Abstract
The acquisition of immunity following subclinical or resolved infection with the intracellular parasite Leishmania donovani suggests that vaccination could prevent visceral leishmaniasis (VL). The LACK (Leishmania homolog of receptors for activated C kinase) antigen is of interest as a vaccine candidate for the leishmaniases because of its immunopathogenic role in murine L. major infection. Immunization of mice with a truncated (24-kDa) version of the 36-kDa LACK antigen, delivered in either protein or DNA form, was found previously to protect against cutaneous L. major infection by redirecting the early T-cell response away from a pathogenic interleukin-4 (IL-4) response and toward a protective Th1 response. The amino acid sequence of the Leishmania p36(LACK) antigen is highly conserved, but the efficacy of this vaccine antigen in preventing disease caused by strains other than L. major has not been determined. We investigated the efficacy of a p36(LACK) DNA vaccine against VL because of the serious nature of this form of leishmaniasis and because it was unclear whether the LACK vaccine would be effective in a model where there was not a dominant pathogenic IL-4 response. We demonstrate here that although the LACK DNA vaccine induced a robust parasite-specific Th1 immune response (IFN-γ but not IL-4 production) and primed for an in vivo T-cell response to inoculated parasites, it did not induce protection against cutaneous or systemic L. donovani challenge. Coadministration of IL-12 DNA with the vaccine did not enhance the strong vaccine-induced Th1 response or augment a protective effect.
Active visceral leishmaniasis (VL) is a progressive, fatal infection characterized by fever, hepatosplenomegaly, cachexia, and pancytopenia and the absence of parasite-specific cell-mediated immune responses (4, 5). In areas of endemic infection, however, a significant number of individuals acquire subclinical infection associated with the development of antigen-specific T-cell responses and gamma interferon (IFN-γ) production, and resistance to visceral infection (3, 42). The importance of parasite-specific Th1 responses in protection has been corroborated by studies in the murine model of VL (19, 27, 44). The acquisition of immunity following subclinical or resolved infection suggests that vaccination, if it induced an appropriate cellular immune response, is a feasible approach to prevent this disease.
Several vaccination strategies against experimental leishmaniasis have been attempted. Immunization of mice with killed Leishmania donovani parasites, crude antigen fractions, and purified L. donovani membrane proteins (15, 19, 31, 35) provided partial protection against parasite challenge. We demonstrated recently that immunization with a multicomponent Leishmania DNA vaccine (which included DNA that encoded histone and other unknown proteins) induced a Th1 immune response and conferred protection against experimental challenge with L. donovani (24). Several other recombinant antigens, including the hydrophilic acylated surface protein B (43), antigens of the LD1 multigene locus (9), and the LCR1 antigen (46), also induced partial protective immunity against L. donovani or L. chagasi in mice. A number of other recombinant antigens also conferred protection against cutaneous infection with L. major or L. amazonensis, (6, 7, 14, 16, 26, 32, 41, 45). These antigens have not been studied for their capacity to induce protection against visceral L. donovani infection. The sustained expression of a recombinant antigen by vaccination with DNA, as has been shown for the gp63, Leishmania homolog of receptors for activated C kinase (LACK), PSA-2, and multicomponent sublibrary vaccines (13, 24, 28, 40, 47), is an attractive approach for protection against leishmaniasis because it mimics the persistent antigenic stimulation of subclinical infection.
The LACK antigen is of particular interest as a vaccine candidate because the prominent role it plays in the immunopathogenesis of experimental L. major infection. During infection of inbred mice that express I-Ad major histocompatibility complex class II molecules (e.g. BALB/c strain), expression of the immunodominant LACK antigen drives the expansion of interleukin-4 (IL-4)-secreting, disease-promoting T cells that express a Vβ4/Vα8 T-cell receptor. Depletion of LACK-reactive T cells diminishes the early IL-4 response, allowing the development of a protective Th1 phenotype (18, 20). Furthermore, immunization of highly susceptible BALB/c mice with a truncated (24-kDa) version of the 36-kDa LACK antigen, delivered in either protein (with recombinant IL-12 adjuvant) or DNA form, confers protection against cutaneous challenge with L. major (12, 13, 26, 37). The protective effect of the LACK vaccine was mediated by IL-12-dependent IFN-γ production, which was sufficient to overcome the early pathogenic IL-4 response observed in this infection model (13).
The efficacy of vaccination with LACK antigen against experimental leishmaniasis caused by strains other than L. major has not been investigated. The LACK amino acid sequence is highly conserved, and the LACK mRNA is expressed by L. donovani (26). However, infection of susceptible (BALB/c) mice with L. donovani is not associated with a highly polarized Th2 phenotype and progressive disease characteristic of L. major infection in this mouse strain. Therefore, the potential for use of the LACK vaccine for protection against L. donovani is unclear. In this report we investigated the utility of a LACK DNA vaccine in an established model of L. donovani infection. Although the LACK DNA vaccine induced a robust parasite-specific Th1 immune response and primed for an in vivo T-cell response to inoculated parasites, it did not induced sustained protection against parasite challenge.
MATERIALS AND METHODS
Parasites and parasite antigens.
