Abstract
A molecular characterization of the main phytochemicals and antioxidant activity of Opuntia robusta (OR) fruit extract was carried out, as well as an evaluation of its hepatoprotective effect against diclofenac (DF)-induced acute liver injury was evaluated. Phenols, flavonoids and betalains were quantified, and antioxidant characterization was performed by means of the ABTS•+, DPPH and FRAP assays. UPLC-QTOF-MS/MS was used to identify the main biocompounds present in OR fruit extract was carried out via. In the in vivo model, groups of rats were treated prophylactically with the OR fruit extract, betanin and N-acteylcysteine followed by a single dose of DF. Biochemical markers of oxidative stress (MDA and GSH) and relative gene expression of the inducible antioxidant response (Nrf2, Sod2, Hmox1, Nqo1 and Gclc), cell death (Casp3) and DNA repair (Gadd45a) were analyzed. Western blot analysis was performed to measure protein levels of Nrf2 and immunohistochemical analysis was used to assess caspase-3 activity in the experimental groups. In our study, the OR fruit extract showed strong antioxidant and cytoprotective capacity due to the presence of bioactive compounds, such as betalain and phenols. We conclude that OR fruit extract or selected components can be used clinically to support patients with acute liver injury.
Keywords: oxidative stress biomarkers, bioactive compounds, antioxidant response, dietary antioxidants, diclofenac, liver failure
1. Introduction
Historically, natural products of plant origin have been used worldwide for medicinal purposes. In Mexico, since pre-Hispanic times, foods, such as corn, maguey and nopal, are part of the population’s diet [1,2]. Interest in studying food consumption and its beneficial effects on health has recently increased [3].
The genus Opuntia spp. is distributed naturally in the southern United States and Latin America and was introduced in Asia, South Africa, Ethiopia, Australia and countries of the Mediterranean basin, such as Italy, Spain and Greece [4]. Mexico is considered the center of the origin and diversity of the genus [5]. The fruits of various species of Opuntia spp. contain compounds with biological activity, such as vitamins B1, A, E and C [6,7], flavonoids (isorhamnetin, kaempferol, quercetin) [6], phenolic compounds (ferulic acid and synapoyl-diglycoside) [6] and pigments (betacyanins and betaxanthins) [8,9].
Likewise, experimental in vivo [10], in vitro and clinical studies have shown that phytochemicals from the fruit of Opuntia spp. have activities, such as: cholesterol-lowering effects (pectin of OR) [11], antiatherogenic effects (betanin and indicaxanthin from O. ficus-indica) [12,13], anticarcinogenic effects (phenolics, flavonoids, betalains from Opuntia spp.) [14,15,16], antigenotoxic and hepatoprotective effects (betacyanins, phenolics and flavonoids in O. ficus-indica, O. streptacantha and O. robusta, respectively) [17,18]. The biological effects of Opuntia spp. fruit consumption have been associated with the antioxidant capacity of phytochemicals via their interaction with different prooxidant mechanisms and the inducible antioxidant response [18].
The O. robusta (OR) species is widely distributed in Mexico, with cultivars in South Africa and the Mediterranean basin [19]. Its fruit (prickly pear) has a purple coloration and contains phenolic compounds, such as p-hydroxy benzoic acid, ferulic acid, pyrogallol [20], vitamins C and E [7] and pigments (betalains, mainly betacyanins) [21].
Diclofenac (DF) is the most widely prescribed over-the-counter (OTC) non-steroidal anti-inflammatory drug (NSAID) in the world [22] and together with ibuprofen accounts for 40% of sales of NSAIDS for osteoarthritis [23] in people aged between 65–85 years with multiple morbidities [24]. It is also prescribed to treat chronic pain related to rheumatoid arthritis, ankylosing spondylitis and acute muscle pain [25]. Its frequent and/or excessive consumption is related to the development of adverse effects in the gastrointestinal tract and liver [26]. Drug-induced liver injury (DILI) is an adverse reaction caused by an increase in cellular oxidative stress, which damages hepatocytes and other liver cells, as a result of drug biotransformation in the liver [27]. This toxicity may be due to the interaction between the drug and special risk factors of the patient (e.g., age, primary pathologies, genetic factors), known as idiosyncrasy (iDILI), or may be dose-dependent, as seen in intrinsic DILI [28,29]. According to Teschke & Danan (2020) [30], diclofenac ranks fifth in the worldwide top ranking of drugs causing DILI cases with a causality assessment performed based on the Roussel Uclaf Causality Assessment Method (RUCAM). Scientometric research reported that of 3312 cases, 46 corresponded to diclofenac iDILI [31,32,33]. Diclofenac acute liver injury is mediated mainly by the isoforms CYP2C9 and CYP3A4 [34]. It generates reactive oxygen species (ROS), reactive nitrogen species (RNS) and electrophilic compounds, such as iminoquinones, which enter redox cycles and produce mitochondrial injury in hepatocytes [35,36,37].
The protective effect of OR fruit extract against the hepatotoxicity induced by thioacetamide (TAA) [7], acetaminophen (APAP) [17,38] and diclofenac [39] has been previously reported, where biomarkers of liver injury were monitored, such as the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes. Moreover, effects on the expression and/or activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [40], mediated by indicaxanthin, and nuclear factor erythroid 2-related factor 2 (Nrf2), mediated by betanin [41], have also been reported.
Therefore, the aim of this study was to complement the characterization of the biocomponents of OR and its antioxidant properties against DF-induced acute liver injury.
2. Materials and Methods
2.1. Chemicals
Gallic acid monohydrate #398225, (+)-catechin hydrate #C1251, DPPH (2,2-diphenyl-1-picrylhydrazyl #D9132), Trolox ((±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid #238813), ABTS•+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid diammonium salt #A1888), diclofenac sodium salt #D6899, N-acetyl-L-cysteine #A7250 and betanin #901266 were provided by Sigma-Aldrich Chemicals (St. Louis, MO, USA).
2.2. Plant Material and Sample Preparation
Samples of wild Opuntia robusta fruits were collected from randomly selected plants in a semi-arid region east of Aguascalientes, México (21°47′04.7″ N 102°06′40.1″ O). Fruits were washed and disinfected, and the peel was separated from the fruit. Sample preparation was carried out following the method described previously [38]. The fruit juice was centrifuged, filtered, lyophilized and stored at −80 °C in a Labconco FreeZone 18 Liter Freeze Dry System (Labconco Corp., Kansas City, MO, USA). For the in vivo experiments, lyophilized extracts were reconstituted with 50 mL of distilled water. All samples were handled under dim-light conditions.
2.3. Phenolic Compound Extraction
In order to evaluate phenolics, flavonoids and antioxidant capacities, extractions were carried out with an aqueous solution of acidified methanol (50% v/v, pH 2.0) and aqueous acetone (70% v/v) prepared according to Osorio-Esquivel et al. [42] with modifications. A 0.25 g sample was shaken first with 10 mL of 50% (v/v) acidified (pH 2.0; HCl) methanol for 1 h at room temperature using an orbital shaker at 200 rpm. The mixture was centrifuged in an EBA 200 Hettich centrifuge (Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany) at 1500× g for 10 min, and the supernatant was recovered (extract A). The insoluble residue was then re-extracted (extract B) with 10 mL of aqueous acetone (70% v/v) following the same procedure described above. Then, both supernatants were mixed (MAA extract) and stored at −20 °C until analysis. Determinations were performed in triplicate.
2.4. Determination of Total Soluble Phenols and Flavonoids
Total phenolics were measured according to the Folin-Ciocalteu method described by Osorio-Esquivel et al. [42] with slight modifications. The absorbance of the reaction was measured at 757 nm using a spectrophotometer (Biomate 3 Thermo Scientific, Waltham, MA, USA). A calibration curve was prepared under similar conditions using gallic acid as the standard, and results were expressed as milligrams of gallic acid equivalents per gram of dry sample. The total flavonoid determination was performed according to the aluminum chloride colorimetric method described by Osorio-Esquivel et al. [42]; the absorbance of the reaction was measured at 510 nm. Catechin was used as a standard for the calibration curve, and the results were expressed as milligrams of catechin equivalents per gram of dry sample.
2.5. Betalain Content
Betacyanin and betaxanthin content was analyzed following the method described by Sumaya-Martínez et al. [43] with slight modifications. Lyophilized extracts were reconstituted in distilled water at a 1:25 dilution and clarified at 12,000× g for 15 min at room temperature. For both pigments, determination was carried out photometrically and reported as milligrams of betanin/indicaxanthin equivalents per liter of clarified juice. Absorbances were measured at 535 nm for betacyanins and 484 nm for betaxanthins, and the content was calculated following the equation:
| Betacyanins or betaxanthins [mg/L] = [(A × DF × MW × 1000/ε × 1)] |
where: A = absorbance at 535 or 484 nm, DF = dilution factor, MW = molecular weight (550 g/mol for betacyanins and 308 g/mol for betaxanthins), and ε = extinction coefficient (60,000 L/(mol cm) for betacyanins and 48,000 L/(mol cm) for betaxanthins).
2.6. Determination of Anti-Free Radical and Ferric Reducing Activities
The free radical-scavenging activity of MAA extract was determined using different methods. The DPPH (2,2-diphenyl-1-picrylhydrazyl) method was performed according to Yap et al. [44] with modifications. The ABTS•+ (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) assay was performed following the method described by Ramlagan et al. [45] with modifications. The chelating activity was evaluated using the FRAP (ferric reducing antioxidant power) method as described by Tenore et al. and Yap et al. [44,46]. Antioxidant activities were expressed as micromoles of Trolox equivalents per gram of dry sample.
2.7. UPLC-QTOF-MS/MS Analysis
2.7.1. Sample Preparation
Betanin standard (Sigma-Aldrich #901266-5G, St. Louis, MO, USA) was prepared at a concentration of 1 mg/mL in ultrapure water. After thawing at 4 °C, 0.11 g of lyophilized extract of OR was resuspended in 1 mL of water with 0.1% formic acid. The sample was vortexed for 60 s and centrifuged for 10 min at 186× g. It was then passed through a 0.22 µm nylon filter and centrifuged again for 5 min at 186× g. Finally, the supernatant was placed in an amber vial for analysis.
2.7.2. Liquid Chromatography and Mass Spectrometry
Chromatographic separation was conducted on a Waters ACQUITY UPLC® Class I (Waters Corporation, Milford, MA, USA) using an Acquity HSS T3 column (2.1 × 100 mm; 1.8 µm, Waters Corporation, Milford, MA USA). The conditions for UPLC were set as follows: column and autosampler were maintained at 30 °C and 6 °C, respectively, flow rate of 0.30 mL/min and the injection volume was 3 μL. The mobile phases consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. The separation of the compounds was in a linear gradient as follows: 1% B (0–3 min), 1–25% B (3–19 min), 25% B (19–20 min), 25–100% B (20–20.10 min), 100% B (20.10–21.10 min), 100–1% B (21.10–21.20 min) and 1% B (21.20–24 min).
