Abstract
Cancer is a primary cause of mortality around the world and imposes a significant physiological, psychological, and financial burden on patients. Lipids regulate cell cycle progression and affect cell proliferation, migration, and apoptosis. Therefore, alterations in serum lipid levels might contribute to carcinogenesis. In this article, we review the relationships between triglyceride (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels and different types of cancer. Then, we examine the association between cancer and familial hypercholesterolemia. Finally, we evaluate the impact of statins on different types of cancer. Increased total cholesterol has been reported to increase cellular proliferation and angiogenesis in tumors and inhibit apoptosis. Increased LDL-C has been reported to induce inflammation and increase susceptibility to oxidative damage. HDL-C has anti-oxidation, anti-inflammatory, and antiproliferative properties. Increased levels of serum TG can induce oxidative stress and a chronic inflammatory state and therefore contribute to the proliferation and progression of cancer cells. Statins decrease downstream products of cholesterol synthesis that are crucial in cell proliferation and growth. Thus, lipid components can have prognostic value in cancer and management of serum lipid levels through lifestyle changes and medical therapy can be beneficial in cancer prevention and treatment.
Keywords: Cancer, cholesterol, dyslipidemia, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, statins
INTRODUCTION
Cancer is a primary cause of mortality around the world and imposes a significant physiological, psychological, and financial burden on patients. According to global cancer statistics, 19.3 million cases of cancer were diagnosed in 2020 worldwide and 10 million patients died of cancer. The global burden of cancer in 2040 is estimated to rise by 47% from 2020 and reach 28.4 million cases.[1]
Lipids including triglycerides (TGs) and cholesterols are necessary for storage of energy and maintenance of homeostasis in body cells. Lipids can be transcription factors’ antagonists or agonists.[2] Other lipids regulate cell cycle progression and affect cell proliferation, migration, and apoptosis.[3] Therefore, alterations in serum lipid levels might contribute to carcinogenesis.
Dyslipidemia is defined as downregulated levels of high-density lipoprotein cholesterol (HDL-C) and upregulated levels of TGs and low-density lipoprotein cholesterol (LDL-C) in the plasma.[4] Dyslipidemia is a recognized cardiovascular disease (CVD) risk factor. CVDs and cancer share several risk factors including unhealthy diet, tobacco smoking, alcohol abuse, and sedentary lifestyle.[5] Dyslipidemia can also affect adaptive immunity by altering the function and development of B cells and CD8+ and CD4+ T-cells. This can affect the immune systems’ antitumor activity.[2] Statins are the most widely used lipid-lowering drugs, which inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase.[6]
Accordingly, the possible association between cancer and plasma lipids has been a subject of many investigations. The [graphical abstract] show various effects of dyslipidemia on cancer incidence and prognosis. This article reviews the relationship between total cholesterol, HDL-C, TG, and LDL-C levels and different types of cancer and addresses some of the molecular mechanisms and clinical implications of this relationship. Then, we examine the association between cancer and familial hypercholesterolemia (FH). Finally, we investigate the effects of statins on different types of cancer.
Graphical abstract.
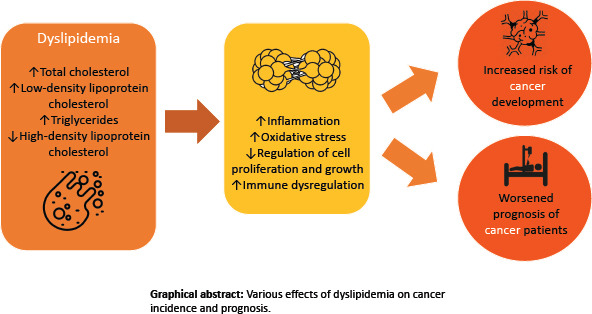
Various effects of dyslipidemia on cancer incidence and prognosis.
We believe that elaboration of the possible impact of lipid components and statins on the risk and prognosis of cancer and their underlying mechanisms in this review would guide physicians and researchers to development of possible therapeutic interventions that can improve cancer patients’ prognosis and outcome. [Figures 1 and 2, Table 1]
Figure 1.
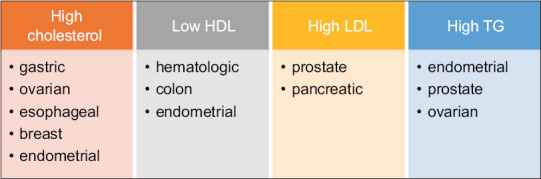
Cancers with increased risk associated with dyslipidemia. HDL: High-density lipoprotein, LDL: Low-density lipoprotein, TG: Triglyceride
Figure 2.
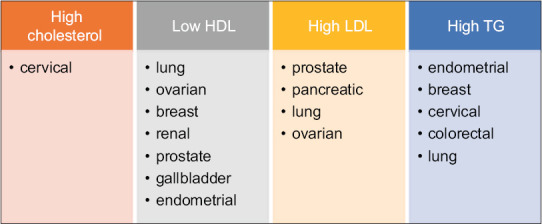
Cancers with worse prognosis associated with dyslipidemia. HDL: High-density lipoprotein, LDL: Low-density lipoprotein, TG: Triglyceride
Table 1.
