Abstract
Background: The COVID-19 pandemic has challenged the treatment of Clostridioides Difficile (CD)-infected patients given the increasing number of co-infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In this context, fecal microbiota transplantation (FMT) shows promise in modulating the immune system’s function and alleviating the burdens associated with this condition. Methods: To achieve this goal, we performed a comparative, retrospective, single-center study on 86 patients (admitted between January 2020 and March 2022). We based our approach on specific inclusion criteria: 1. The study group included 46 co-infected patients (COVID-19 and CD) receiving antibiotics and FMT; 2. In the control group, 40 co-infected patients received antibiotics only. Our results showed no significant group differences in terms of gender, age, risk factors such as cardiovascular and neurological diseases, type 2 diabetes, and obesity (p > 0.05), or in pre-treatment inflammatory status, evaluated by white blood cell (WBC) count and C-reactive protein (CRP) levels. We report a significant decrease in inflammatory syndrome (CRP, WBC) in coinfected patients receiving FMT in addition to antibiotics (p < 0.05), with a lower relapse rate and mitigation of cramping and abdominal pain (91.3%). In addition, a higher level of fibrinogen, persistent moderate abdominal pain (82.5%), and a significantly higher CD infection relapse rate (42.5%) were recorded in co-infected patients treated only with antibiotics (p < 0.05). Conclusion: Our study provides new data to support the multiple benefits of FMT in the case of COVID-19 and CD co-infection by improving patients’ quality of life and inflammatory syndrome.
Keywords: fecal microbiota transferring, co-infection, SARS-CoV2, C. difficile
1. Introduction
The COVID-19 pandemic has challenged the management of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Clostridioides difficile (CD) co-infected patients. Recent studies have highlighted the intestinal microbiota’s crucial role in the immune response to SARS-CoV-2 infection by modulating the inflammatory response [1,2]. In this context, fecal microbiota transplant (FMT) may present itself as a beneficial intervention due to its known capacity for reducing circulating inflammatory markers as well as complications and recurrence rates in CD infections (CDI). These effects might be even more notable in CDI patients co-infected with SARS-CoV-2 as both infections are known to elicit exaggerated inflammatory responses. The spike (S) glycoprotein of SARS-CoV-2 binds to its receptor, angiotensin-converting enzyme 2 (ACE 2), and transmembrane protease serine 2 (TMPRSS2), which, at high levels, activates the viral S protein, cleaves ACE2 receptors, and facilitates viral binding to the host cell membrane, causing dysbiosis. In addition, SARS-CoV-2 can also utilize the phagocytosis or endocytosis functions of host cells to invade certain immune cell types, such as macrophages, thus mediating the coronavirus’s entrance into the host cell through membrane fusion. ACE 2 is found in human cells in the intestines, lungs, heart, and kidneys [3]. Endoscopy studies have revealed the presence of colon damage in these patients. Moreover, a biopsy performed on a COVID-19 patient found evidence of the SARS-CoV-2 protein coat in the stomach, duodenum, and rectum. Consequently, host microbiome interface impairment and dysbiosis, along with the inflammatory response, enhance the risk of pathogenic invasion, which might explain the susceptibility of COVID-19 patients to CDI colitis or other pathogens’ co-infection [3,4,5,6]. Moreover, recent metagenomic analyses in COVID-19 patients revealed a marked dysbiosis [7,8,9,10]. A positive correlation has been found between the disease’s severity (along with its pulmonary complications) and the overgrowth of pathogenic bacteria (Coprobacillus, Clostridium ramnosum, and Clostridium hathewayi) to the detriment of Faecalibacterium prausnitzii bacteria growth [7]. The latter has shown beneficial anti-inflammatory effects in its hosts’ microbiome population. Furthermore, previous studies suggest that FMT, probiotics, and prebiotics may have a positive role in the treatment of CDI by reducing the incidence of fatal outcomes [7,8,9,10,11].
Several reports have highlighted the fact that SARS-CoV-2 patients who are also co-infected with CD complicate clinical management by altering the onset and the clinical course of CDI [1,2,10,12]. In this context, FMT might have a crucial role in the treatment of CDI to improve the long-term evolution and relapse risk [10,11,12,13,14,15,16,17,18,19,20,21]. There is a need for new and innovative treatment approaches to alleviate the inflammatory response. In this sense, further research approaches should be validated by a decrease in inflammatory markers such as C-reactive protein (CRP), white blood cell (WBC) count, procalcitonin, fibrinogen, and d-dimers. Current literature is scarce in this respect. Albeit a few small sample cohort studies have reported FMT outcomes in CDI patients; very few patients with COVID-19 co-infection were enrolled in these studies (case reports on two patients). These studies concluded that FMT appears to be safe and of comparable efficacy in treating recurrent CDI in patients with coexisting COVID-19 and that FMT mitigated more adverse outcomes of SARS-CoV-2 [12,15,22,23,24,25,26].
Moreover, other reports focus mainly on the clinical perspective, providing recommendations for COVID-19 testing (donors and patients) and emphasizing the experience with FMT for recurrent Clostridioides difficile infection (rCDI) [27,28] or as a first-line therapy in primary CDI [11,13]. On the other hand, there are several important reports tackling the inflammatory response in SARS-CoV-2 and CDI-infected patients, albeit solely from the perspective of antibiotic therapy and lacking data on the FMT approach [1,2].
The main objective of our study was to highlight the beneficial effects of FMT in patients with SARS-CoV-2 and CD co-infections. We aimed to point how importance it is to repopulate the gut microbiome in order to restore immune function, decrease systemic inflammation, and reduce the risks of recurrence. In addition, we also wanted to analyze and report the safety and clinical benefits of FMT as a first-line therapy in patients with co-infections of SARS-CoV-2 and CDI in comparison with a control group treated only with antibiotics.
2. Materials and Methods
2.1. Study Design
Our retrospective, single-center study included 86 patients (46 in the study group vs. 40 in the control group) admitted between January 2020 and March 2022 in the Gastroenterology Department of the County Community Emergency Hospital of Sibiu. This study was performed in accordance with the Helsinki Declaration of 1964. Both groups consisted of COVID-19 and CD co-infected patients. The study was approved by the Ethics Committee in Scientific Research of the Lucian Blaga University of Sibiu, approval code nr.15, approval date 14 October 2022.
