Abstract
Background:
The aim of this study was to evaluate the effect of coronary artery calcification on disease severity and prognosis in patients with coronavirus disease-2019 (COVID-19).
Materials and Methods:
One hundred and forty-one patients with COVID-19 were included in this study. The severity of pulmonary involvement and calcification of coronary arteries were assessed by computed tomography scan and calcification was classified by two methods: Weston and segmental. In both the methods, patients were divided into three groups with scores of 0, 1–6, and 7–12, which are called groups 1, 2, and 3, respectively.
Results:
The mean age of patients was 54.26 ± 14.55. Difference in score of pulmonary involvement was reported to be significant between deceased and discharged patients (11.73 ± 5.26 and 7.28 ± 4.47, P = 0.002, respectively). In Weston score system, the chance of recovery of Group 1 patients was significantly higher than Group 3 (odds ratio [OR] =6.72, P = 0.05, 95% confidence interval [CI] =1.901–50.257). Similar results were observed in the segmental scoring system (OR =6.34, P = 0.049, 95% CI =1.814–49.416). Despite the higher chance of severe disease in patients with coronary artery calcification, this increase was not statistically significant in either Weston or segmental methods (OR =0.47, P = 0.23 and OR =0.85, P = 0.79, respectively).
Conclusion:
Coronary artery calcification in patients with COVID-19 has a significant association with poor prognosis. However, no significant relationship was observed between this issue and the severity.
Keywords: Calcification, coronary vessels, coronavirus disease-2019, prognosis
INTRODUCTION
The infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a global pandemic, known as coronavirus disease-2019 (COVID-19), started at December 2019 and its widespread crisis still continues. As of January 5, 2021, there were 86,298,213 confirmed cases and 1,864,933 deaths due to COVID-19.[1] Attempts to find an effective treatment for this disease have so far been unsuccessful, and prevention is currently the only way to deal with it. Recently, numerous studies have been published to describe the epidemiology, clinical manifestations, and clinical and imaging features of COVID-19.[2,3,4,5]
The most common underlying diseases in hospitalized patients with COVID-19 were cardiovascular disease, hypertension, and diabetes with a prevalence of 12.11%, 16.37%, and 7.87%, respectively.[6] Among patients with COVID-19, a high prevalence of preexisting cardiovascular disease has been reported, and this underlying disease can lead to higher mortality rates.[7,8,9,10] One study found that patients with underlying cardiovascular disease are not only more vulnerable to deterioration than other patients, but were also more likely to develop abnormal liver function and elevated lactate dehydrogenase and creatinine. In addition, lymphocyte count, hemoglobin, and albumin in these patients are less than other patients.[11]
Recently, in a review study, prognostic factors were reported in patients with COVID-19. A wide range of factors including demographic characteristics, past medical history and comorbidities, symptoms, vital signs, and laboratory factors are involved in disease severity and survival.[12] However, data on the prognostic role of atherosclerosis and vascular calcification in COVID-19 are very limited. The atherosclerotic process evaluation is possible by examining the extent of vascular calcification in imaging assessments; hence, in this study, we want to evaluate the impact of severity of coronary atherosclerotic disease on COVID-19 severity and survival using coronary calcium score in patients without other possible risk factors (such as diabetes and hypertension).
