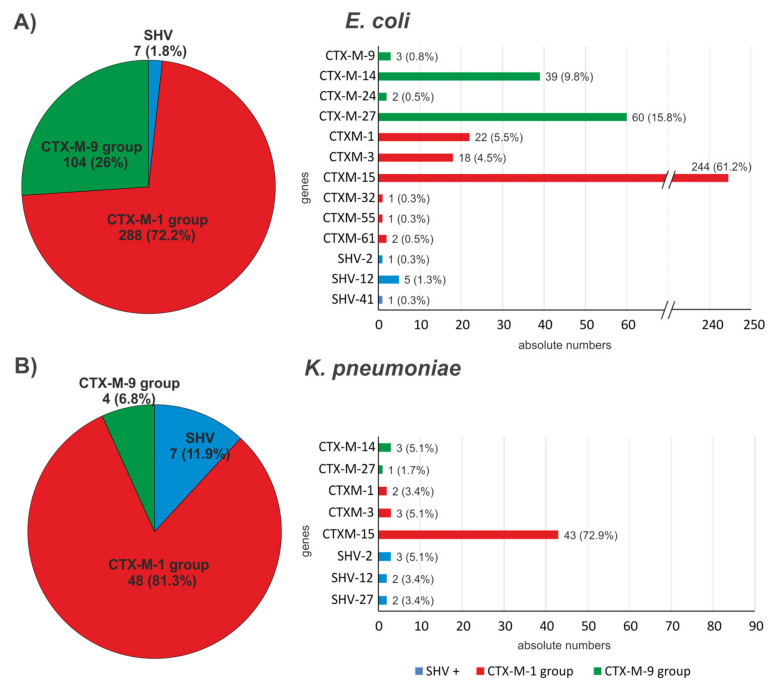
A total of 399 ESBL genes and 148 non-ESBL variants (blaTEM-1, blaSHV-1, blaSHV-11; Table S1) were detected in the 392 E. coli isolates. The most abundant ESBL genes for E. coli were members of the blaCTX-M-1 group (n = 288, 72.18%) (Figure 2). From the 54 K. pneumoniae isolates, 59 ESBL genes, and additionally, 87 non-ESBL variants (blaTEM-1, blaSHV-1, blaSHV-11, blaSHV-28, and blaSHV-76; Table S1) were detected. Forty-eight (81.36%) of the 59 ESBL genes belonged to the blaCTX-M-1 group. Not surprisingly, blaCTX-M-15 (a member of the CTX-M-1 group of genes) was proportionately most common in both species with 61.15% (n = 244) of the ESBL positive E. coli and 72.88% (n = 43) of the ESBL positive K. pneumoniae. ESBL blaSHV gene variants were more abundant in the K. pneumoniae isolates (11.86%) than in the E. coli isolates (1.75%). For the blaCTX-M-9 group, the proportional abundance was higher in E. coli (26.07%) than in K. pneumoniae (6.78%).
Figure 2.
Bla genes coding for ESBL phenotypes isolated from (A) E. coli and (B) K. pneumoniae in absolute numbers and in (%). Left groupwise comparison; right genewise comparison.
CTX-M-15 was also the most widespread enzyme among the other species. BlaVEB-6 was detected three times (all in P. mirabilis isolates) and only one blaCTX-M-2 also in one P. mirabilis isolate was detected, none of those were carrying another ESBL gene (Table S1). There were eight E. coli, four K. pneumoniae, and one E. cloacae, each carrying two different ESBL genes (Table 3).
Table 3.
ESBL coding genes detected and the number of isolates with those genes (n).
