Abstract
Di-, tri-, and tetrasaccharides, synthesized according to the chemical structure of pneumococcal polysaccharide type 3 (PS3), were coupled to the cross-reactive material (CRM197) of modified diphtheria toxin in different molar carbohydrate/protein ratios using the squarate coupling method. To study protective immunity, female BALB/c mice were subcutaneously immunized twice (with a 3-week interval) using the amount of conjugates corresponding to 2.5 μg of oligosaccharide per mouse. The conjugates evoked PS3 binding immunoglobulin G antibodies that lasted for at least 7 weeks after the booster. Immunogenicity was not influenced by the carbohydrate/protein ratio. All mice with PS3-specific antibodies survived the intraperitoneal challenge with Streptococcus pneumoniae type 3. Therefore, synthetic oligosaccharide-protein conjugates might have potential as vaccines.
The encapsulated bacterium Streptococcus pneumoniae is still a major cause of upper and lower respiratory tract infections. About 23 serotypes of the ca. 90 known serotypes cause the majority (∼90%) of pneumococcal infections, such as otitis media, pneumonia, and meningitis (3). S. pneumoniae type 3 strains are related to invasive pneumococcal infection in adults (1, 3) and are often used in experimental meningitis (7) and otitis media models (5) in rabbits and rats. Furthermore, because of its high virulence in mice, S. pneumoniae type 3 offers a good model to study protective immunogenic properties of candidate vaccines (6, 12, 13).
Protection against encapsulated bacteria is primarily mediated by anticapsular antibodies. However, capsular polysaccharides are thymus-independent type 2 antigens and thus induce low-affinity antibodies that display a limited subclass distribution. These antigens evoke no B-cell memory, either. Vaccines consisting of polysaccharides coupled to a protein carrier can circumvent these disadvantages with an increased antibody response to capsular polysaccharides (2, 9, 14).
Neoglycoprotein preparations consisting of polysaccharide or oligosaccharide fragments obtained by degradation of the polysaccharides are sometimes contaminated with other pneumococcal components. They also have an ill-defined structure due to multiple coupling sites or the use of oligosaccharide pools of different chain length, and they lose their reducing end upon conjugation to a carrier. The use of small chemically synthesized oligosaccharides results in a precisely defined conjugate, thereby offering a possibility to evaluate the immunogenic properties of a conjugate vaccine by varying its specific structural parameters, for example, the length of the saccharide fragment and the carbohydrate/protein ratio.
Oligosaccharide-protein conjugates were prepared as follows. The pure synthetic monosaccharides β-d-Glcp-(1→O(CH2)3NH2 and β-d-GlcpA-(1→O(CH2)3NH2, disaccharide β-d-GlcpA-(1→4)-β-d-Glcp-(1→O(CH2)3NH2, trisaccharide β-d-Glcp-(1→3)-β-d-GlcpA-(1→4)-β-d-Glcp-(1→O(CH2)3NH2, and tetrasaccharide β-d-GlcpA-(1→4)-β-d-Glcp-(1→4)-β-d-GlcpA-(1→4)-β-d-Glcp-(1→O(CH2)3NH2 (D. J. LeFeber, J. P. Kamerling, and J. F. G. Vliegenthart, submitted for publication), representing fragments of the repeating unit of the type 3 polysaccharide (PS3), were conjugated to the cross-reactive material (CRM197) of modified diphtheria toxin in different molar carbohydrate/protein ratios using the squarate coupling method (15). 3-Aminopropyl glycoside (1 μmol) was dissolved in 0.1 M sodium phosphate buffer (75 μl, pH 6.95), and a diethyl squarate solution (76.2 μl, 1 μmol) in ethanol (EtOH) (from 24.9 μl of diethyl squarate in 12.8 ml of EtOH) was added. After being stirred for 16 h, EtOH was evaporated by being flushed with N2, and the water layer was diluted with aqueous HO-acetate, pH 4 (6 ml), and charged on a C18 Bakerbond spe column (500 mg). Being rinsed with H2O (10 ml), elution with MeOH (4 ml), and evaporation of the solvent by flushing with N2 afforded the pure elongated fragments. The elongated fragments were dissolved in 0.1 M borate buffer (400 μl, pH 9.55), and the appropriate volume of CRM197 solution (61 mg/ml; Chiron Vaccines, Siena, Italy) required for the target amount of oligosaccharide incorporation was added. After being stirred for 2 to 3 days, the mixture was diluted with 50 mM sodium phosphate (pH 7.2) and dialyzed against the same buffer. The protein concentrations were determined by the Pierce assay (11), and the carbohydrate content was determined by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) analysis. Schematic structures of the conjugates are shown in Fig. 1, and the carbohydrate/protein ratios are given in Table 1.
