Abstract
The corneal epithelium is composed of nonkeratinized stratified squamous cells and has a significant turnover rate. Limbal integrity is vital to maintain the clarity and avascularity of the cornea as well as regeneration of the corneal epithelium. Limbal epithelial stem cells (LESCs) are located in the basal epithelial layer of the limbus and preserve this homeostasis. Proper functioning of LESCs is dependent on a specific microenvironment, known as the limbal stem cell niche (LSCN). This structure is made up of various cells, an extracellular matrix (ECM), and signaling molecules. Different etiologies may damage the LSCN, leading to limbal stem cell deficiency (LSCD), which is characterized by conjunctivalization of the cornea. In this review, we first summarize the basics of the LSCN and then focus on current and emerging bioengineering strategies for LSCN restoration to combat LSCD.
Keywords: limbal stem cells, limbal stem cell deficiency, LSCD, limbal stem cell niche, limbal niche, bioengineering, niche restoration
1. Introduction
The cornea is the transparent structure of the anterior eye and has several critical roles, including separating the inner parts of the eye from the outer environment and properly transmitting light to be focused on the retina. The most superficial layer of the cornea is the epithelium, which is composed of nonkeratinized stratified squamous cells and has a significant turnover rate. The junction between the cornea and the adjacent conjunctiva is an annular transition zone referred to as the limbus [1]. Limbal integrity is vital to maintain the clarity and avascularity of the cornea as well as regeneration of the corneal epithelium. Limbal epithelial stem cells (LESCs) are located in the basal epithelial layer of the limbus and preserve this homeostasis. LESCs show multiple markers, such as K5, K14, K15, Vimentin, Notch-1, TXNIP, ABCB5, and ABCG2, which can help to isolate and identify them [2]. Proper functioning of LESCs is dependent on a specific microenvironment, known as the limbal stem cell niche (LSCN), which demonstrates specific physical, autocrine, and paracrine functions. This structure is made up of various cells, an extracellular matrix (ECM), and signaling molecules. Different etiologies may damage the LSCN, leading to limbal stem cell deficiency (LSCD), which is characterized by conjunctivalization of the cornea [3,4]. A proper understanding of limbal ultrastructure, the limbal microenvironment, and functions of LESCs is fundamental to generating LSCN restoration strategies [5]. In this review, we first summarize the basics of the LSCN and then focus on current and emerging bioengineering strategies for LSCN restoration to combat LSCD.
2. Limbal Niche (LN)
2.1. Stem Cell Niche
Generally, stem cells require particular anatomical sites for preservation and proper functioning [1]. These microenvironments are termed the stem cell niche and contain several components in addition to stem cells, such as supportive cells, several signaling factors, neurovascular inputs, and an ECM. This niche plays a critical role in the terminal differentiation of stem cells into intended tissue cells [2]. While a significant number of cells have the potential to act as stem cells, only a small fraction of them accomplish this task [3].
The niche is critical to limbal stem cell functioning. In one study, total removal of the limbal epithelium with a spared niche was compared to simultaneous injury of the limbal epithelium and niche. In the first group, the epithelium recovered, while the latter group demonstrated corneal neovascularization without healing [4]. Pure injury to the niche without involvement of LESCs may arrest wound healing upon subsequent injury to the limbus [5].
2.2. LN Microstructure and Components
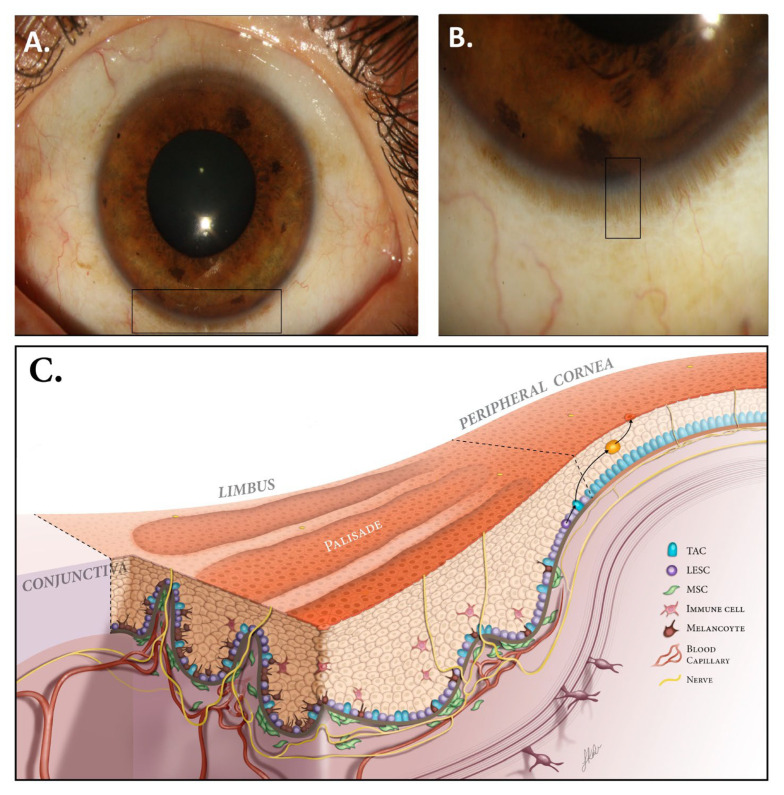
The LSCN is located in the limbal crypts formed from fibrovascular ridges, called the palisades of Vogt [6] (Figure 1). These structures have a length of 0.31 mm and a width of 0.04 mm and are typically more detectable on the superior and inferior sections of the cornea compared to the nasal and temporal regions [7]. Limbal epithelial crypts and focal stromal projections are the other compartments of this area, which promote signal integration from different factors of the niche [6,8]. Limbal epithelial crypts are projections from the undersurface of the limbal epithelium into the stroma. These structures could be parallel or perpendicular to the palisades of Vogt. Focal stromal projections are finger-shaped projections of the stroma containing a central blood vessel, which extend upward into the limbal epithelium [6,8]. Notably, these structures are specific to pigs and humans but no other mammals [9,10]. Multiple cell types, such as nerve cells, vascular cells, immune cells, mesenchymal cells, and melanocytes, are detected in the stroma of the limbus [11]. Melanocytes produce melanin to protect LESCs against UV radiation and scavenge reactive oxygen species (ROS) [12]. Melanocytes and LESCs directly contact each other, which may suggest a supporting role for melanocytes in maintaining the function of the LN and LESCs [13]. Mesenchymal stem cells (MSCs), particularly CD90- and CD105-positive cells, seem to have close interactions with LESCs [14]. In confocal microscopy, these cells were detected adjacent to LESCs, which can be interpreted as evidence for this claim [15]. Additionally, several molecular signaling pathways were identified in this regard, as well as paracrine secretions and intercellular contact [14]. Cells at the base of the corneal limbus are positive for p63, Integrin β1 (CD29), and p75NTR (CD271) [16].
Figure 1.
Normal ocular surface and limbus. (A) The corneoscleral limbus contains the palisades of Vogt (PVs), which have a length of 0.31 mm and a width of 0.04 mm and are typically more detectable on the superior and inferior sections of cornea. (B) Corneoscleral junction with magnification showing PVs. (C) The PVs contain different cells, such as melanocytes, mesenchymal stem cells, and immune cells. These cells, along with neurovasculature, provide growth factors, nutrients, and structural support to promote proper LESC proliferation and differentiation (LESC: limbal epithelial stem cell, TAC: transient amplifying cell, MSC: mesenchymal stem cell). Modified with permission from [14].
2.2.1. ECM of LN
The limbal epithelium basement membrane is composed of type IV collagen, α2 and β2-laminin, vitronectin, fibronectin, Integrin β1 (CD29), and tenascin C, which makes the structure of the limbal ECM completely distinct from that of the corneal stroma [17,18,19]. Overall, ECM components have various important interactions with niche cells. Hyaluronan (HA) is glycosaminoglycan, which makes up another component of the ECM and is produced by hyaluronan synthases (HASs), which have three types: HAS1, HAS2, and HAS3. Notably, all three types of HAS are expressed in the limbal area, and any defects in the expression of each enzyme can decrease the number of epithelial layers and speed of wound repair, as well as changes in the morphology of basal cells [20]. HA may have some role in the maintenance of the stem cell population, as one study showed that defective HAS2 leads to abnormalities in the compartment of LESCs [21]. Hence, HA not only acts as a bed to secure cells but also influences cellular behavior, making it an appropriate scaffold for use in cell or tissue transplantation [22].
2.2.2. Genes and Proteins Implicated in LN Regulation
Several types of interactions have been described to regulate the activity and phenotype of LESCs, including direct cell–cell contact, paracrine signaling, autocrine signaling, and soluble factors [23]. Among these soluble factors, the Wnt signaling pathway is one of the key drivers of differentiation, proliferation, and quiescence of LESCs [24]. It has been shown that exposure of LESCs to high amounts of the Wnt6 ligand can lead to increased proliferation and lower expression of terminal differentiation markers of mature corneal epithelial cells [25]. Aside from the role of Wnt6 expression in the promotion of LESC self-renewal, it seems that the phenotype of LESCs is dependent on the Wnt7a–PAX6 axis [25,26]. Frizzled receptors are key components of Wnt signaling, and the Frizzled 7 (Fz7) receptor is the dominant type in the limbal area [27]. It has been reported that Fz7 receptor knockdown can lead to decreased marker expression and stemness of LESCs [27]. Therefore, manipulation of these signaling pathways could be of interest for clinical applications.
One of the other signaling pathways involved in LESC stemness is Jagged 1 (Jag1)-Notch signaling [28]. It has been reported that activation of this pathway can result in differentiation towards maturity of corneal epithelial cells and decreased LESC stemness. Therefore, therapies that inhibit Jag1-Notch signaling to enhance LESC stemness can be investigated in future studies.
The gene expression profile of inactive LESCs is completely different from that of mature corneal epithelial cells. Single-cell RNA sequencing (scRNA-seq) can help researchers to identify different genes involved in the differentiation and function of LESCs [29,30]. For example, Li and colleagues introduced TSPAN7 and SOX17 as critical factors in maintaining corneal epithelium homeostasis [31]. Additionally, SOX9 expression seems to have some role in the regulation of LESC activation or quiescence [32]. Furthermore, RUNX1, SMAD3, ATF3, ABCB5, H2AX, PBK, and Plk3 are among the other proteins and signaling pathways implicated in the modulation of the function and proliferation of LESCs [26,30,33]. These findings may justify future application of these proteins as potential markers to screen the success rate and outcomes of cultivated LESC transplantation [29]. Overall, these molecules show promising therapeutic applications for the near future, including increasing the transplantation success rate through effects on the self-renewal capacity and stemness of LESCs, introducing new drugs modulating the aforementioned pathways to medically manage partial-LSCD cases, and reprogramming corneal epithelial cells to transdifferentiate into an LESC-like phenotype, a dramatic shortcut to curing bilateral cases of LSCD [29].
