Background:
Symptomatic intracranial hemorrhage (sICH) is a severe complication of reperfusion therapy for ischemic stroke. Multiple models have been developed to predict sICH or intracranial hemorrhage (ICH) after reperfusion therapy. We provide an overview of published models and validate their ability to predict sICH in patients treated with endovascular treatment in daily clinical practice.
Methods:
We conducted a systematic search to identify models either developed or validated to predict sICH or ICH after reperfusion therapy (intravenous thrombolysis and/or endovascular treatment) for ischemic stroke. Models were externally validated in the MR CLEAN Registry (n=3180; Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands). The primary outcome was sICH according to the Heidelberg Bleeding Classification. Model performance was evaluated with discrimination (c-statistic, ideally 1; a c-statistic below 0.7 is considered poor in discrimination) and calibration (slope, ideally 1, and intercept, ideally 0).
Results:
We included 39 studies describing 40 models. The most frequently used predictors were baseline National Institutes of Health Stroke Scale (NIHSS; n=35), age (n=22), and glucose level (n=22). In the MR CLEAN Registry, sICH occurred in 188/3180 (5.9%) patients. Discrimination ranged from 0.51 (SPAN-100 [Stroke Prognostication Using Age and National Institutes of Health Stroke Scale]) to 0.61 (SITS-SICH [Safe Implementation of Treatments in Stroke Symptomatic Intracerebral Hemorrhage] and STARTING-SICH [STARTING Symptomatic Intracerebral Hemorrhage]). Best calibrated models were IST-3 (intercept, −0.15 [95% CI, −0.01 to −0.31]; slope, 0.80 [95% CI, 0.50−1.09]), SITS−SICH (intercept, 0.15 [95% CI, −0.01 to 0.30]; slope, 0.62 [95% CI, 0.38−0.87]), and STARTING−SICH (intercept, −0.03 [95% CI, −0.19 to 0.12]; slope, 0.56 [95% CI, 0.35−0.76]).
Conclusions:
The investigated models to predict sICH or ICH discriminate poorly between patients with a low and high risk of sICH after endovascular treatment in daily clinical practice and are, therefore, not clinically useful for this patient population.
Keywords: endovascular treatment, ischemic stroke, symptomatic intracranial hemorrhage
Reperfusion therapy (ie, intravenous thrombolytics (IVT), endovascular thrombectomy (EVT), or a combination of both) is an effective treatment for ischemic stroke at group level, despite the increased average risk of symptomatic intracranial hemorrhage (sICH).1,2 On an individual level, the occurrence of sICH after reperfusion therapy increases the likelihood of poor functional outcome and death.3 Reliable identification of individual patients with high risk of sICH could be useful to clinicians when therapeutic decisions are made, to inform patients and relatives on prognosis, and to personalize monitoring protocols.4
Several prediction models that aim to identify patients with a high risk of sICH after reperfusion therapy have been published.5–11 Before a prediction model can be implemented in clinical practice, the model should be evaluated thoroughly. External validation is essential in this evaluation because it assesses the generalizability of the model.12 Only a few models to predict sICH have been developed or externally validated in patients receiving EVT for ischemic stroke.9–11,13
We aimed to provide an overview of currently published models to predict sICH or ICH after reperfusion therapy and to externally validate their ability to predict sICH in patients treated with EVT in daily clinical practice.
Methods
Search Strategy and Eligibility of Prediction Models
A search strategy was developed in collaboration with a biomedical information specialist to systematically search PubMed, Embase, Medline, Web of Science, and Cochrane to identify studies reporting on the development or validation of models based on clinical, radiological and treatment-related variables to predict sICH or any ICH after reperfusion therapy for ischemic stroke. We included studies that included at least 2 variables in the model and were published in peer-reviewed journals. The search was restricted to studies published in English and conference abstracts were excluded. The search was conducted in August, 2021. The complete search strategy is listed in Table S1. Two reviewers (NvdE and FK) independently screened all titles and abstracts of the retrieved references. Subsequently, full-text copies of articles that potentially met the criteria were independently reviewed for final inclusion in this study. Consensus was reached with a third reviewer (DD) when needed.
External Validation Cohort
We used data from the MR CLEAN Registry (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands). The MR CLEAN Registry is a prospective, observational study of all patients who underwent EVT for ischemic stroke in the Netherlands. Details on the MR CLEAN Registry were published previously.2 For the present study, we selected patients who were registered between March 16, 2014 and November 1, 2017 and adhered to the following inclusion criteria: age≥18 years; treatment in a center that participated in the MR CLEAN trial; presence of a proximal intracranial occlusion in the anterior circulation confirmed on noninvasive vascular imaging (intracranial carotid artery [internal carotid artery (terminus)], middle cerebral artery [M1/M2], anterior cerebral artery [A1/A2]); and groin puncture within 6.5 hours after symptom onset. The central medical ethics committee of the Erasmus MC University Medical Center Rotterdam, the Netherlands, evaluated the study protocol and granted permission to carry out the study as a registry (MEC-2014-235). In compliance with the General Data Protection Regulation, source data are not available for other researchers. Information about analytic methods, study materials, and scripts of the statistical analyses are available from the corresponding author on reasonable request. The STROBE statement checklist of the study can be found in Table S2.
Predicted Outcome
We externally validated the models for their performance to predict sICH within 90 days after intervention. An ICH was deemed symptomatic if a patient died or deteriorated neurologically and evidence of related ICH on follow-up imaging (non-contrast computed tomography or magnetic resonance imaging).14 To minimize biased reporting, the imaging core laboratory analyzed the follow-up images of patients with sICH and the complication committee made the final decision for reporting a sICH.
Statistical Analysis
Models including predictors that were not included in the MR CLEAN Registry database were reconstructed from available variables if possible. If this was not possible, these models were included in the overview, but excluded for external validation.