The associations between cancer and dyslipidemia: A review of studies
| First author (reference number) | Year | Lipid component | Cancer type | Results |
|---|---|---|---|---|
| Miao and Guan[8] | 2021 | Cholesterol | Gastric | High cholesterol was associated with significantly higher risk (35%) of gastric cancer |
| Zhang et al[9] | 2021 | Cholesterol | Ovarian | High cholesterol had a significant association with increased risk of ovarian cancer |
| Zhao et al[10] | 2021 | Cholesterol | Liver | Higher cholesterol had an inverse association with liver cancer |
| Li et al[13] | 2016 | Cholesterol | Breast | Increased cholesterol had a significant association with increased breast cancer risk |
| Jin et al[14] | 2019 | Cholesterol | Esophageal | Increased cholesterol was significantly associated with increased esophageal cancer risk |
| Gong et al[15] | 2016 | Cholesterol | Endometrial | Increased cholesterol had a significant association with increased endometrial cancer risk |
| Lin et al[16] | 2021 | Cholesterol and TG | Cervical | High cholesterol and high TG had a significant association with poor overall survival in patients with cervical cancer |
| Zhong et al[18] | 2020 | HDL-C | General | HDL-C has a J-shaped dose–response association with cancer mortality |
| Zhou et al[19] | 2018 | HDL-C | General | HDL-C was positively associated with overall survival in patients with cancer |
| Jeong et al[20] | 2021 | HDL-C | Hematologic | Low HDL-C had a significant association with increased hematologic cancers’ risk |
| Ma et al[17] | 2021 | HDL-C and TG | Non-small cell lung cancer | Low HDL-C (≤1.26 mmol/L) and high TG (>1.21 mmol/L) and were associated with shorter overall and disease-free survival in non-small cell lung cancer patients |
| Lin et al[21] | 2021 | HDL-C and LDL-C | Epithelial ovarian | LDL-C had a positive significant association with poor overall survival and HDL-C had a positive significant association with better progression-free survival in epithelial ovarian cancer patients |
| Lofterød et al[22] | 2018 | HDL-C and TG | TNBC | TNBC patients with high HDL-C/total cholesterol ratio (≥0.35) had a 67% decreased risk of overall mortality in comparison with those with a low ratio (≤0.27) Overall mortality was three times higher in TNBC patients with high TG (≥1.23 mmol/l) in comparison with those with low TG |
| Tverdal et al[23] | 2021 | HDL-C | Colon | HDL-C had an inverse association with colon cancer in men |
| Hao et al[25] | 2019 | HDL-C | Clear cell renal | High HDL-C predicted better cancer-specific and overall survival in clear cell renal cancer patients |
| Lebdai et al[26] | 2018 | HDL-C | Prostate | Low HDL-C independently predicted locally advanced prostate cancer |
| Yuan et al[27] | 2019 | HDL-C | Gallbladder | Low HDL-C had a significant association with reduced overall survival in gallbladder cancer patients and also closely associated with distant metastasis |
| Notarnicola et al[28] | 2019 | LDL-C | General | Cancer mortality was significantly associated with elevated levels of small dense LDL-C compared to the control group |
| Jung et al[30] | 2021 | LDL-C | Prostate and pancreatic | LDL-C upregulated the production of various oncogene products and promoted migration, invasion, and proliferation of prostate and pancreatic cancer cells |
| Asare et al[31] | 2019 | LDL-C | Prostate | Assessment of oxidized LDL-C level could help discriminate benign prostatic hyperplasia from prostate cancer with a sensitivity of 69.44% and specificity of 88.24% |
| Wang et al[32] | 2021 | LDL-C | Pancreatic | High LDLR level had a significant correlation with a poor prognosis in pancreatic cancer patients |
| Liu et al[33] | 2021 | LDL-C | Small cell lung | High LDL-C was a predictor of disease progression in limited-stage small cell lung cancer patients and was independently associated with poor progression-free and overall survival |
| Zhang et al[35] | 2018 | LDL-C | General | There was no association between levels of LDL-C and long-term cancer-related death risk in twenty years of follow-up |
| Yarla et al[36] | 2021 | TG | Colorectal | TG was found to be a useful prognostic factor in colorectal cancer patients |
| Chen et al[37] | 2020 | TG | Colorectal | High TG (men≥1.53 mmol/L; women, ≥1.58 mmol/L) had a significant association with decreased overall and disease-free survival in high-risk Stage II or Stage III colorectal cancer patients who underwent surgery |
| Ma et al[38] | 2016 | TG | Prostate and breast | TG had no significant association with breast or prostate cancer risk |
| Cheng et al[39] | 2019 | TG | Prostate | Serum lipid levels including TG had no association with recurrence in prostate cancer patients who underwent radical prostatectomy |
| Arthur et al[40] | 2019 | TG and cholesterol | Prostate | Neither TG nor total cholesterol was associated with prostate cancer mortality |
| Trabert et al[41] | 2021 | TG | Ovarian | TG measured two years before diagnosis had a positive association with ovarian cancer risk |
| Lin et al[16] | 2021 | TG | Cervical | High TG was independently and negatively associated with poor overall survival in cervical cancer patients |
| Luo et al[42] | 2019 | HDL-C and TG | Endometrial | TG/HDL-C ratio≥1.52 independently predicted endometrial cancer and was also positively associated with tumor stage |
TG=Triglycerides, HDL-C=High-density lipoprotein cholesterol, LDL-C=Low-density lipoprotein cholesterol, TNBC=Triple-negative breast cancer, LDLR=LDL receptor
TOTAL CHOLESTEROL
Cholesterol accounts for approximately one-third of lipids in the plasma membrane and regulates its integrity, fluidity, and permeability. Cholesterol is essential for cell cycle progression and differentiation, while cholesterol-depleted cells are arrested. Many critical regulators in cell growth, adhesion, migration, and apoptosis, such as mitogen-activated protein kinase (MAPK) and epidermal growth factor receptor, are located in lipid rafts which are cholesterol-enriched microdomains.[3] Cholesterol metabolism alters immune function by regulating various immunobiologic reactions. For example, T-cell receptors are found in lipid rafts in the cell membrane. Lipid rafts are structures that can be affected by cholesterol homeostasis, which can ultimately alter the function of T-cells. Enrichment of cholesterol in lipid rafts can also induce the proliferation and activation of neutrophils and macrophages.[7] Decreased HDL-C and increased LDL-C and total cholesterol can cause a rise in the levels of inflammatory mediators including tumor necrosis factor-α and interleukin-6 (IL-6).[8]
Thus, abnormal levels of plasma cholesterol can lead to dysfunction of proteins and redox reactions and immune dysregulation. These disruptions increase cellular proliferation and angiogenesis and decrease apoptosis in tumors.[3] Evidence of the associations between different types of cancer and total cholesterol is presented below.
