Abstract
The radiation-induced inflammatory response is involved in radiation damage to the cochlea and causes sensorineural hearing loss (SNHL). NF-κB, as the master switch of the inflammatory response, regulates the expression of many inflammation-related genes and thus the inflammatory response. Therefore, in this study we used a mouse model to determine whether radiation-induced NF-κB activation is involved in damage to the cochlea and to investigate the underlying mechanism. Eventually, we found that NF-κB was activated after radiation of the cochleae and the activation reached a maximum at 2–6 h after radiation. And morphological analysis showed severe damage to the cochleae after radiation, but this damage was significantly ameliorated by JSH-23 (an inhibitor of NF-κB) pretreatment. Along with these morphological changes, the expression levels of proinflammatory molecules (including proinflammatory cytokines IL-6, TNF-α, COX-2 and inflammation-related proteins VCAM-1, MIP-1β) in the cochlear tissues were significantly increased after radiation, but were significantly decreased by JSH-23 pretreatment compared to radiation alone. Therefore, these results indicated that radiation-induced NF-κB activation was involved in damage to the cochleae and resultant SNHL via its promotion of the inflammatory response mediated by overexpression of some proinflammatory molecules in cochlear tissues, and inhibition of radiation-induced NF-κB was conducive to preventing such damage.
Keywords: cochleae, inflammatory response, nuclear factor kappa B (NF-κB), proinflammatory molecules, radiation
INTRODUCTION
Head and neck cancer is one of the most common malignant tumors in the world and radiotherapy is an important treatment. Due to its position adjacent to the primary tumor, the inner ear often receives radiation during radiotherapy for head and neck cancer, especially in cases of nasopharyngeal carcinoma [1,2] and, consequently, radiation damage to the inner ear is often inevitable. As one of the important structures of the inner ear, cochlear involvement and subsequent sensorineural hearing loss (SNHL) are characteristic features of radiation ototoxicity and have been reported widely [3]. For the majority of patients who achieve long-term survival after radiotherapy for head and neck cancer, radiation-induced cochlear damage and SNHL inevitably affect their quality of life.
For a long time, the radiation dose to the inner ear was considered to be responsible for the damage to the cochlea and SNHL [4]. However some studies have reported that the inflammatory response induced by radiation can cause apoptosis of hair cells of the inner ear [5], and a large number of inflammatory cells appear in the inner ear of irradiated experimental animals [6,7]. Our previous experimental study of mice found the proinflammatory resolution mediator Resolvin E1 obviously ameliorated post-radiation damage to the cochlear hair cells [7]. The research indicated that inflammation induced by radiation was involved in damage to the cochlea and SNHL in addition to the direct effects of the radiation. Consequently anti-inflammatory intervention would be conducive to ameliorating radiation-induced cochlear damage and SNHL, and be worthy of further study. However the mechanisms of the inflammatory response induced by radiation, which are involved in damage to the cochlea and SNHL, have not been elucidated.
The inflammatory response is characterized by coordinated activation of various signaling pathways that regulate expression of both pro- and anti-inflammatory mediators in resident tissue cells and leukocytes recruited from the blood [8]. Nuclear factor kappa B (NF-κB) is an evolutionarily-conserved transcription factor that is a major regulator of inflammation and controls the expression of several hundreds of target genes associated with inflammation [9,10]. NF-κB is expressed in almost all cell types and plays a central role in the inflammatory response and in disease [11,12] by regulating leukocyte recruitment, the expression of proinflammatory cytokines, immunoregulatory proteins and factors that regulate cell proliferation and apoptosis [8,11]. NF-κB can be activated by a vast range of stimuli, including microbial components and endogenous damage-associated molecular pathways [13,14]. NF-κB can also be activated by environmental stresses including ultraviolet light, radiation and reactive oxygen species [11,15]. Indeed, that radiation can activate the NF-κB pathway has been well acknowledged, and radiation-induced NF-κB activation is known to be initially triggered by ataxia telangiectasia mutated protein, which is activated by DNA double-strand breaks after radiation [15–17]. Oxidative stress arising from exposure to ionizing radiation might also contribute to NF-κB activation [11,15]. Radiation-induced NF-κB activation involved in damage to normal tissues in radiation fields has been studied, and there are reports that NF-κB activation mediates radiation-induced damage to organs including the lung [18], intestines [19,20] and testis [21]. However there have been no reports of radiation-induced NF-κB activation in relation to damage to the cochlea.
Based on a previous report that the inflammatory response induced by radiation is involved in damage to the cochlea [7], and the central role of NF-κB in the inflammatory response and disease [8–11], we had reasons to believe that radiation-induced NF-κB activation may be involved in damage to the cochlea. Thus we designed this animal experiment to explore the regulatory effects of radiation-induced NF-κB activation in damage to the cochlea and to provide an experimental foundation for cochlear protection during radiotherapy for head and neck cancer.
MATERIALS AND METHODS
Animals and radiation
Two-month-old C57BL/6 female mice were purchased from Jieruikang Biotechnology (Jinan, Shandong, China) for this experiment, and all animal procedures in this study were approved by the Ethics Committee of the Second Hospital of Shandong University (Permit Number: KYLL-2022LW104).
Radiation method
Experimental mice were irradiated with an Elekta Synergy medical linear accelerator (Elekta, Stockholm, Sweden), and 6 MV-X ray was selected for radiation of the inner ear from both sides with the source axis distance (SAD) technique, which required a SAD of 100 cm. The radiation depth of the inner ear was 0.5 cm, which was determined by thin computed tomography scan with a sample mouse. The size of the radiation field was 1 cm × 1 cm, which fully enveloped the inner ear. The thickness of the lead block was 10 cm, and the radiometric rate was 400 cGy/min. All of these radiation procedures were the same as used in our previous studies [7].
