Abstract
Background:
Selenium (Se) can be found in the molecular structure of selenoproteins; including thioredoxin reductase and glutathione peroxidase and also in Type I and II deiodinases. Previous studies have shown that Se deficiency has been linked to autoimmune thyroid disease (AITD). In the present study, we investigated the serum Se levels of patients with Graves’ disease (GD), Hashimoto's thyroiditis (HT), and euthyroid individuals as a control group.
Materials and Methods:
The present study was performed on patients with newly diagnosed AITD (GD and HT). The control group was matched with the case group in terms of parameters such as age and sex. Free thyroxine, free triiodothyronine, thyroid-stimulating hormone, antithyroid peroxidase, antithyroglobulin, and serum Se levels were measured in all participants. These parameters were compared between groups.
Results:
Data from 132 patients with HT, 120 patients with GD, and 120 healthy euthyroid patients as a control group were analyzed. The Se level in patients with HT (104.36 μg/l) and GD (97.68 μg/l) was significantly lower than in the control group (122.63 μg/l) (P < 0.001). The incidence of Se deficiency in patients with HT, GD, and in the control group was 15.2%, 2.5%, and 2.5%, respectively (P < 0.001). In patients with GD, 34 patients (28.33%) had Graves’ orbitopathy. Se levels in patients with orbitopathy were significantly lower than in patients without orbitopathy.
Conclusion:
The serum Se level was significantly lower in newly diagnosed patients with GD and HT than in the control group. Overall, Se deficiency can be considered a risk factor for AITDs.
Keywords: Graves’ disease, Hashimoto's disease, selenium
INTRODUCTION
Autoimmune thyroid diseases (AITD) encompass a wide spectrum of thyroid dysfunctions, ranging from autoimmune hypothyroidism or Hashimoto's thyroiditis (HT) to autoimmune hyperthyroidism or Graves’ disease (GD).[1] Approximately, 1%–2% of the world's population is affected by AITDs, with a higher prevalence reported in 30–50-year-old women. In HT, the thyroid gland is destroyed slowly, causing symptoms, such as weakness, fatigue, weight gain, cold and dry skin, constipation, cold intolerance, muscle pain, and depression, mainly due to the decreased secretion of thyroid hormones.[2] The presence of antibodies against thyroid peroxidase (TPO) and thyroglobulin (Tg) is characteristic of HT.[1] In GD, the antibody against thyroid-stimulating hormone (TSH) receptor is a specific antibody that increases the secretion of thyroid hormones by stimulating the TSH receptor, causing symptoms, such as irritability, increased heart rate, weight loss despite increased appetite, heat intolerance, warm and wet skin, and ocular manifestations (Graves’ orbitopathy).[3]
The pathogenesis of AITD is not established yet. Epidemiological studies have shown that genetic factors play an important role in susceptibility to AITDs.[4] Previous studies have reported that the amount and activity of selenoproteins are associated with single-nucleotide polymorphisms in selenoprotein genes, which may contribute to the risk of disease.[5] There are also some other risk factors, such as age and parity, which play important roles in the development and preservation of thyroid autoimmunity. Furthermore, several environmental factors, including, iodine intake, smoking, allergens, alcohol, estrogen, stress, irradiation, infection, immunotherapy drugs, and selenium (Se) deficiency, have been reported as factors affecting the incidence of AITD.[6]
Se is a trace element that plays an important role in human physiology. It has anti-inflammatory, anti-cancer, and anti-aging properties, as well as protective effects against oxidative stress.[7] This element is available in many tissues, such as the kidney, muscle tissues, and liver, although the highest concentration is found in thyroid tissue. Se can be found in the molecular structure of selenoproteins in the thyroid tissue including glutathione peroxidase and thioredoxin reductase that protect the thyroid against free radicals. They can be also found in the structure of Type I and II deiodinases that participate in the synthesis of thyroid hormones.[8]
Se deficiency is found in 15% of the world's population. The prevalence of Se intake varies significantly in different regions worldwide, which can be attributed to the variable concentration of Se in the soil of different regions and differences in factors that influence the availability of food Se, such as type of Se, organic matter content, and soil pH.[9]
Limited studies have investigated the serum Se concentrations in AITDs.[10,11,12,13,14] The reduced level of serum Se (<70 μg/L) has been reported in patients with AITDs.[11,15,16] Furthermore, a low concentration of Se has been reported in patients with GD.[17] Lower serum level of Se has been reported in patients with Graves’ orbitopathy, compared to those without Graves’ orbitopathy.[18]
In the present study, we investigated the serum Se levels of patients with GD, patients with HT, and euthyroid individuals as a control group (matched in terms of age and sex with the patient groups) to determine the association between serum Se level and thyroid function parameters in AITD.