| Species | CTX-M-1 Group | CTX-M-2 Group | CTX-M-9 Group | SHV | VEB |
|---|---|---|---|---|---|
| E. coli * |
blaCTX-M-1 (22) blaCTX-M-3 (18) blaCTX-M-15 (244) blaCTX-M-32 (1) blaCTX-M-55 (1) blaCTX-M-61 (2) |
- |
blaCTX-M-9 (3) blaCTX-M-14 (39) blaCTX-M-24 (2) blaCTX-M-27 (60) |
blaSHV-2 (1) blaSHV-12 (5) blaSHV-41 (1) |
- |
| K. pneumoniae ** |
blaCTX-M-1 (2) blaCTX-M-3 (3) blaCTX-M-15 (43) |
- |
blaCTX-M-14 (3) blaCTX-M-27 (1) |
blaSHV-2 (3) blaSHV-12 (2) blaSHV-27 (2) |
- |
| P. mirabilis | blaCTX-M-15 (3) | blaCTX-M-2 (1) | blaCTX-M-27 (1) | - | blaVEB-6 (3) |
| E. cloacae *** | blaCTX-M-15 (4) | - | blaCTX-M-9 (1) | blaSHV-12 (1) | - |
| K. oxytoca | blaCTX-M-3 (5) | - | - | - | - |
| C. braakii | - | - | - | blaSHV-12 (1) | - |
| C. freundii | blaCTX-M-15 (1) | - | - | - | - |
| C. koseri | blaCTX-M-15 (1) | - | - | - | - |
| Salmonella spp. | - | - | - | blaSHV-12 (1) | - |
double positive isolates: * four with blaCTX-M-15 and blaCTX-M-14; two with blaCTX-M-15 and blaCTX-M-27; one with blaCTX-M-15 and blaCTX-M-9; one with blaSHV-41 and blaCTX-M-15. ** two with blaSHV-27 and blaCTX-M-15; one with blaSHV-12 and blaCTX-M-15; one with blaCTX-M-15 and blaCTX-M-14. ***one with blaSHV-12 and blaCTX-M-9.
Genes for non-ESBL enzyme TEM-1 were also carried by a large proportion of isolates (across all species), whereas wild-type SHV, apart from two E. coli isolates, was other-wise only found in K. pneumoniae (belongs to the chromosomal wild-type of this species) (Table S1).
3. Discussion
The presence of ESBL-producing strains was documented in Austria for the first time in the 1990s. After the turn of the millennium, many more studies followed that documented and analyzed the presence of ESBL-carrying bacteria in Austria. Most of them focused on isolates from various livestock and wild animals as well as isolates from the environment. ESBL-producing Enterobacteriaceae could be detected in a wide variety of animals, but also on food and different areas of the water cycle [16,19,20,21,22,23,24,25].
However, there are few studies on the distribution of the various ESBL enzymes in the clinical setting. In detail, there are no studies dealing with Enterobacteriaceae species from these types of isolates. Epidemiological studies with large numbers of isolates are also available, but these are unfortunately limited to the phenotype [16,21,22,26].
The aim of the work was to capture the totality of the different ESBL genes at the present time for Austria, to document the occurrence of exotic variants, and thereby provide a basis for comparison for other (non-clinical) studies. Therefore, the limitations of the study are also predefined. The aim of this study was testing a high number of isolates for ESBL genes, strain backgrounds (via the species description) and genetic localization were not.
Due to the high occurrence in infections in general, the high proportion of E. coli and Klebsiella in the ESBL isolates is not surprising. The situation regarding the prevalence of ESBL producers, with about 10.3% of E. coli isolates and 10.5% for Klebsiella pneumonia isolates in 2017, is relatively moderate in Styria compared to other regions [1,27,28,29]
The low number of Enterobacter and Citrobacter in the isolates is somewhat critical. Here, the selection by intrinsic AmpC could have led to a false designation as AmpC hyperproducers, even if an ESBL was the cause of the resistance. Furthermore, it needs to be considered that the prevalence of the ESBL of Enterobacter is also low in other parts of Austria [22,30]. The dominance of the CTX-M-1 group and the CTX-M-15 enzyme, in particular, was not surprising. A comparison with Eisner et al. is a good illustration of the development in Austria over the last 20 years, which is in line with the global situation. Around the turn of the millennium, Eisner et al. found that about one third of the genes in the ESBLs studied encoded for a member of the CTX-M family; now this figure is well over 90%. CTX-M enzymes are dominating the ESBL occurrence worldwide [31,32,33]. Exotic ESBL enzymes, on the other hand, were very rare in the current study. Although there have been reports of CTX-M-2 (group) or GES ESBL being found in Austria in the recent past, none of the isolates examined were found to harbor genes encoding GES enzymes, and only one P. mirabilis harbored a CTX-M-2.
Furthermore, three P. mirabilis isolates carried a gene for VEB-6. These isolates have already been described in a case report, and originate from only one patient. This work was primarily concerned with the carbapenemases. However, since ESBL genes were also present in the isolates, these isolates were also included in this study [19,34].