The L. donovani 1S strain (MHOM/SD/001S-2D) was used for the vaccine challenge experiments and preparation of Leishmania antigen. Other strains used in the cloning of the p36(LACK) cDNA included L. major (Abdou strain; a Syrian isolate kindly provided by H. Louzir, Institut Pasteur, Tunis), L. mexicana (390 strain; a Yucatan isolate kindly provided by F. Andrade-Narvaez, Universidad Autonoma de Yucatan, Merida, Mexico), L. amazonensis (Josefa strain; a Brazilian isolate kindly provided by F. Neva, National Institutes of Health, Bethesda, Md.), and L. braziliensis (HOM/BR/75/M2903; a Brazilian strain kindly provided by B. Travi, CIDEIM, Cali, Colombia). Promastigotes were cultured axenically in M199 medium supplemented with 15% heat-inactivated fetal calf serum, 0.1 mM adenine, 5 μg of hemin per ml, 1 μg of biotin per ml, 2mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (34). We used 4- to 5-day promastigote cultures to prepare soluble L. donovani antigen (SLDA) as previously described (25). The L. donovani strain was continuously maintained by repeated passage through Syrian hamsters. Metacyclic promastigotes were obtained from cultured stationary-phase promastigotes (recently transformed from hamster-derived amastigotes) as described previously (34). Briefly, 5- to 6-day-old stationary-phase cultures were washed and then resuspended in Dulbecco's modified Eagle's medium at 2 × 108/ml. The parasites were incubated with peanut agglutinin (50 μg/ml) for 15 min at room temperature, and the agglutinated parasites were pelleted by centrifugation at 200 × g. The metacyclic promastigotes were then collected from the supernatant, washed, and used immediately for the mouse infections.
Cloning of Leishmania p36(LACK) cDNA.
The p36(LACK) cDNA was cloned from multiple Leishmania spp. by PCR amplification of genomic DNA. Genomic DNA was isolated by lysis of cultured promastigotes in 50 mM NaCl–50 mM EDTA–1% sodium dodecyl sulfate–50 mM Tris-HCl (pH 8.0), treatment with proteinase K and RNase A, and extraction of the DNA with phenol-chloroform. The DNA was precipitated and resuspended in Tris-EDTA buffer, and 0.5 to 1.0 μg was amplified with a mixture (15:1) of Taq and Pfu polymerases. The amplification product was isolated by agarose gel electrophoresis and gel extraction and cloned into the pCR 2.1 vector (Invitrogen). The cDNA insert from each of the plasmids was sequenced using vector-specific primers and an automated, fluorescent DNA sequencer (model 373A; Applied Biosystems). BLAST was used to search the National Center for Biotechnology Information databases to identify previously cloned homologous sequences (2). The entire coding regions of the L. donovani and L. major p36(LACK) cDNAs were subcloned into the HindIII and XbaI sites in the pcDNA3.1 vaccine expression vector (Invitrogen). The pcDNA3.1 plasmid used for these studies is the same as the parent pcDNA3 vector (which was used as the vaccine plasmid in the L. major LACK vaccine studies [13]) except for the addition of several restriction enzyme sites and removal of a SP6 promoter site in the multicloning site.
Construction and analysis of the murine IL-12p40-p35 fusion gene expression vector.
The approach used to construct a functional IL-12p40-p35 fusion molecule was a modification of the method published by Lieschke et al (23). The open reading frames of the murine IL-12 p40 and IL-12 p35 genes were PCR amplified. The forward primer used for the p40 amplification included an EcoRI restriction site and a Kozak sequence, and the reverse primer used for the p35 amplification contained a BamHI site. The reverse primer for the p40 amplification contained a 35-nucleotide linker sequence, and the forward primer of the p35 amplification contained a 36-nucleotide linker sequence that was the reverse complement of the p40 linker sequence. The p40 reverse primer excluded the p40 stop codon, and the p35 forward primer excluded the p35 signal sequence (first 22 codons). Following amplification of the individual subunits, the p40 and p35 products containing the linker sequences were amplified together by overlap PCR using the forward p40 and reverse p35 primers. The amplification product containing the p40 subunit fused in frame with the p35 subunit was cloned into a the TOPO 2.1 vector, its correct sequence was confirmed, and then it was subcloned into the EcoRI and BamHI sites of the pcDNA3.1 eukaryotic expression vector.
The expression of immunoreactive murine IL-12 was determined by measurement of IL-12 in supernatants of transfected L293 cells by enzyme-linked immunosorbent assay (ELISA) (Pharmingen). The biological activity of the IL-12p40-p35 fusion molecule was determined by (i) measurement of IFN-γ in the serum of mice inoculated with 100 μg (once in the footpad) of pcDNA3.1-IL-12p40-p35 or the pcDNA3.1 vector control and (ii) measurement of mitogen (concanavalin A)-induced IFN-γ production by lymph node cells from mice inoculated with the IL-12 or control plasmids.
Immunization and challenge of mice.
The protective efficacy of the p36(LACK) DNA vaccine constructs was determined in a fashion similar to that used in our previous vaccine studies (24). BALB/c mice (Charles River Laboratories) (4 to 6 weeks old; six per group) were immunized with p36(LACK) plasmid DNA or control vector DNA (100 μg in 25 μl of phosphate-buffered saline) by injection in the hind footpad, rump, tail 1 cm distal to the rump, or ear pinna on days 0 and 14. Injections of the ear and rump were true intradermal injections, whereas the injection into the tail and footpad probably delivered the vaccine mostly into the subcutaneous tissue. On day 28, the mice were challenged by intravenous (i.v.) (lateral tail vein) or cutaneous (hind footpad) injection with either 2 × 104 (low-dose) or 1 × 106 (high-dose) purified metacyclic promastigotes. The animals were euthanized 3 or 30 days after challenge and the liver, spleen, and/or lymph node were harvested for determination of parasite burden or in vitro cytokine analysis.
Determination of vaccine-induced cytokine production.
