Abstract
Background:
Numerous studies have explored the correlation of periodontal disease (PD) with the risk of lung cancers, but the findings were inconsistent. Therefore, we did a meta-analysis to ascertain the correlation of PD with the risk of incident lung cancer.
Methods:
The authors searched relevant studies in databases (PubMed, Web of Science, Scopus, Embase, and MEDLINE) till November 2020. We registered the study at the International database of Prospectively Registered Systemic Reviews under the CRD42020198119. The summary relative risk (RR) along with a 95% confidence interval (CI) was calculated using fixed-effects models.
Results:
Twelve studies were included in the qualitative synthesis. The pooled analysis revealed that PD was significantly associated with an increased risk of lung cancer (RR 1.71; 95%CI 1.61–1.81; P < 0.01). Subgroup analysis was performed based on gender distribution, geographic location, and type of studies.
Conclusion:
From this current evidence, PD is a potential risk factor for the development of lung cancer. The risk for incidence of lung cancer is surged twice in the patients with PD, even though age and smoking are controlled in the studies.
Keywords: Lung cancer, meta-analysis, periodontitis, systemic review
Introduction
The mouth is a mirror of the body. The most prevalent oral diseases, dental caries, and periodontal diseases (PD) are of microbial origin. More than 700 bacterial species in PD are accountable for different grades of pathogenesis toward the host. Aforesaid literature betrayed the association between PD and diabetes, respiratory diseases, cardiovascular diseases, mental disorders, and pregnancy complications.[1,2,3,4,5] Moreover, concerning evidence revealed an association between periodontitis and oral cancer, cancer of the head and neck, esophagus cancer, lung, pancreas, colorectal, breast, and hematopoietic cancer.[6,7,8,9,10,11,12,13] In the United States, the prevalence of periodontitis is 47.2% aged 30 years and older and 70.1% aged 65% and older.[12]
Lung cancer is a terminal illness, with a 5-year mortality rate of almost 89%, accounting for approximately 25% of all cancer deaths.[14] The American Cancer Society estimates that the risk of developing lung cancer in men is 1 in 15, whereas in women as 1 in 17 with an estimated incidence rate of 228,820 cancer cases and 135,720 deaths in 2020. Although tobacco is the foremost conviction, 53% of lung cancer cases in women worldwide are not ascribable to smoking, advocating that other factors may independently increase or modify the risk for lung cancer.[15,16] Many cohort and case–control studies were executed since the first association between PD and lung cancer was offered and suggested genetic, microbial, and inflammatory links of pathogenesis between these two. Such connections could be due to several mechanisms: (1). Systemic spread of oral bacteria causes damage to distant organ sites, (2). Increase in systemic inflammatory mediators that initiate and promote tumorigenesis, (3). A change in host immunity by autoimmune response produced during PD.[15,16,17,18] However, no study was able to explain the comprehensive mechanism of interrelation between PD and lung cancer.
Focusing on initial detection and anticipation plans to bring down cancer burden are condemnatory, and oral health seems like a field that may have the future to contribute to cancer deterrence. To assess the role of PD on cancer burden, it is essential to determine whether the association detected in population studies is casual. A meta-analysis performed by Zeng et al., including five cohort studies until 2014, concluded that individuals with PD were associated with a 1.24-fold increased risk of developing lung cancer.[9] Pooled results of the previous studies reinforced the hypothesis of an association between periodontitis and all types of cancer. However, the low methodological quality prohibited us from the punctual implementation of the judgment.[12,17,18] Even though positive relatedness was found, the validity of the result was limited by the inclusion of high-quality and low-quality studies in a single analysis since low-quality studies showed associations. If the corroboration is adequately strong, endorsements can be made to promote prevention through refinements in the dental coverage and enhance awareness of the risk. Therefore, after more recent studies, a more comprehensive meta-analysis is the lifeblood to provide solid authentication of the association.
Methods
Literature and search strategy
The protocol was designed according to preferred reporting items for systemic review and meta-analysis for protocols and recommendations, and registered at the International database of Prospectively Registered Systemic Reviews under the CRD42020198119.[19]
The targeted review question was: Is PD positively associated with lung cancer? The emphasized question obeyed PECO criteria: The population (P) was patients of any age, the Exposure (E) was the presence of periodontitis, the Comparison (C) was an absence of PD, and the Outcome (O) was patients having lung cancer.
Searched methods for the identification of studies
A comprehensive electronic search of the following databases was conducted: PubMed, MEDLINE database through Ovid interface, EMBASE database through Ovid interface, Scopus, and Web of Science between 1952 and 2020. The search was done using keywords along with Boolean operators “OR” and “AND.” The search string from databases was: “Periodontitis” OR “Periodontal disease” OR “Tooth loss” AND “Lung Cancer.” The reference list of all included studies was scanned for relevant review articles and editorial to identify additional pertinent studies. We coped all references by operating reference manager software (EndNote Basics, Clarivate Analytics). The search was completed on November 31, 2020.
