Abstract
The COVID-19 pandemic had cross-cutting impacts on planetary health, quotidian life, and society. Mass vaccination with the current gene-based vaccines has helped control the pandemic but unfortunately it has not shown effectiveness in preventing the spread of the virus. In addition, not all individuals respond to these vaccines, while others develop adverse reactions that cannot be neglected. It is also a fact that some individuals are more susceptible to infection while others develop effective immunization post-infection. We note here that the person-to-person and population variations in vaccine efficacy and side effects have been studied in the field of vaccinomics long before the COVID-19 pandemic. Additionally, the field of adversomics examines the mechanisms of individual differences in the side effects of health interventions. In this review, we discuss the potential of a multi-omics approach for comprehensive profiling of the benefit/risk ratios of vaccines. Vaccinomics and adversomics stand to benefit planetary health and contribute to the prevention of future pandemics in the 21st century by offering precision guidance to clinical trials as well as promoting precision use of vaccines in ways that proactively respond to individual and population differences in their efficacy and safety. This vision of pandemic prevention based on personalized instead of mass vaccination also calls for equity in access to precision vaccines and diagnostics that support a vision and practice of vaccinomics and adversomics in planetary health.
Keywords: COVID-19, vaccines, omics, personalized vaccination, vaccinomics, adversomics, infectious diseases
1. Health Policy in the Management of COVID-19 and Future Pandemics
The beta-coronavirus SARS-CoV-2, the causative agent of the COVID-19 pandemic, is culpable, often in concert with other morbid or predisposing conditions, for the deaths of more than six million worldwide from November 2019 to the present, and is also responsible for the long-lasting morbidity in various organs of infected patients (so-called “Long COVID”). In addition, the COVID-19 pandemic had enormous adverse impacts on quotidian life in society. Even more significant is that the pandemic laid bare the long-standing social injustices in planetary health, not to mention the organizational shortcomings of health systems and services worldwide. If we gaze back over more than two years of pandemic experiences, the measures have so far included confinement of infected areas (lock-down), quarantine of infected individuals, individual protections and social distancing, and mass vaccination. On the one hand, these measures are justified by the goal of the common good and planetary health, but on the other hand, these measures contest individual rights and freedoms while also failing to acknowledge individual and population differences in vaccine efficacy and safety. The responsibility implicit in such health policy choices seems obvious, and it is thus appropriate to reflect on their effectiveness and on how the use of current medical and biotechnological knowledge could help promote more effective management of the pandemic.
In this regard, it should be stressed that there is a risk associated with taking decisions based on the assumption that something is true just because it has not been proved false due to the lack of knowledge (what is called “argumentum ad ignorantiam”), an alert recently raised by the repurposing of drugs for COVID-19 treatment [1], for example, but that holds for COVID-19 management more generally.
In the context of the massive health emergency imposed by an infectious disease that reached the scale of a pandemic, rapid decisions had to be made in a chaotic climate of uncertainty and lack of complete knowledge. It is acceptable (if not understandable) for a generalized lock-down to be implemented while waiting for a vaccine to become available, and then to proceed to mass vaccination while making every effort possible to respect the principles of democracy and the human rights and agencies of citizens and patients. Reconciling individual and collective good is often one of the key challenges in the critical governance of pandemics.
Pandemics and health emergencies also call attention to fostering science and research cultures that enable systems thinking and long-term vision beyond immediacy.
It is noteworthy that many other infectious diseases are (re)appearing on the planetary health scene, foreshadowing or adding to the present health emergencies [2,3,4]. It is likely that the same pattern of management experienced for COVID-19 might be repeated for these emerging planetary health concerns. In fact, in the view of the authors of this perspective, government experts and advisors appear to be in favor of the way COVID-19 has been and is being dealt with becoming the standard protocol for managing upcoming epidemics and pandemics, under the supervision and command of the World Health Organization. This also calls for rethinking the lessons learned so far from COVID-19.
Thus, what have we learned in these two years, and how can we build on our knowledge of COVID-19 and vaccines to better manage upcoming pandemics as well?
2. Omics Studies for Understanding the Immune Response to SARS-CoV-2
Considering that COVID-19 is a novel disease, research on the disease and its short and long-term effects is still emerging [5]. Here we briefly report the omics studies that helped to understand the infection and the development of the disease. From a clinical point of view, COVID-19 presents with a severe acute respiratory syndrome (SARS) that resembles, yet in a milder form and with lower fatality rate, that caused by the two other Betacoronavirus, namely SARS-CoV and MERS-CoV [6]. At present, there are a limited number of genomics and epigenomics studies on COVID-19, and it seems that those few that are available have not yet found their way into the fora of the experts on institutional scientific advisory boards who then determine the health management of the pandemic. To infect cells, the virus primarily (but not exclusively) uses angiotensin-converting enzyme 2 (ACE2), which is widely expressed on the membranes of several cell types and tissues [7]. Genetic linkage studies helped to identify ACE2 polymorphisms linked to individual susceptibility to SARS-CoV-2 infection and risk of developing the hyperinflammatory reaction that may result in thromboembolisms, organ failure, and eventually, patient death [8].
The cytotoxic T- and B-cell immune response to SARS-CoV-2 infection follows the recognition of processed viral peptides bound to the MHC class II of antigen-presenting cells. Next-generation sequencing studies have found that certain MHC-II HLA variants and specific HLA haplotypes are associated with individual immune responses to SARS-CoV-2 and with susceptibility to infection and clinical outcome of COVID-19 [9,10,11]. Whole exome sequencing was used to identify rare variants in a set of genes and HLA alleles that could predispose children with COVID-19 to developing multisystem inflammatory syndrome [12].
The identification of HLA MHC-II variants/haplotypes associated with the immune response to SARS-CoV-2 antigens could help to better stratify patients for a personalized therapeutic regimen and vaccination.
