Abstract
Background:
Preoperative treatment with oral neomycin combined with erythromycin or metronidazole is recommended to decrease the risk of surgical site infections (SSIs) in elective colorectal surgery. However, oral neomycin is not commercially available in Canada, and therefore it is not routinely used. Fluoroquinolones are widely available and have excellent activity against aerobic Gram-negative bacteria. The aim of this systematic review was to identify, critically appraise and summarize the evidence on the efficacy and safety of preoperative use of oral fluoroquinolone antibiotics for the prevention of SSIs in adult patients undergoing elective colorectal resection.
Methods:
Following Cochrane guidelines, we included English-language randomized controlled trials (RCTs) comparing oral fluoroquinolones plus routine preoperative intravenous antibiotics against intravenous antibiotics alone from MEDLINE (Ovid), Embase (Ovid), the Cochrane Central Register of Controlled Trials( Ovid) and ClinicalTrials.gov.
Results:
We included 3 RCTs (1136 patients). Risk of bias was uncertain in 2 trials and high in 1 trial. Preoperative oral fluoroquinolones led to significantly decreased total SSIs (risk ratio [RR] 0.43, 95% confidence interval [CI] 0.32–0.57, I2 = 0%), superficial incisional (RR 0.38, 95% CI 0.22–0.68, I2 = 32%), deep incisional (RR 0.19, 95% CI 0.06–0.65, I2 = 0%) and organ/space SSIs (RR 0.34, 95% CI 0.12–0.90, I2 = 33%). There was also a significant reduction in anastomotic leaks (RR 0.22, 95% CI 0.06–0.87, I2 = 0%). No antibiotic-related adverse events were reported.
Conclusion:
This review suggests that preoperative oral fluoroquinolones with intravenous antibiotics are superior to intravenous antibiotics alone for preventing SSIs after colorectal surgery. If neomycin is unavailable, oral fluoroquinolones should be considered as a reasonable alternative. Future trials are required to further compare the relative efficacy of oral antibiotic regimens.
Abstract
Contexte:
Un traitement préopératoire par néomycine orale associée à de l’érythromycine ou du métronidazole est recommandé pour réduire le risque d’infection du site opératoire (ISO) lors de la chirurgie colorectale non urgente. Or, la néomycine orale n’est pas disponible sur le marché au Canada et par conséquent, n’est pas utilisée. Les fluoroquinolones sont facilement accessibles et exercent une excellente activité contre les bactéries aérobies à Gram négatif. Le but de cette revue systématique était d’identifier, d’évaluer de façon critique et de résumer les données probantes sur l’efficacité et l’innocuité de l’administration préopératoire de fluoroquinolones orales pour la prévention des ISO chez des adultes soumis à une résection colorectale élective.
Méthodes:
Conformément aux lignes directrices Cochrane, nous avons inclus les essais randomisés et contrôlés (ERC) de langue anglaise ayant comparé des fluoroquinolones orales en association avec les antibiotiques intraveineux préopératoires habituels et aux antibiotiques intraveineux seuls, recensés dans les bases de données MEDLINE (Ovid), Embase (Ovid), le Registre central Cochrane des essais contrôlés (Ovid) et ClinicalTrials.gov.
Résultats:
Nous avons inclus 3 ERC (1136 patients). Le risque de biais était incertain pour 2 essais et élevé pour 1 essai. Les fluoroquinolones orales préopératoires ont donné lieu à une diminution significative du nombre total d’ISO (risque relatif [RR] 0,43, intervalle de confiance [IC] de 95 % 0,32–0,7, I2 = 0 %), superficielles (RR 0,38, IC de 95 % 0,22–0,68, I2 =32 %), profondes (RR 0,19, IC de 95 % 0,06–0,65, I2 = 0 %) et des cavités/organes (RR 0,34, IC de 95 % 0,12–0,90, I2 = 33 %). On a aussi noté une réduction significative des fuites anastomotiques (RR 0,22, IC de 95 % 0,06–0,87, I2 = 0 %). Aucun effet indésirable lié à l’antibiothérapie n’a été signalé.
Conclusion:
Selon cette revue, les fluoroquinolones orales préopératoires avec antibiothérapie intraveineuse sont supérieures aux antibiotiques intraveineux seuls pour la prévention des ISO après la chirurgie colorectale. En l’absence de néomycine, les fluoroquinolones orales représentent une solution de rechange acceptable. Il faudra procéder à d’autres essais pour mieux comparer l’efficacité relative des différents schémas d’antibiothérapie orale.