Gastric cancer
A recent systematic review showed that increased intake of cholesterol in the diet can significantly increase gastric cancer risk (P = 0.03).[8]
Ovarian cancer
Results of a systematic review indicated that high blood cholesterol had a significant association with an increased ovarian cancer risk.[9]
Liver cancer
A recent meta-analysis showed that elevated total cholesterol had a significant inverse association with liver cancer. However, genetic factors might have influenced this relationship leading to both hypercholesterolemia and liver cancer. Furthermore, patients with hypercholesterolemia are frequently prescribed statins, affecting the pathogenesis of liver cancer.[10]
Breast cancer
Breast cancer is the most common cancer and the most common reason of cancer-related mortality in women worldwide.[11,12] In a dose–response analysis, a nonlinear association was found between breast cancer and the amount of cholesterol in the diet. This relationship was statistically significant when dietary cholesterol intake became more than 370 mg/dL. The relative risks of breast cancer with 95% confidence intervals for 370, 394, and 480 mg/dL of dietary cholesterol were 1.18 (1.04–1.34), 1.27 (1.10–1.45), and 1.70 (1.34–2.16), respectively. One of the proposed mechanisms for this finding is that a cholesterol metabolite named 27HC can act similar to estrogen and induce the proliferation of breast cancer cells that are estrogen receptor positive.[13]
Esophageal cancer
Findings of a meta-analysis revealed that high cholesterol consumption in the diet can increase the esophageal cancer risk in American and European individuals. The authors suggested that alterations in the levels of apolipoproteins and lipids can lead to inflammation and therefore contribute to carcinogenesis in esophageal cells.[14]
Endometrial cancer
A dose–response meta-analysis showed that increased dietary cholesterol consumption might increase endometrial cancer risk. Cholesterol is the primary substrate for synthesizing steroid hormones, including estrogen. Hence, increased cholesterol concentration may influence endometrial cancer risk by increasing estrogen synthesis. In addition, increased cholesterol consumption in the diet is related to increased oxidative stress.[15]
Cervical cancer
A retrospective study on 583 patients with cervical cancer showed that high cholesterol independently predicted worse overall survival (P = 0.002). The suggested mechanism for this finding was that hypercholesterolemia as a metabolic syndrome could cause constant efflux of chemokines and cytokines and therefore induce a prolonged chronic inflammation status. This can lead to stimulation of myeloid-derived immunosuppressor cells and ultimately cause decreased antitumor cytotoxicity and immune surveillance.[16]
HIGH-DENSITY LIPOPROTEIN CHOLESTEROL
HDL-C causes cholesterol to be reversely transported from peripheral cells to liver. HDL-C can have antitumor activity due to its antiproliferative, anti-inflammatory, and anti-oxidation properties. Disrupted cholesterol metabolism caused by low levels of HDL-C can result in carcinogenesis and cancer progression.[17]
A meta-analysis indicated that HDL-C had a significant dose–response association with mortality from cancer in a J-shaped pattern. The lowest risk was at levels of 64–68 mg/dL. Both higher and lower levels conferred an increased cancer mortality risk.[18] Another systematic review showed that the death risk in cancer patients with higher HDL-C was 37% lower than those with lower HDL-C (hazard ratio [HR] = 0.63 [0.47–0.86]). The relapse risk in cancer patients with higher HDL-C was also 35% lower than those with lower HDL-C (HR = 0.65 [0.48–0.89]).[19] Evidence of the associations between different types of cancer and HDL-C is presented below.
