Abstract
Objectives:
To compare and evaluate the bond durability, surface morphology, and remineralization of the adhesive layer with newer adhesive systems modified with novel bioactive nanoparticles.
Methodology:
Bonding agents evaluated in this study include (a) Conventional dentin bonding agent (CN-DBA) (b) Nanohydroxyapatite (nanoHAP) incorporated dentin bonding agent (NH DBA); (c) Silica doped nanohydroxyapatite (Si nanoHAP) incorporated dentin bonding agent (Si NH DBA). A total of 104 human dentin discs (5 mm × 5 mm × 2 mm) were sectioned. Elemental analysis (Ca/P ratio) and surface morphology of the adhesive layer with different dentin adhesives were evaluated under scanning electron microscopy with energy-dispersive X-ray analysis after speculated storage time of 1 day and 6 months. Microshear bond strength of adhesive restorations with different dentin adhesives was evaluated under universal testing machine and fractographic analysis under scanning electron microscope after speculated storage time of 1 day and 6 months. The results were analyzed using analysis of variance and post hoc analysis.
Results:
Si-NH-DBA showed highest mean microshear bond strength for both 1 day and 6 months, which was significantly higher compared to conventional nanofilled dentin bonding agent (CN-DBA) and NH-DBA. Si-NH-DBA group showed only 10% reduction in bond strength after 6 months, which was less compared to that of other groups. Similarly, Si-NH-DBA showed higher remineralization with stellate-shaped crystals at the adhesive layer after 6 months with hydrolytic resistant hybrid layer, compared to CN-DBA and NH-DBA.
Conclusion:
Silica-doped nanohydroxyapatite proved its efficiency on bond stability, remineralization, and hydrolytic resistance when incorporated into dentin bonding agents because of its bioactivity and carbonate-containing apatite-forming ability.
Keywords: Dentin bonding agents, microshear bond strength, nanohydroxyapatite, remineralization, silica-doped nanohydroxyapatite
Introduction
As restorative dentistry quests for the invention of newer materials and techniques, composite resins occupy a pivotal role in this spectrum of restorative materials owing to their esthetics and adhesion.[1] However, every material has its virtues and blemishes. Acid-etch procedure is usually successful for proper bonding to enamel, but to date, resin–dentin bonds created by infiltration of adhesives into partially demineralized dentin matrix are less perfect.[2]
The structural and chemical limitations associated with dentin bonding include:
Suboptimally infiltrated dentin adhesives after acid etching leaves denuded collagen fibrils at the bottom of the hybrid layer, which are susceptible to matrix metalloproteinase mediated collagenolytic activity[2,3]
Due to the differences in the mechanical properties, stress concentrations will be developed at the junction between mineralized dentin with higher stiffness (20 GPa) and the bottom of the hybrid layer with much lower stiffness (3–4 GPa)[4]
The retention of microscopic amount of residual water in etch and rinse adhesives and intrinsic water in dentin acts as an active medium for hydrolysis of polymerized resin.[4] These issues are critical obstacles for long-term bond durability.
In the pursuit of newer materials and techniques, the science of engineered nanoparticles along with bioactivity offers a noble strategy which facilitates optimal infiltration of adhesive into the collagen matrix, increasing the degree of conversion of adhesives and biomimetic remineralization of resin–dentin bonds to increase the mechanical properties of the hybrid layer[5] and silencing the action of collagenolytic enzymes such as MMPs.[6]
Nanohydroxyapatite (nanoHAP) is chemically and biologically similar to the mineral component of human bone and teeth. It is documented that incorporation of 0.2 wt% HAP into dental adhesives considerably improved the degree of conversion and polymerization rate.[7,8]
Though HAP has good biocompatibility, it has a limited osteogenic ability. In this way, element doping could bring special biological functionalities to HAP. Silicon is an essential trace element and aids in bone mineralization.[9] It is investigated that silica-doped nanohydroxyapatite (Si-nanoHAP) incorporation into polycaprolactone scaffold promotes the proliferation and differentiation of osteoblasts.[10] Some authors demonstrated that Si-nanoHAP coatings provide carbonate-containing apatite layer on dental implants, which acts as nucleation sites for formation of apatite crystals.[11,12] Therefore, Si-nanoHAP might be a versatile material for preparation of new dentin adhesives, satisfying all the strategies.