Colorectal surgical procedures are associated with some of the highest rates of surgical site infections (SSIs) of any intra-abdominal procedures, which are frequently caused by anaerobic bacterial flora and Gram-negative bacilli from the colon.1 Most commonly an SSI results in a subcutaneous abscess at the skin incision. This can be drained easily at the bedside but may require weeks of wound care to heal, leading to patient distress and substantial cost to the medical system.2,3 SSIs are also associated with a doubling of the perioperative mortality risk, higher rates of intensive care unit admission and hospital readmission, and longer hospital lengths of stay.4,5 Owing to the volume of procedures performed, SSIs account for the majority of nosocomial infections in North America.6
Before routine preoperative administration of antibiotics, SSI rates were as high as 36%.7 Preoperative oral antibiotics in combination with mechanical bowel preparation (MBP) and intravenous antibiotics have been used since the 1970s and are proven to reduce SSI rates after colorectal surgery.8 Neomycin in combination with either metronidazole or erythromycin has emerged as the preferred oral antibiotic regimen and is recommended before all major elective colorectal resections in guidelines by both the American College of Surgeons and the American Society of Colon and Rectal Surgeons.9,10 However, despite multiple randomized controlled trials (RCTs) and systematic reviews showing substantial benefit,1,11 there is poor agreement globally on this practice.12
Neomycin, an aminoglycoside antibiotic that is central to the most well-studied preoperative oral antibiotic regimen, is not commercially available in oral formulation in some regions, including Canada and China.4,5 In Canada, preoperative oral antibiotics are not always used before elective colorectal resection, in part because of the feasibility of obtaining the requisite drugs.13 Some groups use specialized compounding pharmacies to create their own neomycin compounds; however, these take time to prepare so must be planned in advance, and this possibility is not widely accessible.14 However, routine preoperative intravenous antibiotics alone are inadequate for preventing SSIs following colorectal surgery.11 Therefore, these patients may benefit from more readily available evidence-based preoperative oral antibiotic options.
Fluoroquinolones are broad-spectrum antibiotics with excellent activity against aerobic Gram-negative bacteria and are frequently used in the management of intraabdominal infections. Furthermore, they are widely available, well tolerated and inexpensive.15 This antibiotic class alone or in combination with another antibiotic with broad anaerobic coverage (e.g., metronidazole) is expected to mimic the beneficial antibiotic activity of the previously studied macrolide–aminoglycoside antibiotic preparations.16 However, the use of oral fluoroquinolones in the preoperative bowel preparation for elective colorectal surgery has not been systematically reviewed in the literature. The aim of this study was to identify and critically appraise evidence on the efficacy and safety of preoperative regimens containing oral fluoroquinolone antibiotics plus routine preoperative intravenous antibiotics, compared with preoperative intravenous antibiotics alone, in adult patients undergoing elective colorectal resection for the prevention of SSIs.
Methods
We registered a protocol for this systematic review in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42021258036). The review was conducted according to guidelines enumerated in the Methodological Expectations of Cochrane Intervention Reviews (MECIR)17,18 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.19 The research question developed a priori was as follows: In adult patients (aged ≥ 18 yr) undergoing elective colorectal resection, what is the effectiveness of preoperative oral fluoroquinolone antibiotics for the prevention of SSIs, compared with intravenous antibiotics alone, and controlling for antibiotic-related adverse events?
Population, interventions, comparators, settings and trial design
We included randomized controlled trials with adult patients (≥ 18 yr) who underwent elective colorectal surgery for any indication. In the case of trials where some patients met the inclusion criteria and others did not (e.g., mix of children and adults), we included in the analysis those where 80% or more of trial participants met the inclusion criteria. Patients must have received preoperative oral fluoroquinolones plus routine preoperative intravenous antibiotics or intravenous antibiotics alone. The use of any antibiotic from this class was included. Co-administration of an oral fluoroquinolone with another oral antibiotic drug was permitted. Both groups must also have received a preoperative intravenous dose of antibiotics. All practice settings were included. Emergent operations and animal trials were also excluded. As the role of mechanical bowel preparation (i.e., osmotic laxatives or enemas) in SSIs is unclear, its use did not affect trial inclusion.11
Outcomes
The primary outcomes were total, superficial incisional, deep incisional and organ space SSIs. Secondary outcomes were anastomotic leaks, mortality, readmissions to hospital, reoperations, postoperative ileus events, urinary tract infections, pulmonary infections and patient-reported quality of life. Safety outcomes included postoperative Clostridium difficile infections, tendinopathy, aortic rupture and allergic reactions. Trials were excluded if none of the outcomes were available from the trial report or through communication with the trial’s corresponding author.
Search strategy
A MEDLINE search strategy was designed and underwent peer review by an independent information professional.20 Database searches were conducted in MEDLINE (Ovid), Embase (Ovid) and the Cochrane Central Register of Controlled Trials (Ovid). Unpublished or ongoing clinical trials were identified by searching ClinicalTrials.gov. The final search strategy is presented in Appendix 1 (available at canjsurg.ca/lookup/doi/10.1503/cjs.019721/tab-related-content). The literature was searched from database inception until June 10, 2021. All retrieved records were imported into Endnote (X9, Thomson Reuters) and deduplicated.