Eligibility criteria
The titles, abstracts, full texts, and appraised studies were independently screened based on the following inclusion criteria: (1). Studies on human subjects, (2). Retrospective and prospective cohorts, and nested case–control studies with available full-text articles, (3). Exposure point is PD regardless of the depth and severity of periodontitis, (4). Description of how confounders were controlled in the analysis, precisely age and smoking, (5). Results reported in terms of adjusted risk ratio (RR) and hazards ratio (HR) associated with a 95% confidence level. In the case of divergence in the article eligibility process, a blinded assessor solved the query.
Data extraction
The data were individualistically collected, and the results were compared. The following data were extracted from each arm of the study: Author's name and year of publication, study design and country of origin, population studied, the sample size in exposure and comparison group, outcome numbers in exposure and comparison group, age and gender, duration of follow-up, methods of determining exposure and outcome, RR or HR associated with 95% confidence interval (CI) and confounder factors controlled for the study.
Outcome measures
The primary outcome was lung cancer risk in patients with periodontitis which was the only outcome measured in the present analysis.
Assessment of methodological quality of studies
The quality of studies was evaluated using the Newcastle-Ottawa Scale.[20] This scale encompasses the assessment of three parts: selection (0–4 stars), compatibility (0–2 stars), and outcome (0–3 stars). The high-quality study consists of 3 or 4 stars in the selection, 1 or 2 in comparison, and 2 or 3 stars in the outcome domain.
Statistical analysis
Relative risk (RR) with a 95% CI was used as a measure of association across the studies. The analysis was performed with the aid of R statistical software version 3.5.3 (Revolution Analytics). Heterogeneity among studies was evaluated by the I2 test (ratio of true heterogeneity to total observed variation) and s2 (among-study variance). I2 values higher than 50% indicate moderate heterogeneity. In the presence of heterogeneity, the effect estimates across studies by use of a random effect model were combined.
All 12 cohorts and case–control studies were included in the final analysis. Because of the increased heterogeneity obtained from the pooled data, separate meta-analyses were carried out, including only high-quality and low-quality trials. The potential heterogeneity was explored by subgroup analysis and sensitivity analysis. Sensitivity analysis was conducted by deleting each study at a time and calculating the summary effect size of the remaining research.
Results
Literature retrieved and study characteristics
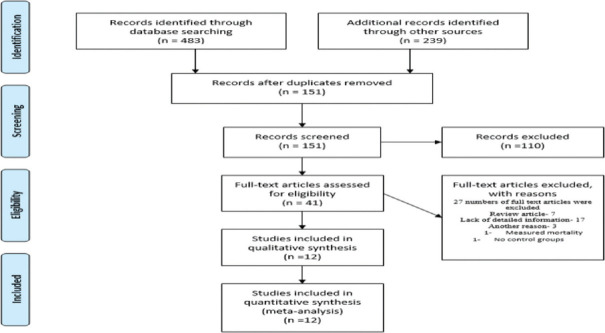
Seven hundred and twenty-two citations were identified from the search in the selected databases. After removing duplicates, the articles were narrowed down to 151 articles. Based on the exclusion criteria, a full text of 41 articles was selected for detailed reading. Evaluating the reference lists in the chosen studies, two articles were included. Twelve studies in total met the eligibility criteria [Figure 1]. The variables with missing values for an adjusting variable were not included in the full model analysis.
Figure 1.
PRISMA flow diagram, PRISMA: preferred reporting items for systemic review and meta-analysis
Table 1 summarizes the detailed characteristics of included studies reporting risk estimates for the correlation between periodontitis and lung cancer.[21,22,23,24,25,26,27,28,29,30,31,32] Eight cohort studies and 1 case–control study of high quality have been reported [Tables 2 and 3]. P < 0.05 was considered statistically significant.
Table 1.