3. Anti-COVID-19 Vaccines in the Omics Era: One Size Fits All?
Because of the high lethality of the infection and the pressure on the health care systems that oversaw hospitalization in the intensive care unit, vaccine discovery and vaccination have been seen as the primary and most appropriate solution. More recently, however, antiviral drugs and theranostics, which refers to the fusion of therapeutics and diagnostics, have come to the fore for pandemic versus endemic COVID-19 management [13]. The age-old maxim ‘one size medicine does not fit all’ applies not only to antivirals and drugs but also to vaccines and health interventions broadly. This vision in effect calls for a personalized/precision medicine approach to planetary health emergencies.
We live in an extraordinary era in which high-throughput omics biotechnologies have revolutionized the way of identifying the cause of human diseases and infections as seen through the lens of precision/personalized medicine [14]. Let us leave aside the risks of relying totally and uncritically on diagnostic hyper-technology and the implicit risk of hyper-care. Instead, let us see how we can make use of omics biotechnologies to better understand the pathology of COVID-19 and other infections, and how vaccines work in order to optimize their efficacy and safety. A new generation of anti-COVID-19 vaccines have been designed according to criteria dictated by genomics and immunomics and make use of recombinant biotechnologies [15,16]. A systems vaccinology approach was used to profile the signature of the immune response to the BNT162b2 mRNA vaccine [17]. mRNA vaccines have been shown to be able to prevent/attenuate the serious damage of SARS-CoV-2 infection [18], yet their efficacy is unfortunately short-lived, on the order of months rather than years [19,20,21]. More disappointingly, concerns about the safety of these vaccines have been raised especially when considering the subject’s age, sex, ethnicity, previous infection, and other genetic factors [22,23].
It is therefore appropriate to reflect on how the use of current multi-omics biotechnologies could help promote more effective and proactive management of the pandemic, with a view to prevent future planetary health crises as well. The responsibility implicit in such health policy choices for the benefit of public and planetary health seems obvious. This is all with a view to move from one-size-fits-all vaccination, which, as mentioned, is justified in times of emergency, to personalized/precision vaccination, including for the management of endemic COVID-19. Personalized/precision vaccines ought to be made available on a planetary scale, as should all essential medicines and health interventions with critical importance for planetary health.
4. Toward Vaccinomics and Adversomics
A vision of precision/personalized vaccines is not in conflict with population vaccination, as such a vision is poised to enhance the efficacy and safety of vaccines. All in all, precision/personalized medicine is a theory for rational therapeutics and prevention that also applies well to vaccines: “the right vaccine, for the right patient, at the right dose, and the right time” [24]. Emerging technologies such as antibody repertoire sequencing, HLA polymorphism genotyping, and high-throughput epitope characterization may assist in designing more effective and safer patient-oriented vaccines [25].
Vaccinomics is concerned with mechanisms of person-to-person and population variations in vaccine efficacy and side effects [26].
Specularly, adversomics makes use of omics and systems biology for investigating the mechanisms of individual differences in the side effects of a given vaccine at genetic and molecular levels [27].
Genomics and systems biology have helped understand the genetic factors that play a role in the adverse effects raised by vaccines [27]. For instance, specific SNPs/haplotypes in the MTHFR (methylenetetrahydrofolate reductase, an enzyme involved in homocysteine metabolism) and IRF1 (interferon regulatory factor-1, which regulates the transcription of IFN type 1 and type 2) genes were significantly associated with systemic adverse events after smallpox vaccination [28]. Immunogenomics has been employed for identifying polymorphisms in cytokine and cytokine receptor genes involved in the immune response to the smallpox vaccine [29].
5. State of the Art in Vaccinomics and Adversomics in COVID-19
Much to our disappointment, there has thus far been a delay in the mainstreaming of vaccinomics and adversomics that could make it possible to optimize the efficacy and safety of vaccines for COVID-19 and other infectious pathogens in planetary health. At the same time, there is ongoing hope for a move toward vaccinomics- and adversomics-guided precision/personalized vaccines [25,30,31,32].
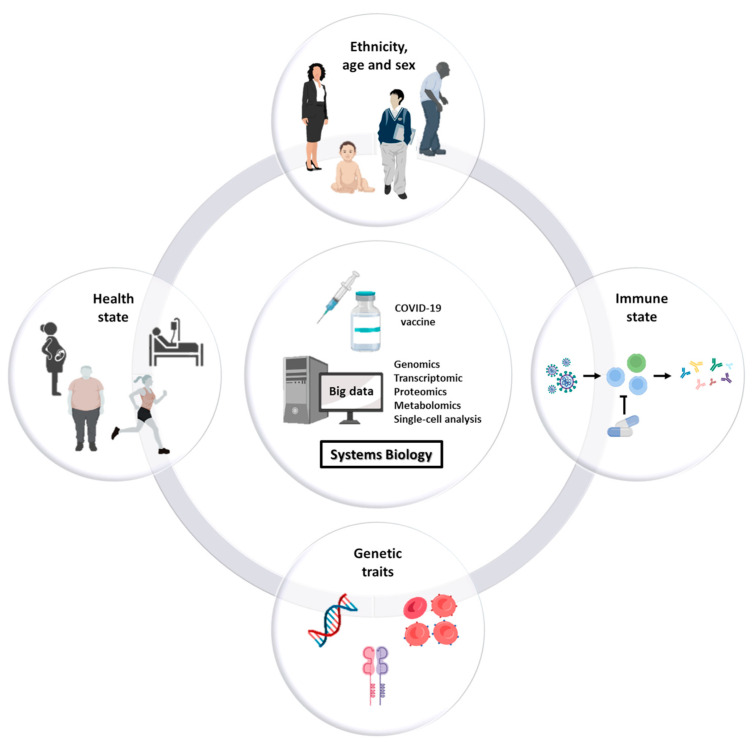
The goal of personalized vaccination is to immunize and protect individuals that are vulnerable to infection, that are responsive to the vaccine and that at the same time are unlikely to develop serious adverse events in the short and long term. High-throughput omics technologies and systems biology for computing big data are instrumental to the identification of the cohort of individuals needing vaccination with a high benefit/risk ratio, taking into account all the factors influencing the efficacy (and safety) of the vaccine, namely sex, age (newborn/infant/adults/elders), immune state (e.g., immunodeficient or immunocompromised vs. immunocompetent; naturally immunized after infection), health state (presence of co-morbidities), immunosuppressive therapies, genetic traits, and others (Figure 1).