Looking at the co-resistance, as expected, ciprofloxacin is a non-beta lactam-antibiotic with the highest resistance rates. The co-occurrence of ESBL (especially CTX-M-15 mediated) and fluoroquinolone resistance is a well-known phenomenon [5,35,36]. Resistance to the last line antibiotic meropenem is below 2% in E. coli and Klebsiella spp. This approximately corresponds to what is found in neighboring countries [28,29,37].
In most cases, ESBL enzymes are sensitive to beta-lactamase inhibitors. However, a high percentage of the isolates examined are resistant to amoxicillin-clavulanic acid, and the proportion of isolates resistant to piperacillin/tazobactam is also in the mid-range for most species, with the high proportion for K. pneumonia being particularly worrying. However, this is most likely not caused by inhibitors of resistant varieties of ESBL; these isolates carry other mechanisms that are likely to give them this additional resistance [3,38,39].
4. Conclusions
The clinical ESBL picture in Austria, especially in the federal state of Styria, shows that it is primarily caused by E. coli and Klebsiella; mainly by the CTX-M-15 enzyme. With the help of the non-invasive isolates analyzed, a good picture can be presented that allows for a better classification of the many studies on ESBL from animals, from the environment, or from individual patients.
5. Materials and Methods
5.1. Collection of Isolates
Between 1 January 2017 and 31 December 2017, all presumptive ESBL Enterobacteriaceae first isolates (n = 1057) from non-invasive infections were collected at the Diagnostic and Research Center for Molecular BioMedicine of the Medical University of Graz and stored at −80 °C in cryotubes (Viabank™, MWE Medical Wire, Corsham, UK). Isolates from every other month (January, March, May, July, September, November), in total 593 isolates, were tested for their ESBL genotype [40]. Different ESBL harboring species from one patient were treated as separated first isolates. From those double (or multiple) isolates from one patient were excluded/included in the statistical analysis following this procedure: All isolates from the same patient belonged to the same species and showed the same antibiotic resistance profile were only listed once (independent if isolated from the same or different body sites). Different ESBL species from the same patient are listed as such. Isolates that did not show any genetic ESBL profile were also not included in the statistical analysis, eight isolates fell into this category. Species and resistance patterns of these isolates are given in Table S2. Following this procedure, a total of 468 different isolates (101 from hospitalized patients and 367 from not hospitalized patients) were finally included in the statistical analysis. These isolates stem from a total of 423 patients as 23 patients were listed twice (14 not hospitalized, five hospitalized, and four were listed once in both groups) three patients three times (all not hospitalized), and two patients (one not hospitalized and one hospitalized) had four infections with different ESBL isolates.
5.2. Antibiotic Susceptibility Test
The isolates were characterized for their resistance pattern to 11 antibiotics by agar diffusion susceptibility testing according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) with ampicillin 10 μg (AM), amoxicillin/clavulanic acid 20 μg/10 μg (AMC), piperacillin/tazobactam 100 μg/10 μg (TZP), cefuroxime 30 μg (CXM), cefotaxime 5 μg (CTX), ceftazidime 10 μg (CAZ), cefepime 30 μg (FEP), meropenem 10 μg (MEM), gentamicin 10 μg (GM), trimethoprim/sulfamethoxazole 1.25 μg/23.75 μg (SXT), and ciprofloxacin 5 μg (CIP) (all with BD BBLTM Sensi-DiscTM paper discs; BD, Sparks, MD, USA). E. coli 25299 was used as the reference strain. The inhibition zone diameters were interpreted according to EUCAST guidelines [41].
5.3. Strain Cultivation
The samples were stored at –80 °C in cryotubes (Viabank™, MWE Medical Wire) until analysis. For MALDI-TOF identification and DNA isolation, strains were recultivated on Luria-Bertani (LB) agar plates (10 g/L tryptone, 10 g/L NaCl, 5 g/L yeast extract, 15 g Agar; all Carl Roth, Karlsruhe, Germany). For each isolate, one beat from the corresponding cryotube was plated on one LB-plate and incubated at 37 °C for 16 h.
