Abstract
Dicyclopropanated 5-vinyl-2-norbornene (dcpVNB) is a strained polycyclic hydrocarbon compound with a high energy content, which makes it promising for the development of propellant components based on it. In this work, the genotoxic properties of dcpVNB were studied using whole-cell lux-biosensors based on Escherichia coli and Bacillus subtilis. It was shown that the addition of dcpVNB to bacterial cells leads to the appearance of DNA damage inducing the SOS response and Dps expression with slight activation of the OxyR-mediated response to oxidative stress. The highest toxic effect of dcpVNB is detected by the following lux-biosensors: E. coli pColD-lux, E. coli pDps, B. subtilis pNK-DinC, and B. subtilis pNK-MrgA, in which the genes of bacterial luciferases are transcriptionally fused to the corresponding promoters: Pcda, Pdps, PdinC, and PmrgA. It was shown that lux-biosensors based on B. subtilis, and E. coli are almost equally sensitive to dcpVNB, which indicates the same permeability to this compound of cell wall of Gram-positive and Gram-negative bacteria. The activation of Pdps after dcpVNB addition maintains even in oxyR mutant E. coli strains, which means that the Pdps induction is only partially determined by the OxyR/S regulon. Comparison of specific stress effects caused by dcpVNB and 2-ethyl(bicyclo[2.2.1]heptane) (EBH), characterized by the absence of cyclopropanated groups, shows that structural changes in hydrocarbons could significantly change the mode of toxicity.
Keywords: lux-biosensor, genotoxicity, strained hydrocarbons, fuel
1. Introduction
Strained cycloalkanes which have extra internal energy due to the deformation of valence bond angles are attractive for high-performance combustion applications, including the development of propellant components [1]. Based on the operating conditions of rocket technology, the development of rocket fuel components involves the selection of compounds that are highly efficient in terms of specific impulse energy but have low toxicity. Unsymmetrical dimethylhydrazine (UDMH) is a classic rocket propellant with a high calorific value; however, it is a highly toxic compound [2,3]. Due to these properties, the use of UDMH is associated with risks of environmental pollution during the operation of rocket technology, and especially in the cases of accidents at launches [4]. There are several studies showing the detection of UDMH in the environment and its impact on the ecosystems and human health [5,6,7]. Numerous studies have demonstrated the genotoxic effect of UDMH and its products of incomplete oxidation on the cell, which is determined by DNA alkylation [8,9,10] and DNA damage by reactive oxygen species (ROS) [11,12]. Strained hydrocarbon compounds based on cycloalkanes have two indisputable advantages: they are energetically more efficient than saturated hydrocarbons [13] and significantly less toxic than nitrogen-containing compounds like UDMH. It was shown in [14,15] that molecules consisting of one or two conjugated norbornanes: 2-ethyl(bicyclo[2.2.1]heptane) (EBH) and 2,2′-bis(bicyclo[2.2.1] heptane), respectively, do not possess the ability to alkylate DNA, but can induce the SOS response caused by cellular DNA damage by reactive oxygen species.
In this study, a strained polycyclic hydrocarbon compound dicyclopropanated 5-vinyl-2-norbornene (dcpVNB) was synthetized and its genotoxic characteristics were evaluated. dcpVNB is based on norbornane with addition of two cyclopropane groups and has an extra internal energy compared to norbornane due to deformation of valence bond angles.
The dcpVNB toxicity evaluation was carried out using semi-specific whole-cell bacterial lux-biosensors. Lux-biosensors used for studying dcpVNB toxicity, were E. coli MG1655 and B. subtilis 168 cells transformed by set of hybrid plasmids in which bacterial luciferase genes (lux-genes) are transcriptionally fused to various stress promoters: Pcda, PoxyS, Pdps, PalkA, PdinC, or PmrgA. These lux-biosensors are semispecific and increase their luminescence in response to damage of cellular components correspondent for each biosensor [9,16,17,18,19]. These biosensors have a higher sensitivity compared to biosensors based on luminescence quenching. Taken together, it allows using these lux-biosensors to study the mechanisms of toxicity of various compounds and to evaluate toxic concentrations for cell. The lux-biosensor E. coli MG1655 pXen7 was used to evaluate the integral toxicity as it contains lux-genes P. luminescens ZM1 under control of its own constitutively transcribed promoter [20], in this case a decrease in the level of bioluminescence shows how compound affects vital functions of the cell.
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
Bacterial strains and plasmids used in the study are listed in Table 1.
Table 1.
Bacterial strains and plasmids used in the study.
| Name | Description | Source | |
|---|---|---|---|
| Strains | |||
| E. coli MG1655 | F- ilvG rfb-50 rph 1 | (VKPM, Russia) | |
| E. coli BW25113 ΔoxyR | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ- rph-1 Δ(rhaD-rhaB)568 hsdR514 ΔoxyR::Km r | [21] | |
| E. coli MK2022 | ΔoxyR::Kmr (obtained by transfer of gene deletion ΔoxyR from BW25113 ΔoxyR to MG1655 using P1 transduction) | This study | |
| B. subtilis 168 | trpC2 | (VKPM, Russia) | |
| Plasmids | |||
| pNK-AlkA | pPL_ABCDExen vector [22] with insertion of the B. subtilis PalkA promoter; PalkA is transcriptionally fused with luxCDABE P. luminescens. Trimethoprim (Tpr), chloramphenicol (Cmr), and ampicillin (Apr) resistance. | [16] | |
| pNK-DinC | As pNK-AlkA, but B. subtilis PdinC promoter is used. Tpr, Cmr, Apr | [16] | |
| pNK-MrgA | As pNK-AlkA, but B. subtilis PmrgA promoter is used. Tpr, Cmr, Apr | [16] | |
| pAlkA-lux | pDEW201 [23] vector with insertion of E. coli PalkA promoter transcriptionally fused with luxCDABE P. luminescens. Apr | [8] | |
| pDps | As pAlkA-lux, but E. coli Pdps promoter is used. Apr | [24] | |
| pOxyR-lux | As pAlkA-lux, but E. coli PoxyS promoter with the gene oxyR is used. Apr | [17] | |
| pColD-lux | As pAlkA-lux, but Pcda from plasmid ColD-CA23 is used. Apr | [17] | |
| pXen7 | pUC18-based plasmid constitutively expressing luxCDABE genes. Apr | [20] | |
E. coli MG1655 and MK2022 and B. subtilis 168 cells were used for obtaining lux-biosensors by transformation with corresponding plasmids.