Figure 1.
Factors influencing the efficacy and safety of vaccines. Studies show that responsiveness as well as potential adverse reactions to vaccines are dependent on several factors including sex, age, ethnicity, comorbidities, immune state, therapies, and the genetic/biological characteristics of the individual. Systems biology is instrumental to differentiate the cohorts of subjects that will benefit from vaccination from the ones that will not and may develop serious adverse effects after vaccination.
For instance, the genetic/biological reason for the lower antibody response after two doses of mRNA COVID-19 vaccine in pregnant compared with non-pregnant women [33] should be investigated at omics level, as should the reason for the rapid and substantial decline in the effectiveness of the vaccine in the general population [10,11,12] and particularly in elders and subjects with a comorbidity [34].
Genome-wide association and epigenome-wide association studies have begun to offer new insights into patients with COVID-19 who are particularly prone to developing severe interstitial pneumonia [35,36,37], while exome and genome sequencing have helped identify a rare variant of TLR7 that may lead to severe COVID-19 outcomes [38]. Genome-wide association studies (GWASs) in COVID-19 patients revealed that risk of intubation and respiratory failure was linked to two critical loci of interest, namely 9q34.2 and 3p21.31; the former locus was associated with the AB0 blood group (and it was found that group A is more susceptible while group 0 is less susceptible) and in the latter locus at least six genes that could play a role in infection and immune response have been defined (SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6, and XCR1) [35]. Specifically, SLC6A20 encodes sodium-proline transporter 1, which can interact with ACE2; CCR9, XCR1 and CXCR6 encode chemokine receptors; and FYCO1 is involved in autophagy (a vesicular process involved in viral assembly and exit). Likewise, exome and genome sequencing revealed that patients with life-threatening COVID-19 pneumonia presented genetic defects at the TLR3 and IRF loci, which are involved in double-stranded RNA sensing and type I IFN immunity [39]. Similarly, GWASs may be useful for identifying the genetic determinants of innate resistance to infection [40]. For instance, prior infections with other coronaviruses may contribute to resistance to SARS-CoV-2 infection due to cross-reactive T-cell-mediated immunity [41], which could be detected by immunocellomics. Immunocellomics and single-cell RNA sequencing were used to determine the causes of an impaired anti-Spike response in tri-vaccinated elderly individuals [42]. The study revealed an enrichment of circulating “atypical” B lymphocytes along with a genetic signature of T-cell exhaustion [42].
Another category of subjects who can benefit from clinical omics profiling is that of immunodepressed (because of concomitant therapies) and immunodeficient patients who will likely not respond to standard vaccination protocols [43,44]. Accordingly, a personalized vaccination strategy has been proposed for rituximab-treated patients in which antibody production is suppressed [45]. Such immunocompromised patients or those on immunosuppressant therapy are given multiple doses to boost and maintain a sufficient level of immunoprotection [44,46]. However, one must then consider the risks of even serious adverse events in the short- and long-term that may become apparent, especially in some susceptible individuals, because of the continuous stresses on the immune system caused by multiple dose vaccinations [47]. In fact, COVID-19 vaccination outcomes demand long-term prospective studies, another area where vaccinomics and adversomics scholarship stands to benefit.
Vaccinomics and adversomics go hand in hand with multi-omics technologies across the biological hierarchy of genomics, proteomics, and metabolomics, not to mention epigenomics. Thus, going forward and contingent on the establishment of a robust evidentiary base in the future, it is likely that one can make use of omics biotechnologies to profile genomic and epigenomic biomarkers associated with the risk of re-infection or of developing adverse events following COVID-19 vaccination. As an example, immunogenomics offers prospects for the identification of MHC-II-related predisposition to re-infection in COVID-19 vaccinated individuals [48]. Similarly, immunocytomics and immunogenomics may identify genes related to T- and NK cell exhaustion and suppression that are associated with adverse reactions to COVID-19 mRNA vaccines [49]. In this context, single-cell mRNA sequencing (scRNA-seq) of peripheral blood lymphocytes and monocytes before and 28 days after vaccination helped to stratify patients with pre-existing clinical disorders that may contribute to adverse reaction to the vaccine [47]. Vaccine-induced thrombosis associated with thrombocytopenia (VITT) has been reported after COVID-19 vaccination, particularly with an adenoviral vector [50], while deep vein thrombosis (DVT) has been reported after lipid nanoparticle-mRNA-based vaccines [51]. The genetic basis of VITT, which though rare is more frequently observed in young females, shares many similarities with SARS-CoV-2-induced coagulopathies, yet it presents unique interactomes associated with the AURKA, CD46 and CD19 genes [52]. Genomic and serological signatures could help identify subjects who are more likely to develop an allergic reaction to specific vaccine components [53,54] or who have a genetic risk of developing venous thromboembolism [55], which may inform and guide preventive interventions.
Many of the reported post-vaccination adverse events are immune-mediated, and it has been hypothesized that certain anti-idiotypes specific to the anti-S primary antibody mimic the original antigen in their potential to produce similar pathological effects in the long-term [56,57]. Omics technologies could be useful for determining the presence and characteristics of the idiotypes of the antibodies induced by the vaccine, and thus help to identify patients potentially at risk of developing adverse effects long after vaccination.
Even more relevant and timely is the possibility offered by the multi-omics technologies in combination with other serological and clinical parameters to profile those individuals for which vaccination would be more beneficial, while excluding those who would not benefit because they are already protected and not particularly susceptible to developing the disease in a severe form and who would be easily treated with available therapies [58]. For instance, immunocytomics could help determine the pool of T and B memory cells that specifically react to common epitopes of previous infection or vaccination and potentially provide protection against a family of viruses sharing those epitopes. These infection-recovered subjects can be further evaluated in light of vaccinomics guidance to determine the most optimal health interventions to prevent or minimize adverse effects [22]. It has been shown that infection-recovered subjects show similar or better protection from COVID-19 than vaccinees in terms of risk of re-infection [58,59,60,61,62], and therefore could be exempted from vaccination. Diverse serious side effects of vaccination in previously naturally immunized subjects have been reported. For instance, myopericarditis was observed following the first dose of the mRNA-COVID-19 vaccine in a patient recovered from mild COVID-19 three months before vaccination [63].