5.4. MALDI-TOF
The exact identification of the bacteria was carried out using MALDI-TOF microflexTM LT/SH (Bruker AXS GmbH, Billerica, MA, USA) at the Institute for Laboratory Diagnostics and Microbiology of the Klinikum Klagenfurt am Wörthersee, Austria. The matrix used was the IVD matrix HCCA-portioned from Bruker.
5.5. DNA Isolation
A distinct colony was stirred into 50 μL of nuclease-free water and heated for 10 min at 100 °C on the heating block. Subsequently, centrifugation was carried out at 21,200 g for 2 min. The supernatant was transferred to a fresh reaction tube and kept in the freezer at −20 °C until further analysis.
5.6. Determination of ESBL Genes
PCR detection and gene identification were performed for different β-lactamase gene families, blaCTX-M-1-group, blaCTX-M-2-group, blaCTX-M-9-group, blaGES, blaSHV, blaTEM, and blaVEB. PCR and sequencing procedures were performed as described previously and carried out for all isolates that showed an ESBL-positive phenotype [42,43,44,45,46,47].
Briefly, for PCR, the Taq * 5x Master Mix (NEB, Ipswich, MA, USA) was used and the PCRs employing the different primer sets (Table 4) were performed on a GeneTouch Thermal Cycler (Biozym, Oldendorf, Germany) initializing with 94 °C for 5 min, 35 cycles at 95 °C for 30 s, 52 °C for 45 s, and 72 °C for 60 s, and closing with a final incubation for 10 min at 72 °C.
Table 4.
Primers used for PCR.
| Gene Families | Primer | Sequence 5’ → 3’ | Reference |
|---|---|---|---|
| bla CTX-M-1-group | f | TCTTCCAGAATAAGGAATCCC | [42] |
| r | CCGTTTCCGCTATTACAAAC | ||
| bla CTX-M-2-group | f | ATGATGACTCAGAGCATTCG | [43] |
| r | TGGGTTACGATTTTCGCCGC | ||
| bla CTX-M-9-group | f | ATGGTGACAAAGAGAGTGCA | [44] |
| r | CCCTTCGGCGATGATTCTC | ||
| bla GES | f | ATGCGCTTCATTCACGC C | [45] |
| r | CTATTTGTCCGTGCTCAGG | ||
| bla SHV | f | TGGTTATGCGTTATATTCGCC | [46] |
| r | GGTTAGCGTTGCCAGTGCT | ||
| bla TEM | f | TCCGCTCATGAGACAATAACC | [42] |
| r | TTGGTCTGACAGTTACCAATGC | ||
| bla VEB | f | GATAGGAGTACAGACATATG | [47] |
| r | TTTATTCAAATAGTAATTCCACG |
bla: beta-lactamase; f: forward, r: reverse.
Gel electrophoresis was performed to identify positive PCR results, and positive PCR samples where purified using the Monarch PCR and DNA Cleanup Kit (NEB, Ipswich, MA, USA). The purified samples were sent for Sanger Sequencing (Eurofins, Luxemburg, Luxemburg or Genewiz, Leipzig, Germany). For this purpose, the corresponding reverse primer and the sample were pipetted into a 96-well PCR plate or in reaction tubes in the ratio requested by the respective company, sealed, and shipped.
Identification of the sequencing results was obtained using the BLAST (Basic Local Alignment Search Tool) program “Standard Nucleotide Blast” of the NCBI (National Center for Biotechnology Information, Bethesda, MD, USA).
5.7. Data Analysis
All data were analyzed using MS Excel (Office 2019) and the figures were prepared using MS Excel and CorelDraw X5 (Corel Corporation, Ottawa, ON, Canada, 2010, version 15.0.0.486).
Acknowledgments
We would like to thank the laboratory team of bacteriology at the Institute of Hygiene, Microbiology, and Environmental Medicine for the collection of the isolates. We thank Christian Petternel and the team at the Institute for Laboratory Diagnostics and Microbiology of the Klinikum Klagenfurt am Wörthersee, Austria, for the use of their MALDI-TOF system and their help in doing so. The authors would also like to thank the respective laboratory staff and colleagues for their support and the fruitful discussions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12010001/s1, Table S1: Abundance of non-ESBL phenotype bla genes.; Table S2: Isolates with the ESBL phenotype but no detected ESBL genes.