3. LESCs’ Functions
3.1. Epithelial Maintenance
The turnover rate of the corneal epithelium is significantly high. Regeneration of the corneal epithelium occurs approximately every 2 weeks based on the XYZ hypothesis. In this theory, X stands for superficial movement of cells from the basal epithelium, Y is representative of centripetal migration of basal cells from the limbus, and Z represents damaged or desquamated lost cells [34]. The hypothesis claims that X + Y = Z, or, in other words, the loss of corneal cells is replenished by basal epithelial and limbal cells. Progenitor cells required for repopulation of the corneal epithelium are produced through division of LESCs located in the limbal basal layer. These progenitor cells, also known as transient amplifying cells (TACs), move centripetally and then superficially for terminal differentiation. In general, LESCs have a highly controlled division pattern: one daughter cell remains in the niche to maintain the LESC population while the other one differentiates into a TAC [35].
3.2. Epithelial Wound Healing
Several studies have reported the response and proliferation of limbal basal epithelial cells following large wounds [36,37]. However, small wounds can be resolved through enlargement of cell clusters of the central cornea [38]. It seems that limbal response starts with a latency period since movement and repopulation of the basal epithelium occurs about 8 h after wounding [23]. In addition to this key role of the limbus (e.g., proliferation of progenitor cells), it may induct a population pressure gradient to lead the migration of wound-edge basal epithelial cells into the wound bed [39].
4. LSCD
Various conditions have been implicated in causing LSCD due to severe damage to the LESCs or LN, among them ocular cicatricial pemphigoid (OCP), Stevens-Johnson syndrome (SJS), thermal or chemical burns, contact lenses, numerous ocular surgeries, local or systemic usage of 5-FU and MMC, and congenital aniridia [29,40]. LSCD is characterized by corneal opacity, neovascularization, and invasion of adjacent conjunctiva. LSCD interferes with corneal wound healing, resulting in subsequent complications such as persistent epithelial defect (PED), corneal ulcers, and even perforation [41]. Diagnosis of this entity is mainly clinical and based on slit-lamp examination findings. However, the gold-standard diagnostic method is impression cytology, which shows goblet or conjunctival cell markers in the corneal area. MUC5AC is used as a marker for goblet cells, and cytokeratin 7 and 13 identify conjunctival cells. Confocal microscopy and optical coherence tomography modalities are other useful diagnostic tools [42,43,44]. Details on this subject are outside of the scope of this review but are discussed in our previous review article [40].
From a microscopic point of view, inflammation is an inseparable part of LSCD, with alteration of several signaling cascades in both the cornea and limbus [45]. It has been reported that levels of pro-inflammatory cytokines (e.g., IL-1 and IL-6) and angiogenic molecules (e.g., vascular endothelial growth factor (VEGF)) are increased in the ocular surface of eyes with conjunctivalization [45]. Prolonged inflammatory conditions can result in unfavorable consequences, including angiogenesis, decreased expression of LESC markers, reduced colony-forming efficiency, and an altered ECM [14].
5. Limbal Stem Cell Transplantation
Several types of limbal transplantation are available based on the source (autologous or allogeneic) and preparation of the harvested tissue (direct or cultivated), including direct autologous transplantation, direct allogeneic transplantation, cultivated autologous transplantation, and cultivated allogeneic transplantation (Figure 2). A meta-analysis on the results of 40 studies was performed in 2020 to assess the outcomes of these 4 methods [46]. The results of this study agree with the superiority of autologous approaches in stabilizing the ocular surface; direct autologous transplantation and cultivated autologous transplantation had the highest success rates at 85.7% and 84.7%, respectively. The success rate of allogeneic methods was considerably lower: 57.8% for direct allogeneic transplantation and 63.2% for cultivated allogeneic methods. Direct autologous limbal transplantation was superior with regard to visual improvement [46]. Although allogeneic transplantation is one of the most successful approaches in the treatment of LSCD, one of the disadvantages of this method is the requirement of a long-term immunosuppressive regimen [47]. Hence, autologous methods are preferable. However, autologous approaches are not applicable in cases with bilateral LSCD [48]. To overcome this limitation, autologous cultivation methods were introduced.
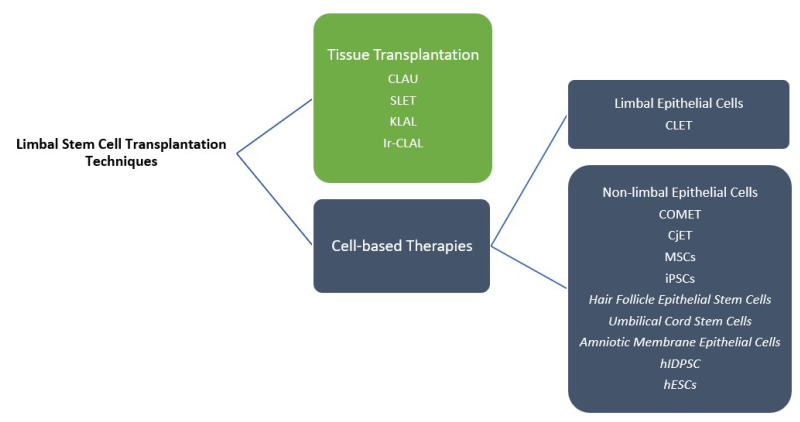
Figure 2.
Different methods of limbal stem cell transplantation (CLAU: conjunctival limbal autograft, CLET: cultivated limbal epithelial transplantation, SLET: simple limbal epithelial transplantation, KLAL: kerato-limbal allograft, Ir-CLAL: living-related conjunctival limbal allograft, COMET: cultivated oral mucosal epithelial transplantation, CjET: conjunctival epithelial transplantation, MSCs: mesenchymal stem cells, iPSCs: induced pluripotent stem cells, hIDPSC: human immature dental pulp stem cells, hESCs: human embryonic stem cells). Currently, only animal studies are available for methods written in italic format in the non-limbal epithelial cells box.
5.1. Tissue Transplantation
LESC transplantation is required in severe cases of LSCD to replace the lost population of stem cells. The severity and extent of involvement are critical factors in choosing the appropriate approach and strategy. In unilateral cases with total involvement, the available options are conjunctival limbal autograft (CLAU) from the fellow eye and simple limbal epithelial transplantation (SLET) [14]. CLAU was introduced in the 1980s [49]. In this technique, two grafts of two clock hours each from the limbus and the adjacent rim of conjunctiva of the patient’s healthy fellow eye, are harvested and transplanted to the diseased eye. A success rate of 75% has been reported for CLAU [50]. SLET is a newer approach that was developed to minimize the risk of iatrogenic LSCD in the fellow healthy eye. In this method, only 1 small 2 × 2 mm (1-clock-hour) specimen from the patient’s normal eye is harvested and divided into smaller segments followed by transplantation to the diseased eye using an amniotic membrane and fibrin glue [51]. A success rate of 76% has been reported for autologous SLET in chemical injuries by Basu and colleagues [52]. In bilateral total LSCD, kerato-limbal allograft (KLAL) and living-related conjunctival limbal allograft (lr-CLAL) are available [53,54,55,56,57,58]. Overall, traditional approaches are based on harvesting a sample of functioning limbal tissue from a healthy eye [49]. More recently, approaches have utilized transplantation of cultivated and expanded LESCs (Table 1) [59].
Table 1.
Advantages, disadvantages, and complications of limbal stem cell transplantation techniques (CLAU: conjunctival limbal autograft, CLET: cultivated limbal epithelial transplantation, SLET: simple limbal epithelial transplantation, KLAL: kerato-limbal allograft, Ir-CLAL: living-related conjunctival limbal allograft, COMET: cultivated oral mucosal epithelial transplantation, PED: persistent epithelial defect, LSCD: limbal stem cell deficiency).
| Technique | Reference | Advantages | Disadvantages | Complications | |
|---|---|---|---|---|---|
| CLAU | [60,61,62] | -Acceptable outcomes -Application of conjunctival patch in ocular surface reconstruction |
Risk of iatrogenic LSCD | -Delayed epithelial healing -PED -Corneal perforation -Progressive conjunctival ingrowth |
|
| CLET | [63,64,65] | -Acceptable outcomes -Requirement of small donor tissue |
-Expense -Technical difficulties -Risk of prion disease transmission via animal product usage during culture |
-Postoperative hemorrhage under the graft -Infection -PED -Corneal perforation |
|
| SLET | [51,66] | -Acceptable outcomes -Requirement of small donor tissue |
-Risk of donor tissue loss | -Focal recurrence of LSCD -Progressive conjunctivalization and symblepharon -Keratitis -PED |
|
| COMET | [67,68] | Applicable in bilateral cases | -Peripheral corneal neovascularization -Suboptimal visual outcomes |
-PED -Corneal perforation -Glaucoma -Infection |
|
| Limbal allografts | lr-CLAL | [54,63] | -Applicable in bilateral cases -Utilizes a large conjunctival patch, which can be used in ocular surface reconstruction |
-Requirement of immunosuppression regimen -Delayed epithelialization -Limited long-term success |
-Rejection -Glaucoma -PED -Corneal melting and perforation -Graft-related issues -Infection -Posterior segment complications such as retinal detachment, vitreous hemorrhage, and cystoid macular edema |
| KLAL | [63,69,70] | -Applicable in bilateral cases -Providing a larger number of LESCs compared to lr-CLAL |
|||
5.2. LESC Culture and Expansion
In cases with unilateral involvement, autologous transplantation possesses the highest rate of success with a low risk of complications. However, the chance of developing iatrogenic LSCD in the healthy fellow eye is a concern [71]. This complication was frequently detected in rabbit models of autologous transplantation in which a 240° arc of limbal tissue was harvested [72,73]. On the other hand, harvesting tissues at a less than 90° arc was associated with transplantation failure [74,75]. So, it seems an intermediate size of tissue should be harvested to balance the risk of these two unfavorable outcomes.