Trial selection and data abstraction
Citations were imported into Rayyan Online (Rayyan),21 and 2 reviewers (G.J., J.Z.) independently screened citations for eligibility in duplicate using a 2-stage approach. First, titles and abstract were reviewed, then potentially relevant full-text articles were examined to determine if they met the inclusion criteria. The rationale for determining that full-text articles were ineligible for inclusion was recorded. Data were extracted by 2 independent reviewers (G.J., J.Z.) using a standardized pilot-tested form. Disagreements at all phases were resolved through consensus or with assistance from a third party (A.M.A.-S.) if consensus could not be achieved.
Risk of bias assessment
The Cochrane Risk of Bias Tool for Randomized Trials (RoB 2)22 was used by 2 independent reviewers (G.J., J.Z.) to assess risk of bias in the included trials.23 Investigators categorized each RCT using the following criteria: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete data, non-comparable groups, performance bias, and detection bias. A final risk of bias assessment was determined for each RCT, with discrepancies between reviewers resolved by discussion and a third investigator (A.M.A.-S.), if necessary.
Statistical analysis
Data were analyzed using RevMan (version 5.3.5). The analysis plan was determined a priori. Pooled continuous data were expressed as mean differences (MDs), or standardized mean differences (SMDs) where multiple scales were used to measure the same outcome, with 95% confidence intervals (CIs). Pooled dichotomous data were presented as risk ratios (RRs), or for rare outcomes as Peto odds ratios (ORs). When the RR was significant, we also calculated the risk difference (RD). Statistical heterogeneity of the data was explored and quantified, using the I2 test.24
One trial used nonstandard language to define the outcomes of superficial and deep SSIs, but it did report “wound” infections.25 The same trial had a high risk of bias owing to excluded outcome data. In this case, we performed a post hoc sensitivity analysis to test the robustness of the results.
We planned to assess publication bias by viewing the overlap of the trial CIs and using funnel plot techniques.26 We also planned to conduct subgroup analyses for the various oral antibiotic regimens that were administered. Owing to the small number of trials retrieved, neither publication bias nor subgroup analyses were performed.
Results
Among the 2644 citations identified in the literature search, 3 RCTs met the inclusion criteria16,25,27 (Figure 1). Summary characteristics of the included trials are shown in Table 1 and Table 2. Ciprofloxacin and levofloxacin were the only oral fluoroquinolone antibiotics used. Each trial used different regimens for oral antibiotics, intravenous antibiotics and MBP. Oral antibiotics were always given the day before surgery. The included trials were conducted in Scotland, Spain and China. All were parallel group RCTs. One trial had pharmaceutical industry sponsorship.25
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Table 1.
Summary of included studies: locations, funding, antibiotic treatments and surgical characteristics
| Study | Country | Funding | Follow- up | Oral antibiotics | IV antibiotics | Mechanical bowel preparation | Surgery indication | Resection type |
|---|---|---|---|---|---|---|---|---|
| Anjum et al.16 | China | National Natural Science Foundation of China | 90 d | Metronidazole 400 mg and levofloxacin 200 mg × 3 (3 pm, 7 pm and 11:30 pm) on the day before surgery | Second-generation cephalosporin and metronidazole, 30–60 min before surgery and repeated every 3 h during surgery; antibiotics continued 24 h after surgery | Sodium phosphate 133 mL BID on the day before surgery | GI tract fistula, abdominal adhesions, iatrogenic injury, IBD, trauma, malignancy | 40 laparoscopic, 150 open |
| Basany et al.27 | Spain | Fundación Asociación Española de Colo- proctología | 4 wk | Metronidazole 250 mg × 3 (12 pm, 6 pm and 12 am), ciprofloxacin 750 mg × 2 (12 pm and 12 am) on the day before surgery | Cefuroxime 1.5 g and metronidazole 1 g at anesthetic induction | None | Malignancy, diverticular disease | 415 laparoscopic, 77 open, 45 conversions |
| Taylor and Lindsay25 | Scotland | Lederle Laboratories* | 6 wk | Ciprofloxacin 500 mg BID on the day before surgery | One dose of piperacillin 4 g IV anesthetic induction | Sodium picosulfate, 1 sachet BID, on the day before surgery | Malignancy, IBD, benign conditions | 381 open, 0 laparoscopic |
BID = twice daily; GI = gastrointestinal; IBD = inflammatory bowel disease; IV = intravenous.
Pharmaceutical company.
Table 2.