Author Contributions
Conceptualization, A.H.P.-F., G.F., and G.Z.; Methodology, A.H.P.-F., N.M., T.H., T.F., G.F., R.B., C.K., and G.Z.; Software, A.H.P.-F., N.M., T.H., T.F., G.F., R.B., C.K., and G.Z.; Validation, A.H.P.-F., G.F., and G.Z.; Formal analysis, N.M., T.H., T.F., and R.B.; Investigation, N.M., T.H., T.F., and R.B.; Resources, A.H.P.-F., C.K., and G.Z.; Data curation, N.M., T.H., T.F., and R.B.; Writing—original draft preparation, A.H.P.-F. and G.Z.; Writing—review and editing, A.H.P.-F., G.F., and G.Z.; Visualization, A.H.P.-F. and G.Z.; Supervision, A.H.P.-F., G.F., C.K., and G.Z.; Project administration, A.H.P.-F. and G.Z.; Funding acquisition, A.H.P.-F. and G.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be obtained from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Adler A., Katz D.E., Marchaim D. The Continuing Plague of Extended-Spectrum β-Lactamase Producing Enterbacterales Infections: An Update. Infect. Dis. Clin. N. Am. 2020;34:677–708. doi: 10.1016/j.idc.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Bush K., Jacoby G.A., Medeiros A.A. A Functional Classification Scheme for Beta-Lactamases and Its Correlation with Molecular Structure. Antimicrob. Agents Chemother. 1995;39:1211–1233. doi: 10.1128/AAC.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drawz S.M., Bonomo R.A. Three Decades of Beta-Lactamase Inhibitors. Clin. Microbiol. Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livermore D.M. Fourteen Years in Resistance. Int. J. Antimicrob. Agents. 2012;39:283–294. doi: 10.1016/j.ijantimicag.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Livermore D.M., Canton R., Gniadkowski M., Nordmann P., Rossolini G.M., Arlet G., Ayala J., Coque T.M., Kern-Zdanowicz I., Luzzaro F., et al. CTX-M: Changing the Face of ESBLs in Europe. J. Antimicrob. Chemother. 2006;59:165–174. doi: 10.1093/jac/dkl483. [DOI] [PubMed] [Google Scholar]
- 6.Carattoli A., Bertini A., Villa L., Falbo V., Hopkins K.L., Threlfall E.J. Identification of Plasmids by PCR-Based Replicon Typing. J. Microbiol. Methods. 2005;63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Woodford N., Carattoli A., Karisik E., Underwood A., Ellington M.J., Livermore D.M. Complete Nucleotide Sequences of Plasmids PEK204, PEK499, and PEK516, Encoding CTX-M Enzymes in Three Major Escherichia Coli Lineages from the United Kingdom, All Belonging to the International O25:H4-ST131 Clone. Antimicrob. Agents Chemother. 2009;53:4472–4482. doi: 10.1128/AAC.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carattoli A. Animal Reservoirs for Extended Spectrum Beta-Lactamase Producers. Clin. Microbiol. Infect. 2008;14((Suppl. S1)):117–123. doi: 10.1111/j.1469-0691.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Migura L., Hendriksen R.S., Fraile L., Aarestrup F.M. Antimicrobial Resistance of Zoonotic and Commensal Bacteria in Europe: The Missing Link between Consumption and Resistance in Veterinary Medicine. Vet. Microbiol. 2014;170:1–9. doi: 10.1016/j.vetmic.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Hasman H., Mevius D., Veldman K., Olesen I., Aarestrup F.M. β-Lactamases among Extended-Spectrum β-Lactamase (ESBL)-Resistant Salmonella from Poultry, Poultry Products and Human Patients in The Netherlands. J. Antimicrob. Chemother. 2005;56:115–121. doi: 10.1093/jac/dki190. [DOI] [PubMed] [Google Scholar]
- 11.Hooban B., Joyce A., Fitzhenry K., Chique C., Morris D. The Role of the Natural Aquatic Environment in the Dissemination of Extended Spectrum Beta-Lactamase and Carbapenemase Encoding Genes: A Scoping Review. Water Res. 2020;180:115880. doi: 10.1016/j.watres.2020.115880. [DOI] [PubMed] [Google Scholar]