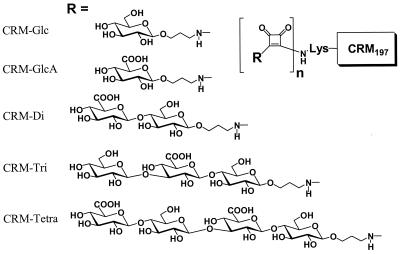
FIG. 1.
Schematic structure of the saccharide-CRM197 conjugates. Presented are the monosaccharides glucose and glucuronic acid and the di-, tri-, and tetrasaccharides, representing fragments of the repeating unit of pneumococci PS3 conjugated to the cross-reactive material (CRM197) using the squarate coupling method. n, oligosaccharide density per CRM molecule; R, structure of the saccharide fragments.
TABLE 1.
Synthetic di-, tri-, and tetrasaccharide fragments of PS3 coupled to CRM197 with different carbohydrate/protein ratios
| Conjugatea | Carbohydrate/protein ratios in:
|
|
|---|---|---|
| μg/mg | mol/mol | |
| CRM Glc | 26 | 8.5 |
| CRM GlcA | 22 | 6.6 |
| CRM di-1 | 19 | 3.1 |
| CRM di-2 | 41 | 6.7 |
| CRM tri-1 | 44 | 4.9 |
| CRM tri-3 | 61 | 6.8 |
| CRM tri-2 | 107 | 12.0 |
| CRM tetra-1 | 35 | 2.9 |
| CRM tetra-4 | 80 | 6.7 |
| CRM tetra-2 | 97 | 8.1 |
After coupling and purification, conjugates were analyzed for protein content by the Pierce assay and for carbohydrate content by MALDI-TOF analysis. di, disaccharide; tri, trisaccharide; tetra, tetrasaccharide.
For the protection study, inbred female BALB/c mice were obtained from the Animal Laboratory of Utrecht University. The Ethics Committee on Animal Experimentation of University Medical Center—Utrecht approved the animal experiments described herein. Mice 8 to 10 weeks old were subcutaneously immunized with the conjugates at four sites (2.5 μg of saccharide per mouse) and received a similar booster after 3 weeks. Control mice were injected with either CRM197 alone, the monosaccharide Glc or GlcA coupled to CRM197, or the buffer used for preparation of the conjugates. Blood samples were taken 1 day before the booster and at weeks 2 and 5 after the booster. The mice were challenged intraperitoneally 2 weeks after the last blood sampling with a 20× 50% lethal dose (400 CFU) of S. pneumoniae type 3 (ATTC 6303; Rockville, Md.). Survival of mice was recorded daily for 14 days, after which blood was withdrawn for evaluation of the immune response after the infection.
Antibodies binding to PS3 were measured by enzyme-linked immunosorbent assay. Plates (Nunc Laboratories, Roskilde, Denmark) were coated with PS3 (1 μg/ml in saline) overnight at 37°C. After being blocked with phosphate-buffered saline (pH 7.4)–3% gelatin, serum dilutions made in phosphate-buffered saline supplemented with 0.05% Tween 20 and 3% Protifar (Nutricia, Zoetermeer, The Netherlands) were transferred to the coated plates and incubated for 1 h at 37°C. After repeat washings the binding of immunoglobulin M (IgM) or IgG antibodies was determined with goat anti-mouse IgM or IgG coupled to horseradish peroxidase (Nordic Immunological Laboratories, El Toro, Calif.). The amount of bound peroxidase was visualized by incubation with a solution of 3,3′,5,5′-tetramethylbenzidine (Sigma Chemical Co., St. Louis, Mo.) and H2O2. After 20 min the reaction was stopped with 0.1 M H2SO4 and optical density was measured at 450 nm with a microplate reader (Bio-Rad model 3550). Antibody titers were defined as the log10 of the dilution giving twice the absorbance value calculated against that of sera of control mice (immunized with the buffer), with a minimum value of 0.2.
