Abstract
Objective
Based on long-term follow-up of patients with COVID-19, to evaluate whether the severity of acute COVID-19 infection affects rehabilitation outcomes.
Design
Observational cohort study.
Subjects
A total of 61 post-acute COVID-19 patients underwent inpatient and outpatient customized rehabilitation treatment.
Methods
The severity of acute COVID-19 infection was measured with the World Health Organization Clinical Progression Scale (WHO-CPS). Motor, cognitive, and functional variables were measured using standard and specified scales 6 months or more after acute illness.
Results
Of the 61 subjects, 65.6% had severe disease according to WHO-CPS. Significant improvement was found in activities of daily living functions (Functional Independence Measure (FIM) at admission 103.7 ± 18.9 vs FIM at discharge 118.7 ± 6.8) (p < 0.00). Of participants, 88% were able to wean off oxygen completely. A significant correlation was found between higher WHO-CPS, prolonged acute hospitalization, and days of ventilation were correlated with lower total and motor FIM at admission, but not with cognitive FIM or Montreal Cognitive Assessment (MoCA). No correlation was found between WHO-CPS, prolonged acute hospitalization and day of ventilation and funnctional level at discharge.
Conclusion
The severity of acute COVID-19 infection affects the functional status of survivors at admission to rehabilitation, but, contrary to expectations, not the functional outcomes at discharge. These findings show that even patients with severe acute COVID-19 infection may improve their daily functioning significantly during rehabilitation program.
LAY ABSTRACT
Many patients worldwide develop severe complications of recent infection with the coronavirus SARS-CoV-2 (COVID-19). These complications include respiratory, motor, cognitive, and functional symptoms. Rehabilitation plays an important role in the recovery of these patients. This study examined whether there is a correlation between the severity of acute COVID-19 infection and the functional level of survivors after rehabilitation. Study participants were 61 post-acute COVID-19 patients who received inpatient and outpatient rehabilitation. Most of the patients improved significantly in daily functions following rehabilitation, and most did not need oxygen support at discharge. Patients with severe COVID-19 infection started the rehabilitation period at a low functional level, but improved significantly during rehabilitation, and at discharge there was no difference between patients with more severe or less severe COVID-19 infections. These findings show that even patients with severe acute COVID-19 infection may improve significantly during rehabilitation program.
Key words: interdisciplinary rehabilitation, COVID-19 infection, activities of daily living, Functional Independence Measure
COVID-19 is considered primarily a respiratory infection, but it is associated with complex multi-organ impairments and long-term physical, cognitive, and psychiatric sequelae (1–5). Although many patients experience long-lasting morbidity, some patients who have severe COVID-19 infection recover well without long-term sequelae. In February 2022, Han et al. published a meta-analysis that systematically evaluated the long-term sequelae of COVID-19 Worldwide. At 1-year follow-up, the most prevalent symptoms were fatigue/weakness (28%), dyspnoea (18%), arthralgia and myalgia (26%), depression (23%), anxiety (22%), memory loss (19%), concentration difficulties (18%), and insomnia (12%) (6).
The many COVID-19 patients needing rehabilitation, together with the backlog of other patients, have challenged rehabilitation services (7). Several policy documents about rehabilitation after COVID-19 were published early in the pandemic, but evidence about the functional outcome of customized rehabilitation programmes for recovered patients is limited (8, 9). Most studies investigated the effect of acute inpatient rehabilitation programmes and found beneficial short-term effects on respiratory function and endurance (10, 11), but only in the short term; most of these studies reported on a 1-month follow-up (12). A few studies reported the effect of multidisciplinary outpatient rehabilitation programmes for COVID-19 patients presenting long-term sequelae (13).
It has been assumed that more severe acute disease that requires ventilation and longer stay in intensive care predicts medical complications and higher risk of sequelae (14). Acquired critical illness polyneuropathy and critical illness myopathy have been reported in almost 25–46% and 48–96% of post-intensive care unit (post-ICU) patients, respectively (15, 16). These are thought to relate to direct inflammatory and hypoperfusion-mediated degradation of muscle fibres and neurones, possibly exacerbated by prolonged immobility, suboptimal glycaemic control, and iatrogenic use of steroids and neuromuscular blocking agents (17–19). Inflammation and hypoperfusion during acute illness were also related to brain injury and subsequent cognitive impairment (20–22). However, it remains unknown how the severity of the acute disease affects functional outcomes after the rehabilitation period.
The aim of this study was to evaluate how the severity of acute COVID-19 infection affects rehabilitation outcomes, and to determine which factors contribute to favourable rehabilitation outcomes, following long-term follow-up of COVID-19 patients. The findings may contribute to improving the rehabilitation of COVID-19 patients and preventing long-term sequelae.