- 12.McEwen S.A., Collignon P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018;6:10. doi: 10.1128/microbiolspec.ARBA-0009-2017. [DOI] [PubMed] [Google Scholar]
- 13.Ramos S., Silva V., Dapkevicius M..d.L.E., Caniça M., Tejedor-Junco M.T., Igrejas G., Poeta P. Escherichia Coli as Commensal and Pathogenic Bacteria Among Food-Producing Animals: Health Implications of Extended Spectrum β-Lactamase (ESBL) Production. Animals. 2020;10:E2239. doi: 10.3390/ani10122239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barišić I., Mitteregger D., Hirschl A.M., Noehammer C., Wiesinger-Mayr H. High Diversity of Beta-Lactamases in the General Hospital Vienna Verified by Whole Genome Sequencing and Statistical Analysis. Infect. Genet. Evol. 2014;27:408–417. doi: 10.1016/j.meegid.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Franiek N., Orth D., Grif K., Ewers C., Wieler L.H., Thalhammer J.G., Würzner R. ESBL-producing E. coli and EHEC in dogs and cats in the Tyrol as possible source of human infection. Berl. Munch. Tierarztl. Wochenschr. 2012;125:469–475. [PubMed] [Google Scholar]
- 16.Huemer H.P., Eigentler A., Aschbacher R., Larcher C. Dominance of CTX-M Group 1 Beta-Lactamase Enzymes in ESBL Producing E. Coli from Outpatient Urines in Neighboring Regions of Austria and Italy. Wien. Klin. Wochenschr. 2011;123:41–44. doi: 10.1007/s00508-010-1527-6. [DOI] [PubMed] [Google Scholar]
- 17.Prelog M., Fille M., Prodinger W., Grif K., Brunner A., Würzner R., Zimmerhackl L.B. CTX-M-1-Related Extended-Spectrum Beta-Lactamases Producing Escherichia Coli: So Far a Sporadic Event in Western Austria. Infection. 2008;36:362–367. doi: 10.1007/s15010-008-7309-7. [DOI] [PubMed] [Google Scholar]
- 18.Zarfel G., Galler H., Feierl G., Haas D., Kittinger C., Leitner E., Grisold A.J., Mascher F., Posch J., Pertschy B., et al. Comparison of Extended-Spectrum-β-Lactamase (ESBL) Carrying Escherichia Coli from Sewage Sludge and Human Urinary Tract Infection. Environ. Pollut. 2013;173:192–199. doi: 10.1016/j.envpol.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Zarfel G., Lipp M., Gürtl E., Folli B., Baumert R., Kittinger C. Troubled Water under the Bridge: Screening of River Mur Water Reveals Dominance of CTX-M Harboring Escherichia Coli and for the First Time an Environmental VIM-1 Producer in Austria. Sci. Total Environ. 2017;593–594:399–405. doi: 10.1016/j.scitotenv.2017.03.138. [DOI] [PubMed] [Google Scholar]
- 20.Bujdáková H., Klimáková J., Allerberger F., Moravcíková M., Bagová M., Hanzen J., Michálková-Papajová D., Dierich M.P., Kettner M. Spectrum and Transferability of Beta-Lactam Resistance in Hospital Strains of Enterobacter Isolated in Bratislava and Innsbruck. Int. J. Antimicrob. Agents. 2000;16:31–36. doi: 10.1016/S0924-8579(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 21.Eisner A., Fagan E.J., Feierl G., Kessler H.H., Marth E., Livermore D.M., Woodford N. Emergence of Enterobacteriaceae Isolates Producing CTX-M Extended-Spectrum Beta-Lactamase in Austria. Antimicrob. Agents Chemother. 2006;50:785–787. doi: 10.1128/AAC.50.2.785-787.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apfalter P., Assadian O., Daxböck F., Hirschl A.M., Rotter M.L., Makristathis A. Extended Double Disc Synergy Testing Reveals a Low Prevalence of Extended-Spectrum Beta-Lactamases in Enterobacter Spp. in Vienna, Austria. J. Antimicrob. Chemother. 2007;59:854–859. doi: 10.1093/jac/dkm060. [DOI] [PubMed] [Google Scholar]