MATERIALS AND METHODS
This case–control study was carried out on newly diagnosed patients with AITD (HT and GD) who were referred to endocrine clinics in Zahedan, Southeastern Iran; an Iodine sufficient area[19] between August 2018 and October 2020.
Patients with at least 18 years of age who were diagnosed with AITD were included in the study. GD was defined as follows: increased free thyroxine (FT4) and free triiodothyronine (FT3) in association with suppressed TSH (normal values: FT4: 0.8–1.8 ng/ml, normal FT3: 2.3–4.2 pg/ml, and normal TSH: 0.4–4.2 mIU/L) and positive TSH Receptor Antibodies (TRAb) (Normal TRAb: Up to 1.75 IU/L). Furthermore, Hashimoto's disease was also defined as follows: HT: decreased FT4 and FT3 in association with elevated TSH ≥10 and positive anti-TPO (up to 40 mIU/L) or anti-Tg (up to 100 mIU/L).[20]
Individuals with a history of thyroid surgery, history of structural thyroid disease, or receiving iodine contrast during the past 6 months were excluded from the study. Furthermore, people with diabetes mellitus, liver, kidney, or heart failure, and psychological disorders that affect thyroid function tests were excluded from the study, too. Patients on hormonal therapy such as oral contraceptives, estrogen replacement therapy, and also medications for chronic diseases such as glucocorticoids, antiepileptic drugs, or amiodarone were excluded from the study. Participants were excluded from the study if they received Se. Women who were pregnant or lactating were also excluded from the study. The EUGOGO classification is used for the diagnosis of Graves’ orbitopathy.[21]
The case group consisted of two groups: Hashimoto's and Graves’ disease. Patients in these two groups were matched based on age and sex. The subjects in the control group were healthy euthyroid individuals who were selected after applying the inclusion and exclusion criteria from the general population. They were excluded from the study in case of any acute or chronic illness. The control group was matched with the case group in terms of age and sex. The geographical area of residence of all participants was the same.
For each participant, a questionnaire that included personal, past medical, and familial history was completed. Participants’ height was measured using a stadiometer and their weight was measured using a digital scale. Body mass index (BMI) was determined by dividing weight in kilograms by height squared in meters.
From all participants, blood samples were recruited and serum fraction was separated by centrifugation at 3000 rpm for 5 min. All blood samples were collected between 8 and 9 a.m., after 12 h of fasting. After collection, serum samples were stored at 70°C until the day of assay. Thyroid function tests, thyroid antibodies, and serum levels of Se were evaluated in Hashimoto, Graves, and the control group.
Measurements of TSH, FT4, and FT3 were performed using immunochemoluminescent method by an automated analyzer (Diagnostic Products LIAISON, 2017, Italy). Anti-TPO (normal range <16 U/ml) and anti-Tg (normal range <100 U/ml) were measured by immunochemoluminescent assays. Se was measured by Atomic Absorption Spectrometer Pg Instruments (Spectra AA500; reference range: 46–143 μg/L) in all samples.
This study was approved by the Zahedan University Ethics Committee for Human Studies (ethical code number: IR. ZAUMS. REC.1399.071). The participants provided written and informed consent.