The study group included patients receiving FMT and antibiotics at the first episode, while the control group was comprised of patients treated only with antibiotics. We selected the following inclusion criteria, similar to [1,2,11,13,29]: 1. patients over 18 years old; 2. COVID-19 patients (diagnosed by SARS-CoV-2 nucleic acid detection by real-time reverse transcriptase polymerase chain reaction from nasopharyngeal and oropharyngeal swabs); 3. microbiological evidence of CDI (A/B positive toxins); 4. patients who have followed the standard treatment protocol in cases of co-infection: a 10-day course of antibiotics with vancomycin 250 mg × 4/day. After 10 days, if needed, metronidazole 500 mg × 3/day i.v. was added along with vancomycin to control the symptoms. This approach has been implemented with good results by Rokas et al., which showed improved mortality in critically ill patients with CDI when i.v. metronidazole was added to oral vancomycin [30]. The control group received only antibiotic therapy, while in the study group, one FMT infusion was employed after the first 10 days of treatment. These patients ceased antibiotic treatment 24 h prior to the instillation procedure. The decision to administer FMT during the first episode of CDI was made in a similar manner to [11,12] and considered the presence of pseudomembranous colitis at endoscopy as an indication in this regard.
2.2. Patient Demographics and Clinical Characteristics
Data collection was procured from the electronic medical records available upon request according to the institutional procedures for clinical retrospective studies. Demographic characteristics included age and gender. We selected the records of patients who had signed informed consent to participate in research studies, similar to [11]. Our focus was to retrieve clinical data related to risk factors, symptomatology, and inflammatory biomarkers. We looked for: (1) comorbidities (malignant neoplasms, diabetes mellitus, cardiovascular diseases, chronic digestive diseases, renal insufficiency, and stroke), (2) clinical manifestations and paraclinical features (number of stools initially assessed and post-transfer of fecal microbiota; abdominal pain which was scaled (mild pain, moderate pain, severe pain), the presence of pseudomembranous colitis at endoscopy—similar or in accordance to data documented in [11,12,21,24,25,26,31,32,33], and biological markers for inflammatory syndromes (WBC count, CRP, and fibrinogen).
The clinical form of COVID-19 infection for the patients included in the study was defined according to World Health Organization (WHO) guidelines as either moderate or severe. There were no patients with mild symptoms admitted, and those with critical disease due to COVID-19 were transferred to the ICU. Moderate pneumonia was defined as the presence of fever, cough, dyspnea, and SpO2 of ≥90% on room air. The severe form of pneumonia was defined by the presence of clinical signs of pneumonia and one or more of the following: a respiratory rate of >30 breaths/min, severe respiratory distress, or an SpO2 of <90% on room air, and increased inflammatory markers (CRP, ESR) [1,2,34].
2.3. Fecal Microbiota Transplantation Procedure
First- or second-degree relatives were selected as potential donors according to the current guidelines [35,36]. The fecal matter samples were tested coproparasitologically and virologically (Ag HBS, ACVHC, HIV, CMV, and CD), as well as for COVID-19. Donors were tested for SARS-CoV-2 (by RT-PCR test) [11,37] and all subjects who tested positive were excluded. In addition, patients with documented autoimmune or other chronic diseases and patients who had undergone major surgery or received blood products 12 months before fecal matter collection were also excluded. Donors over the age of 50 were also excluded. We thoroughly analyzed the eligibility of each donor based on their medical records and a personal interview [35,36].
The day before FMT, the patients were prepared by intestinal lavage with 4 L of PEG (Poly Ethylene Glycol).
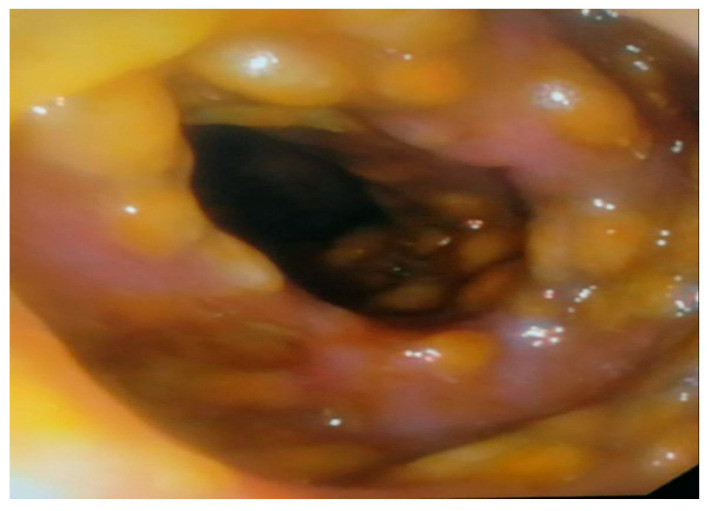
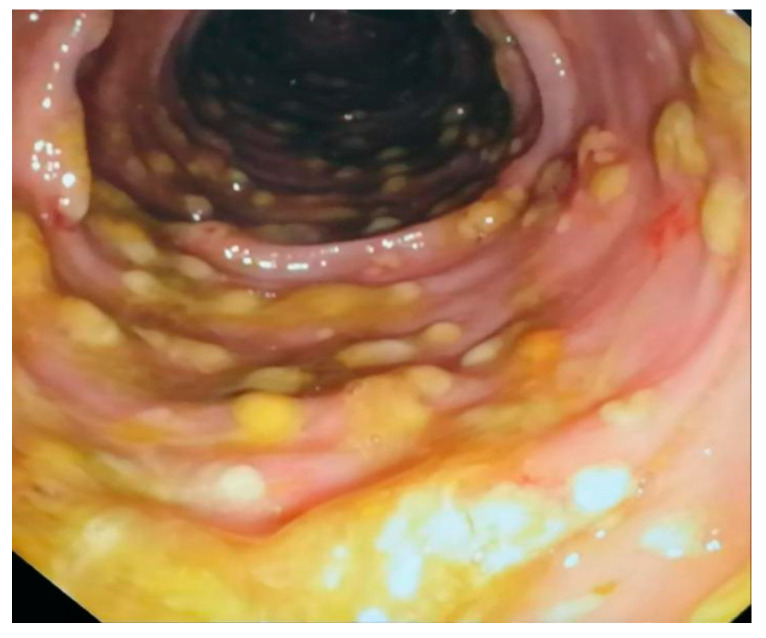
Fecal matter transfer technique involved dissolving 50 g of donated feces (less than 6 h after defecation) in 500 mL of saline 0.9%, mixing to obtain a homogeneous solution, and filtering. Immediately after, a total colonoscopy was performed with terminal ileoscopy, without sedation, in order to preserve anal sphincter control. The suspension, in its fresh state, was introduced from the terminal ileum, with 2/3 of the suspension in the right colon and the rest in the other segments of the colon at withdrawal. Endoscopic appearance of the colon before fecal microbiota transplant was performed, a typical endoscopic appearance of pseudomembranous colitis was outlined at colonoscopy at the moment of the FMT, despite antibiotic treatment. The colonic mucosa presented erased luster, erased vascularization, granular mucosa, and yellow-green pseudomembranous deposits. Classification of pseudomembranous lesions can be made based on the degree and depth of inflammatory changes, with grading of lesions from type 1 (“summit lesions”, focal surface epithelial inflammation or necrosis) (Figure 1 and Figure 2) to type 3 (mucosal necrosis and significant inflammatory debris) [38].