All the plasmids used in the current work (presented in Table 1) were obtained from the National Base of Plasmids (https://nbp.biophystech.ru (accessed on 29 December 2022), BioPhysTech, Russia).
2.2. Culture Medium and Growth Conditions31
E. coli cells were cultured in liquid LB medium with continuous agitation at 200 rpm or on surface of agarized LB medium at 37 °C. The medium was supplemented with appropriate antibiotics (ampicillin 100 μg/mL and/or kanamycin 20 μg/mL).
For bioluminescence measurements overnight cultures of E. coli biosensor strains were diluted 1:100 in fresh LB supplemented with appropriate antibiotics and grown up to OD600 = 0.1 − 0.2, the resulting cultures were used in experiments.
B. subtilis were cultured in liquid BHI medium with continuous agitation at 200 rpm or on surface of agarized BHI medium at 37 °C. Medium was supplemented with 50 mg/L tryptophan and 10 μg/mL chloramphenicol.
For bioluminescence measurements overnight cultures of B. subtilis biosensor strains were diluted 1:100 in fresh LB supplemented with appropriate antibiotics and grown up to OD600 = 0.2 − 0.4, which corresponds to early logarithmic phase, the resulting cultures were used in experiments.
2.3. Measurement of Bioluminescence
Biosensor cells were sampled into the 200 μL subcultures in separate wells of 96-well plates, then 10 μL samples of tested compound (dcpVNB or control) were added. Cells were incubated without shaking at room temperature with regular measurements of total bioluminescence from each well. Bioluminescence was measured using SynergyHT (Biotek Instruments, Winooski, VT, USA). In general, B. subtilis–based biosensors are dimmer in comparison with E. coli-based ones, so more sensitive cuvette-reader Biotox-7BM (BioPhysTech, Russia) was used for validation of their luminescence measurements. Luminescence values were expressed in relative light units (RLU), specific to each luminometer.
2.4. Data Processing
Bioluminescence was measured several times during few hours. All experiments were conducted in three replications. Kinetic curves on graphs are the typical curves for the row of measurements (the kinetic curve which is consistent with the others).
The maximum response amplitude for sample x is calculated due to following equation:
where L(t,x)—the luminescence of probe x in the time point t, L(t,ctrl)—the luminescence of negative control, i.e., biosensor cells without addition of any toxicant at the time point t.
For the determination of threshold concentrations, experiments with each biosensor were carried out with serial dilutions of dcpVNB. The threshold concentration is determined as the minimal concentration x, which gives noticeable induction (L(t, x) was higher than L(t, ctrl) in several time points by at least 2 values of measurement error). Further, threshold concentrations calculated from each experimental replicate were averaged and the standard deviation was calculated.
2.5. P1 Transduction
P1 phage transduction of ΔclpX:Kn gene was performed according to [25]. Liquid P1 lysate was obtained using the donor strain E. coli BW25113 ΔoxyR, final phage stock contained approximately 5*108 pfu/mL. Overnight culture of the recipient strain E. coli MG1655 was mixed with 10 µL of liquid P1 phage lysate in 100 µL of P1 salt solution (10mM MgCl2, 5 mM CaCl2). The final bacterial-phage mix was spread on selective transduction plates containing 10 µm/mL kanamycin and 5 mM sodium citrate.
2.6. Chemicals
All chemicals were of analytical purity. Hydrogen peroxide was obtained from the firm “Ferraine” (Russia). Mitomycin C (MitC), methyl methanesulfonate (MMS), 5-vinyl-2-norbornene, acetic acid, methanol, diethyl ether, N-nitroso-N-methyl urea and other compounds were obtained from Sigma-Aldrich (St. Louis, MO, USA). For comparative measurements, EBH, synthetized earlier in study [14], was used.
2.7. Synthesis of Dicyclopropanated 5-Vinyl-2-Norbornene (dcpVNB)
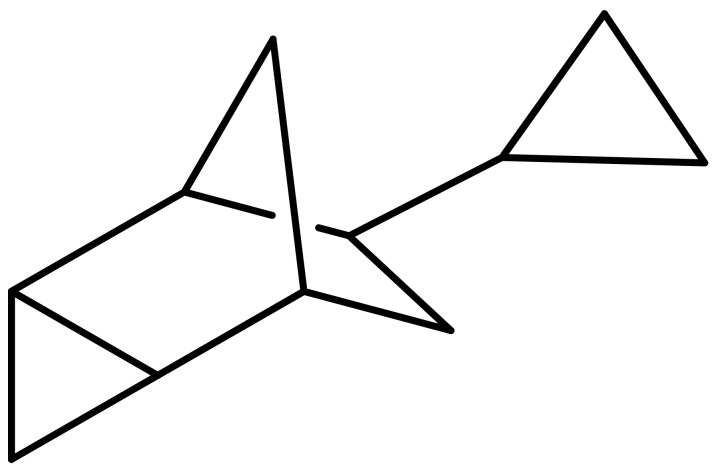
Dicyclopropanated 5-vinyl-2-norbornene (dcpVNB) was obtained according to the published methods [26,27,28,29,30]. The prepared dcpVNB consists of two types of strained cyclic moieties (a norbornane and two cyclopropane rings) and it contains eleven C and sixteen H atoms (Figure 1).