Spleens or lymph nodes from control and immunized mice were harvested, and single-cell suspensions were obtained by homogenization of the tissue between the frosted ends of two glass microscope slides. The erythrocytes were lysed with ammonium chloride lysis buffer (Sigma), and the cells were washed and cultured in complete medium (Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal bovine serum, 100 mM glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 5 × 10−5 M 2-mercaptoethanol) at 2 × 106 cells per ml. The cells were cultured in medium alone (control) or stimulated with 25 μg of SLDA per ml for 48 to 72 h. In some experiments, the spleen or lymph node cells were stimulated with washed, viable L. donovani promastigotes (105 per well). The IL-4 and IFN-γ levels in the supernatants were determined by sandwich ELISA using monoclonal antibodies (capture and detection) (Pharmingen, San Diego, Calif.) as described previously (25). In some experiments the production of IFN-γ in vaccinated and control mice in response to parasite challenge was determined. Spleen and lymph node cells obtained from vaccinated and unvaccinated mice 3 and 30 days postinfection were cultured ex vivo in complete medium in the absence of exogenous antigenic stimulation. The release of IFN-γ into the culture supernatant was measured by ELISA.
Determination of the tissue parasite burden.
Quantitative limiting-dilution culture was performed as described previously (34). Each organ was harvested, and its total weight was determined. A whole lymph node or weighed piece of spleen or liver (20 to 60 mg) was then homogenized between the frosted ends of two sterile glass slides in 1 ml of complete M199 culture medium and diluted with the same medium to a final concentration of 1 mg/ml. Fourfold serial dilutions of the homogenized tissue suspension were then plated in a 96-well tissue culture plate containing a 50-μl blood agar slant and cultured at 26°C for 2 weeks. The wells were examined for motile promastigotes at 3-day intervals, and the reciprocal of the highest dilution which was positive for parasites was considered to be the number of parasites per milligram of tissue. The total organ burden was calculated using the weight of the whole organ.
Statistical analysis.
Data are presented as the mean and standard error of the mean. The significance of differences among groups was determined by Student's two-tailed t test; differences were significant at P ≤ 0.05.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the p36(LACK) DNAs cloned in this study are as follows: L. donovani, AF363974; L. major, AF363975; L. mexicana, AF363976; L. amazonensis, AF363977; L. braziliensis, AF363978.
RESULTS
The p36(LACK) protein is highly conserved among Leishmania spp.
We cloned and sequenced the p36(LACK) cDNA from multiple New and Old World Leishmania spp. As was previously shown for the L. major and L. chagasi p36(LACK) cDNA (26), there is almost complete amino acid sequence identity among the different species, including Old and New World strains (data not shown). The L. donovani p36(LACK) cDNA sequence was determined from several clones obtained by PCR amplification of both genomic DNA or cDNA from the L. donovani 1S strain. The L. donovani p36(LACK) deduced amino acid sequence was identical to the published L. chagasi and L. infantum sequences (GenBank accession numbers U27569 and U49695, respectively and differed at only 1 or 2 amino acids from the L. major, L. mexicana, L. amazonensis, and L. braziliensis sequences we obtained (data not shown). The L. major p36(LACK) immunodominant T-cell epitope (amino acids 158 to 173) shown previously to drive the early IL-4 response in the L. major-infected BALB/c mouse (29) was invariant among the sequences of p36(LACK) from the other Leishmania spp.
Vaccination with L. donovani p36(LACK) DNA induces a strong parasite-specific Th1 response.
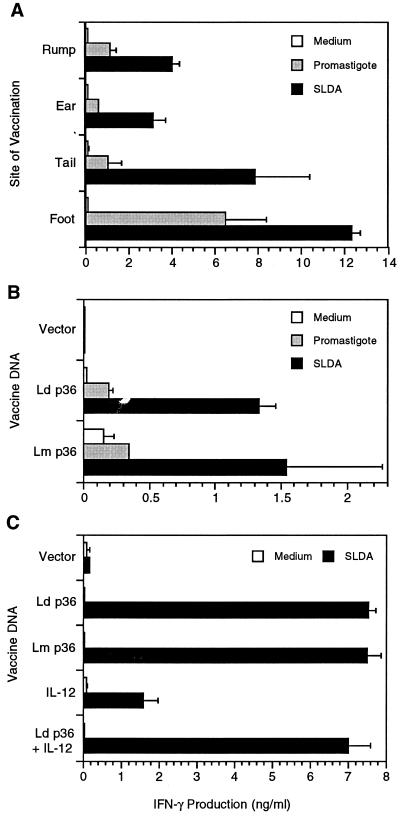
Mice were vaccinated by cutaneous injection of the L. donovani or L. major p36(LACK) DNA cloned into the pcDNA3.1 vector. The cutaneous route was chosen to deliver the DNA to the potent antigen-presenting cells of the skin, epidermal Langerhans' cells and dermal dendritic cells. Since the site of administration may influence the vaccine-induced immune response (10), we initially compared the immunogenicity of the DNA vaccine when delivered at different cutaneous sites. Cutaneous vaccination in the ear, rump, tail, and footpad each induced a spleen cell IFN-γ response to soluble antigen or whole viable parasites (Fig. 1A). Vaccination in the footpad resulted in the strongest antigen (SLDA)-induced IFN-γ response. This difference was statistically significant for the foot compared to the rump (P ≤ 0.001) and ear (P = 0.03) but not compared to the tail. There was also more parasite-induced IFN-γ production in mice vaccinated in the foot compared to other sites, but this difference did not reach statistical significance. There was no vaccine-induced spontaneous IFN-γ production, and no antigen- or parasite-specific IL-4 production could be detected (data not shown). It should be noted that the vaccine was delivered intradermally in the skin of the ear and rump, but intradermal delivery in the footpad and tail is difficult; much of the vaccine at those sites probably localizes to the subcutaneous tissue.
FIG. 1.