Model performance was evaluated with discrimination and calibration. Discrimination was quantified with the concordance (c) statistic, which is identical to the area under the receiver operating curve for binary outcomes. The c statistic varies between 0.5 for a non-informative model and 1 for a perfectly discriminating model.15 A c statistic below 0.7 is considered a poor discriminative ability, a c statistic between 0.7 and below 0.8 is considered acceptable, a c statistic between 0.8 and below 0.9 is considered excellent, and a c statistic of 0.9 and above is considered outstanding.16 Calibration refers to the level of agreement between predicted risks and observed outcome expressed as a calibration intercept and slope. The intercept indicates whether predictions are systematically too high or too low, and should ideally be zero. The calibration slope describes the effect of the predictors in the validation sample and is ideally equal to 1.12 For models developed to predict outcomes other than sICH or ICH, but that have been evaluated for ICH prediction, calibration was only assessed if the regression model was adapted for ICH prediction.
For models that were presented as a risk score, we used the predicted probabilities of each model as published in the original development article. If a study expressed a probability of 0 (0%) for the outcome of interest, this value was adapted to 0.01 (1%), because these probabilities were otherwise excluded by the val.prob.ci.2 function in R.17 If the predicted probabilities were not published, we contacted the corresponding author to provide the predicted probability of the model. For regression models, authors were contacted to provide the regression formula if this was not reported.
Missing values were imputed with multiple imputation (n=5) using the function aregImpute. Confidence intervals of the model performance measures were composed with bootstrapping (200 samples in 5 imputed datasets).
All statistical analyses were performed with R statistical software (version 4.0.5).
Results
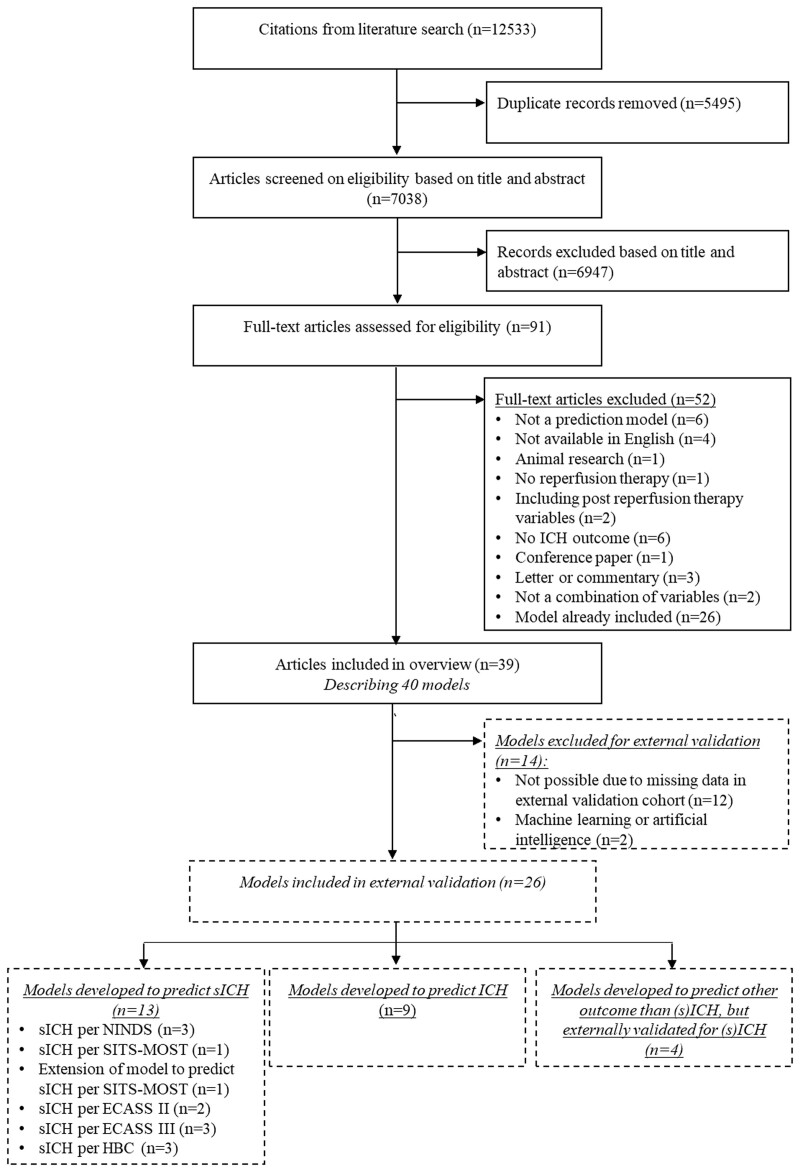
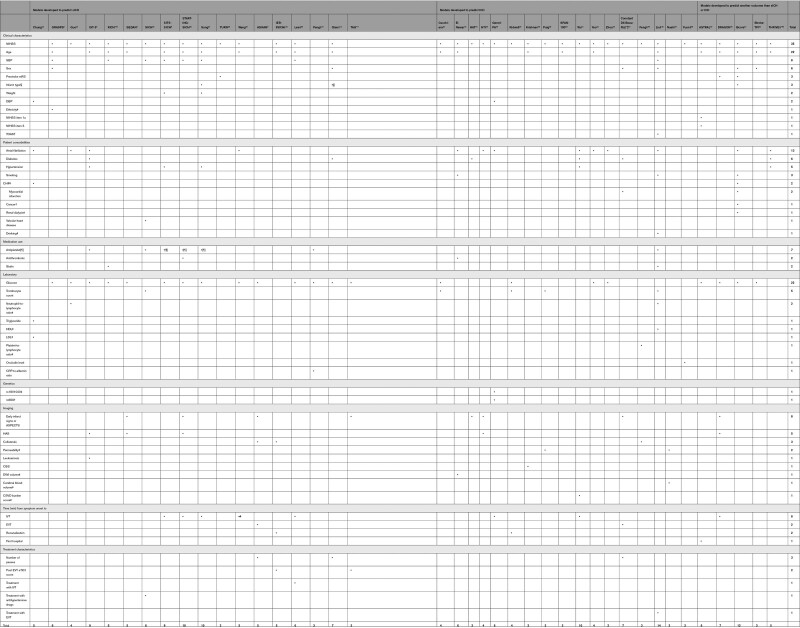
The literature search identified 7038 unique studies. A total of 6947 studies were excluded based on title and abstract. We assessed the full texts of 91 studies and included 39 studies, which described 40 models (Figure 1). Model development characteristics of the included models are shown in Table S3. The number of predictors varied between 2 and 14 predictors. The most frequently used predictors were baseline National Institutes of Health Stroke Scale (NIHSS; n=35), age (n=22), and baseline blood glucose (n=22; Table 1).
