Abstract
Bacteria can form biofilms in natural and clinical environments on both biotic and abiotic surfaces. The bacterial aggregates embedded in biofilms are formed by their own produced extracellular matrix. Staphylococcus aureus (S. aureus) is one of the most common pathogens of biofilm infections. The formation of biofilm can protect bacteria from being attacked by the host immune system and antibiotics and thus bacteria can be persistent against external challenges. Therefore, clinical treatments for biofilm infections are currently encountering difficulty. To address this critical challenge, a new and effective treatment method needs to be developed. A comprehensive understanding of bacterial biofilm formation and regulation mechanisms may provide meaningful insights against antibiotic resistance due to bacterial biofilms. In this review, we discuss an overview of S. aureus biofilms including the formation process, structural and functional properties of biofilm matrix, and the mechanism regulating biofilm formation.
Keywords: Staphylococcus aureus, biofilms, extracellular matrix, quorum sensing, antibiofilm, antibiotic resistance
1. Introduction
Biofilm is an organized bacterial population and refers to the membrane-like extracellular matrix (ECM) formed by the adhesion of bacterial colonies and extracellular polymeric substances (EPS) such as polysaccharides, nucleic acids, and proteins secreted by bacteria during the growth process [1]. The interaction between EPS and bacterial aggregates endows biofilm with cohesion and viscoelasticity [2]. As a result, bacteria can attach to both biotic and abiotic surfaces. The formation of pathogenic biofilm plays an important role in causing chronic persistent infection [3]. Currently, researchers generally believe that more than 80% of chronic infections are mediated by bacterial biofilms [4]. Staphylococcus aureus (S. aureus) is prevalent in hospital environments. It attaches to and persists on host tissues and indwelling medical devices. This may cause skin and soft tissue infection, osteomyelitis, endocarditis, pneumonia, bacteremia, etc. [5,6,7,8]. These infections are difficult to cure due to the biofilm formed that enhances the resistance of S. aureus to antibiotics [9]. Additionally, biofilm formation is considered to be a protected growth mode for bacteria to adapt to harsh environments [10]. The biofilm acts as a barrier to create a stable internal environment for bacterial cell activity and protects bacterial cells from adverse conditions including extreme temperature, nutritional restriction and dehydration, and even antibacterial drugs [11]. Consequently, bacteria can settle quickly and protect themselves from host defense mechanisms and then promote long-term infection by enhancing adhesion to the host surface. Biofilm is therefore the first self-protection line of bacteria. It has been known that biofilm-forming bacteria are resistant to most antibiotics [12]. Most clinical antibiotics are developed targeting planktonic microbial cells. Antibiotics targeting planktonic cells may exert selective pressure on microorganisms, thus giving them a survival advantage over susceptible competitors [13]. Therefore, antibiotic therapy against biofilm usually requires long-term use antibiotics at high doses [14]. However, chronic treatment with such antibiotics may lead to increase the risk of antibiotic resistance and drug toxicity [15]. Due to the highly complex and rapid adaptability of biofilm population [16], an in-depth understanding of biofilm formation mechanism may provide new insights for the development of effective infection control strategy against biofilms [17,18].
2. Biofilm Formation Process
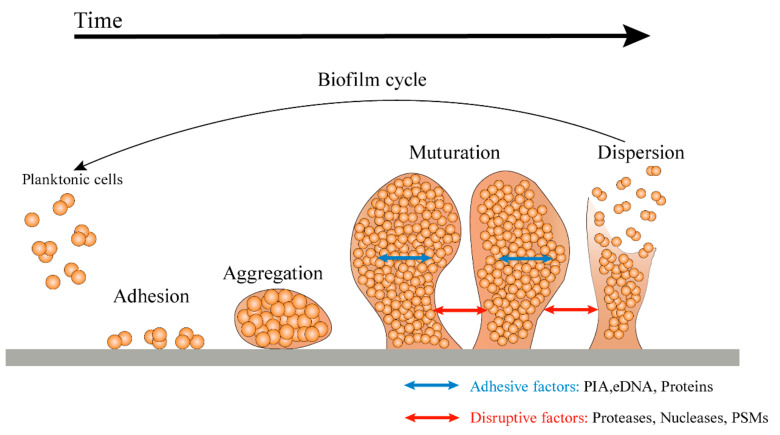
The formation of three-dimensional biofilm by bacteria is a complex process. It is generally divided into four stages: adhesion, aggregation, maturation, and dispersion (Figure 1) [19].
During the adhesion stage, S. aureus planktonic cells use a range of different factors and relevant regulatory mechanisms, such as the expression of cell wall-anchored protein (CWP), adhesin, and eDNA, to promote the combination with the host [20]. The most characteristic of these regulations is the organization of microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) that mainly include fibronectin-binding proteins (FnBPs, including FnbA and FnbB) [21], fibrinogen-binding protein (Fib), clumping factors (ClfA, ClfB) [22], and serine-aspartate repeat family proteins (SdrC, SdrD, and SdrE) [23]. These components mediate bacterial adhesion to natural tissues and biomaterial surfaces. In addition, bacterial appendages, such as flagella, cilia, and pili, allow for more permanent adherence of bacteria to surfaces [24].
After adhering to surfaces, the adherent bacterial cells begin to divide and accumulate in the presence of a sufficient nutrient source [19]. During the aggregation stage, bacteria regulate biofilm formation by sensing environmental signals that trigger regulatory networks and intracellular signal molecules. Then, bacteria continue to proliferate and thicken to form a biofilm [25]. The biofilm formed can provide resistance against the human immune system and antibiotics [26]. Bacterial cells proliferating in the matrix may lose direct contact with the graft surface and host protein and mainly depend on cell–cell and cell–EPS adhesion [27].
During the maturation stage, the structure of biofilm is highly structured and a compact three-dimensional mushroom or tower structure is formed [28]. A large number of pipes around the microcolony is constructed to promote the transport of nutrients to the deep layer of the biofilm [29]. Mature biofilms have diverse and unique metabolic structures that enable them to resist harmful environmental factors and stress drivers [30].
EPS can be attached by many single bacterial cells to form microcolonies, which are the basic units of biofilm structures [31]. Once microcolonies are formed, the bacterial biofilm continues to thicken and disperse the biofilm under the influence of specific genetic regulations or external factors. The process of dispersion may involve multiple steps, including the production of exoenzymes and surfactants to degrade EPS [32] and physiological changes that prepare cells for conditions outside the biofilm [33]. The dispersed bacteria form planktonic bacteria, which in turn can colonize other sites and form biofilm under certain conditions, thus forming a cyclic process.
The dispersion step is the final stage of the biofilm life cycle and the beginning of another life cycle [34]. During the growth and development of biofilm, surfactant phenol-soluble modulins (PSMs) are a key effector molecule for the dispersion and transmission of S. aureus biofilms [35]. PSMs are characterized by amphiphilicity α-helical secondary structure, which gives them surfactant-like properties [36]. These properties can destroy the non-covalent force in the biofilm matrix, forming the necessary channels to transport nutrients to the deeper layer of the biofilm. It also provides the necessary destructive force to spread the biofilm masses to the distal position [37]. PSMs of S. aureus not only exist in soluble form, but also aggregate into amyloid fibers to eliminate the biofilm-degrading activity of monomeric PSMs peptides and stabilize the biofilm structure [38]. In S. aureus, α-PSM1 and α-PSM4 peptides are the main amyloid proteins involved in the α-PSMs fibril production [39].
Figure 1.
A model of the growth cycle of biofilm. In the first step of biofilm formation, planktonic cells attach to the surface via surface-associated proteins. After attachment, the cells gradually aggregate and begin to produce ECM, thus forming microcolonies. With cell division, a mature biofilm is gradually formed. Finally, in the separation stage, enzymes such as protease, nuclease, and a quorum sensing system promote the dispersion of biofilm, allowing the bacterial cells to detach from the biofilm and return to a planktonic state to colonize new ecological niches [40].
3. Biofilm Formation Mechanism
3.1. Polysaccharide Intercellular Adhesion(PIA)-Dependent Mechanism
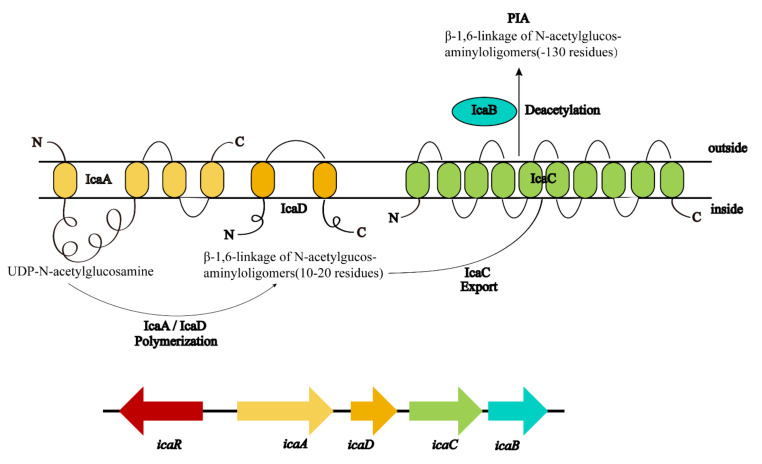
Among the polymeric molecules involved in ECM, polysaccharide intercellular adhesion (PIA) (also known as poly-N-acetylglucosamine; PNAG) is an important factor in S. aureus biofilm formation [41]. PIA has a cationic property (Figure 2) and plays an important role in the adhesion and aggregation stage [42]. In mutual strains lacking PIA, the ability of bacterial cells adhering to each other is decreased significantly. In S. aureus, the mechanism of biofilm formation is controlled by the production of PIA through proteins encoded by icaADBC operon in the ica locus (Figure 3) [43]. In this mechanism, icaA and icaD genes are essential in the regulation of biofilm formation. The product of the icaA gene is N-acetylamino-glucosamine transferase which is a transmembrane protein [44]. The product of the icaD gene is the chaperone protein of icaA. It maintains the correct folding of icaA and increases the specificity of icaA to polymers [45]. The product of the icaC gene is a transmembrane protein that mediates the transfer of the newly synthesized PIA to the cell surface [46]. The product of the icaB gene is a deacetylase responsible for the deacetylation of mature PIA. This deacetylation gives the polymer a net positive charge, which is required for adhesion onto the cell surface and intercellular adhesion [47]. The maximum length of poly N-acetylglucosamine oligomer produced by icaAD is 20 residues. Longer oligomer chains are synthesized only when icaAD is co-expressed with icaC. PIA also increases biofilm retention and its resistance to antimicrobial peptides (AMPs) through deacetylation [48]. Non-deacetylated polyglucosamine in the homogenous icaB mutant cannot adhere to the surface of bacterial cells or mediate the biofilm formation [49]. The icaADBC-mediated polysaccharide production is an important mechanism for biofilm formation and contributes to the early growth of bacteria. Additionally, it is believed that the ica operon is under phase variation, which has a role in slipped strand mispairing and leads to an on/off switch for expressing the products [50].
Figure 2.
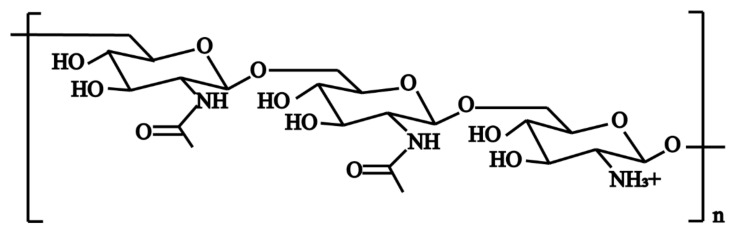
The structure of polysaccharide intercellular adhesin (PIA). PIA is a beta-linear homoglycan composed of 1,6-linked N-acetylglucosamine residues, 15–20% of which are deacetylated and therefore positively charged [41].
Figure 3.