Figure 1.
The molecular structure of dicyclopropanated 5-vinyl-2-norbornene (dcpVNB).
3. Results
3.1. DNA Damage: SOS Response and DNA Alkylation
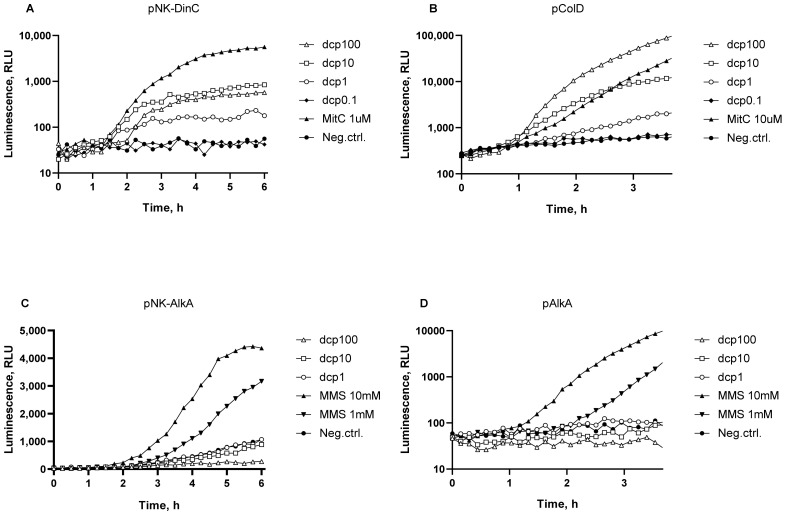
The genotoxic effect of dcpVNB on bacterial cells was studied using E. coli MG1655 pColD-lux and B. subtilis 168 pNK-DinC lux-biosensors, which may detect the SOS response of bacterial cells. The E. coli MG1655 pAlkA-lux and B. subtilis 168 pNK-AlkA biosensors were used to detect the DNA alkylation caused by dcpVNB.
Figure 2 shows the luminescence kinetic curves of biosensor cell cultures after addition of dcpVNB at final concentrations of 0.1–100 mg/mL. The antibiotic MitC was used as a positive control to activate Pcda and PdinC SOS promoters (E. coli MG1655 pColD-lux and B. subtilis 168 pNK-DinC lux-biosensors, respectively). MitC produces DNA crosslinks, then the replication fork stops, single-stranded DNA sections are formed, and, as a result, the SOS response occurs. The alkylating agent MMS was used as a positive control to activate the PalkA promoter.
Figure 2.
Typical kinetic curves of luminescence of B. subtilis 168 pNK-DinC (A), E. coli MG1655 pColD-lux (B), and B. subtilis 168 pNK-AlkA (C), E. coli MG1655 pAlkA-lux (D) cells after addition of dcpVNB. “Neg.ctrl.”—negative control, i.e., biosensor cells without addition of any toxicant; “MitC 1uM” and “MitC 10uM” – mitomycin C added to a final concentration of 1 μM and 10 μM, respectively; “MMS 10mM”, “MMS 1mM”—MMS added to final concentrations of 10 or 1 mM, respectively; “dcp100”, “dcp10”, “dcp1” and “dcp0.1”—dcpVNB added to final concentrations of 100, 10, 1, or 0.1 mg/mL, respectively.
As one can see from the Figure 2, the addition of dcpVNB at concentrations from 0.1% to 10% leads to DNA damage that results in the SOS response both in E. coli and B. subtilis cells (Figure 2A,B). The maximum possible response amplitude of E. coli pColD-lux biosensor is up to thousand [17]. In this experiment, the maximum response amplitude of the biosensor is about 200 during incubation with dcpVNB at concentration of 100 mg/mL. The threshold concentration of dcpVNB is about 0.5 mg/mL, approximately the same for both E. coli and B. subtilis SOS response specific biosensors.
In contrast to the observed SOS response, damage associated with DNA alkylation apparently does not occur as soon as promoter of DNA glycosylase Palka is not induced during cell culture incubation with dcpVNB (Figure 2C,D). A slight decrease in the luminescence of sensitive to alkylation biosensors with dcpVNB added at concentrations of 100 and 10 mg/mL (curves “dcp100” and “dcp10”) is associated with the general toxicity to bacterial cells.
3.2. Oxidative Stress
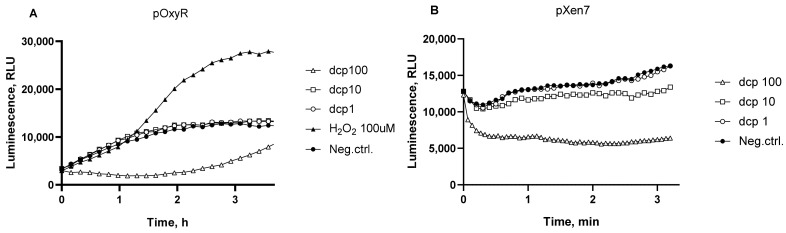
The most sensitive to oxidative stress E. coli-based lux-biosensor is E. coli MG1655 pOxyR-lux, which may detect an activation of the PoxyS promoter of the gene, encoding the regulatory RNA of OxyR/S regulon [9].