Radiation dose
A dose of 20 Gy was selected to irradiate the inner ear from both sides in one fraction. This dose was determined according to the results of the previous animal experiment, and ensured robust survival of all experimental mice along with observable hearing loss and detectable morphological damage to the cochlea, which could be observed via auditory brainstem response, hematoxylin & eosin (H&E) staining and scanning electron microscopy (SEM) [7].
Before radiation, 10% chloral hydrate (the Second Hospital of Shandong University, Jinan, China) was used for enterocelic anesthesia at a dose of 1000 mg/kg, without any signs of peritonitis after administration.
Experimental groups and design
To evaluate whether NF-κB was activated and the activation time, 18 mice were used. Before radiation and at 2, 4, 6, 8 and 10 h after radiation, three mice were randomly chosen for sacrifice to evaluate whether NF-κB was activated, which was assessed by Western blot analysis by detecting translocation of p65 between the cytoplasm and the nucleus.
To evaluate the effects of NF-κB activation on cochlear damage, 48 mice were randomly divided into four groups: wild-type (WT), JSH-23-pretreated wild type (JSH-23), X-ray radiation (Rad) and JSH-23-pretreated X-ray radiation (Rad+ JSH-23). On days 7 and 14 after radiation, six mice in each group were sacrificed to observe any morphological changes of the cochleae, detect changes in the expression of proinflammatory cytokines and analyze changes in expression of inflammation-related proteins downstream of the NF-κB pathway in cochlear tissues.
Activation and inhibition of NF-κB
After radiation, p65 shifts between the cytoplasm and the nucleus were used to evaluate the activation of NF-κB. 4-Methyl-N1-(3-phenyl-propyl)-benzene-1,2-diamine (JSH-23) (MedChemExpress, Monmouth Junction NJ, USA), an inhibitor of NF-κB, which inhibits nuclear translocation of p65 without affecting IκBα degradation, can inhibit NF-κB transcriptional activity with an IC50 of 7.1 μM in lipopolysaccharide (LPS)-stimulated macrophages RAW 264.7 and is used to inhibit NF-κB activation in this study.
For one week before radiation and one week after radiation, JSH-23 was administered every other day via intraperitoneal injection for a total of seven doses. The detailed administration method was as follows: on the day of administration, 10 mg JSH-23 was dissolved in 3.6 mL corn oil (MedChemExpress) mixed with 0.4 mL DMSO (MedChemExpress), and then diluted 10 times with isotonic saline and administered to the mice via intraperitoneal injection at a dose of 5 mg/kg according to the manufacturer’s protocol.
Hematoxylin & eosin staining
H&E staining was used to observe morphological changes to the cochleae before and after radiation. Mice were sacrificed by cervical dislocation, and the cochleae were removed as soon as possible, transferred to precooled 4% paraformaldehyde (PFA) and perfused. After that, the cochlear tissues were placed overnight at 4°C and then washed three times with 1 × PBS for 10 min each, followed by 10% EDTA for decalcification. Three days later, cochleae were washed with 1 × PBS and then soaked successively in 15%, 20% and 30% sucrose for 2 hours each. Next the cochleae were put into a sample mold of 10 mm × 10 mm × 5 mm, and the mold was frozen quickly on a mixture of dry ice and anhydride ethanol. A Leica CM1950 (Leica Microsystems Ltd, Wetzlar, Germany) cryostat was used to cut 8 μm frozen sections. After the slices were dried at 60°C, H&E staining was performed with an H&E staining kit.
Scanning electron microscopy
SEM was used to observe ultrastructural changes in the morphology of cochlear hair cells before and after radiation, and to further confirm the morphological changes of the cochleae observed by H&E staining. Cochleae were decalcified in 10% EDTA for 3 days, soaked in precooled 2.5% glutaraldehyde, and then dissected to obtain the basal membrane. Basal membrane specimens were washed three times with 1 × PBS for 10 min each, then osmium tetroxide was added in a fume hood. Samples were washed again three times with 1 × PBS and four times with sterile distilled water, before dehydrating through an ethanol gradient. Next, the critical point was selected for drying the sample with carbon dioxide for 2 h. Dried specimens were sputter coated with gold for 4 min. Finally, the samples were observed under a scanning electron microscope (Zeiss sigma 300, Carl Zeiss Microscopy GmbH, Jena, Germany).
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was used to detect changes in the expression levels of proinflammatory cytokines in the cochlear tissues before and after radiation. At the indicated days, mice were sacrificed for dissection of the cochleae. RNA was extracted from each cochlear specimen using TRIzol reagent (Tiangen Biotech, Beijing, China) according to the manufacturer’s protocol. After total RNA extraction, the purity and concentration of RNA were quantified using a Nano Drop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) to ensure that total RNA met the requirements for the experiment. Total cDNA was synthesized using a UEIris II RT-PCR System (Yuheng Biotechnology, Suzhou, China). Using cDNA as a template, changes in the expression of proinflammatory cytokines (interleukin [IL]-6, tumor necrosis factor [TNF]-α and cyclooxygenase [COX]-2) in the cochlear tissues were analyzed by qRT-PCR. The qRT-PCR reactions were carried out using a Universal SYBR Green qPCR Supermix (Yuheng Biotechnology) and the reaction conditions were as follows: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 5 s, annealing at 55°C for 15 s and extension at 72°C for 30 s; 40 cycles. Relative gene expressions were calculated using the comparative 2−△△Ct method with the housekeeping gene mus-β-actin as the reference. All primers were synthesized by GENEWIZ (Suzhou, Jiangsu, China) and sequences were as follows: TNF-α forward primer: 5′-AGCCGATGGGTTGTACCTTG-3′, reverse primer: 5′-ATAGCAAATCGGCTGACGGT-3′ (99 bp). IL-6 forward primer: 5′-CTTCTTGGGACTGATGCTGGT-3′, reverse primer: 5′-CTCTGTGAAGTCTCCTCTCCG-3′ (73 bp). COX-2 forward primer: 5′-TGCTGGTGGAAAAACCTCGT-3′, reverse primer: 5′-AAAACCCACTTCGCCTCCAA-3′ (149 bp). β-actin forward primer: 5′-GGCTGTATTCCCCTCCATCG-3′, reverse primer: 5′-CCAGTTGGTAACAATGCCATGT-3′ (154 bp).