Vaccination of mice with L. donovani or L. major p36(LACK) DNA induces a strong in vitro antigen-specific IFN-γ response. (A) Groups of three or four BALB/c mice were vaccinated in the skin of the rump, ear, tail, or hind footpad with 100 μg of purified L. donovani p36(LACK) plasmid DNA on days 0 and 14. On day 28 the mice were sacrificed and the spleen cells were isolated and stimulated in vitro with SLDA (25 μg/ml) or live L. donovani promastigotes (105/well). Culture supernatants were harvested after stimulation for 72 h, and the concentration of IFN-γ was determined by sandwich ELISA. The antigen-induced IFN-γ concentration in culture supernatants is shown as the mean and standard error of the mean for the stimulated (SLDA and promastigotes) and unstimulated (medium) spleen cells. Data shown are from a single immunization experiment. (B) The immunogenicities of the L. donovani (Ld) and L. major (Lm) p36(LACK) DNA vaccines was compared. Groups of three mice were immunized in the hind footpad with the p36(LACK) DNA or vector control, and the spleen cell IFN-γ response to SLDA or viable promastigotes was determined as described above. The data shown are representative of two experiments. (C) The effect of coadministration of IL-12 DNA with the p36(LACK) DNA vaccine was determined. Groups of four mice were immunized as described above with either the L. donovani (Ld) or L. major (Lm) p36(LACK) DNA vaccines, the L. donovani (Ld) p36(LACK) DNA vaccine plus IL-12 DNA (100 μg), the IL-12 DNA alone, or the vector control DNA. Spleen cells were harvested and the SLDA-induced IFN-γ response was determined as described for panel A. The data presented are representative of two experiments.
Because of the strong IFN-γ response induced by administration of the vaccine DNA into the skin of the hind footpad, we used this route of delivery for subsequent experiments. This was also the route of delivery used in the prior L. major LACK vaccine studies (12, 13). Immunization with the vector DNA (lacking Leishmania DNA) did not induce an antigen-specific response in either the draining lymph node cells (Fig. 1B) or spleen cells (Fig. 1C). Vaccination with the p36(LACK) DNA vaccine constructs derived from L. donovani or L. major induced strong antigen-specific Th1 responses in lymph node cells (Fig. 1B) or spleen cells (Fig. 1C). Stimulation of lymph node cells with viable promastigotes also resulted in IFN-γ secretion in the vaccinated but not unvaccinated mice.
Because of previous reports that IL-12 could act as a strong adjuvant with protein and DNA vaccines (1, 38), we postulated that the immunogenicity of the p36(LACK) DNA vaccine might be further enhanced by coadministration of IL-12 DNA. An IL-12p40-p35 fusion cDNA was created by overlap PCR and inserted into the pcDNA3.1 vector. Expression of the IL-12 fusion molecule was confirmed by in vitro transfection of L293 cells and measurement of IL-12 p70 in the transfected cell supernatant by ELISA. The culture supernatant of pcDNA3.1-transfected cells had 0.04 ng of IL-12 per ml, whereas the supernatant of pcDNA3.1–IL-12p40-p35-transfected cells had 11.3 ng of IL-12 per ml. The production of biologically active IL-12 following in vivo delivery of the IL-12 DNA construct was confirmed by finding increased levels of IFN-γ in serum following in vivo injection of 100 μg of plasmid (the IFN-γ concentration in serum 30 days after plasmid inoculation was <125 pg/ml in the vector control mice and 1,410 ± 190 pg/ml in the IL-12 plasmid-injected mice). Furthermore, there was a fivefold enhancement of mitogen-induced lymph node cell IFN-γ production in mice that received the IL-12 plasmid construct compared to mice that received the vector control (788 ± 96 and 165 ± 110 pg/ml, respectively). The in vivo effect persisted for at least 6 weeks after administration of the IL-12 plasmid. Despite production of biologically active murine IL-12, coadministration of the IL-12 expression plasmid did not enhance the spleen cell antigen-specific IFN-γ response induced by the p36(LACK) DNA vaccine (Fig. 1C).
Vaccination with L. donovani p36(LACK) DNA primes for a strong in vivo Th1 response to parasite challenge.
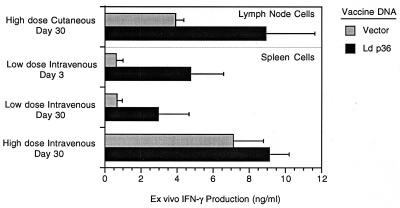
As a further measure of vaccine efficacy, we investigated whether the p36(LACK) DNA vaccine could prime the host to respond to an in vivo parasite challenge. We reasoned the mice should respond to the parasite challenge with enhanced IFN-γ production if they had been primed effectively by the vaccine. In these experiments, vaccinated or control mice were challenged with either a high (1 × 106) or a low (2 × 104) dose of metacyclic L. donovani promastigotes and the IFN-γ response to the infecting parasite was determined 3 and 30 days postinfection. Infection of unvaccinated or sham-vaccinated (vector control) mice resulted in significant production of IFN-γ by spleen cells cultured ex vivo without any exogenous stimulation (Fig. 2). The level of the IFN-γ response to infection in unvaccinated mice was parasite dose dependent; high-dose parasite challenge resulted in greater IFN-γ production than did low-dose challenge (Fig. 2). In response to i.v. challenge with a low dose of parasites, vaccinated mice produced higher levels of IFN-γ than did unvaccinated mice both 3 and 30 days postinfection. Cutaneous challenge with a high dose of parasites similarly resulted in higher levels of production of IFN-γ by lymph node cells in the vaccinated compared to the unvaccinated (vector control) mice. In each of these cases, however, there was considerable variability within the groups, and so the increased level of IFN-γ production by the vaccinated compared to the unvaccinated mice did not reach statistical significance. The level of IFN-γ produced by spleen cells at 30 days postchallenge in control or vaccinated mice that received the high-dose i.v. challenge was similar. In this case, the high parasite burden in the spleen (see Fig. 3), which is itself a strong stimulus for IFN-γ production, appears to have masked any vaccine-induced IFN-γ response. Taken together, these data indicate that immunization with the L. donovani p36(LACK) DNA vaccine primed the host to generate a stronger Th1 response to the inoculated parasite. Codelivery of the IL-12 expression plasmid did not enhance the L. donovani p36(LACK) DNA vaccine priming for parasite-induced IFN-γ (data not shown).