To decrease the mentioned risk of iatrogenic damage, tissue-sparing methods were introduced. Over two decades ago, Pellegrini et al. [59] reported the first application of cultivated autologous transplantation, called cultivated limbal epithelial transplantation (CLET). In this technique, a tiny 2 × 2 mm section of limbal tissue is taken from the healthy eye, followed by ex vivo expansion of LESCs [76]. An amniotic membrane or a suspension is used as a scaffold to expand the harvested stem cells, which lasts 14–21 days [77,78]. A success rate of about 76% has been reported for CLET in chemical-burn-induced LSCD by Rama and colleagues [65]. Notably, it has been shown that the success rate of methods using cultivated stem cells is associated with the percentage of p63+ cells in cultures; Rama et al. reported a success rate of 78% for transplantations containing >3% p63+ cells. Meanwhile, this rate significantly decreases to 11% for transplantation of cultures with <3% p63+ cells [65]. Some studies reported graft survival might decrease over time, which could be related to the absence of a healthy niche [79,80]. In this regard, confocal microscopy has revealed that CLET is not capable of restoring the limbal niche [81]. It should be mentioned that CLET can be performed with autologous or allogeneic grafts. Allogeneic grafts are especially useful for bilateral cases of LSCD. A meta-analysis showed that the graft survival rate and visual improvement were equal for both autologous and allogeneic sources. However, autologous grafts are preferred as they do not require immunosuppression after surgery [82].
A technique offering the benefits of autologous transplantation (e.g., lack of immunosuppression and risk of disease transmission) in bilateral cases of LSCD without a suitable source of LESCs would be a valuable therapeutic tool. Hence, researchers began to use other stem cell lines to transdifferentiate into limbal stem cells, fulfilling this goal and need [29]. Historically, the first attempt in this line, in which the oral mucosa epithelium was cultivated and transplanted, was about two decades ago [48]. A brief review of the available non-limbal sources and relevant studies are provided below.
Oral mucosa epithelium: In 2004, the first usage of the oral mucosa epithelium in LSCD was reported [48]. In this study, six patients were enrolled, three of which were suffering from SJS and three of which had eyes with chemical burn. After 2–3 weeks of culture time, the prepared oral mucosa epithelium was implanted on an amniotic membrane scaffold with a supportive layer of fibroblasts and transplanted onto the diseased eyes. A success rate of about 70% has been reported for cultivated oral mucosal epithelial transplantation (COMET). Mild peripheral corneal neovascularization is the disadvantage of this technique. Moreover, the phenotype of oral epithelium remains unchanged after transplantation, leading to suboptimal visual outcomes due to this type being of a thicker and more opaque nature than the corneal epithelium [83,84].
Conjunctival epithelial cells: Similar to the previous method, conjunctival epithelial cells (CjECs) were used as another autologous source. After 18.5 months of follow-up, conjunctival epithelial transplantation (CjET) showed a 86% success rate in resolving conjunctivalization and corneal opacity [85]. Recovery of the corneal epithelium was approved using confocal microscopy, during which five to six layers of corneal epithelial cells with normal morphology were detected [85]. Overall, data on long-term survival with COMET and CjET grafts are limited.
Hair follicle epithelial stem cells: Follicular epithelial stem cells were reported to be positive for CD29 and CD271 [86]. Transdifferentiating of hair follicle epithelial stem cells to the corneal epithelium was studied in a murine model of LSCD [87]. After isolation and expansion, hair follicle epithelial stem cells were transferred to a medium similar to the limbal niche. Finally, these cells showed markers of corneal-epithelium-like cells, and an 80% success rate of transdifferentiation was observed. Further studies are required to generalize these results to human subjects.
Pluripotent stem cells: These cells are capable of forming a self-formed ectodermal autonomous multi-zone (SEAM), which contains cells of ectodermal lineage that mimic anterior and posterior eye development in vivo [88]. Hongisto et al. studied transdifferentiation of human pluripotent stem cells (PSCs) into human limbal stem cells and achieved over 65% LESCs in 24 days [89]. Additionally, they introduced a protocol to bank human-pluripotent-stem-cell-derived LESCs, which can facilitate further progress in these methods and similar research. Further research is required before implementation of this method in large-scale clinical trials. Recently, a team of scientists from Osaka University reported the results of the first ever trial on iPSC-based corneal transplantation [90]. They performed this trial successfully on four patients without any rejection or tumorigenicity.
Dental pulp: In a rabbit model of LSCD due to chemical injury, grafts containing human immature dental pulp stem cells (hIDPSCs) were transplanted into the limbal niche [91,92]. After 3 months, LESCs markers were detected on hIDPSCs, and the condition of the ocular surface was improved.
Umbilical cord stem cells: Human umbilical cord lining epithelial cells are another potential source for the management of LSCD. Animal models using this type of stem cell are available in the literature [93].
Embryonic stem cells: Human embryonic stem cells (hESCs) are pluripotent stem cells with the capability of differentiating into corneal and limbal epithelial cells [94]. Hence, application of these cells may be beneficial in LSCD. Although challenging, several in vitro models have been successfully used to differentiate hESCs into corneal-epithelial-like cells [95,96,97,98,99].
Amniotic membrane epithelial cells: It seems that expressed markers of amniotic membrane epithelial cells have a significant overlap with mesenchymal and embryonic stem cells. The other advantage of these cells is that they display immunomodulatory characteristics. In rabbit models, these cells have been successfully applied to treat LSCD [100,101].
Mesenchymal stem cells: this alternative source is separately discussed later.
Currently, most culture techniques are based on animal materials, which come with the risk of triggering the host immune system due to the transmission of non-human pathogens [102]. Nevertheless, studies using non-human reagents with acceptable outcomes are available [103,104,105]. Moreover, finding an optimum culture medium to simulate niche conditions in ex vivo is as important as using non-human reagents. In line with this concept, although the presence of supportive feeder cells is not necessary, they can significantly increase clonal efficiency through preserving cell–cell contact [106]. Monolayer irradiated or mitomycin-treated murine 3T3 fibroblasts (mitotically inactive) have been used previously as feeder cells to mimic a more suitable microenvironment. Meanwhile, currently, monolayer limbal mesenchymal cells and human-adipose-derived stem cells and bone marrow stromal cells are successfully applied in three-dimensional (3-D) culture systems [107,108,109].
6. LN Restoration
It seems that the pure transfer of LESCs without restoration of the LN does not lead to good long-term outcomes, especially in severe cases of LSCD [14,52,66,110]. Ongoing inflammation can act as a progressive destructive factor for remaining healthy stem cells. Hence, suppressing inflammation and recovery of LESCs and ECM function compose the foundations of niche restoration strategies (Figure 3) [14].
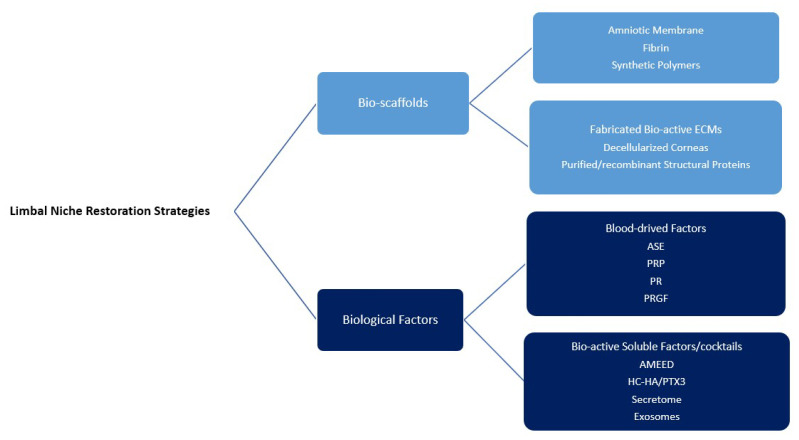
Figure 3.
Different strategies and materials available for limbal niche restoration (ECM: extracellular matrix, ASE: autologous/allogeneic serum eye drops, PRP: platelet-rich plasma, PR: platelet releasate, PRGF: plasma rich in growth factors, AMEED: amniotic membrane extract eye drop).
6.1. Bio-Scaffolds
After successful ex vivo expansion of LESCs, proper carriers should be used to transplant the grafts onto the targets.
6.1.1. Amniotic Membrane
The most commonly used carrier in studies is the human amniotic membrane (HAM). The HAM, which has no vessels or nerves, contains various cytokines and growth factors, as well as collagen types I, III, IV, and V. So, this tissue has the potential to act as either a carrier for cell delivery or a scaffold for bioengineering [111,112]. Mimicking a niche-like environment for LESCs was previously proposed for the HAM [113]. Additionally, this matrix comprises anti-inflammatory, anti-fibrotic, and anti-angiogenic properties. The drawbacks of this agent are its low transparency and tensile strength and the risk of disease transmission [14,114]. Moreover, rapid digestion of the HAM after transplantation may eclipse its long-term outcomes [115].
6.1.2. Fabrication of Bio-Active ECMs
Currently, several materials are used in the fabrication of bio-active ECMs, including decellularized corneas (human or animal) and purified/recombinant structural proteins such as collagen [116]. The process of corneal decellularization is performed via usage of ribonucleases, osmotic solutions, freeze thawing, and detergents to diminish the risk of antigenicity [117]. It should be mentioned that after this process, the ECM remains functional and structured with preservation of healing factors [118]. Decellularized porcine corneas were also transplanted to patients with corneal ulcers [119,120]. In these studies, the most suitable candidates were patients with stromal involvement but an intact epithelium. Hence, application of this method in cases of LSCD can be limited and lead to the development of an alternative option: hydrogel production through digestion of decellularized corneas [14,121,122]. In one study, a thermoresponsive hydrogel was fabricated from a decellularized porcine cornea after digestion using pepsin/HCl [123]. Numerous wound-healing factors were found in this hydrogel. Compatibility of this fabricated hydrogel with corneal cells makes it a proper cell delivery method for 3-D structures [124]. Moreover, further approaches are available to fabricate a bio-active hydrogel, including a silk-film-derived hydrogel with the ability to affect gene expression of the corneal epithelium, a cross-linked collagen hydrogel to substitute the corneal stroma, and a collagen-coupled polymer hydrogel that supports epithelial wound closure [125,126,127]. Regarding the purified/recombinant structural proteins, fabrication of bioengineered limbal crypts is achieved using collagen type I and cast molding [116]. The other approach is using 3-D printing via various bio-inks [128,129]. Collagen type I is the most common type in corneal structures. The biomechanics of collagen can be improved through several methods, such as cross-linking and plastic compression. Its suitable biomechanics, availability, and biocompatibility make collagen a suitable bio-scaffold [130,131].