Western blot analysis
Western blot analysis was used to evaluate whether NF-κB was activated and the activation time, as well as the effects of NF-κB activation on cochlear damage.
To evaluate whether NF-κB was activated and the activation time, translocation of p65 between cytoplasm and nucleus was analyzed. At the indicated time points, mice were sacrificed and the cochlear tissues were dissected. Proteins were extracted from each cochlear tissue specimen using a nuclear and cytoplasmic protein extraction kit (Beyotime Biotechnology, Shanghai, China) as appropriate, according to the manufacturer’s protocol. To ensure that extracted proteins met the requirements for the experiment, the concentration of protein was quantified using a BCA protein determination kit (Boster Biotechnology, Wuhan, China). Then, the proteins were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Sparkjade, Jinan, China) and electrotransferred onto a polyvinylidene difluoride membrane (Pall Corp, Port Washington, NY, USA). The membrane was blocked with 5% skimmed milk for 2 h and incubated with primary antibodies (including anti-NF-κB p65 (RELA) antibody, anti-β-actin antibody and anti-PCNA antibody) (Boster Biotechnology) against target proteins overnight at 4°C, followed by incubation with the corresponding secondary antibodies labeled with horseradish peroxidase (Boster Biotechnology) for 1 h. Finally, bands were visualized by electrochemiluminescence (Merck-Millipore, Darmstadt, Germany) and the densities of the bands were quantified using ImageJ software (NIH, Bethesda, MD, USA). PCNA was used as a reference for nuclear protein and β-actin was used as a reference for cytoplasmic protein.
To evaluate the effects of NF-κB activation on cochlear damage, expression changes of the inflammation-related proteins: vascular cell adhesion molecule-1 (VCAM-1) and macrophage inflammatory protein-1β (MIP-1β) in the pathway downstream of NF-κB were analyzed. At the indicated days, mice were sacrificed and the cochlear tissues were dissected, and then transferred to RIPA lysis buffer (Beyotime Biotechnology) containing a protease inhibitor at a dose of 200 μL per 20 mg of tissue. After samples were fully lysed at 4°C in a homogenizer, the lysate was centrifuged at 12000 × g for 5 min, and the supernatant was taken as the protein solution. The expression of VCAM-1 and MIP-1β was then analyzed by Western blotting as described above.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM SPSS Statistics for Windows, Armonk, NY, USA). The expression levels of proinflammatory cytokines and the densities of the protein bands are expressed as the mean ± standard error of the mean (SEM) and differences among different groups were compared with one-way analysis of variance or t-test, while multiple comparisons among different subgroups were tested using the least significant difference (LSD) test. All P-values are two-sided, and a value of P < 0.05 was considered statistically significant.
RESULTS
Radiation-induced NF-κB activation and activation time
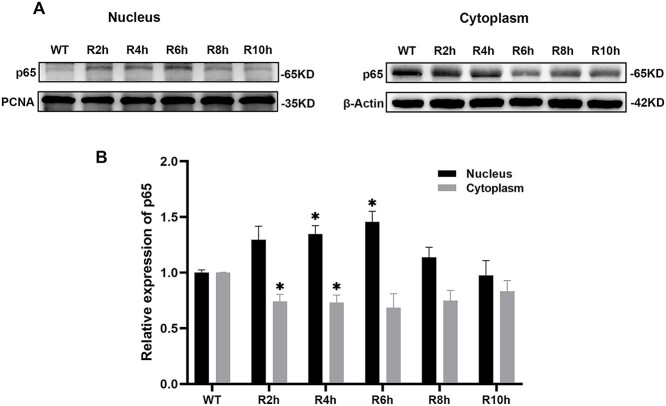
The p65 shifts between cytoplasm and nucleus were used to evaluate the activation of NF-κB. The results showed that the relative expression levels of p65 in the nucleus increased and those in the cytoplasm decreased after radiation of the cochleae. The relative expression peaks of p65 in the nucleus appeared 2–6 h after radiation, and were significantly higher than the relative expression levels in wild type (WT) mice (P < 0.05, Fig. 1 and Table 1). These changes corresponded to a change in the relative expression level of p65 in the cytoplasm, which reached a minimum at the same time, and was also significantly lower than that in WT mice (P < 0.05, Fig. 1 and Table 1). These results indicated that NF-κB was activated after radiation of the cochleae and the activation reached a maximum between 2 and 6 h after radiation.
Fig. 1.
Effect of radiation on the activation of NF-κB in mice cochleae. A: Western blot analysis of p65 in the cytoplasm and nucleus of cochlear tissues. β-actin or PCNA were used as internal controls. β-actin was undetectable in the nuclear fraction and PCNA was undetectable in the cytosolic fraction. B: Relative expression levels of p65 in cytoplasm and nucleus at different time points. The relative expression levels of p65 in the nucleus increased while those in the cytoplasm decreased after radiation of the cochleae compared with WT mice. * = P < 0.05.