METHODS
Study population
This is an observational prospective study of all post-acute COVID-19 patients referred to rehabilitation at Hadassah Medical Center, Jerusalem, Israel, between December 2020 and August 2021. All subjects who agreed to participate in the study and met the inclusion criteria were prospectively recruited. Inclusion criteria were: adults aged 18 years and over, confirmed COVID-19 infection, Mini-Mental State Examination (MMSE) score above 24 according to Institutional Review Board (IRB) request and capable of understanding and signing an informed consent form. Exclusion criteria were: pre-morbidity of mental disorder or dementia or post-COVID-19 cerebral vascular accident with severe cognitive impairment. Ethical approval was granted by the Hadassah Medical Center IRB committee (#0943-20-HMO).
Rehabilitation programme
The rehabilitation programme followed a multidisciplinary approach based on holistic biopsychosocial models of illness (23–25). A total of 61 patients were admitted to rehabilitation at least 4 weeks after diagnosis of acute COVID-19 infection. Twenty-five of the severe patients were admitted to inpatient rehabilitation directly from the acute hospitalization in a COVID-19 ward, after which they joined an outpatient post-COVID-19 customized rehabilitation programme. The remaining 36 patients were admitted directly to the outpatient programme.
Inpatient rehabilitation consisted of a 2-h daily session of physiotherapy and occupational therapy, 5 days a week. The outpatient programme consisted of a 3-h session twice a week. Both rehabilitation programmes included full evaluation and medical care from a rehabilitation professional and further examinations to rule out related treatable post-COVID-19 morbidities, such as pulmonary fibrosis, anaemia, hypothyroidism, glucose intolerance, cardiac arrhythmias, and cardiomyopathy. Patients with moderate-to-severe cognitive impairments were subjected to cerebral magnetic resonance imaging (MRI) with contrast, or computed tomography (CT) scan. Special attention was paid to tailoring the medical treatment for post-COVID-19 pain, insomnia, anxiety, and depression.
The programme consisted of the following activities: exercise that gradually increases cardio-respiratory work and pulmonary rehabilitation, including the active cycle of breathing techniques, torso stretches and mobility, and incentive spirometry. The Borg Rating of Perceived Exertion was used to measure physical activity intensity level at baseline, aiming to gradually achieve a goal of level 5–7. In the course of training, patients had access to a wall-mounted oxygen flow meter when their blood oxygen level dropped below 88%. If their oxygen level did not recover to 92% or more after 1 min rest, the intensity of physical activity was reduced. Muscle strengthening exercises were provided, especially for patients with post-ICU neuropathy or myopathy, and stretching for reduced range of motion for bedridden patients, who were sometimes in a prone position because of mechanical ventilation. Occupational therapy focused on functional daily living skills. Training and cognitive therapy included domain-specific compensatory strategy training and cognitive retraining for memory loss, inattention, and executive functions.
Speech therapy was provided for patients with hoarseness and dysphagia because of intubation or tracheostomy injury to the vocal cords. All patients underwent psychological evaluation for post-traumatic stress disorder, anxiety, or depression, and individualized neuropsychological treatment was provided accordingly. Patients were divided, based on matched characteristics, into support groups to generate group empathy and support based on identification.
Demographic data and acute COVID-19 infection parameters
Demographic data. Data on participants’ demographics and rehabilitation measures were extracted from hospital records. The data included age, sex, comorbidities, duration of acute hospitalization, duration of ventilation, and duration of rehabilitation.
World Health Organization Clinical Progression Scale. The WHO-CPS scale (26) evaluates the severity of acute SARS-CoV-2 infection. It ranges from 0 (uninfected) to 10 (dead). Values 1–9 are divided into ambulatory mild disease (1–3, differing by whether it is asymptomatic and whether it is independent), hospitalized moderate disease (4–5, differing by whether patients used oxygen by mask or nasal prongs), and hospitalized severe disease (6–9, differing based on whether patients received invasive or non-invasive mechanical ventilation, On levels of arterial oxygen partial pressure to fractional inspired oxygen ratio (pO2/FiO2) and Oxygen saturation to fraction of inspired oxygen ratio (SpO(2)/FiO(2)), and on the need for vasopressin, dialysis, or Extracorporeal membrane oxygenation (ECMO).
Measures at admission to rehabilitation
Montreal Cognitive Assessment. The MoCA (27) is a 30-point screening tool that assesses multiple cognitive domains. The suggested cut-off point is 26. Excellent internal consistency (Cronbach’s alpha = 0.78) was found in a stroke population (28).
Visual analogue scale. Participants indicated the perceived level of pain on a VAS (29) ranging from 0 (no pain) to 10 (worst imaginable pain). Excellent test-retest reliability (Interclass Correlation Coefficient (ICC) = 0.97)] and concurrent validity with other pain scale measurements (r = 0.87) were found in several populations (30, 31).
Fatigue Severity Scale. The FSS (32) is a 9-item self-report scale of effects of fatigue on daily functioning, motivation, physical activity, work, family, and social life. Participants were asked to rate how easily they become fatigued and the degree to which this posed a problem for them, on a 7-point Likert scale, ranging from 1 (completely disagree) to 7 (completely agree). Lower scores indicate less fatigue; the recommended cut-off for healthy individuals is 2.3 (33). Adequate internal consistency (Cronbach’s alpha = 0.86) was found in a stroke population (34).