Although there is limited information available on nanoHAP application in dentin adhesives, so far no information is available on Si-nanoHAP in dental application other than dental implants. This paper concerns itself on long-term bond durability, remineralization, and surface morphology of the adhesive layer when novel bioactive nanofillers are incorporated into dentin adhesives.
The null hypothesis of this study was that bond strength, remineralization, and surface morphology would not be affected by incorporation of novel bioactive nanofillers into dentin adhesives.
Methodology
An in vitro experimental study was planned and conducted. Sample size was calculated based on the findings of a preliminary study with a standard deviation value for bond strength of 10% with 80% power and 95% confidence interval. The final sample size was found to be 8 in each group in a total of 104 dentin specimens.
Dentin specimen preparation
One hundred and four square-shaped dentin specimens of size 5 mm × 5 mm × 2 mm were prepared from 52 sound premolars freshly extracted for orthodontic purpose. Ethical approval for extracted human teeth was obtained from the institutional review board and ethical committee with certificate number-IRB/VDC/MDS14 ENDO3 on January 21, 2015. The surface irregularities on dentin specimens were removed with 600 grit silicon-carbide paper (3M, India) (3M ESPE, Bangalore, India).
Preparation of dentin adhesives
Total etch dentin adhesive (Tetric n bond, Ivoclar Vivadent) without addition of bioactive fillers was chosen for the study as conventional nanofilled dentin bonding agent (CN-DBA). The weight of nanoHAP (Sigma Aldrich, India) and Si-nanoHAP powders (Sigma Aldrich, India) was measured using a microbalance. 0.2 wt% nanoHAP-[8] containing dentin bonding agent and 0.2 wt% Si-nanoHAP-containing dentin bonding agents were prepared by mixing 2 mg of these bioactive nanofillers into 1 mL of CN-DBA. The adhesive solutions were homogenized by ultra-sonication using a probe sonicator apparatus (Sonoplus UW2200, Bandelin, Germany) for 1 min.
Baseline Ca/P ratio of sound and acid-etched dentin
Eight dentin specimens were used to measure the baseline Ca/P ratio. The surface of dentin specimens was divided into two halves. One half was subjected to acid etching for 15 s followed by rinsing and air drying. The other half was covered with a teflon tape such that the etchant does not come in contact with the other half. After the acid-etching regimen, the teflon tape was removed. Both the halves were examined for Ca/P ratio under a scanning electron microscope (SEM)-energy-dispersive X-ray (EDX) machine which served as controls.
Acid etching and grouping
The remaining 96 dentin specimens were etched with 37% phosphoric acid gel (D-tech, Ivoclar Vivadent) for 15 s, and were rinsed and blot dried with a foam pellet and were divided into 48 specimens each depending on the parameters to be checked.
Parameter A: Ca/P ratio and surface morphology
Parameter B: Microshear bond strength and fracture pattern analysis.
These specimens were again divided into three groups (n = 16) depending on the type of bonding agent applied.
Group I: CN-DBA
Group II: 0.2wt% nanoHAP-incorporated dentin bonding agent (NH-DBA)
Group III: 0.2wt% Si-nanoHAP-incorporated dentin bonding agent (Si-NH-DBA).
Checking Ca/P ratio and surface morphology (16 dentin specimens in each group – IA, IIA, and IIIA)
After the acid-etching regimen, conventional and experimental bonding agents were applied and light cured with Blue phase light-emitting diode (LED) Light-curing unit (Ivoclar Vivadent, India) with an intensity of 450 mW/cm2 for 20 s. Eight dentin specimens in each group were stored in artificial saliva for 1 day and another eight for 6 months. Artificial saliva was renewed weekly. After the speculated storage time, the dentin specimens were removed from saliva, sputter coated with gold, and observed for surface morphology and calcium phosphate content under environmental SEM fitted with EDX analysis, under ×1000 magnification. Calcium phosphate content readings were converted to Ca/P ratio (wt %) to evaluate the change in mineral density.