Genetic encoding and biosynthesis of PIA. PIA is synthesized from the product of icaADBC operon. IcaA and IcaD are two membrane proteins that polymerize N-acetylglucosamine from the activated precursor UDP-N-acetylglucosamine monomers. This chain may be exported by the membrane protein IcaC. IcaB is an acetylase attached to the outer surface of bacteria. By deacetylation of residues, IcaB introduces a positive charge into the originally neutral PIA molecule [41].
3.2. Protein-Dependent Mechanisms
Some studies reveal that the strains without ica operon can also form biofilm [51,52]. Among the strains forming biofilm, the mutation of the ica gene does not affect the formation of biofilm. When treated with proteases, the biofilm of these strains can be depolymerized [51]. This indicates that there are proteins involved in biofilm formation through other mechanisms independent of the ica operon.
The bap gene of S. aureus encodes a surface protein Bap (biofilm-associated protein) containing 2276 amino acids. Bap was identified as the main determinant of successful surface adhesion and intercellular adhesion during biofilm formation. It promotes the initial adhesion of bacteria to biological and abiotic surfaces and intercellular adhesion through an extracellular polysaccharides-independent mechanism [52]. All S. aureus strains carrying the bap gene have high adhesion and strong biofilm-forming ability, indicating that there is a strong correlation between the existence of this protein and the biofilm-forming ability onto an abiotic surface. The N-terminal region of Bap is released into ECM and assembled into amyloid fibers to help the construction of S. aureus biofilms [53]. The Bap core domain contains C repeats, which are predicted to fold into new structures and participate in cell adhesion. The Bap C-terminal contains a typical cell wall attachment region [54]. During infection, Bap promotes persistence in the mammary gland by enhancing adhesion to epithelial cells and binds to host receptors to prevent cellular internalization, thereby interfering with the FnBPs-mediated invasion pathway [53].
Fibronectin-binding proteins are multi-structural domain glycoproteins (440 kDa), which are found in almost all tissues and organs and biological fluids and play an important role in cell adhesion and migration [55]. The N-terminal A structure of fibronectin-binding proteins A (FnbA) is structurally and functionally related to cohesion factor ClfA and Staphylococcus epidermidis SdrG protein. FnbA binds to fibrinogen at the N2 and N3 ends of the A structural domain through changes in the DLL (dock, lock, and latch) mechanism to form a highly stable complex [56], promotes the accumulation phase and initial adhesion phase of the biofilm, and increases bacterial aggregation [27].
The attachment of clumping factors promotes colonization of S. aureus in the host, facilitates biofilm formation, and causes virulence by binding soluble fibrinogen for immune escape [57]. However, even in the absence of fibrinogen, the biofilm of some strains is dependent on increased ClfB activity in the absence of calcium. ClfB accumulates on the bacterial surface and mediates biofilm formation [58].
S. aureus surface protein G (SasG) causes intercellular adhesion and promotes biofilm formation through zinc-dependent dimerization [59]. The fibrillar nature of SasG can mask the binding of S. aureus MSCRAMM to their ligands and also promote biofilm formation [60]. S. aureus surface protein C (SasC) mediates cell cluster formation, intercellular adhesion, and biofilm formation, but SasC does not mediate the interaction with fibrinogen or fibronectin [61].
The carboxyl terminus of serine–aspartate repeat family proteins contains motifs required for cell-wall anchoring. SdrC mediates strong cellular interactions with hydrophobic surfaces, which may be related to the initial attachment of biological materials, the first stage of biofilm formation [62], while SdrC binds with low-affinity homophilic bonds and promotes cell adhesion as well as biofilm formation [62]. SdrD is an important key factor in the ability of S. aureus to survive and evade the blood’s intrinsic immune system. SdrD promotes S. aureus adhesion to exfoliated nasal epithelial cells [63] and human keratin-forming cells in vitro [64]. It also promotes abscess formation in vivo [65]. SdrE traps the C-terminal tail of complement factor H (CFH) by a unique mechanism and isolates CFH on the surface of S. aureus to evade complement [66].
The collagen-binding adhesin (CAN) was originally reported to be necessary and sufficient for the binding of S. aureus to collagen-rich stromal cartilage [67]. CAN is a virulence factor in several animal models of infectious diseases. It also functions as an adhesin [68,69]. CAN is also a potential complement inhibitor that disrupts the molecular mechanism of complement activation and represents a potential immune evasion strategy that is associated with the development of multiple diseases [70].
3.3. Extracellular DNA (eDNA)-Dependent Mechanism
The mature S. aureus biofilm is sensitive to the external addition of DNase I, indicating that eDNA is a structural component of the biofilm matrix [71]. Due to the negative charge of DNA polymer, eDNA may participate in the early adhesion stage and mature stage of biofilms as an electrostatic polymer and play a basic structural function in the structural integrity of biofilms [72]. Its mechanism is to connect PIA and biofilm-related proteins and other biofilm components to stabilize the biofilm structure [73,74]. At the same time, eDNA also introduces favorable acid-base interactions to enhance adhesion and surface aggregation [75]. The accumulation of eDNA in the biofilm and infection site can acidify the local environment and promote antibiotic resistance phenotype [76]. It was found that eDNA also mediated horizontal gene transfer though conjugation of plasmids between cells in biofilms [77] and neutralized the important effector molecules of innate immunity such as AMPs by binding and isolating cations from the surrounding environment [78]. In Staphylococcus epidermidis, eDNA has also been found to be thermodynamically favorable interacting with positively charged vancomycin. It can reduce the potency of vancomycin, inhibit the transport of vancomycin in the biofilm, and thus protect the bacteria embedded in the biofilms [79]. eDNA is released through cell death and lysis and it is mainly regulated by the cidA gene. cidA encodes a responser of cell wall hydrolase activity and regulates cell death. cidA mediated cell lysis contributes to S. aureus biofilm formation in vivo and in vitro [80]. The inhibition of cidA activity by lgrAB operon can inhibit cell lysis and adhesion in the process of biofilm formation. Mutation of lgrAB operon leads to the formation of more adherent biofilms and higher eDNA contents [81]. Although these are the main components of the biofilm matrix, the exact composition of the biofilm matrix may vary and depends on matrix availability and physical factors.
4. Regulation Mechanism of Biofilm Formation
Biofilm formation is a social group behavior. Each of the steps from initial attachment to the dispersion and transmission of mature biofilm is strictly controlled by multiple regulatory systems or regulators [82].
4.1. Regulation of Quorum-Sensing System-Mediated Biofilm Formation
Intercellular signal transduction, commonly known as quorum sensing, is an internal communication system of bacteria. Bacteria detect the changes in the number of individual bacterial cells or other bacterial populations in the surrounding environment based on the changes in the concentration of a specific signal autoinducer. When the signal molecule reaches a certain threshold, the expression of relevant genes in bacteria is initiated to adapt to the changes in the environment [83]. The quorum sensing system usually involves signal transduction pathways that regulate biofilm formation, virulence, binding, antibiotic resistance, motility, and sporulation [84]. The quorum sensing system of S. aureus includes an accessory regulatory factor (Agr) system and LuxS/AI-2 system [85]. These two systems reduce biofilm formation in two different ways. The Agr system dissociates bacterial biofilm by upregulating the transcription of RNAIII, while the LuxS/AI-2 system reduces the expression of PIA.
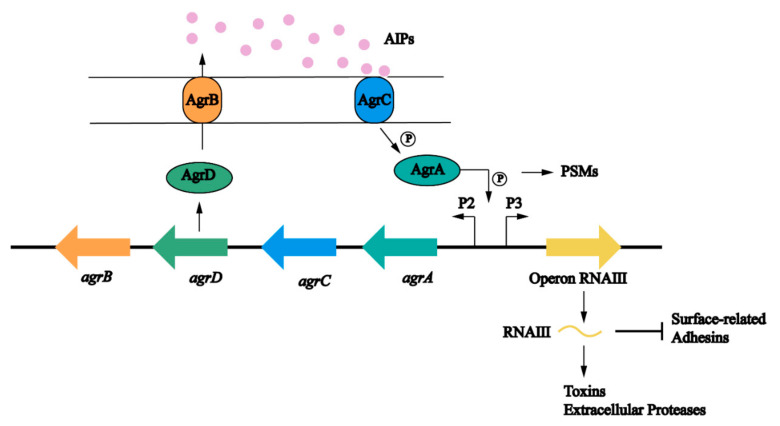
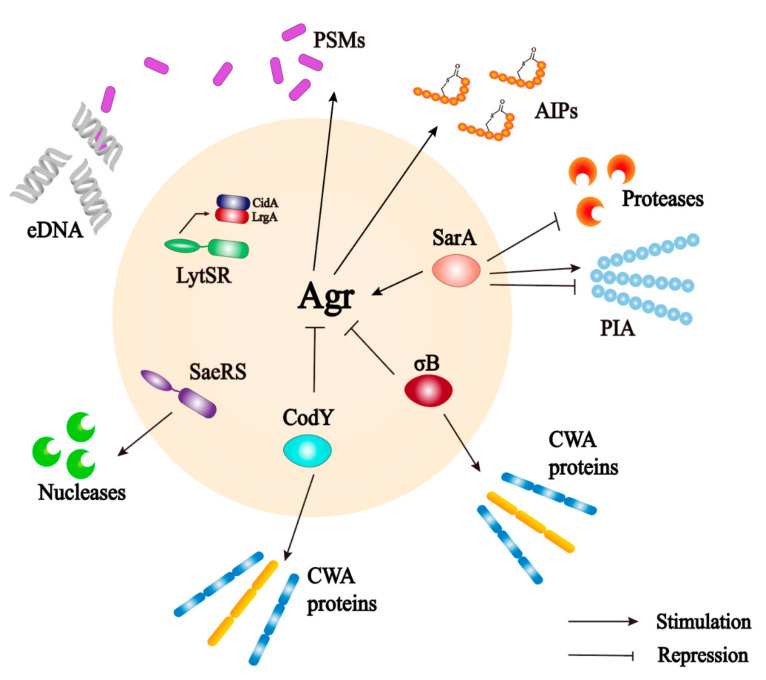
The agr locus is a complex polygenic system (Figure 4). It responds to bacterial cell density and controls the expression of S. aureus adhesion and extracellular protein. The regulation of the Agr system on biofilm is multifaceted and is mainly involved in the adhesion, maturation, and dispersion stages [40]. The Agr system uses an autoinducing peptide (AIP) as the signal molecule of cell density [86]. The agr locus encodes a two-component quorum sensing system consisting of two relatively independent transcription units driven by P2 and P3 promoters [87]. The P2 promoter starts the transcription of RNAII. The RNAII transcript harbors four open reading frames including agrA, agrB, agrC, and agrD, which encode the proteins required for AIP biosynthesis, transport, signal sensing, and regulation of target genes [88]. AgrC is a signal transduction factor with sensor histidine protein kinase activity; AgrA is a response regulator; AgrD is a precursor of the AIP; and AgrB is a multifunctional endopeptidase and chaperone protein. On the one hand, AgrB participates in the processing of AgrD as a protease to make it a mature AIP, but on the other hand, it acts as an oligopeptide transporter that helps secrete mature AIP out of cells [89]. The P3 promoter initiates the transcription of RNAIII. When the concentration of AIP in the environment reaches a certain threshold, it binds to and activates the histidine kinase AgrC, which leads to autophosphorylation and initials the signal transduction process [90]. AgrC phosphorylates AgrA after activation, which in turn induces the P3 promoter to transcriptionally express RNAIII; the main effector molecule of quorum sensing system [88]. RNAIII positively regulates the expression of toxin genes, prevents the translation of the repressor of toxin (Rot) [91], and reduces the expression of several surface adhesins, which are negatively associated with biofilm formation [92]. At the same time, an increased level of the AIP can promote the depolymerization of S. aureus biofilms by increasing the secretion of extracellular protease [85,93]. Different from other targets, the production of PSMs is generated by the direct binding of AgrA to its promoter [94,95]. Upregulation of agr leads to an increase in PSMs and promotes maturation and spreading of biofilm. In addition, some nutrients can affect the biofilm formation through the Agr system. Studies have shown that glucose strongly inhibits the expression of the P3 promoter. In established biofilms, glucose consumption activates the Agr system and leads to biofilm diffusion [93]. In S. aureus, some regulatory systems are interconnected with the Agr system that regulates the response to changes in environmental conditions and the development of biofilms (Figure 5) [20].