Figure 1.
Endoscopic picture before FMT, personal collection.
Figure 2.
Endoscopic picture before FMT, personal collection.
Safety precautions for endoscopy staff during the COVID-19 pandemic were implemented according to current guidelines established by the World Health Organization and international gastroenterology organizations [34,39,40,41,42]. These precautions included practicing strict physical distancing and minimizing the number of staff in the procedure room. Additionally, all of the staff performing colonoscopies on COVID-19 and CDI patients wore full personal protective equipment, including a respirator (FFP2, FFP3, N95, N99, N100, or equivalent), gown, goggles, face shield, gloves, and apron, while also applying standard precautions in providing patient care.
2.4. Outcomes
There were three primary outcomes for our study. The first was based on the reduction of inflammatory markers. The second was related to the resolution of abdominal pain after treatment. Finally, the third outcome concerned disease recurrence, defined in accordance with the European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults [43] as appearing within 8 weeks after a previous episode, after completion of initial treatment [34,43,44]. In consequence, we established an 8-week follow-up period in our study after the patients’ first episode of CDI and subsequent treatment.
The secondary outcome was related to adverse effects attributed to the FMT procedure.
2.5. Data Analysis
We performed univariate analyses of continuous and categorical variables. Continuous variables were represented by age (in years) and inflammatory biomarkers (white blood cell (WBC) count, CRP, and fibrinogen). Categorical variables referred to gender, comorbidity categories (diabetes mellitus, neurological diseases, cardiovascular diseases, obesity), clinical manifestations, and paraclinical features (pseudomembranous colitis at endoscopy, number of stools, abdominal pain). Categorical variable analyses and results were presented as frequencies and percentages, while continuous variables were presented as means and standard deviations. Groups were compared using the chi-square or Fischer test for categorical variables and the independent t-test or Mann-Whitney test for continuous variables. An α-level of 0.05 was considered statistically significant.
3. Results
3.1. Study Population and CDI Risk Factors
There was a total of 235 patients with SARS-CoV-2 and CD co-infection documented during the two-year period of our retrospective search. 149 cases were excluded due to one or more of the following: missing informed consent to participate in research studies; missing data (particularly on recurrence rate); death during hospitalization or transfer to ICU; significant comorbidities or demographic characteristics which would have created inhomogeneities between groups or which would have had an intrinsic influence on the determined outcome variables.
We recorded a notable difference in patients coming from rural areas (60.5%) vs. the patients from urban environments (39.5%), however, with the same distribution pattern in both groups (p > 0.05). On the same note, both groups were homogeneous, with the same percentage of patients (60.4%), hypertension as a factor of severity, and diabetes mellitus type 2. Then, in both groups, there was a close distribution of neurological and metabolic risk factors. Around 7% of the cases presented a stroke in their medical history, and obesity as a risk factor was noted in a slightly higher percentage for the study group (17.4% vs. 15%) (p > 0.05) (Table 1).
Table 1.
Demographic and clinical characteristics of the patients.
| Variable | FMT Group (n = 46) |
Control Group (n = 40) |
Chi-Square Test p-Value |
|---|---|---|---|
| Age (years) | 67.63 | 67.68 | 0.917 |
| Male gender Female gender |
23 (50%) 23 (50%) |
20 (50%) 20 (50%) |
0.585 0.585 |
| Rural areas Urban |
33 (71.73%) 13 (28.26%) |
19 (47.5%) 21 (52.5%) |
0.28 0.28 |
| Cardiovascular disease | 6 (13%) | 7 (17.5%) | 0.817 |
| Hypertension | 14 (30.4%) | 12 (30%) | 0.817 |
| Neurological disease | 3 (6.5%) | 3 (7.5%) | 0.817 |
| Diabetes | 14 (30.4%) | 12 (30%) | 0.817 |
| Obesity | 8 (17.4%) | 6 (15%) | 0.815 |
| Antibiotics | |||
| Vancomycin | 45 (97.82%) | 11 (27.5%) | 0.001 |
| Vancomycin and metronidazole | 1 (2.17%) | 29 (72.5%) | 0.001 |
3.2. Clinical Manifestations and Inflammatory Status Prior to Treatment
There were no significant differences between patients with regards to their symptoms (i.e., moderate vs severe abdominal pain and number of stools per day) or the inflammatory status (quantified by WBC count and CRP) prior to treatment, as shown in Table 2.
Table 2.