Table 1.
Relative expression levels of p65 in cytoplasm and nucleus at different times
| Group | cytoplasm | nucleus | ||
|---|---|---|---|---|
| mean ± SEM | P value * | mean ± SEM | P value * | |
| WT | 1.000 ± 0.004 | 1.000 ± 0.024 | ||
| R2h | 0.741 ± 0.063 | 0.026 | 1.297 ± 0.121 | 0.134 |
| R4h | 0.729 ± 0.070 | 0.030 | 1.346 ± 0.077 | 0.046 |
| R6h | 0.684 ± 0.125 | 0.086 | 1.457 ± 0.093 | 0.039 |
| R8h | 0.748 ± 0.093 | 0.073 | 1.139 ± 0.088 | 0.255 |
| R10h | 0.831 ± 0.097 | 0.182 | 0.974 ± 0.134 | 0.865 |
*: all compared with WT mice (wild-type).
SEM: standard error of mean.
R: X-ray radiation.
Morphological changes of the cochleae after radiation-induced NF-κB activation and inhibition
Cochlear tissues of each group were collected on days 7 and 14 after radiation. Morphological changes of the basement membrane hair cells in the cochlea were observed by H&E staining (Fig. 2), which showed obvious loss of basement membrane hair cells in the cochlea on days 7 and 14 after radiation. The points of loss are indicated by arrows. JSH-23 pretreatment did not eliminate the damage, but the degree of damage was substantially reduced compared to radiation alone (Fig. 2). These results indicated that activation of NF-κB was involved in the damage caused to cochlear hair cells by radiation, and demonstrated that inhibition of NF-κB had radioprotective effects on cochlear hair cells.
Fig. 2.
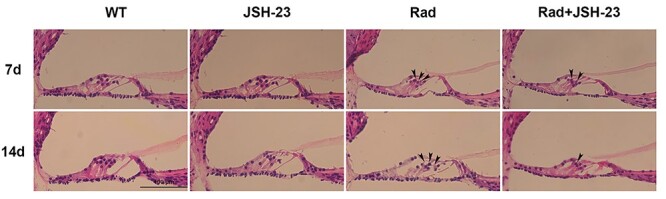
H&E staining showing the changes of cochlear hair cells in different groups. The arrows indicate the positions of the loss of basement membrane hair cells. An obvious reduction in the number of hair cells can be observed at the points indicated by the arrows in the Rad group compared with the WT group. Pretreatment with JSH-23 clearly reduced this damage.
Ultrastructural changes of the cochleae after radiation-induced NF-κB activation and inhibition
At the same time as H&E staining, SEM was also performed to observe ultrastructural changes to the cochlear hair cells after radiation. The results (Fig. 3) also showed obvious loss of the cilia of basement membrane hair cells on days 7 and 14 after radiation; the asterisks indicate the points of the loss. JSH-23 pretreatment alleviated the degree of cochlear hair cell damage compared to radiation alone (Fig. 3). These results further confirmed that activation of NF-κB was involved in the damage to cochlear hair cells after radiation, and established that inhibition of NF-κB had radioprotective effects on cochlear hair cells.
Fig. 3.
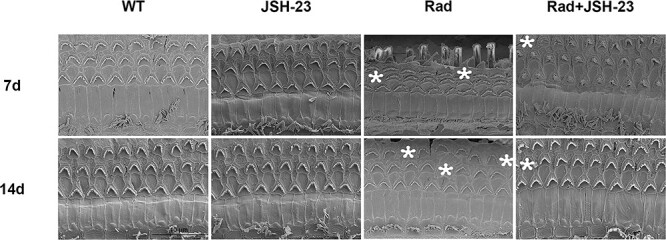
SEM for the changes of the cilia of basement membrane hair cells in different groups. The asterisks indicate the points for the loss of the cilia of basement membrane hair cells, and the number of asterisks represents the frequency of damage, which could be observed on days 7 and 14 after radiation. However, pretreatment with the NF-κB inhibitor JSH-23 obviously reduced this damage.
Expression changes of proinflammatory cytokines in the cochlear tissues after radiation-induced NF-κB activation or inhibition
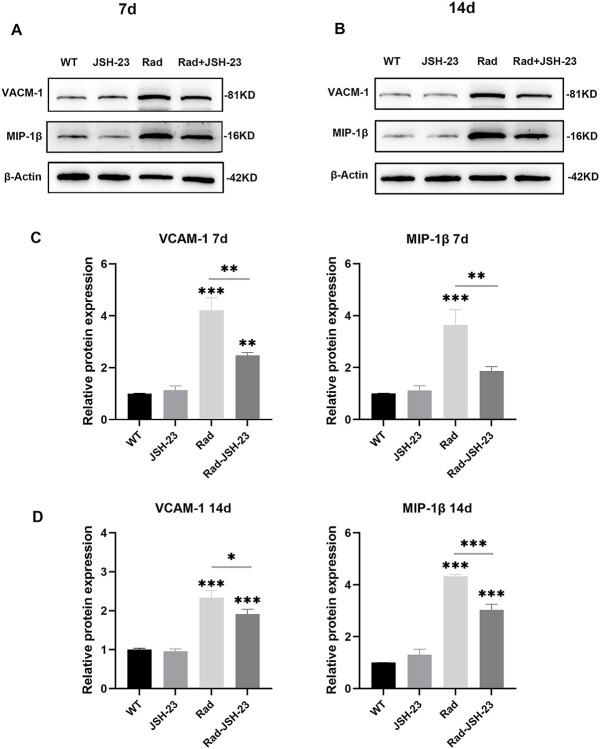
After radiation-induced NF-κB activation or inhibition, changes in expression of the proinflammatory cytokines IL-6, TNF-α and COX-2 in the cochlear tissues of mice were analyzed by qRT-PCR. The results showed that the expression levels of IL-6, TNF-α, and COX-2 in the cochlear tissues were significantly increased on day 7 after radiation, while after pretreatment with JSH-23, these increases were obviously reduced compared to radiation alone (P < 0.05, Fig. 4 and Table 2). On day 14 after radiation, similar expression changes of these proinflammatory cytokines in the cochlear tissues were observed (P < 0.05, Fig. 4 and Table 2), but the levels were lower compared to those on day 7. These results suggested that along with activation of NF-κB after radiation, there were also significant increases in the expression levels of some proinflammatory cytokines in the cochlear tissues, but these increases could be blocked by inhibition of NF-κB.