6.1.3. Others
Synthetic polymers and fibrin are among the other available options. Polyethylene glycol and polymethacrylate are constructed polymers with supportive roles in the cultivation of LESCs. However, they have not been studied in human trials yet [132,133]. Synthetic polymers offer several strengths, such as chemical stability, manipulability, and easy mass production [130]. Fibrin membrane, which is mostly composed of fibrinogen and thrombin, has a long history of safe application as a sealant in ophthalmology [134,135]. Fibrin can be prepared easily and showed an acceptable success rate in trials for LSCD.
6.2. Revitalization of Limbal Niche via Biological Factors
As mentioned before, signaling and cellular contacts are required for proper functioning of the limbal niche. Administration of exogenous factors can be used as an alternative to these signaling pathways [14].
6.2.1. Blood-Derived Factors
Currently, ophthalmologists use autologous/allogeneic serum eye drops (ASEs) in routine practice for various ocular surface disorders, including dry eye disease (DED), PED, and corneal involvement following graft-versus-host disease (GVHD), and Sjögren disease [14]. ASEs are enriched with numerous cytokines and factors, such as TGF-β and EGF, as well as minerals and vitamins helpful in corneal epithelium maintenance and regeneration [136]. These properties justify the usage of ASEs in the management of ocular surface disturbances. Similarly, platelet-derived preparations, including platelet-rich plasma (PRP), platelet releasate (PR), and plasma rich in growth factors (PRGF), contain various growth factors, such as TGF, EGF, IGF-1, and pigment epithelium-derived factor (PEDF), highlighting the potential usefulness of platelet-derived products in limbal niche restoration [137].
6.2.2. Bio-Active Soluble Factors/Cocktails
Different sources can be used to produce bio-active soluble factors/cocktails. One of these sources is amniotic membrane extract eye drops (AMEEDs). One study showed the enhancement of LESC functioning using in vivo cultivation with AMEEDs [138]. The other product extracted from the HAM is HC-HA/PTX3, which has shown to be effective in enhancement of self-renewal capacity of LESCs in 3-D culture systems through influencing the Wnt/BMP signaling pathway [139]. A similar function has been reported for PEDF, a soluble growth factor derived from human plasma, which activates the p38 MAPK and STAT3 signaling pathways [140].
The supernatant layer of in vitro cell cultivation is called secretome since it has all the secreted factors of those cells. Some studies have reported the mesenchymal stem cell (MSC) secretome can also promote LN and ocular surface regeneration. Additionally, MSC secretomes can lead to increased expression of the CD44 receptor and subsequent improvement in hyaluronic acid binding, which can decrease scar formation [141,142]. Other useful factors derived from MSCs include exosomes, which act in cell–cell contact. Corneal-MSC-derived exosomes can enhance wound repair capacity in animal corneas [143]. Additionally, corneal exosomes exhibit anti-inflammatory and immunomodulatory properties, which can address the pathophysiology of LSCD [144]. Furthermore, exosomes can act as a delivery vehicle [145].
Finally, conditioned media from limbal fibroblasts have shown promising results [146]. In an LSCD murine model, using limbal-fibroblast-conditioned media resulted in an increase in corneal-epithelial-like cells as well as lower density of conjunctival goblet cells [146].
6.3. Cell-Based Strategies
Currently, MSCs are the subject of many studies on LN and ocular surface reconstruction due to their formidable properties. Over half of a century has been passed since the initial isolation of these cells from bone marrow specimens [147]. The authors first noted the capability of MSCs in repairing bone defects [147]. The beneficial roles of MSCs are not limited to this finding, as their immunomodulatory functions have made them applicable in the treatment of autoimmune diseases and also organ transplantation [148]. Furthermore, they are also capable of producing ECMs in 3-D culture systems [149]. Application of MSCs in the management of chemical injuries, DED, and LSCD has been studied [150,151,152,153]. MSCs can be obtained from various sources, including bone marrow, adipose tissue and the HAM, limbus, and omentum [154,155,156,157,158]. It has been reported that bone-marrow-derived MSCs can decrease the level of inflammatory cytokines, oxidative stress species, and lipid peroxidation while increasing factors helpful for limbal niche restoration [159,160,161,162,163]. As discussed before, MSCs are one of the most important components found in a normal living LN. The properties that have been reported for limbal-derived MSCs are similar to those found for bone-marrow-derived ones [164,165]. MSCs also offer multiple advantages compared to limbal epithelial cells, including the ability to harvest from multiple tissues through a faster and cheaper process. Moreover, 100% of the MSCs in a transplant are stem cells [37]. In an animal model of chemical burn, local application of limbal-derived MSCs resulted in an increase in corneal transparency, a decreased epithelial defect, and attenuated corneal neovascularization [158]. Similarly, corneal MSCs secrete high levels of antiangiogenic factors [146]. Although data on the clinical application of MSCs are limited, the first clinical trial using allogeneic human-bone-marrow-derived MSCs reported a success rate of 76.5–85.7%, an efficacy similar to that of CLET [166]. Several routes are available to deliver the MSCs, including systemic topical, subconjunctival, sub-tenon, and intrastromal injection [167]. However, there is no general consensus on the optimal route for MSC delivery. Different routes of administration have specific drawbacks. The systemic route of administration may lead to a considerable rate of side effects, while a low number of cells may be delivered to the target site. On the topical route, the cells can be washed out, leading to a short period of cell retainment. In using a scaffold to transplant cells, the number of transferred cells is low, and the cost and risk of surgery should also be considered. Regarding the subconjunctival route, the best cell vehicle solution and cell concentration and also the number and location of injection are still unknown. Moreover, the volume of injection is limited. The intrastromal technique has more technical difficulties [152,159,168]. We conducted a clinical trial to evaluate the safety and maximally tolerated dose of locally delivered allogeneic MSCs. In this study, different doses of bone-marrow-derived MSCs were given using subconjunctival injections to evaluate safety as well as anatomical and functional results in adult cases of neurotrophic keratitis [169]. The results of the first three patients were reported in the annual ARVO 2022 meeting [170]. Overall, MSCs usage can be considered an emerging approach in the management of severe ocular surface disorders with promising results.
7. Conclusions
The presence of a competent limbal niche is completely necessary for proper functioning and homeostasis of LESCs. The limbal niche contains several components, including supportive cells, several signaling factors, neurovascular inputs, and a specialized ECM. Following severe acquired or hereditary injuries to the limbal niche resulting in LSCD, taking action to restore the niche is essential for therapeutic interventions to be successful. In addition to traditional LESC transplantation methods, regenerative approaches such as bio-scaffolds and cell-based therapies have attracted increasing attention. However, further clinical trials and human studies are required to incorporate these novel strategies into clinical practice.
Author Contributions
Conceptualization, A.R.D. and M.S.; writing—original draft preparation, K.C.; writing—review and editing, K.C., R.K. and S.M.B.; supervision, M.S.; project administration, A.R.D.; funding acquisition. A.R.D., M.S. and K.C. should be considered the joint first authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by R01 EY024349 (ARD), UH3 EY031809 (ARD), and the Core Grant for Vision Research EY01792, from NEI/NIH; the Vision Research Program—Congressionally Directed Medical Research Program VR170180 from the Department of Defense; and an unrestricted grant to the department and the Physician-Scientist Award, both from Research to Prevent Blindness.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Tavakkoli F., Eleiwa T.K., Elhusseiny A.M., Damala M., Rai A.K., Cheraqpour K., Ansari M.H., Doroudian M.H., Keshel S., Soleimani M. Corneal stem cells niche and homeostasis impacts in regenerative medicine; concise review. Eur. J. Ophthalmol. 2023:11206721221150065. doi: 10.1177/11206721221150065. [DOI] [PubMed] [Google Scholar]
- 3.Walther V., Graham T.A. Location, location, location! The reality of life for an intestinal stem cell in the crypt. J. Pathol. 2014;234:1–4. doi: 10.1002/path.4370. [DOI] [PubMed] [Google Scholar]
- 4.Nasser W., Amitai-Lange A., Soteriou D., Hanna R., Tiosano B., Fuchs Y., Shalom-Feuerstein R. Corneal-committed cells restore the stem cell pool and tissue boundary following injury. Cell Rep. 2018;22:323–331. doi: 10.1016/j.celrep.2017.12.040. [DOI] [PubMed] [Google Scholar]
- 5.Nubile M., Curcio C., Dua H.S., Calienno R., Lanzini M., Iezzi M., Mastropasqua R., Agnifili L., Mastropasqua L. Pathological changes of the anatomical structure and markers of the limbal stem cell niche due to inflammation. Mol. Vis. 2013;19:516. [PMC free article] [PubMed] [Google Scholar]
- 6.Shortt A.J., Secker G.A., Munro P.M., Khaw P.T., Tuft S.J., Daniels J.T. Characterization of the limbal epithelial stem cell niche: Novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402–1409. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- 7.Townsend W.M. The limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1991;89:721. [PMC free article] [PubMed] [Google Scholar]