Figure 4.
The role of the Agr quorum sensing system in biofilm formation in S. aureus. The Agr system is controlled by agr operon. AgrD is the precursor of self-induced peptides (AIPS), which is modified by AgrB and secreted into the extracellular matrix. AIPs are intracellular signal molecules that respond to cell density. When the bacterial density increases, AIP activates the transmembrane protein AgrC. Phosphorylated AgrC further activates AgrA and finally promotes the expression of target genes. AgrA acts on P2, which regulates the Agr protein, and P3, which can activate RNAIII expression. RNAIII is the effector molecule of agr locus. RNAIII induces the expression of virulence factors, such as protease and toxin. On the other hand, RNAIII also inhibits the expression of surface adhesion proteins [83].
Figure 5.
The interaction between Agr quorum sensing system and some important biofilms regulators (LytSR TCS, SigB, CodY and SarA) [20].
LuxS/AI-2 quorum sensing system is shared by both Gram-negative and Gram-positive bacteria. It is mediated by the signal molecule AI-2 (furanyl borate diester) synthesized by luxS gene (AI-2 synthase). It enables bacteria to make collective decisions about the expression of a specific set of genes. The precursor of AI-2 is 4,5-dihydroxy-2,3-pentanedione (DPD) [96]. LuxS/AI-2 quorum sensing system that exists in S. aureus plays a role in the regulation of biofilm formation [97]. Two early studies have shown that LuxS is a negative regulator of biofilm formation. Biofilm formation is significantly increased in luxS mutant strains compared with wild strains. A study reported that the transcript level of icaR was significantly reduced in luxS mutants, while icaA expression was significantly increased, suggesting that AI-2 represses icaADBC transcription through activation of icaR [98]. Moreover, when a low nanomolar concentration range of DPD was added, the biofilm formation was changed. On the contrary, when higher concentrations of DPD were added, no effect on biofilm formation was observed [98]. Another study found that Rbf was a positive regulator of biofilm. It promotes PIA-dependent biofilm formation in S. aureus by binding to the sarX promoter, upregulating sarX transcription, and indirectly downregulating icaR expression [99]. The transcription level of rbf was increased in luxS mutant strains. The transcription of the rbf gene could also be restored to normal when supplemented with luxS-containing plasmids or effective concentrations of exogenous AI-2. The results suggest that luxS may suppress rbf expression and reduce the transcription level of icaA through AI-2-mediated signaling, thereby reducing PIA-dependent biofilm formation [100]. In contrast to the above study, another study observed upregulation of the icaADBC by AI-2 in S. epidermidis. The expression of the icaADBC was also upregulated when micromolar concentrations of DPD were added [101]. This result suggests that the effect of signal molecule AI-2 may be very different for each species, even depending on the concentration.
4.2. The Global Response Regulator
Staphylococcal accessory regulator (SarA) is a DNA-binding protein encoded by sarA locus [102]. It is the main global regulator of many virulence determinants and directly regulates the expression of some virulence factors [103]. sarA locus is necessary for ica operon transcription, PIA/PNAG production, and biofilm formation of S. aureus [104]. SarA regulates biofilm formation through the agr-dependent pathway, binds to the agr promoter to stimulate transcription of RNAIII, and cascades and regulates downstream target genes [105,106]. SarA can control gene expression by directly interacting with target gene promoters through the agr-independent pathway [104]. In addition, SarA activates the P2 promoter and promotes transcription by bending the DNA region of the agr promoter, thereby enhancing agr dimer interactions to upregulate agr expression [107]. A sequence with 58% homology to the predicted recognition sequence of sarA exists at 70 nt upstream of ica start codon in S. aureus. The purified SarA binds to this sequence with high affinity to upregulate ica operon expression and promote biofilm formation [108]. SarA not only induces the transcription of ica operon but also of its suppressor icaR, indicating that SarA may prevent the excessive production of PIA [109]. Another role of SarA in biofilm formation is the inhibition of extracellular proteases production [110]. sarA mutants have a global impact on the abundance of many S. aureus transcripts, producing high levels of proteases, such as metalloproteinase Aur, serine protease SspA, cysteine protease SspB and ScpA, resulting in the inability to form biofilms [110]. Only by simultaneously eliminating the production of these extracellular proteases, the biofilm formation and virulence of sarA mutants can be fully restored [111]. In summary, SarA is an important regulator controlling the biofilm formation of S. aureus through a variety of mechanisms.
σB is a product of sigB operon and is the main regulator of S. aureus response to environmental stress. It plays an important role in the production of bacterial drug resistance, biofilm formation, and the expression regulation of virulence-related genes [112]. Under osmotic stress, the biofilm formation of S. aureus wild strain MA12 is significantly stimulated, but the sigB deletion mutation eliminates the biofilm formation. After supplying the sigB-containing plasmid, biofilm formation could be restored to normal under conventional conditions or stimulated by osmotic stress [113]. However, another study reported that the sigB deletion mutant still formed biofilm effectively, suggesting that sigB could regulate S. aureus biofilm in a strain-dependent manner [114]. σB also mediates an increase in SarA level and decreases the level of RNA III of the Agr system, leading to growth-stage-dependent differences in some virulence factors [115]. Moreover, it mediates the production of several cell surface proteins related to the early adhesion of biofilms, such as FnbA and ClfA. σB promotes the transcription of fnbA in early growth and significantly upregulates the transcription of clfA in late growth [116]. However, various exoprotein genes that are important for biofilm dispersal are repressed by σB [117].
In S. aureus, the global transcription factor CodY acts mainly as a repressor of metabolic and virulence genes, directly or indirectly regulating more than 200 genes [118]. Under adequate nutritional conditions, CodY interacts with its effector molecules, leading to conformational changes in CodY that enhance the affinity of CodY to its DNA binding sites [119]. CodY strongly inhibits the agr locus [120]. Some studies have shown that CodY may bind to a locus in agrC in vitro [121,122], but this binding does not affect the in vivo transcription of agrA, suggesting that CodY does not control agr gene expression through direct binding within the agr locus [122]. The identification of CodY regulatory Agr system targets reveals undoubtedly a new regulatory pathway affecting most virulence genes in S. aureus. CodY regulates biofilm formation by mediating ica expression and PIA production [117,123]. In the codY deletion mutant, the icaA was overexpressed; in the icaR deletion mutant, the transcript level of icaA gene was extremely low and the amount of biofilm formation was low; in the icaR and codY double mutant, the transcript level of icaA was similar to that of the codY mutant, but the amount of biofilm formation was three times more than that of the codY mutant, indicating that codY may override the icaR-mediated loss of the ica locus repression and that codY is epistatic to icaR [118]. In addition to regulating PIA-dependent biofilm, CodY also represses the exoprotease and thermonuclease (nuc), which are important regulators of biofilm formation [118,124]. nuc is directly controlled by the SaeRS system, and in codY null mutants, nuc overexpression requires SaeR, indicating that the codY overexpression phenotype is at least partially via the SaeRS system [118]. In addition, CodY regulates the sae locus in a Rot-dependent and Rot-independent manner [125].
4.3. ica Operon
The enzyme required for PIA synthesis is encoded by icaADBC. icaR are located upstream of icaADBC and belong to the TetR family encoding transcriptional repressors of the ica locus [126]. IcaR is a DNA-binding protein that negatively regulates icaADBC expression by binding to the upstream region of the icaA start codon [127]. In addition to IcaR, a second icaADBC direct repressor has been identified. This regulator, named TcaR (teicoplanin-associated locus regulator), belongs to the MarR family of transcriptional regulators [128]. The putative binding sequence of TcaR has been identified in the promoter region of the icaADBC operon and has been shown to function as a direct repressor by TcaR [129].
Existing studies have pointed out that S. aureus isolates carrying the deletion of the 5 bp motif “TATTT” between the icaR and icaA intergenic region exhibit a mucoid phenotype, resulting in the increased transcription of ica and inducing excessive production of PIA/PNAG in S. aureus [130]. Through the electrophoretic mobility shift assay and DNaseI footprint assay of recombinant IcaR, it was found that the recombinant IcaR protected a 42 bp region upstream of the icaA gene, but not with the region containing 5 bp motif. It shows that the transcriptional control of 5 bp at ica site is independent of icaR [131]. The effect of the 5 bp motif on icaADBC expression is considered to be controlled by other undetermined repressors. Whole-genome sequencing and microarray analysis revealed that another clinical isolate with a mucus phenotype contained a hypothetical transcriptional regulatory gene with a spontaneous mutation that was expressed at a significantly higher level than in a control strain without biofilm formation [132]. This gene was named “regulator of biofilm” (rob). Rob is a DNA-binding protein that shares homology with the TetR family. Rob was experimentally shown to recognize and bind a 25 bp region between the icaR and icaA intergenic regions (including the 5 bp motif). In the absence of 5 bp, Rob cannot bind to this region, resulting in excessive biofilm formation and is a novel repressor of the ica locus [132]. Rob was first discovered as the gbaAB (glucose-induced biofilm access gene) operon. GbaAB can participate in the regulation of the multicellular aggregation step of glucose responsive S. aureus biofilm formation through ica locus and PIA [133]. However, Rob affects biofilm formation in a glucose-independent manner [132]. Although the strains used in these two studies are different, the potential mechanisms need to be further explored.
4.4. Two-Component Regulatory System
The SrrAB TCS (Staphylococcal respiratory response regulator) is a major regulator of respiratory growth and virulence in S. aureus, which is critical for survival under environment conditions such as hypoxic and oxidative [134]. The oxygen utilization plays an important role in S. aureus virulence by regulating toxin production and biofilm formation [135]. SrrAB TCS sensor kinase SrrB responds to oxygen in the environment by autophosphorylation and the effector molecule SrrA activates or represses the regulation of target genes [136]. Purified phosphorylated SrrA binds to a 100 bp DNA sequence upstream of icaA in a concentration-dependent manner to induce transcription of icaA and PIA production under anaerobic conditions [137]. In particular, the introduction of insertional mutations in srrA resulted in increased PIA production but reduced biofilm formation [138]. Meanwhile, the cysteine residues within the SrrB Cache domain form redox-sensitive disulfide bonds, which are required for biofilm formation and virulence expression under anaerobic and microaerobic conditions [134]. Another study showed that SrrAB-dependent biofilms increased with decreased respiratory activity due to fermenting cells increasing eDNA and proteins in an AtlA murein hydrolase-dependent manner [139]. In srrAB mutants, biofilm formation decreased over time and cell death levels increased under static aeration conditions compared with wild strains [140], indicating that the SrrAB is important for long-term biofilm stability and survival. This difference could be caused by different ways of hypoxia production, different growth media and different culture time. All these factors emphasize the importance of biofilm growth models [141].
The S. aureus exoprotein expression (SaeRS) TCS is a key regulator of toxin and exoprotein, which has critical roles in evasion of innate immunity and pathogenesis [142]. SaeRS TCS is composed of sensor kinase SaeS and response regulator SaeR along with two auxiliary proteins SaeP and SaeQ [143]. SaeP and SaeQ form a protein complex with SaeS, which activates the phosphatase activity of the SaeS and returns to the pre-activation state via a negative feedback mechanism [144]. SaeRS regulates the expression of genes associated with biofilm adhesion proteins such as fnbA, fnbB, and fib [109,145,146]. The accumulation of biofilm-related proteins enhances biofilm formation [147]. SaeRS mutation limits the biofilm formation of S. aureus. However, in S. aureus Newman, a point mutation in SaeS (SaeSP) leads to overexpression of SaeRS activity, preventing this strain from forming a robust biofilm, and exhibiting a SaeRS polymorphism [148]. In addition to SrrAB, SaeRS also regulates fermentation biofilm formation, decreased respiration caused an increasing activity in SaeRS, leading to increased expression of AtlA and FnbA as well as biofilm formation [149]. Recently, overexpression of ScrA (S. aureus clumping regulator A) was found causing an increase in bacterial aggregation [150]. Through proteomics and transcriptomics, strain overexpressing ScrA was found to cause cell aggregation and biofilm formation through activation of SaeRS, resulting in upregulation of multiple adhesins and downregulation of secreted proteases [150].