Statistical method
Continuous and categorical data are presented as mean ± standard deviation and frequency (percentage), respectively. The Shapiro–Wilk test and graphical approaches such as Q–Q plot and histogram were used to examine the normal distribution of variables.
A one-way ANOVA test was used to compare a numerical variable in three study groups. An independent t-test or a Mann–Whitney U-test was used to assess the significance of differences for continuous variables (for normality and nonnormality distributed variables, respectively). The Chi-square or Fisher's exact test was used for the comparison of categorical variables. Bonferroni correction for continuous variables and Chi-square test for categorical variables were used for a post hoc pairwise comparison of these three study groups. The correlation between numerical variables assessed with Pearson correlation coefficient. The P < 0.05 is considered statistically significant. All of the analyses were conducted with Stata statistical software: Release 14. College Station, TX: StataCorp LP.
RESULTS
Data from 132 patients with HT, 120 patients with GD, and 120 healthy euthyroid subjects as a control group were analyzed. In this study, 81.1% of patients in the HT group, 80% in the GD group, and 78.3% in the control group were female. The mean age was 34.42 years in the HT group, 34.07 years in the GD group, and 32.25 years in the control group. Other laboratory characteristics were compared between the three groups in Table 1.
Table 1.
Clinical features and biochemical characteristics by the study group
| Variable | Hashimoto group (n=132) | Graves group (n=120) | Control group (n=120) | P |
|---|---|---|---|---|
| Sex (female) | 107 (81.1) | 96 (80.0) | 94 (78.3) | 0.864 |
| Age (years) | 34.42±10.23 | 34.07±5.96 | 35.25±8.86 | 0.552 |
| BMI (kg/m2) | 24.17±3.54a | 23.77±3.73a,b | 23.02±3.99b | 0.050 |
| FT4 (ng/ml) | 0.45±0.16a | 3.30±0.91b | 1.26±0.20c | <0.001 |
| FT3 (pg/ml) | 1.62±0.50a | 6.69±1.56b | 3.62±0.45c | <0.001 |
| TSH (mIu/L) | 79.70±20.45a | 0.02±0.01b | 1.55±0.78b | <0.001 |
| Anti-TPO (Iu/L) | 475.36±663.34a | 344.16±610.46a | 13.51±19.09b | <0.001 |
| Positive anti-TPO (≥16) | 118 (89.4)a | 97 (80.8)a | 14 (11.7)b | <0.001 |
| Anti-Tg (Iu/L) | 855.11±1287.96a | 545.05±1149.86b | 54.72±49.75c | <0.001 |
| Positive anti-Tg (≥100) | 113 (85.6)a | 97 (80.8)a | 14 (11.7)b | <0.001 |
| Se (µg/l) | 104.36±28.62a | 97.68±20.68a | 122.63±20.03b | <0.001 |
| Se deficiency (<70 µg/l) | 20 (15.2)a | 3 (2.5)b | 3 (2.5)b | <0.001 |
a,b,cPost hoc comparison based on Bonferroni method for continuous variables and Chi-square test for categorical variables. Different superscript letters (a, b, and c) in the same row of variables reflect a significant (P<0.05) difference between the means while the same superscript letters in one row reflect a nonsignificant difference between the means of the three groups. Data are expressed as mean±SD or n (%). SD: Standard deviation; BMI=Body mass index; TPO=Thyroid peroxidase; Tg=Thyroglobulin; TSH=Thyroid-stimulating hormone; FT3=Free triiodothyronine; FT4=Free thyroxine; Se=Selenium
The serum Se level in patients with HT and GD was significantly lower than in the control group [Figure 1]. A comparison of the mean serum Se level in three study groups showed a significant difference. The difference in the mean value between the Hashimoto and the control group (104.4 vs. 122.6), and between the Graves and the control group (97.7 vs. 122.6) was statistically significant [Table 1]. The percentage of Se deficiency in patients with Hashimoto's disease, GD, and control group was 15.2%, 2.5%, and 2.5%, respectively. The incidence of Se deficiency in patients with Hashimoto's disease was statistically significant compared to Graves and control groups (P < 0.001) [Table 1].
