Abstract
Background:
In major depressive disorder (MDD), cognitive dysfunctions strongly contribute to functional impairments but are barely addressed in current therapies. Novel treatment strategies addressing cognitive symptoms in depression are needed. As the gut microbiota–brain axis is linked to depression and cognition, we investigated the effect of a 4-week high-dose probiotic supplementation on cognitive symptoms in depression.
Methods:
This randomized controlled trial included 60 patients with MDD, of whom 43 entered modified intention-to-treat analysis. A probiotic supplement or indistinguishable placebo containing maltose was administered over 31 days in addition to treatment as usual for depression. Participant scores on the Verbal Learning Memory Test (VLMT), Corsi Block Tapping Test, and both Trail Making Test versions as well as brain-derived neurotrophic factor levels were assessed at 3 different time points: before, immediately after and 4 weeks after intervention. Additionally, brain activation changes during working memory processing were investigated before and immediately after intervention.
Results:
We found a significantly improved immediate recall in the VLMT in the probiotic group immediately after intervention, and a trend for a time × group interaction considering all time points. Furthermore, we found a time × group interaction in hippocampus activation during working memory processing, revealing a remediated hippocampus function in the probiotic group. Other measures did not reveal significant changes.
Limitations:
The modest sample size resulting from our exclusion of low-compliant cases should be considered.
Conclusion:
Additional probiotic supplementation enhances verbal episodic memory and affects neural mechanisms underlying impaired cognition in MDD. The present findings support the importance of the gut microbiota–brain axis in MDD and emphasize the potential of microbiota-related regimens to treat cognitive symptoms in depression.
Clinical trial registration:
clinicaltrials.gov identifier NCT02957591.
Introduction
Major depressive disorder (MDD) affects about 264 million people worldwide and is a leading cause of the global disability burden.1 Next to affective symptoms, cognitive symptoms characterize depression.2 Several studies have shown decreased cognitive performance in patients with MDD in domains such as memory and attention.3 In particular, episodic memory deficits are associated with higher depression scores4 and pathological changes such as reduced hippocampus size.5 Low verbal episodic memory performance is considered a premorbid marker of depression.6 Although these depression-related cognitive dysfunctions enormously impair functionality,7 they are barely addressed in current therapies. 8 Usual treatments, including psychotherapy9 and antidepressant medication,10 hardly improve depression-related cognitive impairments. Thus, novel and more efficient treatment strategies addressing affective and cognitive symptoms in depression are required.
Recently, the gut microbiota–brain axis has received increasing attention as a novel treatment target in depression. Studies have linked the gut microbiota to the central nervous system, postulating a bidirectional communication between the gut and brain via several pathways.11,12 Studies have repeatedly reported altered microbiota composition in patients with depressive symptoms compared with controls. 13 Recently, researchers found gut microbiota disturbances to be associated with a decline in anti-inflammatory butyrate-producing bacteria and an increase in proinflammatory bacteria in patients with depression.14 Furthermore, abundances of the butyrate-producing Faecalibacterium and Coprococcus bacteria have been associated with higher quality of life indicators.15 Importantly, 2 independent studies16,17 showed that rodents receiving fecal microbiota transplantation (FMT) from depressed humans had higher inflammation levels and acted in an increased depressive-like manner compared with rodents receiving FMT from healthy volunteers. Supporting the notion of the microbiota’s causal role in MDD, preclinical evaluation in rodents suggests that restoring the gut microbiota ecosystem via probiotics alleviates depressive symptoms by affecting depression-related factors along the gut microbiota–brain axis.18 Indeed, in the main analysis of this randomized controlled trial (RCT), we found significantly reduced depression scores and stabilized microbial diversity and richness after a short-term, high-dose probiotic supplementation in patients with depression.19
Probiotics may also improve several cognitive functions, including verbal episodic memory, in healthy individuals and in patients with MDD, Alzheimer disease, and other disorders. 20 Studies of FMT in rats have underscored the causal role of the gut microbiota in cognitive performance: transplanting microbiota from old to young rats resulted in impaired cognitive performance and reduced expression of brain-derived neurotrophic factor (BDNF),21 a biomarker tightly linked to hippocampal neurogenesis22 and memory,23 whereas transplanting microbiota from young to old rats resulted in improved cognitive performance and changes in the hippocampal metabolome.24 Currently, there is only 1 study investigating the probiotic effect on cognition in a depressed population: Rudzki and colleagues showed that a placebo-controlled supplementation of Lactobacillus plantarum significantly improved verbal memory recall in people with depression.25
This secondary analysis of our RCT19 examined whether a probiotic multistrain supplement add-on therapy would improve cognitive symptoms in patients with depression. Patients took a multistrain probiotic for a 4-week period in addition to treatment as usual, and their performance was assessed in different cognitive domains up to 4 weeks after the intervention. To determine whether probiotics directly affect the neural underpinnings of cognitive impairments in depression, we tested for changes in brain function during a 2-back working memory task and serum BDNF concentrations. Based on previous findings, 25 and because the hippocampus is strongly associated with the gut microbiota–brain axis26 and memory deficits in depression,27 we expected to see beneficial effects of the administered probiotics specifically in hippocampus-related memory tasks (i.e., verbal learning memory task [VLMT], and the hippocampal activation during the 2-back task).
Methods
The present study is a secondary analysis of an RCT in which we investigated the effect of a probiotic add-on treatment in patients with depression, demonstrating alleviation of depressive symptoms along with changes in the gut microbiota and brain activity.19 Data were collected between March 2017 and January 2020 in Basel, Switzerland. The study was approved by the local ethics committee (Ethikkommission Nordwest und Zentralschweiz EKNZ) and conducted in accordance with the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov before the study start (identifier NCT02957591).
Participants
Inpatients with depressive episodes (codes F31.3–F34 in the International Classification of Diseases 10th Revision [ICD-10]) were recruited at the University Hospital of Psychiatry, Basel, Switzerland. Eligible participants had a primary diagnosis of MDD and Hamilton Rating Scale for Depression (HAM-D)28 scores greater than 7 (mild depression29), were 18 years or older, were undergoing treatment as usual for depression and had no psychiatric comorbidities (e.g., schizophrenia, bipolar disorder, substance use disorder). They also had to be able to read and understand the participants’ information materials and give informed consent. Detailed inclusion and exclusion criteria have been described previously.19
Study intervention
In addition to treatment as usual, patients consumed a probiotic supplement (Vivomixx in Europe and Visbiome in the United States) for 4 weeks (31 d). The supplement contained 8 different strains: Streptococcus thermophilus NCIMB 30438, Bifidobacterium breve NCIMB 30441, B. longum NCIMB 30435 (reclassified as B. lactis), B. infantis NCIMB 30436 (reclassified as B. lactis), L. acidophilus NCIMB 30442, L. plantarum NCIMB 30437, L. paracasei NCIMB 30439, and L. delbrueckii subsp and Bulgaricus NCIMB 30440 (reclassified as L. helveticus). The probiotic product contained 9 × 1010 colony forming units (CFU)/g bifidobacteria, 8 × 1010 lactobacilli, and 20 × 1010 of S. salivarius subsp. Thermophilus,30 resulting in a daily dose of 900 billion CFU/d that could be mixed with any cold, noncarbonated drink. In the control group, participants received a placebo containing maltose but no bacteria. The placebo was visually indistinguishable from the probiotic supplement.