MATERIALS AND METHODS
This prospective study was performed on patients with COVID-19 between March 1 and June 1, 2020, in Shahid Labbafinejad and Shahid Modares Hospitals. Consecutive sampling method was performed. One hundred and forty-one patients were enrolled in the study after confirmation of COVID-19 diagnosis by reverse transcription–polymerase chain reaction. The study process was explained to the patients, and written informed consent was obtained. Exclusion criteria included chronic heart disease, coronary stent, diabetes, hypertension, chronic lung disease (asthma and chronic obstructive pulmonary disease), immunodeficiency, and hematologic diseases (such as acute and chronic leukemia). Patients were divided into moderate and severe groups according to the severity of the disease. Severe disease included fever and symptoms of respiratory infection with one of the followings: (1) respiratory rate more than 30 per min, (2) severe respiratory distress, (3) oxygen saturation <93% in room air, and (4) lung infiltrates >50% of the lung field within 24–48 h.[13] Moderate cases were patients with none of the mentioned factors. Severe cases were admitted to the intensive care unit (ICU). Preliminary information and vital signs of patients were collected at the time of admission, and all patients were evaluated by chest computed tomography (CT) scan. In one of the centers, chest CT scans were performed using a 64-slice scanner (SOMATOM Sensation 64; Siemens Medical Solutions; Erlangen, Germany), and the following scanning parameters were applied: gantry rotation time of 0.5 s, 64 mm ×0.6 mm collimation, pitch factor of 1.24, table speed of 45.2 mm/rotation, tube current of 85 mA, and tube kilovoltage of 120 kVp. The scanning characteristics of the other center were as follows: a 64-slice scanner was used (Brilliance 64; Philips Medical Systems; Ohio, USA), gantry rotation time of 0.5 s, 64 mm ×0.625 mm collimation, pitch factor of 1.4, table speed of 45.2 mm/rotation, tube current of 56 mA, and tube kilovoltage of 120 kVp. CT scan images were evaluated using (Extended Brilliance Workspace; Philips Medical Systems; Best, Netherlands) workstation. The maximum time between the initial clinical evaluation and the CT scan of the patients was 1 day. CT scan was performed in full inspiration and in supine position. The results of the CT scan images were interpreted by two radiologists with 17 years and 4 years of experience in cardiothoracic radiology who were unaware of each other's interpretation. We evaluated the following items in CT scans: extent of pulmonary involvement (focal or diffuse and unilateral or bilateral), involvement of different areas of the lung, evidence of ground-glass opacities, and consolidation. To categorize the severity of pulmonary involvement, we classified the volume of involvement in each lobe into the following degrees: 0, no involvement; 1, ≤25%, 2, 26%–50%; 3, 51%–75%; and 4, >75%. Because there were three (upper, middle, and lower) lobes on the right side, we considered three (upper, lingula, and lower) lobes on the left side, so that the involvement score of each lobe was between 0 and 4 and the total score of each patient would be between 0 and 24. Weston[14,15] and a newly defined segmental scoring systems were used to evaluate the severity of coronary calcification. Weston scoring system is as follows: 0, no observation of calcification; 1, observation of calcification only in the form of a high-density pixel; 2, existence of calcification without blooming artifact; and 3, existence of dense calcification which leads to the appearance of blooming artifact. These scores were applied for each coronary artery, separately, and the total score of the patient was calculated by the sum of the individual scores. In segmental method which is defined by us for the first time, coronary arteries were divided into several segments based on the American Heart Association coronary artery segmentation definitions,[16,17] which are shown in [Table 1], and each segment was divided into three parts. The calcification scoring method in each segment is as follows: (1) less than one-third of its length; (2) one-third to two-thirds of its length; and (3) more than two-thirds of the length of the studied segment. To evaluate the effect of coronary artery calcification (segmental and Weston) on the prognosis and severity of the COVID-19, patients were adjusted based on age, sex, and lung involvement. Patients were followed up for 6 months following their CT assessment to evaluate the long-term symptoms of COVID-19.
Table 1.
Coronary artery segments
| Segment | Name | Description |
|---|---|---|
| 1 | Proximal right | From ostium to ½ distance to the acute margin. Gives rise to the conus branch and sinus node branch |
| 2 | Mid right | Up to the acute margin |
| 3 | Distal right | Up to the RPD origin |
| 4 | Right posterior descending | May be absent if left dominant |
| 5 | Left main | Ostium to bifurcation |
| 6 | Proximal left anterior descending | Up to origin of first septal perforator |
| 7 | Mid left anterior descending | Up to half the distance from the first septal perforator to the cardiac apex |
| 8 | Distal left anterior descending | |
| 9 | First diagonal | May include ramus intermedius |
| 10 | Second diagonal | |
| 11 | Proximal circumflex | Up to and including marginal branch origin |
| 12 | Obtuse marginal | Largest branch |
| 13 | Distal circumflex | Distal to marginal origin |
| 14 | Posterolateral | May be small |
| 15 | Left posterior descending | Present in left dominant system |
RPD=Removable partial denture
Statistical analysis
Mean, standard deviation, frequency and percentage were used to describe the data. Distribution of the studied variables was evaluated by Kolmogorov-Smirnov test and Chi-square test was used to compare the qualitative variables between the groups and t-test Independent test and Fisher exact test were used for quantitative variables. To investigate the effect of coronary artery calcification (with two methods: segmental and Weston) on the prognosis of patients with COVID-19, the subjects were divided into three groups in terms of lung involvement. Furthermore, patients were divided into two groups based on outcome and then adjusted based on age, sex, and severity of lung involvement. Using binary logistic regression test, the effect of coronary calcification on severity and prognosis was investigated. All analyzes were performed by SPSS 25.0 (IBM, NY, USA) statistical software and P < 0.05 was considered statistically significant.