FIG. 2.
Ex vivo IFN-γ secretion by spleen or lymph node cells isolated from unvaccinated (vector control) or vaccinated mice after challenge with a high or low dose of parasites. Groups of six BALB/c mice were immunized with L. donovani (Ld) p36(LACK) DNA vaccine or vector control and challenged with either a high dose (1 × 106 metacyclic promastigotes by i.v. or cutaneous [hind footpad] injection) or low dose (2 × 104 metacyclic promastigotes by i.v. injection) of parasites. At either 3 or 30 days postchallenge, the mice were sacrificed and the spleen or draining lymph nodes were harvested. The spleen or lymph node cells were cultured in complete medium in the absence of exogenous antigen stimulation, and the supernatants were collected 72 h later. The concentration of IFN-γ was determined by sandwich ELISA and is shown as the mean and standard error of the mean for the groups of six mice. The data shown are representative of two or three experiments.
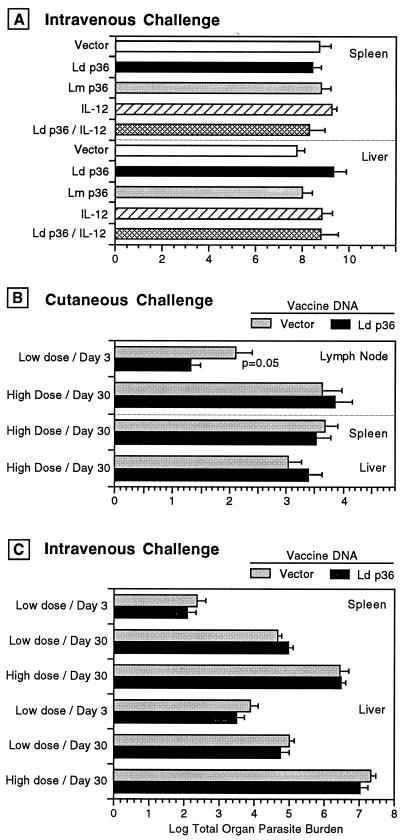
FIG. 3.
Vaccination with L. donovani p36(LACK) does not protect against cutaneous or systemic challenge with a high or low dose of L. donovani metacyclic promastigotes. Groups of five or six BALB/c mice were immunized with either the L. donovani (Ld) or L. major (Lm) p36(LACK) DNA vaccines, the L. donovani (Ld) p36(LACK) DNA vaccine plus IL-12 DNA (100 μg), the IL-12 DNA alone, or the vector control DNA on days 0 and 14. At 14 days later the mice were challenged with metacyclic promastigotes at either a high (1 × 106) or low (2 × 104) dose by IV or cutaneous (hind footpad) injection. At either 3 or 30 days postchallenge, the mice were sacrificed and the lymph node and/or liver and spleen were harvested. The tissue parasite burden was determined by limiting-dilution quantitative culture at 3 or 30 days after challenge and is expressed as the log mean and standard error of the mean for the parasite burden in the whole organ. The data shown are representative of two experiments. (A) Parasite burden in the liver or spleen 30 days after high-dose i.v. challenge. (B) Parasite burden in lymph nodes, liver and spleen at 3 or 30 days after high or low-dose i.v. challenge. There was a statistically significant reduction in the parasite burden in the vaccinated compared to the vector control group only in the lymph nodes 3 days following low-dose cutaneous parasite challenge.
Vaccination with L. donovani p36(LACK) DNA does not protect against parasite challenge.
In spite of the demonstrated ability of the p36(LACK) DNA vaccine to induce an antigen-specific recall response and its ability to prime for a characteristically protective Th1 response in vivo, vaccination did not protect against parasite challenge when the end point for vaccine efficacy was a reduction in tissue parasite burden. In initial experiments we used an immunization model that we had used previously to identify DNA vaccine candidates that protected against L. donovani challenge (24). Mice were immunized with the L. donovani or L. major p36(LACK) DNA vaccines and challenged i.v. with 106 metacyclic promastigotes (high dose), and the parasite burden in the liver and spleen was determined 30 days postchallenge. Under these conditions, vaccination with p36(LACK) DNA did not protect against parasite challenge compared to control mice that received the empty vector (Fig. 3A). The coadministration of the IL-12 expression plasmid did not enhance the protective efficacy of the L. donovani p36(LACK) DNA vaccine. Because there was no protection against parasite challenge despite the strong immunogenicity of the vaccine, we considered that the high dose and direct (i.v.) challenge to the visceral organs could overwhelm a protective response. Therefore, we investigated whether protection could be demonstrated in a model where the mice were challenged either i.v. with a low dose of parasites (2 × 104 metacyclic promastigotes) or by cutaneous inoculation. Both types of infection would be expected to deliver a lower dose of parasites to the reticuloendothelial organs and therefore would more closely mimic a natural infectious challenge.