Study design and procedure
Patients were randomly allocated to the experimental or control group in a 1:1 ratio using a conventional randomization software. An independent psychologist not otherwise involved in the investigation administered the assignments. Both groups were tested at 3 different time points: before, immediately after and 4 weeks after the intervention. Before and immediately after the intervention, participants completed assessments consisting of cognitive measures, brain imaging and blood sampling. Four weeks after the intervention, participants completed a final follow-up assessment including all measures except brain imaging. We collected participant demographic information at baseline. During their inpatient treatment, patients received a standardized diet containing stable amounts of starch and fibres. In the course of the intervention patients’ usual medication was registered.19
Patients had the right to withdraw from the study without giving a reason. Adverse events challenging their health, severe protocol violations or administrative troubles resulted in withdrawal. Investigators and assessors were blinded during data collection and analysis, whereas patients were independently informed of their group allocation after the last assessment.
Cognitive measures
To examine probiotic-induced changes in episodic memory, we administered the VLMT31 immediately after the intervention. Furthermore, we administered the Corsi block-tapping test32 and the Trail Making Test (TMT) A and B33 to assess cognitive components such as memory capacity, working memory, attention and mental flexibility. An overview of all cognitive tests is provided in Table 1.
Table 1.
Brief descriptions of the cognitive tasks used
| Cognitive test | Description | Cognitive domain | Outcome | Measure |
|---|---|---|---|---|
| Verbal Learning Memory Test | A list of 15 nouns is read to the participant and then recalled by the participant after each learning trial (5 times, Dg1–Dg5). Then an interference list of 15 nouns is read out to and recalled by the participant. The original list is recalled immediately after the interference list (Dg6) and 20–30mins after the interference list (Dg7). | Verbal learning and memory | Immediate recall | ∑ Dg1–Dg5 |
| Intermediate recall | Dg5–Dg6 | |||
| Long-term recall | Dg5–Dg7 | |||
| Corsi block tapping test | The clinician indicated a sequence by tapping on blocks in a specific order, beginning with 2 blocks, successively adding more blocks to the sequence. In the forward condition, the participants repeat the sequence after the clinician. In the backward condition, the participants repeat the sequence, but start with the last block. | Visual-spatial short-term memory and working memory | Corsi block forward | Number of correct sequences forward |
| Corsi block backward | Number of correct sequences backward | |||
| Trail Making Test | The participant connects a series of numbers (TMT A) or numbers and letters (TMT B) in the right order as fast as possible without lifting the pen. | Attention and executive functioning | TMT A | Seconds needed for task completion |
| TMT B | ||||
| 2-back task | Participants are presented with a sequence of letters and have to indicate when the current letters match the one from N-steps earlier in the sequence. The 2-back task consists of 3 different conditions with N = 0, N = 1, and N = 2. | Working memory | Reaction time difference | Mean reaction time for correct answers (in seconds) |
| Accuracy | Correctly given answers/number |
TMT = Trail Making Test; VLMT = Verbal Learning Memory Test.
BDNF
For the assessment of serum BDNF levels, blood samples were always drawn at 7 am after overnight fasting according to a standardized protocol, using serum monovette (10 mL) and kept frozen at −80°C until analysis. Detailed BDNF analyses are provided in Appendix, 1 supplemental Methods, available at jpn.ca/lookup/doi/10.1503/jpn.220117/tab-related-content.
Brain function
To reveal probiotic-related brain activation changes during working memory processing, patients underwent a 2-back task using fMRI.34,35 Patients’ performance during scanning was recorded for accuracy (i.e., the number of target letters correctly identified divided by the number of presented target letters, and mean reaction time [RT] to target letters) ( Table 1). Immediately before scanning, all participants received a thorough task instruction with another stimuli set to ensure the task was fully understood. We conducted a whole brain analysis and an a priori–defined region of interest analysis on the hippocampus, as we expected that the hippocampal activation during the 2-back task specifically would be affected by the probiotic supplementation.
Functional imaging data were analyzed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). For further details on the task procedure, image acquisition and data analysis, see Appendix 1, supplemental Methods.
Statistical analyses
To analyze changes in cognition and BDNF, we compared change scores from baseline to post-intervention between groups using 2-sample t tests and the corresponding effect size (Cohen d). Furthermore, we used a random intercept model including all 3 time points (baseline, post-intervention, follow-up) as within-subjects factors and treatment group (probiotics, placebo) as between-subjects factors to address changes in cognition over all 3 time points. A modified intention to treat (ITT) analysis was conducted, excluding missing assessments at the post-intervention time point (n = 13) and noncompliant participants (n = 4). The compliance rate cut-off of greater than 65%36 resulted in the exclusion of 2 patients per group from the statistical analyses (for details see Schaub and colleagues19). To analyze the associations between cognitive performance, brain activation and BDNF levels, we used Pearson correlation, and Fisher z (implemented in the R cocor package) to compare the correlations between groups.37 All analyses regarding cognition and BDNF were completed in RStudio 1.3.1093.
Results
Participants
Of the 60 eligible participants at baseline, 47 completed the intervention, resulting in a dropout rate of 30% in the probiotics group and 13% in the placebo group (Appendix 1, Figure S1). Additionally, 2 participants in each group were excluded owing to noncompliance (compliance ≤ 65 %), resulting in a final sample of 43 participants (mean age 38.56 ± standard deviation [SD] 10.71 yr): 19 in the probiotics group (14 female) and 24 in the placebo group (12 female). One additional participant in each group withdrew from the study between the post-intervention and follow-up assessments. Table 2 presents the demographic and clinical characteristics of all randomized participants (n = 60), participants who completed the study (n = 47) and participants retained for analyses (n = 43). Further sample information (e.g., hospitalization duration) is available in Appendix 1, Tables S1–S3.