Ethics approval
The study was performed in accordance with the Declaration of Helsinki and was approved by Shahid Beheshti University of Medical Sciences’ ethical committee (ethical code: IR.SBMU.MSP.REC.1399.376).
RESULTS
In this study, 141 patients including 98 men and 43 women were evaluated. The mean age of patients was 54.26 ± 14.55 years. Sixty-four patients were admitted to ICU which considered as severe cases and 11 patients, including 8 men and 3 women, died. The most common symptoms at the time of admission were dyspnea, weakness, lethargy, and anorexia. C-reactive protein was significantly higher in severe patients than in moderate patients (P = 0.004). Baseline characteristics of patients are given in Table 2.
Table 2.
Baseline characteristics of patients
| Severe patients (n=64) | Moderate patients (n=77) | P | |
|---|---|---|---|
| Age | 55.45±15.07 | 52.86±13.89 | 0.296* |
| Sex (male) | 44 | 53 | 0.366** |
| Primary symptoms | |||
| Dyspnea | 43 | 29 | <0.001** |
| Lethargy | 54 | 46 | 0.002** |
| Loss of appetite | 19 | 28 | 0.511** |
| Cough | 37 | 26 | 0.007** |
| Fever | 49 | 31 | <0.001** |
| Diarrhea | 10 | 2 | 0.007** |
| Vital signs | |||
| Blood pressure | 130/80±30/10 | 125/80±20/10 | 0.239* |
| Pulse rate | 95.06±27.07 | 92.56±14.43 | 0.485* |
| Respiratory rate | 27.6±9.2 | 23.9±3.6 | 0.485* |
| Temperature | 38.2±1.9 | 37.14±1.2 | 0.0001* |
| Oxygen saturation (%) | 81.26±11.7 | 90.24±8.6 | <0.001* |
| Laboratory data | |||
| CRP | 64.67±34.41 | 47.76±33.52 | 0.004* |
| ESR | 42.84±28.48 | 49.78±29.98 | 0.16* |
| LDH | 728.42±364.91 | 603.11±524.53 | 0.11* |
| WBC (×103 C/ml) | 7.68±3.18 | 7.11±2.77 | 0.26* |
| Hemoglobin (g/dl) | 12.63±2.28 | 13.18±2.12 | 0.16* |
| Platelet (×103 C/ml) | 178.67±73.38 | 188.46±86.24 | 0.47* |
*t-test, **Fisher’s exact test. CRP=C-reactive protein; LDH=Lactate dehydrogenase; ESR=Erythrocyte sedimentation rate; WBC=White blood cells
Pulmonary involvement was assessed in all patients by CT scan. The mean score of pulmonary involvement in severe and moderate patients was not significantly different (7.64 ± 4.57 and 7.63 ± 4.83, P = 0.947, respectively). However, a significant difference was observed in deceased and discharged patients (11.73 ± 5.26 and 7.28 ± 4.47, P = 0.002, respectively) [Figure 1].
Figure 1.