Characteristics of studies on the association of periodontal disease with lung cancer
| Author | Country | Study design | Sample size | Event/total | Assesment method | Population study | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Exposure | No exposure | ||||||||
| Hujoel et al., 2003[21] | USA | Prospective Cohort | 11,328 | 132/4763 | 59/6562 | Oral exam | NHANES I | ||
| Hiraki et al., 2008[22] | JAPAN | Case control study | 15,720 | 235/604 | 674/2123 | Questionnaire | Hospital database | ||
| Michaud et al., 2008[23] | USA | Prospective Cohort | 236/7863 | 442/40,512 | Oral examination | HPFS | |||
| Arora et al., 2010[24] | SWEDEN | Twin cohort study | 15,333 | 14/908 | 175/12,592 | Questionnaire | Swedish Twin registry | ||
| Wen et al., 2014[25] | CHINA | Retrospective cohort study | 148,172 | 243/51,791 Women 88/25,503 Men 155/26,288 |
353/96,375 Women 137/45,583 Men 216/47,522 |
Insurance Claims data ICD 9 | National health insurance program Taiwan | ||
| Mai et al., 2014[26] | USA | Prospective Cohort | 77,485 | 287/19,942 | 467/56,789 | Questionnaire | WHI | ||
| Michaud et al., 2016[27] | USA | Prospective Cohort | 19,933 | 13/1945 | 101/17,988 | Questionnaire | HPFS | ||
| Chrysanthakopoulos, 2016[28] | GREEK | Case control study | 200 | 18/38 | 46/116 | Health questionnaire | Private medical and dental office | ||
| Nwizu et al., 2017[29] | USA | Prospective Cohort | 65,869 | 334/17,103 | 521/48,766 | Self-reporter questionnaire | WHI | ||
| Michaud et al., 2018[30] | USA | Prospective Cohort | 7466 | 192/4923 | 34/2543 | Oral examination | ARIC study | ||
| Tai et al., 2018[31] | TAIWAN | Cohort study | 14,284 | 46/7142 | 21/7142 | Medical records | National health insurance databases | ||
| Yoon et al., 2019[32] | USA | Case control study | 403 | 127/504 | 267/1476 | Intervein questionnaire | SCCS | ||
|
| |||||||||
| Author | Age | Gender | Time period | Follow up | Exposure | Result (95% CI) | Adjustment | ||
|
| |||||||||
| Hujoel et al., 2003[21] | 25-74 | Male/female | 1971-1992 | 10 | PD versus Gingivitis | HR 1.73 (1.01-2.97) | Smoking, alcohol consumption, Vitamin A and Vitamin C intake, age, gender, race, education poverty index and BMI | ||
| Hiraki et al., 2008[22] | 20-80 | Male/female | 2001-2005 | - | OR 1.54 (0.05-2.27) | Age, sex, smoking, alcohol drinking, vegetable and fruit intake, regular exercise, BMI | |||
| Michaud et al., 2008[23] | 40-75 | Male | 1986-2004 | 17.7 | PD versus no PD | HR 1.36 (1.15-1.60) | Race, BMI, physical activity, smoking history, diabetes, region, height, alcohol, Vitamin D and calcium intake | ||
| Arora et al., 2009[24] | 38-77 | Male/female | 1963-2004 | 27 years | PD versus No PD | HR 1.41 (0.81-2.46) | Sex, age, education, smoking status, employment, siblings, diabetes, BMI | ||
| Wen et al., 2014[25] | >20 | Male/female | 1997-2010 | 2 | PD versus gingivitis | HR 1.08 (0.91-1.27) Women HR 1.11 (0.85-1.45) Men HR 1.05 (0.85-1.29) |
Gender, age, diabetes, hypertension, hyperlipidemia | ||
| Mai et al., 2014[26] | 50-79 | Female | 1993 | 5 | PD versus No PD | HR 1.25 (1.06-1.48) | Education, race, BMI, menopausal hormone therapy, recreational physical activity, region of residence, aspirin use, alcohol | ||
| Michaud et al., 2016[27] | 40-75 | Male | 1986-2012 | 8 | PD versus No PD | HR 0.92 (0.49-1.71) | Age, race, alcohol use and physical activity, diabetes, NSAIDs use | ||
| Chrysanthakopoulos, 2016[28] | 45-73 | Male/female | - | Periopocket versus CAL | Pocket depth OR 2.72 (1.05-7.06) | Socioeconomic level, age, gender, smoking status | |||
| Nwizu et al., 2017[29] | 54-86 | Female | 1999-2003 | 8.32 | PD versus no PD | HR 1.31 (1.14-1.51) | Age, race, education, region, family history of cancer, diabetes, physical activity, smoking, alcohol intake, dietary of intake of energy, calcium, Vitamin D, BMI, postmenopausal hormone therapy | ||
| Michaud et al., 2018[30] | 44-66 | Male/female | 1987-2012 | 15 | PD versus no PD versus edentulism | Moderate versus mild HR 1.41 (0.90-2.21) Severe versus mild HR 2.33 (1.51-3.60) | Smoking, age, education, BMI, diabetes, alcohol drinking, sex | ||
| Tai et al., 2019[31] | Any age | Female | 2000-2013 | 13 | PD versus gingivitis | HR 1.90 (1.08-3.35) | Urbanization level, socioeconomic status | ||
| Yoon et al., 2019[32] | 40-79 | Male/female | 2015 | - | PD versus no PD versus tooth loss | OR 1.44 (1.09-1.91) Tooth loss >10 OR 1.64 (1.0-2.69) | BMI, education, household income, COPD, alcohol drinking status and smoking | ||
NHANES I: National Health and Nutrition Examination Survey I, PD: Periodontal disease, HR: Hazard ratio, OR: Odds ratio, HPFS: Health Professional Follow up Study by Harvard University, ICD: International Cancer Disease, BOP: Bleeding on probing, CAL: Clinical attachment Level, WHI: Women’s Health Initiative, ARIC: Atherosclerosis Risk in Communities, SCCS: Southern Community Cohort Study, COPD: Chronic obstructive pulmonary disease, CI: Confidence interval, BMI: Body mass index, NSAIDs: Non-Steroidal Anti-inflammatory drugs
Table 2.