Table 2.
Demographic and clinical characteristics of both study groups at baseline
| Characteristic | Randomized sample n = 60 |
CCA* sample n = 47 |
Modified ITT† sample n = 43 |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Probiotic n = 30 |
Placebo n = 30 |
Group comparison | Probiotic n = 21 |
Placebo n = 26 |
Group comparison | Probiotic n = 19 |
Placebo n = 24 |
Group comparison | |
| Sex, female, n (%) | 20 (66.7) | 16 (53.33) | χ21 = 0.63, p = 0.43 | 14 (67) | 13 (50) | χ21 = 0.73, p = 0.49 | 14 (74) | 12 (50) | χ21 = 1.60, p = 0.21 |
| Age, mean ± SD | 38.03 ± 11.32 | 37.87 ± 10.32 | W = 443, p = 0.92 | 39.43 ± 11.45 | 38.77 ± 10.32 | W = 278, p = 0.92 | 39.21 ± 11.53 | 38.04 ± 10.24 | W = 238.5, p = 0.81 |
| BMI, mean ± SD | 23.53 ± 3.82 | 25.20 ± 3.50 | W = 334, p = 0.13 | 23.50 ± 3.67 | 24.88 ± 3.95 | W = 207, p = 0.23 | 23.83 ± 3.66 | 25.13 ± 4.01 | W = 177, p = 0.30 |
| Smoking, n (%) | |||||||||
| ≥ 1/day | 9 (30) | 12 (40) | χ24 = 3.21, p = 0.52 | 7 (33) | 11 (42) | χ21 = 0.27, p = 0.60 | 5 (26) | 10 (42) | χ21 = 0.87, p = 0.35 |
| NA | 10 (33.3) | 8 (26.7) | 3 (14) | 5 (19) | 3 (16) | 5 (21) | |||
| Hospitalization, n (%) | |||||||||
| 1 | 10 (33) | 13 (43) | W = 274, p = 0.41 | 8 (38) | 12 (46) | W = 224.5, p = 0.45 | 7 (37) | 12 (50) | W = 210.5, p = 0.32 |
| 2 | 4 (13) | 5 (17) | 4 (19) | 5 (19) | 4 (21) | 4 (17) | |||
| 3 | 3 (10) | 3 (10) | 3 (14) | 3 (12) | 3 (16) | 3 (13) | |||
| 4 | 0 (0) | 1 (3) | 0 (0) | 1 (4) | 0 (0) | 1 (4) | |||
| 5 | 3 (10) | 0 (0) | 3 (14) | 0 (0) | 3 (16) | 0 (0) | |||
| ≥ 6 | 1 (3) | 1 (3) | 0 (0) | 1 (4) | 0 (0) | 1 (4) | |||
| NA | 9 (30) | 7 (23) | 3 (14) | 4 (15) | 2 (11) | 3 (13) | |||
| Education, n (%) | |||||||||
| Primary | 9 (30) | 6 (20) | W = 423, p = 0.42 | 7 (33) | 4 (15) | W = 269.5, p = 0.88 | 6 (32) | 4 (17) | W = 237, p = 0.63 |
| Secondary | 3 (10) | 12 (40) | 3 (14) | 12 (46) | 3 (16) | 12 (50) | |||
| Tertiary | 16 (53) | 9 (30) | 11 (52) | 9 (35) | 10 (53) | 7 (29) | |||
| NA | 2 (7) | 3 (10) | 0 (0) | 1 (4) | 0 (0) | 0 (0) | |||
| Medication, DDD, mean ± SD | |||||||||
| Antidepressant equivalents | NA | NA | NA | 1.73 ± 1.30 | 1.79 ± 1.09 | W = 253, p = 0.68 | 1.86 ± 1.30 | 1.82 ± 1.12 | W = 227, p = 0.99 |
| Antipsychotics equivalents | NA | NA | NA | 0.30 ± 0.68 | 0.22 ± 0.30 | W = 278, p = 0.92 | 0.33 ± 0.71 | 0.24 ± 0.31 | W = 241, p = 0.76 |
| Clinical measures, mean ± SD | |||||||||
| HAM-D | 18.90 ± 4.7 | 16.7 ± 4.10 | W = 552, p = 0.08 | 18.93 ± 4.78 | 16.5 ± 4.04 | W = 363, p = 0.05 | 19.13 ± 4.89 | 16.5 ± 4.18 | W = 311, p = 0.04 |
| BDI | 22.5 ± 8.10 | 22.12 ± 9.90 | W = 461.5, p = 0.69 | 22.38 ± 7.54 | 22.33 ± 10.17 | W = 257.5, p = 0.96 | 21.53 ± 7.59 | 22.31 ± 9.94 | W = 218.5, p = 0.96 |
| STAI1 | 42.32 ± 5.525 | 41.10 ± 6.53 | W = 472.5, p = 0.42 | 49.75 ± 13.89 | 52.36 ± 10.40 | W = 229.5, p = 0.65 | 49 ± 14.11 | 51.83 ± 10.61 | W = 191, p = 0.68 |
| GSRS‡ | 30.21 ± 9.94 | 29.53 ± 12.06 | W = 479, p = 0.51 | 28.52 ± 9.48 | 29.83 ± 12.45 | W = 261, p = 0.98 | 28.16 ± 9.65 | 29.96 ± 12.79 | W = 211.5, p = 0.87 |
| Compliance, %, mean ± SD | NA | NA | NA | 83 ± 17.21 | 86 ± 11.72 | W = 231, p = 0.76 | 87 ± 8.44 | 88 ± 8.17 | W = 186, p = 0.84 |
BDI = Beck Depression Inventory; BMI = body mass index; CCA = complete case analysis; DDD = defined daily dose; GSRS = Gastrointestinal Symptom Rating Scale; HAM-D = Hamilton Rating Scale for Depression; ITT = intention to treat; NA = not applicable; SD = standard deviation; STAI1 = State–Trait Anxiety Inventory 1.
Referred to as ITT in Schaub et al.19
Also referred to as modified ITT in Schaub et al.19
Non-normally distributed, b square root–transformed.