Fig. 4.
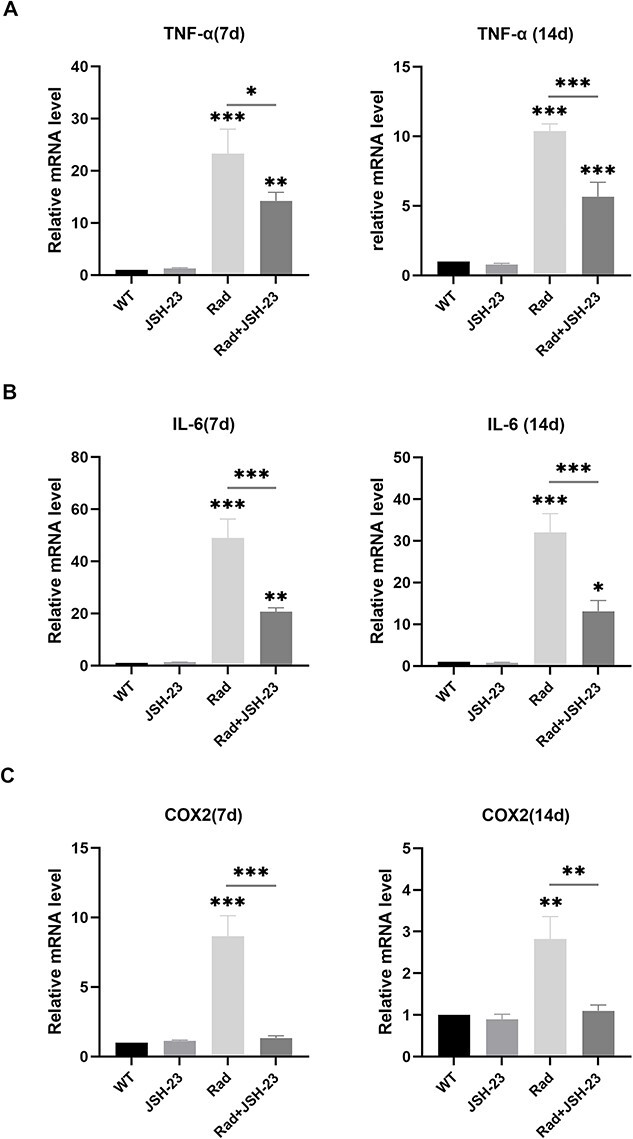
Expression changes of proinflammatory cytokines in cochlear tissues in the different groups. The expression levels of IL-6, TNF-α, and COX-2 in cochlear tissues were all significantly increased on day 7 and day 14 after radiation (all compared with WT groups), and all these significant changes after radiation were ameliorated by JSH-23 pretreatment (comparisons between the Rad and JSH-23 groups) * = P ≤ 0.05; ** = P ≤ 0.01; *** = P ≤ 0.001.
Table 2.
Expression levels of proinflammatory cytokines in cochlear tissues in different groups
| Time | Group | TNF-α | IL-6 | COX-2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SEM | P value | mean ± SEM | P value | mean ± SEM | P value | ||||||||
| Day 7 | WT | 1.000 ± 0.000 | 0.001* | 1.000 ± 0.000 | 0.000* | 1.000 ± 0.000 | 0.000* | ||||||
| JSH-23 | 1.276 ± 0.126 | 0.940** | 1.298 ± 0.059 | 0.956** | 1.130 ± 0.046 | 0.905** | |||||||
| Rad | 23.237 ± 4.759 | 0.000** | 0.035*** | 48.961 ± 7.305 | 0.000** | 0.001*** | 8.646 ± 1.484 | 0.000** | 0.000*** | ||||
| Rad + JSH-23 | 14.231 ± 1.612 | 0.006** | 20.663 ± 1.522 | 0.006** | 1.337 ± 0.153 | 0.758** | |||||||
| Day 14 | WT | 1.000 ± 0.000 | 0.000* | 1.000 ± 0.000 | 0.000* | 1.000 ± 0.000 | 0.004* | ||||||
| JSH-23 | 0.763 ± 0.105 | 0.780** | 0.725 ± 0.172 | 0.943** | 0.895 ± 0.125 | 0.801** | |||||||
| Rad | 10.370 ± 0.522 | 0.000** | 0.000*** | 31.962 ± 4.545 | 0.000** | 0.001*** | 2.821 ± 0.540 | 0.002** | 0.003*** | ||||
| Rad + JSH-23 | 5.660 ± 1.030 | 0.000** | 13.107 ± 2.576 | 0.011** | 1.099 ± 0.142 | 0.084** | |||||||
WT: wild-type group; JSH-23: JSH-23-pretreated wild type group; Rad: X-ray radiation group; Rad+JSH-23: JSH-23-pretreated X-ray radiation group.
TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; COX-2: cyclooxygenase-2.
SEM: standard error of mean.