Figure 1.
Flow chart of the systematic literature search. ECASS indicates European Cooperative Acute Stroke Study; HBC, Heidelberg Bleeding Classification; ICH, intracranial hemorrhage; NINDS, National Institute of Neurological Disorders and Stroke; SICH, symptomatic intracranial hemorrhage; and SITS-MOST, Safe Implementation of Thrombolysis in Stroke-Monitoring Study.
Table 1.
Overview of Predictors Included in the Models
We excluded 14 models for external validation (Figure 1). For 8 included models, 1 predictor was adapted to be able to externally validate the model in the MR CLEAN Registry (Table S4). Of the 26/40 models available for external validation, 7/26 were developed in patients treated with EVT. Calibration of 6 models (GRASPS [GWTG-Stroke sICH Risk], Sung, Kidwell, SPAN-100 [Stroke Prognostication Using Age and National Institutes of Health Stroke Scale], ASTRAL [Acute Stroke Registry and Analysis of Lausanne], DRAGON [dense cerebral artery sign/early infarct signs on admission CT scan, prestroke modified Rankin Scale, age, glucose level at baseline, onset-to-treatment time, and baseline National Institutes of Health Stroke Scale score]) could not be assessed due to missing data in articles.
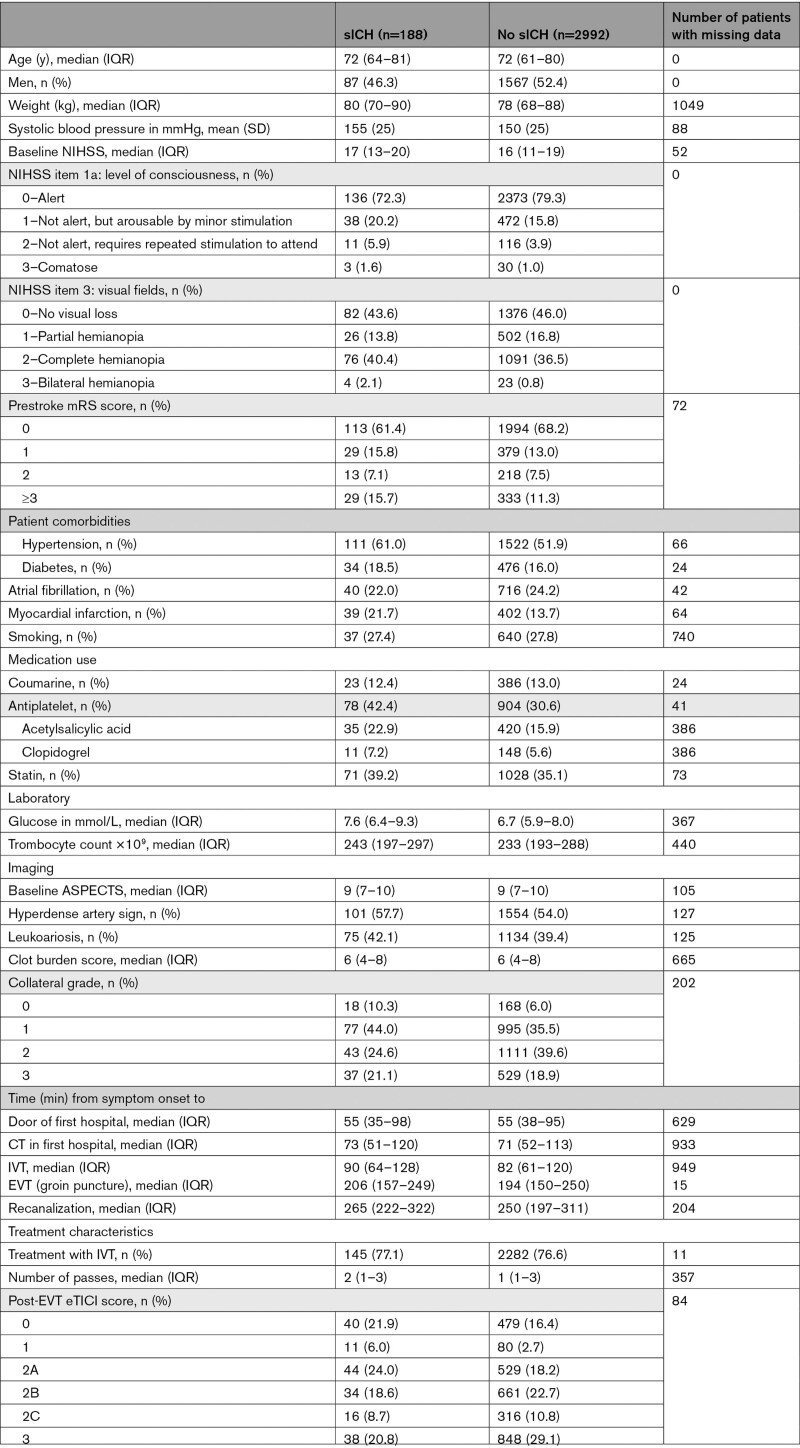
The external validation cohort consisted of 3180 patients. The mean age was 72 years and the median baseline NIHSS score was 16. In total, 188/3180 (5.9%) patients had an sICH (Table 2).