In order to explore the main chemical constituents of OR extract, phytochemical profiling was performed. Mass spectrometry analysis was performed on a Waters Synapt G1 Q-TOF mass spectrometer (Waters Corporation, Milford, MA, USA) equipped with an electrospray ionization (ESI) source operating in both positive and negative ion mode. The MS conditions for the positive ion mode were set as follows: desolvation gas flow of 650 L/h at a temperature of 250 °C. The capillary voltage was 3.5 kV, and the sample and extraction cone voltages were 35 V and 4.5 V, respectively. Trap and transfer collision energies were 6 eV and 4 eV, respectively. For the negative ion mode the conditions were set as follows: desolvation gas flow of 500 L/h at a temperature of 300 °C. The capillary voltage was 2.3 kV, and the sample and extraction cone voltages were 35 V and 3.5 V, respectively. Trap and transfer collision energies were 4 eV and 2 eV, respectively. For both ion modes, the source temperature was 120 °C. The scan interval was 0.1 s, and the range of acquisition was 100–1000 m/z. For an accurate determination of mass, leucine-enkephalin was used as the lock mass, with an m/z value of 556.2771 for ESI (+) and an m/z value of 554.2615 for ESI (−).
Typical chromatograms were processed using the Progenesis QI software to obtain raw data, including precursor and fragment ions. Raw data were compared and classified according to online databases, including The Human Metabolome Database (https://hmdb.ca. accessed on 20 February 2022), ChemSpider (https://www.chemspider.com, accessed on 20 February 2022) and PubChem (https://pubchem.ncbi.nlm.nih.gov, Accessed on 20 February 2022). The compounds were classified according to their chemical structure, and their biological activity was analyzed according to the literature reviewed.
2.8. Animals
Sixty-four Wistar rats weighing 200–250 g were obtained from the animal facility at the Basic Sciences Center of the Autonomous University of Aguascalientes. Rats were housed in polypropylene cages at room temperature (25 ± 2 °C) under a daily 12 h light/day cycle and with free access to water and food. Experiments were performed following the guidelines of the local Ethics Committee from the Autonomous University of Aguascalientes (permission no. CEADI-UAA-02-2021), according to the Mexican governmental guideline NOM-062-ZOO-1999 and the guidelines of the National Institutes of Health for the care and use of Laboratory animals (NIH publications no. 8023).
2.9. Experimental Design
After two weeks of adaptation, animals were divided into 8 groups of 8 animals per group: Control group: untreated rats; DF group: rats who received a single dose of diclofenac (75 mg/kg, i.p.) [39]; OR group: rats who were prophylactically treated with Opuntia robusta fruit extract (800 mg/kg/5 days, orally) [17]; Bet group: rats who were prophylactically treated with betanin (25 mg/kg/5 days, orally) [47]; NAC group: rats who were prophylactically treated with N-acetylcysteine (50 mg/kg/5 days, i.p.) [48]. In the OR + DF, Bet + DF and NAC + DF groups, the antioxidants were administered for 5 days before the induction of acute toxicity with DF on the sixth day. Due to the wide range of doses reported in the literature, a dose–response curve was performed with male Wistar rats (results not shown). The betanin concentration in the administered dose of the OR extract (800 mg/kg) was 4.45 mg of betanin equivalents. Twenty-four hours after the last administration, rats were anesthetized with sodium pentobarbital (40 mg/kg) intraperitoneally, and transcardial perfusion was performed with wash solution (0.9% sodium chloride, 0.5% procaine and 0.1% heparin). Samples of liver tissue were collected, and biomarkers of oxidative stress, gene expression (n = 3) and protein expression (n = 4) were analyzed. For the histochemical study (n = 4), the above procedure was repeated followed by perfusion with 10% formaldehyde buffered to pH 7.0 with phosphate buffer.
2.10. Biomarkers of Oxidative Stress
For biochemical and molecular tests, different fragments of 1 cm3 were isolated and frozen at −80 °C. To determine the concentrations of reduced glutathione (GSH), a colorimetric assay kit was used (#38185, Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions. The final concentration of GSH was determined by the equation:
| GSH = (total glutathione − GSSG) × 2 |
and the ratio of GSSG to GSH was also determined. Malondialdehyde (MDA), a product of lipoperoxidation, was determined according to the method described by Uchiyama & Mihara [49] with modifications.
2.11. Molecular Biomarkers
2.11.1. Quantitative RT-PCR Analysis
After perfusion, total RNA was isolated from 100 mg using the SV Total RNA Isolation System (Promega Corporation, Madison, WI, USA) according to the manufacturer’s protocol. The RNA was quantified using a Biodrop (Isogen Life Science, Barcelona, España) and stored at −80 °C until required. Reverse transcription was performed with 1 µg of total RNA using GoScript™ Reverse Transcriptase (Promega A5000, Madison, WI, USA) according to the manufacturer’s instructions. Subsequently, qPCR was performed using the Maxima SYBR Green/ROX qPCR Master Mix (2×) (K0221, Thermo Scientific, Waltham, MA, USA) using StepOneTM equipment (Applied Biosystems, Waltham, MA, USA) with the following thermocycling conditions: 50 °C for 2 min and 95 °C for 3 min, followed by 40 cycles of 95 °C for 45 s and 59 °C for 35 s and finally 95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s for the melt curve. The oligonucleotide primers are displayed in Supplementary Table S1. Relative expression levels were normalized to those of β-actin, and the differences were determined using the 2–∆∆Ct method [50].
2.11.2. Western Blot
For Western blotting, 40 µg of protein extract was separated on a 10% SDS-PAGE gel, and proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Philadelphia, PA, USA). The membranes were blocked with Tris-buffered saline (TBS) and 5% skimmed milk for 1 h at room temperature. For immunodetection, the membranes were incubated overnight at 4 °C with the primary antibody, a Nrf2 polyclonal antibody (1:1000; Thermo Fisher Scientific, Waltham, MA, USA). Blots were incubated with goat anti-rabbit IgG conjugated HRP antibody (1:500; Thermo Fisher Scientific, Waltham, MA, USA). After the incubation, the membranes were washed with TBST (Tris-buffered saline-0.05% Tween 20), and blots were developed with Clarity Western ECL substrate (Bio-Rad, Hercules, CA, USA) for chemiluminescence imaging. The densitometric analysis of the obtained bands was performed using NIH ImageJ2 version 2.3.0/1.530 software. Intensity values were normalized to those of the internal housekeeping protein GAPDH and expressed as relative protein expression.
2.11.3. Immunohistochemistry (IHC) for Active Caspase-3
To visualize the presence of active caspase-3 in liver tissue, sections were subjected to immunohistochemistry as described previously [51]. Samples were incubated with the primary antibody, a monoclonal antibody against active caspase-3 (1:200; Thermo Fisher Scientific, Waltham, MA, USA), overnight at 4 °C. The secondary antibody, goat anti-mouse HRP (1:100; Thermo Fisher Scientific, Waltham, MA, USA) was incubated for 2 h at room temperature. Slides were washed three times with PBS-Tween 20, and peroxidase activity was developed with diaminobenzidine (DAB) (Thermo Fisher, Waltham, MA, USA) for 5 min. Slides were again washed in 1× PBS and counterstained with hematoxylin (diluted 1:10 in tri-distilled water). For the negative control, the primary antibody was not added. Images were taken on a Leica ICC50W (Leica Biosystems, Buffalo Grove, IL, USA) microscope at 40× magnification and processed using Leica LAS EZ software. Quantification of active caspase-3 was performed by counting the number of positive cells (positive reaction in the cytoplasm) and reported as the mean number of positive cells per mm2. In addition, the reaction area intensity was evaluated based on optical density values of DAB and reported as the reaction area percentage. At least 18 fields per group (1.25 mm2) were evaluated.
2.12. Statistical Analysis
Statistical significance was evaluated by performing ANOVA 1-way (parametric) or Kruskal-Wallis (nonparametric) tests using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). Normal distribution of the data was analyzed with the Shapiro-Wilk test.
For antioxidant analysis of the fruit extract, results are presented as the mean of each determination ± standard deviation (SD). In vivo results are presented as the mean of each group (n = 3 for oxidative stress markers and gene expression, n = 4 for Western blot and immunohistochemical analysis) ± standard error of the mean (SEM). p < 0.05 was considered statistically significant.
3. Results
3.1. Determination of Antioxidant Properties of O. robusta Fruit Extract
As shown in Table 1, the OR fruit extract showed a higher amount of total phenols (1330 ± 1.7 mg GAE/100 g dw) than flavonoids (1090 ± 0.9 mg CatE/100 g dw). With respect to the betalain content (641.1 ± 12.7), betacyanins showed a higher concentration (452.2 ± 9.0 mg BE/L) compared to that of betaxanthins (188.9 ± 3.7 IxE/L).
Table 1.
Total phenols, flavonoids, betacyanins, betaxanthins and total betalain values in O. robusta extract.
| MMA Extraction | H2O Extraction | |||
|---|---|---|---|---|
| Total Phenols (mg GAE/100 g dw) |
Total Flavonoids (mg CatE/100 g dw) |
Betacyanins (mg BE/L) |
Betaxanthins (mg IxE/L) |
Total Betalains (mg betalains/L) |
| 1330 ± 1.7 | 1090 ± 0.9 | 452.2 ± 9.0 | 188.9 ± 3.7 | 641.1 ± 12.7 |
Values represent the mean ± SD of three measurements; GAE—Gallic Acid Equivalents; CatE—Catechin Equivalents; BE—Betacyanin Equivalents; IxE—Indicaxanthin Equivalents; dw—dry weight.
The capacity of OR extract to scavenge the radicals DPPH and ABTS•+ was 30.9 ± 1.3 and 102.6 ± 5.2 µmol TE/g dw, respectively, and the capacity to reduce ferric ions was 95.8 ± 7.3 µmol TE/g dw according to the FRAP assays (Table 2).
Table 2.
Antioxidant activity of O. robusta extract determined based on the DPPH, ABTS●+ and FRAP assays.
| Antioxidant Activity of MAA Extract (μmol TE/g dw) | ||
|---|---|---|
| DPPH | ABTS●+ | FRAP |
| 30.9 ± 1.3 | 102.6 ± 5.2 | 95.8 ± 7.3 |
Values represent the mean ± SD of three measurements; TE—Trolox Equivalents; dw—dry weight.