Measures at admission and discharge from rehabilitation
Functional Independence Measure. The FIM assesses the basic quality of activities of daily living in persons with a disability, using 18 items grouped into motor and cognition subscales (35). Items are scored on a scale ranging from 1 (total assistance or not testable) to 7 (performs independently in a safe and timely manner). Higher scores reflect higher independence in activities of daily living (ADL). Excellent internal consistency (Cronbach’s alpha = 0.5) was found in a general rehabilitation population (36).
10-m walk test. The 10MWT assesses walking speed (m/s) over a 10-m distance (37). The Minimum Clinically Important Difference (MCID) for the geriatric and post-stroke population is 0.1 m/s. Excellent test-retest reliability (ICC = 0.95) (38) and concurrent validity with dependence in Instrumental activities of daily living (IADL) (r = 0.76) (39) was found in a stroke population.
6-min walk test. The 6MWT assesses endurance over a 6-min walk on a paved path (40). The path length is measured in m. All 24 patients who needed oxygen at admission to rehabilitation performed the 6 min test with oxygen, and their blood oxygen saturation level was measured at the beginning and end of the test. Blood Oxygen level cut-off was 88%. The MCID for the geriatric population is 50 m. Excellent test–retest reliability (r = 0.95) and adequate concurrent validity with chair stands (r = 0.67), gait speed (r = 0.73), and standing balance (r = 0.52) were found in a geriatric population (41).
Timed Up and Go test. The TUG examines balance in functional mobility: stand up, walk 3 m, turn, walk back, and sit down (42). Time correlates strongly with level of functional mobility. MCID in the geriatric population is 2.9 s. Excellent test–retest reliability (r = 0.958) and excellent concurrent validity with the Berg Balance Scale (r = –0.66) was found in a geriatric population (43).
Jamar dynamometer for both hands (44). The gold standard tool for hand-grip strength evaluations in the clinic and research is the Jamar dynamometer (45). The MCID for the stroke population is 1.04 and 1.27 kg for dominant and non-dominant hands, respectively (46). Excellent test–retest reliability (ICC = 0.85) was found in a stroke population (47), and excellent concurrent validity between dominant (ICC = 0.99) and non-dominant hand (ICC = 0.98) was found in healthy adults (48).
Box and Blocks Test. The BBT measures unilateral gross manual dexterity (49). Patients move blocks, 1 at a time, from 1 compartment of a box to another compartment of equal size in 60 s. Higher numbers of blocks moved indicate better manual dexterity. MCID for the stroke population is 5.5 blocks. Excellent test–retest reliability (ICC = 0.98) was found in this population (50).
Statistical analyses
Data were entered into a Microsoft Excel file (Microsoft, Redmond, WA, USA) and transferred to a statistical analysis programme (SPSS 26.0, Chicago, IL, USA). A paired sample t-test was used to compare performance measures at the beginning of rehabilitation and discharge. Pearson correlations were performed to examine the relationship between all the variables, and linear regression to examine which variables predicted the effectiveness of rehabilitation, as measured by total FIM and delta total FIM. No MCID data regarding COVID19- patients were found in the literature; therefore it was decided to use the MCID of stroke patients or geriatric patients as a reference point for all the tests.
RESULTS
Demographics and clinical characteristics of patients during acute illness and at admission to rehabilitation
The mean age of the 61 post-COVID-19 participants in the study was 54.1 (SD 15.3) years; 39.3% were over 60 years old; 66% were males; almost 40% had a history of diabetes mellitus; a significant percentage had a history of pulmonary disease or cardiac disturbances (Table I). Of the 61 patients, 80.3% were hospitalized during acute illness, and the mean duration of acute hospitalization was 5.5 (SD 4.2) weeks; 80.3% were treated with oxygen by mask/nasal prongs and 64% needed invasive mechanical ventilation for a mean of 17.4 (SD 19.6) days. Of the patients who were ventilated, 61.5% progressed to tracheostomy, 46.1% were treated with vasopressors, and 20.5% needed dialysis treatment or ECMO. Eleven percent of the cohort was diagnosed with deep vein thrombosis or pulmonary embolism. Patients diagnosed with stroke were excluded from the study. The severity of the initial infection according to the WHO-CPS was 6.3 (SD 2.4, range 2–9); more than 65% of the patients had severe disease (≥ 6).
Table I.