Following cutaneous challenge with high-dose parasites, there was no difference between the vaccinated and unvaccinated groups when the parasite burden was determined at 30 days postinfection in the lymph node, liver, or spleen (Fig. 3B). There was a 0.7-log-unit reduction in the lymph node parasite burden early (3 days) after infection in the vaccinated compared to the unvaccinated mice that received the low-dose intradermal challenge (P = 0.05). As expected, i.v. challenge with a low dose of parasites resulted in a substantially lower (∼2 to 3 log units) visceral parasite burden at 30 days postinfection than did challenge with a high does of parasites (Fig. 3C). Despite this less rigorous systemic parasite challenge, there was no difference in the parasite burdens of the vaccinated and unvaccinated mice at 3 or 30 days after the low-dose i.v. challenge (Fig. 3C).
DISCUSSION
In this study we demonstrated that vaccination with L. donovani p36(LACK) DNA primed for a strong in vitro and in vivo parasite-specific Th1 response but did not confer protection against parasite challenge. This is in contrast to results from earlier studies with a different murine model of cutaneous leishmaniasis, where the L. major LACK(p24) antigen, delivered either as recombinant protein (with IL-12 adjuvant) or as DNA vaccine, was highly effective in protecting against cutaneous L. major challenge (13, 26).
We tested the efficacy of the p36(LACK) DNA vaccine in a model of visceral disease, where there is rapid replication of the parasite in the visceral organs in the few months following i.v. challenge with L. donovani promastigotes. Although vaccine-induced protection has been difficult to achieve in this model (15, 19, 31; A. C. White, Jr., and D. McMahon-Pratt, Letter, J. Infect. Dis. 161:1313–1314, 1990), we demonstrated previously that induction of a Th1 immune response and significant reduction in visceral parasite burden is achievable following immunization with a multicomponent DNA vaccine (24). In the present study the p36(LACK) DNA vaccine induced a similar strongly polarized Th1 response. Therefore, the absence of vaccine efficacy was not due to the lack of a characteristically protective type of immune response or to the generation of a counterprotective cytokine response. In fact, the DNA vaccine induced a strong Th1 response even without coadministration of an adjuvant other than the DNA itself, and this response was not enhanced by codelivery of IL-12 DNA. These data support the findings of previous studies (11, 39) that although the generation of a vaccine-induced Th1 response is necessary for protection against leishmanial infection, it may not be sufficient.
The inability of the p36(LACK) DNA vaccine to induce protection against L. donovani infection was not likely to be due to a difference in the amino acid sequence of the L. donovani protein compared to the L. major sequence. The amino acid sequence differed at only 3 of 212 residues, and the amino acids that comprise the dominant T-cell epitope (amino acids 158 to 173) in the L. major cutaneous infection model were identical. The absence of a protective effect was also not due to a lack of induction of systemic immunity following cutaneous immunization, since strong antigen-specific Th1 responses were observed both in the draining lymph node and spleen.
We postulated initially that in spite of the strong soluble antigen-induced in vitro Th1 response, the lack of protective efficacy might be due to ineffective in vivo priming of the host T-cell response to the invading parasite. Two lines of evidence suggest that this is not the case. First, spleen cells from vaccinated mice secreted IFN-γ following exposure in vitro to whole promastigotes, indicating that viable parasites and not just soluble antigens were a target of the vaccine-induced immune response. Second, vaccination with p36(LACK) DNA augmented the ex vivo production of IFN-γ following in vivo parasite challenge. Thus, in the context of an active in vivo infection, the parasite or its products were recognized by the vaccine-primed T cells. This priming was evident as early as 3 days postinfection and was maintained at least until 30 days postinfection. These data, however, do not exclude the possibility that the vaccine-primed T cells were directed against antigens released by dead or dying parasites rather than against viable parasites present within host macrophages. Recent studies of the L. major LACK antigen would support this possibility. Prina et al. reported that although L. major amastigotes express the LACK antigen, infected macrophages were unable to activate LACK-specific T cells (reactive to the amino acid 158 to 173 epitope) in the absence of exogenous antigen (30). In contrast, LACK-specific T cells could be stimulated only by recently infected macrophages that had been activated by IFN-γ to kill the parasites. Thus, in the L. major model, macrophages that harbored viable amastigotes were not recognized by T cells reactive with the dominant LACK epitope. These findings were extended by Courret et al. who showed that T cells reactive with the immunodominant LACK158-173 peptide could be stimulated only by macrophages that had been infected with nonsurviving, nonmetacyclic promastigotes (8). In our study, T cells from p36(LACK)-vaccinated mice were effectively stimulated in vitro with stationary-phase promastigotes, but this parasite preparation would have contained some noninfective procyclic forms. Likewise, the observed IFN-γ response to in vivo challenge with purified metacyclic promastigotes could have conceivably been directed against a few residual procyclic forms that would have been killed upon entry into the host. Thus, the strong Th1 response and ineffective parasite killing we observed could coexist if the p36(LACK)-activated T cells were directed at antigen from procyclic or killed promastigotes and not at viable intracellular amastigotes. The vaccine-induced transient reduction in parasite burden in the draining lymph nodes early (3 days) after infection may have been related to a bystander effect of the Th1 response to antigens released by dead or dying noninfective promastigotes.
The difference in protection observed between the cutaneous leishmaniasis model and our study may relate to the differences in the role of IL-4 in the pathogenesis of these two infections. The L. major-BALB/c mouse model is unique in that the early dominant T-cell response is directed toward the LACK antigen, resulting in disease-promoting IL-4 production (17, 20, 21). The response is mediated by T cells that express the Vβ4/Vα8 T-cell receptor and respond to a peptide epitope comprising amino acids 158 to 173 of the LACK antigen in the context of major histocompatibility complex class II I-Ad molecules (29, 33). Vaccination with the LACK DNA or LACK protein plus IL-12 was effective because it redirected the early T-cell response away from the pathogenic IL-4 response and toward a protective Th1 response (13, 17, 18). In contrast to the L. major-BALB/c mouse model, IL-4 does not play a primary pathogenic role in murine L. donovani infection (19, 22, 36). Therefore, if the protective effect of the LACK vaccine is mediated solely through redirection of the pathogenic LACK-induced IL-4 response, one would not expect that it would be effective in the murine VL model, where early IL-4 production does not promote susceptibility. If this is the case, then vaccination with this antigen will have little effect outside of the unique L. major-BALB/c mouse model of cutaneous leishmaniasis.