Table 2.
Patient Characteristics in the Validation Cohort According to the Occurrence of Symptomatic Intracranial Hemorrhage
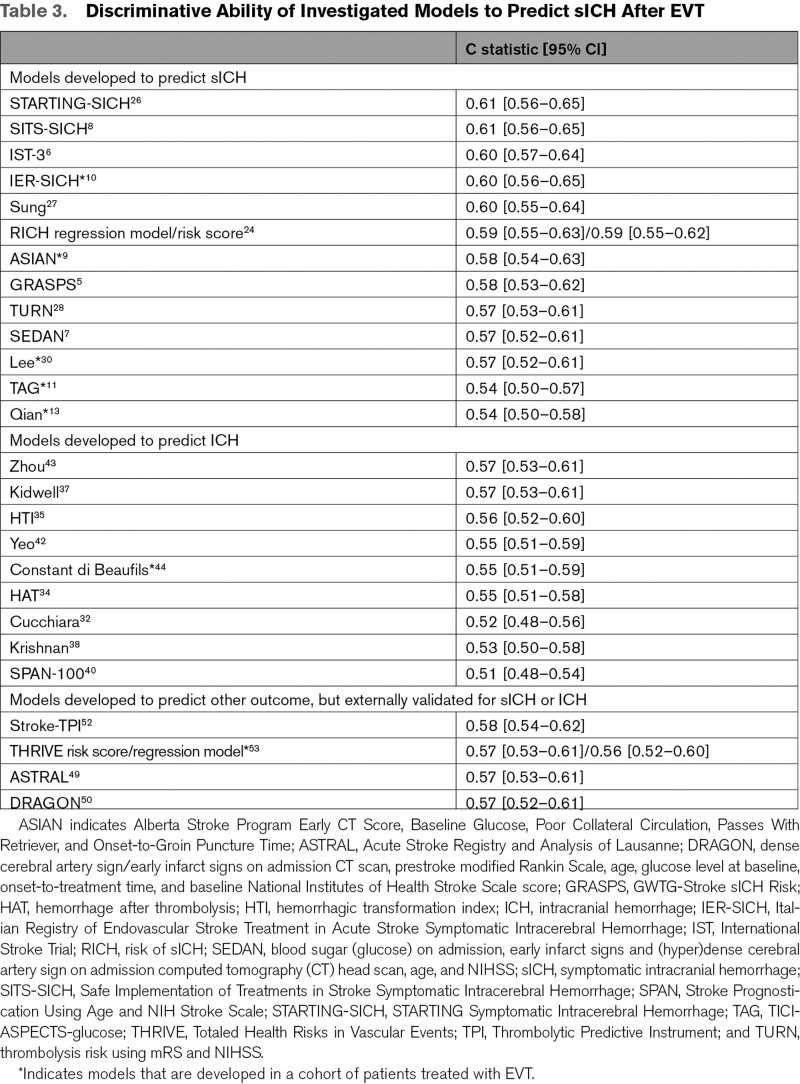
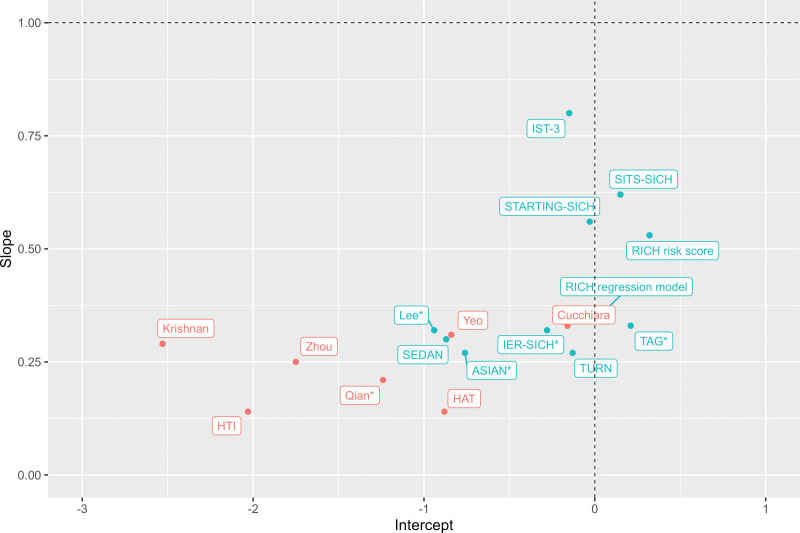
The discriminative ability of the models, expressed as c statistic, ranged from 0.51 (SPAN-100) to 0.61 (SITS-SICH [Safe Implementation of Treatments in Stroke Symptomatic Intracerebral Hemorrhage] and STARTING-SICH [STARTING Symptomatic Intracerebral Hemorrhage]) (Table 3). Model calibration, reported as calibration intercept and slope, varied substantially between studies (Figure 2, Table S4). The values at the extremes of the range for calibration intercept and slope were mainly models developed to predict ICH. Models with the best calibration characteristics were all models developed in a population of patients treated with IVT alone: IST-3 (intercept −0.15 [95% CI, −0.01 to −0.31]; slope 0.80 [95% CI, 0.50–1.09]), SITS-SICH (intercept 0.15 [95% CI, −0.01 to 0.30]; slope 0.62 [95% CI, 0.38–0.87]), and STARTING-SICH (intercept −0.03 [95% CI, −0.19 to 0.12]; slope 0.56 [95% CI, 0.35–0.76]) (Table S5, Figure S1).