Cognitive performance
VLMT
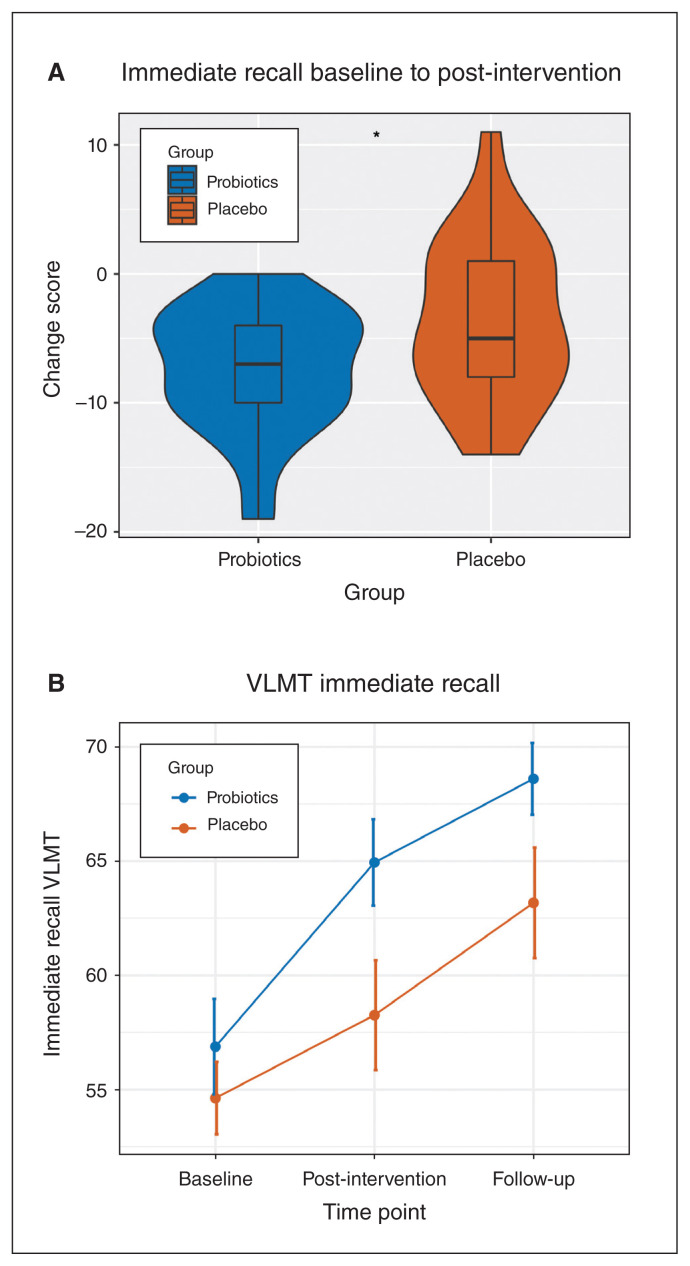
At baseline, the 2 groups did not differ in the immediate recall score (t49.05 = 0.72, p = 0.48). Comparing baseline to the post-intervention assessments, we found a significantly different change score between the groups (t37.86 = −2.16, p = 0.037, d = −0.67; Table 3), with the probiotic group having lower mean ± SD change scores than the placebo group (−7.35 ± 4.86 v. −3.57 ± 6.23). This change indicates a significantly greater improvement in the probiotics group than in the placebo group (Figure 1A). Looking at the immediate memory performance over all 3 time points, we found a significant group effect (F1, 49.04 = 5.03, p = 0.029) and a significant time effect (F2, 75.97 = 58.25, p < 0.001). Moreover, there was a statistical trend for a time × group interaction (F2, 76.12 = 2.55, p = 0.084; Figure 1B). As age significantly improved the fit of the model (χ2 = 18.10, p < 0.001), we included it in the model. Sex (χ2 = 1.27, p = 0.26) and medication (χ2 = 1.49, p = 0.48) did not significantly improve the fit of the model.
Table 3.
Results of cognitive measurements
| Test | Measure | CS* baseline to post-intervention | Random intercept model | Correlation with BDNF (including age) |
|---|---|---|---|---|
| Verbal Learning Memory Test | Immediate recall (Dg1–5) | t37.86 = −2.16, p = 0.037 | F2, 76.12 = 2.55, p = 0.084 | Probiotics: r = 0.26, p = 0.33 Placebo: r = −0.01, p = 0.97 |
| Intermediate recall (Dg5–Dg6) | t37.24 = 0.24, p = 0.82 | F2, 68.32 = 1.29, p = 0.28 | Probiotics: r = 0.04, p = 0.88 Placebo: r = −0.05, p = 0.86 |
|
| Long-term recall (Dg5–Dg7) | t35.47 = −1.79, p = 0.082 | F2, 77.57 = 1.74, p = 0.18 | Probiotics: r = 0.10, p = 0.72 Placebo: r = 0.18, p = 0.52 |
|
| Corsi block tapping test | Correct sequence forward | t35.15 = −0.55, p = 0.59 | F2, 79.51 = 0.09, p = 0.91 | – |
| Correct sequence backward | t37.08 = −1.43, p = 0.16 | F2, 79.92 = 1.44, p = 0.24 | – | |
| Trail Making Test | TMT A time | t29.95 = 0.081, p = 0.94 | F2, 71.57 = 0.06, p = 0.94 | – |
| TMT B time | t28.44 = −1.68, p = 0.10 | F2, 70.92 = 1.02, p = 0.36 | – | |
| 2-back task | Reaction time | t26.97 = 1.037, p = 0.31 | – | Probiotics: r = −0.29, p = 0.34 Placebo: = 0.50, p = 0.08 |
| Accuracy | t22.20 = 0.098, p = 0.92 | – | Probiotics: r = −0.44, p = 0.13 Placebo: r = 0.19, p = 0.53 |
BDNF = brain-derived neurotrophic factor; CS = Change Score; TMT = Trail Making Test.
Calculated by subtracting the post-intervention score from the baseline score.
Figure 1.
(A) Change scores from baseline to post-intervention per group for immediate recall in the VLMT (probiotics: n = 17, placebo: n = 23). (B) Mean trajectory of immediate recall in the VLMT per group from baseline to post-intervention (week 4) and follow-up assessment (week 8). Numbers of participants per time point and per group were as follows: baseline probiotics: n = 19; baseline placebo: n = 24; post-intervention probiotics: n = 17; post-intervention placebo: n = 23; follow-up probiotics: n = 15; follow-up placebo: n = 23. VLMT = Verbal Learning Memory Test.
Analyzing the intermediate recall (Dg5–Dg6; Table 1), we did not find significant differences in change scores between the 2 groups (Table 3). In the intermediate recall, assumptions for mixed model analysis were not met by non-normally distributed residuals. Therefore, we studied the words recalled after the interference list (Dg6) for all 3 time points as a dependent variable and included the score of recalled words immediately before the interference list (Dg5) as a covariate. We did not find any significant time × group interaction for correct recall after interference (F2, 66.55 = 1.29, p = 0.28). The fact that the recall performance of the word list immediately before the interference list (Dg5) significantly improved the model (χ2 = 116.99, p < 0.001) implies that the intermediate recall success significantly relied on the learning success of the immediate recall (Dg5). Again, age significantly improved the model (χ2 = 4.86, p = 0.027).