Hematologic cancers
A recent study showed that reduced HDL-C had a significant association with increased hematologic cancer risk, including different types of leukemias and lymphomas. The authors suggested that low HDL-C can be considered an independent risk marker for these malignancies. Immune-induced inflammatory reactions lead to alterations of oncogenes and leukemogenesis. HDL-C, especially its protein constituent apolipoprotein A-I, may protect against hematologic cancers through its anti-inflammatory and antioxidant properties. HDL-C also inhibits proliferation of myeloid cells through reduction of their progenitors and IL-3 in the bone marrow. In multiple myeloma, IL-6 activates Ras/MAPK pathway which leads to cellular proliferation. Lack of anti-inflammatory activities of HDL-C may result in increased IL-6 which activates this carcinogenic pathway.[20]
Lung cancer
A retrospective review showed that preoperative serum HDL-C independently predicts overall and disease-free survival in non-small cell lung cancer patients. They found that patients with low preoperative HDL-C (≤1.26 mmol/L) had reduced disease-free and overall survival.[17]
Ovarian cancer
Another study indicated that higher HDL-C was significantly associated with increased progression-free survival (HR = 0.491 [0.247–0.975]) in epithelial ovarian cancer patients. Patients with higher HDL-C (≥1.19 mmol/L) had better progression-free survival in comparison with those with lower HDL-C (P = 0.001).[21]
Breast cancer
Another study showed that HDL-C/total cholesterol ratio had a protective effect on mortality in patients with triple-negative breast cancer. IL-6 increases the progression of tumor in these patients. HDL-C levels have been inversely associated with this cytokines’ activity. HDL-C also lowers the expression of vascular endothelial growth factor (VEGF). Expression of VEGF has been previously correlated with the potential of metastasis in triple-negative breast cancer.[22]
Colon cancer
A recent study indicated that HDL-C had an inverse association with colon cancer in men. They contributed this finding to the anti-inflammatory properties of HDL-C.[23]
Gastric cancer
In their retrospective cohort study, Nam et al. demonstrated that low HDL was associated with increased de novo gastric cancer risk (HR = 2.67 [1.14–6.16]).[24]
Renal cancer
Higher HDL-C had a significant association with increased overall (HR = 0.32 [0.13–0.78]) and cancer-specific (HR = 0.42 [0.15–0.99]) survival in clear cell renal cancer patients. The suggested mechanism was that HDL-C can inhibit the synthesis of the membranes of tumor cells through removing cholesterol from membrane lipid rafts.[25]
Prostate cancer
Low HDL-C independently predicted the local advancement prostate cancer in one study. Intracellular cholesterol was previously shown to act as a substrate in de novo androgen synthesis. Therefore, its high levels can contribute to the progression of prostate cancer. HDL-C may subside disease aggression through removal of cholesterol from cancerous cells and delivering them to the liver.[26]
Gallbladder cancer
Low HDL-C had a significant association with decreased survival in gallbladder cancer patients in one study. HDL-C was suggested as a prognostic marker in these patients. Preoperative serum HDL-C also had a significant association with distant metastasis of gallbladder cancer.[27]
LOW-DENSITY LIPOPROTEIN CHOLESTEROL
Particles of LDL-C activate platelets and damage vascular endothelium, thereby initiating and continuing cellular inflammation. Smaller LDL-C molecules transport more easily through the endothelial cells and induce more severe oxidative damage. A previous study showed that people who had died from cancer had significantly elevated levels of small dense LDL-C compared to the control group.[28] Evidence of the associations between different types of cancer and LDL-C is presented below.
Breast cancer
LDL-C can affect cell adhesion, migration, proliferation, and the sensitivity to radiotherapy in breast cancer cells. Patients with breast cancer have increased oxidized LDL in their serum which alters the structure and decreases the repair of DNA and promotes signaling cascades of oncogenes.[29]
Prostate and pancreatic cancers
Results of an in vitro study showed that LDL-C promoted proliferation, invasion, and migration of prostate and pancreatic cancerous cells and upregulated various oncogenic genes’ expression.[30] Asare et al. showed that assessing oxidized LDL-C levels could help discriminate benign prostatic hyperplasia from prostate cancer with a specificity of 88.24% and sensitivity of 69.44%.[31]
Ovarian cancer
Overall survival was found to be longer for patients with epithelial ovarian cancer who had lower LDL-C (<2.76 mmol/L) in comparison with those with higher LDL-C (≥2.76 mmol/L) in another study (P = 0.028).[21]
Pancreatic cancer
A retrospective study indicated that LDL-C receptor (LDLR) was a potential prognostic factor for pancreatic cancer, and high LDLR has a significant correlation with poor outcomes. LDLR increases cellular cholesterol by receptor-mediated endocytosis. Due to the previously mentioned effects of cholesterol on cell cycle progression, increased cholesterol levels may contribute to carcinogenesis.[17,32]
Lung cancer
One study indicated that the amount of increase in LDL-C was a predictor of cancer advancement and the number of sites involved in limited-stage small-cell lung cancer patients, and that elevation of LDL-C could independently predict poor overall survival and progression-free survival.[33] Similar results from another study also showed that higher LDL-C and LDLR had an independent association with poorer overall survival in small-cell lung cancer patients.[34]
In contrast, an epidemiological study was conducted on a large Chinese population and found no association between levels of LDL-C and long-term risk of cancer-related death in 20 years of follow-up.[35]
TRIGLYCERIDES
TGs are lipid components that act as an independent source for oxidation of fatty acids. They are believed to have a role in carcinogenesis through activation of cellular proliferation in tumors.[17] Evidence of the associations between different types of cancer and TG is presented below.