Quality assessment of cohort studies using New Castle Ottawa Scale
| Author | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | The study controls for age, sex and marital status | Study controls for other factors | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | |
| Hujoel et al., 2003[21] | * | * | * | * | * | * | * | * | |
| Michaud et al., 2008[23] | * | * | * | - | * | * | - | * | * |
| Arora et al., 2010[24] | * | * | - | - | * | * | * | * | * |
| Wen et al., 2014[25] | * | * | * | - | * | * | * | * | - |
| Michaud et al., 2016[27] | * | * | * | - | * | * | - | * | * |
| Nwizu et al., 2017[29] | * | * | - | * | * | - | * | * | * |
| Michaud et al., 2018[30] | * | * | * | - | * | * | - | * | * |
| Tai et al., 2019[31] | * | - | * | - | - | * | * | * | * |
*significant and -nonsignificant
Table 3.
Quality assessment of case control studies using New Castle Ottawa Scale
| First author | Selection | Comparability | Exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Case definition is adequate | Consecutive or obviously representative series of cases | Community controls | No history of disease (endpoint) in controls | Study controls for the most important factor | Study controls for any additional factor | Secure record/structured interview blind to case/control status | Same method for ascertainment for cases and controls | Nonresponse rate same for both groups | |
| Hiraki, 2008[22] | - | * | - | * | * | * | - | * | - |
| Mai, 2014[26] | * | * | - | - | - | * | - | * | - |
| Chrysanthakopoulos, 2016[28] | * | * | * | * | * | * | * | * | - |
| Yoon, 2019[32] | - | * | * | * | - | * | - | - | * |
*Significant and -nonsignificant
Overall estimates
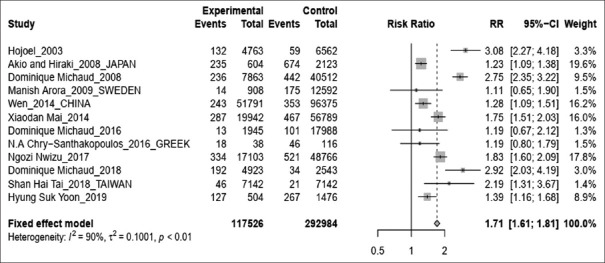
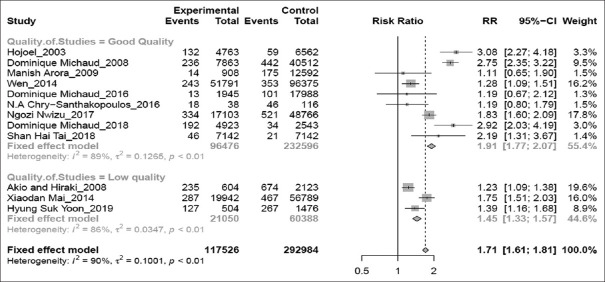
Pooled results showed that subjects with periodontitis have an increased risk of lung cancer by 1.71 times. (RR 1.71; 95%CI 1.61–1.81; P < 0.01) [Figure 2]. Estimates from the high-quality studies indicate the association becomes stronger than the pooled results from low-quality studies (high-quality studies analysis – RR 1.91; 95%CI 1.77–2.07; P < 0.01 vs. low-quality studies meta-analysis – RR1.45; 95%CI 1.33–1.57; P < 0.01) [Figure 3].
Figure 2.
Forest plot for periodontal disease associated with lung risk cancer
Figure 3.
Forest plot of high-quality versus low-quality studies
Subgroup analysis
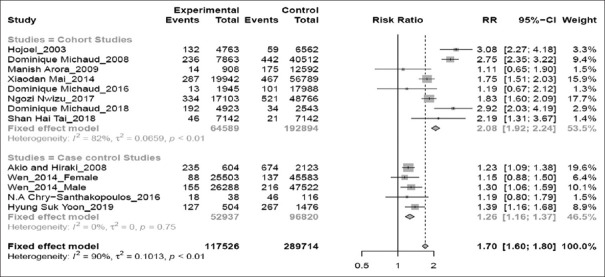
Subgroup analysis according to study design [Figure 4] revealed a significant relation of periodontitis with increased risk of lung cancer in prospective cohorts (RR 2.08; 95%CI 1.92–2.24; P < 0.01) than in case–control studies (RR 1.26; 95% CI 1.16–1.37; P = 0.75).
Figure 4.
Forest plot of cohort versus case–control studies
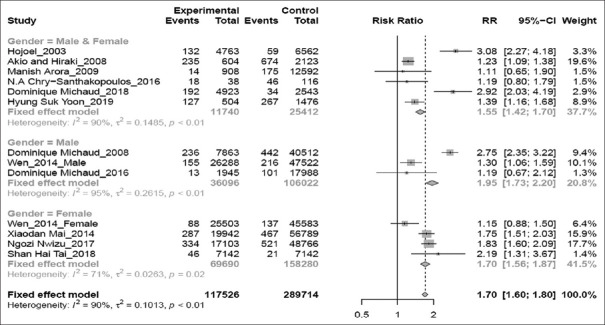
Gender-specific analysis [Figure 5] showed that the association between periodontitis and risk of lung cancer was significant in male and female populations (RR 1.55; 95% CI 1.42–1.70; P < 0.01), women population alone (RR 1.70;95% CI 1.56–1.87; P = 0.02), and men population alone (RR 1.95; 95% CI 1.73–2.20; P < 0.01).