Figure 1.
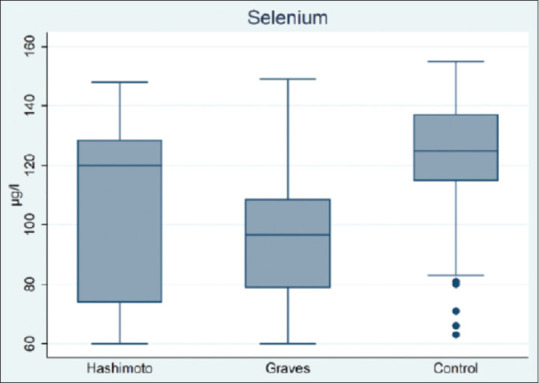
Serum selenium levels in Hashimoto, Graves, and healthy control group
The Se value in none of the study groups (including Hashimoto, Graves, and the control group) had no significant correlation with any of the variables FT4, FT3, TSH, anti-TPO, and anti-Tg.
In patients with GD, 34 patients (28.33%) had Graves’ orbitopathy. The prevalence of Graves’ orbitopathy was significantly higher in men than in women (58.3% vs. 20.8%, P < 0.001). The mean age, BMI, FT4, FT3, TSH, and TRAb in Graves’ patients with and without orbitopathy were not statistically significant. Serum Se levels in patients with orbitopathy were significantly lower than in patients without orbitopathy (86 vs. 102 μg/L; P < 0.001). The mean values of anti-TPO and anti-Tg in Graves’ patients with orbitopathy were significantly higher than in nonorbitopathic patients [Table 2].
Table 2.
Clinical features and biochemical characteristics in patients with and without Graves’ orbitopathy
| Variable | Graves’ orbitopathy | P | |
|---|---|---|---|
|
| |||
| No (n=86) | Yes (n=34) | ||
| Sex | |||
| Male | 10 (41.7) | 14 (58.3) | <0.001 |
| Female | 76 (79.2) | 20 (20.8) | |
| Age (years) | 33.70±6.13 | 35.03±5.49 | 0.272 |
| BMI (kg/m2) | 23.90±3.77 | 23.43±3.67 | 0.535 |
| FT4 (ng/ml) | 3.30±0.91 | 3.29±0.93 | 0.950 |
| FT3 (pg/ml) | 6.56±1.49 | 7.01±1.70 | 0.149 |
| TSH (mIu/L) | 0.02±0.01 | 0.02±0.01 | 0.190 |
| Anti-TPO (Iu/L) | 252.27±496.60 | 576.59±794.33 | 0.032 |
| Anti-Tg (Iu/L) | 365.44±764.10 | 999.35±1722.58 | 0.046 |
| Se (µg/l) | 102.05±20.72 | 86.65±16.15 | <0.001 |
| TRAb (Iu/L) | 0.65±0.38 | 0.68±0.42 | 0.737 |
| Positive anti-TPO (≥16) | |||
| No | 18 (78.3) | 5 (21.7) | 0.435 |
| Yes | 68 (70.1) | 29 (29.9) | |
| Positive anti-Tg (≥100) | |||
| No | 19 (82.6) | 4 (17.4) | 0.195 |
| Yes | 67 (69.1) | 30 (30.9) | |
| Se deficiency (<70 µg/l) | |||
| No | 84 (71.8) | 33 (28.2) | 1.0 |
| Yes | 2 (66.7) | 1 (33.3) | |
Data are shown as n (%) or mean±SD. SD=Standard deviation; BMI=Body mass index; TPO=Thyroid peroxidase; Tg=Thyroglobulin; TSH=Thyroid-stimulating hormone; FT3=Free triiodothyronine; FT4=Free thyroxine; Se=Selenium; TRAb=TSH Receptor Antibodies
DISCUSSION
In this case–control study, the serum Se level was significantly lower in newly diagnosed patients with GD than in the control group. Furthermore, the serum Se level was significantly lower in newly diagnosed patients with HT, compared to the control group. The serum Se level was lower in patients with GD than in patients with HT; however, the difference was not statistically significant. No association was found between the Se concentration and thyroid function parameters, such as FT4, FT3, TSH, and thyroid antibodies.