Figure 3A shows the luminescence kinetic curves of E. coli MG1655 pOxyR-lux biosensor strain after addition of dcpVNB at concentrations from 0.1 to 100 mg/mL. Hydrogen peroxide was used as a positive control to activate OxyR-regulated promoters. Cell cultures were incubated at room temperature without aeration for 4–6 h with periodic measurements of luminescence.
Figure 3.
Typical kinetic curves of luminescence of E. coli MG1655 pOxyR-lux (A) and E. coli MG1655 pXen7 (B) cells after addition of dcpVNB. “Neg.ctrl.”—negative control, i.e., biosensor cells without addition of toxicant; “H2O2 100uM”—hydrogen peroxide added to a final concentration of 100 μM ; “dcp100”, “dcp10”, and “dcp1”—dcpVNB added to a final concentration of 100, 10, and 1 mg/ml, respectively.
As can be seen from the data shown in Figure 3, the addition of dcpVNB to the cell culture causes a relatively weak oxidative stress, which is detected directly by the activation of the PoxyS promoter (Figure 3A). The luminescence is significantly reduced when the dcpVNB concentration is 100 mg/mL, it is apparently associated with the general toxicity to cells. The general toxicity was evaluated with the use of E. coli pXen7 biosensor with constitutive expression of luciferase (Figure 3B). A decrease in constitutive luminescence is observed at concentrations of 100 mg/mL and, to a lesser extent, at 10 mg/mL dcpVNB. On the Figure 3A a gradual rise in the “dcp100” curve can be observed after a fall in the first minutes of experiment, which indicates an activation of the PoxyS promoter, in contrast, there is no rise in luminescence of E. coli pXen7 biosensor in Figure 3B which means that its promoter is not induced.
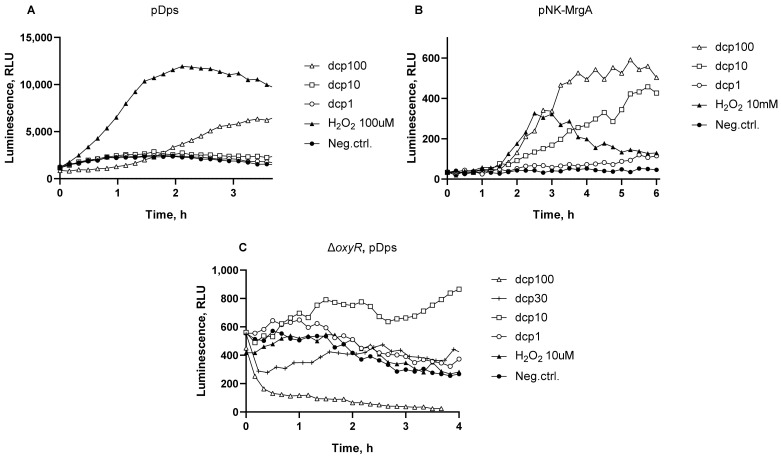
Then, the ability of dcpVNB to induce oxidative stress in bacterial cells was investigated using E. coli MG1655 pDps and B. subtilis 168 pNK-MrgA. These biosensors show an activation of Pdps and PmrgA, promoters regulating genes of DNA-binding ferritin-like proteins [31,32]. These genes are known to be regulated by several factors, including strong OxyR-mediated induction in response to oxidative stress [33,34,35]. Induction of the E. coli Pdps and B. subtilis PmrgA stress promoters by addition of dcpVNB is illustrated in Figure 4. To test the possibility of OxyR-independent Pdpsactivation, we studied the luminescence of the E. coli MK2022 strain with a deletion of the oxyR regulatory gene (Figure 4C).
Figure 4.
Typical kinetic curves of luminescence of E. coli MG1655 pDps (A), B. subtilis 168 pNK-MrgA (B), and E. coli MK2022 pDps (C) cells after addition of dcpVNB. “Neg.ctrl.”—control biosensor cells without addition of toxicant; “H2O2 10uM”, “H2O2 100uM” and “H2O2 10mM”—hydrogen peroxide added to a final concentration of 10 µM, 100 µM and 10 mM, respectively; “dcp100”, “dcp30”, “dcp10”, and “dcp1”—dcpVNB added to a final concentration of 100, 30, 10, and 1 mg/mL, respectively.
The E. coli Pdps promoter and B. subtilis PmrgA have a relatively high response amplitude (Figure 4A,B) and a threshold concentration of dcpVNB for these biosensors is approximately 1 mg/mL. Figure 4C shows that a certain activation of the Pdps promoter also occurs in the ΔoxyR mutant strain. When dcpVNB was added to MK2022 cells, activation was noticeable at concentrations from 1 to 10 mg/mL. dcpVNB at concentration of 100 mg/mL for ΔoxyR strain is more toxic than for the wild type and luminescence of cells is significantly reduced. These results indicate the ability of dcpVNB to activate promoters of DNA-binding ferritin genes in an OxyR-independent manner.