The cell death and lysis of S. aureus are regulated by LytSR TCS through a bacteriophage-like holin/antiholin system [151]. Holin is encoded by cidABC operon, CidA oligomerizes and forms pores in the cytoplasmic membrane, leading to membrane depolarization, activation of murine protein hydrolase activity, and cell lysis [151,152]. The CidA mutant exhibits decreased lysis, resulting in reduced eDNA content and impaired biofilm formation [153]. Antiholin inhibits the activity of CidA, encoded by lrgAB operon [81], which is an inhibitor of these processes [154], counteracts CidA activity by interfering with the ability of CidA to depolarize membranes and cause subsequent death and lysis [77]. The lrgAB operon, together with the cidABC operon, have been shown to be the regulators of cell death and lysis during biofilm development [152]. In S. aureus, LytSR positively regulates lrgAB operon and CidR enhances the expression of cidABC and lrgAB [155]. Agr and SarA, like LytSR, are positively regulating lrgAB expression. Mutations in the agr locus reduce lrgAB expression by approximately sixfold, whereas mutations in the sarA reduce lrgAB expression to undetectable levels [156]. lytS mutation results in more eDNA produced in ECM, leading to a thicker and more adherent biofilm [157]. Some studies have shown that the activity of the lrgAB promoter is mainly expressed in the tower structure of S. aureus biofilms [158], while a small part of the tower structure formed by lytS mutant strain still shows significant lrgAB expression. The results suggest that there is a LytS-independent pathway of LytR activation [159]. Since mutations in srrAB lead to increased cell death during biofilm development, it is reported that SrrAB inhibits cell death by directly suppressing the expression of the cidABC under conditions of glucose overload [160]. The cell death could be due to the effect of cidABC expression leading to increased reactive oxygen species (ROS) accumulation [160].
ArlRS TCS was originally identified as a regulator of autolysis and biofilm formation [161]. MgrA is a global transcriptional regulator downstream of ArlRS that forms a regulatory cascade with ArlRS [162]. ArlRS activates the expression of MgrA and regulates a variety of genes through MgrA, including seven CWPs and virulence [162]. Among the 15 mutants of non-essential TCSs, ArlRS TCS, SrrAB TCS, and Agr are required for biofilm formation, with ArlRS playing a major role in the process [163]. ArlRS enhances the expression of icaADBC by suppressing the expression of icaR, which activates PNAG production, and in arl mutants, the synthesis of PNAG is lost [163]. However, overexpression of MgrA could not restore PNAG expression in ArlRS mutants [164]. In contrast, MgrA can act as a negative regulator of psm expression, negatively regulating biofilm formation and dispersion by directly binding to the psm promoter region to repress transcription of the psm operon in cultures and biofilms [165]. These results suggest that ArlRS is a key TCS for biofilm formation.
4.5. The Second Messenger
Cyclic dinucleotides are highly versatile signaling molecules in prokaryotes involved in the control of various important biological processes [166]. These intracellular signal nucleotides coordinate diverse aspects of bacterial colony behavior, including motility, biofilm formation, and virulence gene expression [167]. The second messenger, cyclic diguanylic acid (c-di-GMP), is synthesized by two molecules of GTP by the diguanylate cyclases (DGCs) and degraded by the c-di-GMP phosphodiesterases (PDEs) [167], which is the main regulator of the conversion between free movement and biofilm of Gram-negative bacteria [168]. A high c-di-GMP level reduces the expression and/or activity of flagella and stimulates the synthesis of adhesins and biofilm-related extracellular polysaccharide [169], promoting biofilm formation [170]. In S. aureus, the second messenger, c-di-AMP, is synthesized by two molecules of ATP by the diadenylyl cyclase (DAC) enzyme DacA and degraded by the c-di-AMP phosphodiesterase GdpP-containing GGDEF domain [171]. Similar to c-di-GMP, in the S. aureus SEJ1 strain with gdpP mutation, the level of c-di-AMP and the amount of biofilm are significantly increased [171]. However, the S. aureus USA300 LAC strain could not form a robust biofilm in this condition [171], indicating that the effect of c-di-AMP on biofilm might be strain dependent. The gdpP mutation also leads to reduced eDNA levels, indicating that c-di-AMP is also involved in the release of eDNA [172]. In addition, The c-di-AMP also interacts with the purine biosynthesis pathway. In purine biosynthesis mutant methicillin-sensitive S. aureus (MRSA) strains, ADP, ATP, c-di-AMP, and eDNA levels were lower, and biofilm formation was less; vancomycin binding and its induced cleavage were increased [173]. In S. aureus, GdpS, a protein containing the GGDEF domain, inhibits early biofilm formation and is independent of autolysis by reducing lrgAB and cidABC-mediated release of eDNA [174]. These results reveal the influence of c-di-AMP on biofilms, which may play an important role in the persistence of S. aureus biofilm infection.
4.6. sRNAs
In addition to regulating transcriptional proteins, RNA molecules are now recognized as key players in gene regulation in all organisms [175]. Small RNAs (sRNAs) are usually non-coding and function at the level of transcription, translation, or RNA degradation [176], playing a key role in biofilm formation through base pairing with target mRNAs or interactions with regulatory proteins, resulting in both positive and negative regulatory mechanisms [177,178]. sRNA RsaA inhibits the synthesis of MgrA and enhances biofilm formation by binding to two regions of the mRNA of mgrA [179]. In addition, the synthesis of RsaA is controlled by SigB. Thus, RsaA functionally connects the global regulators SigB and MgrA [179]. The sRNA RsaF binds the hyaluronate lyase HsyA and serine protease-like protein SplD; the disruption of RsaF leads to a decreasing activity in HysA, which in turn increases biofilm formation [180]. sRNA RsaI (or RsaOG) depress the formation of biofilms at high glucose concentrations by binding to the 3′-UTR of icaR. Inhibition of IcaR synthesis by stabilization of mRNA recycles and/or by counteracts binding to activators of IcaR mRNA translation, but the exact molecular mechanism has not been determined. The unwinding of biofilm formation occurs by binding to the 3′-UTR of icaR. This result corroborates with previous reports of increased biofilm formation observed in hysA mutants [181]. The 5′-untranslated region of sarA contains two sRNAs, named teg49 and teg48, which are detectable in the P3-P1 and P1 regions of the sarA promoter, respectively [182]. sRNA teg58 plays an important role in regulating arginine biosynthesis and biofilm formation in S. aureus. teg58 inhibits arginine synthesis; the arginine biosynthesis gene (argGH) is upregulated in teg58 mutants. Biofilm formation is reduced in parental strains after supplementation with exogenous arginine or endogenous argGH [183]. However, teg49 does not affect biofilm formation. Biofilm-associated genes (such as ica locus) are not affected in the case of teg49 inactivation or overexpression [184]. Transcriptomic analysis suggests that teg49 may post-transcriptionally affect the SaeRS and LytRS TCS, but the exact mechanism is unclear [184]. The sRNA SprX is encoded in the pathogenicity island of the MRSA Newman strain. SprX1 is one of three copies of SprX. SprX1 interacts directly with mRNAs encoding ClfB and Hld. Cells overexpressing SprX1 exhibited increased intercellular aggregation and biofilm formation [185]. In addition, SprX may modulate its effect on biofilms by increasing the stability of RNAIII [185]. In general, the mechanism of sRNA in the regulatory pathway of biofilm formation is not very clear at present. Further exploration is still needed.
5. Conclusions and Future Perspectives
The presence of S. aureus and most bacteria in the form of a biofilm is a considerable challenge for the medical community. Biofilms are a survival strategy for bacteria, making them extremely difficult to treat due to their inherent immune response and antibiotic resistance. Therefore, it is necessary to further study the formation and regulation mechanism of S. aureus biofilm for research and development of anti-biofilm drugs that inhibit biofilm formation. The process of biofilm formation is complex and involves the co-expression of multiple genes. S. aureus relies on a broad network of regulatory systems that coordinate biofilm formation in a complementary or opposing way. At present, although progress has been made in the regulatory mechanism of the formation of S. aureus biofilm, researchers have not yet understood the synergy between different regulatory networks. Moreover, the regulation mechanism of biofilm is dynamic. For example, different growth times and different environments may have different regulatory genes. How to realize these signals thus needs further investigation. At the same time, little is known about whether the regulation mechanism of biofilm in vivo is the same as in vitro. Human organoid models may be one of the most exciting tools to understand the regulation mechanism of S. aureus biofilm. On the other hand, the experimental results of reference strains may not be similar to the results of clinical isolates. The results obtained from reference strains may have some differences from that of clinical isolates. Therefore, different clinical isolates can be used for further research to provide better clinical significance. In addition, in clinical infection, S. aureus can also form mixed species biofilm with other bacteria and/or fungi. It is also important to include mixed species biofilm in the study.