Figure 5.
Forest plot of gender-based subgroup analysis
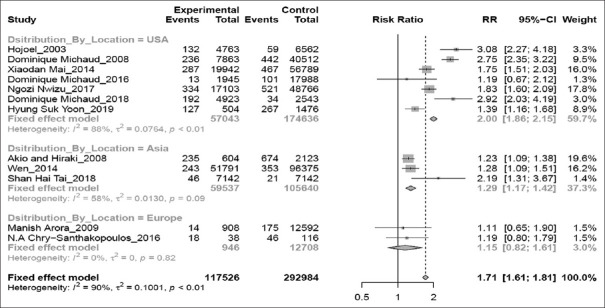
Seven studies were performed in America, two in Europe, and four in Asia. For subgroup analysis by geographic region [Figure 6], the association observed for the America (RR 2.00; 95% CI 1.86–2.15; P < 0.01) and the Asia (RR 1.29; 95% CI 1.17–1.42; P = 0.09) was significant, however for Europe (RR 1.15; 95% CI 0.82–1.61; P = 0.82), the association was not significant.
Figure 6.
Forest plot of location-based subgroup analysis
Sensitivity analysis
In sensitivity analysis using the leave-one-out approach, the result showed that no individual study altered the pooled RR for lung cancer demonstrating that meta-analysis results were robust.
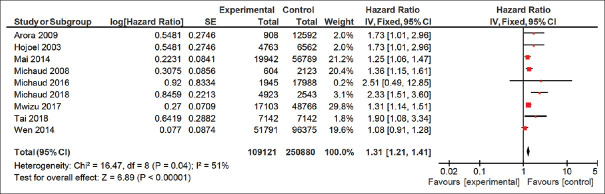
Survival plot analysis
Based on the results obtained by the survival plot analysis for patients with lung cancer with or without periodontitis included in the present analysis, it was found that patients with periodontitis have 1.31 times more chance of getting lung cancer as compared to patients without periodontitis [Figure 7].
Figure 7.
Survival plot analysis
Discussion
The present meta-analysis of eight cohorts and four case–control studies revealed that individuals with PD are associated with a 1.71-fold increased risk for developing lung cancer. Furthermore, the separate meta-analysis of high-quality studies stipulated around 2-fold intensified in risk of developing lung cancer (RR 1.91; 95%CI 1.77–2.07; P < 0.01). Of the twelve included studies, nine were adjusted for smoking status, ten were adjusted for age, and nine were adjusted for alcohol drinking status, the critical risk factors for lung cancer incidence.
The proposed hypothesis of the mechanism of association between lung cancer and periodontitis is as follows: chronic inflammation is a risk factor in 25% of cancer. Bacteria produce inflammatory mediators and detrimental effects on fibroblasts, epithelium, endothelium, and extracellular matrix by activating immunologic and inflammatory reactions. Rybojad et al. identified microorganisms from the bronchial secretion in 30 lung cancer patients. The most frequently isolated microorganisms were Actinomycosis spp., Peptostreptococcos spp., followed by Eubacterium Lentum, Veillonella Parvula, Provetella spp., Bacteroides Spp., Lactobacillus.[33]
Scannapieco proposed four mechanisms through which periodontal bacteria enter the lung and enhance infection and cancer;first, aspiration: periodontal bacteria can aspirate directly to the lower respiratory tract from salivary secretion. Lung abscesses contain the most type of facultative anaerobic bacteria, enhancing mucosal adhesion where the periodontal bacterial enzymes can modify the mucosal surface, enhance attachment and colonization, and then get aspirated into the lung. Second, poor oral hygiene leads to an increased risk of infection. Porphyromonas gingivalis and Spirochetes alter mucosal epithelium to increase adhesion and colonization of respiratory pathogens. They also secrete enzymes such as mannosidase, fructokinase, and hexosaminidase, which are responsible for increasing the adhesion of bacteria to the mucosa by exposing adhesin receptors on mucosa epithelium. Third, it can also inhibit bacterial clearance as the periodontal bacterial enzymes destroy salivary pellicles on bacteria and resist its clearance. Finally, the cytokines and pro-inflammatory mediators alter respiratory epithelium to enhance bacteria adhesion and decrease its removal.[34]
P. gingivalis causes the production of pro-inflammatory cytokines including interleukin (IL)-1, IL-6, Il-8, tumor necrosis factor α, C-reactive protein (CRP), matrix metalloproteinases. Pine et al. evaluated the association between IL-6, IL-8, and CRP in 123 lung cancer patients using logistic regression models. High serum IL-8 level predicts the subsequent diagnosis of the disease, as it was present 5 years before the diagnosis.[35] IL-6 levels increased in patients who developed lung cancer. IL-6 and IL-8 directly act on lung epithelium via β1 (Nuclear Factor of Kappa light polypeptide gene enhancer in B-cells 1) pathways and induce tumorigenesis.[36] IL-6 plays an important role in tumor initiation and progression. IL-6 causes an increase in reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI), thus capable of promoting tumor initiation by altering the epigenetics of certain genes. IL-6 activates tumorigenic-related transcription factors and causes tumor progression.[37]
Periodontal bacteria cause an increase in fibronectin expression, especially in nonsmall-cell carcinoma, by increasing the adhesion of lung carcinoma cells to fibronectin and enhancing tumorigenesis.[38] Fibronectin also interacts with vertebrate androgen receptors by stimulating gonadal steroids and controlled the expression of cyclin D. Cyclin D is a factor in a cell cycle. Due to this mechanism, fibronectin may enhance tumor growth and its resistance to therapy.[39]