Checking microshear bond strength and fracture pattern analysis (μSBS) (16 dentin specimens in each group – IB, IIB, and IIIB)
Conventional and experimental dentin bonding agents were applied using fully saturated micro applicator tips with slight agitation and were gently air-dried for 1–3 s, to allow the solvent to evaporate, then light cured with Blue phase LED light-curing unit (Ivoclar vivadent, India) with an intensity of 450 mW/cm2 for 20 s according to the manufacturer's instructions. Synthetic polyvinylchloride (PVC) tubes with 1 mm internal diameter and 2 mm height were precut and were placed perpendicular to the dentinal surface at the middle third equidistant from all the surfaces. Composite (Tetric n ceram) restoration was done and light cured for 40 s. PVC tubes were removed with a Bard-Parker blade. Eight samples in each group were stored in artificial saliva for 1 day and another 8 samples for 6 months in an air-tight container. The artificial saliva was renewed weekly.
Shear stress application
After the speculated storage time in each group, each specimen was mounted in an acrylic resin. Shear bond strength test was performed under universal testing machine with a chisel of 2 mm width by fixing to the upper jig. The specimens were fixed to the lower jig such that the cutting edge of the chisel was held parallel to the composite–dentin interface. Load was applied at a rate of 1 mm/min until the bond failure occurred. Data were recorded in Newton. Bond strength in Mpa was calculated by using the following formulae:
μSBS (MPa) = Shear force (N)/cross-sectional area (mm2)
Area is calculated using the following formulae: -A=πr2 = 0.785
Where π = 22/7 = 3.146; r = radius of bonded surface (0.5 mm).
Fracture pattern analysis
After obtaining the fractured samples, they were sputter coated with gold and analyzed under SEM for the type of failure, at ×150 magnification. The type of failure that occurred at the tooth/adhesive system/restoration interface was classified according to Pilo, with modifications:[13]
Adhesive: Failure at the interface
Cohesive: Failure in the composite
Mixed 50–50%: Failure approximately 50% at the interface and 50% in composite
Mixed – mainly adhesive: Failure mostly at the interface (>50%)
Mixed – mainly cohesive: Failure mostly in the composite (>50%).
Statistical analysis
The results were analyzed statistically using the Statistical Package for the Social Sciences (SPSS), version 17.0 (SPSS Inc., Chicago IL, USA). For each group, mean and standard deviation were calculated. To find whether the bond strength values in the all groups is homogenous, analysis of variance was conducted. To find significance between two groups, Tukey's post hoc analysis was conducted. Descriptive data analysis was applied to evaluate the type of failure that occurred between each group and it is represented by means of graphs.
Results
Ca/P ratio
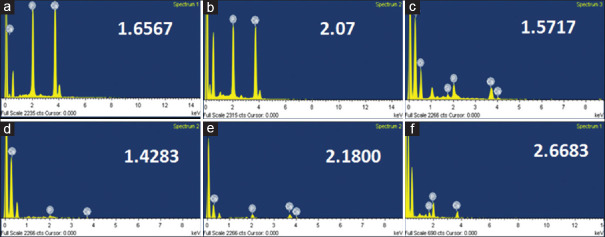
Baseline Ca/P ratio for sound and acid-etched dentin were 1.94 and 1.46, respectively. Table 1 shows mean Ca/P ratio of all groups after 1 day and 6 months storage. Figure 1 shows elemental analysis of adhesive surface after application of bonding agents.
Table 1.
Mean Ca/P ratio and microshear bond strength of all groups after 1 day and 6 months storage with respective statistical analysis
| Storage Time | Groups | Ca/P ratio | P | Pair-Wise Comparison | MPa | P | Pair-Wise Comparison |
|---|---|---|---|---|---|---|---|
| 1 Day | CN-DBA (I) | 1.65±0.16 | 0.001* | I vs II* | 26.54±2.21 | 0.001* | I vs II* |
| NH-DBA (II) | 2.07±0.25 | I vs III (NS) | 38.16±6.51 | I vs III* | |||
| Si-NH-DBA (III) | 1.57±0.14 | II vs III* | 56.56±4 | II vs III* | |||
| 6 Months | CN-DBA (I) | 1.42±0.30 | 0.002* | I vs II* | 20.69±3.58 | 0.001* | I vs II* |
| NH-DBA (II) | 2.18±0.50 | I vs III* | 32.40±2.07 | I vs III* | |||
| Si-NH-DBA (III) | 2.66±0.58 | II vs III (NS) | 50.86±5.36 | II vs III* |
*P≤0.05 considered statistically significant; (NS) – No significant difference between pairs upon Pairwise comparison using Tukey’s Post-Hoc Test
Figure 1.