Author Contributions
Conceptualization, Q.P.; writing—original draft preparation, Q.P., W.D. and X.T.; writing—review and editing, W.Y. and N.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant No. 81703333), Natural Science Foundation of Guangdong Province (grant No. 2020A1515011326 and 2021A1515011360), Guangzhou Science and Technology Project (grant No. 202102080469), Guangzhou Health Science and Technology Project (grant No. 2020A010015), and Medical Scientific Research Foundation of Guangdong Province (grant No. C2021077).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Karygianni L., Ren Z., Koo H., Thurnheer T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020;28:668–681. doi: 10.1016/j.tim.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Di Martino P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018;4:274–288. doi: 10.3934/microbiol.2018.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilhen C., Forestier C., Balestrino D. Biofilm dispersal: Multiple elaborate strategies for dissemination of bacteria with unique properties. Mol. Microbiol. 2017;105:188–210. doi: 10.1111/mmi.13698. [DOI] [PubMed] [Google Scholar]
- 4.Jamal M., Ahmad W., Andleeb S., Jalil F., Imran M., Nawaz M.A., Hussain T., Ali M., Rafiq M., Kamil M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018;81:7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Vestby L.K., Grønseth T., Simm R., Nesse L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics. 2020;9:59. doi: 10.3390/antibiotics9020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khatoon Z., McTiernan C.D., Suuronen E.J., Mah T.-F., Alarcon E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4:e01067. doi: 10.1016/j.heliyon.2018.e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowy F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y., He L., Asiamah T.K., Otto M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018;20:3141–3153. doi: 10.1111/1462-2920.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J., Zilm P.S., Kidd S.P. Novel Research Models for Staphylococcus aureus Small Colony Variants (SCV) Development: Co-pathogenesis and Growth Rate. Front. Microbiol. 2020;11:321. doi: 10.3389/fmicb.2020.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Genet. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 11.Guo H., Tong Y., Cheng J., Abbas Z., Li Z., Wang J., Zhou Y., Si D., Zhang R. Biofilm and Small Colony Variants—An Update on Staphylococcus aureus Strategies toward Drug Resistance. Int. J. Mol. Sci. 2022;23:1241. doi: 10.3390/ijms23031241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P., Lee J.-H., Beyenal H., Lee J. Fatty Acids as Antibiofilm and Antivirulence Agents. Trends Microbiol. 2020;28:753–768. doi: 10.1016/j.tim.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Beloin C., Renard S., Ghigo J.-M., Lebeaux D. Novel approaches to combat bacterial biofilms. Curr. Opin. Pharmacol. 2014;18:61–68. doi: 10.1016/j.coph.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Girish V.M., Liang H., Aguilan J.T., Nosanchuk J.D., Friedman J.M., Nacharaju P. Anti-biofilm activity of garlic extract loaded nanoparticles. Nanomedicine. 2019;20:102009. doi: 10.1016/j.nano.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donne J., Dewilde S. The Challenging World of Biofilm Physiology. In: Poole R.K., editor. Recent Advances in Microbial Oxygen-Binding Proteins. Volume 67. Academic Press; London, UK: 2015. pp. 235–292. (Advances in Microbial Physiology). [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro S.M., Felício M.R., Boas E.V., Gonçalves S., Costa F.F., Samy R.P., Santos N.C., Franco O.L. New frontiers for anti-biofilm drug development. Pharmacol. Ther. 2016;160:133–144. doi: 10.1016/j.pharmthera.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Idrees M., Sawant S., Karodia N., Rahman A. Staphylococcus aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies. Int. J. Environ. Res. Public Health. 2021;18:7602. doi: 10.3390/ijerph18147602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moormeier D.E., Bayles K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017;104:365–376. doi: 10.1111/mmi.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schilcher K., Horswill A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020;84:e00026-19. doi: 10.1128/MMBR.00026-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neill E., Pozzi C., Houston P., Humphreys H., Robinson D.A., Loughman A., Foster T.J., O’Gara J.P. A Novel Staphylococcus aureus Biofilm Phenotype Mediated by the Fibronectin-Binding Proteins, FnBPA and FnBPB. J. Bacteriol. 2008;190:3835–3850. doi: 10.1128/JB.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolz C., Goerke C., Landmann R., Zimmerli W., Fluckiger U. Transcription of Clumping Factor A in Attached and Unattached Staphylococcus aureus In Vitro and during Device-Related Infection. Infect. Immun. 2002;70:2758–2762. doi: 10.1128/IAI.70.6.2758-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabat A., Melles D.C., Martirosian G., Grundmann H., van Belkum A., Hryniewicz W. Distribution of the Serine-Aspartate Repeat Protein-Encoding sdr Genes among Nasal-Carriage and Invasive Staphylococcus aureus Strains. J. Clin. Microbiol. 2006;44:1135–1138. doi: 10.1128/JCM.44.3.1135-1138.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaussart A., Feuillie C., El-Kirat-Chatel S. The microbial adhesive arsenal deciphered by atomic force microscopy. Nanoscale. 2020;12:23885–23896. doi: 10.1039/D0NR07492F. [DOI] [PubMed] [Google Scholar]
- 25.Mangwani N., Kumari S., Das S. Bacterial biofilms and quorum sensing: Fidelity in bioremediation technology. In: Mayes S., Harding S.E., editors. Biotechnology and Genetic Engineering Reviews. Volume 32. Taylor & Francis Ltd.; Abingdon, UK: 2016. pp. 43–73. (Biotechnology & Genetic Engineering Reviews). [DOI] [PubMed] [Google Scholar]
- 26.Otto M. Staphylococcal biofilms. In: Romeo T., editor. Bacterial Biofilms. Volume 322. Springer; Berlin/Heidelberg, Germany: 2008. p. 207. (Current Topics in Microbiology and Immunology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman-Bausier P., El-Kirat-Chatel S., Foster T.J., Geoghegan J.A., Dufrêne Y.F. Staphylococcus aureus Fibronectin-Binding Protein A Mediates Cell-Cell Adhesion through Low-Affinity Homophilic Bonds. mBio. 2015;6:e00413-15. doi: 10.1128/mBio.00413-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasamiravaka T., Labtani Q., Duez P., El Jaziri M. The Formation of Biofilms by Pseudomonas aeruginosa: A Review of the Natural and Synthetic Compounds Interfering with Control Mechanisms. BioMed Res. Int. 2015;2015:759348. doi: 10.1155/2015/759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Toole G., Kaplan H.B., Kolter R. Biofilm Formation as Microbial Development. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 30.Gupta P., Sarkar S., Das B., Bhattacharjee S., Tribedi P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016;198:1–15. doi: 10.1007/s00203-015-1148-6. [DOI] [PubMed] [Google Scholar]
- 31.Costerton J. Introduction to biofilm. Int. J. Antimicrob. Agents. 1999;11:217–221; discussion 237–239. doi: 10.1016/S0924-8579(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 32.Lister J.L., Horswill A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014;4:178. doi: 10.3389/fcimb.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boles B.R., Horswill A.R. Staphylococcal biofilm disassembly. Trends Microbiol. 2011;19:449–455. doi: 10.1016/j.tim.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandana, Das S. Genetic regulation, biosynthesis and applications of extracellular polysaccharides of the biofilm matrix of bacteria. Carbohydr. Polym. 2022;291:119536. doi: 10.1016/j.carbpol.2022.119536. [DOI] [PubMed] [Google Scholar]
- 35.Periasamy S., Joo H.-S., Duong A.C., Bach T.-H.L., Tan V.Y., Chatterjee S.S., Cheung G.Y.C., Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA. 2012;109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung G.Y., Joo H.-S., Chatterjee S.S., Otto M. Phenol-soluble modulins—Critical determinants of staphylococcal virulence. FEMS Microbiol. Rev. 2014;38:698–719. doi: 10.1111/1574-6976.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le K.Y., Dastgheyb S., Ho T.V., Otto M. Molecular determinants of staphylococcal biofilm dispersal and structuring. Front. Cell. Infect. Microbiol. 2014;4:167. doi: 10.3389/fcimb.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz K., Syed A.K., Stephenson R.E., Rickard A.H., Boles B.R. Functional Amyloids Composed of Phenol Soluble Modulins Stabilize Staphylococcus aureus Biofilms. PLoS Pathog. 2012;8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marinelli P., Pallares I., Navarro S., Ventura S. Dissecting the contribution of Staphylococcus aureus α-phenol-soluble modulins to biofilm amyloid structure. Sci. Rep. 2016;6:34552. doi: 10.1038/srep34552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otto M. Staphylococcal Infections: Mechanisms of Biofilm Maturation and Detachment as Critical Determinants of Pathogenicity. Annu. Rev. Med. 2013;64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen H.T., Nguyen T.H., Otto M. The staphylococcal exopolysaccharide PIA—Biosynthesis and role in biofilm formation, colonization, and infection. Comput. Struct. Biotechnol. J. 2020;18:3324–3334. doi: 10.1016/j.csbj.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mack D., Fischer W., Krokotsch A., Leopold K., Hartmann R., Egge H., Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: Purification and structural analysis. J. Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cramton S.E., Gerke C., Schnell N.F., Nichols W.W., Götz F. The Intercellular Adhesion (ica) Locus Is Present in Staphylococcus aureus and Is Required for Biofilm Formation. Infect. Immun. 1999;67:5427–5433. doi: 10.1128/IAI.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Gara J.P. ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 2007;270:179–188. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 45.Gerke C., Kraft A., Süßmuth R., Schweitzer O., Götz F. Characterization of the N-Acetylglucosaminyltransferase Activity Involved in the Biosynthesis of the Staphylococcus epidermidis Polysaccharide Intercellular Adhesin. J. Biol. Chem. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 46.Atkin K.E., MacDonald S.J., Brentnall A.S., Potts J.R., Thomas G.H. A different path: Revealing the function of staphylococcal proteins in biofilm formation. FEBS Lett. 2014;588:1869–1872. doi: 10.1016/j.febslet.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Vuong C., Kocianova S., Voyich J.M., Yao Y., Fischer E.R., DeLeo F.R., Otto M. A Crucial Role for Exopolysaccharide Modification in Bacterial Biofilm Formation, Immune Evasion, and Virulence. J. Biol. Chem. 2004;279:54881–54886. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- 48.Sedarat Z., Taylor-Robinson A.W. Biofilm Formation by Pathogenic Bacteria: Applying a Staphylococcus aureus Model to Appraise Potential Targets for Therapeutic Intervention. Pathogens. 2022;11:388. doi: 10.3390/pathogens11040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marques V.F., Santos H.A., Santos T.H., Melo D.A., Coelho S.M., Coelho I.S., Souza M.M. Expression of icaA and icaD genes in biofilm formation in Staphylococcus aureus isolates from bovine subclinical mastitis. Pesquisa Veterinária Brasileira. 2021;41:e06645. doi: 10.1590/1678-5150-pvb-6645. [DOI] [Google Scholar]