Demographics and clinical characteristics of 61 patients during acute illness
| Variable | n | % |
|---|---|---|
| Sex, male | 40 | 66 |
| Past medical history | ||
| Diabetes | 24 | 39.3 |
| Pulmonary diseases | 14 | 23.3 |
| Heart diseases | 7 | 11.5 |
| Ventilated | 24 | 39.3 |
| Mean WHO-CPS | ||
| Mild | 12 | 19.7 |
| Moderate | 9 | 14.7 |
| Severe | 40 | 65.6 |
| Needs oxygen | 25 | 43 |
| Critical illness myopathy | 40 | 65.6 |
| Critical illness polyneuropathy or other neuropathy | 26 | 44.3 |
| Reported pain | 37 | 61 |
| MoCA <26 | 36 | 62 |
WHO-CPS: World Health Organization Clinical Progression Scale; MoCA: Montreal Cognitive Assessment.
Mean duration from acute illness to rehabilitation was 3.7 (SD 2.3) months. The mean duration of the inpatient and outpatient rehabilitation programme was 3.2 (SD 2.1) months, and the mean follow-up time from acute illness to discharge was 7.2 (SD 3.2) months. At admission to rehabilitation, more than 40% of patients needed continuous oxygen therapy. 61.7% reported some pain, with a perceived level of 3.59 ± 3.3 on the VAS, and a median of 4. All patients reported fatigue levels above the recommended cut-off for the healthy population (2.3), with mean fatigue severity of 5.6 (SD 1.1) according to the FSS. The mean cognitive status, measured by MoCA, was 23.6 (SD 3.7), with more than 60% of patients below the normal recommended cut-off of 26.
Motor and functional rehabilitation outcomes
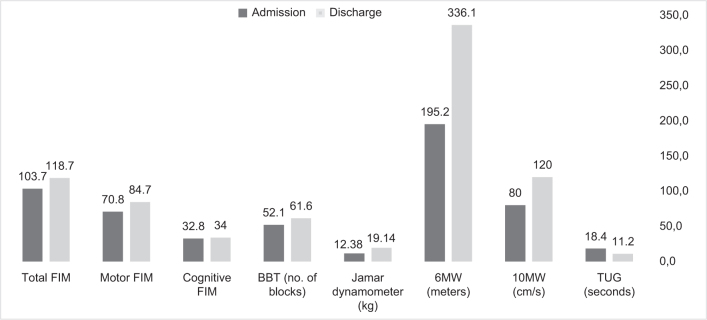
All functional variables improved significantly during the rehabilitation period (Fig. 1). Of the 61 participants, 88% were able to wean off oxygen completely at discharge. More than half improved beyond the MCID for BBT, dynamometer, 6MWT, 10MWT, and TUG. On other tests, although most participants did not reach the threshold of MCID, the overall difference between rehabilitation admission and discharge was statistically significant.
Fig. 1.
Comparison of motor and functional parameters at admission to rehabilitation and at discharge. Values are mean. FIM: Functional Independence Measure; BBT: Box and Blocks Test of both hands; Jamar dynamometer: hand grip force of both hands; 6MWT: 6-min walk test; 10MWT: 10-m walk test; TUG: Timed Up and Go test.
Correlations between parameters of acute infection and measurements at admission to rehabilitation
Significant correlations were found between WHO-CPS, duration of acute hospitalization and days of ventilation, and lower motor and total FIM at admission (Table II). The duration of acute hospitalization in weeks correlated with lower motor measurement scores, including BBT, dynamometer, 6MWT, and 10MWT. Higher WHO-CPS correlated with lower fatigue level (FSS) at admission. A significant correlation was found between duration of ventilation (in days) and lower pain level (VAS). No correlations were found between severity of disease or duration of hospitalization and days of ventilation and cognitive measurements at admission (MoCA and cognitive FIM).
Table II.
Correlation between World Health Organization Clinical Progression Scale (WHO-CPS), duration of acute hospitalization and ventilation, and measurements at admission to rehabilitation
| VAS | MoCA | FSS | Motor FIM | Cognitive FIM | Total FIM | BBT | Dynamometer | 6MWT | 10MWT | TUG | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO-CPS | –0.10 | –0.064 | –0.33 * | –0.47 ** | 0.056 | –0.45 ** | –0.27 | –0.19 | –0.24 | –0.25 | 0.23 |
| Weeks of acute hospitalization | –0.18 | 0.038 | –0.15 | –0.67 ** | –0.124 | –0.67 ** | –0.38 ** | –0.34 * | –0.41 ** | –0.35 * | 0.30* |
| Days of ventilation | –0.27 * | 0.099 | –0.03 | –0.47 ** | 0.115 | –0.44 ** | –0.31 * | –0.24 | –0.24 | –0.04 | 0.12 |
Pearson’s correlation coefficient.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
VAS: visual analogue scale; MoCA: Montreal Cognitive Assessment; FSS: Fatigue Severity Scale; FIM: Functional Independence Measure; BBT: Box and Blocks Test of both hands; Jamar dynamometer: hand grip force of both hands; 6MWT: 6-min walk test; 10MWT: 10-m walk test; TUG: Timed Up and Go test.