Table 3.
Discriminative Ability of Investigated Models to Predict sICH After EVT
Figure 2.
Calibration intercept and slope of the models in the MR CLEAN Registry (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands). The models in blue are developed to predict symptomatic intracranial hemorrhage (sICH), and the models in red are developed to predict intracranial hemorrhage. The vertical dotted line indicates the ideal calibration intercept and the horizontal dotted line indicates the perfect calibration slope. A calibration intercept >0 indicates systematic underestimation of sICH risk, and an intercept<0 indicates systematic overestimation of sICH risk. A calibration slope <1 reflects that predictions were too extreme: low predictions too low, and high predictions too high. *Indicates models that are developed in a cohort of patients treated with endovascular thrombectomy. For SITS-SICH (Safe Implementation of Treatments in Stroke Symptomatic Intracerebral Hemorrhage), predicted probabilities according to several definitions of sICH are available in the article. In this Figure, calibration was based on predicted probabilities according to sICH per ECASS II (European Cooperative Acute Stroke Study) because this percentage of hemorrhages approximated the percentage in our dataset most and we therefore expected best calibration of the model with these predicted probabilities.
Discussion
We conducted a systematic search to provide an overview of published models to predict sICH or ICH after reperfusion therapy and externally validated their ability to predict sICH in patients treated with EVT in daily clinical practice. Investigated models to predict sICH or ICH discriminated poorly between patients with a low and high risk of sICH after EVT.
The IST-3, SITS-SICH, and STARTING-SICH showed overall the best predictive performance in terms of discrimination and calibration in stroke patients treated with EVT in daily clinical practice. The models had reasonable calibration characteristics, but the discriminative performance was poor. Even if a model would have perfect calibration characteristics (ie, predicted risk of the outcome for patients is equal to the observed risk), it is useless if it does not discriminate between patients with a low and high risk of the outcome.12
The poor discriminative performance of all models can be explained by several reasons. We included models that were developed to predict sICH or ICH according to different definitions, and this may affect strength and nature of the predictors. Another explanation is that most models were developed in patients treated with IVT alone. Although the incidence of sICH in patients treated with IVT alone is similar to patients treated with EVT,18 predictors of sICH or the predictive value of predictors may differ. For example, endovascular-procedure–related factors, such as number of passes or reperfusion at the end of the procedure, are important predictors of sICH.19,20 Moreover, generalizability of the models might be limited due to different selection criteria for treatment with IVT and/or EVT. Also, the use of antihypertensive medication or antithrombotics could influence the risk of sICH.
The predictive performance of models developed in patients treated with EVT in terms of both discrimination (n=7) and calibration (n=5) was poor. This could be explained because the population in which the models were developed was different from our external validation cohort. For example, TAG was developed in patients treated with EVT within 24 hours and ASIAN was developed in Chinese patients, who are at increased risk of sICH.9 Predictors or the prognostic value of predictors in the Asian population may differ from our population. The poor predictive performance of all models in our study emphasizes the importance of external validation.
External validation studies provide the best insight into the performance of a model, indicating how useful it might be in other participants, centers, regions, or settings.21 Therefore, it is efficient to evaluate the performance and usefulness of published models before a new model is developed. Because the predictive performance of all models we evaluated was poor, further research should focus on identifying predictors of sICH in patients treated with EVT, including interaction of predictors, before a new model should be developed and externally validated.12
Our study has several limitations. First, we evaluated the external validity of models to predict sICH in patients treated with EVT within 6.5 hours of symptom onset. Therefore, we cannot draw any conclusions about the usefulness of these models to predict sICH in patients treated after 6.5 hours of symptom onset or treated with IVT alone. Secondly, some variables were not available in our dataset. Therefore, we were not able to evaluate the external validity of all models: for example, models that included magnetic resonance imaging parameters. For some models, we imputed a missing variable with a value of 0, which might underestimate the performance of a model. For example, GRASPS included Asian ethnicity as predictor, which was not available in our dataset. We assigned all patients a score of 0 (ie, non-Asian ethnicity), because the number of people with Asian ethnicity in the Netherlands is relatively low and we believe this variable could not be imputed based on other variables. However, this might have influenced the predictive performance of GRASPS. Lastly, the predicted outcome in this study was sICH according to the Heidelberg Bleeding Classification. Therefore, the performance of models developed for other outcome measures or other sICH definitions, might be underestimated. However, being able to predict sICH accurately is more relevant than all ICH, because it is more strongly associated with poor functional outcome.
To conclude, the investigated models to predict sICH or ICH discriminate poorly between patients with a low and high risk of sICH after EVT in daily clinical practice. Therefore, these models are not clinically useful for this patient population.
Article Information
Acknowledgments
We gratefully appreciate the support of our biomedical information specialist, Wichor M. Bramer, Erasmus MC, who contributed to the literature search. We thank the MR CLEAN Registry investigators listed in Table S5.
Sources of Funding
The MR CLEAN Registry was partly funded by Stichting Toegepast Wetenschappelijk Instituut voor Neuromodulatie (TWIN), Erasmus MC University Medical Center, Maastricht University Medical Center, and Amsterdam University Medical Center.
Disclosures
Drs Dippel and van der Lugt report unrestricted grants from Stryker, Penumbra, Medtronic, Cerenovus, Thrombolytic Science, LLC, Dutch Heart Foundation, Brain Foundation Netherlands, The Netherlands Organization for Health Research and Development, and Health Holland Top Sector Life Sciences & Health for research, paid to institution. Dr Majoie received funds from TWIN Foundation (related to this project, paid to institution), CVON/Dutch Heart Foundation, Stryker, European Commission, Health Evaluation Netherlands (unrelated to this project; all paid to institution) and is shareholder of Nicolab. Dr Postma received an institutional grant from Siemens Healthineers. The other authors report no conflicts.