- 50.Brooks J.L., Jefferson K.K. Phase Variation of Poly-N-Acetylglucosamine Expression in Staphylococcus aureus. PLoS Pathog. 2014;10:e1004292. doi: 10.1371/journal.ppat.1004292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Neill E., Pozzi C., Houston P., Smyth D., Humphreys H., Robinson D.A., O’Gara J.P. Association between Methicillin Susceptibility and Biofilm Regulation in Staphylococcus aureus Isolates from Device-Related Infections. J. Clin. Microbiol. 2007;45:1379–1388. doi: 10.1128/JCM.02280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cucarella C., Tormo M.A., Ubeda C., Trotonda M.P., Monzón M., Peris C., Amorena B., Lasa I., Penadés J.R. Role of Biofilm-Associated Protein Bap in the Pathogenesis of Bovine Staphylococcus aureus. Infect. Immun. 2004;72:2177–2185. doi: 10.1128/IAI.72.4.2177-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taglialegna A., Navarro S., Ventura S., Garnett J.A., Matthews S., Penades J.R., Lasa I., Valle J. Staphylococcal Bap Proteins Build Amyloid Scaffold Biofilm Matrices in Response to Environmental Signals. PLoS Pathog. 2016;12:e1005711. doi: 10.1371/journal.ppat.1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arrizubieta M.J., Toledo-Arana A., Amorena B., Penadés J.R., Lasa I. Calcium Inhibits Bap-Dependent Multicellular Behavior in Staphylococcus aureus. J. Bacteriol. 2004;186:7490–7498. doi: 10.1128/JB.186.22.7490-7498.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pietrocola G., Campoccia D., Motta C., Montanaro L., Arciola C.R., Speziale P. Colonization and Infection of Indwelling Medical Devices by Staphylococcus aureus with an Emphasis on Orthopedic Implants. Int. J. Mol. Sci. 2022;23:5958. doi: 10.3390/ijms23115958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCourt J., O’Halloran D.P., McCarthy H., O’Gara J.P., Geoghegan J.A. Fibronectin-binding proteins are required for biofilm formation by community-associated methicillin-resistant Staphylococcus aureus strain LAC. FEMS Microbiol. Lett. 2014;353:157–164. doi: 10.1111/1574-6968.12424. [DOI] [PubMed] [Google Scholar]
- 57.Herman-Bausier P., Labate C., Towell A.M., Derclaye S., Geoghegan J.A., Dufrêne Y.F. Staphylococcus aureus clumping factor A is a force-sensitive molecular switch that activates bacterial adhesion. Proc. Natl. Acad. Sci. USA. 2018;115:5564–5569. doi: 10.1073/pnas.1718104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abraham N.M., Jefferson K.K. Staphylococcus aureus clumping factor B mediates biofilm formation in the absence of calcium. Microbiology. 2012;158:1504–1512. doi: 10.1099/mic.0.057018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Formosa-Dague C., Speziale P., Foster T.J., Geoghegan J.A., Dufrêne Y.F. Zinc-dependent mechanical properties of Staphylococcus aureus biofilm-forming surface protein SasG. Proc. Natl. Acad. Sci. USA. 2016;113:410–415. doi: 10.1073/pnas.1519265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corrigan R.M., Rigby D., Handley P., Foster T.J. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology. 2007;153:2435–2446. doi: 10.1099/mic.0.2007/006676-0. [DOI] [PubMed] [Google Scholar]
- 61.Schroeder K., Jularic M., Horsburgh S.M., Hirschhausen N., Neumann C., Bertling A., Schulte A., Foster S., Kehrel B.E., Peters G., et al. Molecular Characterization of a Novel Staphylococcus Aureus Surface Protein (SasC) Involved in Cell Aggregation and Biofilm Accumulation. PLoS ONE. 2009;4:e7567. doi: 10.1371/journal.pone.0007567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feuillie C., Formosa-Dague C., Hays L.M.C., Vervaeck O., Derclaye S., Brennan M.P., Foster T.J., Geoghegan J.A., Dufrêne Y.F. Molecular interactions and inhibition of the staphylococcal biofilm-forming protein SdrC. Proc. Natl. Acad. Sci. USA. 2017;114:3738–3743. doi: 10.1073/pnas.1616805114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corrigan R.M., Miajlovic H., Foster T.J. Surface proteins that promote adherence of Staphylococcus aureusto human desquamated nasal epithelial cells. BMC Microbiol. 2009;9:22. doi: 10.1186/1471-2180-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Askarian F., Ajayi C., Hanssen A.-M., van Sorge N., Pettersen I., Diep D.B., Sollid J.U.E., Johannessen M. The interaction between Staphylococcus aureus SdrD and desmoglein 1 is important for adhesion to host cells. Sci. Rep. 2016;6:22134. doi: 10.1038/srep22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Askarian F., Uchiyama S., Valderrama J.A., Ajayi C., Sollid J.U.E., van Sorge N.M., Nizet V., van Strijp J.A.G., Johannessen M. Serine-Aspartate Repeat Protein D Increases Staphylococcus aureus Virulence and Survival in Blood. Infect. Immun. 2017;85:e00559-16. doi: 10.1128/IAI.00559-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y., Wu M., Hang T., Wang C., Yang Y., Pan W., Zang J., Zhang M., Zhang X. Staphylococcus aureus SdrE captures complement factor H’s C-terminus via a novel ‘close, dock, lock and latch’ mechanism for complement evasion. Biochem. J. 2017;474:1619–1631. doi: 10.1042/BCJ20170085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patti J.M., Boles J.O., Hook M. Identification and biochemical characterization of the ligand binding domain of the collagen adhesin from Staphylococcus aureus. Biochemistry. 1993;32:11428–11435. doi: 10.1021/bi00093a021. [DOI] [PubMed] [Google Scholar]
- 68.Singh K.V., Nallapareddy S.R., Sillanpää J., Murray B.E. Importance of the Collagen Adhesin Ace in Pathogenesis and Protection against Enterococcus faecalis Experimental Endocarditis. PLoS Pathog. 2010;6:e1000716. doi: 10.1371/annotation/1ccae8f8-d274-4ff8-a295-815037ce9cc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakano K., Hokamura K., Taniguchi N., Wada K., Kudo C., Nomura R., Kojima A., Naka S., Muranaka Y., Thura M., et al. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat. Commun. 2011;2:485. doi: 10.1038/ncomms1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang M., Ko Y.-P., Liang X., Ross C.L., Liu Q., Murray B.E., Höök M. Collagen-binding Microbial Surface Components Recognizing Adhesive Matrix Molecule (MSCRAMM) of Gram-positive Bacteria Inhibit Complement Activation via the Classical Pathway. J. Biol. Chem. 2013;288:20520–20531. doi: 10.1074/jbc.M113.454462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mann E.E., Rice K.C., Boles B.R., Endres J.L., Ranjit D., Chandramohan L., Tsang L.H., Smeltzer M.S., Horswill A.R., Bayles K.W. Modulation of eDNA Release and Degradation Affects Staphylococcus aureus Biofilm Maturation. PLoS ONE. 2009;4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campoccia D., Montanaro L., Arciola C.R. Extracellular DNA (eDNA). A Major Ubiquitous Element of the Bacterial Biofilm Architecture. Int. J. Mol. Sci. 2021;22:9100. doi: 10.3390/ijms22169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blakeman J.T., Morales-García A.L., Mukherjee J., Gori K., Hayward A.S., Lant N.J., Geoghegan M. Extracellular DNA Provides Structural Integrity to a Micrococcus luteus Biofilm. Langmuir. 2019;35:6468–6475. doi: 10.1021/acs.langmuir.9b00297. [DOI] [PubMed] [Google Scholar]
- 74.Payne D.E., Boles B.R. Emerging interactions between matrix components during biofilm development. Curr. Genet. 2016;62:137–141. doi: 10.1007/s00294-015-0527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das T., Sharma P., Busscher H.J., van der Mei H.C., Krom B.P. Role of Extracellular DNA in Initial Bacterial Adhesion and Surface Aggregation. Appl. Environ. Microbiol. 2010;76:3405–3408. doi: 10.1128/AEM.03119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilton M., Charron-Mazenod L., Moore R., Lewenza S. Extracellular DNA Acidifies Biofilms and Induces Aminoglycoside Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016;60:544–553. doi: 10.1128/AAC.01650-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall C.W., Mah T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017;41:276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- 78.Batoni G., Maisetta G., Esin S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta. 2016;1858:1044–1060. doi: 10.1016/j.bbamem.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 79.Doroshenko N., Tseng B.S., Howlin R.P., Deacon J., Wharton J.A., Thurner P.J., Gilmore B.F., Parsek M.R., Stoodley P. Extracellular DNA Impedes the Transport of Vancomycin in Staphylococcus epidermidis Biofilms Preexposed to Subinhibitory Concentrations of Vancomycin. Antimicrob. Agents Chemother. 2014;58:7273–7282. doi: 10.1128/AAC.03132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rice K.C., Firek B.A., Nelson J.B., Yang S.-J., Patton T.G., Bayles K.W. The Staphylococcus aureus cidAB Operon: Evaluation of Its Role in Regulation of Murein Hydrolase Activity and Penicillin Tolerance. J. Bacteriol. 2003;185:2635–2643. doi: 10.1128/JB.185.8.2635-2643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groicher K.H., Firek B.A., Fujimoto D.F., Bayles K.W. The Staphylococcus aureus lrgAB Operon Modulates Murein Hydrolase Activity and Penicillin Tolerance. J. Bacteriol. 2000;182:1794–1801. doi: 10.1128/JB.182.7.1794-1801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parsek M.R., Greenberg E. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 83.Lu L., Hu W., Tian Z., Yuan D., Yi G., Zhou Y., Cheng Q., Zhu J., Li M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019;14:11. doi: 10.1186/s13020-019-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sionov R.V., Steinberg D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms. 2022;10:1239. doi: 10.3390/microorganisms10061239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Le K.Y., Otto M. Quorum-sensing regulation in staphylococci—An overview. Front. Microbiol. 2015;6:1174. doi: 10.3389/fmicb.2015.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dunman P.M., Murphy E., Haney S., Palacios D., Tucker-Kellogg G., Wu S., Brown E.L., Zagursky R.J., Shlaes D., Projan S.J. Transcription Profiling-Based Identification of Staphylococcus aureus Genes Regulated by the agr and/or sarA Loci. J. Bacteriol. 2001;183:7341–7353. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harraghy N., Kerdudou S., Herrmann M. Quorum-sensing systems in staphylococci as therapeutic targets. Anal. Bioanal. Chem. 2006;387:437–444. doi: 10.1007/s00216-006-0860-0. [DOI] [PubMed] [Google Scholar]
- 88.Ji G., Beavis R., Novick R.P. Bacterial Interference Caused by Autoinducing Peptide Variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 89.Gordon C.P., Olson S.D., Lister J.L., Kavanaugh J.S., Horswill A.R. Truncated Autoinducing Peptides as Antagonists of Staphylococcus lugdunensis Quorum Sensing. J. Med. Chem. 2016;59:8879–8888. doi: 10.1021/acs.jmedchem.6b00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Novick R.P., Geisinger E. Quorum Sensing in Staphylococci. Annu. Rev. Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 91.Mootz J.M., Benson M.A., Heim C.E., Crosby H.A., Kavanaugh J.S., Dunman P.M., Kielian T., Torres V.J., Horswill A.R. Rot is a key regulator of Staphylococcus aureus biofilm formation. Mol. Microbiol. 2015;96:388–404. doi: 10.1111/mmi.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yarwood J.M., Bartels D.J., Volper E.M., Greenberg E.P. Quorum Sensing in Staphylococcus aureus Biofilms. J. Bacteriol. 2004;186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boles B.R., Horswill A.R. agr-Mediated Dispersal of Staphylococcus aureus Biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Queck S.Y., Jameson-Lee M., Villaruz A.E., Bach T.-H.L., Khan B.A., Sturdevant D.E., Ricklefs S.M., Li M., Otto M. RNAIII-Independent Target Gene Control by the agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le K.Y., Villaruz A.E., Zheng Y., He L., Fisher E.L., Nguyen T.H., Ho T.V., Yeh A.J., Joo H.-S., Cheung G.Y., et al. Role of Phenol-Soluble Modulins in Staphylococcus epidermidis Biofilm Formation and Infection of Indwelling Medical Devices. J. Mol. Biol. 2019;431:3015–3027. doi: 10.1016/j.jmb.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schauder S., Shokat K., Surette M.G., Bassler B.L. The LuxS family of bacterial autoinducers: Biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 97.Xu L., Li H., Vuong C., Vadyvaloo V., Wang J., Yao Y., Otto M., Gao Q. Role of the luxS Quorum-Sensing System in Biofilm Formation and Virulence of Staphylococcus epidermidis. Infect. Immun. 2006;74:488–496. doi: 10.1128/IAI.74.1.488-496.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu D., Zhao L., Xue T., Sun B. Staphylococcus aureus autoinducer-2 quorum sensing decreases biofilm formation in an icaR-dependent manner. BMC Microbiol. 2012;12:288. doi: 10.1186/1471-2180-12-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cue D., Lei M.G., Lee C.Y. Activation of sarX by Rbf Is Required for Biofilm Formation and icaADBC Expression in Staphylococcus aureus. J. Bacteriol. 2013;195:1515–1524. doi: 10.1128/JB.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma R., Qiu S., Jiang Q., Sun H., Xue T., Cai G., Sun B. AI-2 quorum sensing negatively regulates rbf expression and biofilm formation in Staphylococcus aureus. Int. J. Med. Microbiol. 2017;307:257–267. doi: 10.1016/j.ijmm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Xue T., Ni J., Shang F., Chen X., Zhang M. Autoinducer-2 increases biofilm formation via an ica- and bhp-dependent manner in Staphylococcus epidermidis RP62A. Microbes Infect. 2015;17:345–352. doi: 10.1016/j.micinf.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 102.Selvaraj A., Jayasree T., Valliammai A., Pandian S.K. Myrtenol Attenuates MRSA Biofilm and Virulence by Suppressing sarA Expression Dynamism. Front. Microbiol. 2019;10:2027. doi: 10.3389/fmicb.2019.02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balamurugan P., Krishna V.P., Bharath D., Lavanya R., Vairaprakash P., Princy S.A. Staphylococcus aureus Quorum Regulator SarA Targeted Compound, 2-[(Methylamino)methyl]phenol Inhibits Biofilm and Down-Regulates Virulence Genes. Front. Microbiol. 2017;8:1290. doi: 10.3389/fmicb.2017.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valle J., Toledo-Arana A., Berasain C., Ghigo J.-M., Amorena B., Penadés J.R., Lasa I. SarA and not σB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 2003;48:1075–1087. doi: 10.1046/j.1365-2958.2003.03493.x. [DOI] [PubMed] [Google Scholar]