Colorectal cancer
According to a recent study, most molecules that mediate the TG anabolic pathways are overexpressed or overactivated in the process of colorectal cancer development. In contrast, most molecules that mediate the TG anabolic pathways are inactivated or downregulated. Enzymes that are involved in the metabolism of TG and fatty acids also play a role in colorectal cancer proliferation and progression. Therefore, TG could be useful as a diagnostic and prognostic marker in colorectal cancer.[36] Another study showed that high TG (women: ≥1.58 mmol/L and men: ≥1.53 mmol/L) had a significant association with decreased overall and disease-free survival in high-risk Stage II or III colorectal cancer patients who had undergone surgical resection. The mechanism that the authors suggested for this finding was that hypertriglyceridemia-induced chronic inflammatory response could stimulate the proliferation of cancerous cells. TG levels also have a positive association with cytotoxic fecal bile acids which can cause DNA damage and cause colorectal cancer progression.[37]
Lung cancer
In a retrospective review, researchers found that preoperative serum TG can independently predict overall and disease-free survival non-small cell lung cancer patients. They found that patients who had higher preoperative TG (>1.21 mmol/L) had shorter overall and disease-free survival. The proposed mechanism for this finding is that high TG upregulates signaling cascades in the cells which accelerate the formation of reactive oxygen species, resulting in increased oxidative stress and cancer progression.[17]
Prostate and breast cancer
TG levels correlate with levels of androgens, sex hormone-binding globulin, and testosterone. These hormones have a primary role in the formation and advancement of prostate cancer. Therefore, TG may alter the risk of prostate cancer. High levels of estrogen receptor in the serum cause TG levels to rise, and elevated TG can increase the levels of free estrogen receptor, thereby increasing the risk of breast cancer.[38] However, results of a dose–response analysis showed that TG levels did not have a significant association with breast and prostate cancer.[38] Another meta-analysis also showed that levels of serum lipids including TG in patients who had undergone radical prostatectomy did not have a significant association with the recurrence of prostate cancer.[39] In a cohort study performed by Arthur et al. on prostate cancer patients, neither TG nor cholesterol had a significant association with prostate cancer mortality.[40]
Results of a study indicated that the overall mortality of patients with triple-negative breast cancer with higher TG (≥1.23 mmol/L) was three times higher in comparison with those with lower TG (≤0.82 mmol/L) (HR = 2.99 [1.17–7.63]). The 5-year survival was 19% lower in the former group. TGs are a source of oxidation for fatty acids which are energy fuels for cellular proliferation. Overexpression of fatty acid oxidation pathways leads to increased aggression of cancerous cells. Metastatic triple-negative breast cancer cells also produce a major portion of their required ATP from oxidation of fatty acids.[22]
Ovarian cancer
A nested case–control study in two cohorts in the United Kingdom and the United States showed that the levels of TGs measured 2 years before diagnosis might have a positive association with ovarian cancer risk.[41]
Cervical cancer
A retrospective study on 583 patients with cervical cancer showed that TG levels independently and negatively predicted overall survival (P = 0.001). The suggested mechanism for this finding was that hypertriglyceridemia could induce the constant production of inflammatory cytokines and lead to chronic inflammation, which can ultimately result in the suppression of antitumor immune cells.[16]
Endometrial cancer
A study on 631 postmenopausal women indicated that TG/HDL-C ratio ≥1.52 independently predicted the incidence of endometrial cancer (HR = 4.123, P < 0.001). TG/HDL-C ratio also had a positive association with tumor stage. TG is the main lipid in adipose cells and high TG causes these cells’ size and number to increase. The adipose tissue can produce aromatase, an enzyme that converts androstenedione to estrogen. This reaction provides estrogen in postmenopausal women, which induces proliferation of endometrial cells. Lack of progesterone in these women leads to unchecked cellular proliferation, thereby increasing the risk of endometrial cancer.[42]
Gastric cancer
In one study, TG/HDL-C ratio independently predicted the 5-year mortality of gastric cancer patients.[43]
FAMILIAL HYPERCHOLESTEROLEMIA
FH is a disease with codominant autosomal inheritance caused by defects in proteins that facilitate the clearance of LDL-C in the liver. These proteins include the LDL receptor which clears the LDL-C from plasma (most common), apolipoprotein B, and PCSK9 which is an enzyme that degrades the LDL receptor. These mutations accumulate LDL in the plasma and lead to an increased risk of CVD.[44] It was shown in a previous study that after a median follow-up of 8.7 years, the risk of smoking-related cancer was decreased by 20% in patients with FH; however, the risk of total cancer was the same compared to controls. The incidence of alcohol or obesity-related cancers was also similar in the two groups. Smoking was less prevalent in patients with FH compared to controls in this study, which might explain the reduced smoking-related cancer risk.[5] However, A systematic review on FH comorbidities, showed that cancer had a lower or similar prevalence in FH patients with respect to the general population.[45]
Another study showed that high cholesterol level affect on platelet activity as an inflammatory marker in FH patients which could be related to increase risk of cancer in these patients.[46]
STATINS
Statins are the most used cholesterol-lowering medication around the world. They act through inhibition of HMG-CoA reductase, an enzyme that synthetizes cholesterol. Statins can exert anticancer effects via two signal pathways: dependent or independent of HMG-CoA reductase.[6]
In the dependent pathway, inhibition of HMG-CoA reductase by statins leads to blockade of conversion of HMG-CoA into mevalonate, thereby reducing downstream products including Ras/Rho families of the mevalonate pathway. These products are essential for regulation of signaling cascades that mediate cellular proliferation. In the independent pathway, statins disrupt the interaction between intercellular adhesion molecule-1 and lymphocyte function-associated molecule-1. This action blocks the migration and invasion of cancerous cells. Statins can also inhibit proteasomes, limiting the destruction of cyclin-dependent kinase inhibitors p27 and p21. The reactions mentioned in these two pathways also have immunomodulatory and anti-inflammatory consequences.[6] Evidence of the associations between different types of cancer and statins is presented below.