Clinical manifestations and inflammatory status prior to treatment.
| Variable | FMT Group (n = 46) |
Control Group (n = 40) |
t-Test Chi-Square Test |
|---|---|---|---|
| CRP pre-treatment (mean) | 46.8 mg/L | 56.2 mg/L | 0.18 |
| WBC count pre-treatment (mean) | 17,000 cells/mL | 16,850 cells/mL | 0.795 |
| Moderate abdominal pain pre-treatment | 26 (56.5%) | 18 (45%) | 0.28 |
| Severe abdominal pain pre-treatment | 20 (43.5%) | 22 (55%) | 0.28 |
| 3–5 stools/day pre-treatment | 28 (60.9%) | 24 (60%) | 0.93 |
| >5 stools/day pre-treatment | 18 (39.1%) | 16 (40%) | 0.93 |
With regards to COVID-19 disease severity, there were 20 patients (50%) in the control group with moderate disease and 20 patients with severe disease. In the FMT group, 23 patients presented with a moderate form (50%), and 23 had a severe form.
3.3. FMT’s Role in Decreasing the Inflammatory Syndrome, Abdominal Pain, and Risk of Recurrence
We recorded a statistically significant improvement in inflammatory syndrome (CRP, WBC count) after FMT, p < 0.05. In the control group, 29 patients (72.5%) received Metronidazole and Vancomycin, in comparison with the study group, where this combination of antibiotics was used only in 1 patient (2.17%). There was only one case of relapse (2.17%) out of the 46 patients receiving an FMT, in contrast to 17 cases of recurrence (42.5% relapse rate) in the control group (p < 0.05).
Post-treatment more than 91% of FMT patients had no abdominal pain while a significant number of patients treated only with antibiotics (82.5%) had persistent moderate pain (p < 0.05). (Table 3). FMT alleviated abdominal pain and lowered the relapse rate (p < 0.05).
Table 3.
Relapse rate, inflammatory markers, and abdominal pain after treatment between study groups.
| Variable | FMT Group (n = 46) |
Control Group (n = 40) |
T Test Chi-Square Test * Fisher Test |
|---|---|---|---|
| Fibrinogen | 420 mg/dl | 452.55 mg/dl | 0.02 |
| CRP post-treatment (mean) | 5.67 mg/L | 9.18 mg/l | 0.001 |
| Leucocyte post-treatment (mean) | 7695 cells/ml | 85,751 cells/mL | 0.001 |
| No abdominal pain post-treatment | 42 (91.3%) | 7 (17.5%) | 0.0001 |
| Moderate abdominal pain post-treatment | 4 (8.7%) | 33 (82.5%) | 0.001 |
| Relapse | 1 (2.2%) | 17 (42.5%) | 0.001 * |
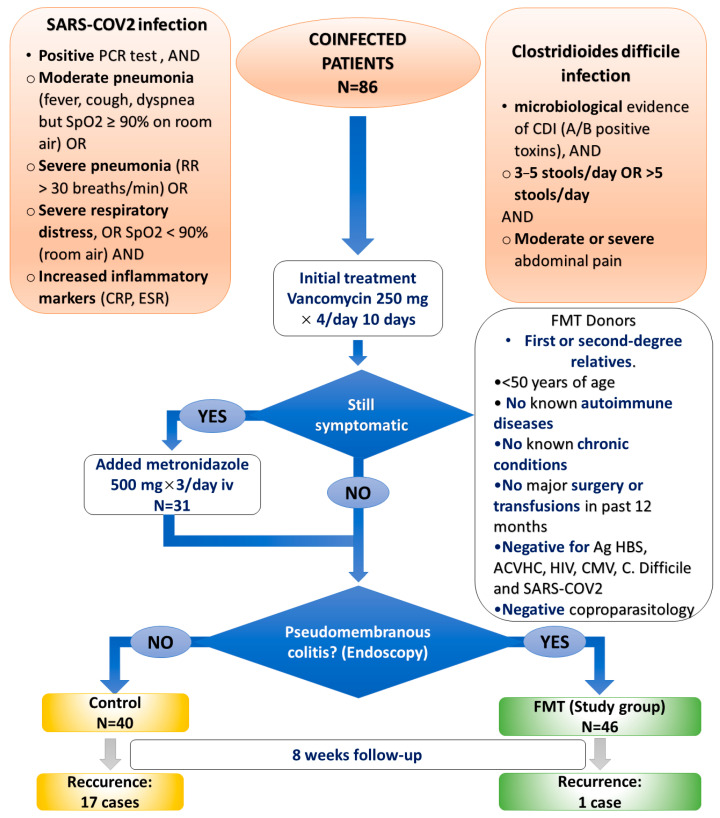
The study design and a representation of the results concerning disease recurrence are given in Figure 3.
Figure 3.
Study design and results regarding disease recurrence.
With regards to our secondary outcome, we did not record any severe adverse effects during or after the FMT procedure for the 46 patients that underwent this intervention.
4. Discussion
In this paper, we analyzed the importance of FMT in alleviating gut microbiome dysbiosis and improving immune system function in SARS-CoV-2 and C. difficile co-infected patients. We hypothesized a decrease in systemic inflammation along with improvements in clinical condition and a reduction in the recurrence risk for these patients. To achieve this goal, we analyzed and reported the safety of FMT and its clinical benefits as a first-line therapy for the aforementioned patients, in comparison to the control group treated only with antibiotics.
Our results showed that in more than 90% of the whole cohort (study and control groups), specific risk factors such as stroke, diabetes, obesity, and hypertension were present as important comorbidities for both infections, which is consistent with the current literature [11,12,16,17,19]. Our results are in line with Kovacevic et. al. [45] who recorded similar risk factors for co-infection (cardiovascular disease (11.96%) and diabetes (30.37%)).
Clostridioides difficile preferentially affects elderly patients and causes a high mortality rate, especially in co-infection. In the literature, the co-infected patients’ profile has a mean of 61.22 years of age, comes from urban areas, and has a higher chance of being female (62.5%). In contrast, our study noted homogeneity in gender prevalence in the included population. In addition, a higher percentage of patients were from rural areas, albeit without reaching statistical significance (p > 0.05) [1,2,16,22]. Rajib et al. outlined smoking rates and obesity rates explain, to some extent, the geographic disparities in COVID-19 prevalence. Their study noted a higher obesity rate in rural areas and a higher percentage of adult smokers, increasing the risk for cardiorespiratory diseases, but further studies are needed in terms of epidemiology [46]. However, our study did not assess smoking status and showed a homogenous distribution of the studied comorbidities.