For long-term recall, there were also no significant differences in change scores between the 2 groups and no significant group × time interactions (mixed model: F2, 77.57 = 1.74, p = 0.18; Table 3). Age significantly improved the fit of the mixed model (χ2 = 7.66, p = 0.0056).
Corsi Block and TMT
We did not find any significant differences in change scores between the groups in the Corsi block tapping test or the TMT A and B, nor did we find any significant group × time interactions (Table 3).
BDNF
The mean ± SD change score of BDNF levels from baseline to the post-intervention assessment was − 0.28 ± 12.01 in the probiotics group, which indicates a slight increase in BDNF levels. Conversely, the mean ± SD change score in the placebo group indicated a decrease (1.53 ± 13.51). However, the group difference was not significant (t108.57 = −0.75, p = 0.45, d = −0.14). Also, when we included all 3 time points in our model, we did not find a significant difference between groups over time in BDNF levels (F2, 66.89 = 2.15, p = 0.12). Furthermore, we did not find a significant correlation between any recall type of the VLMT and BDNF (Table 3).
Brain activation during working memory processing
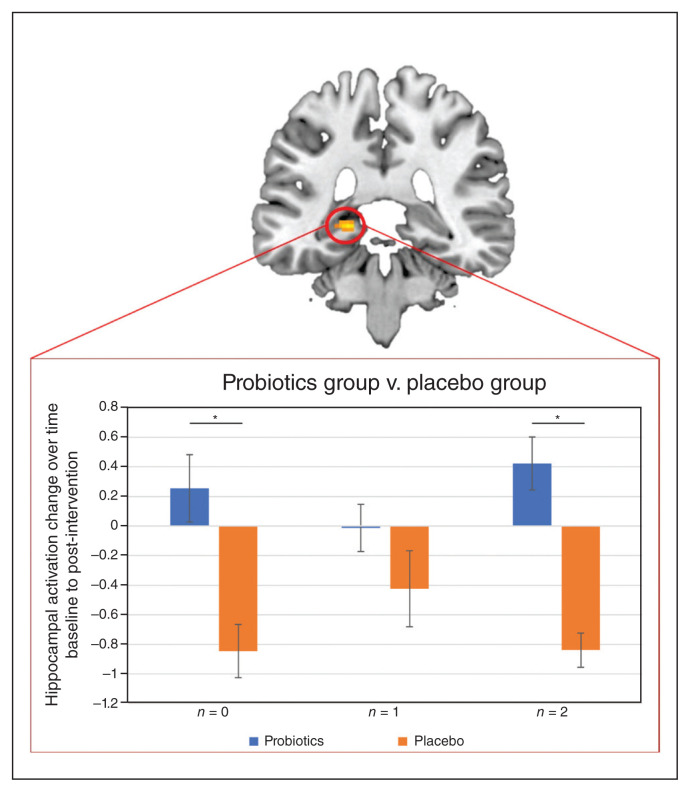
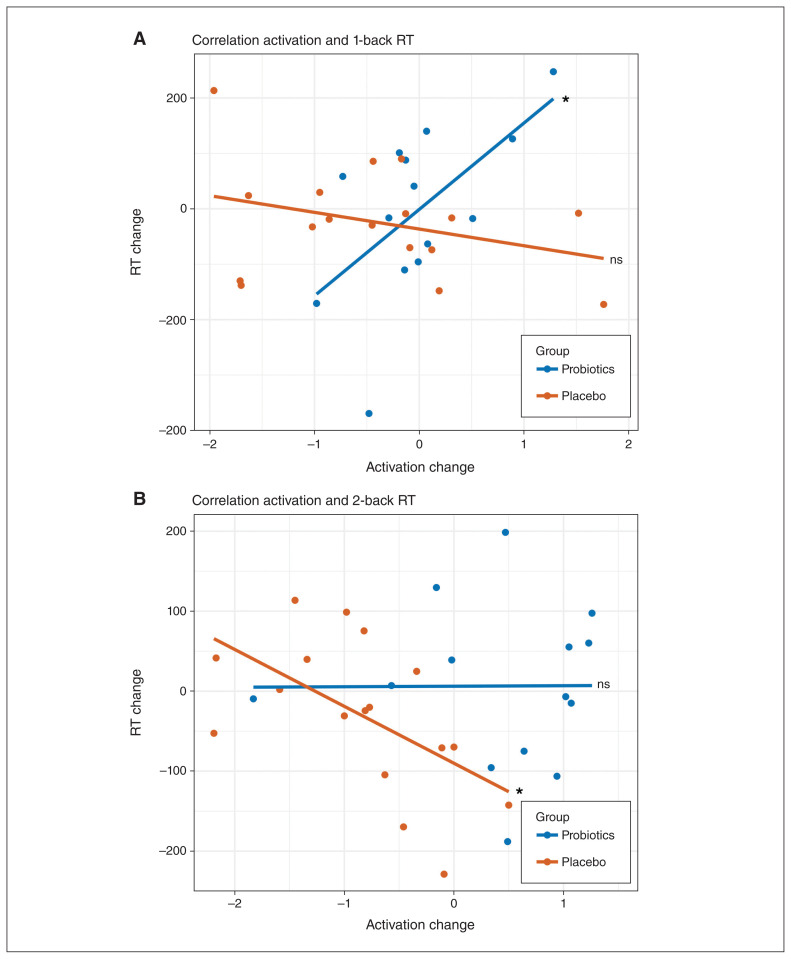
Looking at the task effect of the 2-back task at baseline, we found typical activation patterns involved in working memory tasks: increased activation in regions associated with the cognitive control circuit, including frontal, temporal and subcortical areas (Appendix 1, Table S4; see Niendam and colleagues38) and decreased activation in regions associated with the default mode network, including ventromedial prefrontal cortex, the posterior cingulum and precuneus (Appendix 1, Table S4; see Alves and colleagues39). At the whole-brain level, we did not find a significant main effect of group or group × time interaction for the 2-back condition (Appendix 1, Tables S4–S7). However, considering the hippocampus, we found significant differences in activation changes over time between groups in the left hippocampus for the 0-back condition (Montreal Neurological Institute [MNI] space x, y, z = −20, −34, −2; k = 29; T = 5.80, ppeak(FWE) = 0.001, small volume corrected [SVC]) and the 2-back condition (MNI space x, y, z = −20, −36, −2; k = 13; T = 4.26, ppeak(FWE) = 0.031, SVC). For both conditions, we saw an activation decrease for the probiotics group and an activation increase for the placebo group after the intervention (Figure 2). For the 1-back condition, we did not find a significant difference, but a similar pattern of activation changes over time (Figure 2). For all conditions, the activation changes did not correlate with the BDNF changes. On the behavioural level, we did not find any accuracy or RT differences over time or between groups (Table 3). When we correlated the functional changes in the hippocampus with behaviour (i.e., accuracy and RT differences) for each level of complexity within the 2-back task, we found a significant correlation between activation changes and RT differences (baseline – post-intervention) in the 1-back condition for the probiotics group (rage = 0.64; p = 0.019; Figure 3A) and in the 2-back condition for the placebo group (rage = −0.56; p = 0.024; Figure 3B). In both cases, the correlations significantly differed between groups (1-back: z = 2.65, p = 0.008; 2-back: z = 2.14, p = 0.033). A positive correlation indicates that decreased hippocampal activation after the intervention is associated with faster RT after the intervention, whereas the negative correlation indicates that increased hippocampal activation after the intervention is associated with faster RT after the intervention.