Groups of four mice were immunized subcutaneously with oligosaccharide-protein conjugates (2.5 μg of carbohydrate per mouse). After the first immunization no IgM or IgG PS3 binding antibodies were detected (data not shown). IgG antibodies were present 2 weeks after the second immunization (data not shown), and titers did not change after another 3 weeks. As shown in Table 2, all mice immunized with the tri- and tetrasaccharide-CRM197 conjugates developed PS3 binding IgG antibodies. In each of the two disaccharide-CRM197 immunized groups there was one mouse in which antibodies were not detectable, even after the booster. The mice immunized with the tenfold-lower dose of the tetrasaccharide-2 conjugate developed a slightly lower level of antibodies.
TABLE 2.
Antibody development and outcome of infection in individual micea
| Oligosaccharide conjugate | Log IgG titer before infection for mouse no.:
|
Survival time (days) for mouse no.:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| CRM | <2.0 | <2.0 | <2.0 | NT | 2 | 3 | 3 | NT |
| CRM Glc | <2.0 | <2.0 | <2.0 | <2.0 | 3 | 3 | 3 | 3 |
| CRM GlcA | <2.0 | <2.0 | <2.0 | <2.0 | 3 | >14 | >14 | 3 |
| Buffer | <2.0 | <2.0 | <2.0 | NT | 3 | 3 | 3 | NT |
| CRM di-1 | 4.0 | 4.3 | <2.0 | 4.2 | >14 | >14 | 4 | >14 |
| CRM di-2 | 2.8 | 3.1 | <2.0 | 3.2 | >14 | >14 | 2 | >14 |
| CRM tri-1 | 4.6 | 4.0 | 3.4 | 3.7 | >14 | >14 | >14 | >14 |
| CRM tri-2 | 4.7 | 4.0 | 4.7 | 4.5 | >14 | >14 | >14 | >14 |
| CRM tri-3 | 4.5 | 4.3 | 4.4 | 4.3 | >14 | >14 | >14 | >14 |
| CRM tetra-1 | 4.5 | 4.6 | 4.1 | 4.3 | >14 | >14 | >14 | >14 |
| CRM tetra-2 | 4.5 | 4.4 | 3.9 | 3.8 | >14 | >14 | >14 | >14 |
| CRM tetra-2 (0.25 μg) | NT | <2.0 | 2.6 | 3.4 | NT | >14 | >14 | >14 |
| CRM tetra-4 | 3.4 | 4.1 | 4.7 | 3.4 | >14 | >14 | >14 | >14 |
Groups of four female BALB/c mice were immunized subcutaneously with 2.5 μg of saccharide as a protein conjugate with a different saccharide epitope density. Control mice received CRM197 alone, CRM197 coupled to the monosaccharide Glc or GlcA, or the buffer solution. One group received a tenfold-lower dose of the CRM197 tetrasaccharide (tetra)-2 conjugate (0.25 μg of saccharide). A similar booster was given 3 weeks after the first injection. Blood samples were taken 2 weeks before the challenge, and individual sera were tested for the presence of IgG antibodies recognizing PS3. An intraperitoneal challenge with a 20× 50% lethal dose of type 3 S. pneumoniae was given 7 weeks after the booster. Survival was recorded daily for 14 days. di, disaccharide; tri, trisaccharide; NT, not tested.
Upon intraperitoneal challenge with a lethal dose of S. pneumoniae type 3, all mice with PS3-specific antibodies survived (Table 2). All control mice died within 4 days, except for two mice in the group injected with GlcA-CRM197. Two mice in each of the disaccharide-CRM197 immunized groups, with no detectable antibodies, also died. There was no influence of the saccharide density on the immunogenic capacity of the conjugate vaccines.