Elemental analysis of adhesive surface after application of different bonding agents at both 1 day and 6 months (a and d) Elemental analysis of conventional nanofilled dentin bonding agent group after 1 day and 6 months, respectively. (b and e) Elemental analysis of NH-DBA group after 1 day and 6 months, respectively. (c and f) Elemental analysis of Si- nanoHAP incorporated dentin bonding agent group after 1 day and 6 months, respectively
There was a reduction of Ca/P ratio below the levels of acid-etched dentin after 6 months storage in the CN-DBA group when compared to 1 day in the CN-DBA group. It was slightly increased in the NH-DBA group whereas there was a significant increase in Ca/P ratio after 6 months in the Si-NH-DBA group [Table 2].
Table 2.
Comparison between 1 day and 6 months values of Ca/P ratio and microshear bond strength of all groups and their respective statistics
| Groups | Storage Time | n | Ca/P Ratio | P | MPa | P |
|---|---|---|---|---|---|---|
| CN-DBA | Day 1 | 8 | 1.65±0.16 | 0.132 | 26.54±2.21 | 0.007* |
| 6 Months | 8 | 1.42±0.3 | 20.69±3.5 | |||
| NH-DBA | Day 1 | 8 | 2.07±0.25 | 0.643 | 38.16±6.51 | 0.066 |
| 6 Months | 8 | 2.18±0.5 | 32.40±2.07 | |||
| Si-NH-DBA | Day 1 | 8 | 1.57±0.14 | 0.001* | 56.56±3.10 | 0.064 |
| 6 Months | 8 | 2.66±0.58 | 50.86±2.24 |
*P≤0.05 considered statistically significant
Surface morphology
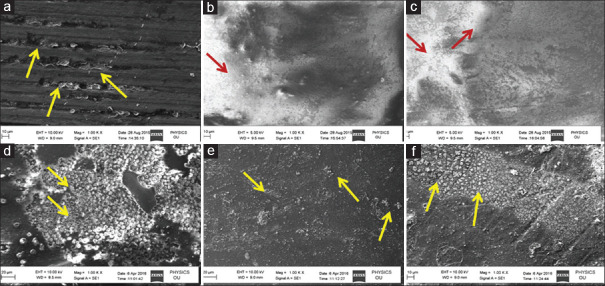
CN-DBA did not penetrate completely into all the dentinal tubules and after 6 months of storage the dentin bonding agent completely disappeared. In NH DBA group, it was observed that bonding agent was completely penetrated into all the dentinal tubules and after 6 months storage, bonding agent was intact with minute amount of mineral/crystalline sites. In Si-NH-DBA, bonding agent was completely penetrated with thick homogenous distribution of nanoparticles on the adhesive layer and after 6 months of storage, stellate-shaped insoluble complexes of silica-rich domains were observed on the surface [Figure 2].
Figure 2.
Scanning electron microscope images of surface micromorphology of adhesive layers at the end of 1 day and 6 months. In (a) conventional nanofilled dentin bonding agent did not penetrate completely into the acid-etched dentin, leaving some open dentinal tubules (arrows). (d) At the end of 6 months conventional nanofilled dentin bonding agent was disappeared maximum number of tubules were devoid of resin plugs (arrows). In (b) nanoHAP incorporated dentin bonding agent penetrated completely with thin homogenous distribution of the nanoparticles (arrows). (e) After 6 months, storage in nanoHAP-incorporated dentin bonding agent group showed intact bonding agent with minute amount of mineral/crystalline (arrows). (c) Si-nanoHAP-incorporated dentin bonding agent penetrated completely with thick homogenous distribution (arrows) of the nanoparticles on the adhesive layer. (f) After 6 months Si-nanoHAP-incorporated dentin bonding agent adhesive layer showed stellate-shaped insoluble complexes of silica-rich domains on the surface (arrow)
Microshear bond strength
Si-NH-DBA group showed highest mean μSBS at both 1 day and 6 months’ storage followed by NH-DBA and lowest for group CN-DBA [Table 1].