Figure 2.
Pattern of activation change from baseline to post-intervention per group and condition based on the hippocampal cluster revealed in the group contrast (probiotics group > placebo group) for the 0-back task (probiotics: n = 14; placebo: n = 18). Probiotics group v. placebo group
Figure 3.
(A) Correlation between left hippocampal activation changes and RT differences in the 1-back condition. (B) Correlation between the left hippocampal activation changes and RT differences in the 2-back condition (probiotics: n = 14; placebo: n = 18). RT = reaction time.
Discussion
In this secondary analysis of an RCT,19 we investigated the effect of a 4-week high-dose probiotic supplementation on cognition, serum BDNF levels and brain activation during a working memory task in patients with depression. Our main finding on cognition was a significantly improved immediate recall after 4 weeks of probiotic supplementation. This finding is in line with those of previous studies reporting a positive probiotics effect on episodic memory,25,40 global cognition, 40 executive functions and attention,25,40 and spatial learning.41 Rudzki and colleagues, who investigated the effect of the probiotic L. plantarum as add-on therapy for cognitive functions in patients with MDD, also found an improved immediate verbal memory recall in the probiotic group compared with the placebo group.25 Their assessment of cognition took place before and after an 8-week intervention of probiotics or placebo. Memory was measured with the California Verbal Learning Test (CVLT), which comprises equivalent variables to the VLMT used in our study.
Nevertheless, results of probiotic effects on cognition are rather heterogeneous.20,40,42–44 A recent meta-analysis including 1500 individuals from 22 different studies on several diseases and disorders, including MDD, showed that the results were not consistent across studies.43 This heterogeneity might be caused by heterogeneous patient samples and methodological differences. A major methodological concern is the variety of tasks used to assess cognition.20 Probiotic effects on cognition might be very domain-specific, causing heterogeneous results depending on which cognitive domain is assessed. This could explain why we found different results across different cognitive domains relying on the neural mechanisms that are stimulated by probiotics. Also, the mode of supplementation (multistrain v. monostrain) and bacterial combinations vary across studies. While we found similar effects as Rudzki and colleagues,25 who used a single-strain probiotic, it has been suggested that single-strain interventions are more effective than multistrain supplements.20 Last, the applied intervention period is a methodological concern; the intervals usually vary between 3 and 12 weeks.43 Rudzki and colleagues25 chose an 8-week intervention, whereas we chose a 4-week intervention. Both studies present a significant positive effect of probiotics on cognition after the intervention, indicating that cognitive symptoms improved during regular probiotic intake. However, it is still unclear how long such positive effects last and whether the intervention period influences the long-lasting effect of probiotic supplements. Contrary to Rudzki and colleagues,25 we included a follow-up assessment and found a trend for a long-lasting probiotic effect 4 weeks after the intervention. Nevertheless, there is still the question whether a longer intervention period would have led to greater long-lasting probiotic effects. Generally, we assume that the heterogeneous results reported in the literature are caused by the methodological differences outlined here and that probiotics may be a reasonable add-on therapy to support the improvement of affective and cognitive symptoms in depression. Future research should address these methodological concerns and investigate the optimal duration of probiotic supplementation to gain long-term cognitive benefits in MDD.
On the neural level, we found significant differences in activation changes over time between the probiotic and placebo groups in our a priori–defined region of interest, the hippocampus — particularly the left hippocampus. The revealed hippocampal deactivation over time in the probiotics group is assumed to reflect the beneficial effect of the probiotics on depression-related cognitive impairments. Commonly, the hippocampus appears hyperactive in patients with depressive symptoms45 and hyperresponsive during the N-back task while performance is maintained.46 Such disruptions of the hippocampal function might contribute to deficits in concentration and memory, both of which have been identified as relevant diagnostic features of MDD.27 As the hippocampus has strong neural connections to the prefrontal cortex (PFC)47 and belongs to the key structures regulating PFC functions,48 hippocampal hyperactivity during working memory tasks in depression has been assumed to reflect difficulties in switching off self-referential default-mode processing.49 Specifically, patients with depression tend to show an aberrant interaction between the task-positve and default-mode networks.49 The reduced hippocampal activation seen during the 2-back task after probiotic supplementation can be interpreted as a remediated balance between the task-positive and default-mode networks during the working memory task and, thus, as enhancing cognition. This explanation is further supported by the significant positive correlation we found for the 1-back condition in the probiotic group. The positive direction of this correlation indicates that reduced hippocampal activation after the intervention is accompanied by faster RT after the intervention. Eventually, our interpretation dovetails with previous findings, reporting a positive impact of probiotics on the hippocampus,26 and with evident effects of common antidepressants.46,50 Our findings might reveal a mechanism by which probiotics directly affect the neural underpinnings of cognitive deficits in depression.