- 23.Loncaric I., Stalder G.L., Mehinagic K., Rosengarten R., Hoelzl F., Knauer F., Walzer C. Comparison of ESBL--and AmpC Producing Enterobacteriaceae and Methicillin-Resistant Staphylococcus Aureus (MRSA) Isolated from Migratory and Resident Population of Rooks (Corvus Frugilegus) in Austria. PLoS ONE. 2013;8:e84048. doi: 10.1371/journal.pone.0084048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desvars-Larrive A., Ruppitsch W., Lepuschitz S., Szostak M.P., Spergser J., Feßler A.T., Schwarz S., Monecke S., Ehricht R., Walzer C., et al. Urban Brown Rats (Rattus Norvegicus) as Possible Source of Multidrug-Resistant Enterobacteriaceae and Meticillin-Resistant Staphylococcus Spp., Vienna, Austria, 2016 and 2017. Euro Surveill. 2019;24:1900149. doi: 10.2807/1560-7917.ES.2019.24.32.1900149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarfel G., Galler H., Luxner J., Petternel C., Reinthaler F.F., Haas D., Kittinger C., Grisold A.J., Pless P., Feierl G. Multiresistant Bacteria Isolated from Chicken Meat in Austria. Int. J. Environ. Res. Public Health. 2014;11:12582–12593. doi: 10.3390/ijerph111212582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberhart M., Grisold A., Lavorato M., Resch E., Trobisch A., Resch B. Extended-Spectrum Beta-Lactamase (ESBL) Producing Enterobacterales in Stool Surveillance Cultures of Preterm Infants Are No Risk Factor for Necrotizing Enterocolitis: A Retrospective Case-Control Study over 12 Years. Infection. 2020;48:853–860. doi: 10.1007/s15010-020-01453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bundesministeriums für Arbeit, Soziales, Gesundheit und Konsumentenschutz . Resistenzbericht Österreich AURES 2017. AURES Druckerei des Bundesministeriums für Arbeit, Soziales, Gesundheit und Konsumentenschutz; Wien, Austria: 2018. [Google Scholar]
- 28.Rohde A.M., Zweigner J., Wiese-Posselt M., Schwab F., Behnke M., Kola A., Obermann B., Knobloch J.K.-M., Feihl S., Querbach C., et al. Incidence of Infections Due to Third Generation Cephalosporin-Resistant Enterobacteriaceae—A Prospective Multicentre Cohort Study in Six German University Hospitals. Antimicrob. Resist. Infect. Control. 2018;7:159. doi: 10.1186/s13756-018-0452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fils P.E.L., Cholley P., Gbaguidi-Haore H., Hocquet D., Sauget M., Bertrand X. ESBL-Producing Klebsiella Pneumoniae in a University Hospital: Molecular Features, Diffusion of Epidemic Clones and Evaluation of Cross-Transmission. PLoS ONE. 2021;16:e0247875. doi: 10.1371/journal.pone.0247875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meini S., Tascini C., Cei M., Sozio E., Rossolini G.M. AmpC β-Lactamase-Producing Enterobacterales: What a Clinician Should Know. Infection. 2019;47:363–375. doi: 10.1007/s15010-019-01291-9. [DOI] [PubMed] [Google Scholar]
- 31.Fiolić Z., Bosnjak Z., Bedenić B., Budimir A., Mareković I., Cetkovic H., Kalenić S. Nationwide Survey of Klebsiella Pneumoniae Strains Producing CTX-M Extended-Spectrum β-Lactamases in Croatia. Coll. Antropol. 2015;39:947–951. [PubMed] [Google Scholar]
- 32.Castanheira M., Simner P.J., Bradford P.A. Extended-Spectrum β-Lactamases: An Update on Their Characteristics, Epidemiology and Detection. JAC Antimicrob. Resist. 2021;3:dlab092. doi: 10.1093/jacamr/dlab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giufrè M., Mazzolini E., Cerquetti M., Brusaferro S. CCM2015 One-Health ESBL-producing Escherichia coli Study Group Extended-Spectrum β-Lactamase-Producing Escherichia Coli from Extraintestinal Infections in Humans and from Food-Producing Animals in Italy: A “One Health” Study. Int. J. Antimicrob. Agents. 2021;58:106433. doi: 10.1016/j.ijantimicag.2021.106433. [DOI] [PubMed] [Google Scholar]