Recent clinical trials mention the role of FMT in inflammatory bowel disease (IBD), multiple sclerosis, and Parkinson’s disease. This suggests not only a local modulating intestinal effect but also a systemic immunological response to FMT with an impact on the gut-lung and gut-brain axes [3,46]. The immunological response after FMT was associated with a substantial reduction in the colonic mucosal CD8+ T cell density and a decrease in serum concentrations of IL-6, and IP-10. Serum levels of IL-6 and VCAM-1 were all significantly correlated with CRP and ESR, as has been highlighted in the study by Yanzhi et al. on FMT’s role in ulcerative colitis treatment [47]. Given the excessive inflammatory response in SARS-CoV2 and CDI infections, with elevated levels of IL-6, TNF-α, IL-1β, and IP-10, as well as associated dysbiosis mentioned in the literature [3,4,48], FMT might be a key factor in CDI treatment or more severe clinical scenarios like SARS-CoV-2 and CDI co-infections. FMT could improve the clinical outcome of these patients.
Clinical evolution for CDI and/or SARS-CoV-2 patients is related to the associated inflammatory response. According to Clinical Practice Guidelines for Clostridioides difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA), an important cut-off point is leukocytosis (>15.000 cells/mL). Our baseline mean in leukocytosis (16.613 cells/mL) could be regarded as a crucial predictor for the clinical outcome and survival [16,19,22,23]. Moreover, Konturek et. al. outlined a significant CRP reduction after FMT therapy in all treated patients [20]. Likewise, our data showed normalization of inflammatory markers: CRP 5.67 mg/dl mean, WBC mean 7695 cells/mL, and fibrinogen 420 mg/dl in FMT patients (p < 0.05).
It is acknowledged that SARS-CoV-2 and CDI co-infection challenge clinical management in the sense that abdominal cramping and pain may be more severe and longer lasting after the disease’s resolution [20,33,38]. We recorded a statistically significant correlation between FMT and the reduction of abdominal pain for our patients at discharge (91.3%, p < 0.005). In a similar approach, other studies focusing on CDI alone pointed out a significant reduction in abdominal pain and bloating at 4–8 weeks. Furthermore, a retrospective clinical review regarding FMT for CDI infection also highlighted a significant improvement in abdominal pain and cramping from baseline at 6-month follow-up [17,22,45]. These aspects, in addition to the data presented in our study, suggest that restoring a normal microbiota through fecal instillation relents abdominal pain and improves patients’ quality of life.
On the same note, the FMT group needed only one class of antibiotic (vancomycin) in most of the cases (97.82%) with a better clinical evolution, while the control group, in 75% of the cases, received a second antibiotic class (metronidazole) associated with properly managing the case. Similarly, previous studies on fecal microbiota transplantation using colonoscopy pointed out a significantly more effective approach than antibiotherapy (vancomycin) per se or vancomycin and fidaxomicin to abate the clinical symptoms of CDI [18]. The literature is scarce, however, when it comes to SARS-CoV-2 patients and CDI. To the best of our knowledge, there are only a few reports that address SARS-CoV-2 and CDI co-infections (case series). Further clinical trials assessing a personalized therapeutic management for these cases could shed light in regards to survival rates, antibiotic use reduction, antibiotic associations, and their side effects. Consequently, our study adds important information to the literature concerning the treatment methodology of SARS-CoV-2 and CDI.
Moreover, many research papers highlighted that CDI was associated with a high risk of relapsing, especially in patients with comorbidities and/or co-infections. In the latest reports, early fecal transplantation was associated with a significantly reduced mortality rate for recurrent CDI. Furthermore, in a Cox model proposed by Lagier et al., early transplantation was the only independent predictor of survival in severe CDI (hazard ratio 0.18, p = 0.006) [15].
Our previous results showed important FMT benefits from the first episode in cases of severe colitis, which correlated with the risk of relapsing [11]. In this study, we recorded only one case of CDI recurrence after 6 weeks in the FMT group. Our patient presented with severe leukocytosis, more than six stools/day, and associated diabetes and obesity. In contrast, there were multiple cases of recurrence in the control group—17 patients (42.5%). Similar results were presented by Marinescu et al. outlining the recurrence in 19 co-infected patients treated only with antibiotics [2,18]. Roshan et al. in their retrospective study mentioned relapse in one case 5 weeks after the FMT procedure [17,20,22]. These reports further add importance in providing arguments for FMT being subsequently employed after monotherapy to prevent CDI recurrence [18].
An important overall observation that should be highlighted is the 97.82% success rate that we recorded, regardless of age and comorbidities. In addition, very few adverse effects are generally directly attributable to the procedure. Most reported adverse events in the literature have been self-limiting gastrointestinal symptoms, comprising abdominal cramps, nausea, and constipation. Fever, gram-negative bacteremia, and bowel perforation are very rare adverse effects [19,49,50]. The patients enrolled in our study were informed about these possible adverse effects. We did not, however, record any severe adverse effects during or after the procedure for the 46 patients that received FMT. Recent studies discuss a new method to further improve the safety of FMT, called washed microbiota preparation. This approach is based on the use of an automatic microfiltration machine and subsequent repeated centrifugation. In a study with patients who underwent either washed microbiota transplantation (WMT) or crude FMT in the same FMT center with the same indications, fewer adverse effects were recorded in the WMT group. However, further studies are needed to understand the mechanisms behind these findings [51,52,53]. Consequently, FMT might be regarded as an alternative therapeutic solution, especially in older patients with comorbidities, modulating the immune system’s functionality and increasing the survival rate.
Nevertheless, these results must be interpreted with caution, and several limitations should be kept in mind, as this is a single-center research report and a retrospective study with the possibility of residual confounding based on the data gathered during the COVID-19 pandemic. Further research using multicentric randomized control trials is needed to establish a treatment protocol for practitioners in co-infection cases.