Correlations between parameters of acute infection and measurements at admission to rehabilitation and functional improvement at discharge
No correlation was found between total FIM at discharge and any of the parameters of acute infection and age, VAS, MoCA, or FSS (Table III). Significant correlations were found between change in total FIM and change in motor FIM and higher WHO-CPS, as well as longer duration of acute hospitalization and ventilation. Motor FIM at discharge correlated negatively with older age and positively with higher pain level (VAS) at admission. A significant correlation was found between MoCA and cognitive FIM at discharge, and no correlation was found between functional improvement (FIM at discharge and change in FIM) and fatigue level.
Table III.
Correlation between functional improvement during rehabilitation (measured by FIM at discharge, delta FIM, and motor parameters at discharge) and severity of acute illness (measured by WHO-CPS and duration of acute hospitalization and ventilation)
| Admission Discharge | Age | CPS | Weeks of acute hospitalization | Days of ventilation | VAS | MoCA | FSS |
|---|---|---|---|---|---|---|---|
| Length of rehabilitation | –0.16 | 0.11 | 0.28 * | 0.15 | 0.29 * | –0.04 | –0.04 |
| Cognitive FIM | 0.13 | 0.24 | 0.19 | 0.19 | –0.20 | 0.43 ** | –0.07 |
| Motor FIM | –0.26 * | –0.19 | –0.21 | –0.08 | 0.27 * | 0.1 | 0.18 |
| Total FIM | –0.23 | –0.14 | –0.17 | –0.04 | 0.23 | 0.22 | 0.17 |
| Delta cognitive FIM | –0.22 | 0.08 | 0.25 * | –0.02 | –0.01 | –0.04 | 0.02 |
| Delta motor FIM | 0.04 | 0.46 ** | 0.68 ** | 0.50 ** | –0.03 | 0.22 | 0.10 |
| Delta total FIM | 0.00 | 0.45 ** | 0.68 ** | 0.47 ** | –0.03 | 0.20 | 0.10 |
| BBT | –0.34 * | –0.24 | –0.30 * | –0.16 | 0.10 | 0.26 | 0.02 |
| Dynamometer | –0,19 | –0.00 | –0.14 | –0.07 | 0.045 | 0.22 | –0.23 |
| 6MW | –0.43 * | 0.16 | –0.18 | –0.02 | 0.03 | 0.21 | 0.02 |
| 10MW | –0.28 * | 0.02 | 0.04 | 0.25 | 0.03 | 0.17 | 0.06 |
| TUG | 0.29 * | 0.10 | 0.12 | –0.05 | –0.08 | 0.0 | –0.09 |
Pearson's correlation coefficient.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
WHO-CPS: World Health Organization Clinical Progression Scale. VAS: Visual Analogue Scale. MoCA: Montreal Cognitive Assessment. FSS: Fatigue Severity Scale. FIM: Functional Independence Measure. BBT: Box and Blocks of both hands. Jamar dynamometer: hand grip force of both hands. 6MW: six-minute walk test. 10MW: 10-meter walk test. TUG: Time Up and Go.
Linear regression to predict functional improvement
Linear regression was conducted to predict functional improvement after rehabilitation. The predicted variables were total FIM and change in total FIM. Constant variables included in the regression were age, sex, duration of acute hospitalization in weeks, days of ventilation, WHO-CPS, MoCA, FSS, and history of diabetes. The model was not significant in explaining the variance in total FIM. It explained 69% of the variance in change in total FIM, and was significant at a level of 0.00. Duration of acute hospitalization and history of diabetes (data not shown) contributed significantly to the model.
DISCUSSION
This study evaluated the correlation between parameters of acute COVID-19 infection and the functional outcome of patients who underwent inpatient and outpatient rehabilitation. Over the course of 3 months of rehabilitation, all motor and cognitive parameters improved significantly, and most patients were able to wean off oxygen. There was a significant correlation between the severity of the acute illness and lower functional and motor levels at admission to rehabilitation, but no correlation between these parameters and functional level at discharge. Patients who had higher severity of acute disease and started the rehabilitation with lower functional and motor levels reached the same functional level at discharge as patients with less severe disease. Patients who had higher severity of acute disease reported lower levels of fatigue and pain during rehabilitation.
Many studies have discussed the need for early inpatient rehabilitation for COVID-19 survivors (51–53), investigating mostly the effect of short-term and acute rehabilitation programmes on mobility and independence in ADL. Patel et al. (54) showed that an interdisciplinary rehabilitation programme of 106 post-COVID-19 patients with an a mean length of stay of 17 days improved ambulatory distance and the percentage of the patients who were able to breathe room air. Similar to the results of the current study, greater functional improvement was associated with younger age and longer intubation duration. Vickory et al. (55) showed that a short inpatient rehabilitation programme improved the functional status of 30 severe post-COVID-19 patients. Similarly, the current study demonstrated that such a programme can improve mobility and limitations in ADL, and increase walking capacity and pulmonary function.