Prostate cancer
Results of a meta-analysis showed that statin use provided no significant benefit for preventing low-grade prostate cancer. However, statin use was significantly associated with a decreased risk of high-grade prostate cancer (risk ratio [RR] = 0.83 [0.66–0.99]). This was explained by the effect of statins on apoptosis of prostate cancer cells. Statins were able to reduce the advancement of existing cancer, but not prevent cancer initiation. This study also showed that the risk of prostate cancer was not affected by long-term use of statins.[47]
Breast cancer
A meta-analysis of cohort studies revealed that statins significantly decreased cancer-specific mortality and recurrence in breast cancer patients. The proposed mechanisms for these findings include: (1) high expression of HMG-CoA reductase has an association with worse disease-free survival. Statins inhibit this enzyme. (2) Inhibition of HMG-CoA reductase by statins can lead to disruption of prenylation of proteins, which is a process that occurs in carcinogenic signaling pathways. (3) Atorvastatin induces to autophagy in cancerous cells of breast. (4) Lovastatin can cause death of cancerous cells through interacting with p53 signaling pathway. (5) Simvastatin impedes pro-angiogenic factors that are induced by hypoxia-inducible factor-1α, thereby inhibiting angiogenesis in breast tumors.[48]
Ovarian cancer
A meta-analysis concluded that statins have an association with decreased ovarian cancer risk. This effect is different according to the type and duration of statin use and cancer type. Inflammatory conditions such as obesity and endometriosis increase endometrioid ovarian cancer risk. Smoking also increases mucinous ovarian cancer risk through induction of inflammation. Therefore, statins can counteract these risk factors through their anti-inflammatory actions.[49]
Esophageal cancer
A meta-analysis indicated that the use of statins was correlated with lower mortality risk in esophageal cancer patients independent of time of medication use and cancer subtype. HMG-CoA reductase has been shown to have an essential part in the tumorigenicity of esophageal squamous cell carcinoma cells. Moreover, the mevalonate pathway is upregulated in these cells. Esophageal cancerous cells’ growth and viability and the expression of critical metastatic markers are decreased by statins. Statins can also increase the sensitivity to radiation in these cells.[6]
Colon cancer
A study on more than 19,000 patients with colon cancer undergoing elective surgery showed that statin therapy had a significant association with decreased cancer-specific and all-cause mortality 5 years after surgery.[50]
Pancreatic cancer
A cohort study was performed in Japan and it was shown that statin use had a significant association with reduced pancreatic cancer incidence (HR = 0.84 [0.72–0.99]), suggesting that statins may have a role in pancreatic cancer prevention.[51]
Statin in FH patients
A prospective study showed that lipid-lowering therapy with statins reduced cancer-related mortality by 37% in heterozygous FH patients.[52]
CONCLUSIONS
Cholesterol, HDL-C, LDL-C, and TG levels and statins can affect the incidence, progression, and prognosis of different types of cancer, such as lung, prostate, ovary, breast, and gastrointestinal cancers. Management of serum lipid levels through lifestyle changes and medical therapy can be beneficial in cancer prevention and treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kim D, Chung H, Lee JE, Kim J, Hwang J, Chung Y. Immunologic aspects of dyslipidemia: A critical regulator of adaptive immunity and immune disorders. J Lipid Atheroscler. 2021;10:184–201. doi: 10.12997/jla.2021.10.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Škara L, Huđek Turković A, Pezelj I, Vrtarić A, Sinčić N, Krušlin B, et al. Prostate cancer-focus on cholesterol. Cancers (Basel) 2021;13:4696. doi: 10.3390/cancers13184696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su X, Cheng Y, Zhang G, Wang B. Novel insights into the pathological mechanisms of metabolic related dyslipidemia. Mol Biol Rep. 2021;48:5675–87. doi: 10.1007/s11033-021-06529-0. [DOI] [PubMed] [Google Scholar]
- 5.Krogh HW, Svendsen K, Igland J, Mundal LJ, Holven KB, Bogsrud MP, et al. Lower risk of smoking-related cancer in individuals with familial hypercholesterolemia compared with controls: A prospective matched cohort study. Sci Rep. 2019;9:19273. doi: 10.1038/s41598-019-55682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng HY, Lan X, Zheng X, Zha P, Zhou J, Wang RL, et al. The association between statin use and survival of esophageal cancer patients: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e16480. doi: 10.1097/MD.0000000000016480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Zhao W, Li X, He Y. Cholesterol metabolism as a potential therapeutic target and a prognostic biomarker for cancer immunotherapy. Onco Targets Ther. 2021;14:3803–12. doi: 10.2147/OTT.S315998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao P, Guan L. Association of dietary cholesterol intake with risk of gastric cancer: A systematic review and meta-analysis of observational studies. Front Nutr. 2021;8:722450. doi: 10.3389/fnut.2021.722450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, Xi Y, Feng Y. Ovarian cancer risk in relation to blood lipid levels and hyperlipidemia: A systematic review and meta-analysis of observational epidemiologic studies. Eur J Cancer Prev. 2021;30:161–70. doi: 10.1097/CEJ.