3.2. Identification of the Main Bioactive Compounds in O. robusta Fruit Extract
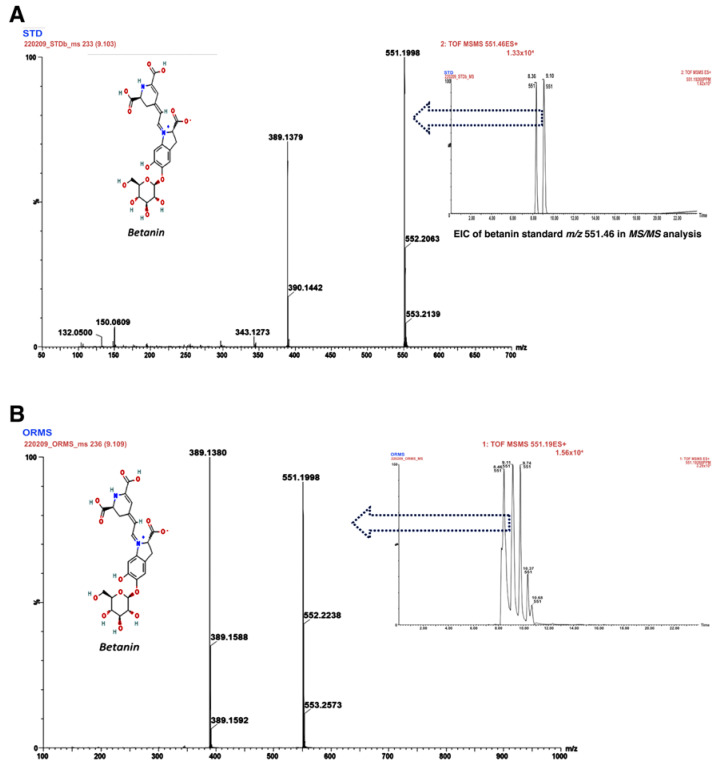
Figure 1A shows the mass spectrum of betanin with a [M+H]+ peak at m/z 551.1998 and a retention time of 9.10 min, as well as the extracted ion chromatogram (EIC). For the MS/MS analysis, the precursor of betanin molecular ion [M+H]+ was filtered to obtain the spectrum and a molecular ion of m/z 389.1379. Figure 1B shows the mass spectrum of the OR fruit extract with the same fragmentation pattern as that of the betanin standard. This result confirms the presence of betanin in the OR fruit extract.
Figure 1.
Mass spectra of betanin standard and Opuntia robusta extract. (A) Extracted ion chromatogram (EIC) and mass spectrum of betanin standard. (B) Mass spectrum of O. robusta sample. The EIC and mass spectra were analyzed via UPLC–QTOF–MS/MS.
In total, 50 tentative compounds, including 15 organo-oxygen compounds, 9 betalains and 26 other compounds, were determined in the OR fruit extract. In Table 3, 12 compounds are listed for the positive ion mode, including the presence of vitamin C (m/z 11.0387 and retention time of 5.197 min) and betalains: betalamic acid (m/z 212.055), indicaxanthin (m/z 309.0984), neobetanin (m/z 549.1382), gomphrenin-I (m/z 551.1633) and betanin (m/z 551.1498). In Table 4, 22 compounds are listed for the negative ion mode, including phenols, such as 5-hydroxyconiferyl alcohol (m/z 195.382), flavonoids, such as hesperetin 5-O-glucoside (m/z 463.1337), and betalains, such as vulgaxanthin I (m/z 384.1014).
Table 3.
Compounds with biological activity identified in O. robusta extract in positive ion mode.
| Compound | Formula | Adduct | m/z | Retention Time (min) |
|---|---|---|---|---|
| Oxanes | ||||
| 1,5-Anhydro-D-fructose | C6H10O5 | M+H-H2O | 145.0488 | 0.919 |
| Cinnamic acids and derivatives | ||||
| Cinnamic acid | C9H8O2 | M+H-H2O | 131.0495 | 14.037 |
| Phenylpropanoic acids | ||||
| Hydrocinnamic acid | C9H10O2 | M+H-H2O | 133.0641 | 16.460 |
| Benzene and derivatives | ||||
| 3,4-O-Dimethylgallic acid | C9H10O5 | M+NH4 | 216.0867 | 5.119 |
| Indanes | ||||
| 1-Indanone | C9H8O | M+H | 133.0634 | 11.692 |
| Vitamins | ||||
| Vitamin C | C6H8O6 | M+H | 177.0397 | 5.197 |
| Carboxylic acids and derivatives | ||||
| Betaine | C5H11NO2 | M+H | 118.0821 | 0.919 |
| Betalains | ||||
| Betalamic acid | C9H9NO5 | M+H | 212.055 | 1.048 |
| Indicaxanthin | C14H16N2O6 | M+H | 309.0984 | 8.830 |
| Neobetanin | C24H24N2O13 | M+H | 549.1382 | 11.613 |
| Gomphrenin-I | C24H26N2O13 | M+H | 551.1633 | 10.764 |
| Betanin | C24H26N2O13 | M+H | 551.1498 | 9.887 |
Table 4.
Compounds with biological activity identified in O. robusta extract in negative ion mode.
| Compound | Formula | Adduct | m/z | Retention Time (min) |
|---|---|---|---|---|
| Lactones | ||||
| D-Glucaro-1,4-lactone | C6H8O7 | M-H | 191.0223 | 1.616 |
| Cinnamic acids and derivatives | ||||
| 1-O-Sinapoylglucose | C17H22O10 | M-H | 385.1205 | 11.743 |
| Benzene and derivatives | ||||
| Vanillic acid | C8H8O4 | M-H | 167.0382 | 7.284 |
| 2-O-Galloyl-1,4-galactarolactone | C13H12O11 | M+K-2H | 380.9824 | 21.898 |
| Carboxylic acids and derivatives | ||||
| 2-O-Caffeoylhydroxycitric acid | C15H14O11 | M+Na-2H | 391.0282 | 1.486 |
| Organooxygen compounds | ||||
| Cis-5-Caffeoylquinic acid | C16H18O9 | M+K-2H | 391.0376 | 1.099 |
| trans-o-Coumaric acid 2-glucoside | C15H18O8 | M+H-H2O | 309.0968 | 6.176 |
| trans-p-Coumaric acid 4-glucoside | C15H18O8 | M+H-H2O | 309.0969 | 6.176 |
| 6-Caffeoylsucrose | C21H28O14 | M-H | 503.138 | 7.902 |
| Gentiobiosyl 2-methyl-6-oxo-2E,4E-heptadienoate | C20H30O13 | M-H | 477.159 | 9.398 |
| Glucocaffeic acid | C15H18O9 | M-H | 341.0926 | 10.455 |
| Coumarins and derivatives | ||||
| Rutaretin 9-rutinoside | C26H34O14 | M+FA-H | 615.202 | 6.665 |
| Fatty acyls | ||||
| 1-Hexanol arabinosylglucoside | C17H32O10 | M+Na-2H | 417.171 | 7.902 |
| Flavonoids | ||||
| Hesperetin 5-O-glucoside | C22H24O11 | M-H | 463.1337 | 9.038 |
| Phenol lipids | ||||
| Caryoptosidic acid | C16H24O11 | M-H2O-H | 373.119 | 9.578 |
| Hydroxyisonobilin | C20H26O6 | M-H | 361.1717 | 17.079 |
| Furanoid lignans | ||||
| Divanillyltetrahydrofuran ferulate | C30H32O8 | M+FA-H | 565.197 | 16.022 |
| Phenols | ||||
| 5-Hydroxyconiferyl alcohol | C10H12O4 | M-H | 195.0701 | 16.382 |
| Betalains | ||||
| Betalamic acid | C9H9NO5 | 2M-H | 211.0525 | 4.321 |
| Vulgaxanthin I | C14H17N3O7 | M+FA-H | 384.1014 | 6.744 |
| Betanin | C24H26N2O13 | 2M-H | 1099.287 | 7.852 |
| Betalamic acid | C9H9NO5 | M-H2O-H | 192.0344 | 8.909 |
Other compounds with possible biological activity are listed in Supplementary Tables S2 and S3.
3.3. Biomarkers of Oxidative Stress
Figure 2 shows the oxidative stress biomarkers present in tissue homogenates. There was a significant increase of 50.85% in MDA levels in the group treated with DF (59.92 ± 5.33 nmol/100 mg) compared to those in the control group (39.72 ± 6.75 nmol/100 mg) (p < 0.05) (Figure 2A). Pretreatment with OR fruit extract, Bet and NAC followed by DF administration significantly reduced MDA levels (19.43% for OR + DF, 23.79% for Bet + DF and 17.84% for NAC + DF) compared to those with DF alone (p < 0.05).
Figure 2.
Oxidative stress biomarkers in livers of rats that received DF treatment and pretreatments with O. robusta extract (OR + DF), betanin (Bet + DF) and NAC (NAC + DF). (A) MDA concentrations, (B) GSH levels and (C) GSSG/GSH ratio. Bar graphs show the mean (n = 3) ± SEM; p > 0.05, **: p ≤ 0.01, ****: p ≤ 0.0001 vs. control group. #: p ≤ 0.05, ##: p ≤ 0.01, ###: p ≤ 0.001, ####: p ≤ 0.0001 vs. DF group.
DF administration induced a significant decrease in GSH concentrations (Figure 2B) of 65% (62.90 ± 0.42 μmol/L) compared to those in the control group (96.21 ± 3.31 μmol/L) (p < 0.05). The prophylactic administration of OR, Bet and NAC maintained GSH concentrations at levels comparable to those in the control group with no statistical difference, but concentrations were significantly (p < 0.05) higher (40.23, 124.2 and 44.25% respectively) when they were administered to the DF groups compared to those with DF alone. We also measured the ratio of GSSG to reduced GSH in the liver after DF challenge (Figure 2B), and there was a significant decrease in the DF group pretreated with Bet (141.03 ± 8.63 μmol/L) compared to that in the DF group.
3.4. Relative Expression of Genes Related to the Constitutive and Inducible Antioxidant Response
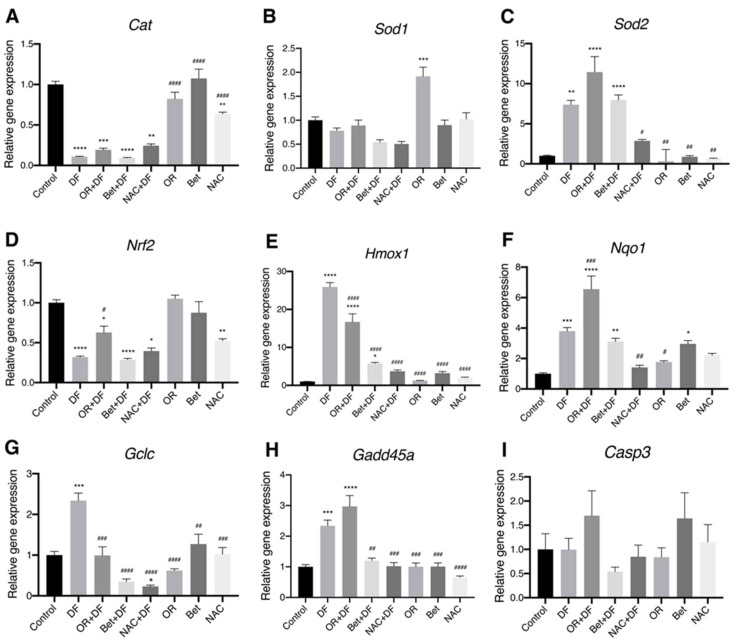
The relative gene expression of catalase was significantly decreased, compared to that in the control group, in the DF (0.8-fold; p < 0.0001), OR + DF (0.8-fold; p < 0.001), Bet + DF (0.9-fold; p < 0.0001), NAC + DF (0.7-fold; p < 0.01) and NAC (0.3-fold; p < 0.01) groups (Figure 3A). Sod1 showed a significant increase in its expression only in the OR group (0.9-fold higher; p < 0.001: Control vs. OR) (Figure 3B). Sod2 expression was increased in the DF (6.3-fold; p < 0.01: Control vs. DF), OR + DF (10.4-fold; p < 0.0001: Control vs. OR + DF) and Bet + DF (6.9-fold; p < 0.0001: Control vs. Bet + DF) groups (Figure 3C). Nrf2 expression was decreased in the DF (0.3-fold; p < 0.0001: Control vs. DF), OR + DF (0.6-fold; p < 0.05: Control vs. OR + DF), Bet + DF (0.7-fold; p < 0.0001: Control vs. Bet + DF), NAC + DF (0.6-fold; p < 0.05: Control vs. NAC + DF) and NAC (0.4-fold; p < 0.01: Control vs. NAC) groups (Figure 3D). Heme-oxygenase 1 (Hmox1) expression was significantly induced in the DF (24.9-fold; p < 0.0001: Control vs. DF), OR + DF (15.7-fold; p < 0.0001: Control vs. OR + DF) and Bet + DF (4.7-fold; p < 0.05: Control vs. Bet + DF) groups (Figure 3E). The expression of NAD(P)H dehydrogenase [quinone] 1 (Nqo1) was significantly increased in the DF (2.7-fold; p < 0.001: Control vs. DF), OR + DF (5.5-fold; p < 0.0001: Control vs. OR + DF), Bet + DF (2-fold; p < 0.01: Control vs. Bet + DF) and Bet (2-fold; p < 0.05: Control vs. Bet) groups (Figure 3F). The expression of glutamate-cysteine ligase catalytic subunit (Gclc) was significantly increased only in the group treated with DF (1.3-fold; p < 0.001: Control vs. DF). Finally, expression of the DNA damage-inducible growth arrest gene (Gadd45a) was significantly increased in the DF (2.3-fold; p < 0.001: Control vs. DF) and OR + DF (2.9-fold; p < 0.0001: Control vs. OR + DF) groups. No significant differences were observed in the relative expression of caspase-3 (Casp3 gene) (Figure 3I).