Although antibody-dependent enhancement (ADE) of viral infection seems to be unlikely at the population level [64], it is advisable to exempt individuals possessing anti-COVID-19 antibodies from vaccination according to the precaution principle [65,66,67].
It is now possible to profile immunized (post-infection and post-vaccination) subjects by rapidly and efficiently testing the level and specificity of anti-SARS-CoV-2 antibodies with microfluidic technology [68] and multiplex assays [69].
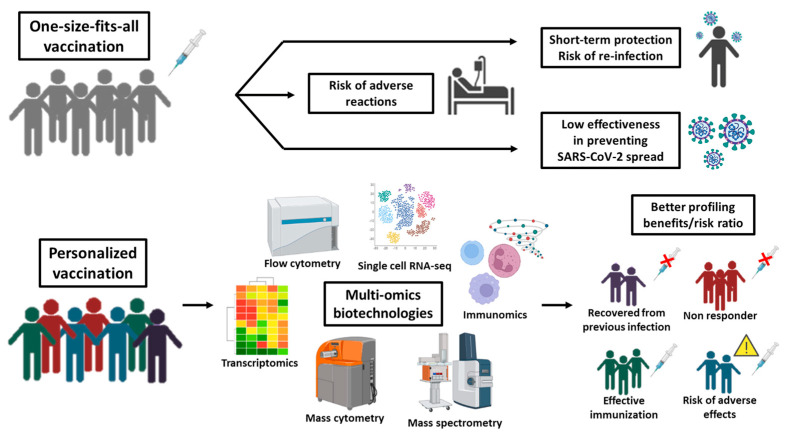
Overall, emerging studies suggest how we could harness multi-omics knowledge to narrow the pool of patients who are most likely to benefit from vaccines while also alerting to the likelihood of adverse events (Figure 2). Going forward, precision/personalized vaccines developed by vaccinomics and adversomics would allow for a more accurate assessment of the benefit/risk ratio of vaccination. This is a formidable opportunity for improving the safety profile of vaccination programs in vulnerable populations and especially in children and adolescents [70].
Figure 2.
Mass versus personalized vaccination in the omics era. When the vaccine is administered to a large population without considering the individual pathophysiological condition(s), it is highly probable to observe inefficacy and/or immediate/late adverse effects in part of the vaccinated. Omics-biotechnologies allow a better subject profiling by investigating all the variables influencing the efficacy and safety of vaccines in view of personalized vaccination that targets the individuals who can benefit of it with negligible side effects while excluding those who may not respond (because immunocompromised) or are already immunized (by prior infection) or are predisposed to severe reactions (e.g., allergy).
Thus, it is desirable that the necessary investments in omics biotechnologies be made in the immediate future to exploit their full potential to develop personalized antivirals and vaccines for COVID-19 that would also help future pandemic-preparedness [71,72,73,74].
6. Conclusions and Perspectives
Although anti-COVID-19 mass vaccination has helped to control the pandemic in terms of reduced severity of the disease, these vaccines were found to be ineffective in preventing the spread of the virus [75]. Mass vaccination may have different unpredictable outcomes that include the risk of serious immediate or late adverse events. This calls for proactive surveillance and registration of post-vaccination adverse effects [76]. This is particularly true for children, who are subjected to several vaccines in a relatively short period. Particularly, it is questionable whether mass vaccination of children with mRNA-based COVID-19 vaccines is an appropriate approach [77,78].
A personalized approach based on the use of omics technologies allows for categorization of the subjects that are more likely to benefit from vaccination, excluding the ones who may not need it (because they are protected) or who may not respond (because of immunologic impairment), and at the same time helps identify the subjects that could develop adverse effects in the short or long term after vaccination. For instance, scRNA-seq of peripheral mononuclear cells in vaccinated individuals revealed pathophysiological changes in coagulation profiles, renal function, and glucose metabolism along with increased NF-κB signaling and reduced type I interferon responses, suggesting caution in vaccinating frail patients with renal dysfunction, diabetes, and coagulation disorders [47]. Compared to males, females have shown a more vigorous immune response to vaccines and may require a lower dose of the vaccine to minimize adverse effects [24]. ADE reactions could develop after vaccination of immune subjects, which calls for determination of the cut-off for circulating microbe-specific antibodies [79]. Although rare, COVID-19 mRNA vaccines have been associated with several other adverse effects, including myocarditis and pericarditis, Guillain–Barré syndrome, Bell’s palsy, and stroke, among others. These clinical manifestations are likely linked to the ectopic expression of the modified (open state) Spike protein and/or of the lipid nanoparticle carrier [80]. Thus, alternative vaccine formulations might be safer [81]. Exuberant immune and inflammatory responses resulting in autoimmune and systemic reactions may be linked to specific HLA molecules to which the vaccine epitope binds or to specific polymorphisms in genes regulating the immune-inflammatory response [12].
To learn more in view of a personalized vaccinology, it is desirable that DNA, RNA, non-coding RNA, and protein samples from the blood and tissues of vaccinated (with and without adverse effects) and unvaccinated donors are collected and stored in biobanks and made available for omics and systems biology analyses. These studies will help elucidate the mechanisms of the vaccine adverse effects and could be exploited for the design of new vaccines or alternative strategies that minimize or avoid such events.
Vaccines must be safer than the disease they are supposed to prevent because vaccines are given to a large population of otherwise healthy people. Such a vision of planetary health and pandemic prevention also calls for equity in access to precision vaccines and diagnostics.