5. Conclusions
The fecal microbiota transplantation approach shows promise regarding the safety and efficiency of the treatment of CDI in patients with co-existing COVID-19. It offers a personalized therapeutic management strategy with multiple benefits like decreasing inflammatory syndrome, limiting the use of antibiotics, reducing relapse risk, and alleviating symptomatology.
Author Contributions
Conceptualization, A.B., B.N., F.B., S.B., M.O.N., D.B., C.I.M., C.M., H.D., M.D.R. and S.R.F.; Data curation, A.B., B.N., C.T., C.I.M., C.B. and A.H.; Formal analysis, B.N. and S.R.F.; Funding acquisition, A.B., C.M. and S.R.F.; Investigation, A.B., F.B., S.B., C.T., D.B., C.I.M., C.M. and S.R.F.; Methodology, A.B., B.N., C.I.M., C.M., H.D., C.B. and S.R.F.; Project administration, A.B., H.D., A.H., M.D.R. and S.R.F.; Resources, A.B., D.B. and C.M.; Software, B.N.; Supervision, A.B., B.N., C.T., C.B., M.D.R. and M.O.N.; Validation, A.B., B.N., S.B., F.B. and M.O.N.; Visualization, A.B. and M.D.R.; Writing—original draft, A.B., B.N., F.B. and S.B., Writing—review and editing A.B., B.N., H.D., C.M., S.R.F., M.O.N., A.M., D.B. and C.B. had a substantial role in conceiving the manuscript and should be regarded as one of the main authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethics Committee in Scientific Research of the Lucian Blaga University of Sibiu, approval code nr.15, approval date 14 October 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Ministry of Research, Innovation, and Digitization through Program 1—Development of the national research-development system, Subprogram 1.2—Institutional performance—Projects for financing excellence in RDI, contract no. 28PFE/30.12.2021.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cojocariu C., Girleanu I., Trifan A., Olteanu A., Muzica C.M., Huiban L., Chiriac S., Singeap A., Cuciureanu T., Sfarti C., et al. Did the severe acute respiratory syndrome-coronavirus 2 pandemic cause an endemic Clostridium difficile infection? World J. Clin. Cases. 2021;9:10180. doi: 10.12998/wjcc.v9.i33.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marinescu A., Laza R., Musta V.F., Cut T.G., Dumache R., Tudor A., Porosnicu M., Lazureanu V.E., Licker M. Clostridium Difficile and COVID-19: General Data, Ribotype, Clinical Form, Treatment-Our Experience from the Largest Infectious Diseases Hospital in Western Romania. Medicina. 2021;57:1099. doi: 10.3390/medicina57101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang B., Zhang L., Wang Y., Dai T., Qin Z., Zhou F., Zhang L. Alterations in microbiota of patients with COVID-19: Potential mechanisms and therapeutic interventions. Sig. Transduct. Target. Ther. 2022;7:143. doi: 10.1038/s41392-022-00986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linares-García L., Hernández-Ceballos W., Pérez-Solano C.S., Morales-Guzmán A.S., Miller D.S., Schmulson M. Bacterial and Fungal Gut Dysbiosis and Clostridium difficile in COVID-19: A Review. J. Clin. Gastroenterol. 2022;56:285. doi: 10.1097/MCG.0000000000001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramzan K., Shafiq S., Raees I., Mustafa Z.U., Salman M., Khan A.H., Meyer J.C., Godman B. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalized with COVID-19 during the First Five Waves of the Pandemic in Pakistan; Findings and Implications. Antibiotics. 2022;11:789. doi: 10.3390/antibiotics11060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granata G., Petrosillo N., Al Moghazi S., Caraffa E., Puro V., Tillotson G., Cataldo M.A. The burden of Clostridioides difficile infection in COVID-19 patients: A systematic review and meta-analysis. Anaerobe. 2022;74:102484. doi: 10.1016/j.anaerobe.2021.102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao Z., Xiaojian W., Weiping W., Ping L. Gut Microbiome Alterations in COVID-19. Genom. Proteom. Bioinform. 2021;19:679. doi: 10.1016/j.gpb.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma S., Zhang F., Zhou F., Li H., Ge W., Gan R., Nie H., Li B., Wang Y., Wu M., et al. Metagenomic analysis reveals oropharyngeal microbiota alterations in patients with COVID-19. Sig. Transduct. Target. Ther. 2021;6:191. doi: 10.1038/s41392-021-00614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna S., Kraft C.S. The interplay of SARS-CoV-2 and Clostridioides difficile infection. Future Microbiol. 2021;16:439–443. doi: 10.2217/fmb-2020-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laszkowska M., Kim J., Faye A.S., Joelson A.M., Ingram M., Truong H., Silver E.R., May B., Greendyke W.G., Zucker J., et al. Prevalence of Clostridioides difficile and Other Gastrointestinal Pathogens in Patients with COVID-19. Dig. Dis. Sci. 2021;66:4398. doi: 10.1007/s10620-020-06760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popa D., Neamtu B., Mihalache M., Boicean A., Banciu A., Banciu D.D., Moga D.F.C., Birlutiu V. Fecal Microbiota Transplant in Severe and Non-Severe Clostridioides difficile Infection. Is There a Role of FMT in Primary Severe CDI? J. Clin. Med. 2021;13:5822. doi: 10.3390/jcm10245822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biliński J., Winter K., Jasiński M., Szczęś A., Bilinska N., Mullish B.H., Małecka-Panas E., Basak G.W. Rapid resolution of COVID-19 after faecal microbiota transplantation. Gut. 2022;71:230. doi: 10.1136/gutjnl-2021-325010. [DOI] [PubMed] [Google Scholar]
- 13.Fischer M., Kao D., Mehta S.R., Martin T., Dimitry J., Keshteli A.H., Cook G.K., Phelps E., Sipe B.W., Xu H., et al. Predictors of Early Failure After Fecal Microbiota Transplantation for the Therapy of Clostridium Difficile Infection: A Multicenter Study. Am. J. Gastroenterol. 2016;111:1024. doi: 10.1038/ajg.2016.180. [DOI] [PubMed] [Google Scholar]