- 8.Dua H.S., Shanmuganathan V., Powell-Richards A., Tighe P., Joseph A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005;89:529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grieve K., Ghoubay D., Georgeon C., Thouvenin O., Bouheraoua N., Paques M., Borderie V. Three-dimensional structure of the mammalian limbal stem cell niche. Exp. Eye Res. 2015;140:75–84. doi: 10.1016/j.exer.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Notara M., Schrader S., Daniels J.T. The porcine limbal epithelial stem cell niche as a new model for the study of transplanted tissue-engineered human limbal epithelial cells. Tissue Eng. Part A. 2011;17:741–750. doi: 10.1089/ten.tea.2010.0343. [DOI] [PubMed] [Google Scholar]
- 11.Vantrappen L., Geboes K., Missotten L., Maudgal P., Desmet V. Lymphocytes and Langerhans cells in the normal human cornea. Investig. Ophthalmol. Vis. Sci. 1985;26:220–225. [PubMed] [Google Scholar]
- 12.Polisetti N., Gießl A., Zenkel M., Heger L., Dudziak D., Naschberger E., Stich L., Steinkasserer A., Kruse F.E., Schlötzer-Schrehardt U. Melanocytes as emerging key players in niche regulation of limbal epithelial stem cells. Ocul. Surf. 2021;22:172–189. doi: 10.1016/j.jtos.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Dziasko M.A., Tuft S.J., Daniels J.T. Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments. Exp. Eye Res. 2015;138:70–79. doi: 10.1016/j.exer.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Yazdanpanah G., Haq Z., Kang K., Jabbehdari S., l Rosenblatt M., Djalilian A.R. Strategies for reconstructing the limbal stem cell niche. Ocul. Surf. 2019;17:230–240. doi: 10.1016/j.jtos.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathews S., Chidambaram J.D., Lanjewar S., Mascarenhas J., Prajna N.V., Muthukkaruppan V., Chidambaranathan G.P. In vivo confocal microscopic analysis of normal human anterior limbal stroma. Cornea. 2015;34:464. doi: 10.1097/ICO.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto N., Hirano K., Kojima H., Sumitomo M., Yamashita H., Ayaki M., Taniguchi K., Tanikawa A., Horiguchi M. Cultured human corneal epithelial stem/progenitor cells derived from the corneal limbus. In Vitro Cell. Dev. Biol.-Anim. 2010;46:774–780. doi: 10.1007/s11626-010-9344-9. [DOI] [PubMed] [Google Scholar]
- 17.Ljubimov A.V., Burgeson R.E., Butkowski R.J., Michael A.F., Sun T.-T., Kenney M.C. Human corneal basement membrane heterogeneity: Topographical differences in the expression of type IV collagen and laminin isoforms. Lab. Investig. J. Tech. Methods Pathol. 1995;72:461–473. [PubMed] [Google Scholar]
- 18.Schlötzer-Schrehardt U., Dietrich T., Saito K., Sorokin L., Sasaki T., Paulsson M., Kruse F. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp. Eye Res. 2007;85:845–860. doi: 10.1016/j.exer.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Yeung A.M.-H., Schlötzer-Schrehardt U., Kulkarni B., Tint N.L., Hopkinson A., Dua H.S. Limbal epithelial crypt: A model for corneal epithelial maintenance and novel limbal regional variations. Arch. Ophthalmol. 2008;126:665–669. doi: 10.1001/archopht.126.5.665. [DOI] [PubMed] [Google Scholar]
- 20.Sun M., Puri S., Mutoji K.N., Coulson-Thomas Y.M., Hascall V.C., Jackson D.G., Gesteira T.F., Coulson-Thomas V.J. Hyaluronan derived from the limbus is a key regulator of corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2019;60:1050–1062. doi: 10.1167/iovs.18-25920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gesteira T.F., Sun M., Coulson-Thomas Y.M., Yamaguchi Y., Yeh L.-K., Hascall V., Coulson-Thomas V.J. Hyaluronan rich microenvironment in the limbal stem cell niche regulates limbal stem cell differentiation. Investig. Ophthalmol. Vis. Sci. 2017;58:4407–4421. doi: 10.1167/iovs.17-22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yazdani M., Shahdadfar A., Jackson C.J., Utheim T.P. Hyaluronan-based hydrogel scaffolds for limbal stem cell transplantation: A review. Cells. 2019;8:245. doi: 10.3390/cells8030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seyed-Safi A.G., Daniels J.T. The limbus: Structure and function. Exp. Eye Res. 2020;197:108074. doi: 10.1016/j.exer.2020.108074. [DOI] [PubMed] [Google Scholar]
- 24.Clevers H., Loh K.M., Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang H., Xue Y., Lin Y., Zhang X., Xi L., Patel S., Cai H., Luo J., Zhang M., Zhang M. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature. 2014;511:358–361. doi: 10.1038/nature13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M., Huang H., Li L., He C., Zhu L., Guo H., Wang L., Liu J., Wu S., Liu J. Core transcription regulatory circuitry orchestrates corneal epithelial homeostasis. Nat. Commun. 2021;12:420. doi: 10.1038/s41467-020-20713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei H., Nakatsu M.N., Baclagon E.R., Deng S.X. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem Cells. 2014;32:938–945. doi: 10.1002/stem.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González S., Halabi M., Ju D., Tsai M., Deng S.X. Role of Jagged1-mediated Notch signaling activation in the differentiation and stratification of the human limbal epithelium. Cells. 2020;9:1945. doi: 10.3390/cells9091945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masood F., Chang J.-H., Akbar A., Song A., Hu W.-Y., Azar D.T., Rosenblatt M.I. Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions. Cells. 2022;11:3247. doi: 10.3390/cells11203247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan N., Wang J., Wray B., Patel P., Yang W., Peng H., Lavker R.M. Single-cell RNA transcriptome helps define the limbal/corneal epithelial stem/early transit amplifying cells and how autophagy affects this population. Investig. Ophthalmol. Vis. Sci. 2019;60:3570–3583. doi: 10.1167/iovs.19-27656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D.-Q., Kim S., Li J.-M., Gao Q., Choi J., Bian F., Hu J., Zhang Y., Li J., Lu R. Single-cell transcriptomics identifies limbal stem cell population and cell types mapping its differentiation trajectory in limbal basal epithelium of human cornea. Ocul. Surf. 2021;20:20–32. doi: 10.1016/j.jtos.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menzel-Severing J., Zenkel M., Polisetti N., Sock E., Wegner M., Kruse F.E., Schlötzer-Schrehardt U. Transcription factor profiling identifies Sox9 as regulator of proliferation and differentiation in corneal epithelial stem/progenitor cells. Sci. Rep. 2018;8:10268. doi: 10.1038/s41598-018-28596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ksander B.R., Kolovou P.E., Wilson B.J., Saab K.R., Guo Q., Ma J., McGuire S.P., Gregory M.S., Vincent W.J., Perez V.L. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014;511:353–357. doi: 10.1038/nature13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thoft R.A. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–1443. [PubMed] [Google Scholar]
- 35.Ahmad S., Kolli S., Lako M., Figueiredo F., Daniels J.T. Stem cell therapies for ocular surface disease. Drug Discov. Today. 2010;15:306–313. doi: 10.1016/j.drudis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Seyed-Safi A.G., Daniels J.T. A validated porcine corneal organ culture model to study the limbal response to corneal epithelial injury. Exp. Eye Res. 2020;197:108063. doi: 10.1016/j.exer.2020.108063. [DOI] [PubMed] [Google Scholar]
- 37.Cotsarelis G., Cheng S.-Z., Dong G., Sun T.-T., Lavker R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 38.Amitai-Lange A., Altshuler A., Bubley J., Dbayat N., Tiosano B., Shalom-Feuerstein R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells. 2015;33:230–239. doi: 10.1002/stem.1840. [DOI] [PubMed] [Google Scholar]
- 39.Park M., Richardson A., Pandzic E., Lobo E.P., Whan R., Watson S.L., Lyons J.G., Wakefield D., Di Girolamo N. Visualizing the contribution of keratin-14+ limbal epithelial precursors in corneal wound healing. Stem Cell Rep. 2019;12:14–28. doi: 10.1016/j.stemcr.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elhusseiny A.M., Soleimani M., Eleiwa T.K., ElSheikh R.H., Frank C.R., Naderan M., Yazdanpanah G., Rosenblatt M.I., Djalilian A.R. Current and Emerging Therapies for Limbal Stem Cell Deficiency. Stem Cells Transl. Med. 2022;11:259–268. doi: 10.1093/stcltm/szab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonnet C., Roberts J.S., Deng S.X. Limbal stem cell diseases. Exp. Eye Res. 2021;205:108437. doi: 10.1016/j.exer.2021.108437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabatabaei S.A., Soleimani M., Mirshahi R., Zandian M., Ghasemi H., Hashemian M.N., Ghomi Z. Selective localized tenonplasty for corneal burns based on the findings of ocular surface fluorescein angiography. Cornea. 2017;36:1014–1017. doi: 10.1097/ICO.0000000000001256. [DOI] [PubMed] [Google Scholar]
- 43.Barbaro V., Ferrari S., Fasolo A., Pedrotti E., Marchini G., Sbabo A., Nettis N., Ponzin D., Di Iorio E. Evaluation of ocular surface disorders: A new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br. J. Ophthalmol. 2010;94:926–932. doi: 10.1136/bjo.2009.164152. [DOI] [PubMed] [Google Scholar]
- 44.Garcia I., Etxebarria J., Boto-de-Los-Bueis A., Díaz-Valle D., Rivas L., Martínez-Soroa I., Saenz N., López C., Del-Hierro-Zarzuelo A., Méndez R. Comparative study of limbal stem cell deficiency diagnosis methods: Detection of MUC5AC mRNA and goblet cells in corneal epithelium. Ophthalmology. 2012;119:923–929. doi: 10.1016/j.ophtha.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Moore J.E., McMullen T.C., Campbell I.L., Rohan R., Kaji Y., Afshari N.A., Usui T., Archer D.B., Adamis A.P. The inflammatory milieu associated with conjunctivalized cornea and its alteration with IL-1 RA gene therapy. Investig. Ophthalmol. Vis. Sci. 2002;43:2905–2915. [PubMed] [Google Scholar]