3.3. Comparison of SOS Response and Oxidative Stress in Cells from dcpVNB and EBH
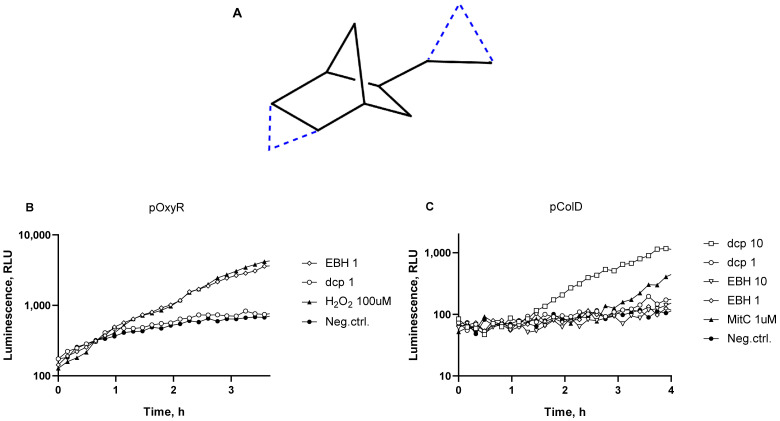
In our previous work, the toxicity of EBH, a compound with a structure quite similar to dcpVNB but without cyclopropane groups (Figure 5A), was investigated [14]. We noted that dcpVNB produce very little amount of oxidative stress and strongly induce the SOS response, while EBH showed presumably oxidative damage of cells. To investigate this difference, we compared the genotoxic effects of dcpVNB and EBH in one experiment with the use of the following biosensors: E. coli MG1655 pOxyR-lux and E. coli MG1655 pColD-lux.
Figure 5.
(A)—Molecular structure of EBH (black solid lines). The difference in structure of dcpVNB and EBH (additional two carbons and four bonds) is given in blue dashed lines. (B,C)—Typical kinetic curves of luminescence of E. coli MG1655 pOxyR-lux (B) and E. coli MG1655 pColD-lux (C) cells after addition of dcpVNB and EBH. “Neg.ctrl.”—negative control, biosensor cells without addition of toxicant; “H2O2 100uM”—hydrogen peroxide added to a final concentration of 100 µM; “MitC 1uM”—mitomycin C added to a final concentration of 1 µM; “dcp10” and “dcp1”—dcpVNB added; “EBH 1” and “EBH 10”—EBH added to final concentrations of 10 and 1 mg/mL, respectively.
Compounds dcpVNB and EBH were added to biosensor cells in various concentrations, and then the samples were incubated at room temperature without aeration for 3–4 h with periodic measurements of bioluminescence (Figure 5).
As can be seen from Figure 5, EBH possesses a high ability to activate the oxidative stress in the cells, while dcpVNB possesses a low one (Figure 5B), and vice versa, while the SOS response is activated in the cells, dcpVNB has high efficiency to induce SOS response, while the EBH ability to damage DNA is relatively low (Figure 5C).
Collected data was processed and the values for threshold concentrations were summarized in the Table 2.
Table 2.
Threshold concentrations for dcpVNB, EBH, and UDMH detectable with lux-biosensors, used in this study. * Concentrations were obtained earlier [10,36]. ** ”nd”—not determined. ***“ne”- not evaluated.
| Biosensors | dcpVNB, mM | EBH, mM | UDMH *, mM | Note |
|---|---|---|---|---|
| E. coli pAlkA-lux | nd ** | nd | 2*10−2 | Alkylation of DNA |
| B. subtilis pNK-AlkA | nd | ne *** | ne | |
| E. coli pColD-lux | 2.3 ± 0.7 | 5.6 ± 1.9 | 8*10−3 | DNA damage, leading to SOS response |
| B. subtilis pNK-DinC | 3.5 ± 1.2 | ne | ne | |
| E. coli pDpS | 54 ± 8 | ne | ne | Oxidative stress |
| B. subtilis pNK-MrgA | 6.0 ± 2.1 | ne | ne | |
| E. coli pOxyR-lux | 470 ± 50 | 4.2 ± 1.8 | 3*10−3 | Oxidation by hydrogen peroxide |
| E. coli pXen7 | 67 ± 15 | 43 ± 7 | 2 | Total toxicity, decrease in luminescence correlates with the number of living cells |
4. Discussion
Incomplete oxidation of most hydrocarbon compounds leads to the appearance of various reactive oxygen species in the aquatic environment, in particular alkyl hydroperoxides, alkyl radicals, and other compounds capable of causing oxidative stress in both pro- and eukaryotic cells [37,38]. Compounds with strained bonds, as shown in [1,13], are oxidized mainly by the radical mechanism. The radical mechanism of oxidation along with the increased reaction energy suggests that the genotoxic effect of strained compounds should be determined mainly by reactions of reactive oxygen species formation [15]. For the EBH compound, these statements are generally confirmed by experiments on E. coli [14], demonstrating the activation of the OxyR/S regulon. In contrast to EBH, the addition of dcpVNB to the biosensor cell culture weakly activates the PoxyS promoter, which means almost no oxidative damage occurs. Moreover, the data presented in Figure 5 demonstrate that there is a fundamental difference in the mechanisms of genotoxicity of EBH and dcpVNB compounds. EBH is characterized by the formation of ROS, which cause DNA damage, that is consistent with the data [10], while dcpVNB has a ROS-independent SOS response, characterized by a high response amplitude of Pcda, though the oxidative stress at the same concentrations of dcpVNB is absent. The ROS-independent toxic effect of dcpVNB on bacterial cells is supported by data demonstrating the activation of E. coli Pdps and B. subtilis PmrgAferritin promoters. This result is somewhat unexpected, since it was assumed that the key contribution to the activation of Pdps and PmrgA is brought by OxyR/S. The activation of the Pdpspromoter in the ΔoxyR strain explains this phenomenon by the participation of other regulators. It is known that, in addition to OxyR/S, these ferritin promoters can be activated by several other regulators, in particular, CsrA, FIS, H-NS, σ38, and by the sporulation signal in bacilli [33,34,37,38]. It appears that the ROS-independent toxic effect of dcpVNB induces the activation of some of these regulators.