- 14.Sandu A., Tillotson G., Polistico J., Salimnia H., Cranis M., Moshos J., Cullen L., Jabbo L., Diebel L., Chopra T. Clostridioides difficile in COVID-19 Patients, Detroit, Michigan, USA. Emerg. Infect. Dis. 2020;26:2272. doi: 10.3201/eid2609.202126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hocquart M., Lagier J.C., Cassir N., Saidani N., Eldin C., Kerbaj J., Delord M., Valles C., Brouqui P., Raoult D., et al. Early Fecal Microbiota Transplantation Improves Survival in Severe Clostridium difficile Infections. Clin. Infect. Dis. 2018;66:645. doi: 10.1093/cid/cix762. [DOI] [PubMed] [Google Scholar]
- 16.Vargas E., Apewokin S., Madan R. Role of the leukocyte response in normal and immunocompromised host after Clostridium difficile infection. Anaerobe. 2017;45:101. doi: 10.1016/j.anaerobe.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roshan N., Clancy A.K., Borody T.J. Faecal Microbiota Transplantation is Effective for the Initial Treatment of Clostridium difficile Infection: A Retrospective Clinical Review. Infect. Dis. Ther. 2020;9:935–942. doi: 10.1007/s40121-020-00339-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hvas C.L., Dahl Jørgensen S.M., Jørgensen S.P., Storgaard M., Lemming L., Lemming M.M., Erikstrup C., Dahlerup J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology. 2019;156:1324. doi: 10.1053/j.gastro.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 19.McDonald L.C., Gerding D.N., Johnson S., Bakken J.S., Carroll K.C., Coffin S.E., Dubberke E.R., Garey K.W., Gould C.V., Kelly C., et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin. Infect. Dis. 2018;66:e1. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konturek P.C., Koziel J., Dieterich W., Haziri D., Wirtz S., Glowczyk I., Konturek K., Neurath M.F., Zopf Y. Successful therapy of Clostridium difficile infection with fecal microbiota transplantation. J. Physiol. Pharmacol. 2016;67:859. doi: 10.1016/S0016-5085(17)31403-8. [DOI] [PubMed] [Google Scholar]
- 21.Song J.H., Kim Y.S. Recurrent Clostridium difficile Infection: Risk Factors, Treatment, and Prevention. Gut Liver. 2019;13:16. doi: 10.5009/gnl18071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer M.P., Hensgens M.P., Miller M., Gerding D.N., Wilcox M.H., Dale A.P., Fawley W.N., Kuijper E.J., Gorbach S.L. Renal failure and leukocytosis are predictors of a complicated course of Clostridium difficile infection if measured on day of diagnosis. Clin. Infect. Dis. 2012;55:149. doi: 10.1093/cid/cis340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C.C., Lee J.C., Chiu C.W., Tsai P.J., Ko W.C., Hung Y.P. Neutrophil Ratio of White Blood Cells as a Prognostic Predictor of Clostridioides difficile Infection. J. Inflamm. Res. 2022;15:1943. doi: 10.2147/JIR.S353814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponce-Alonso M., Sáez de la Fuente J., Rincón-Carlavilla A., Moreno-Nunez P., Martínez-García L., Escudero-Sánchez R., Pintor R., García-Fernández S., Cobo J. Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection. Infect. Control Hosp. Epidemiol. 2021;42:406. doi: 10.1017/ice.2020.454. Correction in: Infect. Control Hosp. Epidemiol. 2021, 42, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira E.O., Penna B., Yates E.A. Should We Be Worried About Clostridioides difficile During the SARS-CoV2 Pandemic? Front. Microbiol. 2020;11:581343. doi: 10.3389/fmicb.2020.581343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malfertheiner P., Bornschein J., Ricciardiello L. COVID-19: Don’t Neglect the Gastrointestinal Tract! Dig. Dis. 2020;38:259–260. doi: 10.1159/000508289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granata G., Bartoloni A., Codeluppi M., Contadini I., Cristini F., Fantoni M., Ferraresi A., Fornabaio C., Grasselli S., Lagi F., et al. The Burden of Clostridioides Difficile Infection during the COVID-19 Pandemic: A Retrospective Case-Control Study in Italian Hospitals (CloVid) J. Clin. Med. 2020;9:3855. doi: 10.3390/jcm9123855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer M., Sipe B.W., Rogers N.A., Cook G.K., Robb B.W., Rex V.D.K. Faecal microbiota transplantation plus selected use of vancomycin for severe-complicated Clostridium difficile infection: Description of a protocol with high success rate. Aliment. Pharmacol. Ther. 2015;42:470. doi: 10.1111/apt.13290. [DOI] [PubMed] [Google Scholar]
- 29.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J., Li P., Hu B., Wang J., Hu C., et al. Clinical Characteristics of COVID-19 Patients with Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020;115:766. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rokas K.E., Johnson J.W., Beardsley J.R., Ohl C.A., Luther V.P., Williamson J.C. The Addition of Intravenous Metronidazole to Oral Vancomycin is Associated with Improved Mortality in Critically Ill Patients with Clostridium difficile Infection. Clin Infect. Dis. 2015;61:934. doi: 10.1093/cid/civ409. [DOI] [PubMed] [Google Scholar]
- 31.Doll M., Marra A.R., Apisarnthanarak A., Al-Maani A.S., Abbas S., Rosenthal V.D. Prevention of Clostridioides difficile in hospitals: A position paper of the International Society for Infectious Diseases. Int. J. Infect. Dis. 2021;102:188. doi: 10.1016/j.ijid.2020.10.039. [DOI] [PubMed] [Google Scholar]
- 32.Marra A.R., Perencevich E.N., Nelson R.E., Samore M., Khader K., Chiang H., Chorazy M.L., Herwaldt L.A., Diekema D.J., Kuxhausen M.F., et al. Incidence and Outcomes Associated with Clostridium difficile Infections: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2020;3:e1917597. doi: 10.1001/jamanetworkopen.2019.17597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magill S.S., Edwards J.R., Bamberg W., Beldavs S., Dumyati G., Kainer M.A., McAllister-Hollod L., Nadle J., Ray S.M., Thompson D.L., et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370:1198. doi: 10.1056/NEJMoa1306801. Correction in: N. Engl. J. Med. 2022, 386, 2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization (WHO) Living Guidance for Clinical Management of COVID-19: Living Guidance, 23 November 2021. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 16 December 2022)]. Available online: https://apps.who.int/iris/handle/10665/349321. [Google Scholar]