As mentioned earlier, besides the presence of Se as a component of deiodinase structure and its role in the production of thyroid hormones, it neutralizes the dissemination and activity of hydrogen peroxide and maintains the integrity of thyrocytes. CD4+ T cells can differentiate into Th1 or Th2 cells, depending on pro-Th1 or pro-Th2 activation. Se promotes the activity of CD4+/CD25 and T regulatory cells while suppressing the secretion of cytokines; therefore, it prevents follicular cell apoptosis and causes protection against thyroiditis.[22]
However, the serum Se level reported in the current study (122 μg/L) is different from another study[23] that reported a serum Se level of 103 μg/L. Significant differences in the Se concentration of foods and soil in different regions can explain the significant difference in the serum levels of the study populations. Besides, other factors, such as the type of Se, the organic content of the soil, and soil pH may explain the different levels of Se.[9,24] Se intake is sourced from proteinaceous food such as meat, shellfish, fish, grains, cereals, and offal.[7,25] However, there is no consensus on whether the serum Se concentration is an indicator of the body's Se status or is merely a normal reference value. Overall, glutathione peroxidase activity is optimal at a serum Se level of 100 μg/L, while a serum concentration ≤70 μg/L is defined as Se deficiency.[26]
The evaluation of Se status has some challenges, as Se measurements cannot accurately represent the tissue concentration of Se, and a normal Se concentration does not reject the possibility of low Se content in the thyroid tissue.[16] It has been also shown that inflammatory cytokines downregulate the Se level and reduce the selenoprotein expression.[27]
In addition to various genetic and environmental factors, Se deficiency has been suggested as a risk factor for AITD.[28] The association between Se level and AITD has been investigated in several studies. In this regard, Kucharzewski et al. reported 18 cases of GD in women, who had lower serum Se levels than patients with multinodular goiter or thyroid cancer.[16] In another cross-sectional study, an inverse association was found between the serum Se level and thyroid hypoechogenecity, as an autoimmune thyroid marker.[29] Furthermore, a study in Denmark investigated 97 newly diagnosed patients with GD, 96 patients with HT, 92 euthyroid patients with TPO antibody levels >1500 U/mL, and 830 randomly selected individuals as the control group. They reported that patients with GD and HT had significantly lower Se levels.[11] Moreover, in a study conducted in Australia, Se levels were significantly lower in patients with autoimmune hypothyroidism, compared to the healthy control group.[23] Another study on 41 patients with relapsed GD reported that the number of patients with normal thyroid antibody levels was significantly higher in the Se -recipient group at the end of the study.[30] Moreover, a large-scale study of Se status among 6000 residents of two regions in China indicated the protective effects of Se adequacy by comparing regions with Se deficiency and adequacy. After adjustments for confounding factors, the prevalence of thyroid diseases was significantly lower in the region with Se adequacy (18.0 vs. 30.5%; P < 0.001).[15] The results of our study are consistent with another study[18] in which, Se depletion was associated with the presence and severity of Graves’ orbitopathy.
This study has several limitations. First, to determine the Se status of the patient group, the serum Se concentration was measured, which cannot exactly reflect the tissue concentration of Se second, this cross-sectional study could not indicate the association between Se concentration and AITD. On the other hand, the inclusion of a euthyroid control group, matched in terms of age and sex with the patient group, and a relatively large sample size are the strengths of this study.