After 6 months storage in artificial saliva, all the groups showed reduction in bond strength values which was not significant in Si-NH-DBA and NH-DBA groups, whereas statistically significant reduction was observed in CN-DBA group [Table 2].
Fracture pattern analysis
Figure 3 shows the graphical representation of failure modes of all groups. Figure 4 shows the surface morphology of the different failure modes of all groups. Examination of the fractured specimens under SEM revealed that most of the failure modes were cohesive and predominantly cohesive failures in resin (~60%) in all the three groups. The number of cohesive fractures decreased with storage period. Very few fractures were classified as adhesive (~3%). The remaining fractures were mixed and predominantly adhesive. In CN-DBA group, most of the dentin specimens showed predominantly adhesive and mixed fractures, whereas in NH-DBA and Si-NH-DBA most of the fractures were cohesive and predominantly cohesive fractures in resin.
Figure 3.
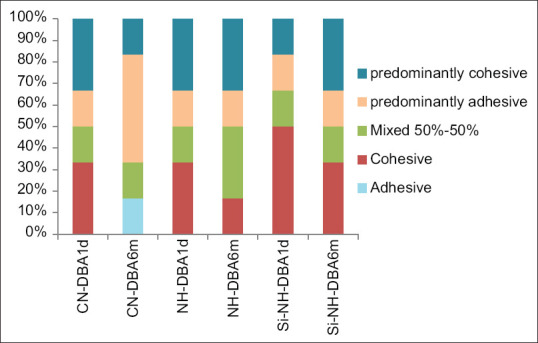
Graphical representation of % of fractures in all the groups
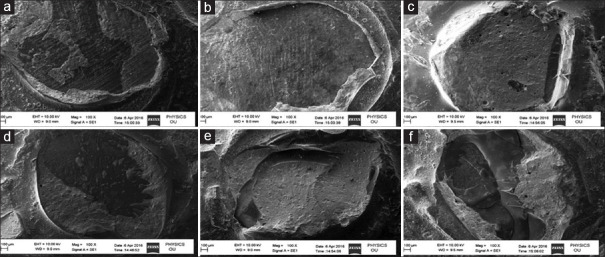
Figure 4.
Scanning electron microscope images showing representative of failure modes of all groups. (a) mixed failure-mainly adhesive (conventional nanofilled dentin bonding agent -1 day), (b) adhesive failure at the interface (conventional nanofilled dentin bonding agent - 6 months) (c) cohesive in the composite (nanoHAP-incorporated dentin bonding agent - 1 day) (d) mixed 50%–50% (nanoHAP-incorporated dentin bonding agent - 6 months) (e) cohesive failure in the composite (Si-nanoHAP-incorporated dentin bonding agent - 1 day) (f) mixed-mainly cohesive (Si-nanoHAP-incorporated dentin bonding agent - 6 months)
Discussion
In the current study, single bonding agent “Tetric N-Bond total etch” was used in order to study the efficacy of the adhesive interface with inclusion of different fillers by excluding the variation of different bonding systems. It contains ethanol as a solvent, thereby increasing the infiltrative capacity of the adhesive.[14]
0.2 wt% nanoHAP and Si-nanoHAP were incorporated into the adhesive and homogenized by ultra-sonication to prevent the formation of agglomerates in the adhesive solution. According to previous studies, 0.2 wt% of nanoHAP fillers significantly improved the properties of dentin adhesives.[8]
In the current study, EDX was used to analyze the mineral content of the specimens. EDX spectroscopy is an analytical technique used for chemical and elemental analysis at the ultra-structural level. The ratio of Ca to P is an indicative of chemical changes, since a change can suggest that the mineral phase was altered or significant substitution of ions may have occurred.[15]
The philosophy behind the bond strength testing is that higher the bonding capacity of an adhesive, the better it will resist such stresses and longer the restorations will survive in vivo.[16] μSBS test employed as large bonded areas of macroshear specimens have a higher chance to include critical flaws at the interfaces, resulting in lower bond strength value compared to microshear specimens.[17] In the current study, a flat dentin surface was preferred, as it would provide a wider area of dentin to be treated.[18] Chisel method at a cross head speed of 1 mm/min was used in this study, due to its superior sensitivity to slight differences, such as stress distribution, loading configuration, and material stiffness.[19]
Interpretation of results
The baseline Ca/P ratio of sound dentin was 1.9402. This ratio was decreased to 1.4635 after acid etching for 15 s due to loss of minerals such as HAP (Ca, P).