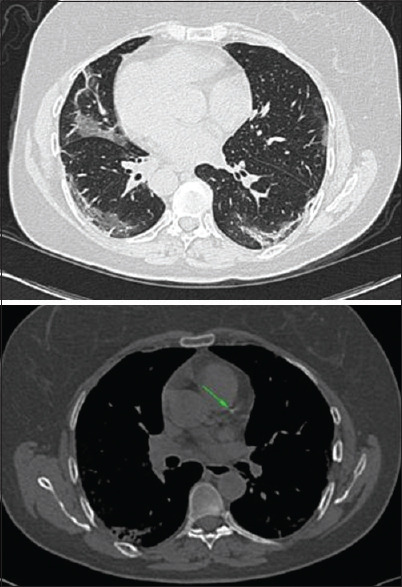
A 55-year-old male presented with 3-day history of fever and dry cough without any comorbidity with initial lung CT involvement score of twelve. The score of involvement of the right lung lobes was as follows: right lower lobe: 2, right upper lobe: 2, right middle lobe: 1. In the left lung lobes, the involvement scores were as follows: left lower lobe: 2, lingula: 3, left upper lobe: 2. Furthermore, the score of coronary calcification based on Weston and segmental methods was 2, 1, and 3, respectively. The arrow shows calcification in the coronary arteries. CT = computed tomography
As shown in Table 3, the difference in the rate of coronary artery calcification was insignificant between severe and moderate cases, but this difference was significantly different between survived and nonsurvived patients. To more accurately investigate the adjusted effect of coronary artery calcification on disease severity and survival in patients with COVID-19, patients were divided into three groups of 0, 1–6, and 7–12 based on their Weston score and were named Weston groups 1, 2, and 3, respectively [Table 4]. Regarding Weston's method, it was found that patients in Group 3 had a slight increase in risk compared to the other two groups for severe disease, but this increase in risk was not statistically significant. However, Group 1 patients had a better survival rate than Group 3 patients. The odds of recovery in this group was 6.72 times the group 3 (P = 0.05). Furthermore, in Group 3, the odds of death were 4.47 times higher than Group 2. However, this increase in risk was statistically borderline (P = 0.065). Similarly, segmental scoring revealed that patients in Group 3 did not have a significantly increased risk of developing severe disease and hospitalization and ICU admission compared to groups 1 and 2. However, in relation to the survival, it was observed that the chance of discharge of patients in Group 1 of segmental classification was 6.34 times the Group 3 (P = 0.049). Although the survival rate of Group 3 was lesser than Group 2 and the probability of death in this group was 3.40 times the Group 2, this increment of risk was not statistically significant.
Table 3.
Comparison of lung involvement and coronary artery calcification scores
| Total | Severe patients (n=64) | Moderate patients (n=77) | P | Deceased | Discharged | P | |
|---|---|---|---|---|---|---|---|
| Mean score of lung involvement | 7.63±4.67 | 7.63±4.83 | 7.64±4.57 | 0.947 | 11.73±5.26 | 7.28±4.47 | 0.002 |
| Mean score of Weston method | 2.16±2.92 | 2.3±3.26 | 2.04±2.62 | 0.603 | 4.64±3.29 | 1.95±2.8 | 0.003 |
| Mean score of segmental method | 2.4±3.6 | 2.4±3.29 | 2.39±3.96 | 0.984 | 5.91±5.61 | 2.1±3.23 | 0.001 |
Table 4.
Relationship between Weston and segmental subgroups with disease severity and prognosis
| Total (%) | Severity | Prognosis | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| OR | 95% CI | P* | OR | 95% CI | P* | ||
| Weston classification | |||||||
| 0 | 71 (50.4) | 0.473 | 0.137–1.636 | 0.237 | 6.728 | 1.901–50.257 | 0.05 |
| 1–6 | 53 (37.6) | 0.451 | 0.144–1.414 | 0.172 | 4.474 | 0.913–21.911 | 0.065 |
| 7–12 | 17 (12.1) | ||||||
| Segmental classification | |||||||
| 0 | 71 (50.4) | 0.854 | 0.26–2.801 | 0.794 | 6.341 | 1.814–49.416 | 0.049 |
| 1–6 | 53 (37.6) | 0.831 | 0.271–2.549 | 0.747 | 3.407 | 0.658–17.628 | 0.144 |
| 7–12 | 17 (12.1) | ||||||
*Adjusted for age, sex, and lung involvement. OR=Odds ratio; CI=Confidence interval
Patients were followed up for 6 months after their first CT for the evaluation of long-term complications of COVID-19. Three patients complained of fatigue and one patient complained of shortness of breath. One patient had lower extremity deep vein thrombosis (DVT) 2 weeks after discharge and was medically treated.
The findings indicate that radiologists can estimate the prognosis of patients with COVID-19 in the same age, gender, and pulmonary involvement status and extent by assessing coronary artery calcification in the mediastinal view of CT scans. Patients with a coronary artery calcification score of more than 7 need more care and attention, as this group has been shown to have higher mortality rates.