- 105.Chien Y.-T., Cheung A.L. Molecular Interactions between Two Global Regulators, sar and agr, in Staphylococcus aureus. J. Biol. Chem. 1998;273:2645–2652. doi: 10.1074/jbc.273.5.2645. [DOI] [PubMed] [Google Scholar]
- 106.Cheung A.L., Nishina K., Manna A.C. SarA of Staphylococcus aureus Binds to the sarA Promoter To Regulate Gene Expression. J. Bacteriol. 2008;190:2239–2243. doi: 10.1128/JB.01826-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reyes D., Andrey D.O., Monod A., Kelley W.L., Zhang G., Cheung A.L. Coordinated Regulation by AgrA, SarA, and SarR To Control agr Expression in Staphylococcus aureus. J. Bacteriol. 2011;193:6020–6031. doi: 10.1128/JB.05436-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chien Y.-T., Manna A.C., Projan S.J., Cheung A.L. SarA, a Global Regulator of Virulence Determinants in Staphylococcus aureus, Binds to a Conserved Motif Essential for sar-dependent Gene Regulation. J. Biol. Chem. 1999;274:37169–37176. doi: 10.1074/jbc.274.52.37169. [DOI] [PubMed] [Google Scholar]
- 109.Xiong Y.Q., Willard J., Yeaman M.R., Cheung A.L., Bayer A.S. Regulation of Staphylococcus aureus α-Toxin Gene (hla) Expression by agr, sarA, and sae In Vitro and in Experimental Infective Endocarditis. J. Infect. Dis. 2006;194:1267–1275. doi: 10.1086/508210. [DOI] [PubMed] [Google Scholar]
- 110.Zielinska A.K., Beenken K.E., Mrak L.N., Spencer H.J., Post G.R., Skinner R.A., Tackett A.J., Horswill A.R., Smeltzer M.S. sarA-mediated repression of protease production plays a key role in the pathogenesis of Staphylococcus aureus USA300 isolates. Mol. Microbiol. 2012;86:1183–1196. doi: 10.1111/mmi.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loughran A.J., Atwood D.N., Anthony A.C., Harik N.S., Spencer H.J., Beenken K.E., Smeltzer M.S. Impact of individual extracellular proteases on Staphylococcus aureus biofilm formation in diverse clinical isolates and their isogenic sarA mutants. MicrobiologyOpen. 2014;3:897–909. doi: 10.1002/mbo3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kullik I., Giachino P., Fuchs T. Deletion of the Alternative Sigma Factor ς B in Staphylococcus aureus Reveals Its Function as a Global Regulator of Virulence Genes. J. Bacteriol. 1998;180:4814–4820. doi: 10.1128/JB.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rachid S., Ohlsen K., Wallner U., Hacker J., Hecker M., Ziebuhr W. Alternative Transcription Factor ς B Is Involved in Regulation of Biofilm Expression in a Staphylococcus aureus Mucosal Isolate. J. Bacteriol. 2000;182:6824–6826. doi: 10.1128/JB.182.23.6824-6826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Valle J., Echeverz M., Lasa I. σB Inhibits Poly-N-Acetylglucosamine Exopolysaccharide Synthesis and Biofilm Formation in Staphylococcus aureus. J. Bacteriol. 2019;201:e00098-19. doi: 10.1128/JB.00098-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bischoff M., Entenza J.M., Giachino P. Influence of a Functional sigB Operon on the Global Regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 2001;183:5171–5179. doi: 10.1128/JB.183.17.5171-5179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Entenza J.-M., Moreillon P., Senn M.M., Kormanec J., Dunman P.M., Berger-Bächi B., Projan S., Bischoff M., Aguilar-Be I., Zardo R.D.S., et al. Role of σ B in the Expression of Staphylococcus aureus Cell Wall Adhesins ClfA and FnbA and Contribution to Infectivity in a Rat Model of Experimental Endocarditis. Infect. Immun. 2005;73:990–998. doi: 10.1128/IAI.73.2.990-998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Atwood D.N., Loughran A.J., Courtney A.P., Anthony A.C., Meeker D.G., Spencer H.J., Gupta R.K., Lee C.Y., Beenken K.E., Smeltzer M.S. Comparative impact of diverse regulatory loci on Staphylococcus aureus biofilm formation. MicrobiologyOpen. 2015;4:436–451. doi: 10.1002/mbo3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Waters N.R., Samuels D.J., Behera R.K., Livny J., Rhee K.Y., Sadykov M.R., Brinsmade S.R. A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 2016;101:495–514. doi: 10.1111/mmi.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stenz L., Francois P., Whiteson K., Wolz C., Linder P., Schrenzel J. The CodY pleiotropic repressor controls virulence in gram-positive pathogens. FEMS Immunol. Med. Microbiol. 2011;62:123–139. doi: 10.1111/j.1574-695X.2011.00812.x. [DOI] [PubMed] [Google Scholar]
- 120.Majerczyk C.D., Sadykov M.R., Luong T.T., Lee C., Somerville G.A., Sonenshein A.L. Staphylococcus aureus CodY Negatively Regulates Virulence Gene Expression. J. Bacteriol. 2008;190:2257–2265. doi: 10.1128/JB.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Majerczyk C.D., Dunman P.M., Luong T.T., Lee C.Y., Sadykov M.R., Somerville G.A., Bodi K., Sonenshein A.L. Direct Targets of CodY in Staphylococcus aureus. J. Bacteriol. 2010;192:2861–2877. doi: 10.1128/JB.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roux A., Todd D.A., Velázquez J.V., Cech N.B., Sonenshein A.L. CodY-Mediated Regulation of the Staphylococcus aureus Agr System Integrates Nutritional and Population Density Signals. J. Bacteriol. 2014;196:1184–1196. doi: 10.1128/JB.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mlynek K.D., Bulock L.L., Stone C.J., Curran L.J., Sadykov M.R., Bayles K.W., Brinsmade S.R. Genetic and Biochemical Analysis of CodY-Mediated Cell Aggregation in Staphylococcus aureus Reveals an Interaction between Extracellular DNA and Polysaccharide in the Extracellular Matrix. J. Bacteriol. 2020;202:e00593-19. doi: 10.1128/JB.00593-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rom J.S., Atwood D.N., Beenken K.E., Meeker D.G., Loughran A.J., Spencer H.J., Lantz T.L., Smeltzer M.S. Impact of Staphylococcus aureus regulatory mutations that modulate biofilm formation in the USA300 strain LAC on virulence in a murine bacteremia model. Virulence. 2017;8:1776–1790. doi: 10.1080/21505594.2017.1373926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mlynek K.D., Sause W.E., Moormeier D.E., Sadykov M.R., Hill K.R., Torres V.J., Bayles K.W., Brinsmade S.R. Nutritional Regulation of the Sae Two-Component System by CodY in Staphylococcus aureus. J. Bacteriol. 2018;200:e00012-18. doi: 10.1128/JB.00012-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Conlon K.M., Humphreys H., O’Gara J.P. icaR Encodes a Transcriptional Repressor Involved in Environmental Regulation of ica Operon Expression and Biofilm Formation in Staphylococcus epidermidis. J. Bacteriol. 2002;184:4400–4408. doi: 10.1128/JB.184.16.4400-4408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoang T.-M., Zhou C., Lindgren J.K., Galac M.R., Corey B., Endres J.E., Olson M.E., Fey P.D. Transcriptional Regulation of icaADBC by both IcaR and TcaR in Staphylococcus epidermidis. J. Bacteriol. 2019;201:e00524-18. doi: 10.1128/JB.00524-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jefferson K.K., Pier D.B., Goldmann D.A., Pier G.B. The Teicoplanin-Associated Locus Regulator (TcaR) and the Intercellular Adhesin Locus Regulator (IcaR) Are Transcriptional Inhibitors of the ica Locus in Staphylococcus aureus. J. Bacteriol. 2004;186:2449–2456. doi: 10.1128/JB.186.8.2449-2456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang Y.-M., Jeng W.-Y., Ko T.-P., Yeh Y.-J., Chen C.K.-M., Wang A.H.-J. Structural study of TcaR and its complexes with multiple antibiotics from Staphylococcus epidermidis. Proc. Natl. Acad. Sci. USA. 2010;107:8617–8622. doi: 10.1073/pnas.0913302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schwartbeck B., Birtel J., Treffon J., Langhanki L., Mellmann A., Kale D., Kahl J., Hirschhausen N., Neumann C., Lee J.C., et al. Dynamic in vivo mutations within the ica operon during persistence of Staphylococcus aureus in the airways of cystic fibrosis patients. PLoS Pathog. 2016;12:e1006024. doi: 10.1371/journal.ppat.1006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jefferson K.K., Cramton S.E., Götz F., Pier G. Identification of a 5-nucleotide sequence that controls expression of the ica locus in Staphylococcus aureus and characterization of the DNA-binding properties of IcaR. Mol. Microbiol. 2003;48:889–899. doi: 10.1046/j.1365-2958.2003.03482.x. [DOI] [PubMed] [Google Scholar]
- 132.Yu L., Hisatsune J., Hayashi I., Tatsukawa N., Sato’O Y., Mizumachi E., Kato F., Hirakawa H., Pier G.B., Sugai M. A Novel Repressor of the ica Locus Discovered in Clinically Isolated Super-Biofilm-Elaborating Staphylococcus aureus. mBio. 2017;8:e02282-16. doi: 10.1128/mBio.02282-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.You Y., Xue T., Cao L., Zhao L., Sun H., Sun B. Staphylococcus aureus glucose-induced biofilm accessory proteins, GbaAB, influence biofilm formation in a PIA-dependent manner. Int. J. Med. Microbiol. 2014;304:603–612. doi: 10.1016/j.ijmm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 134.Tiwari N., López-Redondo M., Miguel-Romero L., Kulhankova K., Cahill M.P., Tran P.M., Kinney K.J., Kilgore S.H., Al-Tameemi H., Herfst C.A., et al. The SrrAB two-component system regulates Staphylococcus aureus pathogenicity through redox sensitive cysteines. Proc. Natl. Acad. Sci. USA. 2020;117:10989–10999. doi: 10.1073/pnas.1921307117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park M.K., Myers R.A.M., Marzella L. Oxygen Tensions and Infections: Modulation of Microbial Growth, Activity of Antimicrobial Agents, and Immunologic Responses. Clin. Infect. Dis. 1992;14:720–740. doi: 10.1093/clinids/14.3.720. [DOI] [PubMed] [Google Scholar]
- 136.Pragman A.A., Yarwood J.M., Tripp T.J., Schlievert P.M. Characterization of Virulence Factor Regulation by SrrAB, a Two-Component System in Staphylococcus aureus. J. Bacteriol. 2004;186:2430–2438. doi: 10.1128/JB.186.8.2430-2438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ulrich M., Bastian M., Cramton S.E., Ziegler K., Pragman A.A., Bragonzi A., Memmi G., Wolz C., Schlievert P., Cheung A., et al. The staphylococcal respiratory response regulator SrrAB induces ica gene transcription and polysaccharide intercellular adhesin expression, protecting Staphylococcus aureus from neutrophil killing under anaerobic growth conditions. Mol. Microbiol. 2007;65:1276–1287. doi: 10.1111/j.1365-2958.2007.05863.x. [DOI] [PubMed] [Google Scholar]