Figure 3.
Relative gene expression of antioxidant genes: (A) Cat, (B) Sod1, (C) Sod2, detoxifying genes: (D) Nrf2, (E) Hmox1, (F) Nqo1 and (G) Gclc, the DNA damage-inducible gene: (H) Gadd45a and the cell death gene (I): Casp3 after DF treatment and OR, Bet and NAC treatments and pretreatments in rat livers. Bar graphs show the mean (n = 3) ± SEM; p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001, ****: p ≤ 0.0001 vs. Control group. #: p ≤ 0.05, ##: p ≤ 0.01, ###: p ≤ 0.001, ####: p ≤ 0.0001 vs. DF group.
3.5. Nrf2 Protein Expression
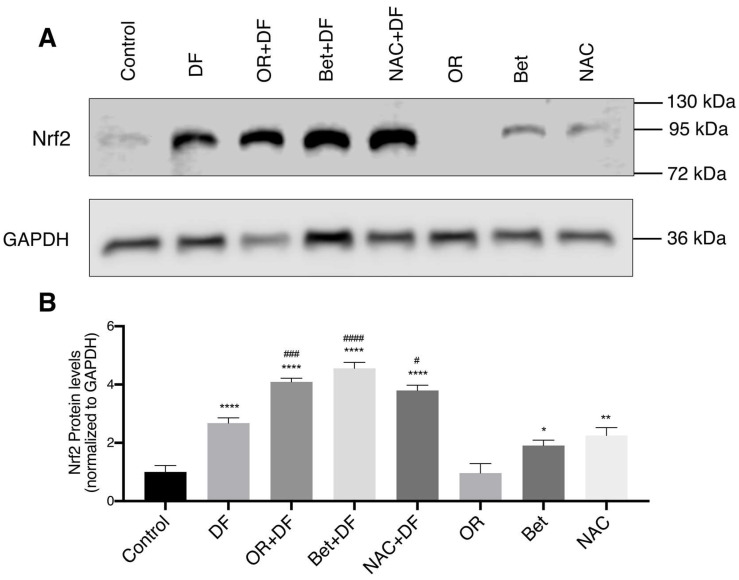
Figure 4A shows the Western blot for the Nrf2 protein in liver tissue. Densitometric analysis (Figure 4B) showed a significant increase in Nrf2 protein expression in the DF (1.6-fold; p < 0.0001), OR + DF (3-fold; p < 0.0001), Bet + DF (3.5-fold; p < 0.0001), NAC + DF (2.7-fold; p < 0.0001), Bet (0.9-fold; p < 0.05) and NAC (1.2-fold; p < 0.001) group, compared to that in the control group. It should be noted that the highest expression was observed in the OR + DF (1.4-fold, p < 0.001), Bet + DF (1.8-fold, p < 0.0001) and NAC + DF (1.1-fold, p < 0.05) groups when compared to that in the DF group.
Figure 4.
Western blot analysis and quantification of Nrf2 expression in rat liver after DF treatment and OR, Bet and NAC treatments and pretreatments in rat livers. (A) Nrf2 and GAPDH blots; (B) graphs showing the relative Nrf2 protein levels normalized to GAPDH from triplicate samples. Bar graphs show the mean (n = 4) ± SEM; p > 0.05, *: p ≤ 0.05, **: p ≤ 0.01, ****: p ≤ 0.0001 vs. Control group. #: p ≤ 0.05, ###: p ≤ 0.001, ####: p ≤ 0.0001 vs. DF group.
3.6. Apoptosis and Active Caspase-3 Evaluation
Immunohistochemical analysis showed a significant increase in the number of active caspase-3-positive cells (641%) in the group treated with diclofenac, equivalent to 441 cells on average/mm2 (p < 0.0001) (Figure 5A,B), with an increase in reaction intensity of 20.15% (p < 0.0001) (Figure 5C) with respect to that in the control group (69 cells/mm2). The groups treated prophylactically with OR fruit extract, Bet and NAC showed a number of active caspase-3 positive cells similar to that in the control group, with mean values of 48, 60 and 36 (cells/mm2), respectively.
Figure 5.
Immunohistochemical staining and quantification of active caspase-3 in rat liver after DF treatment and OR, Bet and NAC treatments and pretreatments in rat livers. (A) Representative tissue sections are shown. 400×. Scale bar = 30 µm. (B,C) Photographs of 18–22 fields (1.25 mm2) per group were taken, and the number of positive hepatocytes was determined, in addition to the intensity of the reaction area, using NIH ImageJ ver 2.3.0/1.530 software. Bar graphs show the mean (n = 4) ± SEM. Control group. p > 0.05, ***: p ≤ 0.001, ****: p ≤ 0.0001 vs. Control group. ####: p ≤ 0.0001 vs. DF group.
4. Discussion
Phytochemicals are bioactive compounds that have beneficial effects on health. Their biological activity depends on their chemical structure, and they occur in a variety of foods. The nutraceutical value of a food is based on the amount and type of phytochemicals it contains [52]. It has been reported that OR pulp contains antioxidants, such as phenolics, flavonoids, ascorbic acid (vitamin C) and betalains [17,38,43].
In our study, the amount of phenolic compounds obtained (1330 ± 1.7 mg GAE/100 g dw) in the OR fruit extract was similar to that reported by pulido-Hornedo et al. [7] and higher than that in O. ficus-indica (89.2 ± 3.6 mg GAE/100 g) [53] and O. streptacantha (104.66 ± 1.51 mg GAE/100 g) [54]. Phenolic compounds are phytochemicals that have an aromatic ring with at least one hydroxyl substituent (phenol), which gives them important antioxidant characteristics. The three main mechanisms of action are derived from their direct reaction with free radicals: (1) the transfer of a hydrogen atom (HAT), (2) single electron transfer (SET), and (3) chelation of free metal ions, such as Fe(III). Based on these interactions, an antioxidant can act as a scavenger when the final product generates another less reactive radical or as a quencher when it completely neutralizes the radical, generating stable molecules [55].
Flavonoids are phenols widely distributed in plant foods, and their concentration (1090 ± 0.9 mg CatE/100 g dw) in the OR fruit extract was higher than that reported for OR pulp (793 mg CAE/100 g dw) [7] or O. ficus-indica (red cultivar, 35.19 ± 2.08 mg CATE/100 g dw) [56]. The basic structure of these compounds is the flavan nucleus (2-phenyl-benzo-c-pyran) and a system of two benzene rings (A and B) linked by an oxygen-containing pyran ring (C), which gives rise to the subclasses: flavones, isoflavonones, flavanols, flavonols and flavanones. These phytochemicals can inhibit the enzyme nitric oxide synthase (NOS) that generates the nitric oxide radical (•NO), preventing the formation of radicals, such as peroxynitrite, which is highly reactive with essential macromolecules [57].
The high concentrations of betacyanins (452.2 ± 9.0 mg BE/L) and betaxanthins (188.90 ± 3.69 mg IxE/L) in the OR fruit extract coincide with previous reports [7,17]. Betalains are a group of pigments that occur in 13 families of Caryophyllales, including the Cactaceae family [58]. These immonium derivatives of betalamic acid are divided intro betacyanins, responsible for the red–purple coloration (λ ≈ 535 nm), and betaxanthins, responsible for the yellow coloration (λ ≈ 480 nm) [59]. These pigments are more abundant in the fruits with red–purple colorations. Our results confirm that the OR fruit has the highest concentrations of betalains, mainly betacyanins, within the Cacti family [17]. Czapski et al. [60] suggest a strong correlation between antioxidant capacity and red pigment content; therefore, OR fruit represents a rich source of antioxidant pigments.
Since the initial analysis of phytochemicals showed that the OR extract is a rich source of betalains, molecular analysis was performed via UPLC-QTOF-MS/MS. Betalamic acid was identified, and this compound has been reported in O. ficus-indica [61] and O. stricta [8]; its antioxidant activity is due to the fact that it reduces two Fe(III) ions into Fe(II) [62], preventing the Fenton reaction and the generation of the hydroxyl radical (•OH). Two betaxanthins were also identified: indicaxanthin and vulgaxanthin I. These pigments are the product of the condensation of betalamic acid with amines or their derivatives [58], and it has been suggested that their antioxidant activity is due the presence of 2-3 imino groups (=NH) and 1-2 hydroxyl groups (-OH) in the amines or amino acid moiety [63].
Indicaxanthin is found in large quantities in O. ficus-indica [64], and in addition to its antioxidant activity, it has been shown in in vitro studies: (1) to modulate the expression of the intercellular adhesion molecule ICAM-1 in human umbilical vein endothelial cells [65]; (2) to prevent atherogenic formation by inhibiting the overexpression of NADPH oxidase 4 (NOX-4); (3) to inhibit the activation of nuclear enhancing factor of NF-κB; and (4) to prevent the apoptosis of cells of a human monocytic cell line (THP-1) [40]. Vulgaxanthin I has been found in O. ficus-indica and Beta vulgaris (yellow beet) and in Amaranthaceae [21,63,66].