Supplemental Material
Tables S1–S6
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- EVT
- endovascular thrombectomy
- IVT
- intravenous thrombolytics
- MR CLEAN
- Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands
- sICH
- symptomatic intracranial hemorrhage
The MR CLEAN Registry Investigators are listed in Table S6.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.122.040065.
Contributor Information
Femke C.C. Kremers, Email: Femke.kremers@gmail.com.
Wouter van der Steen, Email: w.vandersteen@erasmusmc.nl.
Esmee Venema, Email: e.venema@erasmusmc.nl.
Manon Kappelhof, Email: m.kappelhof@amc.uva.nl.
Charles B.L.M. Majoie, Email: c.b.majoie@amsterdamumc.nl.
Alida A. Postma, Email: l.jacobi@mumc.nl.
Jelis Boiten, Email: j.boiten@haaglandenmc.nl.
Ido R. van den Wijngaard, Email: i.van.den.wijngaard@haaglandenmc.nl.
Aad van der Lugt, Email: a.vanderlugt@erasmusmc.nl.
Diederik W.J. Dippel, Email: d.dippel@erasmusmc.nl.
Bob Roozenbeek, Email: b.roozenbeek@erasmusmc.nl.
References
- 1.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database of Syst Rev. 2014;CD000213 doi: 10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansen IGH, Mulder M, Goldhoorn RB, investigators MCR. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN registry). BMJ. 2018;360:k949. doi: 10.1136/bmj.k949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier B, Desilles JP, Mazighi M. Intracranial hemorrhage after reperfusion therapies in acute ischemic stroke patients. Front Neurol. 2020;11:599908. doi: 10.3389/fneur.2020.599908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, Kim LJ, Mayer SA, Sheth KN, Schwamm LH, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Quality of Care and Outcomes Research. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e343–e361. doi: 10.1161/STR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 5.Menon BK, Saver JL, Prabhakaran S, Reeves M, Liang L, Olson DM, Peterson ED, Hernandez AF, Fonarow GC, Schwamm LH, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke. 2012;43:2293–2299. doi: 10.1161/STROKEAHA.112.660415 [DOI] [PubMed] [Google Scholar]
- 6.Whiteley WN, Thompson D, Murray G, Cohen G, Lindley RI, Wardlaw J, Sandercock P, IST-3 Collaborative Group. Targeting recombinant tissue-type plasminogen activator in acute ischemic stroke based on risk of intracranial hemorrhage or poor functional outcome: An analysis of the third international stroke trial. Stroke. 2014;45:1000–1006. doi: 10.1161/STROKEAHA.113.004362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strbian D, Engelter S, Michel P, Meretoja A, Sekoranja L, Ahlhelm FJ, Mustanoja S, Kuzmanovic I, Sairanen T, Forss N, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: The SEDAN score. Ann Neurol. 2012;71:634–641. doi: 10.1002/ana.23546 [DOI] [PubMed] [Google Scholar]
- 8.Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D, Wahlgren N, Ahmed N, Investigators S. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: Safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012;43:1524–1531. doi: 10.1161/STROKEAHA.111.644815 [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Xie Y, Wang H, Yang D, Jiang T, Yuan K, Gong P, Xu P, Li Y, Chen J, et al. Symptomatic intracranial hemorrhage after mechanical thrombectomy in Chinese ischemic stroke patients: the ASIAN score. Stroke. 2020;51:2690–2696. doi: 10.1161/STROKEAHA.120.030173 [DOI] [PubMed] [Google Scholar]
- 10.Cappellari M, Mangiafico S, Saia V, Pracucci G, Nappini S, Nencini P, Konda D, Sallustio F, Vallone S, Zini A, et al. ; Listing of IER Collaborators. IER-SICH nomogram to predict symptomatic intracerebral hemorrhage after thrombectomy for stroke. Stroke. 2019;50:909–916. doi: 10.1161/STROKEAHA.118.023316 [DOI] [PubMed] [Google Scholar]
- 11.Montalvo M, Mistry E, Chang AD, Yakhkind A, Dakay K, Azher I, Kaushal A, Mistry A, Chitale R, Cutting S, et al. Predicting symptomatic intracranial hemorrhage after mechanical thrombectomy: the tag score. J Neurol Neurosurg Psychiatry. 2019;90:1370–1374. doi: 10.1136/jnnp-2019-321184 [DOI] [PubMed] [Google Scholar]
- 12.Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an abcd for validation. Eur Heart J. 2014;35:1925–1931. doi: 10.1093/eurheartj/ehu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian Y, Qian ZT, Huang CH, Wang HY, Lu X, Cao K, Sun JY, Li QY. Predictive factors and nomogram to evaluate the risk of symptomatic intracerebral hemorrhage for stroke patients receiving thrombectomy. World Neurosurg. 2020;144:e466–e474. doi: 10.1016/j.wneu.2020.08.181 [DOI] [PubMed] [Google Scholar]
- 14.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 15.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosmer D, Lemeshow S, Sturdivant R. Applied Logistic Regression. Wiley, 2013:173–182. [Google Scholar]
- 17.Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: from utopia to empirical data. J Clin Epidemiol. 2016;74:167–176. doi: 10.1016/j.jclinepi.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 18.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, et al. ; HERMES Collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 19.Hao Y, Yang D, Wang H, Zi W, Zhang M, Geng Y, Zhou Z, Wang W, Xu H, Tian X, et al. ; ACTUAL Investigators (Endovascular Treatment for Acute Anterior Circulation Ischemic Stroke Registry). Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke. 2017;48:1203–1209. doi: 10.1161/STROKEAHA.116.016368 [DOI] [PubMed] [Google Scholar]