CONCLUSION
The serum Se level was significantly lower in newly diagnosed patients with GD than in the control group. Furthermore, the serum Se level was significantly lower in patients with HT than in the control group. The results showed that the level of Se was lower in patients with GD than those with HT; however, the difference was not statistically significant. In patients with GD, 28.33% had Graves’ orbitopathy. Serum Se levels in patients with orbitopathy were significantly lower than in patients without orbitopathy. Overall, Se deficiency can be considered a risk factor for AITDs.
Financial support and sponsorship
This study was funded by Zahedan University of Medical Sciences (grant number: 1805003).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank all the participants in this study. This study was supported by Zahedan University of Medical Sciences (Ethical approval number: IR. ZAUMS. REC.1399.071).
REFERENCES
- 1.Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: Autoimmune thyroid disease: Old and new players. Eur J Endocrinol. 2014;170:R241–52. doi: 10.1530/EJE-14-0047. [DOI] [PubMed] [Google Scholar]
- 2.Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–40. doi: 10.1080/08916930410001705394. [DOI] [PubMed] [Google Scholar]
- 3.Brent GA. Clinical practice. Graves’ disease. N Engl J Med. 2008;358:2594–605. doi: 10.1056/NEJMcp0801880. [DOI] [PubMed] [Google Scholar]
- 4.Ban Y, Tomer Y. Genetic susceptibility in thyroid autoimmunity. Pediatr Endocrinol Rev. 2005;3:20–32. [PubMed] [Google Scholar]
- 5.Santos LR, Durães C, Mendes A, Prazeres H, Alvelos MI, Moreira CS, et al. A polymorphism in the promoter region of the selenoproteins gene (SEPS1) contributes to Hashimoto's thyroiditis susceptibility. J Clin Endocrinol Metab. 2014;99:E719–23. doi: 10.1210/jc.2013-3539. [DOI] [PubMed] [Google Scholar]
- 6.Duntas LH. Environmental factors and thyroid autoimmunity. Ann Endocrinol (Paris) 2011;72:108–13. doi: 10.1016/j.ando.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–68. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 8.Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34–44. doi: 10.1017/S0029665118001192. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CC, Fordyce FM, Rayman MP. Symposium on ‘geographical and geological influences on nutrition’: Factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc Nutr Soc. 2010;69:119–32. doi: 10.1017/S0029665109991807. [DOI] [PubMed] [Google Scholar]
- 10.Duntas LH. The evolving role of selenium in the treatment of Graves’ disease and ophthalmopathy. J Thyroid Res 2012. 2012 doi: 10.1155/2012/736161. 736161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bülow Pedersen I, Knudsen N, Carlé A, Schomburg L, Köhrle J, Jørgensen T, et al. Serum selenium is low in newly diagnosed Graves’ disease: A population-based study. Clin Endocrinol (Oxf) 2013;79:584–90. doi: 10.1111/cen.12185. [DOI] [PubMed] [Google Scholar]
- 12.Nordio M, Pajalich R. Combined treatment with Myo-inositol and selenium ensures euthyroidism in subclinical hypothyroidism patients with autoimmune thyroiditis. J Thyroid Res 2013. 2013 doi: 10.1155/2013/424163. 424163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasiliu I, Preda C, Serban IL, Strungaru SA, Nicoară M, Plăvan G, et al. Selenium status in autoimmune thyroiditis. Rev Med Chir Soc Med Nat Iasi. 2015;119:1037–44. [PubMed] [Google Scholar]
- 14.Preda1 C, Mihalache L, Armasu I, Serban IL, Serban DN, Ciobanu DG, et al. Selenium – Essential antioxidant element. The example of autoimune thyroiditis. Revista de Chimie (Bucharest) 2017;68:1617–21. [Google Scholar]
- 15.Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, Gao C, et al. Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab. 2015;100:4037–47. doi: 10.1210/jc.2015-2222. [DOI] [PubMed] [Google Scholar]