- 46.Le Q., Chauhan T., Yung M., Tseng C.-H., Deng S.X. Outcomes of limbal stem cell transplant: A meta-analysis. JAMA Ophthalmol. 2020;138:660–670. doi: 10.1001/jamaophthalmol.2020.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Buenaga R., Aiello F., Zaher S.S., Grixti A., Ahmad S. Twenty years of limbal epithelial therapy: An update on managing limbal stem cell deficiency. BMJ Open Ophthalmol. 2018;3:e000164. doi: 10.1136/bmjophth-2018-000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura T., Inatomi T., Sotozono C., Amemiya T., Kanamura N., Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004;88:1280–1284. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keivyon K.R., Tseng S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–723. doi: 10.1016/S0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 50.Shanbhag S.S., Nikpoor N., Donthineni P.R., Singh V., Chodosh J., Basu S. Autologous limbal stem cell transplantation: A systematic review of clinical outcomes with different surgical techniques. Br. J. Ophthalmol. 2020;104:247–253. doi: 10.1136/bjophthalmol-2019-314081. [DOI] [PubMed] [Google Scholar]
- 51.Sangwan V.S., Basu S., MacNeil S., Balasubramanian D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012;96:931–934. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 52.Basu S., Sureka S.P., Shanbhag S.S., Kethiri A.R., Singh V., Sangwan V.S. Simple limbal epithelial transplantation: Long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology. 2016;123:1000–1010. doi: 10.1016/j.ophtha.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 53.Cheung A.Y., Holland E.J. Keratolimbal allograft. Curr. Opin. Ophthalmol. 2017;28:377–381. doi: 10.1097/ICU.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 54.Daya S.M., Ilari F.L. Living related conjunctival limbal allograft for the treatment of stem cell deficiency. Ophthalmology. 2001;108:126–133. doi: 10.1016/S0161-6420(00)00475-9. [DOI] [PubMed] [Google Scholar]
- 55.Shanbhag S.S., Saeed H.N., Paschalis E.I., Chodosh J. Keratolimbal allograft for limbal stem cell deficiency after severe corneal chemical injury: A systematic review. Br. J. Ophthalmol. 2018;102:1114–1121. doi: 10.1136/bjophthalmol-2017-311249. [DOI] [PubMed] [Google Scholar]
- 56.Solomon A., Ellies P., Anderson D.F., Touhami A., Grueterich M., Espana E.M., Ti S.-E., Goto E., Feuer W.J., Tseng S.C. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109:1159–1166. doi: 10.1016/S0161-6420(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 57.Espana E., Di Pascuale M., Grueterich M., Solomon A., Tseng S. Keratolimbal allograft in corneal reconstruction. Eye. 2004;18:406–417. doi: 10.1038/sj.eye.6700670. [DOI] [PubMed] [Google Scholar]
- 58.Tsubota K., Shimmura S., Shinozaki N., Holland E.J., Shimazaki J. Clinical application of living-related conjunctival-limbal allograft. Am. J. Ophthalmol. 2002;133:134–135. doi: 10.1016/S0002-9394(01)01208-9. [DOI] [PubMed] [Google Scholar]
- 59.Pellegrini G., Traverso C.E., Franzi A.T., Zingirian M., Cancedda R., De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 60.Baradaran-Rafii A., Eslani M., Jamali H., Karimian F., Tailor U.A., Djalilian A.R. Postoperative complications of conjunctival limbal autograft surgery. Cornea. 2012;31:893–899. doi: 10.1097/ICO.0b013e31823f095d. [DOI] [PubMed] [Google Scholar]
- 61.Singh V., Tiwari A., Kethiri A.R., Sangwan V.S. Current perspectives of limbal-derived stem cells and its application in ocular surface regeneration and limbal stem cell transplantation. Stem Cells Transl. Med. 2021;10:1121–1128. doi: 10.1002/sctm.20-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daya S.M. Conjunctival–limbal autograft. Curr. Opin. Ophthalmol. 2017;28:370–376. doi: 10.1097/ICU.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 63.Yin J., Jurkunas U. Seminars in Ophthalmology. Taylor & Francis; Oxford, UK: 2018. Limbal stem cell transplantation and complications; pp. 134–141. [DOI] [PubMed] [Google Scholar]
- 64.Basu S., Ali H., Sangwan V.S. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am. J. Ophthalmol. 2012;153:643–650.e642. doi: 10.1016/j.ajo.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 65.Rama P., Matuska S., Paganoni G., Spinelli A., De Luca M., Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 66.Vazirani J., Ali M.H., Sharma N., Gupta N., Mittal V., Atallah M., Amescua G., Chowdhury T., Abdala-Figuerola A., Ramirez-Miranda A. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: Multicentre results. Br. J. Ophthalmol. 2016;100:1416–1420. doi: 10.1136/bjophthalmol-2015-307348. [DOI] [PubMed] [Google Scholar]
- 67.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E., Nagai S., Kikuchi A., Maeda N., Watanabe H. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 68.Cabral J.V., Jackson C.J., Utheim T.P., Jirsova K. Ex vivo cultivated oral mucosal epithelial cell transplantation for limbal stem cell deficiency: A review. Stem Cell Res. Ther. 2020;11:301. doi: 10.1186/s13287-020-01783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han E.S., Wee W.R., Lee J.H., Kim M.K. Long-term outcome and prognostic factor analysis for keratolimbal allografts. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011;249:1697–1704. doi: 10.1007/s00417-011-1760-3. [DOI] [PubMed] [Google Scholar]
- 70.Baradaran-Rafii A., Eslani M., Djalillian A.R. Complications of keratolimbal allograft surgery. Cornea. 2013;32:561–566. doi: 10.1097/ICO.0b013e31826215eb. [DOI] [PubMed] [Google Scholar]
- 71.Liang L., Sheha H., Li J., Tseng S. Limbal stem cell transplantation: New progresses and challenges. Eye. 2009;23:1946–1953. doi: 10.1038/eye.2008.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J., Tseng S. Corneal epithelial wound healing in partial limbal deficiency. Investig. Ophthalmol. Vis. Sci. 1990;31:1301–1314. [PubMed] [Google Scholar]
- 73.Chen J., Tseng S. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Investig. Ophthalmol. Vis. Sci. 1991;32:2219–2233. [PubMed] [Google Scholar]
- 74.Moldovan S., Borderie V., Baudrimont M., Laroche L. Treatment of unilateral limbal stem cell deficiency syndrome by limbal autograft. J. Fr. D’ophtalmologie. 1999;22:302–309. [PubMed] [Google Scholar]
- 75.Rao S.K., Rajagopal R., Sitalakshmi G., Padmanabhan P. Limbal autografting: Comparison of results in the acute and chronic phases of ocular surface burns. Cornea. 1999;18:164–171. doi: 10.1097/00003226-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Atallah M.R., Palioura S., Perez V.L., Amescua G. Limbal stem cell transplantation: Current perspectives. Clin. Ophthalmol. (Auckl. NZ) 2016;10:593. doi: 10.2147/OPTH.S83676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghareeb A.E., Lako M., Figueiredo F.C. Recent advances in stem cell therapy for limbal stem cell deficiency: A narrative review. Ophthalmol. Ther. 2020;9:809–831. doi: 10.1007/s40123-020-00305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shortt A.J., Secker G.A., Rajan M.S., Meligonis G., Dart J.K., Tuft S.J., Daniels J.T. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology. 2008;115:1989–1997. doi: 10.1016/j.ophtha.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 79.Behaegel J., Zakaria N., Tassignon M.-J., Leysen I., Bock F., Koppen C., Dhubhghaill S.N. Short-and long-term results of xenogeneic-free cultivated autologous and allogeneic limbal epithelial stem cell transplantations. Cornea. 2019;38:1543. doi: 10.1097/ICO.0000000000002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borderie V.M., Ghoubay D., Georgeon C., Borderie M., Sousa C., Legendre A., Rouard H. Long-term results of cultured limbal stem cell versus limbal tissue transplantation in stage III limbal deficiency. Stem Cells Transl. Med. 2019;8:1230–1241. doi: 10.1002/sctm.19-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pedrotti E., Passilongo M., Fasolo A., Nubile M., Parisi G., Mastropasqua R., Ficial S., Bertolin M., Di Iorio E., Ponzin D. In vivo confocal microscopy 1 year after autologous cultured limbal stem cell grafts. Ophthalmology. 2015;122:1660–1668. doi: 10.1016/j.ophtha.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Mishan M.A., Yaseri M., Baradaran-Rafii A., Kanavi M.R. Systematic review and meta-analysis investigating autograft versus allograft cultivated limbal epithelial transplantation in limbal stem cell deficiency. Int. Ophthalmol. 2019;39:2685–2696. doi: 10.1007/s10792-019-01092-x. [DOI] [PubMed] [Google Scholar]
- 83.Kolli S., Ahmad S., Mudhar H.S., Meeny A., Lako M., Figueiredo F.C. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells. 2014;32:2135–2146. doi: 10.1002/stem.1694. [DOI] [PubMed] [Google Scholar]
- 84.Ilmarinen T., Laine J., Juuti-Uusitalo K., Numminen J., Seppänen-Suuronen R., Uusitalo H., Skottman H. Towards a defined, serum-and feeder-free culture of stratified human oral mucosal epithelium for ocular surface reconstruction. Acta Ophthalmol. 2013;91:744–750. doi: 10.1111/j.1755-3768.2012.02523.x. [DOI] [PubMed] [Google Scholar]
- 85.Ricardo J.R.S., Cristovam P.C., Pedro Filho A., Farias C.C., de Araujo A.L., Loureiro R.R., Covre J.L., de Barros J.N., Barreiro T.P., dos Santos M.S. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea. 2013;32:221–228. doi: 10.1097/ICO.0b013e31825034be. [DOI] [PubMed] [Google Scholar]
- 86.Inoue K., Aoi N., Sato T., Yamauchi Y., Suga H., Eto H., Kato H., Araki J., Yoshimura K. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab. Investig. 2009;89:844–856. doi: 10.1038/labinvest.2009.48. [DOI] [PubMed] [Google Scholar]
- 87.Blazejewska E.A., Schlötzer-Schrehardt U., Zenkel M., Bachmann B., Chankiewitz E., Jacobi C., Kruse F.E. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27:642–652. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayashi R., Ishikawa Y., Sasamoto Y., Katori R., Nomura N., Ichikawa T., Araki S., Soma T., Kawasaki S., Sekiguchi K. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature. 2016;531:376–380. doi: 10.1038/nature17000. [DOI] [PubMed] [Google Scholar]
- 89.Hongisto H., Vattulainen M., Ilmarinen T., Mikhailova A., Skottman H. Efficient and scalable directed differentiation of clinically compatible corneal limbal epithelial stem cells from human pluripotent stem cells. J. Vis. Exp. 2018;140:e58279. doi: 10.3791/58279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Japan Team Proves iPS-Based Cornea Transplant Safe in World-1st Trial. [(accessed on 13 December 2022)]. Available online: https://english.kyodonews.net/news/2022/04/c8af6b7913b2-japan-team-proves-ips-based-cornea-transplant-safe-in-world-1st-trial.html.