- 20.van der Steen W, van der Ende NAM, van Kranendonk KR, Chalos V, van Oostenbrugge RJ, van Zwam WH, Roos Y, van Doormaal PJ, van Es A, Lingsma HF, et al. Determinants of symptomatic intracranial hemorrhage after endovascular stroke treatment: a retrospective cohort study. Stroke. 2022;53:2818–2827. doi: 10.1161/STROKEAHA.121.036195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, Reitsma JB, Collins GS. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the charms checklist. PLoS Med. 2014;11:e1001744. doi: 10.1371/journal.pmed.1001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung CC, Chan L, Bamodu OA, Hong CT, Chiu HW. Artificial neural network based prediction of postthrombolysis intracerebral hemorrhage and death. Sci Rep. 2020;10:20501. doi: 10.1038/s41598-020-77546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H, Xu W, Zhang X, Zhang S, Dai Z, Li S, Xie Y, Li Y, Xue J, Liu X. A nomogram to predict symptomatic intracranial hemorrhage after intravenous thrombolysis in Chinese patients. Neuropsychiatr Dis Treat. 2021;17:2183–2190. doi: 10.2147/NDT.S320574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erdur H, Polymeris A, Grittner U, Scheitz JF, Tutuncu S, Seiffge DJ, Audebert HJ, Nolte CH, Engelter ST, Rocco A. A score for risk of thrombolysis-associated hemorrhage including pretreatment with statins. Front Neurol. 2018;9:74. doi: 10.3389/fneur.2018.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lokeskrawee T, Muengtaweepongsa S, Patumanond J, Tiamkao S, Thamangraksat T, Phankhian P, Pleumpanupat P, Sribussara P, Kitjavijit T, Supap A, et al. Prediction of symptomatic intracranial hemorrhage after intravenous thrombolysis in acute ischemic stroke: the symptomatic intracranial hemorrhage score. J Stroke Cerebrovasc Dis. 2017;26:2622–2629. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.030 [DOI] [PubMed] [Google Scholar]
- 26.Cappellari M, Turcato G, Forlivesi S, Zivelonghi C, Bovi P, Bonetti B, Toni D. STARTING-SICH nomogram to predict symptomatic intracerebral hemorrhage after intravenous thrombolysis for stroke. Stroke. 2018;49:397–404. doi: 10.1161/STROKEAHA.117.018427 [DOI] [PubMed] [Google Scholar]
- 27.Sung SF, Chen SC, Lin HJ, Chen CH, Tseng MC, Wu CS, Hsu YC, Hung LC, Chen YW. Oxfordshire community stroke project classification improves prediction of post-thrombolysis symptomatic intracerebral hemorrhage. BMC Neurol. 2014;14:39. doi: 10.1186/1471-2377-14-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asuzu D, Nystrom K, Amin H, Schindler J, Wira C, Greer D, Chi NF, Halliday J, Sheth KN. Turn: a simple predictor of symptomatic intracerebral hemorrhage after iv thrombolysis. Neurocrit Care. 2015;23:166–171. doi: 10.1007/s12028-015-0131-z [DOI] [PubMed] [Google Scholar]
- 29.Wang F, Huang YHQ, Xia Y, Zhang W, Fang K, Zhou XY, Yu XF, Cheng X, Li G, Wang XP, et al. Personalized risk prediction of symptomatic intracerebral hemorrhage after stroke thrombolysis using a machine-learning model. Ther Adv Neurol Diso. 2020;13:1756286420902358. doi: 10.1177/1756286420902358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JS, Kim CK, Kang J, Park JM, Park TH, Lee KB, Lee SJ, Cho YJ, Ko J, Seo J, et al. A novel computerized clinical decision support system for treating thrombolysis in patients with acute ischemic stroke. J Stroke. 2015;17:199–209. doi: 10.5853/jos.2015.17.2.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng Q, Hou J, Wang S, Zhou F, E Y, Wang W, Huang T, Wang M, Huang S, Zhou J, et al. Hypersensitive c-reactive protein-albumin ratio predicts symptomatic intracranial hemorrhage after endovascular therapy in acute ischemic stroke patients. BMC Neurol. 2021;21:47. doi: 10.1186/s12883-021-02066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cucchiara B, Tanne D, Levine SR, Demchuk AM, Kasner S. A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2008;17:331–333. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 33.El Nawar R, Yeung J, Labreuche J, Chadenat ML, Duong DL, De Malherbe M, Cordoliani YS, Lapergue B, Pico F. MRI-based predictors of hemorrhagic transformation in patients with stroke treated by intravenous thrombolysis. Front Neurol. 2019;10:897. doi: 10.3389/fneur.2019.00897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lou M, Safdar A, Mehdiratta M, Kumar S, Schlaug G, Caplan L, Searls D, Selim M. The hat score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology. 2008;71:1417–1423. doi: 10.1212/01.wnl.0000330297.58334.dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalinin MN, Khasanova DR, Ibatullin MM. The Hemorrhagic Transformation Index score: a prediction tool in middle cerebral artery ischemic stroke. BMC Neurol. 2017;17:177. doi: 10.1186/s12883-017-0958-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrera C, Cullell N, Torres-Aguila N, Muino E, Bustamante A, Davalos A, Lopez-Cancio E, Ribo M, Molina CA, Giralt-Steinhauer E, et al. ; Spanish Stroke Genetic Consortium. Validation of a clinical-genetics score to predict hemorrhagic transformations after rtPA. Neurology. 2019;93:e851–e863. doi: 10.1212/WNL.0000000000007997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kidwell CS, Saver JL, Carneado J, Sayre J, Starkman S, Duckwiler G, Gobin YP, Jahan R, Vespa P, Villablanca JP, et al. Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke. 2002;33:717–724. doi: 10.1161/hs0302.104110 [DOI] [PubMed] [Google Scholar]