* results of one-way analysis of variance.
** all compared with WT groups
*** comparisons between the Rad and JSH-23 groups
Expression changes of inflammation-related proteins in cochlear tissues after radiation-induced NF-κB activation or inhibition
After radiation-induced NF-κB activation or inhibition, changes in the expression of some inflammation-related proteins in the cochlear tissues were also analyzed by Western blotting. The results showed that the levels of the inflammation-related proteins VCAM-1 and MIP-1β in the cochlear tissues were significantly increased on days 7 and 14 after radiation, while after pretreatment with JSH-23, expression of these inflammation-related proteins was obviously decreased compared to radiation alone (P < 0.05, Fig. 5 and Table 3). These results suggested that as well as the activation of NF-κB observed after radiation, the expression levels of some inflammation-related proteins in the cochlear tissues were significantly increased, but that inhibition of NF-κB suppressed the elevation of these inflammation-related proteins.
Fig. 5.
Expression changes of inflammation-related proteins in cochlear tissues in the different groups. A and B: Western blot analysis of the expressions of VCAM-1 and MIP-1β in cochlear tissues on days 7 and 14 in the different groups. C and D: Relative expression levels of VCAM-1 and MIP-1β in cochlear tissues on days 7 and 14 in the different groups. The two inflammation-related proteins were significantly increased on days 7 and 14 after radiation (all compared with WT groups), and all these significant changes after radiation were ameliorated by JSH-23 pretreatment (this was compared between the Rad and JSH-23 groups) * = P ≤ 0.05; ** = P ≤ 0.01; *** = P ≤ 0.001. VCAM-1: vascular cell adhesion molecule-1; MIP-1β: macrophage inflammatory protein-1β.
Table 3.
Expression levels of inflammation-related proteins in cochlear tissues in different groups
| Time | Group | VCAM-1 | MIP-1β | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mean ± SEM | P value | mean ± SEM | P value | ||||||
| Day 7 | WT | 1.000 ± 0.013 | 0.000* | 1.000 ± 0.015 | 0.001* | ||||
| JSH-23 | 1.125 ± 0.171 | 0.747** | 1.110 ± 0.189 | 0.816** | |||||
| Rad | 4.203 ± 0.490 | 0.000** | 0.002*** | 3.637 ± 0.597 | 0.000** | 0.005*** | |||
| Rad + JSH-23 | 2.471 ± 0.111 | 0.004** | 1.861 ± 0.172 | 0.098** | |||||
| Day 14 | WT | 1.000 ± 0.036 | 0.000* | 1.000 ± 0.004 | 0.000* | ||||
| JSH-23 | 0.954 ± 0.064 | 0.786** | 1.308 ± 0.210 | 0.197** | |||||
| Rad | 2.334 ± 0.180 | 0.000** | 0.033*** | 4.325 ± 0.069 | 0.000** | 0.000*** | |||
| Rad + JSH-23 | 1.915 ± 0.123 | 0.000** | 3.030 ± 0.411 | 0.000** | |||||
WT: wild-type group; JSH-23: JSH-23-pretreated wild type group; Rad: X-ray radiation group; Rad+JSH-23: JSH-23-pretreated X-ray radiation group.
VCAM-1: vascular cell adhesion molecule 1; MIP-1β: macrophage inflammatory protein 1 beta.
SEM: standard error of mean.
*results of one-way analysis of variance.
**all compared with WT groups
***comparisons between the Rad and JSH-23 groups
DISCUSSION
Damage to the cochleae and SNHL are characteristic features of radiation ototoxicity subsequent to radiotherapy for head and neck cancer, and have been reported widely [3]. Inevitably these side-effects reduce the quality of life of the cancer survivors. For a long time, the radiation dose received by the cochleae was considered to be responsible for the damage [4]. In our previous study [7], we found that the radiation-induced inflammatory response was involved in the damage to the cochleae and subsequent SNHL, but the mechanisms were not completely elucidated. It is well known that radiation activates NF-κB, which is considered to be the master switch of the inflammatory response and involved in the regulation of many inflammation-related genes [9,10]. Radiation-induced NF-κB activation is known to be involved in the damage to a lot of normal tissues [18–21], but damage to the cochleae caused by the NF-κB-mediated inflammatory response had not been reported. Consequently we designed this animal experiment to explore the regulatory effects of an NF-κB-mediated inflammatory response in radiation-induced damage to the cochlea, in the hope of providing an experimental foundation for cochlear protection during radiotherapy for head and neck cancer.
It is well known that radiation activates NF-κB, that RelA (p65) comprises the predominant NF-κB transcriptional activity and consequently is responsible for the expression of a large number of proinflammatory mediators, and that the nuclear translocation of p65 is considered as an indicator of NF-κB activation, all of which have been reported widely [11,15,22–24]. However, large differences between different tissues and cells have been reported in regard to the radiation dose used to activate NF-κB and the activation time after radiation [15]. Thus in this study, we also chose the p65 shifts between cytoplasm and nucleus to evaluate activation of NF-κB, and first explored whether a radiation dose of 20 Gy activated NF-κB in the cochlear tissues and ascertained the activation time. This radiation dose ensured robust survival of all experimental mice while still causing observable hearing loss and detectable morphological damage to the cochlea as described in our previous animal experiment [7]. The results confirmed that NF-κB was widely activated in cochlear tissues and revealed that the activation reached a maximum at 2–6 h after 20 Gy radiation to the cochleae. The use of this radiation dose to activate NF-κB in the cochlear tissues and the activation time had not been reported previously. These confirmations of NF-κB activation in the cochlear tissues guaranteed that subsequent experiments could be implemented smoothly and that the results obtained would be reliable.