Conventional nanofilled dentin bonding agent
When acid-etched dentin was infiltrated with CN-DBA and stored in artificial saliva for 1 day, this ratio was increased to 1.6567. This could be due to minerals present in the artificial saliva. However, after 6 months storage in artificial saliva, this ratio decreased to 1.4283 which is below the level of acid-etched dentin [Figure 1]. This finding is in accordance with the SEM observation, i.e., after 6 months’ storage, bonding agent was completely disappeared with exposed dentinal tubules [Figure 2d]. This might be an indication of hydrolytic degradation of the adhesive when stored in artificial saliva.[20,21] As a result of this hydrolytic degradation, there might be loss of mineral content, which leads to decreased Ca/P ratio after 6 months’ storage.
In CN-DBA group, there is 23% reduction in the bond strength after 6 months. According to previous literatures, remaining uncured monomer may be a path way for hydrolytic degradation of the adhesive and loss of bond strength over time[4] with increased percentage of mixed and predominantly adhesive failures.
NanoHAP incorporated dentin bonding agent
When acid-etched dentin was infiltrated with NH-DBA and stored in artificial saliva for 1 day Ca/P ratio was increased to 2.07. Nanosized particles as well as calcium arising from storage medium should have followed concentration gradient, leading to remineralization.[22] After 6 months of storage this ratio was increased to 2.18, i.e., 5% increase compared to 1 day [Figure 1b and e]. This finding is in accordance with the SEM observation, i.e., after 6 months’ storage, intact and slightly rough adhesive surface with the presence of minute amount of mineral/crystalline sites [Figure 2e]. This finding portrays that NH-DBA infiltrated adhesive surface is more resistant to hydrolytic degradation. HAP particles may be biomineralized on the surface of collagen network through hydrogen bonding between COOH, OH, NH2 groups of the dentin collagen and OH group of HAP might lead to bio-mineralization at resin–dentin interface after 6 months storage.[8]
In NH-DBA group, there was 15% reduction in the μSBS after 6 months compared to 1 day. However, this reduction was less compared to CN-DBA group. Both 1 day and 6 months μSBS values of NH-DBA group were higher compared to 1 day values of CN-DBA group. These results are in accordance with study done by Leitune et al.[23] NHAP from the adhesive resin might have leached and got deposited around the denuded collagen, leading to less reduction in μSBS.[23] Another reason could be as the volumetric fraction of inorganic matrix increase there is tapering in organic portion, leading to minimal polymerization shrinkage and increased modulus of elasticity of adhesive layer with more number of cohesive and predominantly cohesive failures.[24]
Si-nanoHAP-incorporated dentin bonding agent
There was a significant increase (70%) in Ca/P ratio of Si-NH-DBA group after 6 months. After 6 months, surface morphology showed stellate-shaped insoluble complexes of silica rich domains, which might act as nucleation sites for remineralization [Figure 2f].
Si-NH-DBA group showed significantly higher μSBS compared to CN-DBA and NH-DBA groups at both 1 day (56.5618 MPa) and 6 months (50.8614) with more number of cohesive failures in resin. Although there is a slight reduction in bond strength, i.e., 10% reduction after 6 months, this reduction is less compared to that of other groups [Table 1].