Betacyanins are divided into betanin-type, gomphrenin-type and amaranthine-type. Gomphrenin-I has been reported in species, such as Gomphrena globosa [67], and was reported for the first time in O. robusta by Stintzing et al. (2005) [66]. It has a chemical structure similar to that of betanin, and it has been suggested that its high antioxidant capacity is due to the presence of the -OH group at carbon 5 of betanidin (general structure of betacyanins), unlike betanin, which presents it at C-6 [63,68]. Likewise, the UPLC-QTOF-MS/MS analysis showed the presence of betanin in OR fruit extract, consistent with previous reports [17,21,69]. Other studies show the same fragmentation pattern (parent ion m/z 551.19 and daughter ion m/z 389.13) for species such as B. vulgaris [70], Hylocereus polyrhizus [71] and O ficus-indica [61]. The antioxidant effects of betanin have been widely described: the inhibition of lipoperoxidation and LDL [64,72,73,74], as well as the increase in GSH synthesis [59]. It has been reported that the antioxidant activity of betanin is due to its capacity to donate electrons and hydrogen atoms, which comes from the phenolic hydroxyl group attached to C-6. The resulting product is a betanin radical that can be broken down into betalamic acid and the stable cyclo-DOPA 5-O-B-glucoside radical [75].
The presence of vitamin C is in line with what has been reported previously for OR [38]. Recently, Pulido-Hornedo et al. [7] quantified vitamin C (141.14 mg/100 g) in the OR fruit extract via HPLC-UV. This vitamin is part of a series of redox cycles in which non-enzymatic reducers (vitamin E, glutathione and NADPH) and enzymatic reducers (glutaredoxin and glutathione reductase) participate, stopping the propagation of peroxidative processes and repairing peroxidized lipids in biological membranes [76,77]. On the other hand, it neutralizes ROS, such as superoxide anion and hydroxyl radical [78].
Phenolic acids are phenols that have a carboxylic acid functionality that depending on the constitutive carbon framework they contain are divided into hydroxycinnamic acids and hydroxybenzoic acids [79]. We identified 3,4-O-dimethylgallic acid (m/z 216.08) and cinnamic acid (m/z 131.04), 2-O-galloyl-1,4-galactarolactone (m/z 380.98) and vanillic acid (m/z 167.03), which are compounds for which the antioxidant capacity is due to the number and position of the phenolic hydroxyl groups, in addition to the methoxy and carboxylic acid groups [80]. Likewise, 5-hydroxyconiferyl alcohol was identified, which is an intermediate in the synthesis of lignans; its antioxidant activity has been described, although little has been explored [81,82,83]. The flavonoid identified, hesperetin 5-O-glucoside (m/z 463.13), has antidiabetic activity owing to its glycosylated portion at the C-5 position [84].
Antioxidant activity is one of the most described biological effects of phytochemicals, determined using in vitro assays where the ability to quench radicals, such as ABTS•+ and DPPH, is measured [63,64], as well as reducing ferric ions [85]. In the present study, the OR fruit extract showed a higher antioxidant capacity (102.6 ± 5.2 μmol TE/g dw) in the ABTS•+ assay compared to that reported for the OR pulp (62.2 ± 5.0 μmol TE/g dw) [7] and the Spanish prickly pear O. ficus-indica, (6.70 ± 0.73 μmol TE/g) [86]. Likewise, the value obtained (30.09 ± 1.4 μmol TE/g dw) in the DPPH assay was higher than that reported for the Spanish prickly pear (5.22 ± 0.89 μmol TE/g) [86], indicating that the phytochemicals in the OR fruit extract are good electron and hydrogen atom donors. The FRAP assay value of the OR fruit extract (95.8 ± 7.3 μmol TE/g dw) was higher than that of the OR pulp (62.2 ± 5.0 μmol TE/g) [7] and less than that of the ethyl ethanolic extract of blueberry (Rubus spp.): 258.9 ± 11.69 μmol TE/g [87].
Our results suggest through the different antioxidant mechanisms described that the beneficial effects of the OR fruit extract can be attributed to the biological diversity of phytochemicals present in the fruit, apart from betanin, such as gomphrenin I, vulgaxanthin I, indicaxanthin and phenolic acids, as well as other important bioactive compounds that have not been studied in detail (Supplemental Tables S2 and S3).
In our in vivo model, the OR fruit extract protected the liver from damage caused by a high dose of diclofenac (DF). The hydroxylation of DF, mediated by the cytochrome P450 enzymes CYP2C9 and CYP3A4 [34,39], generates the metabolites 4′-OH-DF and 5-OH-DF, respectively, which can be oxidized to form the electrophilic intermediates DF-1,′4′-iminoquinone and DF-2,5-iminoquinone [37] and induces redox cycles that increase ROS, RNS generation and cellular oxidative stress [35,36,37]. ROS (e.g., O2•−, •OH) can initiate lipid peroxidation that results in the formation of lipid aldehydes, such as malondialdehyde (MDA) [88]. In our model, prophylactic administration with the antioxidants OR, Bet and NAC decreased the concentration of MDA to a level comparable to that in the control group, indicating that they prevent lipid peroxidation caused by the reactive metabolites derived from DF. In another study, a decrease in hepatic MDA levels was also reported after pretreatment with OR in a model of acute hepatotoxicity induced by APAP [17].
Reduced glutathione (GSH) is a non-enzymatic endogenous antioxidant that maintains the redox balance [89], and its oxidized form (GSSG) increases under oxidative stress. The bioactive compounds of the OR fruit extract can directly interact with GSSG and reduce it to GSH. Esatbeyoglu et al. [90] suggested that betanin induces GSH synthesis via the Nrf2 pathway, which explains the high hepatic concentrations of GSH in the group pretreated with betanin. GSH is an important antioxidant since it is also a substrate for glutathione peroxidase (GPx), which reduces lipid hydroperoxide radicals into hydroxy-fatty acids. Likewise, this reaction causes the reduction of vitamin C, which in turn reduces vitamin E [76,91].
The antioxidant enzymes CAT, SOD1 and SOD2 are constitutively expressed in peroxisomes, the cytoplasm and mitochondria, respectively. In our study, the increase in gene expression of the enzyme Sod2 in the group treated with DF shows that this drug induces mitochondrial injury by increasing the generation of superoxide anions [35,37]. We show that the OR fruit extract protects against this oxidative damage by increasing the expression of Sod2, in line with previous in vitro studies [39]. Sod2 gene expression is regulated by transcription factors such as NF-κB and Nrf2, important regulators of the antioxidant response. In basal conditions, Nrf2 is inactive in the cytosol and bound to Keap1. Nrf2 activators cause its uncoupling from Keap1 via different pathways, the most important one being the oxidation of Keap1 cysteine residues mediated by electrophilic compounds and other oxidants [92]. Although a decrease in Nrf2 gene expression was observed, our Western blot results show its post-translational activation. Once uncoupled from Keap1, Nrf2 translocates to the nucleus where it heterodimerizes with sMaf proteins and binds to electrophilic response gene (EpRE) motifs that encode detoxifying enzymes (e.g., Nqo1), antioxidant enzymes (e.g., Sod2, Cat), heme detoxifying enzymes (e.g., Hmox1) and enzymes involved in GSH synthesis (e.g., Gclc) [93,94].
Bioactive compounds are also capable of activating and/or enhancing cellular antioxidant responses. It has been reported that betanin induces the antioxidant response mediated by the Nrf2 pathway and its downstream proteins NQO1 (Nqo1 gene) and HO-1 (Hmox1 gene) in HepG2 hepatoma cells [90], as well as the induction of phase II detoxifying enzymes, such as glutathione S-transferase P (GSTP), glutathione S-transferase mu (GSTM) and NQO1, via the activation of mitogen-activated protein kinases [41]. In another study, polysaccharides, extracted from the cladode of O. milpa alta, protected pancreatic β-cells against alloxan-induced apoptosis and oxidative stress via the Nrf2-mediated induction of γ-glutamyl-cysteine synthetase (γ-GCSc) [95]. We have previously evaluated the effect of OR fruit extract on Sod2, Hmox1 and Gclc gene expression in an in vivo model of APA-induced acute hepatotoxicity [17]. In this study, we observed that pretreatment with OR fruit extract increased the expression of the Sod2, Hmox1 and Nqo1 genes via the post-translational activation of Nrf2. As expected, acute DF toxicity induced the activation of Nrf2; however, the protein levels of Nrf2 were higher in the groups subjected to prophylactic treatments with OR, Bet and NAC, with Bet being the antioxidant that induced the highest expression levels of Nrf2.
Hmox1 is a downstream target gene of Nrf2. It encodes a protein that catalyzes the oxidative degradation of the heme group, resulting in the generation of anti-inflammatory and cytoprotective products: bilirubin, biliverdin and CO [90,96]. Under stress conditions, the induction of Hmox1 expression is modulated by intracellular GSH depletion [97,98,99]. Our results showed an increase in the expression of Hmox1 in the DF group and a significant diminution after pretreatments with OR, Bet and NAC, similar to that reported with APAP-induced liver injury [17]. As previously described, GSH levels decreased in the group treated with DF, while they were restored in the groups that received the prophylactic treatments (OR + DF, Bet + DF, NAC + DF).
In our study, the prophylactic administration of OR fruit extract induced high expression of Nqo1, indicating that iminoquinones are one of the major metabolites generated from DF and that OR fruit extract can detoxify these metabolites. Iminoquinones are capable of damaging proteins and other biomolecules. The protein that Nqo1 encodes is responsible for catalyzing the reduction of quinones to hydroquinones, facilitating their excretion [41].
In order to elucidate whether antioxidants present in OR fruit extract induce GSH synthesis, the expression of Gclc, the gene that encodes the enzyme that is important for GSH synthesis, was determined [100]. No significant changes in the expression of this gene were observed. These results indicate that the OR fruit extract has different mechanisms of action and that the biocomponents that it contains can act directly on the reactive chemical species and/or directly restore GSSG to its reduced state (GSH). On the other hand, since the prophylactic administration of Bet resulted in a significant increase in hepatic GSH concentrations, it is likely that this component can also induce its synthesis [90].
We recently reported that OR fruit extract protects against apoptotic cell death in a model of hepatocyte injury [39]. Our present results are in line with this cytoprotective effect. Although no differences were observed in the gene expression of caspase-3 in the experimental groups, our immunohistochemical analysis showed a significant increase in the number of cells positive for active caspase-3 in the group treated with DF. In normal conditions, caspase proteins are present as inactive procaspases [101]. The increase in the number of caspase-3-positive cells demonstrates increased apoptotic cell death in the DF toxicity model. DNA damage can activate apoptosis, which can be caused by the peroxynitrite radical (ONOO-) [102].
Ferroptosis is a type of cell death related to an increase in Fe2+, the Fenton reaction that produces the •OH radical, lipid peroxidation, GSH depletion and induction of the Nrf2-mediated antioxidant response [103,104,105,106,107,108]. Our results suggest that the OR extract may have a protective effect against this type of death due to the chelating power of iron, the decrease in MDA, the restoration of GSG and the increase in the expression of Nrf2 and Nqo1 [107,108].
Cells have DNA damage-sensing mechanisms. One of these is the DNA damage-inducible growth arrest gene (Gadd45a), which was induced by the prophylactic administration of OR fruit extract. Gadd45a expression is rapid and dose-dependent, and it has been reported to be involved in DNA repair [109,110]. This gene can be induced via different pathways, one of them being mediated by p53. Since we previously reported that OR fruit extract downregulates the expression of p53 in vitro [39], it is suggested that in the present study, the regulation of GADD45a by OR fruit extract is mediated by a p53-independent pathway, e.g., via the MAP kinase pathway [110].