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 10.Zhao L, Deng C, Lin Z, Giovannucci E, Zhang X. Dietary fats, serum cholesterol and liver cancer risk: A systematic review and meta-analysis of prospective studies. Cancers (Basel) 2021;13:1580. doi: 10.3390/cancers13071580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omidi Z, Koosha M, Nazeri N, Khosravi N, Zolfaghari S, Haghighat S. Status of breast cancer screening strategies and indicators in Iran: A scoping review. J Res Med Sci. 2022;27:21. doi: 10.4103/jrms.jrms_1390_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iraji Z, Jafari Koshki T, Dolatkhah R, Asghari Jafarabadi M. Parametric survival model to identify the predictors of breast cancer mortality: An accelerated failure time approach. J Res Med Sci. 2020;25:38. doi: 10.4103/jrms.JRMS_743_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Yang L, Zhang D, Jiang W. Systematic review and meta-analysis suggest that dietary cholesterol intake increases risk of breast cancer. Nutr Res. 2016;36:627–35. doi: 10.1016/j.nutres.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Jin Y, Yang T, Li D, Ding W. Effect of dietary cholesterol intake on the risk of esophageal cancer: A meta-analysis. J Int Med Res. 2019;47:4059–68. doi: 10.1177/0300060519865632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong TT, Li D, Wu QJ, Wang YZ. Cholesterol consumption and risk of endometrial cancer: A systematic review and dose-response meta-analysis of observational studies. Oncotarget. 2016;7:16996–7008. doi: 10.18632/oncotarget.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin F, Zheng R, Yu C, Su Y, Yan X, Qu F. Predictive role of serum cholesterol and triglycerides in cervical cancer survival. Int J Gynecol Cancer. 2021;31:171–6. doi: 10.1136/ijgc-2020-001333. [DOI] [PubMed] [Google Scholar]
- 17.Ma C, Wang X, Guo J, Liu P. Prognostic significance of preoperative serum triglycerides and high-density lipoproteins cholesterol in patients with non-small cell lung cancer: A retrospective study. Lipids Health Dis. 2021;20:69. doi: 10.1186/s12944-021-01492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong GC, Huang SQ, Peng Y, Wan L, Wu YQ, Hu TY, et al. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: A pooled analysis of 37 prospective cohort studies. Eur J Prev Cardiol. 2020;27:1187–203. doi: 10.1177/2047487320914756. [DOI] [PubMed] [Google Scholar]
- 19.Zhou P, Li B, Liu B, Chen T, Xiao J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clin Chim Acta. 2018;477:94–104. doi: 10.1016/j.cca.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Jeong SM, Choi T, Kim D, Han K, Kim SJ, Rhee SY, et al. Association between high-density lipoprotein cholesterol level and risk of hematologic malignancy. Leukemia. 2021;35:1356–64. doi: 10.1038/s41375-020-01081-5. [DOI] [PubMed] [Google Scholar]
- 21.Lin Q, Liu W, Xu S, Sun L. Associations of preoperative serum high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels with the prognosis of ovarian cancer. Arch Gynecol Obstet. 2022;305:683–91. doi: 10.1007/s00404-021-06215-3. [DOI] [PubMed] [Google Scholar]
- 22.Lofterød T, Mortensen ES, Nalwoga H, Wilsgaard T, Frydenberg H, Risberg T, et al. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancer recurrence and survival by breast cancer subtypes. BMC Cancer. 2018;18:654. doi: 10.1186/s12885-018-4568-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tverdal A, Høiseth G, Magnus P, Næss Ø, Selmer R, Knudsen GP, et al. Alcohol consumption, HDL-cholesterol and incidence of colon and rectal cancer: A prospective cohort study including 250,010 participants. Alcohol Alcohol. 2021;56:718–25. doi: 10.1093/alcalc/agab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam SY, Park BJ, Nam JH, Kook MC. Effect of Helicobacter pylori eradication and high-density lipoprotein on the risk of de novo gastric cancer development. Gastrointest Endosc. 2019;90:448–56.e1. doi: 10.1016/j.gie.2019.04.232. [DOI] [PubMed] [Google Scholar]
- 25.Hao B, Peng X, Bi B, Yu M, Sang C, Chen Z. Preoperative serum high-density lipoprotein cholesterol as a predictor of poor survival in patients with clear cell renal cell cancer. Int J Biol Markers. 2019;34:168–75. doi: 10.1177/1724600819831404. [DOI] [PubMed] [Google Scholar]
- 26.Lebdai S, Mathieu R, Leger J, Haillot O, Vincendeau S, Rioux-Leclercq N, et al. Metabolic syndrome and low high-density lipoprotein cholesterol are associated with adverse pathological features in patients with prostate cancer treated by radical prostatectomy. Urol Oncol. 2018;36:80.e17–24. doi: 10.1016/j.urolonc.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Yuan B, Fu J, Yu WL, Fu XH, Qiu YH, Yin L, et al. Prognostic value of serum high-density lipoprotein cholesterol in patients with gallbladder cancer. Rev Esp Enferm Dig. 2019;111:839–45. doi: 10.17235/reed.2019.6201/2019. [DOI] [PubMed] [Google Scholar]
- 28.Notarnicola M, DE Nunzio V, Tutino V, Veronese N, Guerra V, Osella AR, et al. Integrated small dense low-density lipoprotein profile in cardiovascular disease and cancer: A longitudinal study. Anticancer Res. 2019;39:6035–9. doi: 10.21873/anticanres.13809. [DOI] [PubMed] [Google Scholar]
- 29.Campion O, Al Khalifa T, Langlois B, Thevenard-Devy J, Salesse S, Savary K, et al. Contribution of the low-density lipoprotein receptor family to breast cancer progression. Front Oncol. 2020;10:882. doi: 10.3389/fonc.2020.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung YY, Ko JH, Um JY, Chinnathambi A, Alharbi SA, Sethi G, et al. LDL cholesterol promotes the proliferation of prostate and pancreatic cancer cells by activating the STAT3 pathway. J Cell Physiol. 2021;236:5253–64. doi: 10.1002/jcp.30229. [DOI] [PubMed] [Google Scholar]