Compared to pure nanoHAP which has limited osteogenic ability, Si-nanoHAP was reported to effectively upregulate osteogenic-related gene expressions of osteoblastic cells, including alkaline phosphatase, bone morphogenetic protein 2, and type I collagen, thus suggesting good osteogenic ability.[9] Moreover, an important property of Si-nanoHAP compared to pure HAP is its role in the formation of carbonate-containing apatite layer on its surface in the presence of simulated body fluids. This carbonate has a special property of increasing the chemical reactivity of apatite by providing higher number of nucleation sites for the formation of apatite crystallites helps in chemical bonding.[11] Also, it is investigated that surface charge of Si–HAP was much lower than the pure HA. Therefore, once the particles have infiltrated the demineralized dentin, they will remain embedded in the subsurface collagen matrix that acts as a scaffold retaining the particles and favors Ca2+ adsorption, thus showing a better bioactivity.[25] In the current study, the so-formed adhesive layer was more resistant to hydrolytic degradation compared to NH-DBA and CN-DBA as this layer was not degraded on long-term storage and also provides higher nucleation sites for formation of apatite crystals.
The higher μSBS and remineralization with Si-NH-DBA could be better explained by a principle that re-incorporation of mineral into the partially demineralized dentin matrix will provide a right environment as the mineral precipitated may act as a site for further nucleation and the remineralized tissue may be more resistant to degradation.[26] In the current study, Si-NHAP is not only a mineral which is in nano-form but is also more bioactive compared to pure HAP which has considerable osteogenic ability.
However, to employ this material in clinical situations, other properties such as colloidal stability and effect on collagenolytic activity need to be assessed in further investigations. In vivo conditions such as cycling loading, thermal variations, and pH dynamics need to be simulated in this study. Hence, further ex vivo, clinical and randomized controlled trials are advocated.
Conclusion
Among the bonding agents used in the study, Si-NH-DBA showed its highest efficacy for remineralization because of its high infiltrative capacity along with bioactivity and carbonate-containing apatite-forming ability as it provides suitable scaffold of silica rich domains which acts nucleation sites for mineral growth
This remineralized layer is hydrolytically resistant which shows highest mean microshear bond strength values at both 1 day and 6 months with no significant reduction in bond strength after 6 months when compared to NH-DBA and CN-DBA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Authors thank Professor Dr. Ramesh, Department of public health dentistry Vishnu Dental College, Bhimavaram, Andhra Pradesh, India for providing his valuable time for statistical analysis.
References
- 1.Nagpal R, Manuja N, Tyagi SP, Singh UP. In vitro bonding effectiveness of self-etch adhesives with different application techniques: A microleakage and scanning electron microscopic study. J Conserv Dent. 2011;14:258–63. doi: 10.4103/0972-0707.85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besinis A, van Noort R, Martin N. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent Mater. 2012;28:1012–23. doi: 10.1016/j.dental.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. Strategies to prevent hydrolytic degradation of the hybrid layer – A review. Dent Mater. 2013;29:999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besinis A, van Noort R, Martin N. Remineralization potential of fully demineralized dentin infiltrated with silica and hydroxyapatite nanoparticles. Dent Mater. 2014;30:249–62. doi: 10.1016/j.dental.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Osorio R, Yamauti M, Sauro S, Watson TF, Toledano M. Experimental resin cements containing bioactive fillers reduce matrix metalloproteinase-mediated dentin collagen degradation. J Endod. 2012;38:1227–32. doi: 10.1016/j.joen.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro . Biomed Mater. 2009;4:034104. doi: 10.1088/1748-6041/4/3/034104. [DOI] [PubMed] [Google Scholar]