DISCUSSION
COVID-19 pandemics has now become a global crisis that has affected more than 200 countries around the world and has led to the collapse of health-care systems in many parts of the world.[18,19] SARS-Cov-2, like SARS and Middle East respiratory syndrome, causes respiratory infections and can lead to viral pneumonia and acute respiratory distress syndrome in involved patients.[20] In addition, uncontrolled SARS-Cov-2 infection can cause cytokine storm and multiorgan damage by proinflammatory cytokines.[21] Currently, studies have shown that both susceptibility and poor prognosis of COVID-19 are strongly associated with cardiovascular disease.[22,23] In addition, COVID-19 can cause development of cardiovascular disorders such as myocardial injury, arrhythmia, acute coronary syndrome, and venous thromboembolism.[24,25] However, the role of atherosclerosis and vascular calcification in the prognosis of patients with COVID-19 has not been accurately evaluated. So far, very limited and scattered studies have been performed on coronary calcification in patients with COVID-19 and its relationship with prognosis.
In this study, the correlation between coronary artery calcification in patients with COVID-19 with their disease severity and prognosis was evaluated. The severity of coronary calcification was assessed by Weston and segmental methods. Although coronary calcification was not significantly different between severe and moderate patients, this difference was significant between deceased and discharged patients. In both the methods, it was observed that the odds of discharge and recovery of Group 1 patients were significantly higher than Group 3. Although the odds of recovery of patients with a score of 1–6 were higher than patients with that of 7–12, this increase in the probability of recovery was not statistically significant. However, no significant association was observed between coronary artery calcification and disease severity.
Dillinger et al. investigated the effect of coronary calcification on the initial outcome, including the need for invasive or noninvasive mechanical ventilation or death in the first 30 days of hospitalization in patients with COVID-19. It was observed that the incidence of initial outcome in patients with coronary artery calcification was significantly higher than patients without calcification (P < 0.001). Furthermore, Cox analysis revealed that coronary artery calcification, regardless of age, sex, hypertension, diabetes, and smoking, was associated with initial outcome.[26] Although in our study, coronary artery calcification was associated with poor prognosis and mortality, no significant association was found between this variable and the severity of disease. This may be due to the presence of other comorbidities, such as chronic lung disease, which were not excluded in the previous studies and can affect the severity of COVID-19, independent of coronary artery calcification.
Alhaj et al. examining 1720 patients with pulmonary emphysema by electron beam CT found that there was a significant relationship between coronary calcification score above 100 and pulmonary emphysema (P = 0.013). In addition, age, sex, hypertension, and smoking play a role in the development of coronary calcification, independently and significantly.[27]
In the follow-up of patients for long-term complications of COVID-19, fatigue and shortness of breath were observed in 3 and 1 patients, respectively. In the study of Carfì et al., the most common long-term complications reported in patients with COVID-19 were fatigue and shortness of breath. These two symptoms were reported in 53.1% and 43.4% of patients, respectively.[28] These results were similar to the findings of our study. However, the reduction in the long-term symptom rate in our study may be due to the follow-up time difference of the patients, which was 2 months in the mentioned study and 6 months in ours.
Left lower extremity DVT was reported in one of our patients, approximately 2 weeks after discharge from the hospital and was medically treated. Coagulopathy is a common finding in patients with COVID-19 due to systemic inflammatory response syndrome. Approximately 20%–50% of patients admitted with COVID-19 have hematologic changes in coagulation tests (elevated D-dimer, prolonged prothrombin time, thrombocytopenia, and/or low fibrinogen levels). These changes are more associated with thrombotic events than hemorrhagic ones.[29] In addition, COVID-19 infection can cause venous or arterial thrombosis due to increased inflammatory response.[30]
There were limitations in this study. The lack of information about active or passive smoking and pulmonary function tests was the most important limitation of this study.
CONCLUSION
It was found that coronary artery calcification in patients with COVID-19 has a significant association with poor prognosis and mortality. Given the high agreement between the interpretations of the two radiologists, it can be concluded that the assessment of coronary artery calcification on CT scan can also be performed accurately by novice radiologists. The chance of discharge and recovery of patients without calcification of coronary arteries is significantly higher than patients with calcification (odds ratio =6.72, P = 0.05). However, no significant relationship was observed between this variable and the severity of the disease. Coronary artery calcification is a prognostic factor in COVID-19, and by reporting it on CT scan results, it is possible to draw more attention to patients with this factor (especially with a score above 7). Therefore, by providing more effective medical care to patients with COVID-19 and presence of coronary artery calcification, it is possible to reduce mortality in these patients. Based on our findings, it might be recommended that radiologists who encounter extensive and heavily calcified coronary artery plaques when interpreting the chest CT of COVID-19 patients mention the high coronary calcification in their reports to let clinicians know about the higher risk of mortality and poor prognosis in their patients and to provide a more intense care.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the legal guardian has given her consent for images and other clinical information to be reported in the journal. The guardian understands that her names and initials will not be published and due efforts will be made to conceal the patient's identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge all patients who participated in our study.