Author Contributions
Conceptualization, C.I.; writing—review and editing, A.F. and C.I.; visualization, A.F. and C.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.López Reboiro M.L., Sardiña González C., López Castro J. COVID-19 y Argumentum ad ignorantiam o «no todo vale» [COVID-19 and Argumentum ad ignorantiam or «not everything goes»] Rev. Clínica Española. 2020;220:457. doi: 10.1016/j.rce.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesan P. Re-emergence of infectious diseases associated with the past. Lancet Microbe. 2021;2:e140. doi: 10.1016/S2666-5247(21)00066-5. [DOI] [PubMed] [Google Scholar]
- 3.Branswell H. As COVID Precautions Disappear, Other Viruses Are Cropping Up in Unexpected Ways. [(accessed on 27 November 2022)]. Available online: https://www.pbs.org/newshour/health/as-covid-precautions-disappear-other-viruses-are-cropping-up-in-unexpected-ways.
- 4.Smitham E., Glassman A. The Next Pandemic Could Come Soon and Be Deadlier. [(accessed on 27 November 2022)]. Available online: https://www.cgdev.org/blog/the-next-pandemic-could-come-soon-and-be-deadlier.
- 5.Friedman M.J., Lee H., Kwon Y.-C., Oh A.S. Dynamics of Viral and Host 3D Genome Structure upon Infection. J. Microbiol. Biotechnol. 2022;32:1515–1526. doi: 10.4014/jmb.2208.08020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muus C., Luecken M.D., Eraslan G., Sikkema L., Waghray A., Heimberg G., Kobayashi Y., Vaishnav E.D., Subramanian A., Smillie C., et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat. Med. 2021;27:546–559. doi: 10.1038/s41591-020-01227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devaux C.A., Camoin-Jau L. An update on angiotensin-converting enzyme 2 structure/functions, polymorphism, and duplicitous nature in the pathophysiology of coronavirus disease 2019: Implications for vascular and coagulation disease associated with severe acute respiratory syndrome coronavirus infection. Front. Microbiol. 2022;13:1042200. doi: 10.3389/fmicb.2022.1042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Mayordomo V., Montero-Escribano P., Matías-Guiu J.A., González-García N., Porta-Etessam J., Matías-Guiu J. Clinical exacerbation of SARS-CoV2 infection after fingolimod withdrawal. J. Med. Virol. 2021;93:546–549. doi: 10.1002/jmv.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pisanti S., Deelen J., Gallina A.M., Caputo M., Citro M., Abate M., Sacchi N., Vecchione C., Martinelli R. Correlation of the two most frequent HLA haplotypes in the Italian population to the differential regional incidence of COVID-19. J. Transl. Med. 2020;18:352. doi: 10.1186/s12967-020-02515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer J.C., Balz V., Jazmati D., Bölke E., Freise N.F., Keitel V., Feldt T., Jensen B.-E.O., Bode J., Lüdde T., et al. Prognostic markers for the clinical course in the blood of patients with SARS-CoV-2 infection. Eur. J. Med Res. 2022;27:255. doi: 10.1186/s40001-022-00864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos-Rebouças C.B., Piergiorge R.M., Ferreira C.D.S., Zeitel R.D.S., Gerber A.L., Rodrigues M.C.F., Guimarães A.P.D.C., Silva R.M., Fonseca A.R., Souza R.C., et al. Host genetic susceptibility underlying SARS-CoV-2-associated Multisystem Inflammatory Syndrome in Brazilian Children. Mol. Med. 2022;28:153. doi: 10.1186/s10020-022-00583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Taie A., Denkdemir F.R., Sharief Z., Buyuk A.S., Şardaş S. The Long View on COVID-19 Theranostics and Oral Antivirals: Living with Endemic Disease and Lessons from Molnupiravir. OMICS A J. Integr. Biol. 2022;26:324–328. doi: 10.1089/omi.2022.0045. [DOI] [PubMed] [Google Scholar]
- 14.Visvikis-Siest S., Theodoridou D., Kontoe M.-S., Kumar S., Marschler M. Milestones in Personalized Medicine: From the Ancient Time to Nowadays-the Provocation of COVID-19. Front. Genet. 2020;11:569175. doi: 10.3389/fgene.2020.569175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotugno N., Ruggiero A., Santilli V., Manno E.C., Rocca S., Zicari S., Amodio D., Colucci M., Rossi P., Levy O., et al. OMIC Technologies and Vaccine Development: From the Identification of Vulnerable Individuals to the Formulation of Invulnerable Vaccines. J. Immunol. Res. 2019;2019:8732191. doi: 10.1155/2019/8732191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J.W., Lagniton P.N., Liu Y., Xu R.-H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021;17:1446–1460. doi: 10.7150/ijbs.59233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arunachalam P.S., Scott M.K.D., Hagan T., Li C., Feng Y., Wimmers F., Grigoryan L., Trisal M., Edara V.V., Lai L., et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature. 2021;596:410–416. doi: 10.1038/s41586-021-03791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federico M. How Do Anti-SARS-CoV-2 mRNA Vaccines Protect from Severe Disease? Int. J. Mol. Sci. 2022;23:10374. doi: 10.3390/ijms231810374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrotri M., Krutikov M., Nacer-Laidi H., Azmi B., Palmer T., Giddings R., Fuller C., Irwin-Singer A., Baynton V., Tut G., et al. Duration of vaccine effectiveness against SARS-CoV-2 infection, hospitalisation, and death in residents and staff of long-term care facilities in England (VIVALDI): A prospective cohort study. Lancet Healthy Longev. 2022;3:e470–e480. doi: 10.1016/S2666-7568(22)00147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Florentino P.T.V., Millington T., Cerqueira-Silva T., Robertson C., Oliveira V.D.A., Júnior J.B.S., Alves F.J.O., Penna G.O., Katikireddi S.V., Boaventura V.S., et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: A test-negative case-control study. Lancet Infect. Dis. 2022;22:1577–1586. doi: 10.1016/S1473-3099(22)00451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z., Mateus J., Coelho C.H., Dan J.M., Moderbacher C.R., Gálvez R.I., Cortes F.H., Grifoni A., Tarke A., Chang J., et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell. 2022;185:2434–2451.e17. doi: 10.1016/j.cell.2022.