- 34.Valentin T., Feierl G., Masoud-Landgraf L., Kohek P., Luxner J., Zarfel G. Proteus Mirabilis Harboring Carbapenemase NDM-5 and ESBL VEB-6 Detected in Austria. Diagn. Microbiol. Infect. Dis. 2018;91:284–286. doi: 10.1016/j.diagmicrobio.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Nicolas-Chanoine M.-H., Bertrand X., Madec J.-Y. Escherichia Coli ST131, an Intriguing Clonal Group. Clin. Microbiol. Rev. 2014;27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzariol A., Bazaj A., Cornaglia G. Multi-Drug-Resistant Gram-Negative Bacteria Causing Urinary Tract Infections: A Review. J. Chemother. 2017;29:2–9. doi: 10.1080/1120009X.2017.1380395. [DOI] [PubMed] [Google Scholar]
- 37.Mancuso G., Midiri A., Zummo S., Gerace E., Scappatura G., Biondo C. Extended-Spectrum β-Lactamase & Carbapenemase-Producing Fermentative Gram-Negative Bacilli in Clinical Isolates from a University Hospital in Southern Italy. New Microbiol. 2021;44:227–233. [PubMed] [Google Scholar]
- 38.Bush K. Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 2018;62:e01076-18. doi: 10.1128/AAC.01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chong Y., Shimoda S., Shimono N. Current Epidemiology, Genetic Evolution and Clinical Impact of Extended-Spectrum β-Lactamase-Producing Escherichia Coli and Klebsiella Pneumoniae. Infect. Genet. Evol. 2018;61:185–188. doi: 10.1016/j.meegid.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Poulou A., Grivakou E., Vrioni G., Koumaki V., Pittaras T., Pournaras S., Tsakris A. Modified CLSI Extended-Spectrum β-Lactamase (ESBL) Confirmatory Test for Phenotypic Detection of ESBLs among Enterobacteriaceae Producing Various β-Lactamases. J. Clin. Microbiol. 2014;52:1483–1489. doi: 10.1128/JCM.03361-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 7.1. 2017. [(accessed on 31 October 2022)]. Available online: http://www.eucast.org.
- 42.Stürenburg E., Kühn A., Mack D., Laufs R. A Novel Extended-Spectrum Beta-Lactamase CTX-M-23 with a P167T Substitution in the Active-Site Omega Loop Associated with Ceftazidime Resistance. J. Antimicrob. Chemother. 2004;54:406–409. doi: 10.1093/jac/dkh334. [DOI] [PubMed] [Google Scholar]
- 43.Asai T., Masani K., Sato C., Hiki M., Usui M., Baba K., Ozawa M., Harada K., Aoki H., Sawada T. Phylogenetic Groups and Cephalosporin Resistance Genes of Escherichia Coli from Diseased Food-Producing Animals in Japan. Acta Vet. Scand. 2011;53:52. doi: 10.1186/1751-0147-53-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnamurthy V., Vijaykumar G.S., Sudeepa Kumar M., Prashanth H.V., Prakash R., Nagaraj E.R. Phenotypic and Genotypic Methods for Detection of Extended Spectrum β Lactamase Producing Escherichia Coli and Klebsiella Pneumoniae Isolated from Ventilator Associated Pneumonia. J. Clin. Diagn. Res. 2013;7:1975–1978. doi: 10.7860/JCDR/2013/6544.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vourli S., Giakkoupi P., Miriagou V., Tzelepi E., Vatopoulos A.C., Tzouvelekis L.S. Novel GES/IBC Extended-Spectrum Beta-Lactamase Variants with Carbapenemase Activity in Clinical Enterobacteria. FEMS Microbiol. Lett. 2004;234:209–213. doi: 10.1016/j.femsle.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 46.Naas T., Poirel L., Karim A., Nordmann P. Molecular Characterization of In50, a Class 1 Integron Encoding the Gene for the Extended-Spectrum Beta-Lactamase VEB-1 in Pseudomonas Aeruginosa. FEMS Microbiol. Lett. 1999;176:411–419. doi: 10.1111/j.1574-6968.1999.tb13691.x. [DOI] [PubMed] [Google Scholar]
- 47.Pasterán F., Rapoport M., Petroni A., Faccone D., Corso A., Galas M., Vázquez M., Procopio A., Tokumoto M., Cagnoni V. Emergence of PER-2 and VEB-1a in Acinetobacter Baumannii Strains in the Americas. Antimicrob. Agents Chemother. 2006;50:3222–3224. doi: 10.1128/AAC.00284-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data can be obtained from the corresponding author upon reasonable request.