Our results confirm our hypothesis that the hippocampus, as a crucial structure for depression-related cognitive impairments, benefits most from add-on probiotic supplementation. This finding is in accordance with those of numerous studies demonstrating a strong association between the gut microbiota and hippocampus-dependent learning, memory and behaviour.41,51 Accumulating findings suggest that the gut microbiota influences hippocampus-dependent behaviour by neurochemical, neurotrophic and transcriptional factors, neurogenesis, and the plasticity of pyramidal and granular cells.26 In the present study, we found an increased performance in the immediate recall of the VLMT, which is a typical hippocampus-dependent verbal learning task.31 Furthermore, we found a slight but nonsignificant increase in BDNF levels in our probiotic group and a decrease in our placebo group after the 4-week intervention. The lack of significant findings in our study could be explained by the general heterogeneity of BDNF results. Different factors, such as treatment response52 or sampling techniques,53 are thought to be responsible for the heterogeneity. Nevertheless, the direction of the BDNF change is congruent to the findings of other studies reporting significantly increased BDNF levels when probiotics were given in addition to antidepressant treatment,54 but not given as stand-alone treatment.54 Finally, the close link between the gut microbiota–brain axis and the hippocampus is shown by our imaging results, revealing reduced hippocampal activation during the 2-back task after probiotic supplementation. A possible explanation for the specific hippocampus-related effects of probiotics in our RCT is that the hippocampal structure and functioning is altered by a dysregulated hypothalamic–pituitary–adrenal (HPA) axis due to chronic stress and increased inflammation, resulting in impaired adaptation and memory.55 The stress response and inflammation play major roles in the vulnerability to and the recovery from depression55,56 and are strongly related to the gut microbiota–brain axis.11,57 For instance, chronic stress and inflammasome signalling change the intestinal permeability and alter the composition and stability of the gut microbiota.58,59 The gut microbiota, on the other hand, influence the host’s stress and inflammation responses. 58 For example, researchers found gut microbiota disturbances to be related to a decline of anti-inflammatory butyrate-producing bacteria and an increase of proinflammatory bacteria in patients with depression.13,14 Moreover, there is evidence that the administration of probiotics can reduce the response of the HPA axis to stress.60 Hence, we suggest that targeting the gut microbiota with probiotics affects the regulation of the HPA axis and, thus, hippocampal functioning. This interpretation also explains why we found an improvement only in the VLMT and not in other cognitive tasks, which test for different PFC-dependent executive functions than hippocampus-dependent learning and memory (Table 1).
Limitations
Although we found improved cognitive performance, our study has some limitations. The exclusion of noncompliant participants resulted in a relatively small sample size. Thus, our results should be validated and replicated in future studies. While we held diet consistent in the inpatient setting, we did not investigate dietary influences in more detail. Future probiotics research should assess dietary effects, as diet could also impact the viability and long-lasting effects of probiotics. Furthermore, the probiotics were administered in addition to treatment as usual and contained a higher dose of bacteria than commercial probiotic supplementation does. It is still open to discussion whether antidepressants work in synergy with the probiotics if the administered dose has a strong influence on the treatment response. Future studies should therefore address the influence of the dose and medication in more detail and ideally test the effects of probiotics on medication-naïve patients.
Furthermore, it would be interesting to examine whether a longer intervention period increases long-term effects of probiotics on cognitive symptoms in depression. Perhaps an 8-week supplementation might ameliorate deficits in the PFC-dependent executive functions and reveal prefrontal activation changes during the N-back task, both mediated by improved hippocampal function. To fully confirm our hypothesis that the hippocampus-dependent cognition benefits most from probiotic supplementation, future studies should assess several hippocampal-dependent learning and memory tasks (e.g., associative memory tasks) to examine cognitive improvement with probiotic supplementation. Especially for exploring the neural basis of the probiotic effects, the examination of activation changes during hippocampus-specific cognitive tasks would be promising. Finally, we used an modified ITT analysis to test the as-received treatment effect of probiotics on cognition and related biological markers. More large-scale RCTs are needed to evaluate the effectiveness of probiotics in light of inevitable nonadherence.
Conclusion
Additional probiotic supplementation improves verbal episodic memory and affective symptoms,19 both of which are highly relevant in MDD. Probiotic supplementation has a direct effect on the neural mechanisms underlying cognitive impairments in depression by balancing the altered hippocampal function during the 2-back task. The present findings support the claim of the importance of the gut microbiota–brain axis in MDD and emphasize the potential of microbiota-related treatment approaches as accessible, nonstigmatizing and holistic therapy, treating affective and cognitive symptoms in depression simultaneously.
Footnotes
Competing interests: None declared.
Contributors: N. Schweinfurth, L. Mählmann, S. Brand, S. Borgwardt, U. Lang and A. Schmidt designed the study. J. Doll, N. Schweinfurth, A.-C. Schaub, N. Varghese, L. Mählmann, S. Brand, A. Eckert and A. Schmidt acquired the data, which E. Schneider, J. Doll, C. Kettelhack, G. Yamanbaeva, S. Borgwardt, U. Lang and A. Schmidt analyzed. E. Schneider, J. Doll, S. Brand and A. Schmidt wrote the article, which all authors reviewed. All authors approved the final version to be published, agree to be accountable for all aspects of the work and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Funding: This work was supported by the Gertrud Thalmann-Fonds (SBo, UEL), Seerave Foundation (UEL), Kämpf-Bötschi Stiftung (UEL) and Research Fund Junior Researchers of the University of Basel (AS).