In earlier studies, Snippe et al. (13) demonstrated that a hexasaccharide, coupled without a spacer to keyhole limpet hemocyanin (KLH) and so, in fact, reduced to a pentasaccharide, was able to induce protective immunity against type 3 pneumococci in mice. A small amount of IgM was measured 1 week after the first immunization, and high levels of IgG were detected after booster injections. Furthermore, a protective immunity has been observed in mice after several injections with polysaccharide type 6B-related synthetic spacered tetrasaccharide-KLH conjugates, whereas the di- and trisaccharide-KLH conjugates induced a low antibody response with no or partial protection against a lethal dose of type 6B pneumococci (W. T. M. Jansen, S. Hogenboom, M. J. Thijssen, J. P. Kamerling, J. F. G. Vliegenthart, J. Verhoef, H. Snippe, and A. F. M. Verheul, submitted for publication). Rabbits, immunized with these conjugates, developed antibodies which could passively protect mice. Our results show that for type 3 pneumococci, the disaccharide conjugate is already large enough to generate protection in mice without the use of adjuvant.
The study of Laferrière et al. (4) demonstrated that conjugation of a different carbohydrate chain length (with an average length of 8, 16, 27, and 37 repeating units) derived from PS3 to tetanus toxoid (TT) did not result in a distinct immune response in rabbits. However, it should be noted that pools of average chain lengths were used, which complicates interpretation of the results. Rabbits immunized with those conjugates developed PS3 binding IgG antibodies, but the sera showed a low opsonophagocytic capacity, suggesting that there were no protective antibodies, since protection against pneumococci by type-specific antibodies is mediated by opsonization and phagocytosis of the bacteria. In a previous study conjugates with carbohydrate chains of pneumococcal strains 6A, 18C, 19F, and 23F induced in rabbits high levels of polysaccharide binding serum antibodies with an effective opsonophagocytic capacity. Only in the case of the 19F-TT conjugate was a decrease in immunogenicity with increasing saccharide chain length observed. It was also demonstrated by Paoletti et al. (8) that, in rabbits immunized with (on average) 6, 14, and 25 repeating units of the polysaccharide of group B Streptococcus type III conjugated to TT, an opsonophagocytic immune response could be evoked. These rabbit sera could passively protect mice against a lethal dose of type III group B Streptococcus, although full protection was seen only with the serum of 14-TT immunized rabbits. The study of Pozsgay et al. (10) on Shigella dysenteriae type 1 using precisely defined synthetic oligosaccharide-human serum albumin (HSA) conjugates shows a different influence of the chain length with different oligosaccharide loading, stressing the importance of varying only one parameter. In our study we found a correlation between PS3-specific antibodies and protective capacity of all the conjugates. The mechanism of the observed protection is not yet certain, since the opsonophagocytic capacities of these sera are still under investigation.
In our study, a change in carbohydrate/protein ratios of the conjugate vaccines did not influence their immunogenic capacity. Also in the study of Laferrière et al. (4), with PS3-derived oligosaccharide-TT conjugates there was no relationship between the number of polysaccharide chains incorporated and the immunogenicity of the conjugates. Observations made by Pozsgay et al. (10) with synthetic tetra-, octa-, dodeca-, and hexadecasaccharide fragments of the lipopolysaccharide of S. dysenteriae type 1 (HSA) conjugates showed high levels of lipopolysaccharide binding IgG antibodies in mice after three injections, except for the tetrasaccharide conjugate, which showed a low immunogenicity and was not further evaluated. The influence of the carbohydrate/protein ratio was different for the three remaining conjugates. The octasaccharide-HSA conjugate with the highest density evoked a good immune response, while the dodeca- and hexadecasaccharide conjugates with the median density were the optimal immunogens. They explain this as the covering effect of the protein carrier by the saccharide chains, which is related to the size of the saccharide and influences the availability of antigenic peptides to associate with major histocompatibility complex class II molecules on B cells and thus influences the stimulation of T cells. This could explain our results with short synthetic saccharides with a different carbohydrate/protein ratio that showed no differences in immunogenicity, although in the present study a different carrier protein was used.
Studies performed with different oligosaccharide conjugate vaccines show that they are good immunogens but that the immunogenicity of these conjugates is not clearly determined by chain length and saccharide density. This indicates that the immunogenicity of saccharide conjugates depends on the immunological properties of the saccharide and protein used. Furthermore, oligosaccharides derived by acid hydrolysis of polysaccharides are mixtures of oligosaccharides, differing in reducing and nonreducing ends. Also, the coupling methodology and the structure of the reducing monosaccharide after reduction can influence the immunogenic properties of the conjugate. Synthesis of saccharides and coupling of them to the carrier protein in a well-controlled process, thereby varying only one parameter as we did in our study, make it possible to study the influence of two parameters, chain length and density, that determine the immunogenicity of conjugate vaccines.