According to our results, the mechanisms underlying OR extract activity that provide hepatoprotection against oxidative stress are as follows: (1) chelation of iron and thus prevention of the Fenton reaction and lipid peroxide formation, (2) promotion of the reduction of peroxidized lipids (L-OOH) to lipid alcohols (L-OH) by restoring GSH levels, (3) direct interactions with ROS as scavengers or quenchers, (4) induction of the expression of antioxidant response genes regulated by NRf2, and (5) prevention of cell death mediated by apoptosis and possibly by ferroptosis.
Our study did not address iDILI caused by DF, and it did not explore risk factors that increase the development of liver damage due to DF consumption, due to age and the presence of previous pathologies, such as diabetes and hypertension present in a significant sector of the population older than 60 years, in which there is an increase in the incidence of liver damage caused by DF. However, cellular oxidative stress is a key factor involved in the pathogenesis of DILI, which was addressed in this study.
5. Conclusions
We observed that OR fruit extract is a rich source of beneficial, health promoting biological compounds with strong antioxidant and cytoprotective effects against mitochondrial damage and oxidative stress generated by DF. Both Bet and OR fruit extract exert their effects via a direct interaction with reactive species, the restoration of GSH levels and indirect effects via the regulation of genes involved in the antioxidant response mediated by Nrf2. The protective effects of the OR fruit extract and betanin are similar to those of NAC, which is currently used to treat acute liver injury, suggesting its potential use in clinical practice as a complement or alternative to treat acute liver injury related to cellular oxidative stress.
Acknowledgments
To Fernando Abiram García García, Andres Quezada Duron and Yunuen Reyes Castro for their technical and academic support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12010113/s1, Table S1: Primer sequences for RT-PCR; Table S2: Compounds identified in O. robusta extract in positive ion mode; Table S3: Compounds identified in O. robusta extract in negative ion mode.
Author Contributions
Conceptualization, G.S.V.-J., H.M., H.A.G.-P. and M.C.M.-S.; methodology: G.S.V.-J., H.A.G.-P., M.B.-H., F.G.-L., E.S.-A., S.L.M.-H., J.V.-J., M.H.M.-O. and M.C.M.-S.; software, G.S.V.-J. and H.A.G.-P.; validation, G.S.V.-J., F.G.-L., S.L.M.-H., J.V.-J., M.H.M.-O. and M.C.M.-S.; formal analysis, G.S.V.-J., H.A.G.-P., F.G.-L. and M.C.M.-S.; investigation, G.S.V.-J., H.A.G.-P. and M.C.M.-S.; resources, M.C.M.-S.; data curation G.S.V.-J. and H.A.G.-P.; writing—original draft preparation, G.S.V.-J.; writing—review and editing, H.M., F.J.A.-G., H.A.G.-P., F.G.-L., E.S.-A., S.L.M.-H., J.V.-J., M.H.M.-O. and M.C.M.-S.; visualization, G.S.V.-J., H.A.G.-P., F.G.-L., E.S.-A., S.L.M.-H., J.V.-J., M.H.M.-O. and M.C.M.-S.; project administration, F.J.A.-G. and M.C.M.-S.; funding acquisition, M.C.M.-S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study was conducted according to the guidelines of the Ethics Committee of Universidad Autónoma de Agascalientes: Comité de Ética para el uso de Animales en la docencia e Investigación (CECADI), protocol code: PIT19-2.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the data is contained within the article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Gloria Stephanie Villa Jaimes was supported by a doctoral grant from CONACYT, no. 715428, and internal support by the Universidad Autónoma de Aguascalientes with key project PIT19-2.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Conaculta . People of Corn Mexico’s Ancestral Cuisine. Conaculta; Mexico City, Mexico: 2004. [Google Scholar]
- 2.Zizumbo-Villarreal D., Flores-Silva A., Colunga-García P.M. The Archaic Diet in Mesoamerica: Incentive for Milpa Development and Species Domestication. Econ. Bot. 2012;66:328–343. doi: 10.1007/s12231-012-9212-5. [DOI] [Google Scholar]
- 3.Torres-Maravilla E., Méndez-Trujillo V., Hernández-Delgado N.C., Bermúdez-Humarán L.G., Reyes-Pavón D. Looking inside Mexican Traditional Food as Sources of Synbiotics for Developing Novel Functional Products. Fermentation. 2022;8:123. doi: 10.3390/fermentation8030123. [DOI] [Google Scholar]
- 4.Sipango N., Ravhuhali K.E., Sebola N.A., Hawu O., Mabelebele M., Mokoboki H.K., Moyo B. Prickly Pear (Opuntia spp.) as an Invasive Species and a Potential Fodder Resource for Ruminant Animals. Sustainability. 2022;14:3719. doi: 10.3390/su14073719. [DOI] [Google Scholar]
- 5.Illoldi-Rangel P., Ciarleglio M., Sheinvar L., Linaje M., Sánchez-Cordero V., Sarkar S. Opuntia in México: Identifying Priority Areas for Conserving Biodiversity in a Multi-Use Landscape. PLoS ONE. 2012;7:e36650. doi: 10.1371/journal.pone.0036650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madrigal-Santillán E., Portillo-Reyes J., Madrigal-Bujaidar E., Sánchez-Gutiérrez M., Mercado-Gonzalez P.E., Izquierdo-Vega J.A., Vargas-Mendoza N., Álvarez-González I., Fregoso-Aguilar T., Delgado-Olivares L., et al. Opuntia genus in Human Health: A Comprehensive Summary on Its Pharmacological, Therapeutic and Preventive Properties. Part 1. Horticulturae. 2022;8:88. doi: 10.3390/horticulturae8020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulido-Hornedo N.A., Ventura-Juárez J., Guevara-Lara F., González-Ponce H.A., Sánchez-Alemán E., Buist-Homan M., Moshage H., Martínez-Saldaña M.C. Hepatoprotective effect of Opuntia robusta fruit biocomponents in a rat model of thioacetamide-induced liver fibrosis. Plants. 2022;10:1757. doi: 10.3390/plants11152039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellar R., Obón J.M., Alacid M., Fernández-López J.A. Color Properties and Stability of Betacyanins from Opuntia Fruits. J. Agric. Food Chem. 2003;51:2772–2776. doi: 10.1021/jf021045h. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan S.P., Sheth N.R., Rathod I.S., Suhagia B.N., Maradia R.B. Analysis of betalains from fruits of Opuntia species. Phytochem. Rev. 2012;12:35–45. doi: 10.1007/s11101-012-9248-2. [DOI] [Google Scholar]
- 10.Attanzio A., Tesoriere L., Vasto S., Pintaudi A.M., Livrea M.A., Allegra M. Short-term cactus pear [Opuntia ficus-indica (L.) Mill] fruit supplementation ameliorates the inflammatory profile and is associated with improved antioxidant status among healthy humans. Food Nutr. Res. 2018;62 doi: 10.29219/fnr.v62.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfram R.M., Kritz H., Efthimiou Y., Stomatopoulos J., Sinzinger H. Effect of prickly pear (Opuntia robusta) on glucose- and lipid-metabolism in non-diabetics with hyperlipidemia-a pilot study. Wien Klin Wochenschr. 2002;114:840–846. [PubMed] [Google Scholar]
- 12.Allegra M., D’Acquisto F., Tesoriere L., Attanzio A., Livrea M.A. Pro-oxidant activity of indicaxanthin from Opuntia ficus indica modulates arachidonate metabolism and prostaglandin synthesis through lipid peroxide production in LPS-stimulated RAW 264.7 macrophages. Redox Biol. 2014;2:892–900. doi: 10.1016/j.redox.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva D.V.T.D., Baiao D.D.S., Ferreira V.F., Paschoalin V.M.F. Betanin as a multipath oxidative stress and inflammation modulator: A beetroot pigment with protective effects on cardiovascular disease pathogenesis. Crit. Rev. Food Sci. Nutr. 2021;62:539–554. doi: 10.1080/10408398.2020.1822277. [DOI] [PubMed] [Google Scholar]
- 14.Chavez-Santoscoy R.A., Gutierrez-Uribe J.A., Serna-Saldívar S.O. Phenolic composition, antioxidant capacity and in vitro cancer cell cytotoxicity of nine prickly pear (Opuntia spp.) juices. Plant Foods Hum. Nutr. 2009;64:146–152. doi: 10.1007/s11130-009-0117-0. [DOI] [PubMed] [Google Scholar]
- 15.Hahm S.W., Park J., Oh S.Y., Lee C.W., Park K.Y., Kim H., Son Y.S. Anticancer properties of extracts from Opuntia humifusa against human cervical carcinoma cells. J Med. Food. 2015;18:31–44. doi: 10.1089/jmf.2013.3096. [DOI] [PubMed] [Google Scholar]
- 16.Allegra M., De Cicco P., Ercolano G., Attanzio A., Busà R., Cirino G., Tesoriere L., Livrea M.A., Ianaro A. Indicaxanthin from Opuntia ficus indica (L. Mill) impairs melanoma cell proliferation, invasiveness, and tumor progression. Phytomedicine. 2018;50:19–24. doi: 10.1016/j.phymed.2018.09.171. [DOI] [PubMed] [Google Scholar]
- 17.González-Ponce H.A., Martínez-Saldaña M.C., Tepper P.G., Quax W.J., Buist-Homan M., Faber K.N., Moshage H. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res. Int. 2020;137:09461. doi: 10.1016/j.foodres.2020.109461. [DOI] [PubMed] [Google Scholar]
- 18.Tsafantakis N., Katsanou E.S., Kyriakopoulou K., Psarou E.-C., Raptaki I., Skaltsounis A.L., Audebert M., Machera K.A., Fokialakis N. Comparative UHPLC-HRMS Profiling, Toxicological Assessment, and Protection Against H2O2-Induced Genotoxicity of Different Parts of Opuntia ficus indica. J. Med. Food. 2019;22:1280–1293. doi: 10.1089/jmf.2019.0032. [DOI] [PubMed] [Google Scholar]
- 19.Walters M., Figueiredo E., Crouch N., Winter P., Smith G., Zimmermann H., Mashope-Potgieter B. Naturalised and Invasive Succulents of Southern Africa. SANBI; Cape Town, South Africa: 2011. [Google Scholar]
- 20.Kıvrak Ş., Kıvrak İ., Karababa E. Analytical evaluation of phenolic compounds and minerals of Opuntia robusta J.C. Wendl. and Opuntia ficus-barbarica A. Berger. Int. J. Food Prop. 2018;21:229–241. doi: 10.1080/10942912.2018.1451342. [DOI] [Google Scholar]
- 21.Stintzing F.C., Schieber A., Carle R. Identification of Betalains from Yellow Beet (Beta vulgaris L.) and Cactus Pear [Opuntia ficus-indica (L.) Mill.] by High-Performance Liquid Chromatography−Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2002;50:2302–2307. doi: 10.1021/jf011305f. [DOI] [PubMed] [Google Scholar]