- 8.Sadat-Shojai M, Atai M, Nodehi A, Khanlar LN. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent Mater. 2010;26:471–82. doi: 10.1016/j.dental.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Qiu ZY, Noh IS, Zhang SM. Silicate-doped hydroxyapatite and its promotive effect on bone mineralization. Front Mater sci. 2013;7:40–50. [Google Scholar]
- 10.Wang G, Qian G, Zan J, Qi F, Zhao Z, Yang W, et al. A co-dispersion nanosystem of graphene oxide@ silicon-doped hydroxyapatite to improve scaffold properties. Mater Des. 2021;199:109399. [Google Scholar]
- 11.Thian ES, Huang J, Best SM, Barber ZH, Bonfield W. Novel silicon-doped hydroxyapatite (Si-HA) for biomedical coatings: An in vitro study using acellular simulated body fluid. J Biomed Mater Res B Appl Biomater. 2006;76:326–33. doi: 10.1002/jbm.b.30368. [DOI] [PubMed] [Google Scholar]
- 12.Dalgic AD, Alshemary AZ, Tezcaner A, Keskin D, Evis Z. Silicate-doped nano-hydroxyapatite/graphene oxide composite reinforced fibrous scaffolds for bone tissue engineering. J Biomater Appl. 2018;32:1392–405. doi: 10.1177/0885328218763665. [DOI] [PubMed] [Google Scholar]
- 13.Pilo R, Brosh T, Geron V, Levartovsky S, Eliades G. Effect of silane reaction time on the repair of a nanofilled composite using tribochemical treatment. J Adhes Dent. 2016;18:125–34. doi: 10.3290/j.jad.a35907. [DOI] [PubMed] [Google Scholar]
- 14.Al-Agha EI, Alagha MI. Nanoleakage of class V resin restorations using two nanofilled adhesive systems. J Int Oral Health. 2015;7:6–11. [PMC free article] [PubMed] [Google Scholar]
- 15.Hegde MN, Moany A. Remineralization of enamel subsurface lesions with casein phosphopeptide-amorphous calcium phosphate: A quantitative energy dispersive X-ray analysis using scanning electron microscopy: An in vitro study. J Conserv Dent. 2012;15:61–7. doi: 10.4103/0972-0707.92609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumari RV, Siddaraju K, Nagaraj H, Poluri RK. Evaluation of shear bond strength of newer bonding systems on superficial and deep dentin. J Int Oral Health. 2015;7:31–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong S, Geraldeli S, Maia R, Raposo LH, Soares CJ, Yamagawa J. Adhesion to tooth structure: A critical review of “micro” bond strength test methods. Dent Mater. 2010;26:e50–62. doi: 10.1016/j.dental.2009.11.155. [DOI] [PubMed] [Google Scholar]
- 18.Makkar S, Goyal M, Kaushal A, Hegde V. Effect of desensitizing treatments on bond strength of resin composites to dentin – An in vitro study. J Conserv Dent. 2014;17:458–61. doi: 10.4103/0972-0707.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz MA, Baggio R, Mendes YB, Gomes GM, Luque-Martinez I, Loguercio AD, et al. The effect of the loading method and cross-head speed on resin – Dentin microshear bond strength. Int J Adhes Adhes. 2014;50:136–41. [Google Scholar]
- 20.Hass V, Luque-Martinez IV, Gutierrez MF, Moreira CG, Gotti VB, Feitosa VP, et al. Collagen cross-linkers on dentin bonding: Stability of the adhesive interfaces, degree of conversion of the adhesive, cytotoxicity and in situ MMP inhibition. Dent Mater. 2016;32:732–41. doi: 10.1016/j.dental.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Vale MR, Afonso FA, Borges BC, Freitas AC, Jr, Farias-Neto A, Almeida EO, et al. Preheating impact on the degree of conversion and water sorption/solubility of selected single-bottle adhesive systems. Oper Dent. 2014;39:637–43. doi: 10.2341/13-201-L. [DOI] [PubMed] [Google Scholar]
- 22.Tschoppe P, Zandim DL, Martus P, Kielbassa AM. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J Dent. 2011;39:430–7. doi: 10.1016/j.jdent.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Leitune VC, Collares FM, Trommer RM, Andrioli DG, Bergmann CP, Samuel SM. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J Dent. 2013;41:321–7. doi: 10.1016/j.jdent.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Dumont VC, Silva RM, Almeida-Junior LE, Roa JP, Botelho AM, Santos MH. Characterization and evaluation of bond strength of dental polymer systems modified with hydroxyapatite nanoparticles. J Mater Sci Chem Eng. 2013;1:13–23. [Google Scholar]
- 25.Botelho CM, Lopes MA, Gibson IR, Best SM, Santos JD. Structural analysis of Si-substituted hydroxyapatite: Zeta potential and X-ray photoelectron spectroscopy. J Mater Sci Mater Med. 2002;13:1123–7. doi: 10.1023/a:1021177601899. [DOI] [PubMed] [Google Scholar]
- 26.Osorio R, Osorio E, Medina-Castillo AL, Toledano M. Polymer nanocarriers for dentin adhesion. J Dent Res. 2014;93:1258–63. doi: 10.1177/0022034514551608. [DOI] [PMC free article] [PubMed] [Google Scholar]