- 38.Krishnan P, Saposnik G, Ovbiagele B, Zhang L, Symons S, Aviv R. Contribution and additional impact of imaging to the SPAN-100 score. AJNR Am J Neuroradiol. 2015;36:646–652. doi: 10.3174/ajnr.A4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puig J, Blasco G, Daunis IEP, van Eendendburg C, Carrillo-Garcia M, Aboud C, Hernandez-Perez M, Serena J, Biarnes C, Nael K, et al. High-permeability region size on perfusion ct predicts hemorrhagic transformation after intravenous thrombolysis in stroke. PLoS One. 2017;12:e0188238. doi: 10.1371/journal.pone.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC. Stroke prognostication using age and NIH stroke scale: SPAN-100. Neurology. 2013;80:21–28. doi: 10.1212/WNL.0b013e31827b1ace [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y, Chen H, Liu X, Cai X, Kong Y, Wang H, Zhou Y, Zhu J, Zhang L, Fang Q, et al. A new nomogram for individualized prediction of the probability of hemorrhagic transformation after intravenous thrombolysis for ischemic stroke patients. BMC Neurol. 2020;20:426. doi: 10.1186/s12883-020-02002-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeo LLL, Chien SC, Lin JR, Liow CW, Lee JD, Peng TI, Luen TH, Sharma V, Chan B, Lee TH, et al. ; SRICHS Group. Derivation and validation of a scoring system for intravenous tissue plasminogen activator use in Asian patients. J Stroke Cerebrovasc Dis. 2017;26:1695–1703. doi: 10.1016/j.jstrokecerebrovasdis.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, Yin X, Niu Q, Liang S, Mu C, Zhang Y. Risk factors and a nomogram for predicting intracranial hemorrhage in stroke patients undergoing thrombolysis. Neuropsychiatr Dis Treat. 2020;16:1189–1197. doi: 10.2147/NDT.S250648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Constant Dit Beaufils P, Preterre C, De Gaalon S, Labreuche J, Mazighi M, Di Maria F, Sibon I, Marnat G, Gariel F, Blanc R, et al. ; Endovascular Treatment in Ischemic Stroke (ETIS) Research Investigators. Prognosis and risk factors associated with asymptomatic intracranial hemorrhage after endovascular treatment of large vessel occlusion stroke: A prospective multicenter cohort study. Eur J Neurol. 2021;28:229–237. doi: 10.1111/ene.14539 [DOI] [PubMed] [Google Scholar]
- 45.Feng X, Ye G, Cao R, Qi P, Lu J, Chen J, Wang D. Identification of predictors for hemorrhagic transformation in patients with acute ischemic stroke after endovascular therapy using the decision tree model. Clin Interv Aging. 2020;15:1611–1624. doi: 10.2147/CIA.S257931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Wang Y, Jin Y, Guo W, Song Q, Wei C, Li J, Zhang S, Liu M. Prediction of hemorrhagic transformation after ischemic stroke: development and validation study of a novel multi-biomarker model. Front Aging Neurosci. 2021;13:667934. doi: 10.3389/fnagi.2021.667934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nael K, Knitter JR, Jahan R, Gornbein J, Ajani Z, Feng L, Meyer BC, Schwamm LH, Yoo AJ, Marshall RS, et al. Multiparametric magnetic resonance imaging for prediction of parenchymal hemorrhage in acute ischemic stroke after reperfusion therapy. Stroke. 2017;48:664–670. doi: 10.1161/STROKEAHA.116.014343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan S, Li W, Hou C, Kang H, Ma Q, Ji X, Qi Z, Liu KJ. Serum occludin level combined with NIHSS score predicts hemorrhage transformation in ischemic stroke patients with reperfusion. Front Cell Neurosci. 2021;15:714171. doi: 10.3389/fncel.2021.714171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ntaios G, Faouzi M, Ferrari J, Lang W, Vemmos K, Michel P. An integer-based score to predict functional outcome in acute ischemic stroke: the astral score. Neurology. 2012;78:1916–1922. doi: 10.1212/WNL.0b013e318259e221 [DOI] [PubMed] [Google Scholar]
- 50.Strbian D, Meretoja A, Ahlhelm FJ, Pitkaniemi J, Lyrer P, Kaste M, Engelter S, Tatlisumak T. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the dragon score. Neurology. 2012;78:427–432. doi: 10.1212/WNL.0b013e318245d2a9 [DOI] [PubMed] [Google Scholar]
- 51.Saposnik G, Demchuk A, Tu JV, Johnston SC; Stroke Outcomes Research Canada (SORCan) Working Group; Stroke Outcomes Research Canada Working Group. The iscore predicts efficacy and risk of bleeding in the National Institute of Neurological Disorders and Stroke Tissue Plasminogen Activator Stroke Trial. J Stroke Cerebrovasc Dis. 2013;22:876–882. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 52.Kent DM, Ruthazer R, Decker C, Jones PG, Saver JL, Bluhmki E, Spertus JA. Development and validation of a simplified stroke-thrombolytic predictive instrument. Neurology. 2015;85:942–949. doi: 10.1212/WNL.0000000000001925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flint AC, Rao VA, Chan SL, Cullen SP, Faigeles BS, Smith WS, Bath PM, Wahlgren N, Ahmed N, Donnan GA, et al. ; SITS International and VISTA-plus investigators. Improved ischemic stroke outcome prediction using model estimation of outcome probability: The thrive-c calculation. Int J Stroke. 2015;10:815–821. doi: 10.1111/ijs.12529 [DOI] [PubMed] [Google Scholar]