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.RRomero-Ibarguengoitia M.E., González-Cantú A., Pozzi C., Levi R., Mollura M., Sarti R., Sanz-Sánchez M., Rivera-Salinas D., Hernández-Ruíz Y.G., Armendariz-Vázquez A.G., et al. Analysis of immunization time, amplitude, and adverse events of seven different vaccines against SARS-CoV-2 across four different countries. Front. Immunol. 2022;13:894277. doi: 10.3389/fimmu.2022.894277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urakawa R., Isomura E.T., Matsunaga K., Kubota K., Ike M. Impact of age, sex and medical history on adverse reactions to the first and second dose of BNT162b2 mRNA COVID-19 vaccine in Japan: A cross-sectional study. BMC Infect. Dis. 2022;22:179. doi: 10.1186/s12879-022-07175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein S.L., Poland G.A. Personalized vaccinology: One size and dose might not fit both sexes. Vaccine. 2013;31:2599–2600. doi: 10.1016/j.vaccine.2013.02.070. [DOI] [PubMed] [Google Scholar]
- 25.Brooks B.D., Beland A., Aguero G., Taylor N., Towne F.D. Moving beyond Titers. Vaccines. 2022;10:683. doi: 10.3390/vaccines10050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poland G.A., Ovsyannikova I.G., Kennedy R.B., Haralambieva I.H., Jacobson R.M. Vaccinomics and a new paradigm for the development of preventive vaccines against viral infections. OMICS A J. Integr. Biol. 2011;15:625–636. doi: 10.1089/omi.2011.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitaker J.A., Ovsyannikova I.G., Poland G.A. Adversomics: A new paradigm for vaccine safety and design. Expert Rev. Vaccines. 2015;14:935–947. doi: 10.1586/14760584.2015.1038249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reif D.M., McKinney B.A., Motsinger A.A., Chanock S.J., Edwards K.M., Rock M.T., Moore J.H., Crowe J.J.E. Genetic basis for adverse events after smallpox vaccination. J. Infect. Dis. 2008;198:16–22. doi: 10.1086/588670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ovsyannikova I.G., Haralambieva I.H., Kennedy R.B., Pankratz V.S., Vierkant R.A., Jacobson R.M., Poland G.A. Impact of cytokine and cytokine receptor gene polymorphisms on cellular immunity after smallpox vaccination. Gene. 2012;510:59–65. doi: 10.1016/j.gene.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soni D., Van Haren S.D., Idoko O.T., Evans J.T., Diray-Arce J., Dowling D.J., Levy O. Towards Precision Vaccines: Lessons From the Second International Precision Vaccines Conference. Front. Immunol. 2020;11:590373. doi: 10.3389/fimmu.2020.590373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omersel J., Kuželički N.K. Vaccinomics and Adversomics in the Era of Precision Medicine: A Review Based on HBV, MMR, HPV, and COVID-19 Vaccines. J. Clin. Med. 2020;9:3561. doi: 10.3390/jcm9113561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de la Fuente J., Contreras M. Vaccinomics: A future avenue for vaccine development against emerging pathogens. Expert Rev. Vaccines. 2021;20:1561–1569. doi: 10.1080/14760584.2021.1987222. [DOI] [PubMed] [Google Scholar]
- 33.Blakeway H., Amin-Chowdhury Z., Prasad S., Kalafat E., Ismail M., Abdallah F.N., Rezvani A., Amirthalingam G., Brown K., Le Doare K., et al. Evaluation of immunogenicity and reactogenicity of COVID-19 vaccines in pregnant women. Ultrasound Obstet. Gynecol. 2022;60:673–680. doi: 10.1002/uog.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petráš M., Máčalík R., Janovská D., Čelko A.M., Dáňová J., Selinger E., Doleček J., Neradová S., Franklová M., Dlouhý P., et al. Risk factors affecting COVID-19 vaccine effectiveness identified from 290 cross-country observational studies until February 2022: A meta-analysis and meta-regression. BMC Med. 2022;20:461. doi: 10.1186/s12916-022-02663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., Fernández J., Prati D., Baselli G., Asselta R., et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N. Engl. J. Med. 2020;383:1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Y., Ye F., Li K., Xu P., Tan W., Feng Q., Rao S. Genome and epigenome editing identify CCR9 and SLC6A20 as target genes at the 3p21.31 locus associated with severe COVID-19. Signal Transduct. Target. Ther. 2021;6:85. doi: 10.1038/s41392-021-00519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Moura M.C., Davalos V., Planas-Serra L., Alvarez-Errico D., Arribas C., Ruiz M., Aguilera-Albesa S., Troya J., Valencia-Ramos J., Vélez-Santamaria V., et al. Epigenome-wide association study of COVID-19 severity with respiratory failure. Ebiomedicine. 2021;66:103339. doi: 10.1016/j.ebiom.2021.103339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler-Laporte G., Povysil G., Kosmicki J.A., Cirulli E.T., Drivas T., Furini S., Saad C., Schmidt A., Olszewski P., Korotko U., et al. Exome-wide association study to identify rare variants influencing COVID-19 outcomes: Results from the Host Genetics Initiative. PLoS Genet. 2022;18:e1010367. doi: 10.1371/journal.pgen.1010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreakos E., Abel L., Vinh D.C., Kaja E., Drolet B.A., Zhang Q., O’Farrelly C., Novelli G., Rodríguez-Gallego C., Haerynck F., et al. A global effort to dissect the human genetic basis of resistance to SARS-CoV-2 infection. Nat. Immunol. 2022;23:159–164. doi: 10.1038/s41590-021-01030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mateus J., Grifoni A., Tarke A., Sidney J., Ramirez S.I., Dan J.M., Burger Z.C., Rawlings S.A., Smith D.M., Phillips E., et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira I.A.T.M., Lee C.Y.C., Foster W., Abdullahi A., Tuong Z.K., Stewart B.J., Ferdinand J.R., Guillaume S., Potts M.O.P., Perera M., et al. Atypical B Cells and Impaired SARS-CoV-2 Neutralisation Following Booster Vaccination in the Elderly. [(accessed on 27 November 2022)]. Available online: https://www.medrxiv.org/content/10.1101/2022.10.13.22281024v1.