- 22.Altman R., Bosch B., Brune K., Patrignani P., Young C. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs. 2015;75:859–877. doi: 10.1007/s40265-015-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conaghan P.G. A turbulent decade for NSAIDs: Update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol. Int. 2011;32:1491–1502. doi: 10.1007/s00296-011-2263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freytag A., Quinzler R., Freitag M., Bickel H., Fuchs A., Hansen H., Hoefels S., König H.-H., Mergenthal K., Riedel-Heller S., et al. Gebrauch und potenzielle Risiken durch nicht verschreibungspflichtige Schmerzmittel. Schmerz. 2014;28:175–182. doi: 10.1007/s00482-014-1415-5. [DOI] [PubMed] [Google Scholar]
- 25.Grillo M.P., Ma J., Teffera Y., Waldon D.J. A Novel Bioactivation Pathway for 2-[2-(2,6-Dichlorophenyl)aminophenyl]ethanoic Acid (Diclofenac) Initiated by Cytochrome P450-Mediated Oxidative Decarboxylation. Drug Metab. Dispos. 2008;36:1740–1744. doi: 10.1124/dmd.108.021287. [DOI] [PubMed] [Google Scholar]
- 26.Bjarnason I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int. J. Clin. Pract. 2013;67:37–42. doi: 10.1111/ijcp.12048. [DOI] [PubMed] [Google Scholar]
- 27.Andrade R.J., Chalasani N., Björnsson E.S., Suzuki A., Kullak-Ublick G.A., Watkins P.B., Devarbhavi H., Merz M., Lucena M.I., Kaplowitz N., et al. Drug-induced liver injury. Nat. Rev. Dis. Prim. 2019;5:58. doi: 10.1038/s41572-019-0105-0. [DOI] [PubMed] [Google Scholar]
- 28.Dragoi D., Benesic A., Pichler G., Kulak N.A., Bartsch H.S., Gerbes A.L. Proteomics Analysis of Monocyte-Derived Hepatocyte-Like Cells Identifies Integrin Beta 3 as a Specific Biomarker for Drug-Induced Liver Injury by Diclofenac. Front. Pharmacol. 2018;9:699. doi: 10.3389/fphar.2018.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teschke R. Top-ranking drugs out of 3312 drug-induced liver injury cases evaluated by the Roussel Uclaf Causality Assessment Method. Expert Opin. Drug Metab. Toxicol. 2018;14:1169–1187. doi: 10.1080/17425255.2018.1539077. [DOI] [PubMed] [Google Scholar]
- 30.Teschke R., Danan G. Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993–Mid 2020: A Comprehensive Analysis. Medicines. 2020;7:62. doi: 10.3390/medicines7100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Björnsson E., Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 32.Andrade R.J., Lucena M.I., Fernández M.C., Pelaez G., Pachkoria K., García-Ruiz E., García-Muñoz B., González-Grande R., Pizarro A., Durán J.A., et al. Drug-Induced Liver Injury: An Analysis of 461 Incidences Submitted to the Spanish Registry Over a 10-Year Period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Andrade R.J., Lucena M.I., Kaplowitz N., García-Muņoz B., Borraz Y., Pachkoria K., García-Cortés M., Fernández M.C., Pelaez G., Rodrigo L., et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–1588. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- 34.González-Ponce H.A., Rincón-Sánchez A.R., Jaramillo-Juárez F., Moshage H. Natural Dietary Pigments: Potential Mediators against Hepatic Damage Induced by Over-The-Counter Non-Steroidal Anti-Inflammatory and Analgesic Drugs. Nutrients. 2018;10:117. doi: 10.3390/nu10020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boelsterli U.A. Diclofenac-induced liver injury: A paradigm of idiosyncratic drug toxicity. Toxicol. Appl. Pharmacol. 2003;192:307–322. doi: 10.1016/S0041-008X(03)00368-5. [DOI] [PubMed] [Google Scholar]
- 36.Syed M., Skonberg C., Hansen S.H. Mitochondrial toxicity of diclofenac and its metabolites via inhibition of oxidative phosphorylation (ATP synthesis) in rat liver mitochondria: Possible role in drug induced liver injury (DILI) Toxicol. Vitr. 2016;31:93–102. doi: 10.1016/j.tiv.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Tang W. The Metabolism of Diclofenac-Enzymology and Toxicology Perspectives. Curr. Drug Metab. 2003;4:319–329. doi: 10.2174/1389200033489398. [DOI] [PubMed] [Google Scholar]
- 38.González-Ponce H.A., Martínez-Saldaña M.C., Rincón-Sánchez A.R., Sumaya-Martínez M.T., Buist-Homan M., Faber K.N., Moshage H., Jaramillo-Juárez F. Hepatoprotective Effect of Opuntia robusta and Opuntia streptacantha Fruits against Acetaminophen-Induced Acute Liver Damage. Nutrients. 2016;8:607. doi: 10.3390/nu8100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villa-Jaimes G.S., Aguilar-Mora F.A., González-Ponce H.A., Avelar-González F.J., Martínez Saldaña Ma C., Buist-Homan M., Moshage H. Biocomponents from Opuntia robusta and Opuntia streptacantha fruits protect against diclofenac-induced acute liver damage in vivo and in vitro. J. Funct. Foods. 2022;89:104960. doi: 10.1016/j.jff.2022.104960. [DOI] [Google Scholar]
- 40.Tesoriere L., Attanzio A., Allegra M., Gentile C., Livrea M.A. Phytochemical indicaxanthin suppresses 7-ketocholesterol-induced THP-1 cell apoptosis by preventing cytosolic Ca2+ increase and oxidative stress. Br. J. Nutr. 2013;110:230–240. doi: 10.1017/S000711451200493X. [DOI] [PubMed] [Google Scholar]
- 41.Krajka-Kuźniak V., Paluszczak J., Szaefer H., Baer-Dubowska W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013;110:2138–2149. doi: 10.1017/S0007114513001645. [DOI] [PubMed] [Google Scholar]
- 42.Osorio-Esquivel O., Moreno A.O., Álvarez V.B., Dorantes-Álvarez L., Giusti M.M. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 2011;44:2160–2168. doi: 10.1016/j.foodres.2011.02.011. [DOI] [Google Scholar]
- 43.Sumaya-Martínez M.T., Cruz-Jaime S., Madrigal-Santillán E., García-Paredes J.D., Cariño-Cortés R., Cruz-Cansino N., Valadez-Vega C., Martinez-Cardenas L., Alanís-García E. Betalain, Acid Ascorbic, Phenolic Contents and Antioxidant Properties of Purple, Red, Yellow and White Cactus Pears. Int. J. Mol. Sci. 2011;12:6452–6468. doi: 10.3390/ijms12106452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yap C.H., Junit S.M., Aziz A.A., Kong K.W. Multiple extraction conditions to produce phytochemical- and antioxidant-rich Alternanthera sessilis (red) extracts that attenuate lipid accumulation in steatotic HepG2 cells. Food Biosci. 2019;32:100489. doi: 10.1016/j.fbio.2019.100489. [DOI] [Google Scholar]
- 45.Ramlagan P., Rondeau P., Planesse C., Neergheen-Bhujun V.S., Fawdar S., Bourdon E., Bahorun T. Punica granatum L. mesocarp suppresses advanced glycation end products (AGEs)- and H 2 O 2 -induced oxidative stress and pro-inflammatory biomarkers. J. Funct. Foods. 2017;29:115–126. doi: 10.1016/j.jff.2016.12.007. [DOI] [Google Scholar]
- 46.Tenore G.C., Novellino E., Basile A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods. 2012;4:129–136. doi: 10.1016/j.jff.2011.09.003. [DOI] [Google Scholar]
- 47.Motawi T.K., Ahmed S.A., El-Boghdady N.A., Metwally N.S., Nasr N.N. Protective effects of betanin against paracetamol and diclofenac induced neurotoxicity and endocrine disruption in rats. Biomarkers. 2019;24:645–651. doi: 10.1080/1354750X.2019.1642958. [DOI] [PubMed] [Google Scholar]
- 48.Nissar A.U., Farrukh M.R., Kaiser P.J., Rafiq R.A., Afnan Q., Bhushan S., Adil H.S., Subhash B.C., Tasduq S.A. Effect of N-acetyl cysteine (NAC), an organosulfur compound from Allium plants, on experimentally induced hepatic prefibrogenic events in wistar rat. Phytomedicine. 2013;20:828–833. doi: 10.1016/j.phymed.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Uchiyama M., Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 50.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 51.Munro B. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. Pathology. 1971;3:249. doi: 10.1016/S0031-3025(16)39410-7. [DOI] [Google Scholar]
- 52.Patil B.S., Jayaprakasha G.K., Murthy K.N.C., Vikram A. Bioactive Compounds: Historical Perspectives, Opportunities, and Challenges. J. Agric. Food Chem. 2009;57:8142–8160. doi: 10.1021/jf9000132. [DOI] [PubMed] [Google Scholar]
- 53.Albano C., Negro C., Tommasi N., Gerardi C., Mita G., Miceli A., De Bellis L., Blando F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants. 2015;4:269–280. doi: 10.3390/antiox4020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mabrouki L., Boutheina Z., Bendhifi M., Borgi M.A. Evaluation of antioxidant capacity, phenol and flavonoid contents of Opuntia streptacantha and Opuntia ficus indica fruits pulp. Nat. Technol. 2015;13:2. [Google Scholar]
- 55.Leopoldini M., Russo N., Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011;125:288–306. doi: 10.1016/j.foodchem.2010.08.012. [DOI] [Google Scholar]
- 56.Bargougui A., Tag H.M., Bouaziz M., Triki S. Antimicrobial, Antioxidant, Total Phenols and Flavonoids Content of Four Cactus (Opuntia ficus-indica) Cultivars. Biomed. Pharmacol. J. 2019;12:1353–1368. doi: 10.13005/bpj/1764. [DOI] [Google Scholar]
- 57.Shutenko Z., Henry Y., Pinard E., Seylaz J., Potier P., Berthet F., Girard P., Sercombe R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem. Pharmacol. 1998;57:199–208. doi: 10.1016/S0006-2952(98)00296-2. [DOI] [PubMed] [Google Scholar]
- 58.Miguel M.G. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants. 2018;7:53. doi: 10.3390/antiox7040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esatbeyoglu T., Wagner A.E., Schiniatbeyo V.B., Rimbach G. Betanin-A food colorant with biological activity. Mol. Nutr. Food Res. 2014;59:36–47. doi: 10.1002/mnfr.201400484. [DOI] [PubMed] [Google Scholar]
- 60.Czapski J., Mikołajczyk-Bator K., Kaczmarek M. Relationship between antioxidant capacity of red beet juice and contents of its betalain pigments. Pol. J. Food Nutr. Sci. 2009;59:119–122. [Google Scholar]
- 61.García-Cayuela T., Gómez-Maqueo A., Guajardo-Flores D., Welti-Chanes J., Cano M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019;76:1–13. doi: 10.1016/j.jfca.2018.11.002. [DOI] [Google Scholar]
- 62.Gandía-Herrero F., Escribano J., García-Carmona F. Purification and Antiradical Properties of the Structural Unit of Betalains. J. Nat. Prod. 2012;75:1030–1036. doi: 10.1021/np200950n. [DOI] [PubMed] [Google Scholar]
- 63.Cai Y., Sun M., Corke H. Antioxidant Activity of Betalains from Plants of the Amaranthaceae. J. Agric. Food Chem. 2003;51:2288–2294. doi: 10.1021/jf030045u. [DOI] [PubMed] [Google Scholar]