This study found that patients who spent a longer time in acute hospitalization had lower functional levels at admission to rehabilitation, but not at discharge, and needed longer rehabilitation to reach similar functional independence to that of patients who spent less time in acute hospitalization. Similarly, a study comparing the rehabilitation outcomes of 43 COVID-19 patients with 247 non-COVID-19 patients found that COVID-19 patients had greater deficits at admission, but eventually reached similar functional outcomes as non-COVID-19 patients (56), possibly because more severe disease and longer time in intensive care, or more ventilating days caused severe muscle wasting, weakness, and other complications. But when these patients arrived at rehabilitation, they showed good potential to improve and reach functional independence at discharge if they were given appropriate and extensive rehabilitation. A study in Brazil also found that duration of treatment correlated positively with improvement in FIM scores (57).
The current study is one of a few that have reported on long-term outcomes (over 6 months) of post-COVID-19 patients who have undergone acute inpatient and outpatient rehabilitation. In Spain, FIM scores were measured before and after 2 months of outpatient rehabilitation for COVID-19 patients (n = 43) (12). The study showed a significant improvement of 4 points in motor FIM, whereas in the current study the delta motor FIM was 13.9 points after 3.2 months of rehabilitation. These findings emphasize the importance of long-term rehabilitation and follow-up of post-COVID-19 patients.
Patients with more severe illness reported lower levels of fatigue and pain in the current study, and no correlations were found between severity of disease or duration of hospitalization and days of ventilation and cognitive measurements, such as MoCA, and cognitive FIM at admission and discharge from rehabilitation. Similarly, in a study conducted in Italy with 87 post-COVID-19 patients, the cognitive status of patients who underwent sedation and ventilation was less compromised (58), possibly because cognitive evaluation may have been influenced by emotional status and higher level of pain.
Study limitations
The current study has several limitations. The number of participants was relatively small, although larger than in previous studies. There was no control group of patients who received no rehabilitation, but because at least 65% of the patients had the highest severity of the disease in at least 65% of the patients, we considered it unethical not to provide them with the optimal rehabilitation programme we could. Compared with other studies, which included only patients with mild disease, in the current study the majority of patients were coping with severe illness. Despite the lack of a control group, our data compared 2 time-points in the course of COVID-19 infections, at the acute disease stage and during rehabilitation, and also compared between mild and severe cases of COVID-19. Finally, this study did not measure the levels of pain, fatigue, and MoCA at discharge from rehabilitation.
CONCLUSION
The severity of acute COVID-19 infection affects the functional status of survivors at admission to rehabilitation, but, contrary to expectation, not at discharge. Even patients recovering from severe COVID-19 improved their functional ability and participation if they spent an appropriate period in rehabilitation. These findings show that even patients with severe acute COVID-19 infection may improve their daily functioning significantly during rehabilitation program.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Datta SD, Talwar A, Lee JT. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA 2020; 324: 2251–2252. 10.1001/jama.2020.22717 [DOI] [PubMed] [Google Scholar]
- 2.Prescott HC, Girard TD. Recovery from severe COVID-19: leveraging the lessons of survival from sepsis. JAMA 2020; 324: 739–740. 10.1001/jama.2020.14103 [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26: 1017–1032. 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serafim RB, Póvoa P, Souza-Dantas V, Kalil AC, Salluh JIF. Clinical course and outcomes of critically ill patients with COVID-19 infection: a systematic review. Clin Microbiol Infect 2021; 27: 47–54. 10.1016/j.cmi.2020.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daste C, Ficarra S, Dumitrache A, Cariou A, Lefèbvre A, Pène F, et al. Post-intensive care syndrome in patients surviving COVID-19. Ann Phys Rehabil Med 2021; 64: 101549. 10.1016/j.rehab.2021.101549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 2022; 19: 269. 10.3390/pathogens11020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips M, Turner-Stokes L, Wade D, Walton K. Rehabilitation in the wake of Covid-19-a Phoenix from the ashes. British Society of Rehabilitation Medicine. 2020;1:1–20. [Google Scholar]
- 8.Chartered Society of Physiotherapy Rehabilitation and Covid-19 – CSP policy statement. CSP, 2020. [Accessed 4 May 2020]. https://www.csp.org.uk/professional-clinical/improvement-innovation/community-rehabilitation/rehab-covid-19-policy-statement. [Google Scholar]