- 16.Kucharzewski M, Braziewicz J, Majewska U, Góźdź S. Concentration of selenium in the whole blood and the thyroid tissue of patients with various thyroid diseases. Biol Trace Elem Res. 2002;88:25–30. doi: 10.1385/BTER:88:1:25. [DOI] [PubMed] [Google Scholar]
- 17.Wertenbruch T, Willenberg HS, Sagert C, Nguyen TB, Bahlo M, Feldkamp J, et al. Serum selenium levels in patients with remission and relapse of graves’ disease. Med Chem. 2007;3:281–4. doi: 10.2174/157340607780620662. [DOI] [PubMed] [Google Scholar]
- 18.Khong JJ, Goldstein RF, Sanders KM, Schneider H, Pope J, Burdon KP, et al. Serum selenium status in Graves’ disease with and without orbitopathy: A case-control study. Clin Endocrinol (Oxf) 2014;80:905–10. doi: 10.1111/cen.12392. [DOI] [PubMed] [Google Scholar]
- 19.Delshad H, Azizi F. Review of iodine nutrition in iranian population in the past quarter of century. Int J Endocrinol Metab. 2017;15:e57758. doi: 10.5812/ijem.57758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kratzsch J, Fiedler GM, Leichtle A, Brügel M, Buchbinder S, Otto L, et al. New reference intervals for thyrotropin and thyroid hormones based on national academy of clinical biochemistry criteria and regular ultrasonography of the thyroid. Clin Chem. 2005;51:1480–6. doi: 10.1373/clinchem.2004.047399. [DOI] [PubMed] [Google Scholar]
- 21.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves’ orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 2016;5:9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Liu S, Mao J, Piao S, Qin J, Peng S, et al. Serum trace elements profile in Graves’ disease patients with or without orbitopathy in Northeast China. Biomed Res Int 2018. 2018 doi: 10.1155/2018/3029379. 3029379. Doi: 10.1155/2018/3029379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wimmer I, Hartmann T, Brustbauer R, Minear G, Dam K. Selenium levels in patients with autoimmune thyroiditis and controls in lower Austria. Horm Metab Res. 2014;46:707–9. doi: 10.1055/s-0034-1377029. [DOI] [PubMed] [Google Scholar]
- 24.Sturniolo G, Mesa J. Selenium supplementation and autoimmune thyroid diseases. Endocrinol Nutr. 2013;60:423–6. doi: 10.1016/j.endonu.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Navarro-Alarcon M, Cabrera-Vique C. Selenium in food and the human body: A review. Sci Total Environ. 2008;400:115–41. doi: 10.1016/j.scitotenv.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Lymbury R, Tinggi U, Griffiths L, Rosenfeldt F, Perkins AV. Selenium status of the Australian population: Effect of age, gender and cardiovascular disease. Biol Trace Elem Res. 2008;126(Suppl 1):S1–10. doi: 10.1007/s12011-008-8208-6. [DOI] [PubMed] [Google Scholar]
- 27.Nichol C, Herdman J, Sattar N, O’Dwyer PJ, St J O’Reilly D, Littlejohn D, et al. Changes in the concentrations of plasma selenium and selenoproteins after minor elective surgery: Further evidence for a negative acute phase response? Clin Chem. 1998;44:1764–6. [PubMed] [Google Scholar]
- 28.Duntas LH. Environmental factors and autoimmune thyroiditis. Nat Clin Pract Endocrinol Metab. 2008;4:454–60. doi: 10.1038/ncpendmet0896. [DOI] [PubMed] [Google Scholar]
- 29.Derumeaux H, Valeix P, Castetbon K, Bensimon M, Boutron-Ruault MC, Arnaud J, et al. Association of selenium with thyroid volume and echostructure in 35-to 60-year-old French adults. Eur J Endocrinol. 2003;148:309–15. doi: 10.1530/eje.0.1480309. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Wang B, Chen SR, Hou X, Wang XF, Zhao SH, et al. Effect of selenium supplementation on recurrent hyperthyroidism caused by Graves’ disease: A prospective pilot study. Horm Metab Res. 2016;48:559–64. doi: 10.1055/s-0042-110491. [DOI] [PubMed] [Google Scholar]