- 31.Asare GA, Owusu-Boateng E, Asiedu B, Amoah BY, Essendoh E, Otoo RY. Oxidised low-density lipoprotein, a possible distinguishing lipid profile biomolecule between prostate cancer and benign prostatic hyperplasia. Andrologia. 2019;51:e13321. doi: 10.1111/and.13321. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Huang L, Zhang J, Fan J, Wu H, Xu J. Dyslipidemia in Chinese pancreatic cancer patients: A two-center retrospective study. J Cancer. 2021;12:5338–44. doi: 10.7150/jca.60340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T, Zhou T, Luo F, Yang Y, Zhao S, Huang Y, et al. Clinical significance of kinetics of low-density lipoprotein cholesterol and its prognostic value in limited stage small cell lung cancer patients? Cancer Control. 2021;28:10732748211028257. doi: 10.1177/10732748211028257. Doi: 10.1177/10732748211028257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou T, Zhan J, Fang W, Zhao Y, Yang Y, Hou X, et al. Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC) BMC Cancer. 2017;17:269. doi: 10.1186/s12885-017-3239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Liu J, Wang M, Qi Y, Sun J, Liu J, et al. Twenty-year epidemiologic study on LDL-C levels in relation to the risks of atherosclerotic event, hemorrhagic stroke, and cancer death among young and middle-aged population in China. J Clin Lipidol. 2018;12:1179–89.e4. doi: 10.1016/j.jacl.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Yarla N, Madka V, Rao C. Targeting triglyceride metabolism for colorectal cancer prevention and therapy. Curr Drug Targets. 2022;23:628–35. doi: 10.2174/1389450122666210824150012. [DOI] [PubMed] [Google Scholar]
- 37.Chen XQ, Wu PW, Liu DH, Yan SJ, Shen XM, Yang LY. Prognostic significance of high triglyceride and apolipoprotein B levels in patients with stage III and high-risk stage II colorectal cancer undergoing curative surgery. Oncol Lett. 2020;20:705–14. doi: 10.3892/ol.2020.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma HQ, Cui LH, Li CC, Yu Z, Piao JM. Effects of serum triglycerides on prostate cancer and breast cancer risk: A meta-analysis of prospective studies. Nutr Cancer. 2016;68:1073–82. doi: 10.1080/01635581.2016.1206582. [DOI] [PubMed] [Google Scholar]
- 39.Cheng S, Zheng Q, Ding G, Li G. Influence of serum total cholesterol, LDL, HDL, and triglyceride on prostate cancer recurrence after radical prostatectomy. Cancer Manag Res. 2019;11:6651–61. doi: 10.2147/CMAR.S204947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arthur R, Møller H, Garmo H, Häggström C, Holmberg L, Stattin P, et al. Serum glucose, triglycerides, and cholesterol in relation to prostate cancer death in the Swedish AMORIS study. Cancer Causes Control. 2019;30:195–206. doi: 10.1007/s10552-018-1093-1. [DOI] [PubMed] [Google Scholar]
- 41.Trabert B, Hathaway CA, Rice MS, Rimm EB, Sluss PM, Terry KL, et al. Ovarian cancer risk in relation to blood cholesterol and triglycerides. Cancer Epidemiol Biomarkers Prev. 2021;30:2044–51. doi: 10.1158/1055-9965.EPI-21-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo YZ, Yang Z, Qiu YL, Li XH, Qin LQ, Su QS, et al. Pretreatment triglycerides-to-high density lipoprotein cholesterol ratio in postmenopausal women with endometrial cancer. Kaohsiung J Med Sci. 2019;35:303–9. doi: 10.1002/kjm2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun H, Huang X, Wang Z, Zhang G, Mei Y, Wang Y, et al. Triglyceride-to-high density lipoprotein cholesterol ratio predicts clinical outcomes in patients with gastric cancer. J Cancer. 2019;10:6829–36. doi: 10.7150/jca.35939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaseghi G, Taheri M, Heshmat-Ghahdarijani K, Rayati M, Zarfeshani S, Pourmoghaddas A, et al. Familial Hypercholesterolemia (FH) in Iran: Findings from the Four-Year FH Registry. J Lipids 2021. 2021 Jun 11; doi: 10.1155/2021/9913969. 9913969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaseghi G, Javanmard SH, Heshmat-Ghahdarijani K, Sarrafzadegan N, Amerizadeh A. Comorbidities with Familial Hypercholesterolemia (FH): A Systematic Review. Curr Probl Cardiol. 2022:101109. doi: 10.1016/j.cpcardiol.2022.101109. [DOI] [PubMed] [Google Scholar]
- 46.Vaseghi G, Heshmat-Ghahdarijani K, Taheri M, Ghasempoor G, Hajian S, Haghjooy-Javanmard S, et al. Hematological Inflammatory Markers in Patients with Clinically Confirmed Familial Hypercholesterolemia. Biomed Res Int 2022. 2022 doi: 10.1155/2022/5051434. 5051434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan P, Wei S, Tang Z, Gao L, Zhang C, Nie P, et al. LDL-lowering therapy and the risk of prostate cancer: A meta-analysis of 6 randomized controlled trials and 36 observational studies. Sci Rep. 2016;6:24521. doi: 10.1038/srep24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv H, Shi D, Fei M, Chen Y, Xie F, Wang Z, et al. Association between statin use and prognosis of breast cancer: A meta-analysis of cohort studies. Front Oncol. 2020;10:556243. doi: 10.3389/fonc.2020.556243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irvin S, Clarke MA, Trabert B, Wentzensen N. Systematic review and meta-analysis of studies assessing the relationship between statin use and risk of ovarian cancer. Cancer Causes Control. 2020;31:869–79. doi: 10.1007/s10552-020-01327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pourlotfi A, Ahl Hulme R, Forssten MP, Sjolin G, Bass GA, Cao Y, et al. Statin therapy and its association with long-term survival after colon cancer surgery. Surgery. 2022;171:890–6. doi: 10.1016/j.surg.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Saito K, Sato Y, Nakatani E, Kaneda H, Yamamoto S, Miyachi Y, et al. Statin exposure and pancreatic cancer incidence: A Japanese Regional Population-Based Cohort Study, the Shizuoka Study. Cancer Prev Res (Phila) 2021;14:863–72. doi: 10.1158/1940-6207.CAPR-21-0123. [DOI] [PubMed] [Google Scholar]
- 52.Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: A prospective registry study. Eur Heart J. 2008;29:2625–33. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]