- 9.Daynes E, Gerlis C, Chaplin E, Gardiner N, Singh SJ. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition – a cohort study. Chron Respir Dis 2021; 18: 14799731211015691. 10.1177/14799731211015691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein J, Visco CJ, Barbuto S:. Rehabilitation medicine response to the COVID-19 pandemic. Am J Phys Med Rehabil 2020; 99: 573–579. 10.1097/PHM.0000000000001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korupolu R, Francisco GE, Levin H, Needham DM. Rehabilitation of critically ill COVID-19 survivors. J Int Soc Phys Rehabil Med 2020; 3: 45. 10.4103/jisprm.jisprm_8_20 [DOI] [Google Scholar]
- 12.Tay MRJ, Ong PL, Puah SH, Acute functional outcomes in critically ill COVID-19 patients. Front Med 2021; 7: 615–997. 10.3389/fmed.2020.615997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albu S, Rivas Zozaya N, Murillo N, García-Molina A, Figueroa Chacón CA, Kumru H. Multidisciplinary outpatient rehabilitation of physical and neurological sequelae and persistent symptoms of covid-19: a prospective, observational cohort study. Disabil Rehabil 2021; 24: 1–8. 10.1080/09638288.2021.1977398. [DOI] [PubMed] [Google Scholar]
- 14.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021; 18: e1003773. 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zorowitz RD. ICU-acquired weakness: a rehabilitation perspective of diagnosis, treatment, and functional management. Chest 2016; 150: 966–971. 10.1016/j.chest.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM, et al. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med 2007; 33:1876–1891. 10.1007/s00134-007-0772-2 [DOI] [PubMed] [Google Scholar]
- 17.Herridge MS, Moss M, Hough CL, Hopkins RO, Rice TW, Bienvenu OJ, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med 2016; 42: 725–738. 10.1007/s00134-016-4321-8 [DOI] [PubMed] [Google Scholar]
- 18.Schweickert WD, Hall J. ICU-acquired weakness. Chest 2007; 131: 1541–1549. 10.1378/chest.06-2065 [DOI] [PubMed] [Google Scholar]
- 19.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304: 1787–1794. 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikkelsen ME, Lanken PE, Biester R, et al. Conservative fluide strategy is associated with neurocognitive deficits in survivors of acute lung injury. Paper presented at: American Thoracic Society; May 21, 2008; Toronto, ON. [Google Scholar]
- 21.Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA 2004; 292: 2901–2908. 10.1001/jama.292.23.2901 [DOI] [PubMed] [Google Scholar]
- 22.Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol 2005; 4: 371–380. 10.1016/S1474-4422(05)70099-5 [DOI] [PubMed] [Google Scholar]
- 23.Wade DT, Halligan PW. The biopsychosocial model of illness: a model whose time has come. Clin Rehabil 2017; 31:995–1004. 10.1177/0269215517709890 [DOI] [PubMed] [Google Scholar]
- 24.Wade DT. A teamwork approach to neurological rehabilitation. In: Dietz V, Ward NS, editors. Oxford textbook on neurorehabilitation. Oxford: Oxford University Press; 2020, p. 9–22. [Google Scholar]
- 25.Rehabilitation for patients in the acute care pathway following severe disabling illness or injury: BSRM core standards for specialist rehabilitation. London: British Society of Rehabilitation Medicine; 2014. [Google Scholar]
- 26.Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20: 192–197. 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 28.Toglia J, Fitzgerald KA, O’Dell MW, Mastrogiovanni AR, Lin CD. The Mini-Mental State Examination and Montreal Cognitive Assessment in persons with mild subacute stroke: relationship to functional outcome. Arch Phys Rehabil Med 2011; 92: 792–798. 10.1016/j.apmr.2010.12.034 [DOI] [PubMed] [Google Scholar]
- 29.Carlsson, AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983; 16: 87–101. 10.1016/0304-3959(83)90088-X [DOI] [PubMed] [Google Scholar]
- 30.Boonstra AM, Schiphorst Preuper HR, Reneman MF, Posthumus JB, Stewart RE. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res 2008; 31:165–169. 10.1097/MRR.0b013e3282fc0f93 [DOI] [PubMed] [Google Scholar]
- 31.Alghadir AH, Anwer S, Iqbal A, Iqbal ZA. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res 2018; 11: 851–856. 10.2147/JPR.S158847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurology 1989; 46: 1121–1123. 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 33.Grace J, Mendelsohn A, Friedman JHA. Comparison of fatigue measures in Parkinson’s disease. Parkinsonism Relat Disord 2007; 13: 443–445. 10.1016/j.parkreldis.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 34.Lerdal A, Kottorp A. Psychometric properties of the Fatigue Severity Scale – Rasch analyses of individual responses in a Norwegian stroke cohort. Int J Nurs Stud 2011; 48: 1258–1265. 10.1016/j.ijnurstu.2011.02.019 [DOI] [PubMed] [Google Scholar]
- 35.Ottenbacher KJ., Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil 1996; 77: 1226–1232. 10.1016/S0003-9993(96)90184-7 [DOI] [PubMed] [Google Scholar]