In subsequent experiments, the effects of NF-κB on radiation-induced damage to the cochleae were first investigated. An inhibitor of p65, JSH-23, which is generally accepted as an inhibitor of NF-κB, was used to inhibit NF-κB activation. The results of H&E staining and SEM demonstrated that NF-κB inhibition considerably ameliorated radiation-induced damage to the cochlear hair cells and confirmed that activation of NF-κB in cochlear tissues was involved in the damage to cochlear hair cells after radiation. These results provided the evidence that it was feasible to protect cochlear hair cells from radiation-induced damage by inhibiting the activation of NF-κB in cochlear tissues.
NF-κB-induced genes regulate the expression of a variety of factors including chemokines, cytokines, adhesion molecules, inflammatory mediators and apoptosis inhibitors [9], and the main function of NF-κB in controlling the inflammatory response is as a major regulator of inflammation. To investigate whether the activation of NF-κB in cochlear tissues was involved in radiation-induced damage of the cochleae by mediating the inflammatory response as previously reported [5–7], the expression of the proinflammatory cytokines and inflammation-related proteins TNF-α, IL-6, COX-2, VCAM-1 and MIP-1β in cochlear tissues was investigated. The results showed that together with the activation of NF-κB after radiation, the expression levels of these proinflammatory molecules in the cochlear tissues were also significantly increased, while inhibition of NF-κB effectively inhibited this elevation, and all of these changes agreed with the corresponding morphological changes of the cochleae as described above. These results implied that activation of NF-κB was involved in radiation-induced damage to the cochleae by promoting an inflammatory response mediated by proinflammatory molecules in the cochlear tissues. All of these data confirmed our hypothesis.
Among the proinflammatory molecules, TNF-α is produced by monocytes, and can specifically bind to TNF receptor 1, which is expressed in almost all cells, resulting in inflammation, apoptosis, reactive oxygen species generation, cell proliferation and cell survival [25]. IL-6 can be generated by immune-mediated cells, mesenchymal cells, endothelial cells, fibroblasts and many other cells, and exerts complicated biological effects associated with inflammation and immunity [26]. COX-2 is less widely expressed; however, it can be induced in type 2 macrophages by different stimuli, and can exert proinflammatory effects by production of prostaglandin E2 [27,28]. VCAM-1 is an adhesion molecule, is inducible and predominantly expressed in endothelial cells, but can also be expressed on the surface of other cells, including tissue macrophages, dendritic cells, bone marrow fibroblasts and myoblasts [25]. As a major regulator of leukocyte adhesion and transendothelial migration, VCAM-1 recruits a subset of leukocytes to inflammatory sites [25]. MIP-1β as a chemokine, produced by various cells including monocytes, lymphocytes, dendritic cells, neutrophils and vascular smooth muscle cells. It induces leukocyte chemotaxis and transendothelial migration, leading to the recruitment of leukocytes to inflammatory sites [29]. All these cytokines, adhesion protein and chemokine are important proinflammatory molecules downstream of NF-κB. In the cochlear tissues after radiation, the cells which produce these proinflammatory molecules are widely present, and radiation-induced NF-κB activation in these cells induces production of these proinflammatory molecules. In fact, as the transcriptional outputs of radiation-activated NF-κB, the production of these proinflammatory molecules has been widely reported [15,30–34]. At the same time, some interaction and induction among these proinflammatory molecules and the positive feedbacks to NF-κB activation have also been widely reported [11,25–29]. All of these factors lead to the persistent presence of these proinflammatory molecules in cochlear tissues after radiation where they activate the inflammatory cascade reaction, which inevitably causes a persistent inflammatory response in the cochlear tissues and resultant damage to the cochleae. In fact, in this study, the expression of these proinflammatory molecules in the cochlear tissues remained significantly elevated up to day 14 after radiation. Thus it is not difficult to explain why radiation-induced NF-κB activation in the cochlear tissues can lead to damage to the cochleae and result in SNHL.
In summary, radiation to the cochleae induces NF-κB activation in cochlear tissues, which is involved in damage to the cochleae and causes SNHL, and might be induced by promoting an inflammatory response mediated by the overexpression of some proinflammatory molecules in the cochlear tissues. Radiation-induced NF-κB inhibition decreases the expression of these proinflammatory molecules in cochlear tissues, alleviating the damage to the cochleae and SNHL.
FUNDING
This work was supported by the Natural Science Foundation of Shandong Province (grant no. ZR2019MH017). And the funding bodies had no roles in the study.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Contributor Information
Jiaojiao Tong, Cancer Center, the Second Hospital of Shandong University, Jinan, Shandong Province 250033, China.
Chunhui Hu, Cancer Center, the Second Hospital of Shandong University, Jinan, Shandong Province 250033, China.
Yuqian Wu, Cancer Center, the Second Hospital of Shandong University, Jinan, Shandong Province 250033, China.
Qin Liu, Cancer Center, the Second Hospital of Shandong University, Jinan, Shandong Province 250033, China.
Dianshui Sun, Cancer Center, the Second Hospital of Shandong University, Jinan, Shandong Province 250033, China.