In spite of the absence of sustained p36(LACK) vaccine-induced protection against parasite challenge, it is possible that the induction of a LACK antigen-specific Th1 response to early infection could contribute to the protective response generated by a multicomponent vaccine. Even if p36(LACK)- reactive T cells responded only to procyclic or dead parasite antigens contained in an infectious sand fly inoculum, the local IFN-γ production could potentially contribute to protection by either activating recently infected macrophages through a local bystander effect or driving the T-cell response directed at other vaccine antigens toward a Th1 phenotype. This possibility deserves further investigation.
ACKNOWLEDGMENTS
This work was supported by a Merit Review Grant from the U.S. Department of Veterans Affairs.
We thank Hector Flores for excellent technical assistance and Sunil Ahuja, Seema Ahuja, and Marlon Quinones for helpful discussions.
REFERENCES
- 1.Afonso L C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Badaro R, Jones T C, Carvalho E M, Sampaio D, Reed S G, Barral A, Teixeira R, Johnson W D., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho E M, Bacellar O, Barral A, Badaro R, Johnson W D., Jr Antigen-specific immunosuppression in visceral leishmaniasis is cell mediated. J Clin Investig. 1989;83:860–864. doi: 10.1172/JCI113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho E M, Teixeira R S, Johnson W D., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981;33:498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champsi J, McMahon-Pratt D. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun. 1988;56:3272–3279. doi: 10.1128/iai.56.12.3272-3279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell N D, Medina-Acosta E, McMaster W R, Bloom B R, Russell D G. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci USA. 1993;90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courret N, Prina E, Mougneau E, Saraiva E M, Sacks D L, Glaichenhaus N, Antoine J C. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur J Immunol. 1999;29:762–773. doi: 10.1002/(SICI)1521-4141(199903)29:03<762::AID-IMMU762>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Dole V S, Raj V S, Ghosh A, Madhubala R, Myler P J, Stuart K D. Immunization with recombinant LD1 antigens protects against experimental leishmaniasis. Vaccine. 2000;19:423–430. doi: 10.1016/s0264-410x(00)00207-3. [DOI] [PubMed] [Google Scholar]
- 10.Forg P, von Hoegen P, Dalemans W, Schirrmacher V. Superiority of the ear pinna over muscle tissue as site for DNA vaccination. Gene Ther. 1998;5:789–797. doi: 10.1038/sj.gt.3300628. [DOI] [PubMed] [Google Scholar]
- 11.Gicheru M M, Olobo J O, Anjili C O, Orago A S, Modabber F, Scott P. Vervet monkeys vaccinated with killed Leishmania major parasites and interleukin-12 develop a type 1 immune response but are not protected against challenge infection. Infect Immun. 2001;69:245–251. doi: 10.1128/IAI.69.1.245-251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurunathan S, Prussin C, Sacks D L, Seder R A. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat Med. 1998;4:1409–1415. doi: 10.1038/4000. [DOI] [PubMed] [Google Scholar]
- 13.Gurunathan S, Sacks D L, Brown D R, Reiner S L, Charest H, Glaichenhaus N, Seder R A. Vaccination with DNA encoding the immunodominant. LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handman E, Symons F M, Baldwin T M, Curtis J M, Scheerlinck J P. Protective vaccination with promastigote surface antigen 2 from Leishmania major is mediated by a TH1 type of immune response. Infect Immun. 1995;63:4261–4267. doi: 10.1128/iai.63.11.4261-4267.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe C L, Rachamim N, Sarfstein R. Characterization of two proteins from Leishmania donovani and their use for vaccination against visceral leishmaniasis. J Immunol. 1990;144:699–706. [PubMed] [Google Scholar]
- 16.Jardim A, Alexander J, Teh H S, Ou D, Olafson R W. Immunoprotective Leishmania major synthetic T cell epitopes. J Exp Med. 1990;172:645–648. doi: 10.1084/jem.172.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julia V, Glaichenhaus N. CD4(+) T cells which react to the Leishmania major LACK antigen rapidly secrete interleukin-4 and are detrimental to the host in resistant B10.D2 mice. Infect Immun. 1999;67:3641–3644. doi: 10.1128/iai.67.7.3641-3644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 19.Kaye P M, Curry A J, Blackwell J M. Differential production of Th1–7 and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991;146:2763–2770. [PubMed] [Google Scholar]
- 20.Launois P, Maillard I, Pingel S, Swihart K G, Xenarios I, Acha-Orbea H, Diggelmann H, Locksley R M, MacDonald H R, Louis J A. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 21.Launois P, Swihart K G, Milon G, Louis J A. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–3324. [PubMed] [Google Scholar]
- 22.Lehmann J, Enssle K H, Lehmann I, Emmendorfer A, Lohmann-Matthes M L. The capacity to produce IFN-gamma rather than the presence of interleukin-4 determines the resistance and the degree of susceptibility to Leishmania donovani infection in mice. J Interferon Cytokine Res. 2000;20:63–77. doi: 10.1089/107999000312748. [DOI] [PubMed] [Google Scholar]