- 91.Gomes J.Á.P., Monteiro B.G., Melo G.B., Smith R.L., da Silva M.C.P., Lizier N.F., Kerkis A., Cerruti H., Kerkis I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Investig. Ophthalmol. Vis. Sci. 2010;51:1408–1414. doi: 10.1167/iovs.09-4029. [DOI] [PubMed] [Google Scholar]
- 92.Monteiro B., Serafim R., Melo G., Silva M., Lizier N., Maranduba C., Smith R., Kerkis A., Cerruti H., Gomes J. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009;42:587–594. doi: 10.1111/j.1365-2184.2009.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reza H.M., Ng B.-Y., Gimeno F.L., Phan T.T., Ang L.P.-K. Umbilical cord lining stem cells as a novel and promising source for ocular surface regeneration. Stem Cell Rev. Rep. 2011;7:935–947. doi: 10.1007/s12015-011-9245-7. [DOI] [PubMed] [Google Scholar]
- 94.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 95.Hanson C., Hardarson T., Ellerström C., Nordberg M., Caisander G., Rao M., Hyllner J., Stenevi U. Transplantation of human embryonic stem cells onto a partially wounded human cornea in vitro. Acta Ophthalmol. 2013;91:127–130. doi: 10.1111/j.1755-3768.2011.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu J., Zhang K., Sun Y., Gao X., Li Y., Chen Z., Wu X. Reconstruction of functional ocular surface by acellular porcine cornea matrix scaffold and limbal stem cells derived from human embryonic stem cells. Tissue Eng. Part A. 2013;19:2412–2425. doi: 10.1089/ten.tea.2013.0097. [DOI] [PubMed] [Google Scholar]
- 97.da Mata Martins T.M., da Silva Cunha P., Rodrigues M.A., de Carvalho J.L., de Souza J.E., de Carvalho Oliveira J.A., Gomes D.A., de Goes A.M. Epithelial basement membrane of human decellularized cornea as a suitable substrate for differentiation of embryonic stem cells into corneal epithelial-like cells. Mater. Sci. Eng. C. 2020;116:111215. doi: 10.1016/j.msec.2020.111215. [DOI] [PubMed] [Google Scholar]
- 98.Ahmad S., Stewart R., Yung S., Kolli S., Armstrong L., Stojkovic M., Figueiredo F., Lako M. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25:1145–1155. doi: 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- 99.Zhang C., Du L., Pang K., Wu X. Differentiation of human embryonic stem cells into corneal epithelial progenitor cells under defined conditions. PLoS ONE. 2017;12:e0183303. doi: 10.1371/journal.pone.0183303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He Y.-G., Alizadeh H., Kinoshita K., McCulley J.P. Experimental transplantation of cultured human limbal and amniotic epithelial cells onto the corneal surface. Cornea. 1999;18:570–579. doi: 10.1097/00003226-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 101.Fatimah S.S., Ng S.L., Chua K.H., Hayati A.R., Tan A.E., Tan G.C. Value of human amniotic epithelial cells in tissue engineering for cornea. Hum. Cell. 2010;23:141–151. doi: 10.1111/j.1749-0774.2010.00096.x. [DOI] [PubMed] [Google Scholar]
- 102.Hernáez-Moya R., González S., Urkaregi A., Pijoan J.I., Deng S.X., Andollo N. Expansion of Human Limbal Epithelial Stem/Progenitor Cells Using Different Human Sera: A Multivariate Statistical Analysis. Int. J. Mol. Sci. 2020;21:6132. doi: 10.3390/ijms21176132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kolli S., Ahmad S., Lako M., Figueiredo F. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells. 2010;28:597–610. doi: 10.1002/stem.276. [DOI] [PubMed] [Google Scholar]
- 104.Brejchova K., Trosan P., Studeny P., Skalicka P., Utheim T.P., Bednar J., Jirsova K. Characterization and comparison of human limbal explant cultures grown under defined and xeno-free conditions. Exp. Eye Res. 2018;176:20–28. doi: 10.1016/j.exer.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 105.Bobba S., Chow S., Watson S., Di Girolamo N. Clinical outcomes of xeno-free expansion and transplantation of autologous ocular surface epithelial stem cells via contact lens delivery: A prospective case series. Stem Cell Res. Ther. 2015;6:23. doi: 10.1186/s13287-015-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.González S., Deng S.X. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Exp. Eye Res. 2013;116:169–176. doi: 10.1016/j.exer.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mei H., González S., Nakatsu M.N., Baclagon E.R., Chen F.V., Deng S.X. Human adipose-derived stem cells support the growth of limbal stem/progenitor cells. PLoS ONE. 2017;12:e0186238. doi: 10.1371/journal.pone.0186238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakatsu M.N., González S., Mei H., Deng S.X. Human limbal mesenchymal cells support the growth of human corneal epithelial stem/progenitor cells. Investig. Ophthalmol. Vis. Sci. 2014;55:6953–6959. doi: 10.1167/iovs.14-14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.González S., Mei H., Nakatsu M.N., Baclagon E.R., Deng S.X. A 3D culture system enhances the ability of human bone marrow stromal cells to support the growth of limbal stem/progenitor cells. Stem Cell Res. 2016;16:358–364. doi: 10.1016/j.scr.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Djalilian A.R., Mahesh S.P., Koch C.A., Nussenblatt R.B., Shen D., Zhuang Z., Holland E.J., Chan C.-C. Survival of donor epithelial cells after limbal stem cell transplantation. Investig. Ophthalmol. Vis. Sci. 2005;46:803–807. doi: 10.1167/iovs.04-0575. [DOI] [PubMed] [Google Scholar]
- 111.Niknejad H., Yazdanpanah G., Ahmadiani A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell Tissue Res. 2016;363:599–608. doi: 10.1007/s00441-016-2364-3. [DOI] [PubMed] [Google Scholar]
- 112.Ma D.H.-K., Chen H.-C., Ma K.S.-K., Lai J.-Y., Yang U., Yeh L.-K., Hsueh Y.-J., Chu W.-K., Lai C.-H., Chen J.-K. Preservation of human limbal epithelial progenitor cells on carbodiimide cross-linked amniotic membrane via integrin-linked kinase-mediated Wnt activation. Acta Biomater. 2016;31:144–155. doi: 10.1016/j.actbio.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 113.Tsai R.J.-F., Tsai R.Y.-N. From stem cell niche environments to engineering of corneal epithelium tissue. Jpn. J. Ophthalmol. 2014;58:111–119. doi: 10.1007/s10384-014-0306-8. [DOI] [PubMed] [Google Scholar]
- 114.Zhou Q., Liu X.-Y., Ruan Y.-X., Wang L., Jiang M.-M., Wu J., Chen J. Construction of corneal epithelium with human amniotic epithelial cells and repair of limbal deficiency in rabbit models. Hum. Cell. 2015;28:22–36. doi: 10.1007/s13577-014-0099-6. [DOI] [PubMed] [Google Scholar]
- 115.Konomi K., Satake Y., Shimmura S., Tsubota K., Shimazaki J. Long-term results of amniotic membrane transplantation for partial limbal deficiency. Cornea. 2013;32:1110–1115. doi: 10.1097/ICO.0b013e31828d06d2. [DOI] [PubMed] [Google Scholar]
- 116.Levis H.J., Daniels J.T. Recreating the human limbal epithelial stem cell niche with bioengineered limbal crypts. Curr. Eye Res. 2016;41:1153–1160. doi: 10.3109/02713683.2015.1095932. [DOI] [PubMed] [Google Scholar]
- 117.Shafiq M.A., Gemeinhart R.A., Yue B.Y., Djalilian A.R. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng. Part C Methods. 2012;18:340–348. doi: 10.1089/ten.tec.2011.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Amin S., Jalilian E., Katz E., Frank C., Yazdanpanah G., Guaiquil V.H., Rosenblatt M.I., Djalilian A.R. The Limbal Niche and Regenerative Strategies. Vision. 2021;5:43. doi: 10.3390/vision5040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi Y., Bikkuzin T., Song Z., Jin X., Jin H., Li X., Zhang H. Comprehensive evaluation of decellularized porcine corneal after clinical transplantation. Xenotransplantation. 2017;24:e12338. doi: 10.1111/xen.12338. [DOI] [PubMed] [Google Scholar]
- 120.Shi W., Xie L. Focus on the clinical application of the first artificial bioengineered cornea in China. [Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2016;52:161–163. doi: 10.3760/cma.j.issn.0412-4081.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 121.Ahearne M., Lynch A.P. Early observation of extracellular matrix-derived hydrogels for corneal stroma regeneration. Tissue Eng. Part C Methods. 2015;21:1059–1069. doi: 10.1089/ten.tec.2015.0008. [DOI] [PubMed] [Google Scholar]
- 122.Lu Y., Yao Q.K., Feng B., Yan C.X., Zhu M.Y., Chen J.Z., Fu W., Fu Y. Characterization of a hydrogel derived from decellularized corneal extracellular matrix. J. Biomater. Tissue Eng. 2015;5:951–960. doi: 10.1166/jbt.2015.1410. [DOI] [Google Scholar]
- 123.Yazdanpanah G., Jiang Y., Rabiee B., Omidi M., Rosenblatt M.I., Shokuhfar T., Pan Y., Naba A., Djalilian A.R. Fabrication, Rheological, and Compositional Characterization of Thermoresponsive Hydrogel from Cornea. Tissue Eng. Part C Methods. 2021;27:307–321. doi: 10.1089/ten.tec.2021.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duan X., Sheardown H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: Mechanical properties and corneal epithelial cell interactions. Biomaterials. 2006;27:4608–4617. doi: 10.1016/j.biomaterials.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 125.Myung D., Farooqui N., Zheng L.L., Koh W., Gupta S., Bakri A., Noolandi J., Cochran J.R., Frank C.W., Ta C.N. Bioactive interpenetrating polymer network hydrogels that support corneal epithelial wound healing. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009;90:70–81. doi: 10.1002/jbm.a.32056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee H.J., Fernandes-Cunha G.M., Na K.S., Hull S.M., Myung D. Bio-orthogonally crosslinked, in situ forming corneal stromal tissue substitute. Adv. Healthc. Mater. 2018;7:1800560. doi: 10.1002/adhm.201800560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kang K.B., Lawrence B.D., Gao X.R., Luo Y., Zhou Q., Liu A., Guaiquil V.H., Rosenblatt M.I. Micro-and nanoscale topographies on silk regulate gene expression of human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2017;58:6388–6398. doi: 10.1167/iovs.17-22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sorkio A., Koch L., Koivusalo L., Deiwick A., Miettinen S., Chichkov B., Skottman H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials. 2018;171:57–71. doi: 10.1016/j.biomaterials.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 129.Dehghani S., Rasoulianboroujeni M., Ghasemi H., Keshel S.H., Nozarian Z., Hashemian M.N., Zarei-Ghanavati M., Latifi G., Ghaffari R., Cui Z. 3D-Printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: An in vitro & in vivo study. Biomaterials. 2018;174:95–112. doi: 10.1016/j.biomaterials.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 130.Nguyen K.N., Bobba S., Richardson A., Park M., Watson S.L., Wakefield D., Di Girolamo N. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater. 2018;65:21–35. doi: 10.1016/j.actbio.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 131.Glowacki J., Mizuno S. Collagen scaffolds for tissue engineering. Biopolym. Orig. Res. Biomol. 2008;89:338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 132.Ma A., Zhao B., Bentley A.J., Brahma A., MacNeil S., Martin F.L., Rimmer S., Fullwood N.J. Corneal epithelialisation on surface-modified hydrogel implants. J. Mater. Sci. Mater. Med. 2011;22:663–670. doi: 10.1007/s10856-011-4244-4. [DOI] [PubMed] [Google Scholar]