Summary of included studies: study arms and patient characteristics
| Study | Segment resected | Arm | No. of patients | Mean age | BMI | ASA score | ||
|---|---|---|---|---|---|---|---|---|
| Total | Men | Women | ||||||
| Anjum et al.16 | Small bowel 39, right 67, left 50, rectum 34 | MBP + OA | 95 | 61 | 34 | 46.3 ± 14.4 | 21.3 ± 3.7 | I: 0 II: 80 III: 15 |
| MBP | 95 | 59 | 36 | 45.2 ± 15.6 | 21.7 ± 4.8 | I: 2 II:76 III:17 |
||
| Basany et al.27 | Right 268, left 240, total colectomy 25, segment 3, other 2 | OA | 283† | 142 | 125 | 70.0 ± 11.9 | 27.6 ± 4.1 | I–II: 145 III–IV: 122 |
| Control | 282‡ | 152 | 117 | 71.7 ± 13.3 | 27.6 ± 4.1 | I–II:129 III–IV: 140 |
||
| Taylor and Lindsay25 | Right 93, left 168, Hartmann 6, APR 43, other 17* | OA + MBP | 189§ | 79 | 80 | 66.7 ± 13.0 | NR | NR |
| MBP | 192¶ | 79 | 89 | 66.5 ± 12.9 | NR | NR | ||
APR = abdominoperineal resection; ASA = American Society of Anesthesiologists; BMI = body mass index; MBP = mechanical bowel preparation; NR = not reported; OA = oral antibiotics.
Not reported for 54 cases.
Not reported for 16 cases.
Not reported for 12 cases.
The sex of 30 patients was unknown.
The sex of 24 patients was unknown.
Risk of bias
The risk of bias assessment for the primary outcomes is shown in Figure 2. In all 3 trials, appropriate randomization processes were followed; however, it was unclear if the allocation sequence was concealed until participants were enrolled and assigned to interventions. All trials were also limited by the lack of placebo use, which would be expected to affect blinding. In 1 trial, patients were excluded if they experienced an anastomotic leak. This occurred much more frequently in the control arm and could have biased the rates of SSIs reported in that group. However, the data for total SSIs, anastomotic leaks and mortality were available for the excluded patients, so they were included in the meta-analysis.
Fig. 2.
Cochrane risk of bias tool (RoB 2) for randomized controlled trials.
Surgical site infections
Among the total of 1136 patients included in the 3 RCTs, 173 SSIs occurred. The use of preoperative oral antibiotics was associated with a significantly decreased total number of SSIs (RR 0.43, 95% CI 0.32–0.57, 3 RCTs, 1136 participants, I2 = 0%), superficial incisional SSIs (RR 0.38, 95% CI 0.22–0.68, 3 RCTs, 1136 participants, I2 = 32%), deep incisional SSIs (RR 0.19, 95% CI 0.06–0.65, 2 RCTs, 755 participants, I2 = 0%) and organ/space SSIs (RR 0.34, 95% CI 0.12–0.90, 3 RCTs, 1136 participants, I2 = 33%, Figure 3; risk differences shown in Appendix 2, available at canjsurg.ca/lookup/doi/10.1503/cjs.019721/tab-related-content). All 3 trials reported SSI rates; however, 1 trial did not separately report superficial and deep incisional SSIs.25 Furthermore, some SSIs were excluded from this trial, with only the total SSI rate reported for those patients. Sensitivity analyses were undertaken with this trial’s results excluded for this variable (Appendix 3, available at canjsurg.ca/lookup/doi/10.1503/cjs.019721/tab-related-content). The overall effect of preoperative oral antibiotics was still significant when this trial was excluded for total and superficial SSIs but not organ/space SSIs.
Fig. 3.
Forest plots for (A) total SSIs, (B) superficial incisional SSIs, (C) deep incisional SSIs and (D) organ/space SSIs, comparing preoperative oral antibiotics plus intravenous antibiotics (experimental) to intravenous antibiotics alone (control). CI = confidence interval; df = degrees of freedom; M-H = Mantel–Haenszel; SSIs = surgical site infections.
Secondary outcomes
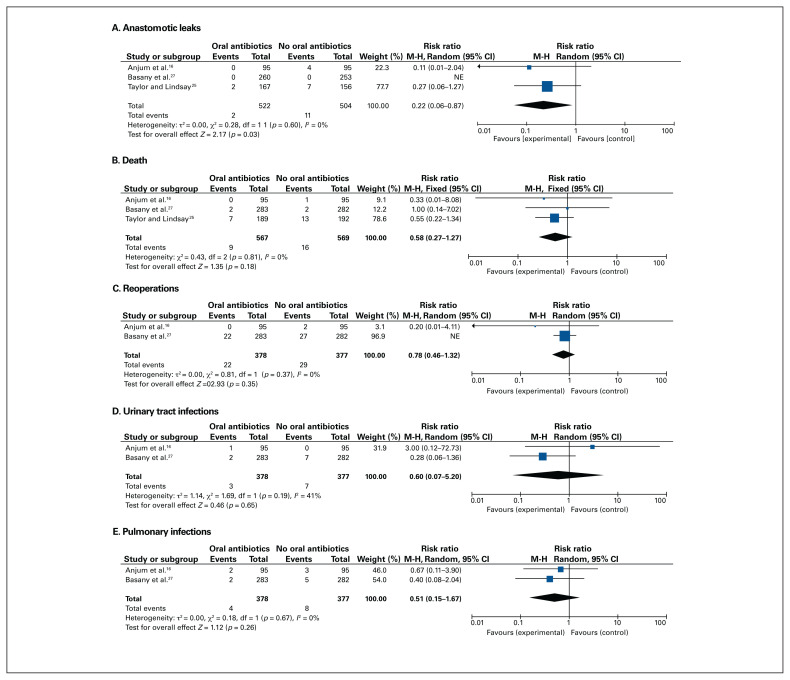
Anastomotic leaks were significantly reduced in patients receiving oral antibiotics compared with no oral antibiotics (RR 0.22, 95% CI 0.06–0.87, 3 RCTs, 1026 participants, I2 = 0%, Figure 4A). Leaks occurred in only 11 patients across the 3 trials. There was no statistically significant difference in mortality, urinary tract infections, pulmonary infections and reoperations between the groups (Figure 4B–4E). Postoperative ileus duration was reported in a single trial,16 and it was not significantly affected by oral antibiotic use (3.96 [standard deviation (SD) 1.26] d v. 4.23 [SD 1.7] d for oral antibiotics and no oral antibiotics, respectively). Hospital readmissions were also reported in 1 trial and were not significantly different between the groups. None of the trials provided data on patient-reported quality of life.