- 35.Cammarota G., Ianiro G., Tilg H., Rajilić-Stojanović M., Kump P., Satokari R., Sokol H., Arkkila P., Pintus C., Hart A., et al. European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aràjol C., Aira Gómez A., González-Suárez B., Casals-Pascual C., Martí S.M., Luzón M.Á.D., Soriano A., Capón J.G., on behalf of the Catalan Group for the Study and Development of Fecal Microbiota Transfer Donor selection for faecal microbiota transplantation. Consensus document of the Catalan Society of Gastroenterology and the Catalan Society of Infectious Diseases and Clinical Microbiology. Selección del donante para la transferencia de microbiota fecal. Documento de posicionamiento de la Societat Catalana de Digestologia y de la Societat Catalana de Malalties Infeccioses i Microbiologia Clínica. Gastroenterol. Hepatol. 2021;44:175. doi: 10.1016/j.gastrohep.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Tariq R., Weatherly R., Kammer P., Pardi D.S., Khanna S. Donor Screening Experience for Fecal Microbiota Transplantation in Patients with Recurrent C. difficile Infection. J. Clin. Gastroenterol. 2018;52:146. doi: 10.1097/MCG.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 38.Farooq P.D., Urrunaga N.H., Tang D.M., von Rosenvinge E.C. Pseudomembranous colitis. Dis. Mon. 2015;61:181. doi: 10.1016/j.disamonth.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization . COVID-19: Occupational Health and Safety for health Workers: Interim Guidance, 2 February 2021. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 16 December 2022)]. Available online: https://apps.who.int/iris/handle/10665/339151. [Google Scholar]
- 40.World Health Organization . Rational Use of Personal Protective Equipment for COVID-19 and Considerations during Severe Shortages: Interim Guidance, 23 December 2020. World Health Organization; Geneva, Switzerland: 2020. [(accessed on 16 December 2022)]. Available online: https://apps.who.int/iris/handle/10665/338033. [Google Scholar]
- 41.Sultan S., Lim J.K., Altayar O., Davitkov P., Feuerstein J.D., Siddique S.M., Falck-Ytter Y., El-Serag H.B. AGA Rapid Recommendations for Gastrointestinal Procedures During the COVID-19 Pandemic. Gastroenterology. 2020;159:739. doi: 10.1053/j.gastro.2020.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chai N., Mei Z., Zhang W., Du C., Wang X., Li L., Ma Y., Zou J., Tang X., Wang N., et al. Endoscopy works during the pandemic of coronavirus COVID-19: Recommendations by the Chinese Society of Digestive Endoscopy. United Eur. Gastroenterol. J. 2020;8:798. doi: 10.1177/2050640620930632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J., Kim J., Kim B., Pai H. Which is the Preferred Regimen for Non-Severe Clostridioides difficile Infection in Korea, Vancomycin or Metronidazole? Infect. Chemother. 2022;54:213. doi: 10.3947/ic.2022.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surawicz C.M., Brandt L.J., Binion D.G., Ananthakrishnan A.N., Curry S.R., Gilligan P.H., McFarland L.V., Mellow M., Zuckerbraun B.S. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013;108:478–499. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 45.Kovačević N., Lendak D., Popović M., Plećaš Đuric A., Pete M., Petrić V., Sević S., Tomić S., Alargić J., Damjanov D., et al. Clinical Presentations, Predictive Factors, and Outcomes of Clostridioides difficile Infection among COVID-19 Hospitalized Patients-A Single Center Experience from the COVID Hospital of the University Clinical Center of Vojvodina, Serbia. Medicina. 2022;58:1262. doi: 10.3390/medicina58091262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul R., Arif A.A., Adeyemi O., Ghosh S., Han D. Progression of COVID-19 From Urban to Rural Areas in the United States: A Spatiotemporal Analysis of Prevalence Rates. J. Rural. Health. 2020;36:591. doi: 10.1111/jrh.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Ren R., Sun G., Peng L., Tian Y., Yang Y. Pilot study of cytokine changes evaluation after fecal microbiota transplantation in patients with ulcerative colitis. Int. Immunopharmacol. 2020;85:106661. doi: 10.1016/j.intimp.2020.106661. [DOI] [PubMed] [Google Scholar]
- 48.Yu H., Chen K., Sun Y., Carter M., Garey K.W., Savidge T.C., Devaraj S., Tessier M.E., von Rosenvinge E.C., Kelly C.P., et al. Cytokines Are Markers of the Clostridium difficile-Induced Inflammatory Response and Predict Disease Severity. Clin. Vaccine Immunol. 2017;24:e00037. doi: 10.1128/CVI.00037-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rapoport E.A., Baig M., Puli S.R. Adverse events in fecal microbiota transplantation: A systematic review and meta-analysis. Ann. Gastroenterol. 2022;35:150. doi: 10.20524/aog.2022.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav D., Khanna S. Safety of fecal microbiota transplantation for Clostridioides difficile infection focusing on pathobionts and SARS-CoV-2. Therap. Adv. Gastroenterol. 2021;14:17562848211009694. doi: 10.1177/17562848211009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fecal Microbiota Transplantation-standardization Study Group. Fa-Ming Z. Nanjing consensus on methodology of washed microbiota transplantation. Chin. Med. J. 2020;133:2330. doi: 10.1097/CM9.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang T., Lu G., Zhao Z., Liu Y., Shen Q., Li P., Chen Y., Yin H., Wang H., Marcella C., et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: Clinical findings, animal studies and in vitro screening. Protein Cell. 2020;11:251. doi: 10.1007/s13238-019-00684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soveral L.F., Korczaguin G.G., Schmidt P.S., Nunes I.S., Fernandes C., Zárate-Bladés C.R. Immunological mechanisms of fecal microbiota transplantation in recurrent Clostridioides difficile infection. World J. Gastroenterol. 2022;28:4762. doi: 10.3748/wjg.v28.i33.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.