- 133.Myung D., Koh W., Bakri A., Zhang F., Marshall A., Ko J., Noolandi J., Carrasco M., Cochran J.R., Frank C.W. Design and fabrication of an artificial cornea based on a photolithographically patterned hydrogel construct. Biomed. Microdevices. 2007;9:911–922. doi: 10.1007/s10544-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 134.Radosevich M., Goubran H., Burnouf T. Fibrin sealant: Scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997;72:133–143. doi: 10.1159/000461980. [DOI] [PubMed] [Google Scholar]
- 135.Atrah H. Fibrin Glue. Volume 308. British Medical Journal Publishing Group; London, UK: 1994. pp. 933–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Azari A.A., Rapuano C.J. Autologous serum eye drops for the treatment of ocular surface disease. Eye Contact Lens. 2015;41:133–140. doi: 10.1097/ICL.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 137.Kim K.M., Shin Y.-T., Kim H.K. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn. J. Ophthalmol. 2012;56:544–550. doi: 10.1007/s10384-012-0175-y. [DOI] [PubMed] [Google Scholar]
- 138.Baradaran-Rafii A., Asl N.S., Ebrahimi M., Jabbehdari S., Bamdad S., Roshandel D., Eslani M., Momeni M. The role of amniotic membrane extract eye drop (AMEED) in in vivo cultivation of limbal stem cells. Ocul. Surf. 2018;16:146–153. doi: 10.1016/j.jtos.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 139.Chen S.-Y., Han B., Zhu Y.-T., Mahabole M., Huang J., Beebe D.C., Tseng S.C. HC-HA/PTX3 purified from amniotic membrane promotes BMP signaling in limbal niche cells to maintain quiescence of limbal epithelial progenitor/stem cells. Stem Cells. 2015;33:3341–3355. doi: 10.1002/stem.2091. [DOI] [PubMed] [Google Scholar]
- 140.Ho T.-C., Chen S.-L., Wu J.-Y., Ho M.-Y., Chen L.-J., Hsieh J.-W., Cheng H.-C., Tsao Y.-P. PEDF promotes self-renewal of limbal stem cell and accelerates corneal epithelial wound healing. Stem Cells. 2013;31:1775–1784. doi: 10.1002/stem.1393. [DOI] [PubMed] [Google Scholar]
- 141.Fernandes-Cunha G.M., Na K.-S., Putra I., Lee H.J., Hull S., Cheng Y.-C., Blanco I.J., Eslani M., Djalilian A.R., Myung D. Corneal wound healing effects of mesenchymal stem cell secretome delivered within a viscoelastic gel carrier. Stem Cells Transl. Med. 2019;8:478–489. doi: 10.1002/sctm.18-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tirassa P., Rosso P., Iannitelli A. Neurotrophic Factors. Springer; Berlin/Heidelberg, Germany: 2018. Ocular nerve growth factor (NGF) and NGF eye drop application as paradigms to investigate NGF neuroprotective and reparative actions; pp. 19–38. [DOI] [PubMed] [Google Scholar]
- 143.Samaeekia R., Eslani M., Putra I., Rabiee B., Shen X., Park Y.J., Hematti P., Djalilian A.R. Role of Human Corneal Mesenchymal Stromal Cell-derived Exosomes in Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018;59:3454. doi: 10.1167/iovs.18-24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harting M.T., Srivastava A.K., Zhaorigetu S., Bair H., Prabhakara K.S., Toledano Furman N.E., Vykoukal J.V., Ruppert K.A., Cox C.S., Jr., Olson S.D. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36:79–90. doi: 10.1002/stem.2730. [DOI] [PubMed] [Google Scholar]
- 145.Shtam T.A., Kovalev R.A., Varfolomeeva E.Y., Makarov E.M., Kil Y.V., Filatov M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eslani M., Putra I., Shen X., Hamouie J., Afsharkhamseh N., Besharat S., Rosenblatt M.I., Dana R., Hematti P., Djalilian A.R. Corneal mesenchymal stromal cells are directly antiangiogenic via PEDF and sFLT-1. Investig. Ophthalmol. Vis. Sci. 2017;58:5507–5517. doi: 10.1167/iovs.17-22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic transplants of bone marrow. Transplantation. 1968;6:230–247. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 148.Neofytou E., Deuse T., Beygui R.E., Schrepfer S. Mesenchymal stromal cell therapy: Different sources exhibit different immunobiological properties. Transplantation. 2015;99:1113–1118. doi: 10.1097/TP.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 149.McCorry M.C., Puetzer J.L., Bonassar L.J. Characterization of mesenchymal stem cells and fibrochondrocytes in three-dimensional co-culture: Analysis of cell shape, matrix production, and mechanical performance. Stem Cell Res. Ther. 2016;7:39. doi: 10.1186/s13287-016-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Amirjamshidi H., Milani B., Sagha H., Movahedan A., Shafiq M., Lavker R., Yue B., Djalilian A. Limbal fibroblast conditioned media: A non-invasive treatment for limbal stem cell deficiency. Mol. Vis. 2011;17:658. [PMC free article] [PubMed] [Google Scholar]
- 151.Ye J., Yao K., Kim J. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: Engraftment and involvement in wound healing. Eye. 2006;20:482–490. doi: 10.1038/sj.eye.6701913. [DOI] [PubMed] [Google Scholar]
- 152.Cejkova J., Trosan P., Cejka C., Lencova A., Zajicova A., Javorkova E., Kubinova S., Sykova E., Holan V. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp. Eye Res. 2013;116:312–323. doi: 10.1016/j.exer.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 153.Lee M.J., Ko A.Y., Ko J.H., Lee H.J., Kim M.K., Wee W.R., Khwarg S.I., Oh J.Y. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol. Ther. 2015;23:139–146. doi: 10.1038/mt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Holan V., Trosan P., Cejka C., Javorkova E., Zajicova A., Hermankova B., Chudickova M., Cejkova J. A comparative study of the therapeutic potential of mesenchymal stem cells and limbal epithelial stem cells for ocular surface reconstruction. Stem Cells Transl. Med. 2015;4:1052–1063. doi: 10.5966/sctm.2015-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Espandar L., Caldwell D., Watson R., Blanco-Mezquita T., Zhang S., Bunnell B. Application of adipose-derived stem cells on scleral contact lens carrier in an animal model of severe acute alkaline burn. Eye Contact Lens. 2014;40:243. doi: 10.1097/ICL.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bu P., Vin A.P., Sethupathi P., Ambrecht L.A., Zhai Y., Nikolic N., Qiao L., Bouchard C.S. Effects of activated omental cells on rat limbal corneal alkali injury. Exp. Eye Res. 2014;121:143–146. doi: 10.1016/j.exer.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 157.Acar U., Pinarli F.A., Acar D.E., Beyazyildiz E., Sobaci G., Ozgermen B.B., Sonmez A.A., Delibasi T. Effect of allogeneic limbal mesenchymal stem cell therapy in corneal healing: Role of administration route. Ophthalmic Res. 2015;53:82–89. doi: 10.1159/000368659. [DOI] [PubMed] [Google Scholar]
- 158.Zeng W., Li Y., Zeng G., Yang B., Zhu Y. Transplantation with cultured stem cells derived from the human amniotic membrane for corneal alkali burns: An experimental study. Ann. Clin. Lab. Sci. 2014;44:73–81. [PubMed] [Google Scholar]
- 159.Lan Y., Kodati S., Lee H.S., Omoto M., Jin Y., Chauhan S.K. Kinetics and function of mesenchymal stem cells in corneal injury. Investig. Ophthalmol. Vis. Sci. 2012;53:3638–3644. doi: 10.1167/iovs.11-9311. [DOI] [PubMed] [Google Scholar]
- 160.Wen L., Zhu M., Madigan M.C., You J., King N.J., Billson F.A., McClellan K., Sutton G., Petsoglou C. Immunomodulatory effects of bone marrow-derived mesenchymal stem cells on pro-inflammatory cytokine-stimulated human corneal epithelial cells. PLoS ONE. 2014;9:e101841. doi: 10.1371/journal.pone.0101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ke Y., Wu Y., Cui X., Liu X., Yu M., Yang C., Li X. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS ONE. 2015;10:e0119725. doi: 10.1371/journal.pone.0119725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ma Y., Xu Y., Xiao Z., Yang W., Zhang C., Song E., Du Y., Li L. Reconstruction of chemically burned rat corneal surface by bone marrow–derived human mesenchymal stem cells. Stem Cells. 2006;24:315–321. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- 163.Cejka Č., Cejkova J., Trosan P., Zajicova A., Sykova E., Holan V. Transfer of mesenchymal stem cells and cyclosporine A on alkali-injured rabbit cornea using nanofiber scaffolds strongly reduces corneal neovascularization and scar formation. Histol. Histopathol. 2016;31:969–980. doi: 10.14670/HH-11-724. [DOI] [PubMed] [Google Scholar]
- 164.Eslani M., Putra I., Hamouie J., Shen X., Afsharkhamseh N., Hematti P., Djalilian A.R. The Effect of Corneal-Derived Versus Bone Marrow-Derived Mesenchymal Stromal Cell Secretome on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018;59:4421. doi: 10.1167/iovs.18-24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Polisetti N., Agarwal P., Khan I., Kondaiah P., Sangwan V.S., Vemuganti G.K. Gene expression profile of epithelial cells and mesenchymal cells derived from limbal explant culture. Mol. Vis. 2010;16:1227. [PMC free article] [PubMed] [Google Scholar]
- 166.Calonge M., Pérez I., Galindo S., Nieto-Miguel T., López-Paniagua M., Fernández I., Alberca M., García-Sancho J., Sánchez A., Herreras J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2019;206:18–40. doi: 10.1016/j.trsl.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 167.Galindo S., de la Mata A., López-Paniagua M., Herreras J.M., Pérez I., Calonge M., Nieto-Miguel T. Subconjunctival injection of mesenchymal stem cells for corneal failure due to limbal stem cell deficiency: State of the art. Stem Cell Res. Ther. 2021;12:60. doi: 10.1186/s13287-020-02129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mittal S.K., Omoto M., Amouzegar A., Sahu A., Rezazadeh A., Katikireddy K.R., Shah D.I., Sahu S.K., Chauhan S.K. Restoration of corneal transparency by mesenchymal stem cells. Stem Cell Rep. 2016;7:583–590. doi: 10.1016/j.stemcr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Safety of Locally Delivered Allogeneic Mesenchymal Stromal Cells. [(accessed on 13 December 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04626583.
- 170.Margolis M., Jung R., Tu G., An S., Dana R., Jeng B.H., Basu S., Rosenblatt M., Hematti P., Mahmud N. Phase I Study of the Safety of Locally Delivered Allogeneic Mesenchymal Stem Cells for Promoting Corneal Repair: Early Results. Investig. Ophthalmol. Vis. Sci. 2022;63:91-A0189. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.