Fig. 4.
Forest plots for the secondary outcomes comparing preoperative oral antibiotics and intravenous antibiotics (experimental) to intravenous antibiotics alone (control) for (A) anastomotic leaks, (B) death, (C) reoperations, (D) urinary tract infections and (E) pulmonary infections. CI = confidence interval; df = degrees of freedom; M-H = Mantel–Haenszel; SSIs = surgical site infections.
Safety outcomes
Safety outcomes are shown in Table 3. There were no adverse events reported in any trial attributable to the oral antibiotic regimens used. However, 1 trial did not report any information related to safety outcomes.25 Two trials specifically reported no instances of C. difficile infections across a total of 755 patients.16,27 The authors of 1 trial commented that there were no allergic reactions.27 No trials reported on tendinopathy or aortic rupture. Owing to a lack of reported adverse events, a meta-analysis was not performed for safety outcome data.
Table 3.
Safety outcomes
Discussion
This review demonstrates that preoperative oral fluoroquinolones in combination with intravenous antibiotics are superior to intravenous antibiotics alone for the prevention of SSIs after colorectal surgery. Rates of total, superficial, deep and organ/space SSIs, in addition to anastomotic leaks, were all significantly reduced by antibiotic regimens containing oral fluoroquinolones without a corresponding increase in reported antibiotic-related adverse events. While data on the use of preoperative oral antibiotics have been synthesized multiple times in past meta-analyses,1,11,28 the previous literature has focused on regimens containing oral macrolides or aminoglycosides. To our knowledge, this is the first systematic review to examine the role of oral fluroquinolones specifically.
The overall effect of the preoperative oral fluoroquinolone regimens on total SSIs determined in this meta-analysis (RR 0.43, 95% CI 0.32–0.57) is similar to that in a recent meta-analysis examining the effect of preoperative oral macrolide–aminoglycoside antibiotics (RR 0.45, 95% CI 0.34–0.60).28 Risk reduction for incisional SSIs is also comparable between our meta-analysis (RR 0.38, 95% CI 0.22–0.68) and theirs (RR 0.38, 95% CI 0.26–0.56). However, in our analysis we found that preoperative fluoroquinolones led to a reduced risk of organ/space infections and anastomotic leaks, which has not previously been observed in meta-analysis of RCTs for oral neomycin.28 Despite these promising results, to our knowledge a modern oral neomycin-containing antibiotic preparation has never been compared with an oral fluoroquinolone regimen for this indication. A large randomized comparative trial is needed to determine the relative efficacy of these 2 antibiotics.
A strength of this systematic review compared with previous ones is the inclusion of more contemporary trials. Two of the 3 included trials were conducted within the past 5 years. Most of the included patients had laparoscopic surgery. A major limitation of past systematic reviews on this topic using neomycin-containing antibiotic regimens is the reliance on RCTs predating the advent of laparoscopic surgery.1,11,28
Three distinct oral fluoroquinolone antibiotic regimens are represented in the literature. There was minimal statistical heterogeneity between trials and a universal positive effect on total SSI rates. Ciprofloxacin in particular was used in 2 of the 3 trials,25,27 alone in 1 trial and in combination with metronidazole in the other. Levofloxacin was the other fluoroquinolone antibiotic used, and it was also combined with metronidazole.16 Both drugs appeared to perform equally well, although each trial used different antibiotic combinations, preventing meaningful subgroup analysis. Future trials could aim to compare fluoroquinolone antibiotic regimens for superiority. However, the results of this current meta-analysis would suggest that the difference between these particular regimens is quite small.