REFERENCES
- 1.COVID-19 Coronavirus Pandemic. [Last accessed on 2020 Jan 28]. Available from: https://www.worldometers.info/coronavirus .
- 2.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–23. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–4. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alahyari S, Rajaeinejad M, Jalaeikhoo H, Chegini L, Almasi Aghdam M, Asgari A, et al. Immunological evaluation of patients with 2019 novel coronavirus pneumonia: CD4+ and CD16+ cells may predict severity and prognosis. PLoS One. 2022;17:e0268712. doi: 10.1371/journal.pone.0268712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrishami A, Eslami V, Arab-Ahmadi M, Alahyari S, Azhideh A, Sanei-Taheri M. Prognostic value of inflammatory biomarkers for predicting the extent of lung involvement and final clinical outcome in patients with COVID-19. J Res Med Sci. 2021;26:115. doi: 10.4103/jrms.JRMS_1160_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: A systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 7.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–58. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushita K, Ding N, Kou M, Hu X, Chen M, Gao Y, et al. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: A systematic review and meta-analysis. Glob Heart. 2020;15:64. doi: 10.5334/gh.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15:e0243191. doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Lu S, Wang X, Jia X, Li J, Lei H, et al. Do underlying cardiovascular diseases have any impact on hospitalised patients with COVID-19? Heart. 2020;106:1148–53. doi: 10.1136/heartjnl-2020-316909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS One. 2020;15:e0241955. doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 14.Chandra D, Gupta A, Leader JK, Fitzpatrick M, Kingsley LA, Kleerup E, et al. Assessment of coronary artery calcium by chest CT compared with EKG-gated cardiac CT in the multicenter AIDS cohort study. PLoS One. 2017;12:e0176557. doi: 10.1371/journal.pone.0176557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirsch J, Buitrago I, Mohammed TL, Gao T, Asher CR, Novaro GM. Detection of coronary calcium during standard chest computed tomography correlates with multi-detector computed tomography coronary artery calcium score. Int J Cardiovasc Imaging. 2012;28:1249–56. doi: 10.1007/s10554-011-9928-9. [DOI] [PubMed] [Google Scholar]
- 16.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 17.Young PM, Gerber TC, Williamson EE, Julsrud PR, Herfkens RJ. Cardiac imaging: Part 2, normal, variant, and anomalous configurations of the coronary vasculature. AJR Am J Roentgenol. 2011;197:816–26. doi: 10.2214/AJR.10.7249. [DOI] [PubMed] [Google Scholar]
- 18.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–9. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: A review. JAMA Cardiol. 2020;5:831–40. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 23.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–60. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–10. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dillinger JG, Benmessaoud FA, Pezel T, Voicu S, Sideris G, Chergui N, et al. Coronary artery calcification and complications in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2468–70. doi: 10.1016/j.jcmg.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alhaj EK, Alhaj NE, Bergmann SR, Hecht H, Matarazzo TJ, Smith S, et al. Coronary artery calcification and emphysema. Can J Cardiol. 2008;24:369–72. doi: 10.1016/s0828-282x(08)70598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carfì A, Bernabei R, Landi F Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–5. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-Mesa JE, Galindo-Coral S, Montes MC, Muñoz Martin AJ. Thrombosis and coagulopathy in COVID-19. Curr Probl Cardiol. 2021;46:100742. doi: 10.1016/j.cpcardiol.2020.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivas D, Roldán V, Esteve-Pastor MA, Roldán I, Tello-Montoliu A, Ruiz-Nodar JM, et al. Recommendations on antithrombotic treatment during the COVID-19 pandemic. Position statement of the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology. Rev Esp Cardiol. 2020;73:749–57. doi: 10.1016/j.rec.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