Actinomycosis Actinomycetemocomitans produced cytolethal toxins, which directly affect the G2M phase of the cell cycle. Bacterial toxins can disturb the cell cycle, resulting in altered cell growth, abnormal mitosis, and apoptosis.[40]
P. gingivalis plays an important role in the development of lung cancer by acting on (Grainyhead-like 2 [GRHL2] transcription factor). GRHL2 maintains epithelial plasticity and stemness, including self-renewal capacity. The majority of GRHL2 roles are shown to be implicated with carcinogenesis action.[41] It can directly bind to the promoter region of (Ras homology Growth [RhoG] related) and plays an important role in proliferation and metastasis. RhoG is responsible for the regulation of cell shape, attachment, and mobility. GRHL2 enhances cell growth and colony formation by inhibiting cell migration and invasion.[42] GRHL2 causes regulation of epithelial-to-mesenchymal transition (EMT), and it suppresses EMT through inhibiting ZEB1 promoter transactivation. EMT is controlled by a complex network of the transcription factors ZEB1, and ZEB2. The event of cancer progression, metastasis, chemoresistance, and phenotypic plasticity is governed by EMT. Overexpression of GRHL2 causes a reduction of expression of ZEB1 and ZEB2, and increased expression of Octamer-binding transcription factor 4 (Oct-4), which was confirmed by Chen et al., who showed that GRHL2 binds with miR-200 promoter and proximal region of Oct-4 gene promoter to regulate the expression of ZEB and the occurrence of EMT.[43]
During the inflammatory reactions, the cytokines and chemokines increase the expression of NADPH oxidase and nitric oxide synthase, leading to increase production of ROS and RNI. Increased expression of oxygen and nitrogen species identified in cancer.[44]
Treatment for periodontal infection can reduce markers of systemic inflammation and endothelial dysfunction within 2–6 months. Hwang et al. found that the anti-inflammation treatment of PD significantly reduces the risk of developing lung cancer (HR 0.45; 95%CI 0.38, 0.54 P < 0.05).[45,46]
The present meta-analysis has some possible limitations. First, although most included studies were adjusted for common covariates, including age, smoking, and alcohol consumption, other unmeasured confounding factors such as stress, socioeconomic status, and genetics might affect lung cancer and PD. Second, the result had a wide CI in three studies, which limited the power of analyses.[24,27,31] Third, moderate heterogeneity was detected in the pooled analysis. Thus, the subgroup results should be interpreted with caution. Assessment techniques of periodontitis were heterogeneous in included studies, and definitions for categories of PD severity were diverse between the studies. Three studies used an oral examination of periodontal tissue by dentists for assessment of PD, two studies used insurance data to evaluate PD patients, seven studies used health questionnaire forms, which might affect the heterogeneity and precision of our result.[21,22,23,24,25,26,27,28,29,30,31,32] Furthermore, in the literature search for the present analysis, no studies identified with safety outcomes related to lung cancer in periodontitis patients. However, this could be attributed to the focus of the analysis being only on the primary outcome measuring the lung cancer risk in patients with periodontitis.
However, the present meta-analysis has numerous critical strengths. The most significant strength is the large sample size of patients in exposure and control groups included in the cumulative analysis. Second, according to the Newcastle-Ottawa Quality Assessment score, most studies included in the analysis were high-quality studies that controlled for significant confounders such as age, sex, smoking, alcohol, diet, and other comorbidities. Six of the nine cohorts have more than 10 years of follow-up duration. Sufficiently long follow-up is necessary because most lung cancers have a long subclinical period. The results were separately investigated according to the methodological quality of the studies. Third, the most fascinating remark is that association becomes stronger (1.91-fold) when the data were pooled separately from high-quality studies compared to the 1.4-fold increase in the development of lung cancer according to the data pooled from low-quality studies. This is in accordance with a large meta-epidemiological study, which distinctly endorses that systemic reviewers should present meta-analysis confined to studies at low risk of bias for each outcome.[46] One should be attentive when illustrating the data from low-quality studies. Fourth, even though the present meta-analyses employed a moderate heterogeneity, subgroup and sensitivity analysis disclosed coherent results for the connection between PD and lung cancer, which infer the robustness and consistency of the findings.