Patient-reported outcomes may be 1 way to differentiate between antibiotic regimens. For example, oral metronidazole can be associated with gastrointestinal symptoms.30 Patients may also find it easier to manage 2 split doses of oral antibiotics administered at the same time as their purgative, as described by Taylor and Lindsay,25 compared with 3 doses at different intervals, as described by Anjum and colleagues.16 If MBP (i.e., referring to the cathartic agent only) can be omitted, as suggested by Basany and colleagues, this may further improve the patient experience.27 A recent network meta-analysis examining primarily oral aminoglycoside or macrolide antibiotic preoperative regimens found only a small beneficial effect of MBP on the effectiveness of oral antibiotics in reducing SSIs.11 How these data apply to the current fluoroquinolone antibiotic regimens is not clear. Unfortunately, none of the trials in the present review examined patient perspectives.
To compound the issue, patient preference and SSI rates are not the only factors relating to MBP use that should be considered. Some surgeons prefer a clean colon for construction of their anastomosis, particularly for low rectal surgery.30 MBP also facilitates intraoperative tumour localization via endoscopy should a preoperative localization error occur, or if preoperative tumour marking is not visible.30 Localization errors have been reported at a rate of 15.4%,31 although this can be somewhat reduced with repeat preoperative endoscopy.32
No adverse events related to the oral antibiotics were reported in any of the included trials. No C. difficile infections occurred across 2 trials and 755 patients. This is consistent with a recent large systematic review and meta-analysis, which showed that Clostridium infection was a rare event following elective colorectal surgery, occurring no more frequently following use of oral antibiotics than following use of intravenous antibiotics alone.1 No allergic reactions related to fluoroquinolones were observed, although these were only measured in a single trial.27 Other anticipated fluoroquinolone-specific adverse events such as tendinopathy and aortic rupture were not specifically reported by any trial; however, these events are extremely rare and unlikely to occur after a single dose33,34
Fluoroquinolones are not the only alternative to neomycin studied in the literature. Oral metronidazole alone has had moderate effectiveness in 1 small RCT.35 However, this trial is quite dated, and it examined exclusively open surgery. The applicability of these results to a modern patient population is limited. Furthermore, metronidazole lacks broad Gram-negative coverage; therefore, there is a good theoretical basis for reduced effectiveness compared with combination regimens.29 Oral penicillin–type antibiotics combined with clavulanic acid are another broad-spectrum combination that combines both Gram-negative and anaerobic coverage that could potentially be used. This drug has been compared with standard intravenous prophylaxis for preventing SSI with promising results, but it has not been combined with intravenous antibiotics in an RCT.36–38 Other options include minimally absorbed oral antimicrobials. Oral colistin has been investigated with promising results,39 and a large trial investigating the efficacy of oral rifaximin is underway.40
One important consideration with the use of oral fluoroquinolones in addition to standard intravenous antibiotic prophylaxis for colorectal surgery is that the same concentration of the antibiotic can be achieved with intravenous antibiotics. Both ciprofloxacin and levofloxacin are absorbed in the small intestine and are secreted back into the colon. Colonic excretion also occurs following intravenous administration.25 It remains unclear whether it is the action of ingesting these antibiotics in advance of surgery that leads to their beneficial effects, or whether the addition of fluoroquinolones to existing intravenous antibiotic regimens would have the same effect. Future study is needed to clarify this factor.
Limitations
Despite the importance of our findings, there are some limitations. First, there were only 3 included trials, which limits our ability to detect statistically significant differences between most secondary outcomes. Despite this limitation, more than 1000 patients were included across the 3 trials for primary outcomes, and strong differences were observed between treatment groups. Second, some outcomes were not reported in some trials. For example, both superficial and deep incisional SSIs were reported as wound infections by Taylor and Lindsay,25 probably because this paper was published at approximately the same time as these definitions were first introduced in 1992.41 For these data, it is impossible to differentiate between superficial and deep subtypes. The decision was made by both reviewers to include these events under the heading of superficial SSIs before statistical analysis, as these types of SSI are most common. However, it is possible that some of these events were actually misclassified deep SSIs. Furthermore, patients with anastomotic leaks were excluded in that paper. Only total SSIs, deaths and anastomotic leak rates were reported for excluded patients. Therefore, rates of superficial, deep and organ/space SSI were probably underestimated. Accordingly, sensitivity analysis was performed where this trial was censored for those outcomes. Another limitation is the overall quality of the included trials. All trials had at least unclear risk of bias because of poor reporting of trial methods. Although meta-analyses can increase power and precision, they cannot eliminate any biases that exist in pooled trials. The heterogeneity of the prescribed oral and intravenous antibiotics as well as the bowel preparation regimens used was another limitation. Although statistical heterogeneity was low between the trials, indicating minimal statistically apparent differences between regimens, it is impossible to discern which regimen is superior for the prevention of SSIs from the current literature. In addition, as our search was conducted in English, trials that were published only in other languages were not included in this review.