- 64.BButera D., Tesoriere L., Di Gaudio F., Bongiorno A., Allegra M., Pintaudi A.M., Kohen R., Livrea M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002;50:6895–6901. doi: 10.1021/jf025696p. [DOI] [PubMed] [Google Scholar]
- 65.Gentile C., Tesoriere L., Allegra M., A Livrea M., D’Alessio P. Antioxidant Betalains from Cactus Pear (Opuntia ficus-indica) Inhibit Endothelial ICAM-1 Expression. Ann. N. Y. Acad. Sci. 2004;1028:481–486. doi: 10.1196/annals.1322.057. [DOI] [PubMed] [Google Scholar]
- 66.Stintzing F.C., Herbach K.M., Mosshammer M.R., Carle R., Yi W., Sellappan S., Akoh C.C., Bunch R., Felker P. Color, Betalain Pattern, and Antioxidant Properties of Cactus Pear (Opuntia spp.) Clones. J. Agric. Food Chem. 2004;53:442–451. doi: 10.1021/jf048751y. [DOI] [PubMed] [Google Scholar]
- 67.Cai Y., Sun M., Corke H. Identification and Distribution of Simple and Acylated Betacyanins in the Amaranthaceae. J. Agric. Food Chem. 2001;49:1971–1978. doi: 10.1021/jf000963h. [DOI] [PubMed] [Google Scholar]
- 68.Gandía-Herrero F., Escribano J., García-Carmona F. Structural implications on color, fluorescence, and antiradical activity in betalains. Planta. 2010;232:449–460. doi: 10.1007/s00425-010-1191-0. [DOI] [PubMed] [Google Scholar]
- 69.Castellanos-Santiago E., Yahia E.M. Identification and Quantification of Betalains from the Fruits of 10 Mexican Prickly Pear Cultivars by High-Performance Liquid Chromatography and Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2008;56:5758–5764. doi: 10.1021/jf800362t. [DOI] [PubMed] [Google Scholar]
- 70.Da Silva D.V.T., Dos Santos Baião D., De Oliveira Silva F., Alves G., Perrone D., Del Aguila E.M., Paschoalin V.M.F. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules. 2019;24:458. doi: 10.3390/molecules24030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taira J., Tsuchida E., Katoh M.C., Uehara M., Ogi T. Antioxidant capacity of betacyanins as radical scavengers for peroxyl radical and nitric oxide. Food Chem. 2015;166:531–536. doi: 10.1016/j.foodchem.2014.05.102. [DOI] [PubMed] [Google Scholar]
- 72.Kanner J., Harel S., Granit R. Betalains—A New Class of Dietary Cationized Antioxidants. J. Agric. Food Chem. 2001;49:5178–5185. doi: 10.1021/jf010456f. [DOI] [PubMed] [Google Scholar]
- 73.Tesoriere L., Allegra M., Gentile C., Livrea M.A. Betacyanins as phenol antioxidants. Chemistry and mechanistic aspects of the lipoperoxyl radical-scavenging activity in solution and liposomes. Free Radic. Res. 2009;43:706–717. doi: 10.1080/10715760903037681. [DOI] [PubMed] [Google Scholar]
- 74.Tesoriere L., Allegra M., Butera D., Livrea M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004;80:941–945. doi: 10.1093/ajcn/80.4.941. [DOI] [PubMed] [Google Scholar]
- 75.Sawicki T., Topolska J., Romaszko E., Wiczkowski W. Profile and Content of Betalains in Plasma and Urine of Volunteers after Long-Term Exposure to Fermented Red Beet Juice. J. Agric. Food Chem. 2018;66:4155–4163. doi: 10.1021/acs.jafc.8b00925. [DOI] [PubMed] [Google Scholar]
- 76.Van Kuijk F.J.G.M., Sevanian A., Handelman G.J., Dratz E.A. A new role for phospholipase A2: Protection of membranes from lipid peroxidation damage. Trends Biochem. Sci. 1987;12:31–34. doi: 10.1016/0968-0004(87)90014-4. [DOI] [Google Scholar]
- 77.Winkler B.S., Orselli S.M., Rex T.S. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic. Biol. Med. 1994;17:333–349. doi: 10.1016/0891-5849(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 78.Pehlivan F. Vitamin C: An Antioxidant Agent. Vitamin C. 2017;2:23–35. doi: 10.5772/intechopen.69660. [DOI] [Google Scholar]
- 79.Robbins R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003;51:2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- 80.Chen J., Yang J., Ma L., Li J., Shahzad N., Kim C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020;10:2611. doi: 10.1038/s41598-020-59451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L., Popko J.L., Umezawa T., Chiang V.L. 5-Hydroxyconiferyl Aldehyde Modulates Enzymatic Methylation for Syringyl Monolignol Formation, a New View of Monolignol Biosynthesis in Angiosperms. J. Biol. Chem. 2000;275:6537–6545. doi: 10.1074/jbc.275.9.6537. [DOI] [PubMed] [Google Scholar]
- 82.Prasad K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Mol. Cell. Biochem. 1997;168:117–123. doi: 10.1023/A:1006847310741. [DOI] [PubMed] [Google Scholar]
- 83.Touré A., Xueming X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010;9:261–269. doi: 10.1111/j.1541-4337.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 84.Ali Y., Jannat S., Jung H.-A., Choi J.-S. Structural Bases for Hesperetin Derivatives: Inhibition of Protein Tyrosine Phosphatase 1B, Kinetics Mechanism and Molecular Docking Study. Molecules. 2021;26:7433. doi: 10.3390/molecules26247433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gulcin İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020;94:651–715. doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- 86.Fernández-López J.A., Almela L., Obón J.M., Castellar R. Determination of Antioxidant Constituents in Cactus Pear Fruits. Plant Foods Hum. Nutr. 2010;65:253–259. doi: 10.1007/s11130-010-0189-x. [DOI] [PubMed] [Google Scholar]
- 87.Yin Z.-H., Wang J.-J., Gu X.-Z., Gu H.-P., Kang W.-Y. Antioxidant and a-glucosidase inhibitory activity of red raspberry (Harrywaters) fruits in vitro. Afr. J. Pharm. Pharmacol. 2012;6:3118–3123. doi: 10.5897/AJPP12.408. [DOI] [Google Scholar]
- 88.Islas-Flores H., Gómez-Oliván L.M., Galar-Martínez M., Colín-Cruz A., Neri-Cruz N., García-Medina S. Diclofenac-induced oxidative stress in brain, liver, gill and blood of common carp (Cyprinus carpio) Ecotoxicol. Environ. Saf. 2013;92:32–38. doi: 10.1016/j.ecoenv.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 89.Minich D.M., Brown B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients. 2019;11:2073. doi: 10.3390/nu11092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Esatbeyoglu T., Wagner A.E., Motafakkerazad R., Nakajima Y., Matsugo S., Rimbach G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014;73:119–126. doi: 10.1016/j.fct.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 91.Forman H.J., Zhang H., Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jaiswal A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free. Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 93.Robledinos-Antón N., Fernández-Ginés R., Manda G., Cuadrado A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019;2019:9372182. doi: 10.1155/2019/9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghanim B., Ahmad M., Abdallah Q., Qatouseh L., Qinna N. Modulation of NRF2/ARE pathway- and cell death-related genes during drug-induced liver injury. Hum. Exp. Toxicol. 2021;40:2223–2236. doi: 10.1177/09603271211027947. [DOI] [PubMed] [Google Scholar]
- 95.Li W., Lin K., Zhou M., Xiong Q., Li C., Ru Q. Polysaccharides from Opuntia milpa alta alleviate alloxan-induced INS-1 cells apoptosis via reducing oxidative stress and upregulating Nrf2 expression. Nutr. Res. 2020;77:108–118. doi: 10.1016/j.nutres.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Alcaraz M., Fernandez P., Guillen M. Anti-Inflammatory Actions of the Heme Oxygenase-1 Pathway. Curr. Pharm. Des. 2003;9:2541–2551. doi: 10.2174/1381612033453749. [DOI] [PubMed] [Google Scholar]
- 97.Ryter S.W., Choi A.M. Heme Oxygenase-1: Molecular Mechanisms of Gene Expression in Oxygen-Related Stress. Antioxid. Redox Signal. 2002;4:625–632. doi: 10.1089/15230860260220120. [DOI] [PubMed] [Google Scholar]
- 98.Surh Y.-J., Kundu J.K., Li M.-H., Na H.-K., Cha Y.-N. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch. Pharm. Res. 2009;32:1163–1176. doi: 10.1007/s12272-009-1807-8. [DOI] [PubMed] [Google Scholar]
- 99.Rahman I., MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: Therapeutic approaches. Free Radic. Biol. Med. 2000;28:1405–1420. doi: 10.1016/S0891-5849(00)00215-X. [DOI] [PubMed] [Google Scholar]
- 100.Franklin C.C., Backos D.S., Mohar I., White C.C., Forman H.J., Kavanagh T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Asp. Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu C.-C., Bratton S.B. Regulation of the Intrinsic Apoptosis Pathway by Reactive Oxygen Species. Antioxid. Redox Signal. 2013;19:546–558. doi: 10.1089/ars.2012.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tamura R.E., De Vasconcellos J.F., Sarkar D., Libermann T.A., Fisher P.B., Zerbini L.F. GADD45 Proteins: Central Players in Tumorigenesis. Curr. Mol. Med. 2012;12:634–651. doi: 10.2174/156652412800619978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J., Cao F., Yin H., Huang Z., Lin Z., Mao N., Sun B., Wang G. Ferroptosis: Past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y., Chen Q., Shi C., Jiao F., Gong Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol. Med. Rep. 2019;20:4081–4090. doi: 10.3892/mmr.2019.10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iorga A., Dara L., Kaplowitz N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int. J. Mol. Sci. 2017;18:1018. doi: 10.3390/ijms18051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren Y., Li S., Song Z., Luo Q., Zhang Y., Wang H. The Regulatory Roles of Polysaccharides and Ferroptosis-Related Phytochemicals in Liver Diseases. Nutrients. 2022;14:2303. doi: 10.3390/nu14112303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng K., Dong Y., Yang R., Liang Y., Wu H., He Z. Regulation of ferroptosis by bioactive phytochemicals: Implications for medical nutritional therapy. Pharmacol. Res. 2021;168:105580. doi: 10.1016/j.phrs.2021.105580. [DOI] [PubMed] [Google Scholar]
- 109.Chandramouly G. Gadd45 in DNA Demethylation and DNA Repair. In: Zaidi M.R., Liebermann D.A., editors. Gadd45 Stress Sensor Genes. Springer International Publishing; Cham, Switzerland: 2022. pp. 55–67. [DOI] [PubMed] [Google Scholar]
- 110.Zhan Q. Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat. Res. Mol. Mech. Mutagen. 2005;569:133–143. doi: 10.1016/j.mrfmmm.2004.06.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data is contained within the article and the Supplementary Materials.