Conclusion
From the current evidence, PD is a potential risk factor for the development of lung cancer. The risk for incidence of lung cancer is surged twice in the patients with PD, even though age and smoking are controlled in the studies. We impulse health professionals and the general community to be further aware of the potential association between these two. Furthermore, it is assured that the observation of the contemporary meta-analysis could help increase awareness and significance of oral health preservation, which may lead to a downgrade of the risk for developing lung malignancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2013;40(Suppl 14):S113–34. doi: 10.1111/jcpe.12059. [DOI] [PubMed] [Google Scholar]
- 2.Saini R, Saini S, Sharma S. Periodontitis: A risk factor to respiratory diseases. Lung India. 2010;27:189. doi: 10.4103/0970-2113.68313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz S, Schlitt A, Hofmann B, Schaller HG, Reichert S. Periodontal pathogens and their role in cardiovascular outcome. J Clin Periodontol. 2020;47:173–81. doi: 10.1111/jcpe.13224. [DOI] [PubMed] [Google Scholar]
- 4.Dioguardi M, Crincoli V, Laino L, Alovisi M, Sovereto D, Mastrangelo F, et al. The role of periodontitis and periodontal bacteria in the onset and progression of Alzheimer's disease: A systematic review. J Clin Med. 2020;9:495. doi: 10.3390/jcm9020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polyzos NP, Polyzos IP, Zavos A, Valachis A, Mauri D, Papanikolaou EG, et al. Obstetric outcomes after treatment of periodontal disease during pregnancy: Systematic review and meta-analysis. BMJ. 2010;341:c7017. doi: 10.1136/bmj.c7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao QW, Zhou DS, Peng HJ, Ji P, Liu DS. Association of periodontal disease with oral cancer: A meta-analysis. Tumour Biol. 2014;35:7073–7. doi: 10.1007/s13277-014-1951-8. [DOI] [PubMed] [Google Scholar]
- 7.Wang RS, Hu XY, Gu WJ, Hu Z, Wei B. Tooth loss and risk of head and neck cancer: A meta-analysis. PLoS One. 2013;8:e71122. doi: 10.1371/journal.pone.0071122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Nie S, Zhu Y, Lu M. Teeth loss, teeth brushing and esophageal carcinoma: A systematic review and meta-analysis. Sci Rep. 2015;5:15203. doi: 10.1038/srep15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng XT, Xia LY, Zhang YG, Li S, Leng WD, Kwong JS. Periodontal disease and incident lung cancer risk: A meta-analysis of cohort studies. J Periodontol. 2016;87:1158–64. doi: 10.1902/jop.2016.150597. [DOI] [PubMed] [Google Scholar]
- 10.Maisonneuve P, Amar S, Lowenfels AB. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann Oncol. 2017;28:985–95. doi: 10.1093/annonc/mdx019. [DOI] [PubMed] [Google Scholar]
- 11.Ren HG, Luu HN, Cai H, Xiang YB, Steinwandel M, Gao YT, et al. Oral health and risk of colorectal cancer: Results from three cohort studies and a meta-analysis. Ann Oncol. 2016;27:1329–36. doi: 10.1093/annonc/mdw172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaud DS, Fu Z, Shi J, Chung M. Periodontal disease, tooth loss, and cancer risk. Epidemiol Rev. 2017;39:49–58. doi: 10.1093/epirev/mxx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Shi X, Li Y, Shi X, Gu Y, Qian Q, et al. Hematopoietic and lymphatic cancers in patients with periodontitis: A systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2020;25:e21–8. doi: 10.4317/medoral.23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000. 2020;82:257–67. doi: 10.1111/prd.12323. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Facts & Figures. 2020. [Last accessed on 2021 Dec 23]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer facts-figures/cancer-facts-figures-2020.html .