- 138.Quoc P.H.T., Genevaux P., Pajunen M., Savilahti H., Georgopoulos C., Schrenzel J., Kelley W.L. Isolation and Characterization of Biofilm Formation-Defective Mutants of Staphylococcus aureus. Infect. Immun. 2007;75:1079–1088. doi: 10.1128/IAI.01143-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mashruwala A.A., Van De Guchte A., Boyd J.M. Impaired respiration elicits SrrAB-dependent programmed cell lysis and biofilm formation in Staphylococcus aureus. eLife. 2017;6:e23845. doi: 10.7554/eLife.23845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kinkel T.L., Roux C.M., Dunman P.M., Fang F.C. The Staphylococcus aureus SrrAB Two-Component System Promotes Resistance to Nitrosative Stress and Hypoxia. mBio. 2013;4:e00696-13. doi: 10.1128/mBio.00696-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lamret F., Varin-Simon J., Velard F., Terryn C., Mongaret C., Colin M., Gangloff S.C., Reffuveille F. Staphylococcus aureus Strain-Dependent Biofilm Formation in Bone-Like Environment. Front. Microbiol. 2021;12:714994. doi: 10.3389/fmicb.2021.714994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cho H., Jeong D.-W., Liu Q., Yeo W.-S., Vogl T., Skaar E.P., Chazin W.J., Bae T. Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Staphylococcus aureus Infections. PLoS Pathog. 2015;11:e1005026. doi: 10.1371/journal.ppat.1005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Q., Yeo W., Bae T. The SaeRS Two-Component System of Staphylococcus aureus. Genes. 2016;7:81. doi: 10.3390/genes7100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jeong D.-W., Cho H., Jones M.B., Shatzkes K., Sun F., Ji Q., Liu Q., Peterson S.N., He C., Bae T. The auxiliary protein complex SaePQ activates the phosphatase activity of sensor kinase SaeS in the SaeRS two-component system of Staphylococcus aureus. Mol. Microbiol. 2012;86:331–348. doi: 10.1111/j.1365-2958.2012.08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liang X., Yu C., Sun J., Liu H., Landwehr C., Holmes D., Ji Y. Inactivation of a Two-Component Signal Transduction System, SaeRS, Eliminates Adherence and Attenuates Virulence of Staphylococcus aureus. Infect. Immun. 2006;74:4655–4665. doi: 10.1128/IAI.00322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Olson M.E., Nygaard T.K., Ackermann L., Watkins R.L., Zurek O.W., Pallister K.B., Griffith S., Kiedrowski M.R., Flack C.E., Kavanaugh J.S., et al. Staphylococcus aureus Nuclease Is an SaeRS-Dependent Virulence Factor. Infect. Immun. 2013;81:1316–1324. doi: 10.1128/IAI.01242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mrak L.N., Zielinska A.K., Beenken K.E., Mrak I.N., Atwood D.N., Griffin L.M., Lee C.Y., Smeltzer M.S. saeRS and sarA Act Synergistically to Repress Protease Production and Promote Biofilm Formation in Staphylococcus aureus. PLoS ONE. 2012;7:e38453. doi: 10.1371/journal.pone.0038453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cue D., Junecko J.M., Lei M.G., Blevins J.S., Smeltzer M., Lee C.Y. SaeRS-Dependent Inhibition of Biofilm Formation in Staphylococcus aureus Newman. PLoS ONE. 2015;10:e0123027. doi: 10.1371/journal.pone.0123027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mashruwala A.A., Gries C.M., Scherr T.D., Kielian T., Boyd J.M. SaeRS Is Responsive to Cellular Respiratory Status and Regulates Fermentative Biofilm Formation in Staphylococcus aureus. Infect. Immun. 2017;85:e00157-17. doi: 10.1128/IAI.00157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wittekind M.A., Frey A., Bonsall A.E., Briaud P., Keogh R.A., Wiemels R.E., Shaw L.N., Carroll R.K. The novel protein ScrA acts through the SaeRS two-component system to regulate virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 2022;117:1196–1212. doi: 10.1111/mmi.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ranjit D.K., Endres J.L., Bayles K.W. Staphylococcus aureus CidA and LrgA Proteins Exhibit Holin-Like Properties. J. Bacteriol. 2011;193:2468–2476. doi: 10.1128/JB.01545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rice K.C., Bayles K.W. Molecular Control of Bacterial Death and Lysis. Microbiol. Mol. Biol. Rev. 2008;72:85–109. doi: 10.1128/MMBR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rice K.C., Mann E.E., Endres J.L., Weiss E.C., Cassat J.E., Smeltzer M.S., Bayles K.W. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 2007;104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leroy S., Lebert I., Andant C., Micheau P., Talon R. Investigating Extracellular DNA Release in Staphylococcus xylosus Biofilm In Vitro. Microorganisms. 2021;9:2192. doi: 10.3390/microorganisms9112192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang S.-J., Dunman P.M., Projan S.J., Bayles K.W. Characterization of the Staphylococcus aureus CidR regulon: Elucidation of a novel role for acetoin metabolism in cell death and lysis. Mol. Microbiol. 2006;60:458–468. doi: 10.1111/j.1365-2958.2006.05105.x. [DOI] [PubMed] [Google Scholar]
- 156.Fujimoto D.F., Brunskill E.W., Bayles K.W. Analysis of Genetic Elements Controlling Staphylococcus aureus lrgAB Expression: Potential Role of DNA Topology in SarA Regulation. J. Bacteriol. 2000;182:4822–4828. doi: 10.1128/JB.182.17.4822-4828.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sharma-Kuinkel B.K., Mann E.E., Ahn J.-S., Kuechenmeister L.J., Dunman P.M., Bayles K.W. The Staphylococcus aureus LytSR Two-Component Regulatory System Affects Biofilm Formation. J. Bacteriol. 2009;191:4767–4775. doi: 10.1128/JB.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moormeier D.E., Endres J.L., Mann E.E., Sadykov M.R., Horswill A.R., Rice K.C., Fey P.D., Bayles K.W. Use of Microfluidic Technology To Analyze Gene Expression during Staphylococcus aureus Biofilm Formation Reveals Distinct Physiological Niches. Appl. Environ. Microbiol. 2013;79:3413–3424. doi: 10.1128/AEM.00395-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lehman M.K., Bose J.L., Sharma-Kuinkel B.K., Moormeier D.E., Endres J.L., Sadykov M.R., Biswas I., Bayles K.W. Identification of the amino acids essential for LytSR-mediated signal transduction in Staphylococcus aureus and their roles in biofilm-specific gene expression. Mol. Microbiol. 2015;95:723–737. doi: 10.1111/mmi.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Windham I.H., Chaudhari S.S., Bose J.L., Thomas V.C., Bayles K.W. SrrAB Modulates Staphylococcus aureus Cell Death through Regulation of cidABC Transcription. J. Bacteriol. 2016;198:1114–1122. doi: 10.1128/JB.00954-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fournier B., Hooper D.C. A New Two-Component Regulatory System Involved in Adhesion, Autolysis, and Extracellular Proteolytic Activity of Staphylococcus aureus. J. Bacteriol. 2000;182:3955–3964. doi: 10.1128/JB.182.14.3955-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Crosby H.A., Schlievert P.M., Merriman J.A., King J.M., Salgado-Pabón W., Horswill A.R. The Staphylococcus aureus Global Regulator MgrA Modulates Clumping and Virulence by Controlling Surface Protein Expression. PLoS Pathog. 2016;12:e1005604. doi: 10.1371/journal.ppat.1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burgui S., Gil C., Solano C., Lasa I., Valle J. A Systematic Evaluation of the Two-Component Systems Network Reveals That ArlRS Is a Key Regulator of Catheter Colonization by Staphylococcus aureus. Front. Microbiol. 2018;9:342. doi: 10.3389/fmicb.2018.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Trotonda M.P., Tamber S., Memmi G., Cheung A.L. MgrA Represses Biofilm Formation in Staphylococcus aureus. Infect. Immun. 2008;76:5645–5654. doi: 10.1128/IAI.00735-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang Q., Jin Z., Sun B. MgrA Negatively Regulates Biofilm Formation and Detachment by Repressing the Expression of psm Operons in Staphylococcus aureus. Appl. Environ. Microbiol. 2018;84:e01008-18. doi: 10.1128/AEM.01008-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jenal U., Reinders A., Lori C. Cyclic di-GMP: Second messenger extraordinaire. Nat. Rev. Genet. 2017;15:271–284. doi: 10.1038/nrmicro.2016.190. [DOI] [PubMed] [Google Scholar]
- 167.Ha D.-G., O’Toole G.A. c-di-GMP and its Effects on Biofilm Formation and Dispersion: A Pseudomonas Aeruginosa Review. Microbiol. Spectr. 2015;3:MB-0003-2014. doi: 10.1128/microbiolspec.MB-0003-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kaplan J. Biofilm Dispersal: Mechanisms, Clinical Implications, and Potential Therapeutic Uses. J. Dent. Res. 2010;89:205–218. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xiong Z.-Q., Fan Y.-Z., Song X., Liu X.-X., Xia Y.-J., Ai L.-Z. The second messenger c-di-AMP mediates bacterial exopolysaccharide biosynthesis: A review. Mol. Biol. Rep. 2020;47:9149–9157. doi: 10.1007/s11033-020-05930-5. [DOI] [PubMed] [Google Scholar]
- 170.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 171.Corrigan R.M., Abbott J.C., Burhenne H., Kaever V., Gründling A. c-di-AMP Is a New Second Messenger in Staphylococcus aureus with a Role in Controlling Cell Size and Envelope Stress. PLoS Pathog. 2011;7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.DeFrancesco A.S., Masloboeva N., Syed A.K., DeLoughery A., Bradshaw N., Li G.-W., Gilmore M.S., Walker S., Losick R. Genome-wide screen for genes involved in eDNA release during biofilm formation by Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 2017;114:E5969–E5978. doi: 10.1073/pnas.1704544114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li L., Li Y., Zhu F., Cheung A.L., Wang G., Bai G., Proctor R.A., Yeaman M.R., Bayer A.S., Xiong Y.Q. New Mechanistic Insights into Purine Biosynthesis with Second Messenger c-di-AMP in Relation to Biofilm-Related Persistent Methicillin-Resistant Staphylococcus aureus Infections. mBio. 2021;12:e0208121. doi: 10.1128/mBio.02081-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fischer A., Kambara K., Meyer H., Stenz L., Bonetti E.-J., Girard M., Lalk M., Francois P., Schrenzel J. GdpS contributes to Staphylococcus aureus biofilm formation by regulation of eDNA release. Int. J. Med. Microbiol. 2014;304:284–299. doi: 10.1016/j.ijmm.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 175.Fechter P., Caldelari I., Lioliou E., Romby P. Novel aspects of RNA regulation in Staphylococcus aureus. FEBS Lett. 2014;588:2523–2529. doi: 10.1016/j.febslet.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 176.Storz G., Vogel J., Wassarman K.M. Regulation by Small RNAs in Bacteria: Expanding Frontiers. Mol. Cell. 2011;43:880–891. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Desgranges E., Marzi S., Moreau K., Romby P., Caldelari I. Noncoding RNA. Microbiol. Spectr. 2019;7:7-2. doi: 10.1128/microbiolspec.GPP3-0038-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ghaz-Jahanian M.A., Khodaparastan F., Berenjian A., Jafarizadeh-Malmiri H. Influence of Small RNAs on Biofilm Formation Process in Bacteria. Mol. Biotechnol. 2013;55:288–297. doi: 10.1007/s12033-013-9700-6. [DOI] [PubMed] [Google Scholar]
- 179.Romilly C., Lays C., Tomasini A., Caldelari I., Benito Y., Hammann P., Geissmann T., Boisset S., Romby P., Vandenesch F. A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in Staphylococcus aureus. PLoS Pathog. 2014;10:e1003979. doi: 10.1371/journal.ppat.1003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Patel N., Nair M. The small RNA RsaF regulates the expression of secreted virulence factors in Staphylococcus aureus Newman. J. Microbiol. 2021;59:920–930. doi: 10.1007/s12275-021-1205-6. [DOI] [PubMed] [Google Scholar]
- 181.Ibberson C.B., Parlet C.P., Kwiecinski J., Crosby H.A., Meyerholz D.K., Horswill A.R. Hyaluronan Modulation Impacts Staphylococcus aureus Biofilm Infection. Infect. Immun. 2016;84:1917–1929. doi: 10.1128/IAI.01418-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim S., Reyes D., Beaume M., Francois P., Cheung A. Contribution of teg49 Small RNA in the 5′ Upstream Transcriptional Region of sarA to Virulence in Staphylococcus aureus. Infect. Immun. 2014;82:4369–4379. doi: 10.1128/IAI.02002-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Manna A.C., Leo S., Girel S., González-Ruiz V., Rudaz S., Francois P., Cheung A.L. Teg58, a small regulatory RNA, is involved in regulating arginine biosynthesis and biofilm formation in Staphylococcus aureus. Sci. Rep. 2022;12:14963. doi: 10.1038/s41598-022-18815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Manna A.C., Kim S., Cengher L., Corvaglia A., Leo S., Francois P., Cheung A.L. Small RNA teg49 Is Derived from a sarA Transcript and Regulates Virulence Genes Independent of SarA in Staphylococcus aureus. Infect. Immun. 2018;86:e00635-17. doi: 10.1128/IAI.00635-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kathirvel M., Buchad H., Nair M. Enhancement of the pathogenicity of Staphylococcus aureus strain Newman by a small noncoding RNA SprX1. Med. Microbiol. Immunol. 2016;205:563–574. doi: 10.1007/s00430-016-0467-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.