Thus, compared to EBH, the dicyclopranated form of norbornane, dcpVNB induces a stronger SOS response and less severe oxidative damage to the cell. This is a key difference in the mechanisms of toxicity of two strained cycloalkanes differing only in two cyclopropanated groups. Specific targets in the cell for the molecule of dcpVNB still require further research.
The data in Table 2, comparing the toxicological properties of strained hydrocarbon compounds based on cycloalkanes, shows that the substance dcpVNB studied in this article is averagely 1000 times less toxic than UDMH in terms of its ability to cause oxidative stress in cells and DNA damage that stops the replication fork (SOS response). According to the mechanism of genotoxicity, the disability of dcpVNB to methylate DNA sharply reduces its carcinogenic properties compared to UDMH.
5. Conclusions
In conclusion, it should be noted that, in general, the toxicity of strained cyclic compounds is significantly lower than that of unsymmetrical dimethylhydrazine, which is currently used as the most energy-efficient propellant component.
In this study, we have shown the genotoxic properties of a new compound that has the potential for widespread use as a highly efficient hydrocarbon-based fuel. It is important to note that dcpVNB has no alkylating effect, which means that it has no mutagenic properties, as UDMH has. The oxidizing effect on cell components and DNA damage coupled with formation of single-stranded DNA are not so dangerous for multicellular organisms and do not have a long-lasting effect. Threshold concentrations of strained cycloalkanes causing oxidative stress are much lower than of UDMH, so it makes them less toxic. The use of dcpVNB may provide opportunities for making rocket industry much safer for environment and human health.
We demonstrated here that changes in the structure of the strained cycloalkane caused a sharp change in the mechanism of toxicity. In particular, due to the appearance of two cyclopropane groups in the structure, the ability of the compound to cause oxidative damage to cells was reduced and, at the same time, the ability to damage DNA was increased.
Acknowledgments
The authors are grateful to Gnuchikh Eugeny for the help with creation of the Bacillus-based biosensors platform at MIPT.
Author Contributions
Conceptualization, M.V.B. and I.V.M.; data curation, U.S.N., S.V.B. and I.V.M.; investigation, U.S.N., O.V.K., A.G.K. and A.A.F.; methodology, S.V.B., A.A.K. and I.V.M.; project administration, I.V.M.; resources, A.A.K., M.V.B., S.V.S. and I.V.M.; validation, O.V.K., A.G.K. and U.S.N.; visualization, S.V.B. and U.S.N.; writing—original draft, I.V.M.; writing—review and editing, A.A.K., U.S.N., S.V.B., M.V.B., S.V.S. and I.V.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
the investigation of genotoxic effects with use of inducible semi-specific biosensors was supported by the Russian Science Foundation under grant 22-14-00124. The work of O.V.K. in part of general toxicity assessment was supported by Ministry of Science and Higher Education of the Russian Federation (project 1022060200069-0-1.6.2;1.6.4;1.6.5;1.6.10;1.6.19, “Development of technology for rational and highly productive use of agro- and bioresources, their efficient processing and obtaining safe and high-quality sources of food and non-food products”). Dicyclopropanated 5-vinyl-2-norbornene was prepared and studied by NMR within the State Program of TIPS RAS.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sirjean B., Glaude P.A., Ruiz-Lopez M.F., Fournet R. Detailed Kinetic Study of the Ring Opening of Cycloalkanes by CBS-QB3 Calculations. J. Phys. Chem. A. 2006;110:12693–12704. doi: 10.1021/jp0651081. [DOI] [PubMed] [Google Scholar]
- 2.Back K.C., Carter V.L., Thomas A.A. Occupational Hazards of Missile Operations with Special Regard to the Hydrazine Propellants. Aviat Space Environ. Med. 1978;49:591–598. [PubMed] [Google Scholar]
- 3.Hu C., Zhang Y., Zhou Y., Liu Z.F., Feng X.S. Unsymmetrical Dimethylhydrazine and Related Compounds in the Environment: Recent Updates on Pretreatment, Analysis, and Removal Techniques. J. Hazard Mater. 2022;432:128708. doi: 10.1016/j.jhazmat.2022.128708. [DOI] [PubMed] [Google Scholar]
- 4.Kenessov B., Batyrbekova S. Actual Directions in Study of Ecological Consequences of a Highly Toxic 1,1-Dimethylhydrazine-Based Rocket Fuel Spills. Chem. Bull. Kazakh Natl. Univ. 2012;66:124–131. doi: 10.15328/chemb_2012_2124-131. [DOI] [Google Scholar]