- 36.Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil 1993; 74: 531–536. 10.1016/0003-9993(93)90119-U [DOI] [PubMed] [Google Scholar]
- 37.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006; 54: 743–749. 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 38.Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. Int Disabil Stud 1990; 12: 6–9. 10.3109/03790799009166594 [DOI] [PubMed] [Google Scholar]
- 39.Tyson S, Connell L. The psychometric properties and clinical utility of measures of walking and mobility in neurological conditions: a systematic review. Clin Rehabil 2009; 23: 1018–1033. 10.1177/0269215509339004. [DOI] [PubMed] [Google Scholar]
- 40.Flansbjer UB, Holmbäck AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med 2005; 37: 75–82. 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 41.Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil 1999; 80: 837–841. 10.1016/s0003-9993(99)90236-8. [DOI] [PubMed] [Google Scholar]
- 42.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther 2000; 80: 896–903. 10.1093/ptj/80.9.896 [DOI] [PubMed] [Google Scholar]
- 43.Hofheinz M, Schusterschitz C. Dual task interference in estimating the risk of falls and measuring change: a comparative, psychometric study of four measurements. Clin Rehabil 2010; 24: 831–842. 10.1177/0269215510367993 [DOI] [PubMed] [Google Scholar]
- 44.Mathiowetz V. Comparison of Rolyan and Jamar dynamometers for measuring grip strength. Occup Ther Int 2002; 9: 201–209. 10.1002/oti.165 [DOI] [PubMed] [Google Scholar]
- 45.Werle S, Goldhahns J, Drerup S, Simmen BR, Sprott, H, Herren, DB. Age- and gender-specific normative data of grip and pinch strength in a healthy adult Swiss population. J Hand Surg Eur 2009; 34: 76–84. 10.1177/1753193408096763 [DOI] [PubMed] [Google Scholar]
- 46.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil 2008; 89: 1693–1700. 10.1016/j.apmr.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertrand AM, Mercier C, Bourbonnais D, Desrosiers J, Gravel D. Reliability of maximal static strength measurements of the arms in subjects with hemiparesis. Clin Rehabil 2007; 21: 248–257. 10.1177/0269215506070792. [DOI] [PubMed] [Google Scholar]
- 48.Bellace JV, Healy D, Besser MP, Byron T, Hohman L. Validity of the Dexter Evaluation System’s Jamar dynamometer attachment for assessment of hand grip strength in a normal population. J Hand Ther 2000; 13: 46–51. 10.1016/s0894-1130(00)80052-6. [DOI] [PubMed] [Google Scholar]
- 49.Mathiowetz VG, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther 1985; 39: 386–391. [DOI] [PubMed] [Google Scholar]
- 50.Chen HM, Chen CC, Hsueh IP, Huang SL, Hsieh CL. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair 2009; 23: 435–440. 10.1177/1545968308331146 [DOI] [PubMed] [Google Scholar]
- 51.Puchner B, Sahanic S, Kirchmair R, Pizzini A, Sonnweber B, Wöll E, et al. Beneficial effects of multi-disciplinary rehabilitation in postacute COVID-19: an observational cohort study. Eur J Phys Rehabil Med 2021; 57: 189–198. 10.23736/S1973-9087.21.06549-7 [DOI] [PubMed] [Google Scholar]
- 52.Hameed F, Palatulan E, Jaywant A, Said R, Lau C, Sood V, et al. Outcomes of a COVID-19 recovery program for patients hospitalized with SARS-CoV-2 infection in New York City: a prospective cohort study. PM R 2021; 13: 609–617. 10.1002/pmrj.12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groah S, Pham C, Rounds A, Semel J. COVID patients benefit from inpatient rehabilitation. The Lancet is part of SSRN 2021; 3751322. 10.2139/ssrn.3751322 [DOI] [Google Scholar]
- 54.Patel N, Steinberg C, Patel R, Chomali C, Doulatani G, Lindsay L, et al. Description and functional outcomes of a novel interdisciplinary rehabilitation program for hospitalized patients with COVID-19. Am J Phys Med Rehabil 2021; 100: 1124. 10.1097/PHM.0000000000001897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vickory F, Ridgeway K, Falvey J, Houwer B, Gunlikson J, Payne K, et al. Safety, feasibility, and outcomes of frequent, long-duration rehabilitation in an inpatient rehabilitation facility after prolonged hospitalization for severe COVID-19: an observational study. Phys Ther 2021; 101: 208. 10.1093/ptj/pzab208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abramoff BA, Dillingham TR, Caldera FE, Ritchie MD, Pezzin LE. Inpatient rehabilitation outcomes after severe COVID-19 infections: a retrospective cohort study. Am J Phys Med Rehabil 2021; 100: 1109–1114. 10.1097/PHM.0000000000001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imamura M, Mirisola AR, Pretto LR, De Pretto LR, Alfieri FM, Delgado VR, et al. Rehabilitation of patients after COVID-19 recovery: an experience at the Physical and Rehabilitation Medicine Institute and Lucy Montoro Rehabilitation Institute. Clinics 2021; 76: e2804. 10.6061/clinics/2021/e2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alemanno F, Houdayer E, Parma A, Spina A, Del Forno A, Scatolini A, et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One 2021; 16: e0246590. 10.1371/journal.pone.0246590 [DOI] [PMC free article] [PubMed] [Google Scholar]