References
- 1.Collaborators GDaIIaP. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson L, Adams S. Cognitive deficits in patients with depression. J Nurse Pract 2018;14:437–43. [Google Scholar]
- 3.Bora E, Harrison BJ, Yücel M, et al. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med 2013;43:2017–26. [DOI] [PubMed] [Google Scholar]
- 4.Bierman EJM, Comijs HC, Jonker C, et al. Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry 2005; 13: 686–93. [DOI] [PubMed] [Google Scholar]
- 5.Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behav Brain Res 2010;215:162–71. [DOI] [PubMed] [Google Scholar]
- 6.Airaksinen E, Wahlin Å, Forsell Y, et al. Low episodic memory performance as a premorbid marker of depression: evidence from a 3-year follow-up. Acta Psychiatr Scand 2007;115:458–65. [DOI] [PubMed] [Google Scholar]
- 7.McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety 2013;30:515–27. [DOI] [PubMed] [Google Scholar]
- 8.Buckner JD, Joiner TE, Pettit JW, et al. Implications of the DSM’s emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res 2008;159:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dannehl K, Rief W, Euteneuer F. Effects of cognitive behavioural therapy on verbal learning and memory in major depression: results of a randomized controlled trial. Clin Psychol Psychother 2019; 26: 291–7. [DOI] [PubMed] [Google Scholar]
- 10.Shilyansky C, Williams LM, Gyurak A, et al. Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry 2016;3:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012; 13:701–12. [DOI] [PubMed] [Google Scholar]
- 12.Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol 2017; 595:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuinness AJ, Davis JA, Dawson SL, et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry 2022;27:1920–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolova VL, Smith MRB, Hall LJ, et al. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry 2021;78:1343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 2019;4:623–32. [DOI] [PubMed] [Google Scholar]
- 16.Kelly JR, Borre Y, O’ Brien C, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 2016;82:109–18. [DOI] [PubMed] [Google Scholar]
- 17.Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016;21:786–96. [DOI] [PubMed] [Google Scholar]
- 18.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry 2013;74:720–6. [DOI] [PubMed] [Google Scholar]
- 19.Schaub A-C, Schneider E, Vazquez-Castellanos JF, et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: a randomized controlled trial. Transl Psychiatry 2022;12:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv T, Ye M, Luo F, et al. Probiotics treatment improves cognitive impairment in patients and animals: a systematic review and meta-analysis. Neurosci Biobehav Rev 2021;120:159–72. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Ning L, Yin Y, et al. Age-related shifts in gut microbiota contribute to cognitive decline in aged rats. Aging (Albany NY) 2020; 12:7801–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi C, Angelucci A, Costantin L, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci 2006;24:1850–6. [DOI] [PubMed] [Google Scholar]
- 23.Miranda M, Morici JF, Zanoni MB, et al. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 2019;13:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehme M, Guzzetta KE, Bastiaanssen TFS, et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nature Aging 2021;1:666–76. [DOI] [PubMed] [Google Scholar]
- 25.Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019;100:213–22. [DOI] [PubMed] [Google Scholar]
- 26.Tang W, Meng Z, Li N, et al. Roles of gut microbiota in the regulation of hippocampal plasticity, inflammation, and hippocampus-dependent behaviors. Front Cell Infect Microbiol 2021;10:611014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 2011;16:252–64. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967;6:278–96. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman M, Martinez JH, Young D, et al. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord 2013;150: 384–8. [DOI] [PubMed] [Google Scholar]
- 30.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001;121:580–91. [DOI] [PubMed] [Google Scholar]
- 31.Helmstaedter C, Lendt M, Lux S. Verbaler Lern-und Merkfähigkeitstest: VLMT; Manual. Göttingen: Beltz-Test.; 2001. [Google Scholar]
- 32.Kessels RP, van Zandvoort MJ, Postma A, et al. The Corsi block-tapping task: standardization and normative data. Appl Neuropsychol 2000;7:252–8. [DOI] [PubMed] [Google Scholar]
- 33.Reitan R. Trail-making test. Arizona: Reitan Neuropsychology Laboratory; 1979. [Google Scholar]
- 34.Schmidt A, Smieskova R, Aston J, et al. Brain connectivity abnormalities predating the onset of psychosis: correlation with the effect of medication. JAMA Psychiatry 2013;70:903–12. [DOI] [PubMed] [Google Scholar]
- 35.Owen AM, McMillan KM, Laird AR, et al. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005;25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv 1998;49:196–201. [DOI] [PubMed] [Google Scholar]
- 37.Diedenhofen B, Musch J. cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One 2015;10:e0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niendam TA, Laird AR, Ray KL, et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 2012;12:241–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alves PN, Foulon C, Karolis V, et al. An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun Biol 2019;2:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ceccarelli G, Fratino M, Selvaggi C, et al. A pilot study on the effects of probiotic supplementation on neuropsychological performance and microRNA-29a-c levels in antiretroviral-treated HIV-1-infected patients. Brain Behav 2017;7:e00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rezaei Asl Z, Sepehri G, Salami M. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease. Behav Brain Res 2019;376:112183. [DOI] [PubMed] [Google Scholar]
- 42.Roman P, Estévez AF, Miras A, et al. A pilot randomized controlled trial to explore cognitive and emotional effects of probiotics in fibromyalgia. Sci Rep 2018;8:10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marx W, Scholey A, Firth J, et al. Prebiotics, probiotics, fermented foods and cognitive outcomes: a meta-analysis of randomized controlled trials. Neurosci Biobehav Rev 2020;118:472–84. [DOI] [PubMed] [Google Scholar]
- 44.Kelly JR, Allen AP, Temko A, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun 2017;61:50–9. [DOI] [PubMed] [Google Scholar]
- 45.Videbech P, Ravnkilde B, Pedersen AR, et al. The Danish PET/depression project: PET findings in patients with major depression. Psychol Med 2001;31:1147–58. [DOI] [PubMed] [Google Scholar]
- 46.Smith J, Browning M, Conen S, et al. Vortioxetine reduces BOLD signal during performance of the N-back working memory task: a randomised neuroimaging trial in remitted depressed patients and healthy controls. Mol Psychiatry 2018;23:1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampath D, Sathyanesan M, Newton SS. Cognitive dysfunction in major depression and Alzheimer’s disease is associated with hippocampal-prefrontal cortex dysconnectivity. Neuropsychiatr Dis Treat 2017;13:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci 2000;20: RC106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvey P-O, Fossati P, Pochon J-B, et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage 2005;26:860–9. [DOI] [PubMed] [Google Scholar]
- 50.Kaser M, Deakin JB, Michael A, et al. Modafinil improves episodic memory and working memory cognition in patients with remitted depression: a double-blind, randomized, placebo-controlled study. Biol Psychiatry Cogn Neurosci Neuroimaging 2017;2:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim CS, Cha L, Sim M, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci 2021;76:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arosio B, Guerini FR, Voshaar RCO, et al. Blood brain-derived neurotrophic factor (BDNF) and major depression: Do we have a translational perspective? Front Behav Neurosci 2021;15:626906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polyakova M, Stuke K, Schuemberg K, et al. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord 2015;174:432–40. [DOI] [PubMed] [Google Scholar]
- 54.Nikolova VL, Cleare AJ, Young AH, et al. Updated review and meta-analysis of probiotics for the treatment of clinical depression: adjunctive vs. stand-alone treatment. J Clin Med 2021;10:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dean J, Keshavan M. The neurobiology of depression: an integrated view. Asian J Psychiatr 2017;27:101–11. [DOI] [PubMed] [Google Scholar]
- 56.Troubat R, Barone P, Leman S, et al. Neuroinflammation and depression: a review. Eur J Neurosci 2021;53:151–71. [DOI] [PubMed] [Google Scholar]
- 57.Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- 58.Wong ML, Inserra A, Lewis MD, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry 2016;21:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu F, Tu H, Chen T. The microbiota-gut-brain axis in depression: the potential pathophysiological mechanisms and microbiota combined antidepression effect. Nutrients 2022;14:2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ait-Belgnaoui A, Durand H, Cartier C, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012;37:1885–95. [DOI] [PubMed] [Google Scholar]