References
- 1. Rettig E-M, D'Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am 2015;24:379–96. [DOI] [PubMed] [Google Scholar]
- 2. Alterio D, Marvaso G, Ferrari A et al. Modern radiotherapy for head and neck cancer. Semin Oncol 2019;46:233–45. [DOI] [PubMed] [Google Scholar]
- 3. Theunissen E-A, Bosma S-C, Zuur C-L et al. Sensorineural hearing loss in patients with head and neck cancer after chemoradiotherapy and radiotherapy: a systematic review of the literature. Head Neck 2015;37:281–92. [DOI] [PubMed] [Google Scholar]
- 4. Shi W, Hou X, Bao X et al. Mechanism and protection of radiotherapy induced sensorineural hearing loss for head and neck cancer. Biomed Res Int 2021;2021:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan P-X, Du S-S, Ren C et al. Radiation-induced cochlea hair cell death: mechanisms and protection. Asian Pac J Cancer Prev 2013;14:5631–5. [DOI] [PubMed] [Google Scholar]
- 6. Giese A-P, Guarnaschelli J-G, Ward J-A et al. Radioprotective effect of aminothiol PrC-210 on irradiated inner ear of guinea pig. PLoS One 2015;10:e0143606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang J, Xu A, Niu T et al. A unique radioprotective effect of resolvin E1 reduces irradiation-induced damage to the inner ear by inhibiting the inflammatory response. Radiat Oncol 2020;15(223):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawrence T. The nuclear factor NF-kappa B pathway in inflammation. Cold Spring Harb Perspect Biol 2009;1:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Q, Lenardo M-J, Baltimore D. 30 Years of NF-κB: a blossoming of relevance to human pathobiology. Cell 2017;168:37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharapov M-G, Glushkova O-V, Parfenyuk S-B et al. The role of TLR4/NF-κB signaling in the radioprotective effects of exogenous Prdx6. Arch Biochem Biophys 2021;702:1–16. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell J-P, Carmody R-J. NF-κB and the transcriptional control of inflammation. Int Rev Cell Mol Biol 2018;335:41–84. [DOI] [PubMed] [Google Scholar]
- 12. Baeuerle P-A, Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell 1988;53:211–7. [DOI] [PubMed] [Google Scholar]
- 13. Carmody R-J, Chen Y-H. Nuclear factor-kappa B: activation and regulation during toll-like receptor signaling. Cell Mol Immunol 2007;4:31–41. [PubMed] [Google Scholar]
- 14. Feldman N, Rotter-Maskowitz A, Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res Rev 2015;24:29–39. [DOI] [PubMed] [Google Scholar]
- 15. Hellweg C-E. The Nuclear Factor κB pathway: a link to the immune system in the radiation response. Cancer Lett 2015;368:275–89. [DOI] [PubMed] [Google Scholar]
- 16. Li N, Banin S, Ouyang H et al. ATM is required for IkappaB kinase (IKKk) activation in response to DNA double strand breaks. J Biol Chem 2001;276:8898–903. [DOI] [PubMed] [Google Scholar]
- 17. Durocher D, Jackson S-P. DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell Biol 2001;13:225–31. [DOI] [PubMed] [Google Scholar]
- 18. Xu Y, Zhai D, Goto S et al. Nicaraven mitigates radiation-induced lung injury by downregulating the NF-κB and TGF-β/Smad pathways to suppress the inflammatory response. J Radiat Res 2022;63:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z, Wang Q, Gong L et al. The NF-κB-Regulated miR-221/222/Syndecan-1 Axis and intestinal mucosal barrier function in radiation enteritis. Int J Radiat Oncol Biol Phys 2022;113:166–76. [DOI] [PubMed] [Google Scholar]
- 20. Li J, Zheng X, Li X et al. Study on the protective effect and mechanism of Liriodendrin on radiation enteritis in mice. J Radiat Res 2022;63:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gawish R-A, Fahmy H-A, Abd El Fattah A-I et al. The potential effect of methylseleninic acid (MSA) against γ-irradiation induced testicular damage in rats: Impact on JAK/STAT pathway. Arch Biochem Biophys 2020;679:1–9. [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Ahn K-S, Alharbi S-A et al. Celastrol alleviates gamma irradiation-induced damage by modulating diverse inflammatory mediators. Int J Mol Sci 2020;21:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci U S A 1998;95:13012–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamatani T, Azuma M, Motegi K et al. Cepharanthin-enhanced radiosensitivity through the inhibition of radiation-induced nuclear factor-kappaB activity in human oral squamous cell carcinoma cells. Int J Oncol 2007;31:761–8. [PubMed] [Google Scholar]
- 25. Kong D-H, Kim Y-K, Kim M-R et al. Emerging Roles of Vascular Cell Adhesion Molecule-1(VCAM-1) in Immunological Disorder and Cancer. Int J Mol Sci 2018;19:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hashemi Goradel N, Najafi M, Salehi E et al. Cyclooxygenase-2 in cancer: a review. J Cell Physiol 2019;234:5683–99. [DOI] [PubMed] [Google Scholar]
- 28. Frejborg E, Salo T, Salem A. Role of cyclooxygenase-2 in head and neck tumorigenesis. Int J Mol Sci 2020;21:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev 2002;13:455–81. [DOI] [PubMed] [Google Scholar]
- 30. Liu G-D, Xia L, Zhu J-W et al. Genistein alleviates radiation-induced pneumonitis by depressing Ape1/Ref-1 expression to down-regulate inflammatory cytokines. Cell Biochem Biophys 2014;69:725–33. [DOI] [PubMed] [Google Scholar]
- 31. Schaue D, Kachikwu E-L, McBride W-H. Cytokines in radiobiological responses: a review. Radiat Res 2012;178:505–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghandhi S-A, Yaghoubian B, Amundson S-A. Global gene expression analyses of bystander and alpha particle irradiated normal human lung fibroblasts: synchronous and differential responses. BMC Med Genet 2008;1:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou H, Ivanov V-N, Gillespie J et al. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci U S A 2005;102:14641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao Y, de Toledo S-M, Hu G et al. Connexins and cyclooxygenase-2 crosstalk in the expression of radiation-induced bystander effects. Br J Cancer 2014;111:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]