- 5.Kenessov B., Batyrbekova S., Nauryzbayev M., Bekbassov T., Alimzhanova M., Carlsen L. GC-MS Determination of 1-Methyl-1H-1,2,4-Triazole in Soils Affected by Rocket Fuel Spills in Central Kazakhstan. Chromatographia. 2008;67:421–424. doi: 10.1365/s10337-008-0535-4. [DOI] [Google Scholar]
- 6.Carlsen L., Kenesova O.A., Batyrbekova S.E. A Preliminary Assessment of the Potential Environmental and Human Health Impact of Unsymmetrical Dimethylhydrazine as a Result of Space Activities. Chemosphere. 2007;67:1108–1116. doi: 10.1016/j.chemosphere.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 7.Carlsen L., Kenessov B.N., Batyrbekova S.Y. A QSAR/QSTR Study on the Human Health Impact of the Rocket Fuel 1,1-Dimethyl Hydrazine and Its Transformation Products. Multicriteria Hazard Ranking Based on Partial Order Methodologies. Environ. Toxicol Pharm. 2009;27:415–423. doi: 10.1016/j.etap.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Goryanin I., Kotova V., Krasnopeeva E., Manukhov I., Chubukov P., Balabanov V., Chalkin S., Shatrov T., Zavilgelsky G. Genotoxic Action of the 1,1-Dimethylhydrazine Determined by Alkylating Compounds Appearing in the Result of Oxidation and Hydrogen Peroxide. Tr. MIPT. 2013;5:103–111. [Google Scholar]
- 9.Kessenikh A., Manukhov I., Vagapova E., Vysokikh M., Konopleva M., Kotova V., Gorbunov M., Chalkin S., Zavilgelsky G. Lux-Biosensors Set for Detection of Toxic Products of Incomplete Oxidation of Non-Symmetric Dimethylhydrazine in Medium. RU2626569. Patent. 2015 December 17;
- 10.Manukhov I., Gorbunov M., Degtev I., Zavilgelsky G., Kessenikh A., Konopleva M., Kotova V., Krasnopeeva E., Motovilov K., Osetrova M., et al. The Set of Lux-Biosensors for Determination of Genotoxic Products of Incomplete Oxidation of Unsymmetrical Dimethyl Hydrazine in the Environment. RU2569156. Patent. 2014 December 18;
- 11.Zavilgelsky G., Kotova V., Manukhov I., Kondratyev A., Sambros V., Shatrov Y., Chalkin S. Kit of Lux-Biosensors for Heptyl Detection in Medium. RU229745. Patent. 2007 December 1;
- 12.Zavilgelsky G.B., Kotova V.Y., Manukhov I.V. Action of 1,1-Dimethylhydrazine on Bacterial Cells Is Determined by Hydrogen Peroxide. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007;634:172–176. doi: 10.1016/j.mrgentox.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Dóbé S., Turányi T., Bérces T., Márta F. The Kinetics of Hydroxyl Radical Reactions with Cyclopropane and Cyclobutane. Proc. Indian Acad. Sci. Chem. Sci. 1991;103:499–503. doi: 10.1007/BF02842112. [DOI] [Google Scholar]
- 14.Kessenikh A.G., Manukhov I.V., Yaguzhinsky L.S., Bermeshev M.V., Zisman M.A., Pevgov V.G., Samoilov V.O., Shorunov S.V., Maksimov A.L. Toxic Effect of 2-Ethy l(Bicyclo[2.2.1] Heptane) on Bacterial Cells. Biotekhnologiya. 2019;35:67–72. [Google Scholar]
- 15.Kessenikh A., Gnuchikh E., Bazhenov S., Bermeshev M., Pevgov V., Samoilov V., Shorunov S., Maksimov A., Yaguzhinsky L., Manukhov I. Genotoxic Effect of 2,2’-Bis(Bicyclo[2.2.1] Heptane) on Bacterial Cells. PLoS ONE. 2020;15:e0228525. doi: 10.1371/journal.pone.0228525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessenikh A.G., Novoyatlova U.S., Bazhenov S.V., Stepanova E.A., Khrulnova S.A., Gnuchikh E.Y., Kotova V.Y., Kudryavtseva A.A., Bermeshev M.V., Manukhov I. V Constructing of Bacillus subtilis-Based Lux-Biosensors with the Use of Stress-Inducible Promoters. Int. J. Mol. Sci. 2021;22:9571. doi: 10.3390/ijms22179571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotova V.Y., Manukhov I.v., Zavilgelskii G.B. Lux-Biosensors for Detection of SOS Response, Heat Shock, and Oxidative Stress. Appl. Biochem. Microbiol. 2010;46:781–788. doi: 10.1134/S0003683810080089. [DOI] [Google Scholar]
- 18.Zhu Y., Elcin E., Jiang M., Li B., Wang H., Zhang X., Wang Z. Use of Whole-Cell Bioreporters to Assess Bioavailability of Contaminants in Aquatic Systems. Front. Chem. 2022;10:1018124. doi: 10.3389/fchem.2022.1018124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X., Li B., Schillereff D.N., Chiverrell R.C., Tefsen B., Wells M. Whole-Cell Biosensors for Determination of Bioavailable Pollutants in Soils and Sediments: Theory and Practice. Sci. Total Environ. 2022;811:152178. doi: 10.1016/j.scitotenv.2021.152178. [DOI] [PubMed] [Google Scholar]
- 20.Zavilgelsky G.B., Zarubina A.P., Manukhov I.v. Sequencing and Comparative Analysis of the Lux Operon of Photorhabdus Luminescens Strain Zm1: ERIC Elements as Putative Recombination Hot Spots. Mol. Biol. 2002;36:637–647. doi: 10.1023/A:1020663128043. [DOI] [PubMed] [Google Scholar]