Another consideration is that the trial by Anjum and colleagues included 39 small bowel resections in the analysis.16 Small bowel resections are less likely to lead to wound infections or anastomotic leaks and would therefore have increased the denominator for the total population of patients in both the treatment and the control arms.42 These patients were not reported separately in this study, so the small number of small bowel resections could not be excluded from our meta-analysis. However, there were equal numbers of small bowel resections in both treatment and comparison groups, and this is therefore unlikely to have biased the outcome. As small bowel resections accounted for less than 4% of all patients included in the total meta-analysis (20% of the patients in the study by Anjum and colleagues), and could not be separated from the rest of the data, we felt that it was still prudent to include this trial in the meta-analysis.
Finally, although multiple centres were included in the RCTs, the trials took place only in China or Europe. Antibiotic resistance patterns vary, and it is possible that diminished effectiveness could be seen in areas with predominant fluoroquinolone-resistant pathogens.43 Therefore, the generalizability of these results to those areas is uncertain.
Conclusion
This review suggests that preoperative oral fluoroquinolone antibiotics in combination with intravenous antibiotics are superior to intravenous antibiotics alone for the prevention of SSIs after colorectal surgery. These findings have important implications for care, as they provide compelling support for the use of oral fluoroquinolones, which may be more accessible in regions where commercial access to oral neomycin is limited. However, randomized comparative trials are needed to determine the relative efficacy of antibiotic regimens.
Supplementary Material
Acknowledgements
The authors thank Christine J. Neilson of the University of Manitoba libraries for peer review of the MEDLINE search strategy.
Footnotes
Competing interests: None declared.
Contributors: G. Johnson, R. Helewa and A. Abou-Setta designed the study. J. Ziegler and N. Askin acquired the data, which R. Rabbani analyzed. G. Johnson wrote the article, which J. Ziegler, R. Helewa, N. Askin, R. Rabbani and A. Abou-Setta critically revised. All authors gave final approval of the version to be published.
References
- 1.Khorasani S, Dossa F, McKechnie T, et al. Association between preoperative oral antibiotics and the incidence of postoperative Clostridium difficile infection in adults undergoing elective colorectal resection: a systematic review and meta-analysis. Dis Colon Rectum 2020;63:545–61. [DOI] [PubMed] [Google Scholar]
- 2.Mahmoud NN, Turpin RS, Yang G, et al. Impact of surgical site infections on length of stay and costs in selected colorectal procedures. Surg Infect (Larchmt) 2009;10:539–44. [DOI] [PubMed] [Google Scholar]
- 3.Smith RL, Bohl JK, McElearney ST, et al. Wound infection after elective colorectal resection. Ann Surg 2004;239:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 1999;20:725–30. [DOI] [PubMed] [Google Scholar]
- 5.Badia JM, Casey AL, Petrosillo N, et al. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017;96:1–15. [DOI] [PubMed] [Google Scholar]
- 6.Lewis SS, Moehring RW, Chen LF, et al. Assessing the relative burden of hospital-acquired infections in a network of community hospitals. Infect Control Hosp Epidemiol 2013;34:1229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum ML, Anish DS, Chalmers TC, et al. A survey of clinical trials of antibiotic prophylaxis in colon surgery: evidence against further use of no-treatment controls. N Engl J Med 1981;305:795–9. [DOI] [PubMed] [Google Scholar]
- 8.Nichols RL, Broido P, Condon RE, et al. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann Surg 1973;178:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migaly J, Bafford AC, Francone TD, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the use of bowel preparation in elective colon and rectal surgery. Dis Colon Rectum 2019;62:3–8. [DOI] [PubMed] [Google Scholar]
- 10.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J Am Coll Surg 2017;224:59–74. [DOI] [PubMed] [Google Scholar]
- 11.Toh JWT, Phan K, Hitos K, et al. Association of mechanical bowel preparation and oral antibiotics before elective colorectal surgery with surgical site infection: a network meta-analysis. JAMA Netw Open 2018;1:e183226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zmora O, Wexner SD, Hajjar L, et al. Trends in preparation for colorectal surgery: survey of the members of the American Society of Colon and Rectal Surgeons. Am Surg 2003;69:150–4. [PubMed] [Google Scholar]
- 13.AHS recommended drug regimens for surgical prophylaxis in adult patients. Edmonton: Alberta Health Services; 2018. Available: www.albertahealthservices.ca/assets/info/hp/as/if-hp-as-surgical-prophylaxis.pdf (accessed 2021 Feb. 7). [Google Scholar]
- 14.Provincial clinical knowledge topic ERAS colorectal surgery, adult – inpatient V 1.1. Edmonton: Alberta Health Services; 2019. Available: https://extranet.ahsnet.ca/teams/policydocuments/1/klink/et-klink-ckv-eras-colorectal-surgery-adult-inpatient.pdf (accessed 2021 Apr. 17). [Google Scholar]