- 43.Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amodio D., Ruggiero A., Sgrulletti M., Pighi C., Cotugno N., Medri C., Morrocchi E., Colagrossi L., Russo C., Zaffina S., et al. Humoral and Cellular Response Following Vaccination With the BNT162b2 mRNA COVID-19 Vaccine in Patients Affected by Primary Immunodeficiencies. Front. Immunol. 2021;12:727850. doi: 10.3389/fimmu.2021.727850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Q., Jiang Q., Niu M.M., Fan G.Z., Hu P. COVID-19 vaccination in patients with immune-mediated inflammatory diseases receiving rituximab: A personalized regimen should be formulated. J. Am. Acad. Dermatol. 2022;87:e45–e46. doi: 10.1016/j.jaad.2022.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cotugno N., Franzese E., Angelino G., Amodio D., Romeo E.F., Rea F., Faraci S., Tambucci R., Profeti E., Manno E.C., et al. Evaluation of Safety and Immunogenicity of BNT162B2 mRNA COVID-19 Vaccine in IBD Pediatric Population with Distinct Immune Suppressive Regimens. Vaccines. 2022;10:1109. doi: 10.3390/vaccines10071109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Wang J., Xu J., Xia H., Wang Y., Zhang C., Chen W., Zhang H., Liu Q., Zhu R., et al. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell Discov. 2021;7:99. doi: 10.1038/s41421-021-00329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mentzer A.J., O’Connor D., Bibi S., Chelysheva I., Clutterbuck E.A., Demissie T., Dinesh T., Edwards N.J., Felle S., Feng S., et al. Human leukocyte antigen alleles associate with COVID-19 vaccine immunogenicity and risk of breakthrough infection. Nat. Med. 2022 doi: 10.1038/s41591-022-02078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syenina A., Gan E.S., Toh J.Z.N., de Alwis R., Lin L.Z., Tham C.Y.L., Yee J.X., Leong Y.S., Sam H., Cheong C., et al. Adverse effects following anti-COVID-19 vaccination with mRNA-based BNT162b2 are alleviated by altering the route of administration and correlate with baseline enrichment of T and NK cell genes. PLoS Biol. 2022;20:e3001643. doi: 10.1371/journal.pbio.3001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kircheis R. Coagulopathies after Vaccination against SARS-CoV-2 May Be Derived from a Combined Effect of SARS-CoV-2 Spike Protein and Adenovirus Vector-Triggered Signaling Pathways. Int. J. Mol. Sci. 2021;22:10791. doi: 10.3390/ijms221910791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elrashdy F., Tambuwala M.M., Hassan S.S., Adadi P., Seyran M., El-Aziz T.M.A., Rezaei N., Lal A., Aljabali A.A., Kandimalla R., et al. Autoimmunity roots of the thrombotic events after COVID-19 vaccination. Autoimmun. Rev. 2021;20:102941. doi: 10.1016/j.autrev.2021.102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geronikolou S.A., Takan I., Pavlopoulou A., Mantzourani M., Chrousos G.P. Thrombocytopenia in COVID-19 and vaccine-induced thrombotic thrombocytopenia. Int. J. Mol. Med. 2022;49:35. doi: 10.3892/ijmm.2022.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kounis N.G., Koniari I., de Gregorio C., Velissaris D., Petalas K., Brinia A., Assimakopoulos S.F., Gogos C., Kouni S.N., Kounis G.N., et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines. 2021;9:221. doi: 10.3390/vaccines9030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenhawt M., Shaker M., Golden D.B.K., Abrams E.M., Blumenthal K.G., Wolfson A.R., Stone C.A., Krantz M.S., Chu D.K., Dwamena B.A. Diagnostic accuracy of vaccine and vaccine excipient testing in the setting of allergic reactions to COVID-19 vaccines: A systematic review and meta-analysis. Allergy. 2022 doi: 10.1111/all.15571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee S.D., Lee C.H., Seo M.S., Yoo J.-I. Integrative analyses of genes about venous thromboembolism: An umbrella review of systematic reviews and meta-analyses. Medicine. 2022;101:e31162. doi: 10.1097/MD.0000000000031162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy W.J., Longo D.L. A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination. N. Engl. J. Med. 2022;386:394–396. doi: 10.1056/NEJMcibr2113694. [DOI] [PubMed] [Google Scholar]
- 57.Shukla A.K., Misra S. Clinical implications of anti-idiotype antibodies in COVID-19. J. Basic Clin. Physiol. Pharmacol. 2022;33:727–733. doi: 10.1515/jbcpp-2022-0123. [DOI] [PubMed] [Google Scholar]
- 58.Diani S., Leonardi E., Cavezzi A., Ferrari S., Iacono O., Limoli A., Bouslenko Z., Natalini D., Conti S., Mantovani M., et al. SARS-CoV-2-The Role of Natural Immunity: A Narrative Review. J. Clin. Med. 2022;11:6272. doi: 10.3390/jcm11216272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahman S., Rahman M.M., Miah M., Begum M.N., Sarmin M., Mahfuz M., Hossain M.E., Chisti M.J., Ahmed T., El Arifeen S. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci. Rep. 2022;12:1438. doi: 10.1038/s41598-022-05325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kojima N., Klausner J.D. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect. Dis. 2022;22:12–14. doi: 10.1016/S1473-3099(21)00676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamińska D., Dęborska-Materkowska D., Kościelska-Kasprzak K., Mazanowska O., Remiorz A., Poznański P., Durlik M., Krajewska M. Immunity after COVID-19 Recovery and Vaccination: Similarities and Differences. Vaccines. 2022;10:1068. doi: 10.3390/vaccines10071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Primorac D., Brlek P., Matišić V., Molnar V., Vrdoljak K., Zadro R., Parčina M. Cellular Immunity-The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines. 2022;10:442. doi: 10.3390/vaccines10030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Won T., Gilotra N.A., Wood M.K., Hughes D.M., Talor M.V., Lovell J., Milstone A.M., Steenbergen C., Čiháková D. Increased Interleukin 18-Dependent Immune Responses Are Associated With Myopericarditis After COVID-19 mRNA Vaccination. Front. Immunol. 