- 23.Lieschke G J, Rao P K, Gately M K, Mulligan R C. Bioactive murine and human interleukin-12 fusion proteins which retain antitumor activity in vivo. Nat Biotechnol. 1997;15:35–40. doi: 10.1038/nbt0197-35. [DOI] [PubMed] [Google Scholar]
- 24.Melby P C, Ogden G B, Flores H A, Zhao W, Geldmacher C, Biediger N M, Ahuja S K, Uranga J, Melendez M. Identification of vaccine candidates for experimental visceral leishmaniasis by immunization with sequential fractions of a cDNA expression library. Infect Immun. 2000;68:5595–5602. doi: 10.1128/iai.68.10.5595-5602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melby P C, Yang Y Z, Cheng J, Zhao W. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect Immun. 1998;66:18–27. doi: 10.1128/iai.66.1.18-27.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mougneau E, Altare F, Wakil A E, Zheng S, Coppola T, Wang Z E, Waldmann R, Locksley R M, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 27.Murray H W, Masur H, Keithly J S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982;129:344–350. [PubMed] [Google Scholar]
- 28.Piedrafita D, Xu D, Hunter D, Harrison R A, Liew F Y. Protective immune responses induced by vaccination with an expression genomic library of Leishmania major. J Immunol. 1999;163:1467–1472. [PubMed] [Google Scholar]
- 29.Pingel S, Launois P, Fowell D J, Turck C W, Southwood S, Sette A, Glaichenhaus N, Louis J A, Locksley R M. Altered ligands reveal limited plasticity in the T cell response to a pathogenic epitope. J Exp Med. 1999;189:1111–1120. doi: 10.1084/jem.189.7.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prina E, Lang T, Glaichenhaus N, Antoine J C. Presentation of the protective parasite antigen LACK by Leishmania- infected macrophages. J Immunol. 1996;156:4318–4327. [PubMed] [Google Scholar]
- 31.Rachamim N, Jaffe C L. Pure protein from Leishmania donovani protects mice against both cutaneous and visceral leishmaniasis. J Immunol. 1993;150:2322–2331. [PubMed] [Google Scholar]
- 32.Rafati S, Baba A A, Bakhshayesh M, Vafa M. Vaccination of BALB/c mice with Leishmania major amastigote-specific cysteine proteinase. Clin Exp Immunol. 2000;120:134–138. doi: 10.1046/j.1365-2249.2000.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiner S L, Fowell D J, Moskowitz N H, Swier K, Brown D R, Brown C R, Turck C W, Scott P A, Killeen N, Locksley R M. Control of Leishmania major by a monoclonal alpha beta T cell repertoire. J Immunol. 1998;160:884–889. [PubMed] [Google Scholar]
- 34.Sacks D, Melby P. Animal models for the analysis of immune responses to leishmaniasis. In: Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1998. pp. 19.2.1–19.2.20. [Google Scholar]
- 35.Santos W R, Paraguai de Souza E, Palatnik M, Palatnik de Sousa C B. Vaccination of Swiss Albino mice against experimental visceral leishmaniasis with the FML antigen of Leishmania donovani. Vaccine. 1999;17:2554–2561. doi: 10.1016/s0264-410x(99)00058-4. [DOI] [PubMed] [Google Scholar]
- 36.Satoskar A, Bluethmann H, Alexander J. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect Immun. 1995;63:4894–4899. doi: 10.1128/iai.63.12.4894-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott P, Caspar P, Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990;144:1075–1079. [PubMed] [Google Scholar]
- 38.Sin J I, Kim J J, Arnold R L, Shroff K E, McCallus D, Pachuk C, McElhiney S P, Wolf M W, Pompa-de Bruin S J, Higgins T J, Ciccarelli R B, Weiner D B. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J Immunol. 1999;162:2912–2921. [PubMed] [Google Scholar]
- 39.Sjolander A, Baldwin T M, Curtis J M, Bengtsson K L, Handman E. Vaccination with recombinant parasite surface antigen 2 from Leishmania major induces a Th1 type of immune response but does not protect against infection. Vaccine. 1998;16:2077–2084. doi: 10.1016/s0264-410x(98)00075-9. [DOI] [PubMed] [Google Scholar]
- 40.Sjolander A, Baldwin T M, Curtis J M, Handman E. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J Immunol. 1998;160:3949–3957. [PubMed] [Google Scholar]
- 41.Solioz N, Blum-Tirouvanziam U, Jacquet R, Rafati S, Corradin G, Mauel J, Fasel N. The protective capacities of histone H1 against experimental murine cutaneous leishmaniasis. Vaccine. 1999;18:850–859. doi: 10.1016/s0264-410x(99)00340-0. [DOI] [PubMed] [Google Scholar]
- 42.Southgate B A. Studies in the epidemiology of East African leishmaniasis. 5. Leishmania adleri and natural immunity. J Trop Med Hyg. 1967;70:33–36. [PubMed] [Google Scholar]
- 43.Stager S, Smith D F, Kaye P M. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol. 2000;165:7064–7071. doi: 10.4049/jimmunol.165.12.7064. [DOI] [PubMed] [Google Scholar]
- 44.Stern J J, Oca M J, Rubin B Y, Anderson S L, Murray H W. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988;140:3971–3977. [PubMed] [Google Scholar]
- 45.Webb J R, Campos-Neto A, Ovendale P J, Martin T I, Stromberg E J, Badaro R, Reed S G. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun. 1998;66:3279–3289. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson M E, Young B M, Andersen K P, Weinstock J V, Metwali A, Ali K M, Donelson J E. A recombinant Leishmania chagasi antigen that stimulates cellular immune responses in infected mice. Infect Immun. 1995;63:2062–2069. doi: 10.1128/iai.63.5.2062-2069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu D, Liew F Y. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology. 1995;84:173–176. [PMC free article] [PubMed] [Google Scholar]