- 15.Falagas ME, Matthaiou DK, Bliziotis IA. Systematic review: fluoroquinolones for the treatment of intra-abdominal surgical infections. Aliment Pharmacol Ther 2007;25:123–31. [DOI] [PubMed] [Google Scholar]
- 16.Anjum N, Ren J, Wang G, et al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Dis Colon Rectum 2017;60:1291–8. [DOI] [PubMed] [Google Scholar]
- 17.Cochrane handbook for systematic reviews of interventions. Version 5.0.1. Chichester (UK): John Wiley & Sons; 2008. [Google Scholar]
- 18.Chandler J, Churchill R, Higgins J, et al. Methodological expectations of Cochrane intervention reviews (MECIR): methodological standard for the conduct of new Cochrane intervention reviews. London: Cochrane; 2013. [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGowan J, Sampson M, Salzwedel DM, et al. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- 21.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan — a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Sterne J, Savovic J, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database of Syst Rev 2016; 10(Suppl 1):29–31. [Google Scholar]
- 23.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 25.Taylor EW, Lindsay G. Selective decontamination of the colon before elective colorectal surgery. West of Scotland Surgical Infection Study Group. World J Surg 1994;18:926–31, discussion 931–2. [DOI] [PubMed] [Google Scholar]
- 26.Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 2007;176:1091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basany EE, Solís-Peña A, Pellino G, et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:729–38. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Song X, Chen L-Z, et al. Comparing mechanical bowel preparation with both oral and systemic antibiotics versus mechanical bowel preparation and systemic antibiotics alone for the prevention of surgical site infection after elective colorectal surgery: a meta-analysis of randomized controlled clinical trials. Dis Colon Rectum 2016;59:70–8. [DOI] [PubMed] [Google Scholar]
- 29.Hernández Ceruelos A, Romero-Quezada LC, Ruvalcaba Ledezma JC, et al. Therapeutic uses of metronidazole and its side effects: an update. Eur Rev Med Pharmacol Sci 2019;23:397–401. [DOI] [PubMed] [Google Scholar]
- 30.Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011;9:CD001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acuna SA, Elmi M, Shah PS, et al. Preoperative localization of colorectal cancer: a systematic review and meta-analysis. Surg Endosc 2017;31:2366–79. [DOI] [PubMed] [Google Scholar]
- 32.Al Abbasi T, Saleh F, Jackson TD, et al. Preoperative re-endoscopy in colorectal cancer patients: an institutional experience and analysis of influencing factors. Surg Endosc 2014;28:2808–14. [DOI] [PubMed] [Google Scholar]
- 33.Alves C, Mendes D, Marques FB. Fluoroquinolones and the risk of tendon injury: a systematic review and meta-analysis. Eur J Clin Pharmacol 2019;75:1431–43. [DOI] [PubMed] [Google Scholar]
- 34.Dai X-C, Yang X-X, Ma L, et al. Relationship between fluoroquinolones and the risk of aortic diseases: a meta-analysis of observational studies. BMC Cardiovasc Disord 2020;20:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiddian RV. Prophylaxis in colonic surgery. J Antimicrob Chemother 1978;4:39–47. [DOI] [PubMed] [Google Scholar]
- 36.Kwok SP, Lau WY, Leung KL, et al. Amoxycillin and clavulanic acid versus cefotaxime and metronidazole as antibiotic prophylaxis in elective colorectal resectional surgery. Chemotherapy 1993;39:135–9. [DOI] [PubMed] [Google Scholar]
- 37.Arnaud JP, Bellissant E, Boissel P, et al. Single-dose amoxycillin-clavulanic acid vs. cefotetan for prophylaxis in elective colorectal surgery: a multicentre, prospective, randomized study. The PRODIGE Group. J Hosp Infect 1992;22:23–32. [DOI] [PubMed] [Google Scholar]
- 38.Nyam D, Yeo M, Cheong D, et al. Antibiotic prophylaxis in colorectal surgery: a randomised, double-blind, controlled trial of amoxycillin-clavulanic acid vs ceftriaxone and metronidazole. Asian Journal of Surgery / Asian Surgical Association 1995;18:227–30. [Google Scholar]
- 39.Abis GSA, Stockmann HBAC, Bonjer HJ, et al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg 2019;106:355–63. [DOI] [PubMed] [Google Scholar]
- 40.Theodoropoulos G. Mechanical bowel preparation with or without oral antibiotics for colorectal cancer surgery. NCT03563586. 2018. Jun 20. Available: https://clinicaltrials.gov/ct2/show/NCT03563586 (accessed 2021 May 9).
- 41.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606–8. [PubMed] [Google Scholar]
- 42.Sakr A, Emile SH, Abdallah E, et al. Predictive factors for small intestinal and colonic anastomotic leak: a multivariate analysis. Indian J Surg 2017;79:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaller MA, Cormican M, Flamm RK, et al. Temporal and geographic variation in antimicrobial susceptibility and resistance patterns of enterococci: results from the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum Infect Dis 2019;6:S54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.