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 17.Chung M, York BR, Michaud DS. Oral health and cancer. Curr Oral Health Rep. 2019;6:130–7. doi: 10.1007/s40496-019-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbella S, Veronesi P, Galimberti V, Weinstein R, Del Fabbro M, Francetti L. Is periodontitis a risk indicator for cancer? A meta-analysis. PLoS One. 2018;13:e0195683. doi: 10.1371/journal.pone.0195683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. [Last accessed on 2021 Dec 23]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 21.Hujoel PP, Drangsholt M, Spiekerman C, Weiss NS. An exploration of the periodontitis-cancer association. Ann Epidemiol. 2003;13:312–6. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 22.Hiraki A, Matsuo K, Suzuki T, Kawase T, Tajima K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17:1222–7. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 23.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: A prospective cohort study. Lancet Oncol. 2008;9:550–8. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora M, Weuve J, Fall K, Pedersen NL, Mucci LA. An exploration of shared genetic risk factors between periodontal disease and cancers: A prospective co-twin study. Am J Epidemiol. 2010;171:253–9. doi: 10.1093/aje/kwp340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen BW, Tsai CS, Lin CL, Chang YJ, Lee CF, Hsu CH, et al. Cancer risk among gingivitis and periodontitis patients: A nationwide cohort study. QJM. 2014;107:283–90. doi: 10.1093/qjmed/hct248. [DOI] [PubMed] [Google Scholar]
- 26.Mai X, LaMonte MJ, Hovey KM, Nwizu N, Freudenheim JL, Tezal M, et al. History of periodontal disease diagnosis and lung cancer incidence in the women's health initiative observational study. Cancer Causes Control. 2014;25:1045–53. doi: 10.1007/s10552-014-0405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaud DS, Kelsey KT, Papathanasiou E, Genco CA, Giovannucci E. Periodontal disease and risk of all cancers among male never smokers: An updated analysis of the health professionals follow-up study. Ann Oncol. 2016;27:941–7. doi: 10.1093/annonc/mdw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chrysanthakopoulos NA. Correlation between periodontal disease indices and lung cancer in Greek adults: A case-control study. Exp Oncol. 2016;38:49–53. [PubMed] [Google Scholar]
- 29.Nwizu NN, Marshall JR, Moysich K, Genco RJ, Hovey KM, Mai X, et al. Periodontal disease and incident cancer risk among postmenopausal women: Results from the women's health initiative observational cohort. Cancer Epidemiol Biomarkers Prev. 2017;26:1255–65. doi: 10.1158/1055-9965.EPI-17-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaud DS, Lu J, Peacock-Villada AY, Barber JR, Joshu CE, Prizment AE, et al. Periodontal Disease Assessed using clinical dental measurements and cancer risk in the ARIC study. J Natl Cancer Inst. 2018;110:843–54. doi: 10.1093/jnci/djx278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tai SH, Chen JP, Chang HS, Kuo HC. Periodontitis as a risk factor for lung cancer among women: A nationwide matched cohort study. Kaohsiung J Med Sci. 2019;35:123–4. doi: 10.1002/kjm2.12018. [DOI] [PubMed] [Google Scholar]
- 32.Yoon HS, Wen W, Long J, Zheng W, Blot WJ, Cai Q. Association of oral health with lung cancer risk in a low-income population of African Americans and European Americans in the Southeastern United States. Lung Cancer. 2019;127:90–5. doi: 10.1016/j.lungcan.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybojad P, Los R, Sawicki M, Tabarkiewicz J, Malm A. Anaerobic bacteria colonizing the lower airways in lung cancer patients. Folia Histochem Cytobiol. 2011;49:263–6. doi: 10.5603/fhc.2011.0036. [DOI] [PubMed] [Google Scholar]
- 34.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- 35.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103:1112–22. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley CM, Wang Y, Pal S, Klebe RJ, Harkless LB, Xu X, et al. Fibronectin fragmentation is a feature of periodontal disease sites and diabetic foot and leg wounds and modifies cell behavior. J Periodontol. 2008;79:861–75. doi: 10.1902/jop.2008.070492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosmehl H, Berndt A, Strassburger S, Borsi L, Rousselle P, Mandel U, et al. Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. Br J Cancer. 1999;81:1071–9. doi: 10.1038/sj.bjc.6690809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nougayrède JP, Taieb F, De Rycke J, Oswald E. Cyclomodulins: Bacterial effectors that modulate the eukaryotic cell cycle. Trends Microbiol. 2005;13:103–10. doi: 10.1016/j.tim.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Yan X, Yang M, Liu J, Gao R, Hu J, Li J, et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. 2015;5:3111–22. [PMC free article] [PubMed] [Google Scholar]
- 42.Nair J, Ohshima H, Nair UJ, Bartsch H. Endogenous formation of nitrosamines and oxidative DNA-damaging agents in tobacco users. Crit Rev Toxicol. 1996;26:149–61. doi: 10.3109/10408449609017928. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Yi JK, Shimane T, Mehrazarin S, Lin YL, Shin KH, et al. Grainyhead-like 2 regulates epithelial plasticity and stemness in oral cancer cells. Carcinogenesis. 2016;37:500–10. doi: 10.1093/carcin/bgw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piao JY, Lee HG, Kim SJ, Kim DH, Han HJ, Ngo HK, et al. Helicobacter pylori activates IL-6-STAT3 signaling in human gastric cancer cells: Potential roles for reactive oxygen species. Helicobacter. 2016;21:405–16. doi: 10.1111/hel.12298. [DOI] [PubMed] [Google Scholar]
- 45.Hwang IM, Sun LM, Lin CL, Lee CF, Kao CH. Periodontal disease with treatment reduces subsequent cancer risks. QJM. 2014;107:805–12. doi: 10.1093/qjmed/hcu078. [DOI] [PubMed] [Google Scholar]
- 46.Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta-epidemiological study. BMJ. 2008;336:601–5. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]