2022;13:851620. doi: 10.3389/fimmu.2022.851620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gan L., Chen Y., Tan J., Wang X., Zhang D. Does potential antibody-dependent enhancement occur during SARS-CoV-2 infection after natural infection or vaccination? A meta-analysis. BMC Infect. Dis. 2022;22:742. doi: 10.1186/s12879-022-07735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yahi N., Chahinian H., Fantini J. Infection-enhancing anti-SARS-CoV-2 antibodies recognize both the original Wuhan/D614G strain and Delta variants. A potential risk for mass vaccination? J. Infect. 2021;83:607–635. doi: 10.1016/j.jinf.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang X., Zhang X., Zhao X., Yuan M., Zhang K., Dai J., Guan X., Qiu H.-J., Li Y. Antibody-Dependent Enhancement: “Evil” Antibodies Favorable for Viral Infections. Viruses. 2022;14:1739. doi: 10.3390/v14081739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimizu J., Sasaki T., Koketsu R., Morita R., Yoshimura Y., Murakami A., Saito Y., Kusunoki T., Samune Y., Nakayama E.E., et al. Reevaluation of antibody-dependent enhancement of infection in anti-SARS-CoV-2 therapeutic antibodies and mRNA-vaccine antisera using FcR- and ACE2-positive cells. Sci. Rep. 2022;12:15612. doi: 10.1038/s41598-022-19993-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajsri K.S., McRae M.P., Simmons G.W., Christodoulides N.J., Matz H., Dooley H., Koide A., Koide S., McDevitt J.T. A Rapid and Sensitive Microfluidics-Based Tool for Seroprevalence Immunity Assessment of COVID-19 and Vaccination-Induced Humoral Antibody Response at the Point of Care. Biosensors. 2022;12:621. doi: 10.3390/bios12080621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meyers J., Windau A., Schmotzer C., Saade E., Noguez J., Stempak L., Zhang X. SARS-CoV-2 antibody profile of naturally infected and vaccinated individuals detected using qualitative, semi-quantitative and multiplex immunoassays. Diagn. Microbiol. Infect. Dis. 2022;104:115803. doi: 10.1016/j.diagmicrobio.2022.115803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Connor D. The omics strategy: The use of systems vaccinology to characterize immune responses to childhood immunization. Expert Rev. Vaccines. 2022;21:1205–1214. doi: 10.1080/14760584.2022.2093193. [DOI] [PubMed] [Google Scholar]
- 71.DeMerle K., Angus D.C., Seymour C.W. Precision Medicine for COVID-19: Phenotype Anarchy or Promise Realized? JAMA. 2021;325:2041. doi: 10.1001/jama.2021.5248. [DOI] [PubMed] [Google Scholar]
- 72.Mostafavi E., Dubey A.K., Teodori L., Ramakrishna S., Kaushik A. SARS-CoV-2 Omicron variant: A next phase of the COVID-19 pandemic and a call to arms for system sciences and precision medicine. MedComm. 2022;3:e119. doi: 10.1002/mco2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teodori L., Osimani B., Isidoro C., Ramakrishna S. Mass versus personalized medicine against COVID-19 in the “system sciences” era. Cytom. Part A. 2022;101:995–999. doi: 10.1002/cyto.a.24662. [DOI] [PubMed] [Google Scholar]
- 74.Campos D.M.D.O., da Silva M.K., Barbosa E.D., Leow C.Y., Fulco U.L., Oliveira J.I.N. Exploiting reverse vaccinology approach for the design of a multiepitope subunit vaccine against the major SARS-CoV-2 variants. Biol. Chem. 2022;101:107754. doi: 10.1016/j.compbiolchem.2022.107754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu L., Wisplinghoff H., Kossow A., Hurraß J., Wiesmüller G.A., Grüne B., Hoffmann D., Lüsebrink J., Demuth S., Schildgen O., et al. Limited protection against SARS-CoV-2 infection and virus transmission after mRNA vaccination. J. Infect. 2022;84:94–118. doi: 10.1016/j.jinf.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piché-Renaud P., Morris S.K., Top K.A. A Narrative Review of Vaccine Pharmacovigilance During Mass Vaccination Campaigns: Focus on Myocarditis and Pericarditis after COVID-19 mRNA Vaccination. Br. J. Clin. Pharmacol. 2022 doi: 10.1111/bcp.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paul S., Mishra C.M. Do we need to vaccinate every child against COVID-19: What evidence suggests-A systematic review of opinions. Front. Public Health. 2022;10:1002992. doi: 10.3389/fpubh.2022.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Puspitarani F., Sitaresmi M.N., Ahmad R.A. Adverse events following immunization of COVID-19 vaccine among children aged 6–11 years. Front. Public Health. 2022;10:999354. doi: 10.3389/fpubh.2022.999354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uysal B.B., Yavuzer S., Islamoglu M.S., Cengiz M. Measurement of antibody levels in patients with COVID-19 over time by immunofluorescence assay: A longitudinal observational study. J. Int. Med Res. 2022;50:3000605211069279. doi: 10.1177/03000605211069279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trougakos I.P., Terpos E., Alexopoulos H., Politou M., Paraskevis D., Scorilas A., Kastritis E., Andreakos E., Dimopoulos M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022;28:542–554. doi: 10.1016/j.molmed.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carnell G.W., Ciazynska K.A., Wells D.A., Xiong X., Aguinam E.T., McLaughlin S.H., Mallery D., Ebrahimi S., Ceron-Gutierrez L., Asbach B., et al. SARS-CoV-2 Spike Protein Stabilized in the Closed State Induces Potent Neutralizing Responses. J. Virol. 2021;95:e0020321. doi: 10.1128/JVI.00203-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.