Important questions concerning the observed immune response remain and will be subjects for further study with regard to the nature of the protective immune responses we measured, such as opsonophagocytic capacity, subclass distribution, and change in antigenic specificity, like immune response against proteins and other pneumococcal components in sera taken before and after challenge with viable S. pneumoniae.
REFERENCES
- 1.Butler J C, Shapiro E D, Carlone G M. Pneumococcal vaccines: history, current status, and future directions. Am J Med. 1999;107:69S–76S. doi: 10.1016/s0002-9343(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 2.Jennings H. Further approaches for optimizing polysaccharide-protein conjugate vaccines for prevention of invasive bacterial disease. J Infect Dis. 1992;165(Suppl 1):S156–S159. doi: 10.1093/infdis/165-supplement_1-s156. [DOI] [PubMed] [Google Scholar]
- 3.Kalin M. Pneumococcal serotypes and their clinical relevance. Thorax. 1998;53:159–162. doi: 10.1136/thx.53.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laferrière C A, Sood R K, de Muys J M, Michon F, Jennings H J. The synthesis of Streptococcus pneumoniae polysaccharide-tetanus toxoid conjugates and the effect of chain length on immunogenicity. Vaccine. 1997;15:179–186. doi: 10.1016/s0264-410x(96)00148-x. [DOI] [PubMed] [Google Scholar]
- 5.Magnuson K, Hermansson A, Hellström S. Healing of tympanic membrane after myringotomy during Streptococcus pneumoniae otitis media. An otomicroscopic and histologic study in the rat. Ann Otol Rhinol Laryngol. 1996;105:397–404. doi: 10.1177/000348949610500513. [DOI] [PubMed] [Google Scholar]
- 6.Malley R, Stack A M, Ferretti M L, Thompson C M, Saladino R A. Anticapsular polysaccharide antibodies and nasopharyngeal colonization with Streptococcus pneumoniae in infant rats. J Infect Dis. 1998;178:878–882. doi: 10.1086/597600. [DOI] [PubMed] [Google Scholar]
- 7.Ostergaard C, Benfield T, Gesser B, Kharazmi A, Frimodt M N, Espersen F, Lundgren J D. Pretreatment with granulocyte colony-stimulating factor attenuates the inflammatory response but not the bacterial load in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect Immun. 1999;67:3430–3436. doi: 10.1128/iai.67.7.3430-3436.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paoletti L C, Kasper D L, Michon F, DiFabio J, Jennings H J, Tosteson T D, Wessels M R. Effects of chain length on the immunogenicity in rabbits of group B Streptococcus type III oligosaccharide-tetanus toxoid conjugates. J Clin Investig. 1992;89:203–209. doi: 10.1172/JCI115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poland G A. The burden of pneumococcal disease: the role of conjugate vaccines. Vaccine. 1999;17:1674–1679. doi: 10.1016/s0264-410x(98)00435-6. [DOI] [PubMed] [Google Scholar]
- 10.Pozsgay V, Chu C, Pannell L, Wolfe J, Robbins J B, Schneerson R. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc Natl Acad Sci USA. 1999;96:5194–5197. doi: 10.1073/pnas.96.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 12.Snippe H, van Dam J E, Van Houte A J, Willers J M, Kamerling J P, Vliegenthart J F G. Preparation of a semisynthetic vaccine to Streptococcus pneumoniae type 3. Infect Immun. 1983;42:842–844. doi: 10.1128/iai.42.2.842-844.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snippe H, Van Houte A J, van Dam J E G, De Reuver M J, Jansze M, Willers J M N. Immunogenic properties in mice of hexasaccharide from the capsular polysaccharide of Streptococcus pneumoniae type 3. Infect Immun. 1983;40:856–861. doi: 10.1128/iai.40.3.856-861.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein K E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165(Suppl 1):S49–S52. doi: 10.1093/infdis/165-supplement_1-s49. [DOI] [PubMed] [Google Scholar]
- 15.Tietze L F, Arlt M, Beller M, Glüsenkamp K-H, Jähde E, Rajewski M F. Squaric acid diethyl ester: a new coupling reagent for the formation of drug biopolymer conjugates. Synthesis of squaric acid ester amides and diamides. Chem Ber. 1991;124:1215–1221. [Google Scholar]