- 21.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K.A., Tomita M., Wanner B.L., Mori H. Construction of Escherichia coli K-12 in-Frame, Single-Gene Knockout Mutants: The Keio Collection. Mol. Syst. Biol. 2006;2:2006-0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnuchikh E.Y., Manukhov I.V., Zavilgelsky G. Biosensors for the Determination of Promoters and Chaperones Activity in Bacillus subtilis Cells. Biotekhnologiya. 2020;36:68–77. doi: 10.21519/0234-2758-2020-36-6-68-77. [DOI] [Google Scholar]
- 23.Van Dyk T.K., Rosson R.A. Photorhabdus Luminescens LuxCDABE Promoter Probe Vectors. Methods Mol. Biol. 1998;102:85–95. doi: 10.1385/0-89603-520-4:85. [DOI] [PubMed] [Google Scholar]
- 24.Melkina O.E., Goryanin I.I., Zavilgelsky G.B. The DNA-Mimic Antirestriction Proteins ArdA ColIB-P9, Arn T4, and Ocr T7 as Activators of H-NS-Dependent Gene Transcription. Microbiol. Res. 2016;192:283–291. doi: 10.1016/j.micres.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Thomason L.C., Costantino N., Court D.L. E. coli Genome Manipulation by P1 Transduction. Curr. Protoc. Mol. Biol. 2007;79:1–17. doi: 10.1002/0471142727.mb0117s79. [DOI] [PubMed] [Google Scholar]
- 26.Bell A., Langsdorf L., Burtovyy O. Cycloalkylnorbornene Monomers, Polymers Suitable for Use as Pervaporation Membrane Films. WO25735. U.S. Patent. 2014 February 13;
- 27.Bermeshev M.V., Chapala P.P. Addition Polymerization of Functionalized Norbornenes as a Powerful Tool for Assembling Molecular Moieties of New Polymers with Versatile Properties. Prog. Polym. Sci. 2018;84:1–46. doi: 10.1016/j.progpolymsci.2018.06.003. [DOI] [Google Scholar]
- 28.Ushakov N.V. Selective Hydrogenation of 5-Vinylnorborn-2-Ene and Other Methods for the Synthesis of 2-Vinylnorbornane. Russ. J. Appl. Chem. 2018;91:728–745. doi: 10.1134/S1070427218050026. [DOI] [Google Scholar]
- 29.Zarezin D.P., Rudakova M.A., Shorunov S.V., Sultanova M.U., Samoilov V.O., Maximov A.L., Bermeshev M.V. Design and Preparation of Liquid Polycyclic Norbornanes as Potential High Performance Fuels for Aerospace Propulsion. Fuel Process. Technol. 2022;225:107056. doi: 10.1016/j.fuproc.2021.107056. [DOI] [Google Scholar]
- 30.Shorunov S.V., Zarezin D.P., Samoilov V.O., Rudakova M.A., Borisov R.S., Maximov A.L., Bermeshev M.V. Synthesis and Properties of High-Energy-Density Hydrocarbons Based on 5-Vinyl-2-Norbornene. Fuel. 2021;283:118935. doi: 10.1016/j.fuel.2020.118935. [DOI] [Google Scholar]
- 31.Almiron M., Link A.J., Furlong D., Kolter R. A Novel DNA-Binding Protein with Regulatory and Protective Roles in Starved Escherichia coli. Genes Dev. 1992;6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- 32.Chen L., James L.P., Helmann J.D. Metalloregulation in Bacillus subtilis: Isolation and Characterization of Two Genes Differentially Repressed by Metal Ions. J. Bacteriol. 1993;175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altuvia S., Almiron M., Huisman G., Kolter R., Storz G. The Dps Promoter Is Activated by OxyR during Growth and by IHF and Σs in Stationary Phase. Mol. Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 34.Helmann J.D., Wu M.F.W., Gaballa A., Kobel P.A., Morshedi M.M., Fawcett P., Paddon C. The Global Transcriptional Response of Bacillus subtilis to Peroxide Stress Is Coordinated by Three Transcription Factors. J. Bacteriol. 2003;185:243. doi: 10.1128/JB.185.1.243-253.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sudarev V.V., Dolotova S.M., Bukhalovich S., Bazhenov S.V., Ryzhykau Y.L., Uversky V.N., Bondarev N.A., Osipov S.D., Mikhailov A.E., Kuklina D.D., et al. Ferritin Self-Assembly, Structure, Function, and Biotechnological Applications. Int. J. Biol. Macromol. 2022;224:319–343. doi: 10.1016/j.ijbiomac.2022.10.126. [DOI] [PubMed] [Google Scholar]
- 36.Manukhov I., Balabanov V., Kotova V., Khrulnova S., Melkina O., Kraynov A., Pustovoit K., Krechetov P., Koroleva T., Shatrov T. Use of Lux Biosensors for Detection of UDMH in Soil. Dual Technol. 2008;44:50–56. [Google Scholar]
- 37.Møller P., Scholten R.H., Roursgaard M., Krais A.M. Inflammation, Oxidative Stress and Genotoxicity Responses to Biodiesel Emissions in Cultured Mammalian Cells and Animals. Crit. Rev. Toxicol. 2020;50:383–401. doi: 10.1080/10408444.2020.1762541. [DOI] [PubMed] [Google Scholar]
- 38.Sazykin I., Makarenko M., Khmelevtsova L., Seliverstova E., Rakin A., Sazykina M. Cyclohexane, Naphthalene, and Diesel Fuel Increase Oxidative Stress, CYP153, SodA, and RecA Gene Expression in Rhodococcus erythropolis. Microbiologyopen. 2019;8:e00855. doi: 10.1002/mbo3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is contained within the article.